Regulating solution epitaxy of PbTiO3 film by Ni ions for enhanced visible-light photovoltaic current
Abstract
The bulk photovoltaic effect of perovskite ferroelectric oxides has been widely explored because of its ability to obtain the above-bandgap photovoltage. However, the photovoltaic current in these materials remains low at the nA level in the visible-light range, severely limiting the device applications due to a wide bandgap. Herein, we report a Ni ions-assisted coprecipitation-hydrothermal method to regulate the growth of single-crystal PbTiO3 film with a controlled thickness from 0.7 μm to 2.2 μm. The epitaxial relationship between the tetragonal perovskite film and cubic Nb:SrTiO3 substrate has been characterized to be {001}film || {100}substrate. The film adopts a single-domain structure with a polarization direction pointing to the substrate. Interestingly, the film exhibits a large photovoltaic current under 405 nm irradiation, with values reaching 3.6 mA/cm2, which is ∼ 3.6 times higher than those of the reported ferroelectric materials. Introducing Ni ions as an additive into the precursor solution was investigated to effectively mediate the competitive nucleation and growth processes between the film and the by-product powder, thereby enabling a tunable thickness of the films. An intriguing Ti-vacancy composition gradient was revealed throughout the film and its coupling with the spontaneous polarization generates a polarization gradient and thus a built-in electric field, accounting for the excellent photovoltaic performance reported here.
Keywords
INTRODUCTION
Since the discovery of the bulk photovoltaic (PV) effect in the 1970s, ferroelectric oxides have gained substantial attention due to the distinct polarization-related charge-separation mechanism[1]. Different from the p-n junction effect in silicon PV cells[2,3], ferroelectric PV is a bulk phenomenon[4,5] that exhibits unique characteristics, including above-bandgap photovoltage[6], switchable PV current[7] and inherent light polarization dependence[8,9]. As a classical ferroelectric material among the ABO3 perovskite group, lead titanate [PbTiO3 (PTO)] possesses a high spontaneous polarization that offers various functions such as piezoelectricity[10], ferroelectricity[11], photocatalyst[12], and photovoltaics[13]. A wide bandgap of PTO (~ 3 eV), a common characteristic of many perovskite oxides, however, is not beneficial for enhancing PV current in the visible-light range toward optoelectrical devices.
Different strategies have been developed to enhance the PV current of PTO-based materials. For example, chemical doping and bandgap engineering were employed to address this issue. PTO-based multicomponent solid solution offers flexibility in chemical composition and bandgap adjustment to enhance PV current[14-16], and further increase the current to 6.2 μA/cm2 via designing the composition in the vicinity of the morphotropic phase boundary[17,18]. The substitution of Ni in PTO is thought to work in conjunction with oxygen vacancies to alter the band structure and boost the PV current according to theoretical calculations[19-21]. The preparation of films by solid-state or sol-gel methods to investigate the bandgap narrowing effect in Ni-doped films has been conducted to verify theoretical calculations[22-24] and boost the PV current up to ~ 15.8 μA/cm2[24]. Furthermore, the combination of PTO with a p-n junction realizes the coupling of bulk and junction PV effects, improving the separation of photogenerated carriers and suppressing their non-radiative recombination, thereby increasing the current to 210 μA/cm2[25]. Nonetheless, the studies mentioned above were subjected to polycrystalline films or ceramics, where the defects such as grain boundaries and inconsistent grain sizes could be a center to trap photogenerated carriers and thus increase their recombination, leading to a low PV current. Consequently, constructing efficient channels for the separation and transport of photogenerated carriers in single-phase ferroelectric oxides is particularly desired.
In this work, we developed a high-quality single-crystal PTO film assisted by Ni ions on 0.7 wt.% Nb:SrTiO3 (NSTO) substrate via a coprecipitation-hydrothermal method. Ni ions as an additive effectively regulated the competitive growth of film and by-product powder in the hydrothermal process. The epitaxial relationship of the film with a thickness ranging from 0.7-2.2 μm has been identified to
MATERIALS AND METHODS
Materials
Lead nitrate (Pb(NO3)2, ≥ 99.0%), nickel nitrate hexahydrate [Ni(NO3)2·6H2O, ≥ 98%], tetrabutyl titanate [Ti(OC4H9)4, TBOT, ≥ 98%], potassium hydroxide (KOH, ≥ 85%), ethylene glycol monomethyl ether
Film preparation
The films were synthesized by a coprecipitation-hydrothermal method as follows: NSTO substrate was cleaned ultrasonically with acetone, ethanol, and deionized water before use and then placed in a Teflon holder for drying. Ni(NO3)2·6H2O, Pb(NO3)2, and TBOT were dissolved into ethylene glycol monomethyl ether for the preparation of Ni-Ti-Pb mixed solution. The amount of TBOT was 5 mmol and the Ni/Ti molar ratio was set at 0:100, 1:100, 3:100, 5:100, and 10:100 for film at 0%, 1%, 3%, 5%, and 10% Ni, respectively. The Pb/Ti molar ratio was set at 1.25:1. The mixed metal solution was dripped into excess ammonia water at room temperature. Fine Ni-Ti-Pb coprecipitate could be precipitated in the ammonia solution. After filtering, the Ni-Ti-Pb coprecipitate was added to a 4.5 M potassium hydroxide solution. After 2 h of continuous stirring at room temperature, the NSTO substrate was placed in the solution of the autoclave before hydrothermal treatment at 210 °C for 12 h. NSTO substrate was maintained horizontally 15 mm above the bottom of the autoclave. The resultant products, including film and powder, were washed with water and ethanol several times and subsequently dried at 60 °C in the air.
