Recent advances in gut microbiota-mediated regulation of fat deposition and metabolic disorders
Abstract
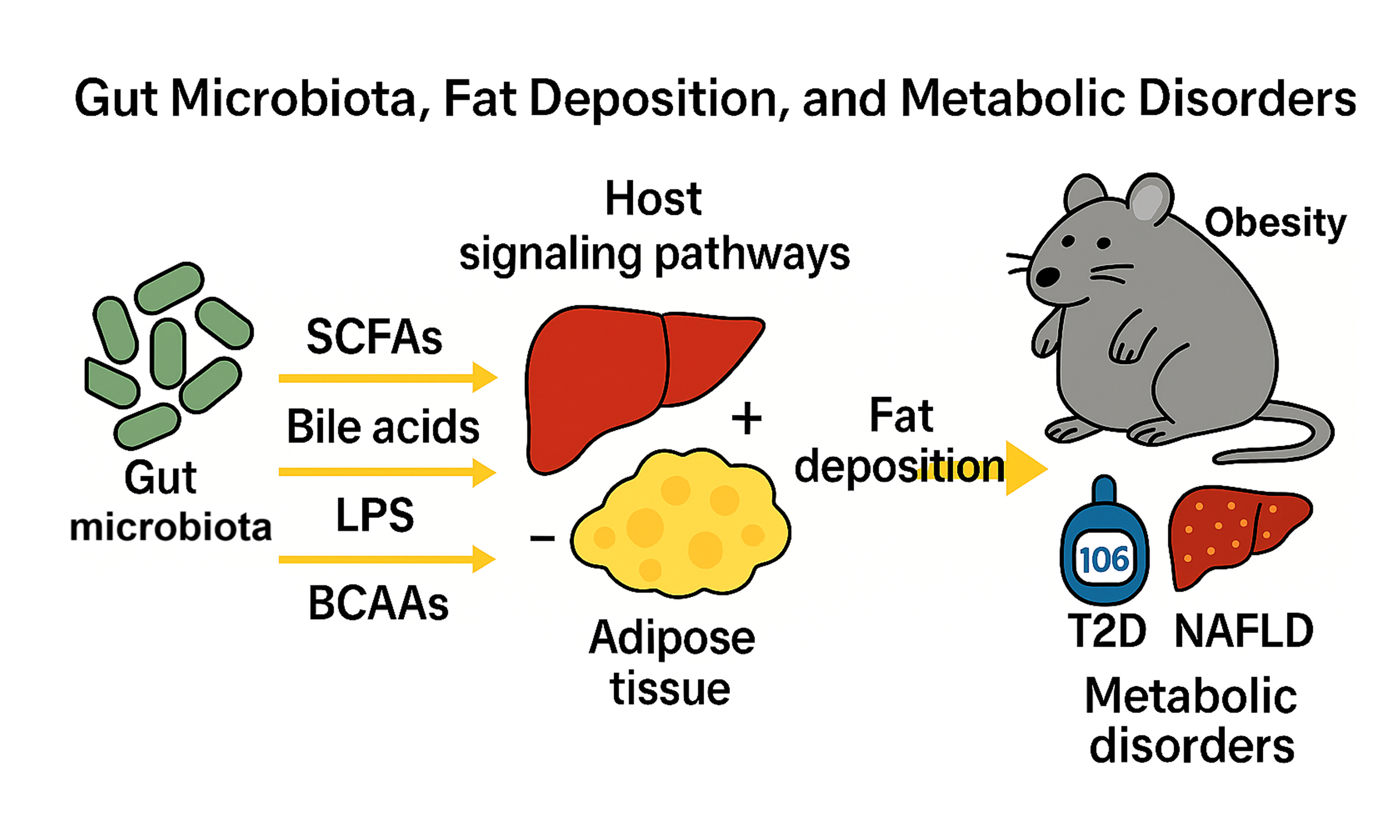
The gut microbiota critically regulates lipid metabolism through microbial metabolites and host signaling pathways. Short-chain fatty acids (SCFAs), derived from dietary fiber fermentation, suppress hepatic lipogenesis via inhibition of SREBP-1c and enhance mitochondrial β-oxidation through GPR41/43 activation. Microbial enzymes convert primary bile acids into secondary bile acids, which activate FXR to inhibit lipogenesis and TGR5 to promote adipose thermogenesis. Lipopolysaccharide (LPS) from dysbiotic microbiota triggers TLR4-NF-κB signaling, exacerbating insulin resistance and adipose inflammation. Branched-chain amino acids (BCAAs), metabolized by gut microbes, drive adipogenesis via mTORC1-PPARγ signaling, with elevated circulating BCAAs linked to obesity. In livestock, microbiota modulation optimizes fat deposition: probiotics in pigs enhance intramuscular fat via Lactobacillus-enriched communities, while dietary succinate or coated sodium propionate reduces abdominal fat in broilers by reshaping cecal microbiota. Fecal microbiota transplantation confirms microbial causality in transferring fat phenotypes. Dysbiosis-associated mechanisms are conserved across species, where SCFAs and bile acids ameliorate metabolic inflammation, whereas LPS and BCAA imbalances worsen lipid dysregulation. Metabolic disorders, including obesity, type 2 diabetes (T2D), and non-alcoholic fatty liver disease (NAFLD), are tightly linked to gut microbiota perturbations. Dysbiosis drives LPS translocation and barrier impairment. These changes, along with altered metabolites, promote inflammation and fat deposition. Future strategies should integrate multi-omics and precision engineering of microbial consortia to advance therapies for both livestock and human metabolic health.
Keywords
INTRODUCTION
The gut microbiota, a dynamic ecosystem of trillions of bacteria, archaea, viruses, and fungi, orchestrates host metabolism and energy homeostasis through its expansive metabolic repertoire[1,2]. Dysbiosis, characterized by shifts in community composition and function, has been strongly linked to aberrant fat deposition and metabolic disorders such as obesity, type 2 diabetes (T2D), and non-alcoholic fatty liver disease (NAFLD)[3]. Emerging evidence highlights the gut-liver axis, microbial metabolites including short-chain fatty acids (SCFAs), bile acids, and lipopolysaccharide (LPS), and immune crosstalk as central mediators of lipid metabolism and inflammation[4,5]. Germ-free and antibiotic-treated murine models have been foundational: colonizing adult germ-free C57BL/6 mice with conventional microbiota induces up to a 60% increase in body fat and insulin resistance within two weeks despite reduced caloric intake, establishing a causal link between microbes and fat deposition[1]. Mechanistic studies reveal that gut microbes enhance monosaccharide absorption and de novo hepatic lipogenesis via suppression of fasting-induced adipose factor and increased adipocyte lipoprotein lipase (LPL) activity[6]. Moreover, fecal microbiota transplantation (FMT) from obese donors transfers adiposity to germ-free or antibiotic-treated recipients, while lean donor FMT protects against high-fat-diet-induced weight gain[1,7]. An elevated Firmicutes/Bacteroidetes ratio commonly observed in obese states correlates with enhanced caloric extraction from complex polysaccharides and increased fat mass in both mice and humans[3].
Concurrently, livestock research has harnessed microbiota manipulation to optimize fat deposition for meat quality without compromising growth performance[8]. In pigs, dietary interventions such as L-glutamate supplementation in Shaziling breeds and targeted probiotic administration have increased intramuscular fat (IMF) and modulated backfat thickness through specific shifts in microbial taxa[9]. In broilers, succinate and coated sodium propionate reshape cecal communities to reduce abdominal fat and enhance carcass traits[10,11]. Furthermore, dietary components (e.g., fibers, probiotics) directly shape microbial composition and function, establishing diet as a primary modulator of microbiota-mediated lipid metabolism across species[12,13]. Together, these integrative findings from murine and agricultural models underscore the translational potential of microbiota-based interventions for controlling fat deposition and mitigating metabolic disorders across species[14].
