IP3 receptors contribute to muscle atrophy and maintain ribosomal RNA content during 3-day hind limb suspension in rats
Abstract
Aim: Skeletal muscle unloading leads to the upregulation of proteolytic gene expression, downregulation of protein synthesis markers, and the development of muscle atrophy. These changes are accompanied by alterations in calcium signaling. We investigated the role of Inositol 1,4,5-triphosphate (IP3) receptors (IP3Rs) in regulating calcium signaling and controlling gene expression in skeletal muscles during unloading.
Methods: Male Wistar rats were randomly assigned to 4 groups (n = 8 per group). C - vivarium control; C+2APB - vivarium control with daily intraperitoneal injections of the IP3 receptor inhibitor 2-aminoethoxydiphenyl borate
Results: Three days of unloading resulted in a significant increase in nuclear phosphorylated Ca2+/calmodulin-dependent protein kinase II (p-CaMK II) content and calcineurin (CaN) expression compared to the C group (P < 0.05); this effect was prevented in the HU+2APB group. In HU+2APB rats, the decline in soleus muscle weight-to-body weight ratio was partially prevented, and the downregulation of protein synthesis markers (as seen in the HU group) was also prevented. However, proteolytic signaling markers were equally upregulated in the HU+2APB and HU groups compared to C.
Conclusion: IP3 receptor inhibition during 3-day hind limb suspension in rats partially prevented the decline in
Keywords
INTRODUCTION
Skeletal muscle unloading leads to atrophy and alterations in muscle function. This phenomenon is observed during bed rest-induced hypokinesia, limb immobilization, and microgravity conditions experienced during spaceflight.
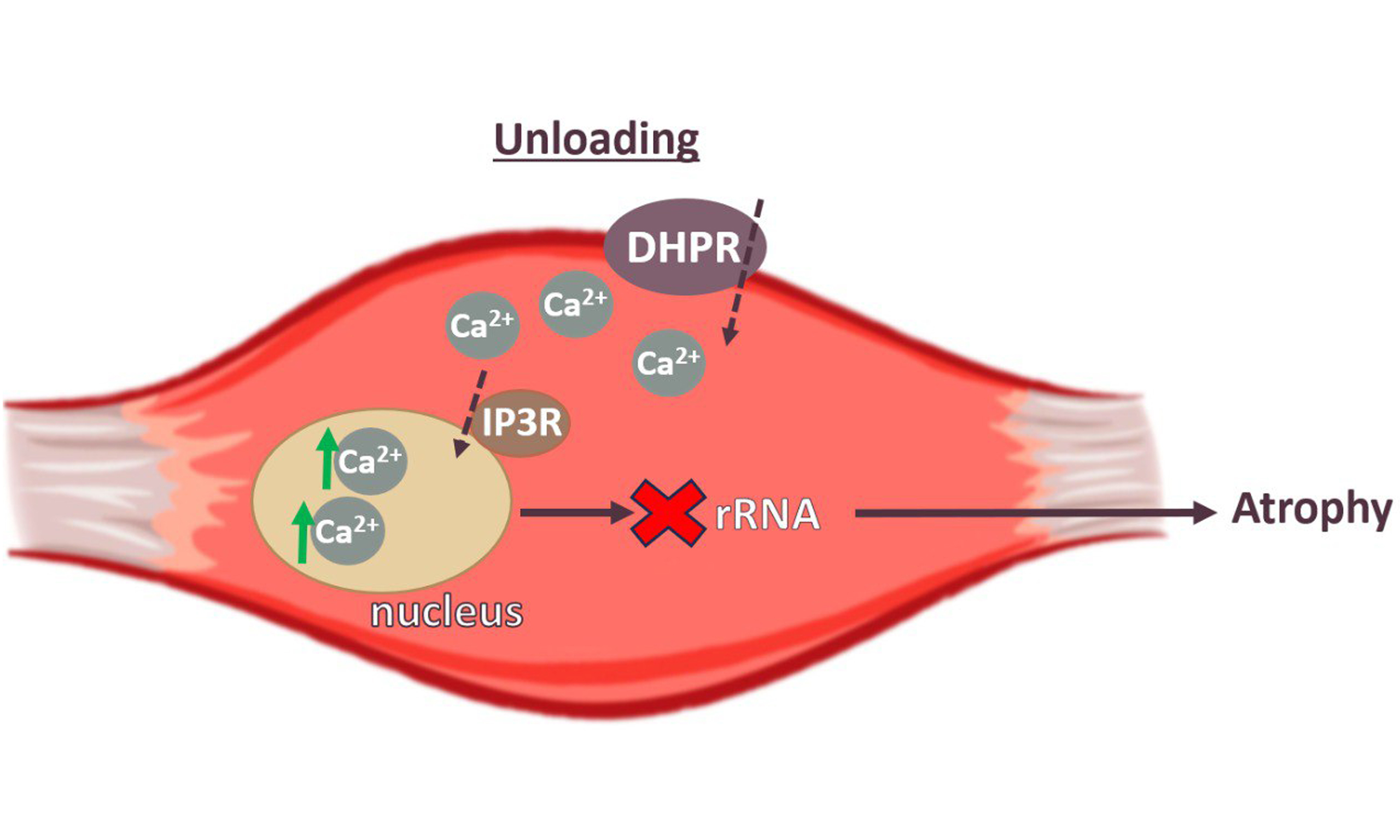
Evidence suggests that calcium signaling may contribute to unloading-induced muscle atrophy. Previous studies have demonstrated an accumulation of Ca2+ ions in the myoplasm[1,2] and myonuclei[3]
In addition to its direct involvement in muscle atrophy through the activation of proteolysis, calcium signaling is also known to regulate gene transcription[6-9]. Muscle unloading leads to a switch off of electrical muscle activity, associated with a decrease in membrane potential within 6-12 h of hindlimb suspension in rats[10-12]. The dihydropyridine receptor (DHPR), normally a voltage-dependent calcium channel, primarily functions in skeletal muscle as a voltage sensor, triggering intracellular calcium release required for excitation-contraction coupling[13]. Inositol 1,4,5-triphosphate receptors (IP3Rs) may participate as downstream targets of DHPR-dependent regulation[6,14], as shown in studies using cultured muscle cells and isolated adult flexor digitorum brevis (FDB) fibers.
IP3R-dependent slow Ca+2 signals may participate in the activation of specific transcriptional programs that determine slow and fast muscle fiber phenotypes[13,15]. Calcium release from the sarcoplasmic reticulum (SR) occurs via both ryanodine receptors (RyRs) and IP3Rs. Notably, IP3Rs are also capable of modulating mitochondrial calcium fluxes[16,17]. The activity of IP3Rs is regulated by Ca2+ ions and IP3, with the strength of the calcium signal varying depending on IP3 concentration[18,19].
To date, the role of IP3Rs in regulating atrophic processes during muscle unloading is unknown. We hypothesize that calcium accumulation in the myoplasm during functional unloading can activate IP3Rs and upregulate nucleoplasmic calcium levels. An increase in nuclear calcium level may, in turn, affect gene expression by activating proteolytic signaling cascades and/or inhibiting the expression of genes related to protein synthesis. To test this hypothesis, we inhibited IP3 receptors during functional unloading of the
METHODS
Ethical approval
All experimental procedures were conducted in accordance with internationally accepted regulations and the ARRIVE guidelines[22]. All experiments and procedures adhered to bioethical principles and were approved by the Biomedical Ethics Commission of the State Scientific Center of the Russian Federation, Institute of Biomedical Problems, Russian Academy of Sciences (Protocol No. 627, dated December 6, 2022).