Characterization
The structures of the sample were characterized by scanning electron microscope (SEM, Hitachi SU-70 Analytical FESEM), X-ray diffraction (XRD, Thermo ARL X’TRA powder diffractometer, Cu Kα radiation, λ = 1.54056 Å), piezoelectric force microscope (PFM, Asylum Research, Cypher S and MFP-3D), and X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB 250Xi). An integrated focused ion beam (FIB, Helios Nanolab 600) and SEM (Hatachi S4700) was used to extract thin-film lamellae from the PTO(Ni)/NSTO sample, and Pt and C layers were deposited on the film surface to protect from damage and increase the conductivity, respectively. Subsequent cross-sectional observations of the film were carried out using an aberration-corrected transmission electron microscope (FEI Titan G2 80-200 Chemi STEM). The variations in composition across the thickness of PTO(Ni) films were characterized by time-of-flight secondary-ion mass spectrometry (TOF-SIMS, IONTOF GmbH TOF.SIMS 5-100).
Photovoltaic measurement
Photovoltaic properties of PTO and PTO(Ni) films were collected using optical units from Thorlabs and source measure units (Keithley 2450). The light from a monochromatic laser passes through a reflector and is vertically incident on the calibration detector (Optical power and energy meter consists of PM100USB and S130VC) or the sample surface. A diaphragm is used to control the on/off state of the light path. Top electrodes (diameter: ~ 1 mm, thickness: ~ 40 nm) were deposited on the film surface by direct current (dc) sputtering (SD-3000). The films were irradiated with light from 405 nm (MDL-III-405) and 375 nm (MDL-III-375) lasers.
RESULTS AND DISCUSSION
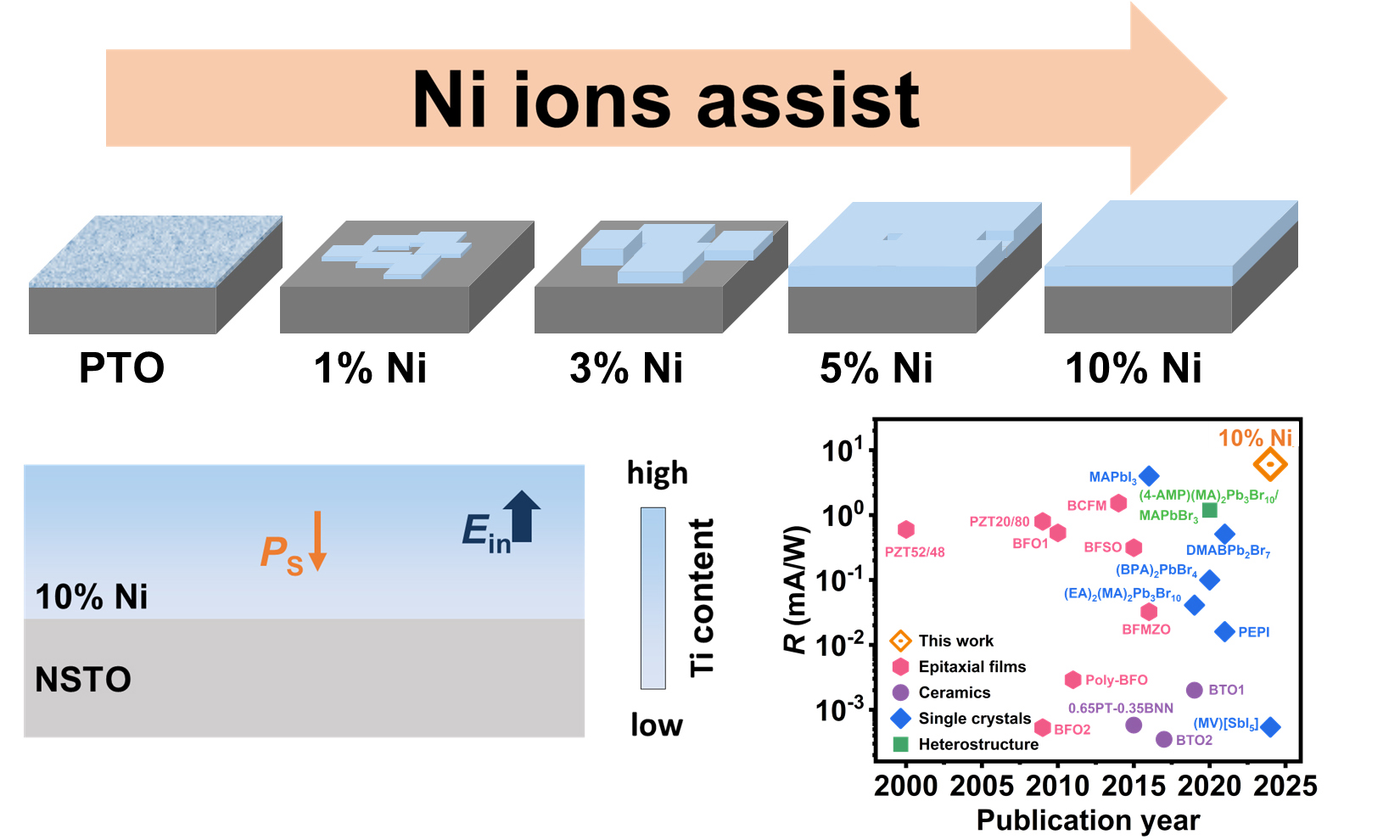
To study the effect of Ni ions on the growth of single-crystal PTO films, different Ni ion concentrations (0, 1, 3, 5 and 10 at.%) were used under the hydrothermal reaction condition. One can identify the epitaxial relationship of {001}PTO(Ni) || {100}NSTO by the X-ray diffraction (XRD) pattern of the tetragonal PTO(Ni) (JCPDS 70-0746) film and cubic NSTO (JCPDS 35-0734) substrate [Supplementary Figure 1A and B]. XRD patterns of the films indicate no slight peak shifts, in contrast to the pronounced peak shift in the by-product powders [Supplementary Figure 1C and D]. This suggests that Ni ions are predominantly localized within the by-product powder rather than the film lattice. We also performed the Rietveld refinement of XRD patterns