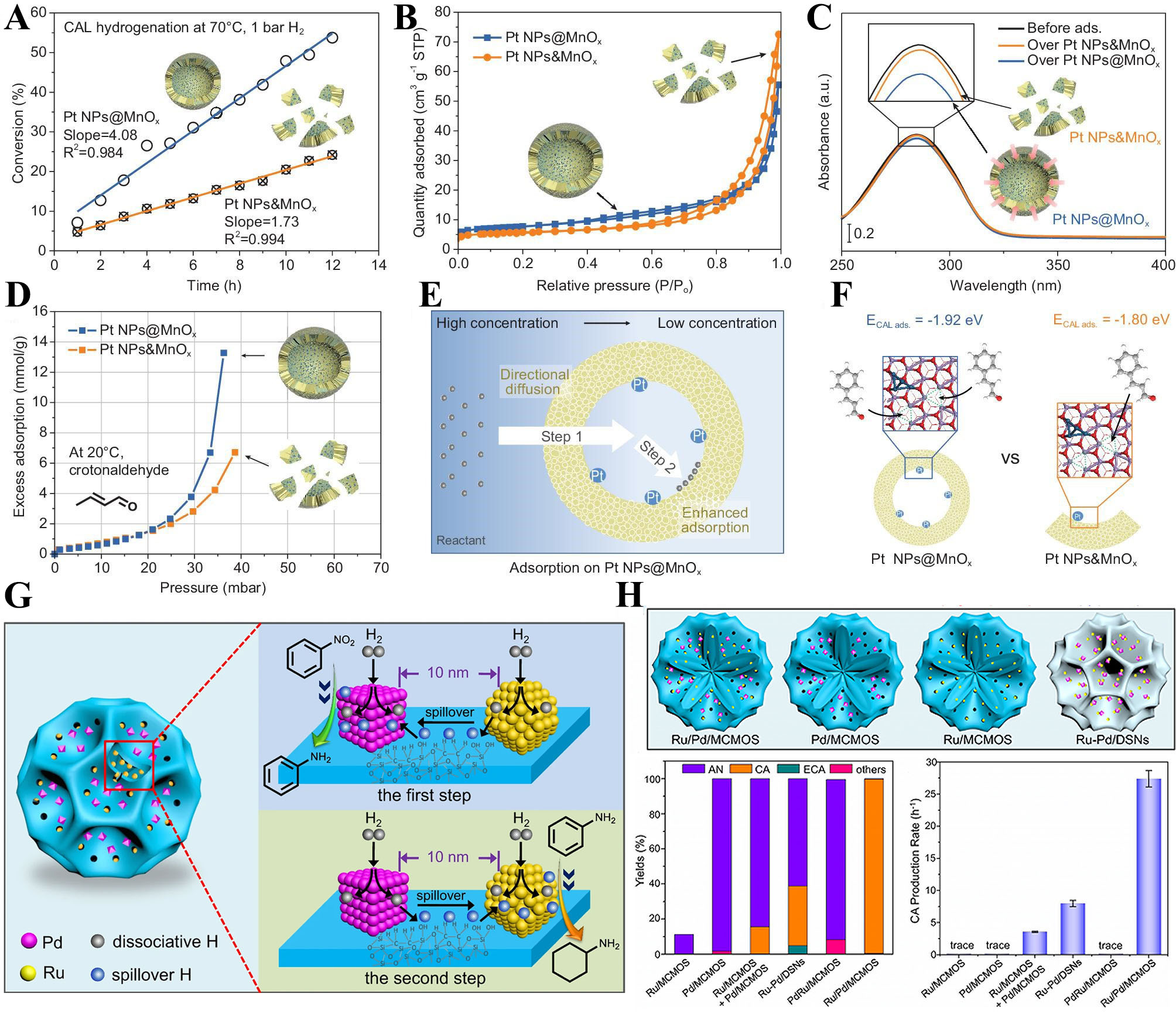
fig5
Figure 5. (A) Hydrogenation performance of CAL at 70 °C, 1 bar H2. Reproduced with permission from ref.[66]. Copyright 2023 National Science Review; (B-D) N2 adsorption-desorption isotherms at 77 K (B), Ultraviolet-visible (UV-vis) spectroscopy spectrum of CAL adsorption experiment (C) In situ gravimetric adsorption analysis profiles (D) over Pt NPs@MnOx and Pt NPs&MnOx, respectively. Reproduced with permission from ref.[66]. Copyright 2023 National Science Review; (E) Schematic illustration of reactant enrichment on Pt NPs@MnOx. Reproduced with permission from ref.[66]. Copyright 2023 National Science Review; (F) The adsorption energy of one CAL adsorbed at one OV site and that of two CAL adsorbed at two OV sites on Pt NPs&MnOx with two OV sites. Reproduced with permission from ref.[66]. Copyright 2023 National Science Review; (G) Schematic illustration for neighboring metal-assisted hydrogenation over Ru/Pd/MCMOS; (H) Schematic illustration of four catalysts including Ru/Pd/MCMOS, Pd/MCMOS, Ru/MCMOS, and Ru-Pd/DMSNs. Product distributions of the sequential hydrogenation over different catalysts. The CA production rate over different catalysts. Reproduced with permission from ref.[60]. Copyright 2021 Springer Nature. CAL: cinnamaldehyde; OV: oxygen vacancy; MCMOS: multicompartmentalized mesoporous organosilica; DMSN: dendritic mesoporous silica nanoparticle; CA: cyclohexylamine.