Experimental design
Muscle unloading was modeled by rat hind limb suspension using the standard Ilyin-Novikov method, modified by Morey-Holton[23]. The suspension was performed so that the hind limbs of the rats did not touch the floor, while their front limbs rested freely on the floor. Thanks to the unique design of the experimental apparatus, the animals were able to move freely. The rats were suspended by their tails on special soft tires at a 45-degree angle to the cage floor. Food and water were provided ad libitum.
Thirty-two male Wistar rats aged 1.5-2 months were divided into four groups (n = 8 each): vivarium control with the administration of placebo (С); vivarium control with the administration of 2-APB (C+2APB); placebo-injected hind limb unloading group (HU); and 2-APB-treated hind limb unloading group (HU+2APB). 2-APB was administered intraperitoneally at a dose of 10 mg/kg b.w. in 5% Dimethyl sulfoxide (DMSO) daily, following previously published in vivo studies[24,25].
After 3 days of the experiment, the rats were anesthetized with avertin (5 mL/kg body weight of a 10% solution), and the m. soleus was isolated, immediately frozen in liquid nitrogen, and stored at -85 °C until analysis. Euthanasia of rats was performed by cervical dislocation under avertin anesthesia.
Electrophoresis and Western blotting
From each m. soleus sample of the experimental animals, sections were cut at a thickness of 20 μm using a Leica microtome-cryostat (total weight: 40-60 mg per sample). The cytoplasmic and nuclear protein fractions were isolated using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Scientific, USA) according to the manufacturer’s instructions. Polyacrylamide gel (PAAG) electrophoresis and Western blot analyses were performed as described previously[26].
The list of primarly antibodies for Western bloting: to detect protein bands, primary antibodies were used against IP3R (ThermoFisher #PA5-96855 1:1,000), Lamin B1 (Cell Signaling #12586 1:1,000), p-CaMKIIb (Cell Signaling, #12716, 1:1,000), CaMKII (Cell Signaling, #3362, 1:1,000), p70 (Cell Signaling #9202, 1:1,000), Akt (Cell Signaling, #2990 1:1,500), phosphorylated eukaryotic elongation factor 2 (p-eEF2) (Cell Signaling, #2331 1:1,000), eukaryotic elongation factor 2 (eEF2) (Cell Signaling, #2332 1:1,000), p-p70S6K (Santa Cruz Biotechnology, sc-11759 1:1,000), Calcineurin A (ThermoFisher # PA5-17446 1:1,000), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ABM, G041 1:10,000).
Protein levels in cytoplasmic fractions were normalized to GAPDH, while nuclear IP3R levels were normalized to lamin B1. Phosphorylated proteins were normalized to their respective total protein levels. The scanned images were then processed using the supplied Image Studio Software (LI-COR) and analyzed as described previously[26].
Real-time reverse transcription polymerase chain reaction
To study gene expression using real-time polymerase chain reaction (PCR), RNA was isolated from muscle tissue samples. RNA extraction was performed using RNeasy Micro columns (Qiagen, Germany) according to the manufacturer’s instructions. Reverse transcription was carried out using reagents from Synthol (Russia). Real-time PCR was performed using a real-time PCR kit with the addition of SYBR Green (Synthol, Russia), following the manufacturer’s recommendations. Expression of the YWHAZ gene was used for normalization. The sequences of the primers used in this study are listed in Table 1. Gene expression levels were quantified using the Pffafl method for PCR data analysis[27]. For each gene, each PCR reaction was conducted at least 3 times.
Primers that were used in the study
| Gene name | Primer sequence |
| MuRF-1 | CCT-GAG-AGC-CAT-TGA-CTT-TGG CTT-CCC-TTC-TGT-GGA-CTC-TTC-CT |
| Atrogin-1 | GCA-GCT-GAA-CAA-CAT-TCA-GAT-CAC CAG-CCT-CTG-CAT-GAT-GTT-CAG-T |
| Calpain-1 | TGT-CGG-AGG-AGA-TCA-TCA-CG TCT-TGG-AGG-AAT-TGG-GAC-CC-C |
| Ulk | GCA-AGG-ACT-CTT-CCT-GTG-ACA-C CCA-CTG-CAC-ATC-AGG-CTG-TCT-G |
| YWHAZ | CCC-ACT-CCG-GAC-ACA-GAA-TA TGT-CAT-CGT-ATC-GCT-CTG-CC |
RNA electrophoresis
Total RNA, isolated as described previously, was mixed with an equal volume of denaturing loading buffer (Thermo Scientific, USA) and heated at 70 °C for 10 min, following the manufacturer’s instructions. Electrophoresis was performed on a 1.2% agarose gel prepared in tris borate EDTA (TBE) buffer and containing ethidium bromide (0.5 μg/mL). The volume of RNA sample for electrophoresis was normalized to the total weight of the tissue sample from which the RNA was extracted. Electrophoresis was carried out in TBE buffer at a voltage of 10 V/cm of gel length. RiboRuler markers (Thermo Scientific, USA) were used to estimate the sizes of RNA bands. The electrophoresis results were visualized using a Gel Doc EZ Imager (Bio-Rad, USA). Image Studio Digits v. 4.0 software was used to calculate the results. The extent of RNA degradation was assessed by calculating the ratio of 28S RNA to 18S RNA.
Statistical data processing
Data were tested for normal distribution, and since all data were normally distributed, differences
RESULTS
After the experiment, body weights of the rats did not differ between groups [Table 2]. Soleus muscle weights were also comparable between the C and C+2APB groups. In contrast, the HU and HU+2APB groups showed significantly reduced soleus muscle weights compared to both the C and C+2APB groups [Table 2]. Soleus muscle weight normalized to rat body weight (soleus weight index) did not differ between the C and C+2APB groups [Table 2]. After 3 days of hind limb unloading, the soleus muscle weight was 15% lower in the HU group than in the C and C+2APB groups (P < 0.05). In the HU+2APB group, the soleus muscle weight index did not significantly differ from any other experimental group.