of by-product powders with different Ni ion concentrations of 0%, 1%, 3%, 5%, and 10% in Supplementary Figure 2A-E. One can observe that all peaks have been well refined. The refined lattice parameters are listed in Supplementary Table 1, which are comparable to those of literature values[26]. Specifically, the refined lattice parameter c decreases from 4.168 to 4.151 Å as the Ni ion concentration increases from 0 to 10%, while a increases slightly from ~ 3.904 to 3.907 Å. The unit cell volumes are found to decrease with increased Ni ion concentration. It could be due to the larger radius of Ni2+ than that of Ti4+. We argue that the introduction of Ni ions is highly likely to play a key role in regulating the nucleation and growth control of the PTO film, rather than merely acting as a doping effect. The morphological changes of films and by-product powders are illustrated in Figure 1A-O, while the corresponding schematic diagrams of the growth are shown in Figure 1P-T. As Figure 1A shows, many fibrous crystallites formed on the substrate in pure PTO condition, with a length and width of ~ 40 nm and ~ 5 nm, respectively. Homogeneous nucleation of particles in the solution is preferred for film growth. In the absence of Ni ions (pure PTO), particle nucleation occurs with a small critical size after a relatively short period (~ 30 min). As the Ni ion concentration increased, the crystallites on the substrate began to extend in the plane, forming a discontinuous film. A possible explanation for this is that when Ni ions were introduced into the coprecipitation process, they could easily enter the lattice of PTO and substitute for Ti ions during the hydrothermal reaction, generating oxygen vacancy defects for a charge balance.
Figure 1. Ni ions-assisted growth of PTO films and by-product powders on NSTO substrate. SEM images of PTO films synthesized via coprecipitation-hydrothermal method from precursor solutions with different Ni ion concentrations of (A) 0%; (B) 1%; (C) 3%; (D) 5%; and (E) 10%. SEM images of by-product powders synthesized from precursor solutions with different Ni ion concentrations of (F) 0%; (G) 1%; (H) 3%; (I) 5%; and (J) 10%. Cross-sectional SEM images of films synthesized from precursor solutions with different Ni ion concentrations of (K) 0%; (L) 1%; (M) 3%; (N) 5%; and (O) 10%. (P-T) The corresponding schematic diagrams of the growth models. PTO: PbTiO3; NSTO: Nb:SrTiO3; SEM: scanning electron microscope.