MICROBIAL COMPOSITION AND FAT DEPOSITION
Obesity-associated dysbiosis in murine models
Obese murine models, including genetically obese ob/ob mice and those fed a high-fat diet (HFD), consistently exhibit an elevated Firmicutes/Bacteroidetes ratio compared to their lean counterparts, suggesting that this microbial shift enhances dietary energy harvest and promotes lipogenesis[15,16]. In particular, the expansion of Firmicutes observed in ob/ob mice is associated with increased production of SCFAs, such as acetate, propionate, and butyrate, which activate G protein-coupled receptors (GPCRs) GPR41 and GPR43 on adipocytes[17]. This activation contributes to adipocyte hypertrophy and greater fat storage by influencing host lipid metabolism and hormonal regulation[13,18]. On the other hand, dietary supplementation with fermentable fibers such as resistant starch and inulin has been shown to remodel the gut microbiota toward SCFA-producing Bacteroidetes, resulting in decreased fat mass and improved glucose tolerance in obese mice[19,20], thus indicating a reversible aspect of diet-induced dysbiosis. Further supporting the causal role of the microbiota in fat deposition, studies using germ-free C57BL/6 mice, which lack all microbial colonization, have shown that these mice accumulate approximately 40% less total body fat compared to conventional mice, despite consuming more calories[1]. This finding implies that the presence of gut microbiota facilitates more efficient extraction of dietary energy and promotes fat accumulation[18].
Similarly, antibiotic treatment of conventional mice leads to reduced weight gain under HFD conditions, and the recolonization of germ-free or antibiotic-treated mice with microbiota derived from either obese or lean donors successfully transfers the corresponding adiposity phenotypes to the recipients, thereby establishing a direct causal link between gut microbial composition and host fat accumulation[18,21]. FMT experiments provide additional evidence for this causality: germ-free or antibiotic-treated mice that receive fecal material from obese donors develop increased white adipose tissue mass, enlarged adipocytes, and impaired insulin sensitivity[1,7]. In contrast, transplantation with microbiota from lean donors protects mice from HFD-induced obesity and its associated metabolic disturbances[18,21]. These observations highlight the critical influence of microbiota-derived signals on systemic energy metabolism. Beyond the structural composition of the gut microbiota, specific microbial metabolites serve as key effectors that modulate host lipid metabolism via diverse signaling pathways. Acetate, one of the primary SCFAs, acts as a substrate for hepatic de novo lipogenesis, whereas propionate has been found to inhibit hepatic cholesterol synthesis. Butyrate, in turn, plays a distinct role by enhancing mitochondrial function in brown adipose tissue, thereby promoting fatty acid oxidation and thermogenesis, which contributes to increased energy expenditure[13,22]. Collectively, the findings from these murine studies illustrate a complex and robust mechanistic framework in which dysbiosis, characterized by an elevated Firmicutes/Bacteroidetes ratio, increased SCFA production, and shifts in microbial metabolic output, directly contributes to pathological fat accumulation and metabolic dysfunction. This body of evidence underscores the gut microbiota’s essential role in regulating fat deposition and its potential as a therapeutic target for obesity and related metabolic disorders.
Specific microbiota in pigs and chickens
Distinct pig breeds harbor characteristic gut microbiota compositions that are closely associated with breed-specific fat deposition traits[23,24]. For instance, the Chinese indigenous Ningxiang pig, known for its high IMF content, possesses a cecal microbiota enriched in Lactobacillus and branched-chain amino acid (BCAA) metabolic pathways[25]. Transplantation of Lactobacillus reuteri isolated from Ningxiang pigs into lean Duroc × Landrace × Yorkshire (DLY) pigs or rats significantly increases circulating BCAA levels and IMF accumulation, highlighting a causal role for this microbial consortium in lipid deposition[23,25]. Moreover, FMT from obese Ningxiang pigs into lean DLY pigs remodels the recipient’s gut microbial community and downregulates the expression of the carnitine transporter SLC22A5 in skeletal muscle, thereby reducing fatty acid oxidation and promoting lipid accumulation[23]. Comparative analyses further reveal that native breeds such as Laiwu or Tibetan pigs display higher microbial diversity and unique taxa enriched in lipid-associated pathways compared to commercial breeds (e.g., Duroc, Landrace, Large White), which tend to harbor gut communities dominated by carbohydrate-fermenting bacteria (e.g., Clostridium, Catenibacterium) and methanogens - features that may underlie differences in energy utilization efficiency and backfat thickness[26,27]. Additionally, gut microbiota composition undergoes dynamic shifts across developmental stages, with lactating piglets dominated by lactic acid bacteria and Bifidobacterium, while post-weaning stages show a rapid increase in fiber-degrading genera such as Prevotella and Roseburia, which in turn modulate host lipid metabolism[28,29]. Importantly, gut microbiota appears to regulate fat deposition in a depot-specific manner; certain microbial taxa and metabolites (e.g., SCFAs) influence subcutaneous fat (e.g., backfat thickness) and IMF differently, through modulation of host lipid metabolism genes such as LPL and ANGPTL4[29]. For example, obese-type pigs exhibit elevated LPL expression and reduced ANGPTL4 levels in muscle, promoting IMF accumulation, while Lactobacillus reuteri from Ningxiang pigs modulates SLC22A5-mediated carnitine transport to enhance IMF specifically[23,29]. Together, these findings underscore the breed-, stage-, and depot-specific roles of the gut microbiota in regulating porcine fat deposition and support the development of precision microbiota-targeted strategies in swine production.
Emerging evidence from poultry research underscores the pivotal role of gut microbiota in modulating fat deposition, largely independent of host genetic background [Table 1]. For instance, specific microbial taxa, including Methanobrevibacter and Mucispirillum schaedleri, exhibit significant correlations with adipose tissue accumulation in chickens[30]. Further investigations reveal that cecal microbiota may modulate abdominal fat deposition through lipid metabolism pathways. Notably, the relative abundance of Parabacteroides, Parasutterella, Oscillibacter, and Anaerofustis shows a positive association with fat deposition, whereas Sphaerochaeta demonstrates an inverse relationship[31]. Additionally, age-dependent dynamics in abdominal fat development correlate with shifts in gut microbiota composition. Studies indicate that Coprobacillus, Shigella, and Butyricicoccus are negatively associated with propionic acid, butyric acid, and abdominal fat mass but positively correlate with isobutyric acid levels[32]. In broilers, dietary succinate (0.4%) reduced abdominal fat deposition by enriching beneficial cecal microbes (e.g., Blautia, Sellimonas) and altering amino acid metabolism linked to lipid handling[10]. Coated sodium propionate supplementation similarly inhibited fat deposition and reduced feed intake, accompanied by decreased adipocyte size and modulation of gut microflora, highlighting the role of propionate as a microbiota-mediated feed additive[11]. Dietary folic acid at 13 mg/kg decreased abdominal fat and increased SCFA-producing taxa, suggesting that vitamin-microbiota synergy can fine-tune carcass composition in broilers[33]. Dietary fiber treatment reduced abdominal fat and altered gut microbiota in yellow-feathered broilers fed corncob meal, decreasing Phascolarctobacterium, Rikenellaceae, and Faecalibacterium while increasing Akkermansia[12]. Studies utilizing FMT demonstrated that folic acid supplementation mitigates abdominal adipose accumulation in broilers, a process potentially mediated by gut microbial shifts. LEfSe analysis identified Lactobacillus, Clostridium, and Dehalobacterium as dominant taxa in the folic acid-treated group, suggesting their role in this regulatory mechanism[34]. Furthermore, dietary inclusion of fermented grape seed meal enhances broiler growth performance while suppressing abdominal fat deposition, likely via modulation of intestinal microbial communities[35]. In parallel, phytosterol supplementation alters gut microbiota composition in broilers, characterized by reduced bacterial alpha diversity and a marked increase in probiotic populations such as Lactobacillus within intestinal digesta[36]. In addition, correlation analysis revealed that many Firmicutes members had a highly positive relationship with blood lipid levels and fat storage capacity, which might contribute to the lower abdominal fat phenotype[37-39]. These findings collectively demonstrate that targeted modulation of gut microbiota through diet or microbial interventions offers a promising strategy for controlling fat accumulation in broilers.