Rat weights, soleus muscle weights and m. soleus weight ratio to animal body weight
| Group | Rat weight, g | Soleus muscle weight, mg | Soleus weight index, mg/g |
| C | 210 ± 5.1 | 83.4 ± 1.9 | 0.4 ± 0.01 |
| C+2APB | 210 ± 5.8 | 81.6 ± 4.1 | 0.37 ± 0.02 |
| HU | 199 ± 4.8 | 66.1 ± 2.2 * ** | 0.33 ± 0.01 * ** |
| HU+2APB | 195 ± 6.0 | 70.7 ± 1.8 * ** | 0.36 ± 0.01 |
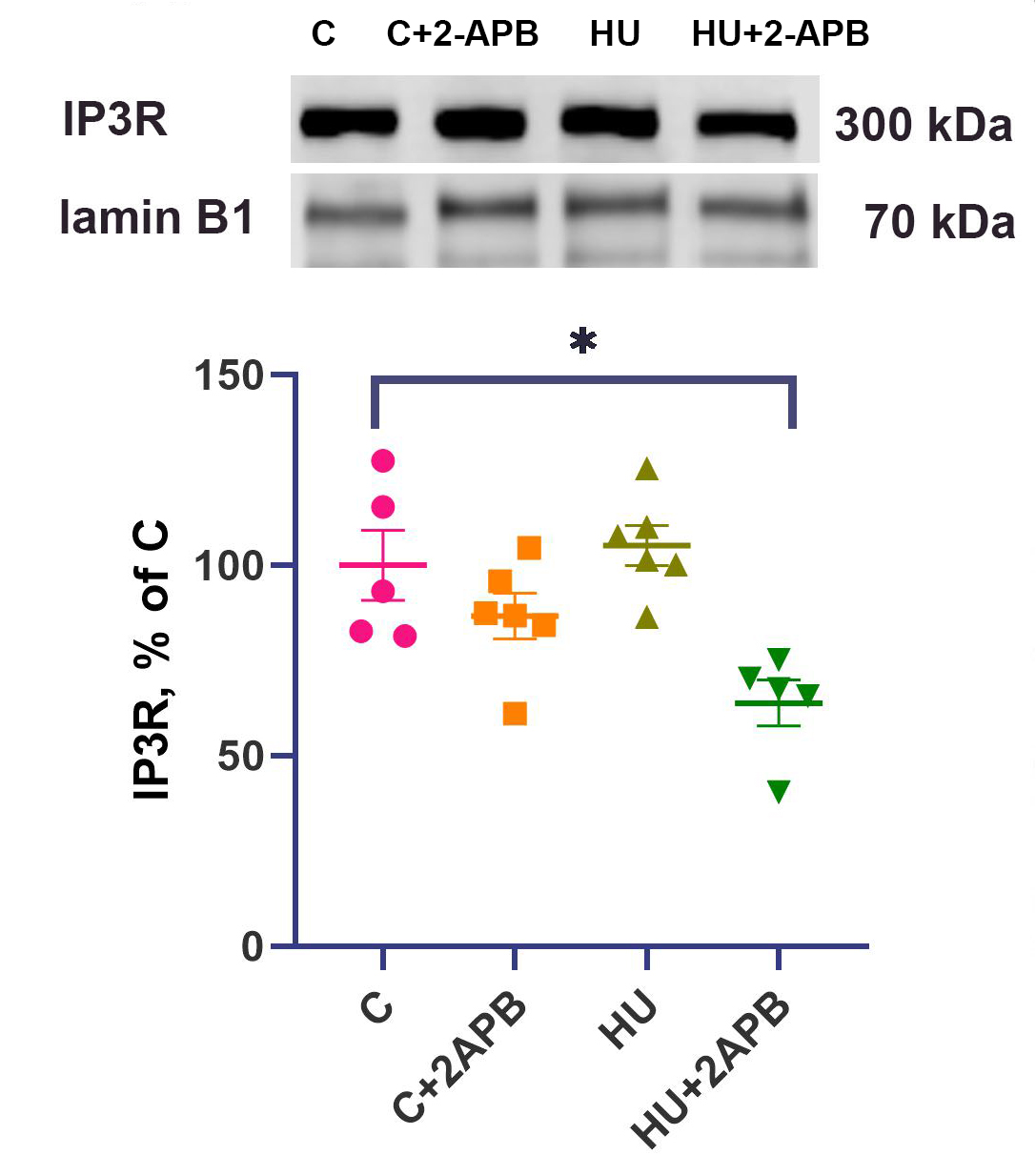
After 3 days of hind limb suspension, the nuclear content of IP3R did not differ significantly between the HU and C groups [Figure 1]. No significant differences in nuclear IP3R content or mRNA expression were observed between the C and C+2APB groups. In contrast, the nuclear content of IP3R in the HU+2APB group was significantly lower than in the C group [Figure 1].
Figure 1. Nuclear IP3R content normalized to lamin B1 content. Data are shown as mean value±SEM. * - significantly different from the C group (P < 0.05). IP3R: Inositol 1,4,5-triphosphate receptors; SEM: standard error of the mean.
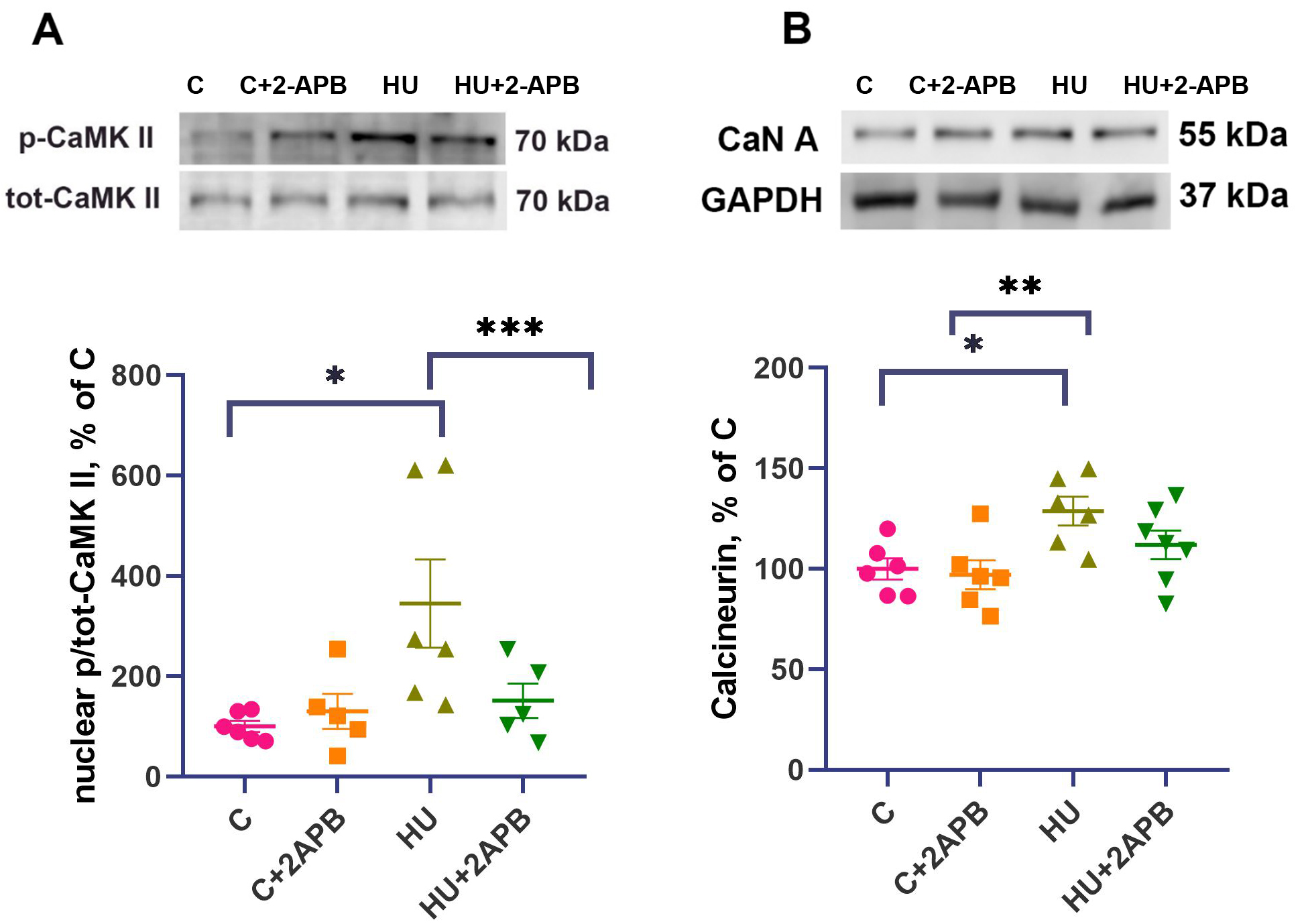
The content of phosphorylated Ca2+/calmodulin-dependent protein kinase II (CaMK II) in the nuclear fraction of soleus muscles was significantly upregulated 4-fold in the HU group compared to the C and C+2APB groups [Figure 2A], while the C and HU+2APB groups did not differ significantly from each other. The phosphorylated Ca2+/calmodulin-dependent protein kinase II (p-CaMK II) content in the HU+2APB group was 71% higher than in the C group but did not differ from any other experimental group. The content of calcineurin A in the cytosolic fraction of soleus muscles did not differ between the C and C+2APB groups, whereas the HU group showed a significantly higher CaN A content than in the C and C+2APB groups [Figure 2B]. CaN A content in the HU+2APB group did not significantly differ from any other experimental group.