The substitution of Ni ions and the resulting oxygen vacancies may create a more complex hydrothermal environment where a larger critical nucleation size in homogeneous nucleation is needed to ensure stability. The defects might also hinder the diffusion of ions involved in the reaction, causing a slower consumption rate of raw materials. As a result, the epitaxial growth of the film is promoted by increasing critical nucleation size and slowing raw material consumption. When the Ni ion concentration increased from 1% to 3%, the in-plane size of the discontinuous film also increased from ~ 0.8 μm to 5-15 μm
Time-dependent experiments in Supplementary Figure 4 also illustrate the growth of PTO assisted by Ni ions. During the initial 2 h of the reaction, there are almost no particles observed on the substrate. When the reaction time was prolonged to 4 h, a few particles of ~ 100 nm were formed. A continuous film with some holes and cracks has been obtained after a reaction of 6 h. Subsequently, the film became smooth and flat after about 12 h. As the reaction time is prolonged, PTO particles coarsen to a continuous film that subsequently undergoes a layer-by-layer-like growth without phase transition
The film obtained from precursor solutions with 10 at.% Ni ion concentration was chosen for microstructure investigation, named as PTO(Ni) film. Figure 2A shows the SEM image of PTO(Ni) film, where the surface of the film is smooth and continuous, with a root-mean-square roughness (Rq) of ~ 0.19 nm [Supplementary Figure 6]. Cross-sectional high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of PTO(Ni) film shows a sharp interface and a flat surface, and the thickness was determined to be ~ 2.2 μm [Figure 2B]. The presence of four peaks at an interval of exactly 90° confirms the single-crystal character of PTO(Ni) film by the in-plane XRD off-axis ϕ scan [Supplementary Figure 7A]. Additionally, we chose three regions at different distances from the interface, marked as 1, 2, and 3 in a circle, for selected area electron diffraction (SAED) analysis [Supplementary Figure 7B-E]. The distinct and intense diffraction spots in the SAED patterns further support the single-crystal nature of PTO(Ni) film. The atomic-scale cross-sectional HAADF-STEM image of the PTO(Ni)/NSTO interface is shown in Figure 2C. The interface is atomic-scale smooth without significant elemental interdiffusion.
Figure 2. Characterization of PTO(Ni) film on NSTO substrate. (A) SEM image of PTO(Ni) film; (B) Cross-sectional HAADF-STEM image of PTO(Ni) film; (C) Atomic-scale cross-sectional HAADF-STEM image of PTO(Ni)/NSTO interface; (D) TOF-SIMS spectra of PTO(Ni) film; (E) Ti L2,3 spectra perpendicular to the interface corresponding to the boxes in (B); (F) Out-of-plane PFM phase image of PTO(Ni) film. PTO: PbTiO3; NSTO: Nb:SrTiO3; SEM: scanning electron microscope; HAADF-STEM: high-angle annular dark-field scanning transmission electron microscopy; TOF-SIMS: time-of-flight secondary-ion mass spectrometry; PFM: piezoelectric force microscope.
Furthermore, a composition distribution of PTO(Ni) film can be investigated by TOF-SIMS analysis [Figure 2D]. PTO(Ni) film exhibits a notable composition gradient of Pb and Ti. Off-axis X-ray reciprocal space mapping (RSM) about the 103-diffraction condition of PTO(Ni) film and NSTO substrate supports that the film is c-axis oriented without forming a-domains [Supplementary Figure 7F]. The 103-diffraction peak of film distinctly extends in Qz, reflecting a gradual change in the lattice spacing of (103) planes, arising from the composition gradient. The presence of Pb-vacancy is attributed to the spontaneous formation during the epitaxial growth process of PTO crystals[13,29]. Usually, the decrease in Ti ions may be associated with the introduction of Ni ions and its subsequent occupation of Ti lattice sites. However, the actual Ni ion concentration in the film is substantially lower than our designed concentration based on the XRD results from both films and by-product powders [Supplementary Figure 1]. In addition, electron energy-loss spectrum (EELS) collected at different regions of the film demonstrates a typical four-peak profile at the Ti L2,3 edges [Figure 2B and E], suggesting that no detectable Ti3+ is present near the interface[30,31] to screen the positively charged polar surface of the film. The O K edge [Supplementary Figure 8] indicates that no clear oxygen vacancies have been determined due to the fact that peak 2 is lower than peak 1 at the interface and NSTO interior[32]. Thus, the Ni2+ ions and oxygen vacancies are not detectable in the film based on the above results. The out-of-plane PFM phase image supports that the film adopts a single-domain structure [Figure 2F]. The polarization direction can be determined to be downward, as all Ti atoms shift upward relative to Pb[11]. Therefore, the PTO(Ni) film retains a similar polarization shielding mechanism to the pure PTO film[13].