Microbial taxa reported to affect abdominal fat deposition in chickens in the past five years
| Influence factors | Sampling ages and breeds | Phenotype | Related gut microbial taxa | Ref. |
| Succinate | 21-day-old yellow-feathered broiler | Abdominal fat deposition | Blautia and Sellimonas | Wang et al. (2024)[10] |
| Coated sodium propionate | 42-day-old broiler | Alistipes, Lactobacillus, Bifidobacterium, Lachnospiraceae and Helicobacter | Wang et al. (2021)[11] | |
| Corncob meal | 135-day-old yellow-feathered broiler | Akkermansia, Phascolarctobacterium, Rikenellaceae, Faecalibacterium | Cui et al. (2022)[12] | |
| Host genetics | 78-day-old yellow-feathered broiler | Methanobrevibacter, Mucispirillum schaedleri | Wen et al. (2019)[30] | |
| Higher and lower abdominal fat | 1, 4, and 12 months of age turpan cockfighting × white leghorn | Sphaerochaeta, Parabacteroides, Parasutterella, Oscillibacter, Anaerofustis | Chen et al. (2023)[31] | |
| Age-associated changes | 14, 28, and 42-day-old broiler | Coprobacillus, Shigella, Butyricicoccus | Liu et al. (2023)[32] | |
| Folic acid | 28-day-old broiler | Alistipes, Oscillospira, Ruminococcus, Clostridium, Dehalobacterium, Parabacteroides | Liu et al. (2023)[33] | |
| Folic acid | 28-day-old broiler | Lactobacillus, Clostridium, Dehalobacterium | Liu et al. (2024)[34] | |
| Fermented grape seed meal | 56-day-old yellow-feathered broiler | Bacteroidetes, Firmicutes | Nan et al. (2022)[35] | |
| Phytosterols | 42-day-old broiler | Lactobacillus | Dai et al. (2023)[36] | |
| Corn resistant starch | 21-day-old broiler | Bacteroidetes, Firmicutes | Zhang et al. (2020)[37] | |
| Anoectochilus roxburghii extract | 63-day-old yellow-feathered broiler | Bacteroidetes, Firmicutes | Wu et al. (2023)[38] | |
| Echinocystic acid | 1-day-old K901 broiler | Bacteroidetes, Firmicutes | Xiao et al. (2025)[39] | |
| Lactococcus G423 | 21, and 42-day-old broiler | Lactobacillus, Firmicutes | Wang et al. (2024)[40] | |
| Lean- and fat-line broilers | 49-day-old broiler | Escherichia coli, Candidatus Acetothermia bacterium, Alistipes sp, Ruminococcaceae bacterium, Clostridiales bacterium, Anaeromassilibacillus sp. | Jing et al. (2021)[41] | |
| Obese and lean chickens | 160-day-old broiler (shouguang, luqin) | Erysipelatoclostridium | Liu et al. (2022)[42] | |
| Two native breeds | 42-day-old AA and 82-day-old beijing-you broiler | Lactobacillus | Lei et al. (2022)[43] | |
| Different abdominal fat deposition | 125-day-old tiannong partridge chicken | Bacteroidetes, Firmicutes, Parabacteroides, B. salanitronis, B. fragilis, P. distasonis, Olsenella, Slackia, Methanobrevibacter | Xiang et al. (2021)[44] |
MECHANISMS OF MICROBIOTA-MEDIATED LIPID METABOLISM
SCFAs
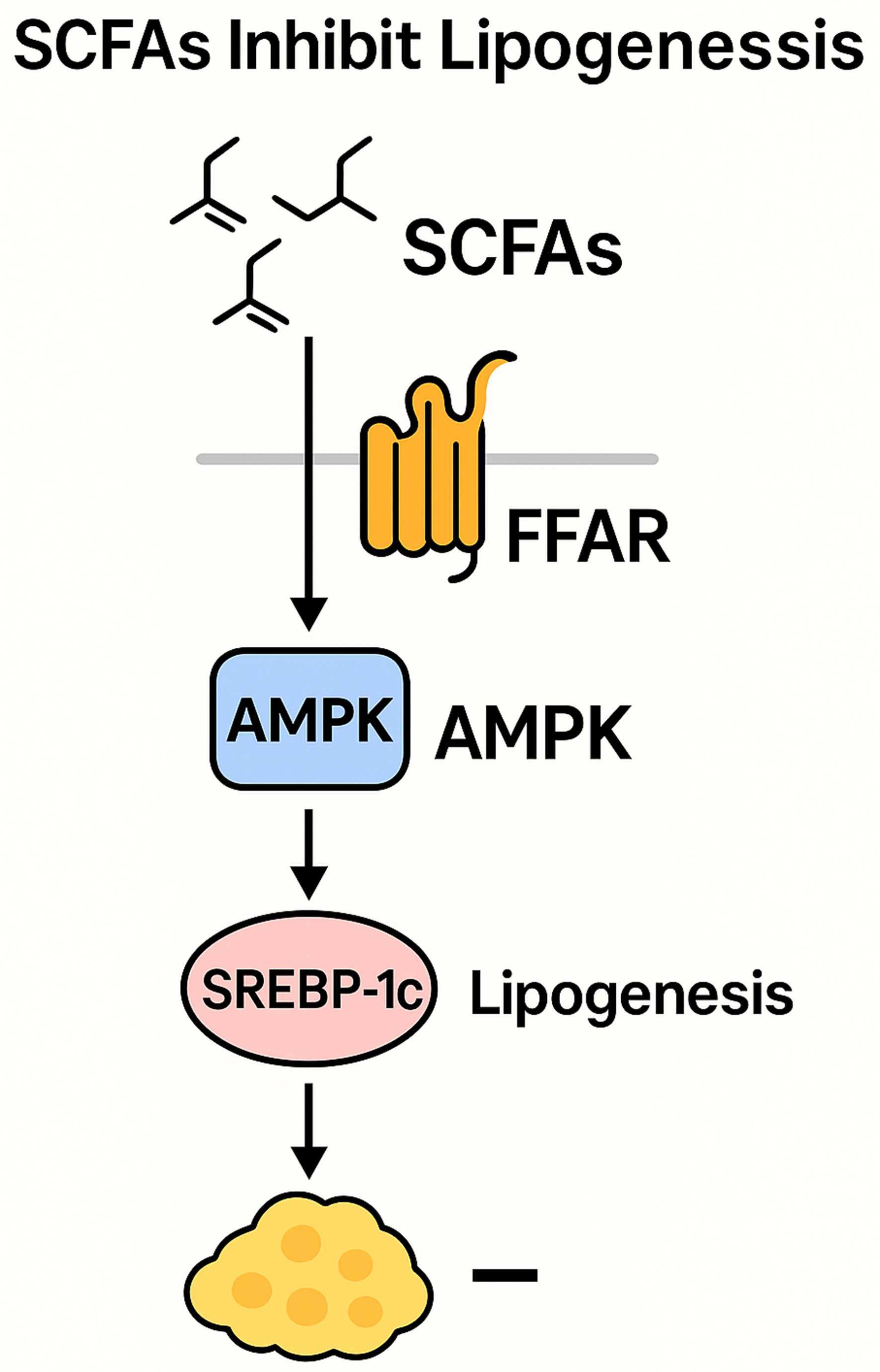
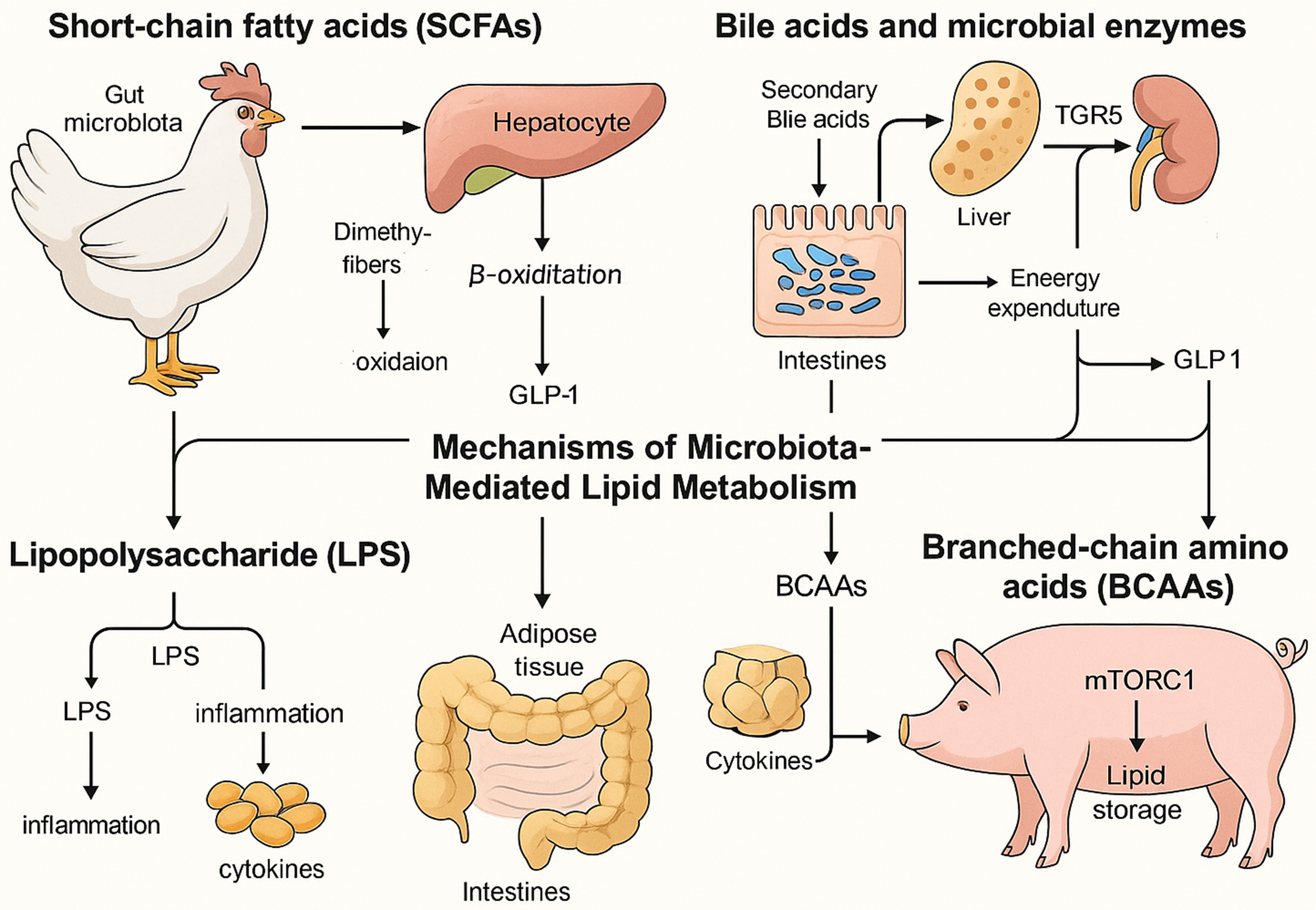