Figure 2. Nuclear p/tot-CaMK II content (A) and calcineurin A content (B). Data are shown as mean value±SEM. * - significant differences from the C group (P < 0.05). ** - significantly different from the C+2APB group, *** - significantly different from the HU group. HU: Hind limb unloading; p/tot-CaMK II: phosphorylated-to-total calcium calmodulin kinase; SEM: standard error of the mean; CaN A: calcineurin A; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
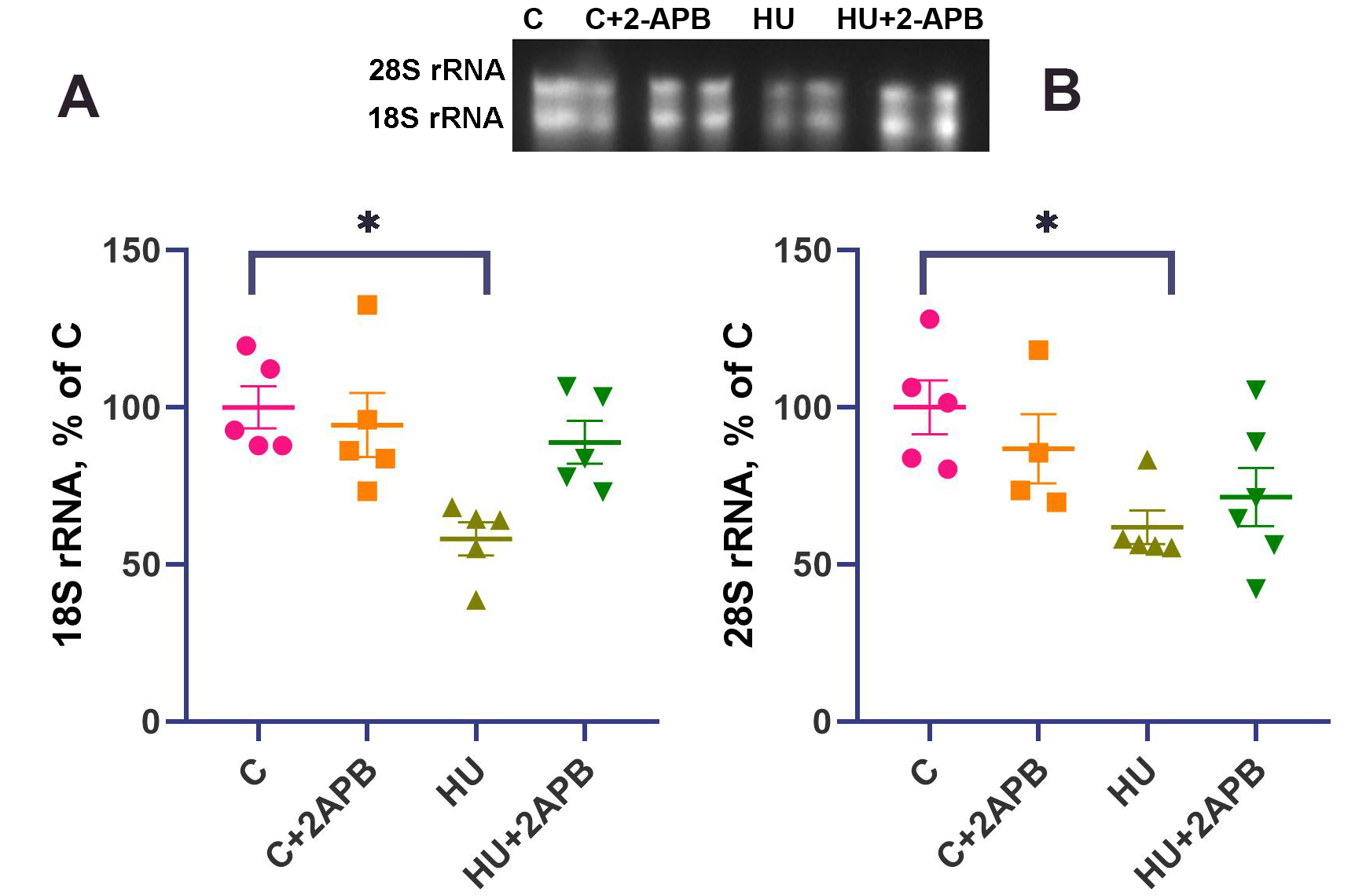
The content of both 18S rRNA and 28S rRNA did not differ between the C and C+2APB groups [Figure 3A and B]. In the HU group, the levels of both 18S and 28S rRNA were decreased by 40% compared to the C group and were significantly lower. In the HU+2APB group, the 18S rRNA and 28S rRNA contents did not significantly differ from those in any other experimental group.
Figure 3. 18S rRNA content (A) and 28S rRNA content (B). Data are shown as mean value±SEM. * - significantly different from the C group (P < 0.05). HU: Hind limb unloading; SEM: standard error of the mean.