For PTO, the displacement of Ti ions is directly proportional to the magnitude of polarization, and the direction of displacement is opposite to the direction of polarization[11]. Herein, the displacements of Ti ions were measured at different areas in Figure 3A using atomic-level HAADF-STEM images [Figure 3B-F]. From Figure 3G, the out-of-plane displacements of Ti ions are suppressed near the interface (δc = 0.00781 ± 0.035 Å at the interface) and gradually increase when moving away from the interface throughout the whole film thickness (δc = 0.190 ± 0.054 Å at the surface). The displacements of Ti ions always remain lower than those of bulk PTO (> 0.3 Å)[27,33]. These results imply that PTO(Ni) film exhibits a polarization gradient from the interface to the surface. In theory, the polarization gradient (
Figure 3. The polarization gradient characterization of PTO(Ni) film. (A) Cross-sectional HAADF-STEM image of PTO(Ni)/NSTO heterostructure, where ∆d represents the distances from the interface; (B-F) Atomic-scale cross-sectional HAADF-STEM images of regions with different ∆d in PTO(Ni) film: (B) ∆d ≈ 2,200 nm; (C) ∆d ≈ 1,000 nm; (D) ∆d ≈ 500 nm; (E) ∆d ≈ 0 nm; (F) ∆d ≈ -500 nm, corresponding to boxes in (A); (G) Experimentally extracted off-center displacements of the Ti ions as a function of the distance from the interface according to B-F. Insets are the schematic illustrations of the average displacement measurement of Ti4+ relative to the center of the four nearest Pb2+ in each of the corresponding regions, where the δc denotes the off-center displacements of Ti4+ along the c-axis. Error bars are indicative of the standard deviations (s.d.) of the displacements; (H) Schematic illustration of the polarization gradient throughout PTO(Ni) film. PS denotes the spontaneous polarization, and Ein denotes the built-in field. PTO: PbTiO3; HAADF-STEM: high-angle annular dark-field scanning transmission electron microscopy; NSTO: Nb:SrTiO3.
We prepared pure PTO film[13] with a thickness of ~ 2.3 μm via a direct hydrothermal method
Figure 4. PV performance between pure PTO and PTO(Ni) film. J-V curve of pure PTO and PTO(Ni) film under the irradiation of 600 mW/cm2Ilight from (A) 405 nm and (B) 375 nm laser; (C) Short-circuit current density (JSC) of pure PTO and PTO(Ni) film at various light intensities. The inset depicts the excellent response of PTO(Ni) film at low light intensities (0.3-4.22 mW/cm2); (D) Photoresponsivity (R) of PTO(Ni) film (this work) compared with previously reported perovskites under blue light. The pink hexagons, purple circles, blue diamonds, and green squares represent epitaxial films, ceramics, single crystals, and heterostructure, respectively. PV: photovoltaic; PTO: PbTiO3.
CONCLUSIONS
In summary, a single-crystal, single-domain PTO(Ni) film on an NSTO substrate was successfully prepared using a coprecipitation-hydrothermal method. The introduction of Ni ions into the precursor solution effectively regulated the competition growth of the film and the by-product powder, enabling the formation of a Ti-vacancy composition gradient. The coupling of the composition gradient with spontaneous polarization generates a polarization-gradient structure, largely enhancing the visible-light PV current up to 3.6 mA/cm2. These findings may establish a unique low-temperature solution-based method, assisted by ions, to prepare single-crystal, single-domain films with gradient structures, thus opening new avenues for the growth of other ferroelectric oxides. Moreover, it may spark interest in developing PTO as a potential material for passive visible light detection devices and novel ferroelectric-based optoelectronic devices[59].
DECLARATIONS
Acknowledgments
Ren, Z. and Han, G. acknowledge funding from the National Key R&D Program of China (2023YFA1406404), the National Natural Science Foundation of China (52272129), the Natural Science Foundation of Zhejiang Province, China (LR21E020004), the Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering (2021SZ-FR007), and Fundamental Research Funds for the Central Universities (226-2023-00064).
Authors’ contributions
Materials synthesis, Photovoltaic measurement: Sun, Y.; Chen, J.; Fu, Y.
Mechanism explanation, data acquisition and analysis: Zhang, R.; Chen, J.; Lin, C; Ren, Z.; Tian, H.
Methodology, initial draft writing, and manuscript revision: Sun, Y.; Zhang, R.; Chen, J.; Lin, C.