SCFAs are produced by microbial fermentation of dietary fibers and serve as pivotal regulators of host lipid metabolism. SCFAs produced by the gut microbiota are absorbed across the intestinal epithelium and metabolized into acetyl-CoA via β-oxidation, playing a pivotal role in systemic lipid metabolism, lipogenesis, gluconeogenesis, and cholesterol synthesis[45]. Additionally, SCFAs function as signaling molecules by binding to and activating free fatty acid receptors (FFARs/GPRs), a class of GPCRs. This activation stimulates the secretion of glucagon-like peptide-1 (GLP-1) and modulates de novo lipogenesis, thereby enhancing glucose and lipid metabolism in adipose tissue and the liver[46]. SCFAs participate in the regulation of multiple signaling pathways associated with lipid metabolism [Figure 1]. On one hand, SCFAs modulate the transcription of key hepatic enzymes involved in lipid synthesis, such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC), and activate the uncoupling protein 2 (UCP2)/adenosine monophosphate-activated protein kinase (AMPK)/ACC signaling pathway, thereby promoting mitochondrial fatty acid oxidation[47]. On the other hand, SCFAs upregulate the expression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), further activating the AMPK signaling cascade, which facilitates fatty acid oxidation while concurrently suppressing lipogenesis[48]. SCFAs, the principal microbial metabolites derived from colonic dietary fiber fermentation, play a pivotal role in modulating host lipid metabolism. Accumulating evidence indicates that SCFAs regulate hepatic lipid homeostasis through multiple molecular mechanisms. A critical pathway involves the inhibition of hepatic de novo lipogenesis via the transcriptional downregulation of sterol regulatory element binding protein-1c (SREBP-1c), the master regulator of lipogenic gene expression. By suppressing SREBP-1c-mediated lipogenic signaling, SCFAs attenuate triglyceride synthesis, thereby ameliorating hepatic steatosis and potentially preventing the pathogenesis of metabolic disorders, including NAFLD[49]. Overall, SCFAs serve as key metabolic integrators that bridge gut microbial activity with host lipid regulation, offering promising targets for therapeutic strategies against metabolic disorders [Figure 2].
Figure 1. Signaling pathway diagram of SCFAs-mediated inhibition of adipogenesis. SCFAs: Short-chain fatty acids.
Figure 2. Mechanisms of gut microbiota regulation of fat deposition in chickens and pigs. This primarily includes four aspects: SCFAs inhibit lipogenesis; secondary bile acids bind TGR5 to promote thermogenesis; LPS triggers TLR4-NF-κB-driven inflammation; and BCAAs activate mTORC1 to stimulate adipogenesis. SCFAs: Short-chain fatty acids; LPS: lipopolysaccharide; NF-κB: nuclear factor-kappa B; BCAAs: branched-chain amino acids; mTORC1: mechanistic target of rapamycin complex 1.