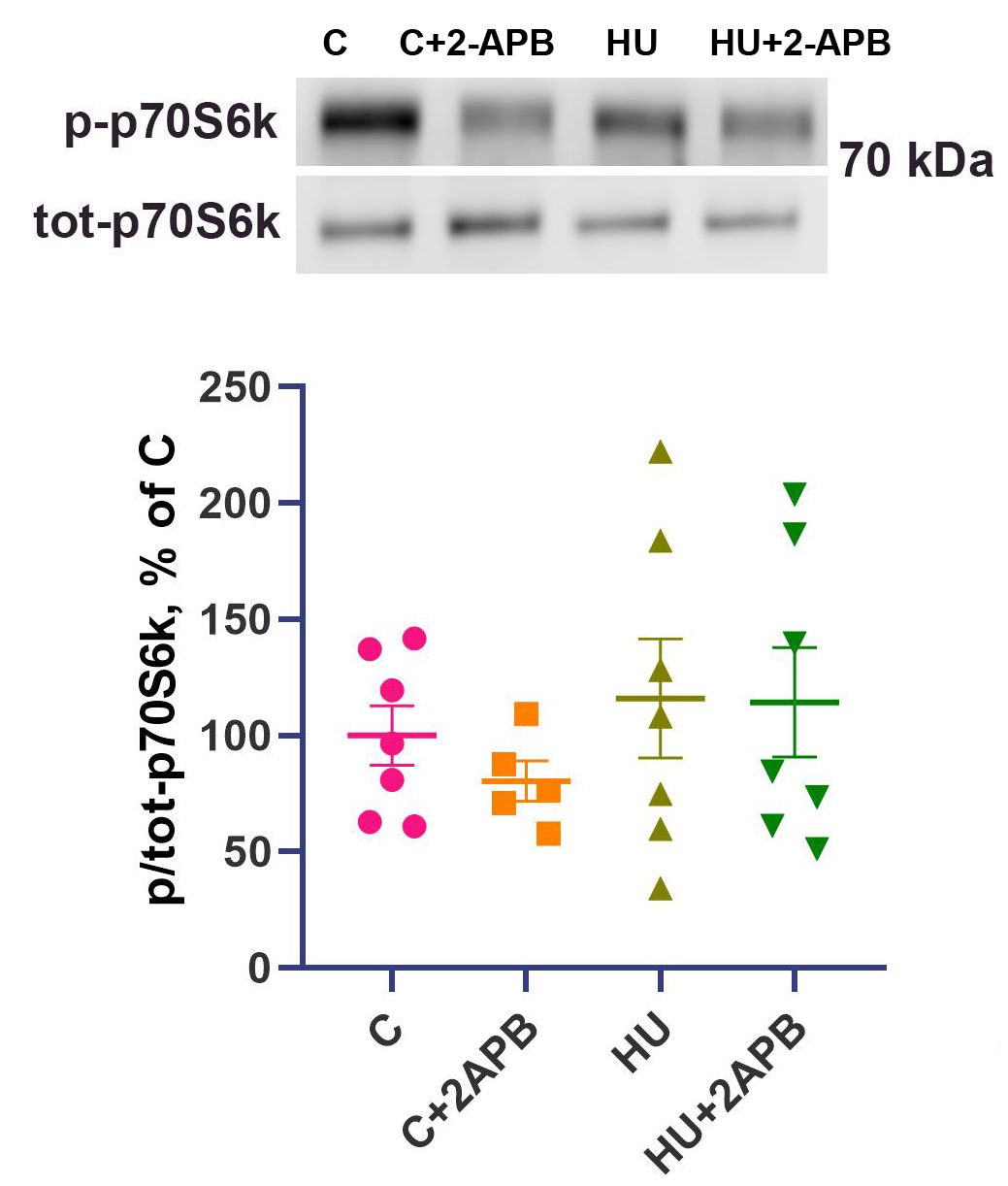
The content of phosphorylated p70 S6 kinase did not differ among the experimental groups [Figure 4].
Figure 4. p/tot-p70 S6 kinase content. Data are shown as mean value±SEM. HU: Hind limb unloading; SEM: standard error of the mean.
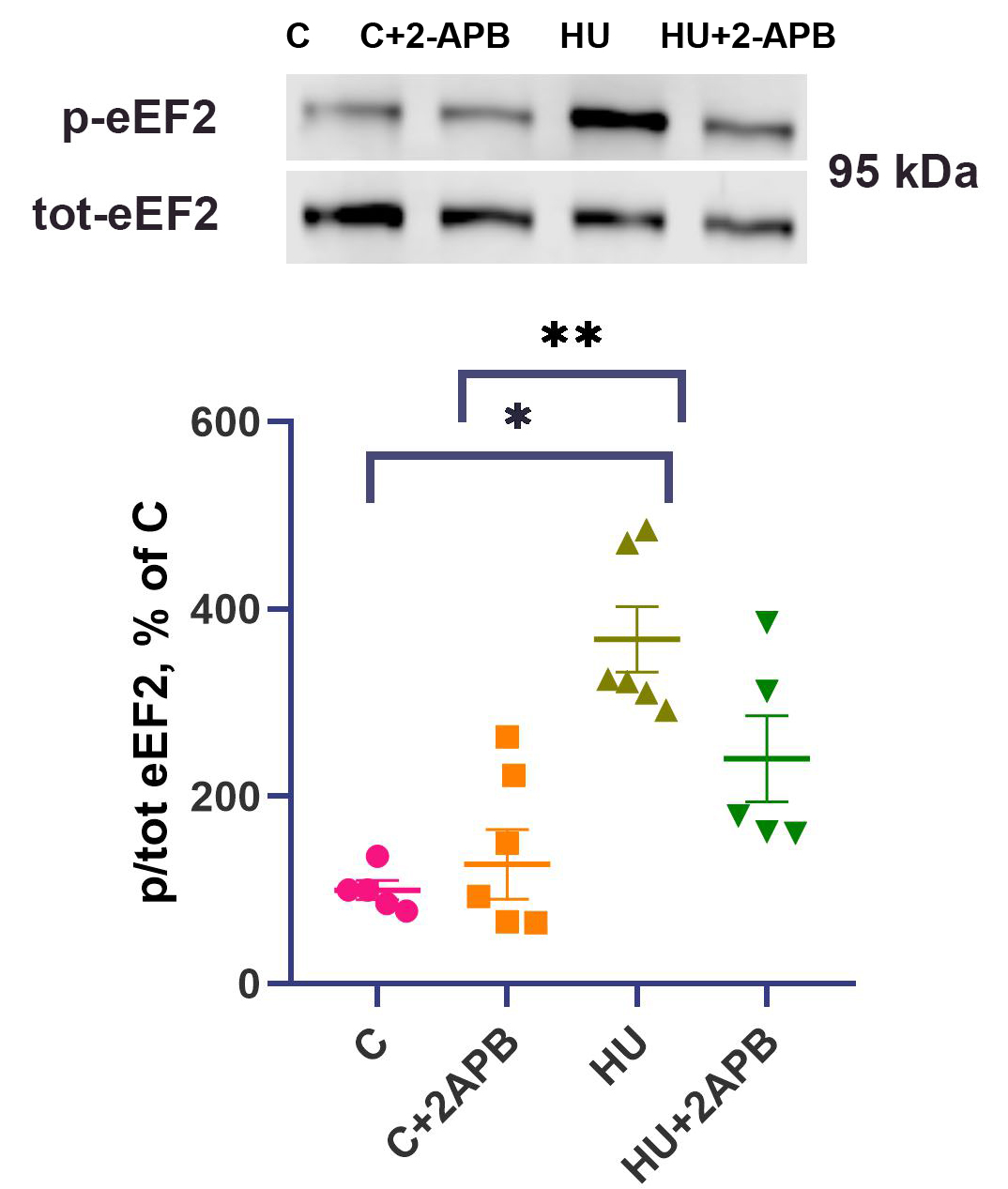
The content of p-eEF2 after 3-day hind limb unloading was 3.5-fold higher in the HU group compared to the C group (P < 0.05) and was also significantly elevated relative to the C+2APB group [Figure 5]. No significant difference was observed between the C and C+2APB groups. In the HU+2APB group, op-eEF2 content was only 2-fold higher than in the C group and did not significantly differ from any other experimental group [Figure 5].
Figure 5. p/tot-eEF2 content. Data are shown as mean value±SEM. * - significantly different from the C group (P < 0.05). ** - significantly different from the C+2APB group. HU: Hind limb unloading; SEM: standard error of the mean; p/tot-eEF2: phosphorylated-to-total eukaryotic elongation factor 2.