Supervision, manuscript revision, and funding acquisition: Ren, Z.; Han, G.
All authors have reviewed and approved the final version of the manuscript.
Availability of data and materials
The rata data supporting the findings of this study are available within this Article and its Supplementary Materials. Further data are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This study was supported by the National Key R&D Program of China (2023YFA1406404), the National Natural Science Foundation of China (52272129), the Natural Science Foundation of Zhejiang Province, China (LR21E020004), the Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering (2021SZ-FR007), and Fundamental Research Funds for the Central Universities (226-2023-00064).
Conflicts of interest
The authors declared that there are no conflicts of interest.
Content of Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
©The Author(s) 2025.
REFERENCES
1. Kreisel, J.; Alexe, M.; Thomas, P. A. A photoferroelectric material is more than the sum of its parts. Nat. Mater. 2012, 11, 260.
2. Aydin, E.; Ugur, E.; Yildirim, B. K.; et al. Enhanced optoelectronic coupling for perovskite/silicon tandem solar cells. Nature 2023, 623, 732-8.
3. Lin, H.; Yang, M.; Ru, X.; et al. Silicon heterojunction solar cells with up to 26.81% efficiency achieved by electrically optimized nanocrystalline-silicon hole contact layers. Nat. Energy. 2023, 8, 789-99.
4. Choi, T.; Lee, S.; Choi, Y. J.; Kiryukhin, V.; Cheong, S. W. Switchable ferroelectric diode and photovoltaic effect in BiFeO3. Science 2009, 324, 63-6.
5. Fridkin, V. M. Bulk photovoltaic effect in noncentrosymmetric crystals. Crystallogr. Rep. 2001, 46, 654-8.
6. Yang, S. Y.; Seidel, J.; Byrnes, S. J.; et al. Above-bandgap voltages from ferroelectric photovoltaic devices. Nat. Nanotechnol. 2010, 5, 143-7.
7. Xiao, Z.; Yuan, Y.; Shao, Y.; et al. Giant switchable photovoltaic effect in organometal trihalide perovskite devices. Nat. Mater. 2015, 14, 193-8.
8. Grinberg, I.; West, D. V.; Torres, M.; et al. Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials. Nature 2013, 503, 509-12.
9. Dalba, G.; Soldo, Y.; Rocca, F.; Fridkin, V. M.; Sainctavit, P. Giant bulk photovoltaic effect under linearly polarized X-ray synchrotron radiation. Phys. Rev. Lett. 1995, 74, 988-91.
10. Yan, Y.; Zhou, J. E.; Maurya, D.; Wang, Y. U.; Priya, S. Giant piezoelectric voltage coefficient in grain-oriented modified PbTiO3 material. Nat. Commun. 2016, 7, 13089.
11. Tang, Y. L.; Zhu, Y. L.; Ma, X. L.; et al. Ferroelectrics. Observation of a periodic array of flux-closure quadrants in strained ferroelectric PbTiO3 films. Science 2015, 348, 547-51.
12. Liu, Y.; Ye, S.; Xie, H.; et al. Internal-field-enhanced charge separation in a single-domain ferroelectric PbTiO3 photocatalyst. Adv. Mater. 2020, 32, e1906513.
13. Lin, C.; Zhang, Z.; Dai, Z.; et al. Solution epitaxy of polarization-gradient ferroelectric oxide films with colossal photovoltaic current. Nat. Commun. 2023, 14, 2341.
14. Liu, H.; Chen, J.; Ren, Y.; et al. Large photovoltage and controllable photovoltaic effect in PbTiO3-Bi(Ni2/3+xNb1/3-x)O3-δ ferroelectrics. Adv. Elect. Mater. 2015, 1, 1400051.
15. Pang, D.; Liu, X.; He, X.; Chen, C.; Zheng, J.; Yi, Z. Anomalous photovoltaic effect in Bi(Ni2/3Ta1/3)O3-PbTiO3 ferroelectric solid solutions. J. Am. Ceram. Soc. 2019, 102, 3448-56.
16. Bobić, J. D.; Katiliute, R. M.; Ivanov, M.; et al. Dielectric, ferroelectric and magnetic properties of La doped Bi5Ti3FeO15 ceramics. J. Mater. Sci:. Mater. Electron. 2016, 27, 2448-54.
17. Wu, L.; Burger, A. M.; Bennett-jackson, A. L.; Spanier, J. E.; Davies, P. K. Polarization-modulated photovoltaic effect at the morphotropic phase boundary in ferroelectric ceramics. Adv. Elect. Materials. 2021, 7, 2100144.