Bile acids and microbial enzymes
There are five main forms of bile acids: conjugated bile acids; primary bile acids, exemplified by cholic acid and chenodeoxycholic acid; secondary bile acids, predominantly represented by deoxycholic acid and lithocholic acid[50]. Gut bacteria express bile salt hydrolases and other enzymes that deconjugate primary bile acids and convert them into secondary bile acids, substantially altering the bile acid pool and host metabolic signaling. Secondary bile acids activate the farnesoid X receptor (FXR) in hepatic tissue to suppress lipogenesis and engage the GPCR TGR5 in adipose tissue to promote energy expenditure via thyroid hormone activation[51,52]. Bile acids can bind to TGR5, leading to improved insulin sensitivity, enhanced glucose tolerance, reduced plasma lipid levels, and alleviation of hepatic steatosis. TGR5 functions mainly through three pathways. First, it activates cyclic adenosine monophosphate (cAMP), which induces type 2 iodothyronine deiodinase (DIO2); DIO2 converts inactive thyroid hormone T4 into active T3, thereby promoting thermogenesis in adipose tissue[53]. Second, TGR5 activation in intestinal L cells promotes the secretion of GLP-1, enhancing insulin secretion and improving glucose homeostasis. Third, TGR5 modulates inflammatory responses by inhibiting the nuclear factor-kappa B (NF-κB) signaling pathway, thereby reducing inflammation associated with metabolic disorders[54]. Additionally, it regulates glucose metabolism and energy balance by releasing GLP-1, inhibits the NF-κB signaling pathway in macrophages, reduces foam cell formation, lowers fat deposition, and suppresses the development of atherosclerosis[55]. Together, these pathways highlight bile acids as critical microbial-derived regulators that orchestrate lipid metabolism, inflammation, and energy homeostasis.
LPS
Metabolic endotoxemia, characterized by low-grade elevation of plasma LPS, triggers TLR4-NF-κB signaling in adipocytes and macrophages, driving pro-inflammatory cytokine release, insulin resistance, and adipose tissue expansion[56,57]. HFDs increase gut permeability, facilitating LPS translocation into the portal circulation; conversely, prebiotic and probiotic interventions that restore epithelial tight junctions lower systemic LPS levels and attenuate adipose inflammation and fat gain[57,58]. CD14-deficient mice resist HFD-induced weight gain and insulin resistance, confirming that LPS-CD14 interactions play a crucial role in setting the tone for metabolic inflammation and the development of obesity[57]. LPS triggers pro-inflammatory cytokine release, immune activation, and chronic inflammation, accelerating atherosclerosis and plaque formation. It also downregulates ATP-binding cassette transporter A1 (ABCA1) in murine macrophages, impairing cholesterol efflux. A HFD increases the abundance of LPS-producing gut bacteria, stimulating tumor necrosis factor-alpha (TNF-α) and NF-κB signaling. Both LPS and TNF-α activate apoptosis signal-regulating kinase 1 (ASK1), a critical suppressor of adipose tissue browning[59]. These findings underscore the pivotal role of microbiota-derived LPS in linking gut barrier dysfunction to systemic inflammation, lipid dysregulation, and metabolic disease progression.
BCAAs
BCAAs, including leucine, isoleucine, and valine, are metabolized by both host and microbial pathways, with dysregulated microbial handling of BCAAs preceding obesity and insulin resistance[60]. Elevated circulating BCAAs correlate with increased fat deposition, and transplantation of BCAA-enriched microbiota from obese donors into germ-free mice raises serum BCAA levels and promotes adipocyte lipid storage[61,62]. The cellular uptake of BCAAs into adipocytes is predominantly regulated by specific amino acid transporters, including solute carrier family 1 member 5 (SLC1A5), solute carrier family 3 member 2 (Slc3a2), and solute carrier family 7 member 5 (Slc7a5). This complex facilitates an antiport mechanism, whereby extracellular BCAAs are exchanged for intracellular glutamine and asparagine. Upon cellular internalization, BCAAs undergo sequential metabolic transformations, culminating in the generation of intermediate acyl-CoA derivatives, including isovaleryl-CoA and 2-methylbutyryl-CoA[63]. These intermediates serve as critical precursors for the biosynthesis of monomethyl branched-chain fatty acids (mmBCFAs). The mitochondrial export of these acyl-CoA species is mediated by carnitine acetyltransferase, followed by their cytosolic elongation catalyzed by FAS. This metabolic cascade highlights the dual role of BCAAs in adipose tissue: not only do they serve as substrates for energy production, but they also contribute to de novo lipogenesis through the generation of mmBCFAs[64]. This pathway underscores the metabolic versatility of adipose tissue in integrating nitrogen and carbon metabolism, further emphasizing its role in systemic BCAA homeostasis and lipid biosynthesis. Green et al. elucidated the pivotal contribution of BCAA degradation to the control of adipocyte differentiation[65]. Their work revealed that increased expression of BCAA-catabolizing enzymes coincides with elevated peroxisome proliferator-activated receptor gamma (PPARγ) levels during the initial phases of adipogenesis, implicating BCAA metabolism in the determination of adipogenic fate. The study further delineated that BCAAs promote adipocyte maturation by stimulating the mechanistic target of rapamycin complex 1 (mTORC1) pathway, with ribosomal protein S6 kinase 1 (S6K1) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) serving as key downstream mediators that ultimately regulate PPARγ function[66]. Moreover, the mitochondrial deacylase SIRT4 was identified as a modulator of BCAA metabolic flux in preadipocytes, acting through PPARγ upregulation - a finding that reinforces the critical crosstalk between mitochondrial metabolic regulation and transcriptional programming in early adipogenic commitment[67]. Enhancing BCAA catabolism, via dietary modulation or next-generation probiotics, improves glucose homeostasis and reduces fat mass in rodent obesity models, offering a novel avenue for metabolic health interventions[68,69]. Altogether, these findings highlight the integral role of BCAA metabolism in adipose tissue development, energy balance, and the pathogenesis of obesity-related metabolic disorders.
GUT MICROBIOTA IN METABOLIC DISORDERS
Obesity
Obese individuals and HFD rodents exhibit a characteristic shift toward an elevated Firmicutes/Bacteroidetes ratio, promoting the extraction of additional calories from complex polysaccharides and increasing fat deposition[70]. Concurrently, depletion of mucin-degrading and barrier-protective taxa such as Akkermansia muciniphila correlates with higher body mass index, while supplementation with
T2D
T2D diabetes is associated with reduced gut microbial diversity, enrichment of opportunistic pathogens, and loss of butyrate-producing taxa (e.g., Faecalibacterium prausnitzii), leading to impaired barrier function and systemic inflammation[76]. Dysbiosis also diminishes GLP-1 release: under healthy conditions, SCFAs and secondary bile acids stimulate enteroendocrine L cells to secrete GLP-1, enhancing insulin secretion and glucose tolerance; in T2D, this axis is blunted, contributing to hyperglycemia[77]. Probiotic and prebiotic interventions (e.g., Lactobacillus rhamnosus, dietary fibers) restore SCFA levels, normalize GLP-1 rhythms, and improve glycemic control in preclinical and clinical studies[78]. Bariatric surgery further underscores microbiota’s role: patients undergoing Roux-en-Y gastric bypass exhibit specific microbial shifts that enhance incretin responses and barrier integrity, correlating with remission of T2D[79]. Next-generation approaches, such as FMT from healthy donors and designer consortia, are under investigation to reprogram dysbiotic communities and reverse insulin resistance[80,81].