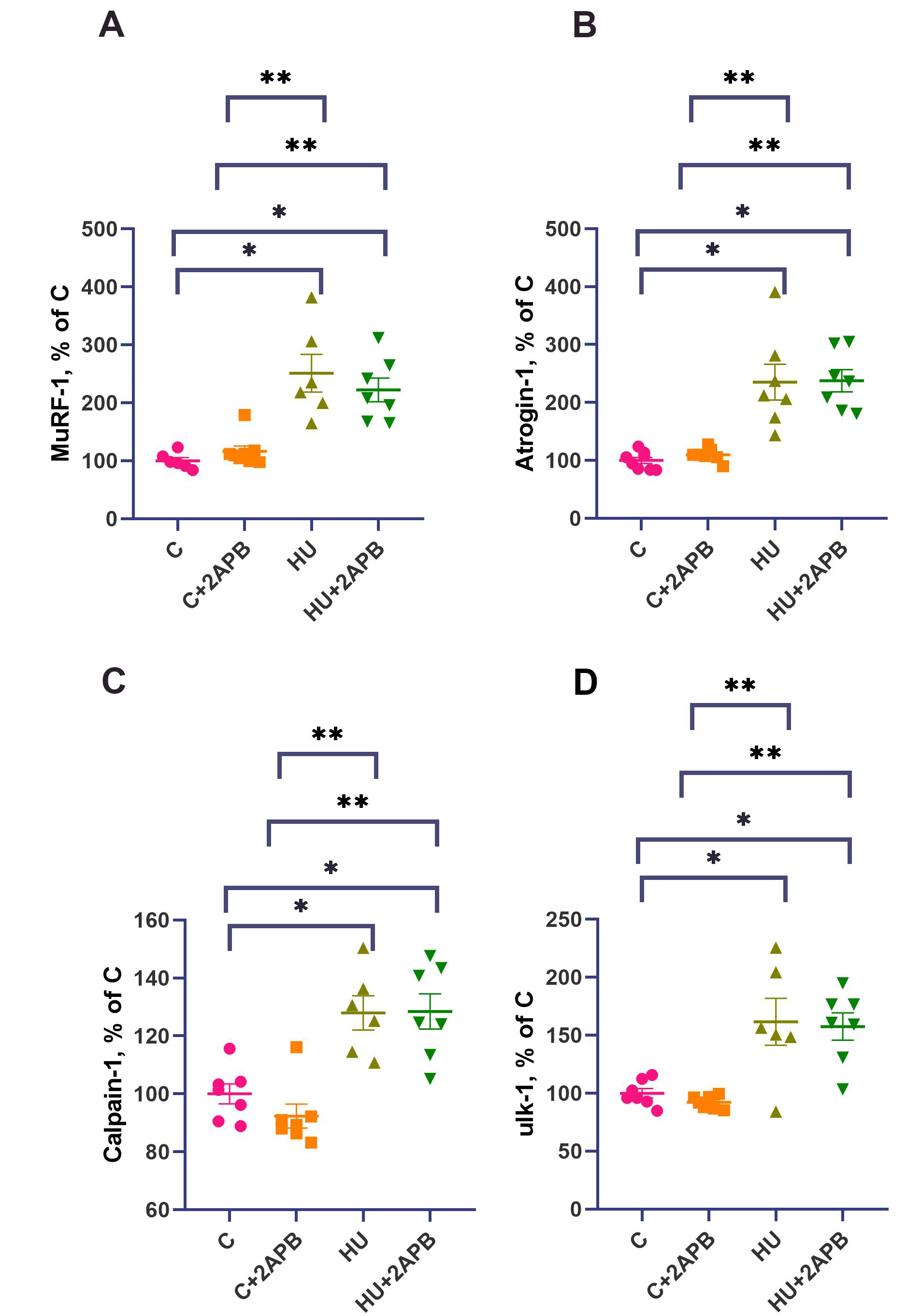
The mRNA expression levels of MuRF-1, atrogin-1, calpain-1, and ulk-1 did not differ between the C and C+2APB experimental groups [Figure 6A-D]. However, MuRF-1, atrogin-1, calpain-1, and ulk-1 mRNA contents were significantly higher in the HU and HU+2APB groups compared to the C and C+2APB groups, while there were no differences between the HU and the HU+2APB groups for these markers
Figure 6. MuRF-1 (A), Atrogin-1 (B), calpain-1 (C) and ulk-1 (D) mRNA content. Data are shown as mean value±SEM. * - significantly different from the C group (P< 0.05). ** - significantly different from the 3CA group (P < 0.05). Gene expression was normalized to YWHAZ. HU: Hind limb unloading; SEM: standard error of the mean.
DISCUSSION
Unloaded m soleus weight upon IP3R inhibition
The soleus muscle weights of the experimental animals, normalized to their body weights (weight index), decreased significantly after 3-day hind limb suspension in the HU group, consistent with previous experimental data[28,29]. Administration of 2-APB did not affect the m. soleus weight index in control animals. However, in the 2-APB-treated hind limb suspended group, the decline in m. soleus weight index was partially prevented, such that it did not differ from any other experimental group, including the C group. Thus, it can be concluded that 2-APB injections partially prevented unloading-induced m. soleus atrophy after 3-day HU. These findings align with the effect of 2-APB on muscle fiber cross-sectional areas after 7 days of rat hind limb unloading[30].
Effects of IP3R inhibition on calcium signaling markers in unloaded soleus muscle
After 3 days of hind limb unloading, we did not detect any significant differences in nuclear IP3R content compared to the C group [Figure 1]. This finding does not align with the data reported by Yang et al., who observed an increase in nuclear IP3R content in unloaded rat soleus muscle. This discrepancy may be due to the different durations of unloading: their study detected increased nuclear IP3R content after 14-day hind limb unloading[3], whereas we analyzed nuclear IP3R content after 3-day HU.
Interestingly, 2-APB administration resulted in a downregulation of nuclear IP3R content compared to the C group. The mechanism by which 2-APB interacts with IP3R, as described in the literature, involves inhibition of IP3R activity rather than downregulation of IP3R expression. Therefore, the observed decrease in nuclear IP3R content in the 2-APB-treated hind limb suspended group remains unclear. Nonetheless, it cannot be denied that this downregulation of IP3R content should be associated with an additional downregulation of IP3R activity, although this is not considered the key mechanism of 2-APB-induced IP3R inhibition[31].
IP3R plays an important role in regulating the nuclear Ca2+ level. Our findings suggest that Ca2+ release from the nuclear envelope via IP3R1 is also decreased in the HU+2APB group compared to HU alone, a conclusion consistent with the assumptions of Yang et al.[3].
Three days of hind limb unloading resulted in a significant increase in p-CaMK II content in the nuclear fraction of m. soleus [Figure 3A]. The 286/287 phosphorylation of CaMK II occurs due to Ca/calmodulin binding[32], indicating calcium accumulation. Therefore, increased p-CaMK II in the nuclei of unloaded rat soleus muscles serves as a marker of an increased nucleoplasmic calcium level. This observation aligns well with the results of Yang et al., who demonstrated myonuclear calcium accumulation after 14-day HU[3]. Administration of 2-APB completely prevented the accumulation of p-CaMK II in nuclei under unloading conditions, suggesting that 2-APB-dependent inhibition of IP3R activity prevents myonuclear calcium accumulation in unloaded soleus muscles. At the same time, 2-APB did not affect p-CaMK II content in the C group. This finding indicates that IP3Rs do not significantly contribute to p-CaMK II content in myonuclei under normal conditions and are only activated during hind limb unloading.
Additionally, 3-day hind limb unloading upregulated calcineurin A protein content in the HU group [Figure 3B]. Calcineurin is a calcium-dependent phosphatase involved in gene expression regulation[33], and its content increases with cytosolic calcium accumulation[34,35], consistent with the previous studies indicating calcium marker upregulation during muscle unloading in humans and animals[26,36,37]. In the
2-APB effects on anabolic and catabolic signaling
Regulation of skeletal muscle protein synthesis depends on both the number of ribosomes per mg of tissue (translational capacity) and the rate of ribosomal activity (translational efficiency)[38].