18. Ke, X.; Wang, D.; Ren, X.; Wang, Y. Polarization spinodal at ferroelectric morphotropic phase boundary. Phys. Rev. Lett. 2020, 125, 127602.
19. Bennett, J. W.; Grinberg, I.; Rappe, A. M. New highly polar semiconductor ferroelectrics through d8 cation-O vacancy substitution into PbTiO3: a theoretical study. J. Am. Chem. Soc. 2008, 130, 17409-12.
20. Gou, G. Y.; Bennett, J. W.; Takenaka, H.; Rappe, A. M. Post density functional theoretical studies of highly polar semiconductive Pb(Ti1-xNix )O3-x solid solutions: effects of cation arrangement on band gap. Phys. Rev. B. 2011, 83.
21. Wang, F.; Young, S. M.; Zheng, F.; Grinberg, I.; Rappe, A. M. Substantial bulk photovoltaic effect enhancement via nanolayering. Nat. Commun. 2016, 7, 10419.
22. Zheng, T.; Deng, H.; Zhou, W.; et al. Bandgap modulation and magnetic switching in PbTiO3 ferroelectrics by transition elements doping. Ceram. Int. 2016, 42, 6033-8.
23. Zhou, W.; Deng, H.; Yu, L.; Yang, P.; Chu, J. Optical band-gap narrowing in perovskite ferroelectric ABO3 ceramics (A = Pb, Ba; B = Ti) by ion substitution technique. Ceram. Int. 2015, 41, 13389-92.
24. Zhao, C.; Luo, B.; Guo, S.; Chen, C. Enhanced electrical and photocurrent characteristics of sol-gel derived Ni-doped PbTiO3 thin films. Ceram. Int. 2017, 43, 7861-5.
25. Li, X.; Wang, X.; Peng, L.; Zhang, K.; Wu, W.; Tang, Y. Ferroelectric thin film on a silicon-based pn junction: Coupling photovoltaic properties. Ferroelectrics 2016, 500, 250-8.
26. Joseph, J.; Vimala, T. M.; Sivasubramanian, V.; Murthy, V. R. K. Structural investigations on Pb(ZrxT1-x)O3 solid solutions using the X-ray Rietveld method. J. Mater. Sci. 2000, 35, 1571-5.
27. Ren, Z.; Wu, M.; Chen, X.; et al. Electrostatic force-driven oxide heteroepitaxy for interface control. Adv. Mater. 2018, 30, e1707017.
28. Luo, B. Role of the defect in determining the properties of PbTi0.9Ni0.1O3 thin films. J. Appl. Phys. 2017, 122, 195104.
29. Zhang, Z.; Wu, P.; Lu, L.; Shu, C. Study on vacancy formation in ferroelectric PbTiO3 from ab initio. Appl. Phys. Lett. 2006, 88, 142902.
30. Ohtomo, A.; Muller, D. A.; Grazul, J. L.; Hwang, H. Y. Artificial charge-modulationin atomic-scale perovskite titanate superlattices. Nature 2002, 419, 378-80.
31. Torres-pardo, A.; Gloter, A.; Zubko, P.; et al. Spectroscopic mapping of local structural distortions in ferroelectric PbTiO3/SrTiO3 superlattices at the unit-cell scale. Phys. Rev. B. 2011, 84.
32. Ryu, J.; Han, G.; Song, T. K.; et al. Upshift of phase transition temperature in nanostructured PbTiO3 thick film for high temperature applications. ACS. Appl. Mater. Interfaces. 2014, 6, 11980-7.
33. Ren, Z.; Zhao, R.; Chen, X.; et al. Mesopores induced zero thermal expansion in single-crystal ferroelectrics. Nat. Commun. 2018, 9, 1638.
34. Mantese, J. V.; Schubring, N. W.; Micheli, A. L.; Catalan, A. B. Ferroelectric thin films with polarization gradients normal to the growth surface. Appl. Phys. Lett. 1995, 67, 721-3.
35. Zhang, J.; Xu, R.; Damodaran, A. R.; Chen, Z.; Martin, L. W. Understanding order in compositionally graded ferroelectrics: flexoelectricity, gradient, and depolarization field effects. Phys. Rev. B. 2014, 89.
36. Marvan, M.; Chvosta, P.; Fousek, J. Theory of compositionally graded ferroelectrics and pyroelectricity. Appl. Phys. Lett. 2005, 86, 221922.
37. Li, H.; Zhang, H.; Wang, Y.; Tang, Y.; Zhu, Y.; Ma, X. Misfit strain-misfit strain phase diagram of (110)-oriented ferroelectric PbTiO3 films: a phase-field study. Microstructures 2024, 4, 2024004.