NAFLD
NAFLD pathogenesis is tightly linked to gut-liver axis perturbations. Dysbiosis elevates gut permeability and LPS translocation, activating hepatic TLR4-mediated inflammation and impairing mitochondrial β-oxidation[82]. Altered microbial metabolism of choline produces toxic intermediates (e.g., δ-valerobetaine) that inhibit the carnitine shuttle and exacerbate triglyceride accumulation in hepatocytes[83]. Conversely, SCFA-enhancing prebiotics expand myeloid-derived suppressor cells that mitigate hepatic steatosis and oxidative stress in rodent NAFLD models[84]. Immune crosstalk also plays a crucial role: gut-derived type 3 innate lymphoid cells migrate to the liver and secrete IL-22, promoting hepatocyte lipid oxidation and reducing fibrosis; strategies that boost the ILC3-IL-22 axis via washed microbiota transplantation have achieved up to 43% reduction in liver fat in early trials[85,86]. Collectively, these insights provide a mechanistic framework for microbiota-based therapies in NAFLD, including probiotics, synbiotics, and targeted microbial metabolite analogs.
Other disorders
In addition to obesity, T2D, and NAFLD, several other well-recognized microbiota-mediated metabolic disorders have been identified, including metabolic syndrome, hypertension, and polycystic ovary syndrome (PCOS). Metabolic syndrome is associated with gut dysbiosis, reduced microbial diversity, impaired metabolism of SCFAs and bile acids, and increased levels of LPS. These alterations contribute to systemic inflammation, insulin resistance, dyslipidemia, and elevated blood pressure[87]. Clinical interventions using prebiotics, probiotics, synbiotics, and postbiotics have shown improvements in metabolic parameters. However, the outcomes remain variable and highlight the importance of personalized therapeutic strategies[88]. In hypertension, gut microbiota dysbiosis is marked by reduced microbial diversity, enrichment of mucin-degrading taxa (Muribaculaceae, Alistipes), and depletion of SCFA-producing genera (Ruminococcus, Eubacterium eligens), as observed in hypertensive cohorts. These changes correlate with altered microbial metabolic pathways (e.g., increased acetate-CoA ligase activity, decreased GPR43 signaling) and contribute to elevated blood pressure via impaired vascular and inflammatory regulation. FMT from hypertensive humans into germ-free mice has causally linked dysbiosis to hypertension development[89,90]. PCOS also displays characteristic features of gut dysbiosis, including lower microbial diversity, a disturbed Firmicutes to Bacteroidetes ratio, increased abundance of Escherichia-Shigella, and reduced levels of Akkermansia. These changes are correlated with insulin resistance, hormonal imbalance, and chronic inflammation[11,91]. Microbiota-targeted interventions, including probiotics, prebiotics, and precision microbiome-based therapies such as designer microbial consortia, have shown potential to improve both metabolic and reproductive outcomes in individuals with PCOS[92,93]. Table 2 summarizes these therapeutic strategies, highlighting the range of microbiota-targeted approaches being investigated for metabolic disorders.
Microbiota-targeted interventions for metabolic disorders
| Disorder | Microbiota features | Mechanisms | Interventions | Reported effects and key references |
| Obesity | ↑ Firmicutes/Bacteroidetes, ↓ Akkermansia | ↑ Caloric extraction, LPS-induced inflammation, ↓ barrier integrity | Akkermansia supplementation, prebiotics | ↓ Fat mass, ↑ GLP-1, ↓ inflammation[70-75] |
| T2D | ↓ Diversity, ↓ butyrate producers (e.g., F. prausnitzii) | ↓ GLP-1 secretion, ↑ inflammation, ↓ insulin sensitivity | Pro-/prebiotics, bariatric surgery, FMT | ↑ Glycemic control, ↑ GLP-1, ↓ insulin resistance[76-81] |
| NAFLD | ↑ Gut permeability, ↑ LPS, altered bile acid metabolism | ↑ Hepatic inflammation, ↓ β-oxidation, ↑ steatosis | Prebiotics, synbiotics, IL-22-based strategies | ↓ Hepatic fat, ↓ oxidative stress, ↑ lipid oxidation[82-86] |
| Metabolic syndrome | ↓ Diversity, ↑ Firmicutes/Bacteroidetes, ↑ LPS | ↑ Inflammation, insulin resistance | Pre-/pro-/synbiotics | Mixed results; personalized strategies suggested[87,88] |
| Hypertension | ↓ Diversity; ↑ Muribaculaceae, Alistipes; ↓ Ruminococcus, Eubacterium eligens | Impaired SCFA signaling (↓ GPR43); ↑ acetate-CoA ligase; promotes inflammation and vascular dysfunction | Probiotics, prebiotics, FMT (preclinical) | FMT from hypertensive donors induces hypertension in mice[89,90] |
| PCOS | ↓ Diversity, ↑ Escherichia-Shigella, ↓ Akkermansia | ↑ Insulin resistance, androgen biosynthesis, inflammation | Pro-/prebiotics, FMT, designer consortia | Improved insulin sensitivity and menstrual regulation[91-93] |
CONCLUSION
The gut microbiota plays a central role in regulating lipid metabolism through its metabolites and interactions with host signaling pathways. SCFAs, produced by microbial fermentation of dietary fiber, suppress hepatic lipogenesis by inhibiting SREBP-1c and activate GPR41/43 receptors to enhance mitochondrial β-oxidation. Microbial enzymes convert primary bile acids into secondary bile acids, which activate FXR to inhibit hepatic triglyceride synthesis and stimulate TGR5 receptors to promote adipose thermogenesis. LPS translocated from dysbiotic microbiota triggers TLR4-NF-κB signaling, driving insulin resistance and adipose tissue inflammation. BCAAs, metabolized by gut microbes, activate the mTORC1-PPARγ axis to promote adipogenesis, with elevated circulating BCAA levels strongly linked to obesity and metabolic dysfunction. In livestock, microbiota-targeted strategies optimize fat deposition for meat quality. For instance, probiotic supplementation in pigs enriches Lactobacillus and BCAA metabolic pathways, enhancing IMF, while dietary succinate or coated sodium propionate reshapes cecal microbiota in chickens to reduce abdominal fat. FMT experiments confirm the causal role of microbial communities in transferring fat deposition phenotypes. Furthermore, the conserved mechanisms linking dysbiosis to metabolic disorders (obesity, T2D, NAFLD) across species highlight the dual role of microbial metabolites: SCFAs and secondary bile acids ameliorate metabolic inflammation, whereas LPS and BCAA imbalances exacerbate lipid dysregulation.
Future research should prioritize precision interventions that target specific microbial pathways - such as engineered probiotics, metabolite analogs, and bacteriophage therapy - to enhance both livestock productivity and human metabolic health. The integration of multi-omics approaches (e.g., metagenomics, metabolomics, transcriptomics) will be essential to uncover functional microbial targets and accelerate the development of effective microbiota-based therapies. However, translating these strategies into clinical and agricultural applications remains challenging due to individual variability in microbiota responses and concerns regarding the long-term safety and stability of engineered microbial consortia.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception of the study and wrote the manuscript: Cui X
Collected materials and reviewed the literature: Yuan Q
Reviewed the literature: Long J, Zhou J
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX24_2293).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-23.