The primary marker of the number of ribosomes in skeletal muscle is the content of 18S and 28S rRNA[39]. After 3-day hind limb unloading, the contents of both 18S and 28S rRNA significantly declined compared to the C group [Figure 3]. These results agree well with the previously observed downregulation of rRNA content after 3-day hind limb suspension in rat soleus muscle[40,41]. Administration of 2-APB to the hind limb unloaded animals resulted in the partial prevention of this decline, such that 18S and 28S rRNA contents in the HU+2APB group did not differ significantly from any other experimental group. However, 2-APB did not upregulate rRNA content in the C+2APB group, indicating that 2-APB-dependent IP3R inhibition specifically affects rRNA content under muscle unloading conditions.
The mechanism by which 2-APB regulates rRNA content may be dependent on nuclear calcium content. Nuclear-localized calcineurin homologous protein CHP1 has been previously shown to interact with upstream binding factor and inhibit ribosomal RNA synthesis; thus, the downregulation of nuclear calcium in the 2-APB-administered, hind limb suspended group may prevent this inhibition and activate rRNA transcription[42].
Translational efficiency is commonly assessed by phosphorylation of p70S6 kinase (p-p70S6k), a downstream target of mTOR. After 3 days of unloading, p-p70S6k levels were unchanged [Figure 4], consistent with previous reports[26,43]. Although mTOR is known to regulate ribosomal RNA expression[44], in our experiment - as well as in a number of studies on unloaded muscles[45,46] - p-p70S6k content did not correlate with changes in 18S and 28S rRNA contents, suggesting that ribosomal RNA content is regulated by an mTOR-independent mechanism during unloading. Consistently, 2-APB treatment did not affect p-p70 content in either control or unloaded animals.
Another marker of translational efficiency is the content of phosphorylated eEF2. Threonine 56 (T56) phosphorylation of eEF2 inhibits its activity, leading to altered translational efficiency and downregulation of protein synthesis[47]. eEF2 phosphorylation occurs under conditions of elevated intracellular calcium[47]. In the HU group, the content of p-eEF2 was significantly increased compared to the C group [Figure 5]. These data align well with previous findings[26] and are in good agreement with the observed cytosolic calcium accumulation during hind limb unloading[2,48]. 2-APB injections did not affect p-eEF2 content in the C+2APB group but partially prevented the upregulation of p-eEF2 under hind limb suspension. Assuming that p-eEF2 is a calcium-dependent negative regulator of protein synthesis[49], we can conclude that 2-APB administration partially prevents the unloading-induced decline in protein synthesis efficiency due to the downregulation of calcium-dependent p-eEF-2 content.
In addition to downregulation of protein synthesis markers, 3-day hind limb unloading led to upregulation of the markers of proteolytic degradation. Specifically, mRNA levels of E3 ubiquitin ligases MuRF-1 and Atrogin-1 [Figure 6A and B], the autophagy marker ulk-1 [Figure 6D], and calpain-1 [Figure 6C] were significantly increased, consistent with previous observations[50-52]. This suggests that IP3Rs primarily affect protein synthesis, not degradation. Notably, 2-APB administration did not affect expression of these markers in either the C+2APB or HU+2APB experimental groups, consistent with previous reports that blocking L-type calcium channels does not alter E3 lygase expression upon muscle unloading[4]. Conversely, SERCA pump activation during 3-day rat hind limb unloading resulted in downregulation of MuRF-1 but not atrogin-1 expression[36]. These data suggest that different calcium-regulating mechanisms selectively influence distinct proteolytic pathways in skeletal muscle under unloading conditions.
Limitations
All data were obtained from the slow-twitch soleus muscle of male Wistar rats subjected to 3-day hind limb suspension. The effects of 2-APB may differ in fast-twitch muscles or following different durations of muscle unloading.
Conclusion
Therefore, 2-APB IP3R inhibitor administration during 3-day hind limb unloading attenuates the decline in soleus muscle weight index and reduces the increase in calcium signaling marker calcineurin A. It also downregulates nuclear IP3R content and prevents the unloading-induced upregulation of nuclear p-CaMK II. Furthermore, it prevents the decline in protein synthesis capacity markers (rRNAs) and the inhibition of translational efficiency marker (eEF2), but does not affect ubiquitin-proteosome signaling factors.
We suggest that IP3Rs regulate nucleoplasmic calcium levels, which in turn control rRNA gene expression during soleus muscle unloading.
DECLARATIONS
Authors’ contributions
Conceived and designed the research: Nemirovskaya TL
Performed the experiments: Belova SP, Zaripova KA, Sharlo KA, Bokov RO
Analyzed the data, interpreted the results of the experiments, prepared the figures, edited and revised the manuscript, approved the final version of the manuscript: Belova SP, Zaripova KA, Sharlo KA, Bokov RO, Nemirovskaya TL
Drafted the manuscript: Belova SP, Zaripova KA, Sharlo KA, Nemirovskaya TL
Availability of data and materials
The data supporting this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This work was supported by the Russian Science Foundation (RSF); Project No. 24-15-00088 (to Nemirovskaya TL).
Conflicts of interest
The authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study was approved by the Ethics Commission of the State Scientific Center of the Russian Federation, Institute of Biomedical Problems, Russian Academy of Sciences (Protocol No. 627, dated December 6, 2022).
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Shenkman BS, Nemirovskaya TL. Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J Muscle Res Cell Motil. 2008;29:221-30.
2. Ingalls CP, Warren GL, Armstrong RB. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol. 1999;87:386-90.
3. Yang H, Wang H, Pan F, et al. New findings: hindlimb unloading causes nucleocytoplasmic Ca2+ overload and DNA damage in skeletal muscle. Cells. 2023;12:1077.
4. Belova SP, Lomonosova YN, Shenkman BS, Nemirovskaya TL. The blockade of dihydropyridine channels prevents an increase in μ-calpain level under m. soleus unloading. Dokl Biochem Biophys. 2015;460:1-3.
5. Sharlo KA, Lvova ID, Tyganov SA, et al. The effect of SERCA activation on functional characteristics and signaling of rat soleus muscle upon 7 days of unloading. Biomolecules. 2023;13:1354.
6. Cárdenas C, Liberona JL, Molgó J, Colasante C, Mignery GA, Jaimovich E. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J Cell Sci. 2005;118:3131-40.
7. Chin ER. Intracellular Ca2+ signaling in skeletal muscle: decoding a complex message. Exerc Sport Sci Rev. 2010;38:76-85.
8. Chin ER. The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc Nutr Soc. 2004;63:279-86.
9. Crabtree GR. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611-4.
10. Chibalin AV, Benziane B, Zakyrjanova GF, Kravtsova VV, Krivoi II. Early endplate remodeling and skeletal muscle signaling events following rat hindlimb suspension. J Cell Physiol. 2018;233:6329-36.
11. Kravtsova VV, Paramonova II, Vilchinskaya NA, et al. Chronic ouabain prevents Na,K-ATPase dysfunction and targets AMPK and IL-6 in disused rat soleus muscle. Int J Mol Sci. 2021;22:3920.
12. Kravtsova VV, Bouzinova EV, Matchkov VV, Krivoi II. Skeletal muscle Na,K-ATPase as a target for circulating ouabain. Int J Mol Sci. 2020;21:2875.