38. Zhang, L.; Chen, J.; Fan, L.; et al. Giant polarization in super-tetragonal thin films through interphase strain. Science 2018, 361, 494-7.
39. Sturman, B. I. Ballistic and shift currents in the bulk photovoltaic effect theory. Phys. -Usp. 2020, 63, 407-11.
40. Li, Y.; Fu, J.; Mao, X.; et al. Enhanced bulk photovoltaic effect in two-dimensional ferroelectric CuInP2S6. Nat. Commun. 2021, 12, 5896.
41. Nakashima, S.; Takayama, K.; Shigematsu, K.; Fujisawa, H.; Shimizu, M. Growth of epitaxial Mn and Zn codoped BiFeO3 thin films and an enhancement of photovoltage generated by a bulk photovoltaic effect. Jpn. J. Appl. Phys. 2016, 55, 10TA07.
42. Ma, N.; Zhang, K.; Yang, Y. Photovoltaic-pyroelectric coupled effect induced electricity for self-powered photodetector system. Adv. Mater. 2017, 29.
43. Ma, N.; Yang, Y. Boosted photocurrent via cooling ferroelectric BaTiO3 materials for self-powered 405 nm light detection. Nano. Energy. 2019, 60, 95-102.
44. Lei, Y.; Hao, W.; Wang, S.; et al. Bulk photovoltaic effect of a hybrid ferroelectric semiconductor. Phys. Rev. B. 2024, 109.
45. Han, S.; Li, M.; Liu, Y.; et al. Tailoring of a visible-light-absorbing biaxial ferroelectric towards broadband self-driven photodetection. Nat. Commun. 2021, 12, 284.
46. Ma, Y.; Wang, J.; Liu, Y.; et al. High performance self-powered photodetection with a low detection limit based on a two-dimensional organometallic perovskite ferroelectric. J. Mater. Chem. C. 2021, 9, 881-7.
47. Zhang, X.; Ji, C.; Liu, X.; et al. Solution-grown large-sized single-crystalline 2d/3d perovskite heterostructure for self-powered photodetection. Adv. Optic. Mater. 2020, 8, 2000311.
48. Ji, C.; Dey, D.; Peng, Y.; Liu, X.; Li, L.; Luo, J. Ferroelectricity-driven self-powered ultraviolet photodetection with strong polarization sensitivity in a two-dimensional halide hybrid perovskite. Angew. Chem. Int. Ed. Engl. 2020, 59, 18933-7.
49. Liu, X.; Wang, S.; Long, P.; et al. Polarization-driven self-powered photodetection in a single-phase biaxial hybrid perovskite ferroelectric. Angew. Chem. Int. Ed. Engl. 2019, 58, 14504-8.
50. Ding, J.; Fang, H.; Lian, Z.; et al. A self-powered photodetector based on a CH3NH3PbI3 single crystal with asymmetric electrodes. CrystEngComm 2016, 18, 4405-11.
51. Fan, Z.; Ji, W.; Li, T.; et al. Enhanced photovoltaic effects and switchable conduction behavior in BiFe0.6Sc0.4O3 thin films. Acta. Materialia. 2015, 88, 83-90.
52. Gupta, S.; Tomar, M.; Gupta, V. Ferroelectric photovoltaic properties of Ce and Mn codoped BiFeO3 thin film. J. Appl. Phys. 2014, 115, 014102.
53. Sharma, S.; Tomar, M.; Gupta, V. Effect of top metal contact on the ferroelectric photovoltaic response of BFO thin film capacitors. Vacuum 2018, 158, 117-20.
54. Ji, W.; Yao, K.; Liang, Y. C. Bulk photovoltaic effect at visible wavelength in epitaxial ferroelectric BiFeO3 thin films. Adv. Mater. 2010, 22, 1763-6.
55. Xu, J.; Cao, D.; Fang, L.; Zheng, F.; Shen, M.; Wu, X. Space charge effect on the photocurrent of Pt-sandwiched Pb(Zr0.20Ti0.80)O3 film capacitors. J. Appl. Phys. 2009, 106, 113705.
56. Yang, S. Y.; Martin, L. W.; Byrnes, S. J.; et al. Photovoltaic effects in BiFeO3. Appl. Phys. Lett. 2009, 95, 062909.
57. Yarmarkin, V. K.; Gol’tsman, B. M.; Kazanin, M. M.; Lemanov, V. V. Barrier photovoltaic effects in PZT ferroelectric thin films. Phys. Solid. State. 2000, 42, 522-7.
58. Shimada, T.; Ueda, T.; Wang, J.; Kitamura, T. Hybrid Hartree-Fock density functional study of charged point defects in ferroelectric PbTiO3. Phys. Rev. B. 2013, 87.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].