2. Valsecchi C, Carlotta Tagliacarne S, Castellazzi A. Gut microbiota and obesity. J Clin Gastroenterol. 2016;50:S157-8.
3. Conterno L, Fava F, Viola R, Tuohy KM. Obesity and the gut microbiota: does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011;6:241-60.
4. Anand S, Mande SS. Host-microbiome interactions: gut-liver axis and its connection with other organs. NPJ Biofilms Microbiomes. 2022;8:89.
5. Cai J, Rimal B, Jiang C, Chiang JYL, Patterson AD. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. 2022;237:108238.
6. Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979-84.
7. Ellekilde M, Selfjord E, Larsen CS, et al. Transfer of gut microbiota from lean and obese mice to antibiotic-treated mice. Sci Rep. 2014;4:5922.
8. Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461-8.
9. Zheng C, Wan M, Guo Q, Duan Y, Yin Y. Glutamate increases the lean percentage and intramuscular fat content and alters gut microbiota in Shaziling pigs. Anim Nutr. 2025;20:110-9.
10. Wang F, Feng J, Yao M, et al. Dietary succinate reduces fat deposition through gut microbiota and lipid metabolism in broilers. Poult Sci. 2024;103:103954.
11. Wang L, Zhou J, Gober HJ, et al. Alterations in the intestinal microbiome associated with PCOS affect the clinical phenotype. Biomed Pharmacother. 2021;133:110958.
12. Cui X, Gou Z, Jiang Z, et al. Dietary fiber modulates abdominal fat deposition associated with cecal microbiota and metabolites in yellow chickens. Poult Sci. 2022;101:101721.
13. Breton J, Galmiche M, Déchelotte P. Dysbiotic gut bacteria in obesity: an overview of the metabolic mechanisms and therapeutic perspectives of next-generation probiotics. Microorganisms. 2022;10:452.
14. Guzzardi MA, La Rosa F, Iozzo P. Trust the gut: outcomes of gut microbiota transplant in metabolic and cognitive disorders. Neurosci Biobehav Rev. 2023;149:105143.
15. Houtman TA, Eckermann HA, Smidt H, de Weerth C. Gut microbiota and BMI throughout childhood: the role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci Rep. 2022;12:3140.
16. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070-5.
17. May KS, den Hartigh LJ. Modulation of adipocyte metabolism by microbial short-chain fatty acids. Nutrients. 2021;13:3666.
18. Cheng Z, Zhang L, Yang L, Chu H. The critical role of gut microbiota in obesity. Front Endocrinol. 2022;13:1025706.
19. Kieffer DA, Piccolo BD, Marco ML, et al. Mice fed a high-fat diet supplemented with resistant starch display marked shifts in the liver metabolome concurrent with altered gut bacteria. J Nutr. 2016;146:2476-90.
20. Zhang L, Ouyang Y, Li H, et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: a randomized crossover trial. Sci Rep. 2019;9:4736.
21. Cho KY. Association of gut microbiota with obesity in children and adolescents. Clin Exp Pediatr. 2023;66:148-54.
22. Wang G, Chen X, Sun C, et al. Gut microbiota and metabolite insights into anti-obesity effect of carboxymethyl pachymaran in high-fat diet mice. J Funct Foods. 2023;111:105898.
23. Yin J, Li Y, Tian Y, et al. Obese Ningxiang pig-derived microbiota rewires carnitine metabolism to promote muscle fatty acid deposition in lean DLY pigs. Innovation. 2023;4:100486.
24. Li C, Zhao X, Zhao G, et al. Comparative analysis of structural composition and function of intestinal microbiota between Chinese indigenous Laiwu Pigs and commercial DLY Pigs. Vet Sci. 2023;10:524.
25. Yang M, Xie Q, Wang J, et al. Ningxiang pig-derived lactobacillus reuteri modulates host intramuscular fat deposition via branched-chain amino acid metabolism. Microbiome. 2025;13:32.
26. Bergamaschi M, Tiezzi F, Howard J, et al. Gut microbiome composition differences among breeds impact feed efficiency in swine. Microbiome. 2020;8:110.
27. Shang P, Wei M, Duan M, Yan F, Chamba Y. Healthy gut microbiome composition enhances disease resistance and fat deposition in Tibetan Pigs. Front Microbiol. 2022;13:965292.
28. Luo Y, Ren W, Smidt H, et al. Dynamic distribution of gut microbiota in pigs at different growth stages: composition and contribution. Microbiol Spectr. 2022;10:e0068821.
29. Wu C, Lyu W, Hong Q, Zhang X, Yang H, Xiao Y. Gut microbiota influence lipid metabolism of skeletal muscle in pigs. Front Nutr. 2021;8:675445.
30. Wen C, Yan W, Sun C, et al. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME J. 2019;13:1422-36.
31. Chen Y, Akhtar M, Ma Z, et al. Chicken cecal microbiota reduces abdominal fat deposition by regulating fat metabolism. NPJ Biofilms Microbiomes. 2023;9:28.
32. Liu X, Wang C, Wang Y, et al. Age-associated changes in the growth development of abdominal fat and their correlations with cecal gut microbiota in broiler chickens. Poult Sci. 2023;102:102900.
33. Liu Y, Yang J, Liu X, et al. Dietary folic acid addition reduces abdominal fat deposition mediated by alterations in gut microbiota and SCFA production in broilers. Anim Nutr. 2023;12:54-62.
34. Liu X, Wang C, Li Y, et al. Fecal microbiota transplantation revealed the function of folic acid on reducing abdominal fat deposition in broiler chickens mediated by gut microbiota. Poult Sci. 2024;103:103392.
35. Nan S, Yao M, Zhang X, et al. Fermented grape seed meal promotes broiler growth and reduces abdominal fat deposition through intestinal microorganisms. Front Microbiol. 2022;13:994033.
36. Dai H, Gao J, Zhang Y, et al. Dietary phytosterols supplementation improves the growth performance and decreases the abdominal fat of broiler chickens by regulating intestinal epithelial structure and microbiota. Anim Feed Sci Technol. 2023;305:115786.
37. Zhang Y, Liu Y, Li J, et al. Dietary corn-resistant starch suppresses broiler abdominal fat deposition associated with the reduced cecal Firmicutes. Poult Sci. 2020;99:5827-37.
38. Wu T, Wang P, Fu Q, et al. Effects of dietary supplementation of Anoectochilus roxburghii extract (ARE) on growth performance, abdominal fat deposition, meat quality, and gut microbiota in broilers. Poult Sci. 2023;102:102842.
39. Xiao L, Liu J, Qin L, et al. Multi-omics reveal the effects and regulatory mechanism of dietary echinocystic acid supplementation on abdominal fat and liver steatosis in broiler chickens. Poult Sci. 2025;104:104981.
40. Wang M, Ma W, Wang C, Li D.
41. Jing Y, Yuan Y, Monson M, et al. Multi-omics association reveals the effects of intestinal microbiome-host interactions on fat deposition in broilers. Front Microbiol. 2021;12:815538.
42. Liu J, Wang J, Zhou Y, et al. Integrated omics analysis reveals differences in gut microbiota and gut-host metabolite profiles between obese and lean chickens. Poult Sci. 2022;101:102165.
43. Lei J, Dong Y, Hou Q, et al. Intestinal microbiota regulate certain meat quality parameters in chicken. Front Nutr. 2022;9:747705.
44. Xiang H, Gan J, Zeng D, et al. Specific microbial taxa and functional capacity contribute to chicken abdominal fat deposition. Front Microbiol. 2021;12:643025.
45. He J, Zhang P, Shen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21:6356.
46. Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364-71.
