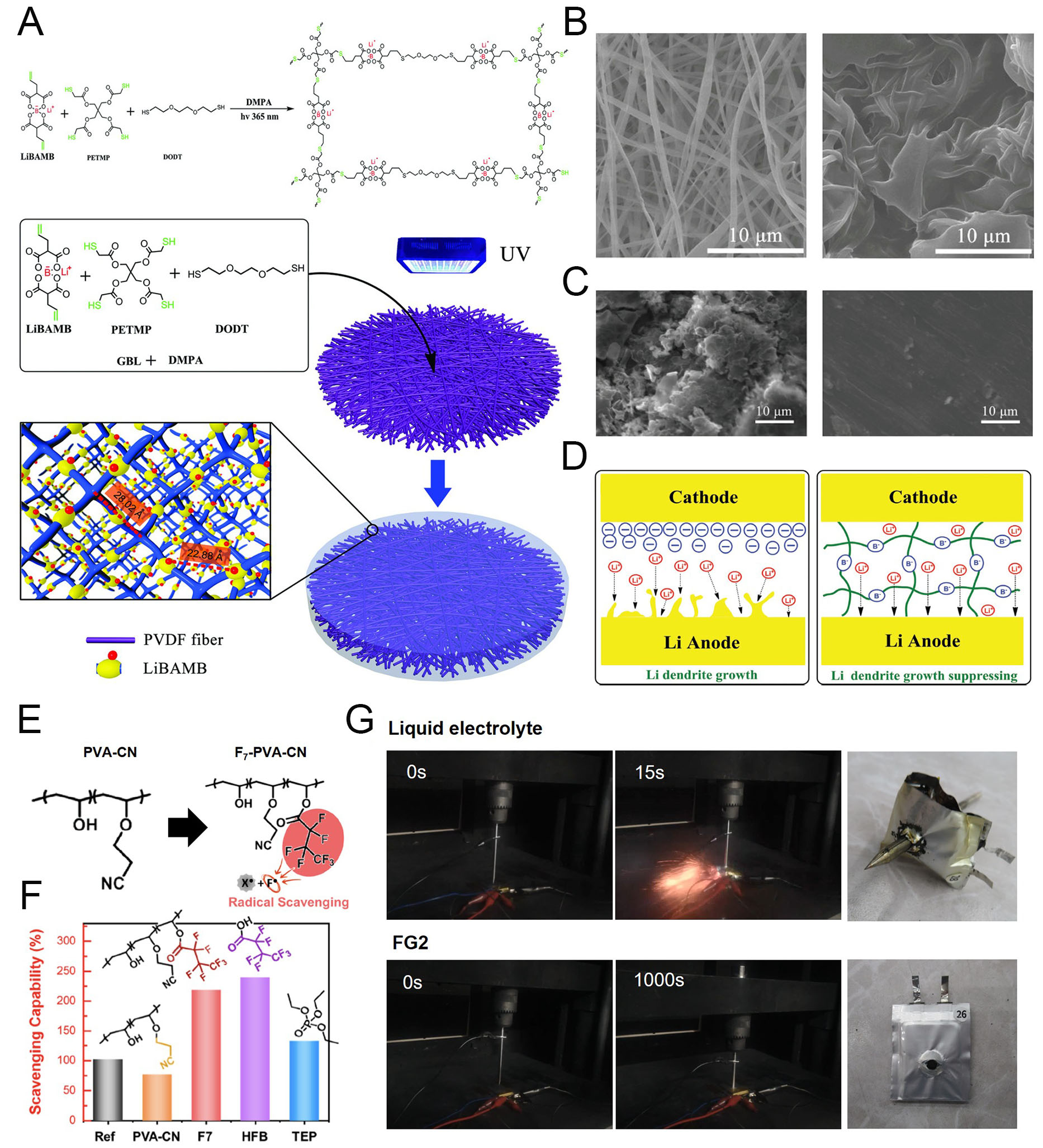
fig11
Figure 11. Polymer electrolyte contribution to electrochemical and thermal stabilization. (A) Schematic illustration of the synthesis and structure of LPD@PVDF SIPEs. (B) SEM images of PVDF (left) and LPD@PVDF SIPE membrane (right). (C) SEM images of cycled Li metal anode after using typical PP (left) and LPD@PVDF SIPE (right). (D) Schematic illustration of the Li metal growth mechanism depending on cell configuration. Reproduced with permission[129]. (E) Fluorine radical (F·) scavenging a flammable reactive radical (X·). F· and X· are generated from F7-PVA-CN and the electrolyte, respectively, via decomposition at elevated temperature, caused by safety issues. (F) Radical-scavenging capabilities (SR) estimated using the α-tocopherol (α-TOH) method. (G) Time-resolved photographs of nail penetration tests for 0.65 Ah pouch cells of graphite||NCM811 and the tested cells. Reproduced with permission[130].