47. den Besten G, Bleeker A, Gerding A, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398-408.
48. Xiao S, Zhang Z, Chen M, et al. Xiexin Tang ameliorates dyslipidemia in high-fat diet-induced obese rats via elevating gut microbiota-derived short chain fatty acids production and adjusting energy metabolism. J Ethnopharmacol. 2019;241:112032.
49. Wang L, Zhang S, Huang Y, Zhou Y, Shan T. Conjugated linoleic acids inhibit lipid deposition in subcutaneous adipose tissue and alter lipid profiles in serum of pigs. J Anim Sci. 2023;101:skad294.
50. di Gregorio MC, Cautela J, Galantini L. Physiology and physical chemistry of bile acids. Int J Mol Sci. 2021;22:1780.
51. Dong Z, Yang S, Tang C, Li D, Kan Y, Yao L. New insights into microbial bile salt hydrolases: from physiological roles to potential applications. Front Microbiol. 2025;16:1513541.
52. Li R, Andreu-Sánchez S, Kuipers F, Fu J. Gut microbiome and bile acids in obesity-related diseases. Best Pract Res Clin Endocrinol Metab. 2021;35:101493.
53. Finn PD, Rodriguez D, Kohler J, et al. Intestinal TGR5 agonism improves hepatic steatosis and insulin sensitivity in Western diet-fed mice. Am J Physiol Gastrointest Liver Physiol. 2019;316:G412-24.
54. Lun W, Yan Q, Guo X, et al. Mechanism of action of the bile acid receptor TGR5 in obesity. Acta Pharm Sin B. 2024;14:468-91.
55. Pols TW, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747-57.
56. Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie. 2016;124:11-20.
57. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-72.
58. Mohammad S, Thiemermann C. Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front Immunol. 2020;11:594150.
59. Ye D, Lammers B, Zhao Y, Meurs I, Van Berkel TJ, Van Eck M. ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis. Curr Drug Targets. 2011;12:647-60.
60. Gojda J, Cahova M. Gut microbiota as the link between elevated BCAA serum levels and insulin resistance. Biomolecules. 2021;11:1414.
61. Vanweert F, Schrauwen P, Phielix E. Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances BCAA metabolism in type 2 diabetes. Nutr Diabetes. 2022;12:35.
62. Daniel N, Nachbar RT, Tran TTT, et al. Gut microbiota and fermentation-derived branched chain hydroxy acids mediate health benefits of yogurt consumption in obese mice. Nat Commun. 2022;13:1343.
63. Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745-51.
64. Wallace M, Green CR, Roberts LS, et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat Chem Biol. 2018;14:1021-31.
65. Green CR, Wallace M, Divakaruni AS, et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12:15-21.
66. Zaganjor E, Yoon H, Spinelli JB, et al. SIRT4 is an early regulator of branched-chain amino acid catabolism that promotes adipogenesis. Cell Rep. 2021;36:109345.
67. Yoneshiro T, Wang Q, Tajima K, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572:614-9.
68. Ejtahed HS, Angoorani P, Soroush AR, Hasani-Ranjbar S, Siadat SD, Larijani B. Gut microbiota-derived metabolites in obesity: a systematic review. Biosci Microbiota Food Health. 2020;39:65-76.
69. Mansoori S, Ho MY, Ng KK, Cheng KK. Branched-chain amino acid metabolism: pathophysiological mechanism and therapeutic intervention in metabolic diseases. Obes Rev. 2025;26:e13856.
70. Van Hul M, Cani PD. The gut microbiota in obesity and weight management: microbes as friends or foe? Nat Rev Endocrinol. 2023;19:258-71.
71. Nemoto S, Kubota T, Ohno H. Exploring body weight-influencing gut microbiota by elucidating the association with diet and host gene expression. Sci Rep. 2023;13:5593.
72. Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev. 2015;36:245-71.
73. Sanmiguel C, Gupta A, Mayer EA. Gut microbiome and obesity: a plausible explanation for obesity. Curr Obes Rep. 2015;4:250-61.
74. Ecklu-Mensah G, Choo-Kang C, Maseng MG, et al. Gut microbiota and fecal short chain fatty acids differ with adiposity and country of origin: the METS-microbiome study. Nat Commun. 2023;14:5160.
75. Visuthranukul C, Sriswasdi S, Tepaamorndech S, et al. Enhancing gut microbiota and microbial function with inulin supplementation in children with obesity. Int J Obes. 2024;48:1696-704.
76. Slouha E, Rezazadah A, Farahbod K, Gerts A, Clunes LA, Kollias TF. Type-2 diabetes mellitus and the gut microbiota: systematic review. Cureus. 2023;15:e49740.
77. Zeng Y, Wu Y, Zhang Q, Xiao X. Crosstalk between glucagon-like peptide 1 and gut microbiota in metabolic diseases. mBio. 2024;15:e0203223.
78. Wu J, Yang K, Fan H, Wei M, Xiong Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front Endocrinol. 2023;14:1114424.
79. Hernández-Montoliu L, Rodríguez-Peña MM, Puig R, et al. A specific gut microbiota signature is associated with an enhanced GLP-1 and GLP-2 secretion and improved metabolic control in patients with type 2 diabetes after metabolic Roux-en-Y gastric bypass. Front Endocrinol. 2023;14:1181744.
80. Byndloss M, Devkota S, Duca F, et al. The gut microbiota and diabetes: research, translation, and clinical applications - 2023 Diabetes, Diabetes Care, and Diabetologia Expert Forum. Diabetes Care. 2024;47:1491-508.
81. Cui X, Chen J, Yang Y. Administration of selenomethionine in combination with serine benefits diabetes via gut microbiota. Front Microbiol. 2022;13:1007814.
82. Su X, Chen S, Liu J, et al. Composition of gut microbiota and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Obes Rev. 2024;25:e13646.
83. Zai W, Chen W, Liu H, Ju D. Therapeutic opportunities of IL-22 in non-alcoholic fatty liver disease: from molecular mechanisms to clinical applications. Biomedicines. 2021;9:1912.
84. Maestri M, Santopaolo F, Pompili M, Gasbarrini A, Ponziani FR. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: effects of current treatments and future strategies. Front Nutr. 2023;10:1110536.
85. Pezzino S, Sofia M, Mazzone C, et al. Gut microbiome in the progression of NAFLD, NASH and cirrhosis, and its connection with biotics: a bibliometric study using dimensions scientific research database. Biology. 2023;12:662.
86. Liu J, Wu A, Cai J, She ZG, Li H. The contribution of the gut-liver axis to the immune signaling pathway of NAFLD. Front Immunol. 2022;13:968799.
87. Alveirinho M, Freitas P, Faleiro ML. Role of gut microbiota in metabolic syndrome: a review of recent evidence. Porto Biomed J. 2020;5:e105.
88. Qureshi W, Dar MA, Rather MY. New therapy for metabolic syndrome: gut microbiome supplementation. World J Diabetes. 2024;15:1833-6.
89. Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14.
90. Nakai M, Ribeiro RV, Stevens BR, et al. Essential hypertension is associated with changes in gut microbial metabolic pathways: a multisite analysis of ambulatory blood pressure. Hypertension. 2021;78:804-15.
91. Li C, Cheng D, Ren H, Zhang T. Unraveling the gut microbiota’s role in PCOS: a new frontier in metabolic health. Front Endocrinol. 2025;16:1529703.
92. Senthilkumar H, Arumugam M. Gut microbiota: a hidden player in polycystic ovary syndrome. J Transl Med. 2025;23:443.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].