13. Araya R, Liberona JL, Cárdenas JC, et al. Dihydropyridine receptors as voltage sensors for a depolarization-evoked, IP3R-mediated, slow calcium signal in skeletal muscle cells. J Gen Physiol. 2003;121:3-16.
14. Arias-Calderón M, Almarza G, Díaz-Vegas A, et al. Characterization of a multiprotein complex involved in excitation-transcription coupling of skeletal muscle. Skelet Muscle. 2016;6:15.
15. Casas M, Buvinic S, Jaimovich E. ATP signaling in skeletal muscle: from fiber plasticity to regulation of metabolism. Exerc Sport Sci Rev. 2014;42:110-6.
16. Atakpa-Adaji P, Ivanova A. IP3R at ER-mitochondrial contact sites: beyond the IP3R-GRP75-VDAC1 Ca2+ funnel. Contact. 2023;6:25152564231181020.
17. Dubinin MV, Chulkov AV, Igoshkina AD, Cherepanova AA, Mikina NV. Effect of 2-aminoethoxydiphenyl borate on the functions of mouse skeletal muscle mitochondria. Biochem Biophys Res Commun. 2024;712-713:149944.
18. Rossi AM, Taylor CW. IP3 receptors - lessons from analyses ex cellula. J Cell Sci. 2018;132:jcs222463.
19. Foskett J, Daniel Mak D. Regulation of IP3R channel gating by Ca2+ and Ca2+ binding proteins. structure and function of calcium release channels. Curr Top Membr. 2010;66:235-72.
20. Mirzoev T, Tyganov S, Vilchinskaya N, Lomonosova Y, Shenkman B. Key markers of mTORC1-dependent and mTORC1-independent signaling pathways regulating protein synthesis in rat soleus muscle during early stages of hindlimb unloading. Cell Physiol Biochem. 2016;39:1011-20.
21. Belova SP, Mochalova EP, Kostrominova TY, Shenkman BS, Nemirovskaya TL. P38α-MAPK signaling inhibition attenuates soleus atrophy during early stages of muscle unloading. Int J Mol Sci. 2020;21:2756.
22. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412.
23. Morey-holton E, Globus RK, Kaplansky A, Durnova G. The Hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. experimentation with animal models in space. Adv Space Biol Med. 2005;10:7-40.
24. Lin L, Zhao X, Yan W, Qi W. Influence of orai1 intervention on mouse airway epithelium reactions in vivo and in vitro. Ann Allergy Asthma Immunol. 2012;108:103-12.
25. Wang G, Zhang J, Xu C, Han X, Gao Y, Chen H. Inhibition of SOCs attenuates acute lung injury induced by severe acute pancreatitis in rats and PMVECs injury induced by lipopolysaccharide. Inflammation. 2016;39:1049-58.
26. Belova SP, Zaripova K, Sharlo K, Kostrominova TY, Shenkman BS, Nemirovskaya TL. Metformin attenuates an increase of calcium-dependent and ubiquitin-proteasome markers in unloaded muscle. J Appl Physiol. 2022;133:1149-63.
27. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
28. Giger JM, Bodell PW, Zeng M, Baldwin KM, Haddad F. Rapid muscle atrophy response to unloading: pretranslational processes involving MHC and actin. J Appl Physiol. 2009;107:1204-12.
29. Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1-12.
30. Zaripova KA, Bokov RO, Sharlo KA, Belova SP, Nemirovskaya TL. Blocking IP3 receptors with 2-APB alters cellular signaling during 7-day soleus unloading in rats. J Evol Biochem Phys. 2024;60:1795-806.
31. Saleem H, Tovey SC, Molinski TF, Taylor CW. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br J Pharmacol. 2014;171:3298-312.
32. Rostas JAP, Skelding KA. Calcium/calmodulin-stimulated protein kinase II (CaMKII): different functional outcomes from activation, depending on the cellular microenvironment. Cells. 2023;12:401.
33. Hudson MB, Price SR. Calcineurin: a poorly understood regulator of muscle mass. Int J Biochem Cell Biol. 2013;45:2173-8.
34. Fajardo VA, Rietze BA, Chambers PJ, et al. Effects of sarcolipin deletion on skeletal muscle adaptive responses to functional overload and unload. Am J Physiol Cell Physiol. 2017;313:C154-61.
35. Bai H, Zhu H, Yan Q, et al. TRPV2-induced Ca2+-calcineurin-NFAT signaling regulates differentiation of osteoclast in multiple myeloma. Cell Commun Signal. 2018;16:68.
36. Zaripova KA, Belova SP, Sharlo KA, et al. SERCA activation prevents Ca2+ and ATP upregulation during 3-day soleus muscle unloading in rats. Am J Physiol Regul Integr Comp Physiol. 2025;329:R108-22.
37. Sharlo KA, Lvova ID, Vilchinskaya NA, et al. Molecular signaling effects of electrical stimulation of the soleus muscle during 6 days of dry immersion. Sports Med Health Sci. 2025; doi: 10.1016/j.smhs.2025.06.002.
38. Moinard C, Fontaine E. Direct or indirect regulation of muscle protein synthesis by energy status? Clin Nutr. 2021;40:1893-6.
39. Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS. Relationship between protein synthesis and RNA content in skeletal muscle. Nature. 1973;241:204-5.
40. Tyganov SA, Mochalova EP, Belova SP, et al. Effects of plantar mechanical stimulation on anabolic and catabolic signaling in rat postural muscle under short-term simulated gravitational unloading. Front Physiol. 2019;10:1252.
41. Rozhkov SV, Sharlo KA, Mirzoev TM, Shenkman BS. Temporal changes in the markers of ribosome biogenesis in rat soleus muscle under simulated microgravity. Acta Astronautica. 2021;186:252-8.
42. Jiménez-Vidal M, Srivastava J, Putney LK, Barber DL. Nuclear-localized calcineurin homologous protein CHP1 interacts with upstream binding factor and inhibits ribosomal RNA synthesis. J Biol Chem. 2010;285:36260-6.
43. Roberson PA, Shimkus KL, Welles JE, et al. A time course for markers of protein synthesis and degradation with hindlimb unloading and the accompanying anabolic resistance to refeeding. J Appl Physiol. 2020;129:36-46.
44. Figueiredo VC, McCarthy JJ. Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology. 2019;34:30-42.
45. Kotani T, Tamura Y, Kouzaki K, Kato H, Isemura M, Nakazato K. Percutaneous electrical stimulation-induced muscle contraction prevents the decrease in ribosome RNA and ribosome protein during pelvic hindlimb suspension. J Appl Physiol. 2022;133:822-33.
46. Rozhkov SV, Sharlo KA, Shenkman BS, Mirzoev TM. Inhibition of mTORC1 differentially affects ribosome biogenesis in rat soleus muscle at the early and later stages of hindlimb unloading. Arch Biochem Biophys. 2022;730:109411.
47. Rose AJ, Alsted TJ, Jensen TE, et al. A Ca2+-calmodulin-eEF2K-eEF2 signalling cascade, but not AMPK, contributes to the suppression of skeletal muscle protein synthesis during contractions. J Physiol. 2009;587:1547-63.
48. Ingalls CP, Wenke JC, Armstrong RB. Time course changes in [Ca2+]i, force, and protein content in hindlimb-suspended mouse soleus muscles. Aviat Space Environ Med 2001;72:471-6.
49. Hizli AA, Chi Y, Swanger J, et al. Phosphorylation of eukaryotic elongation factor 2 (eEF2) by cyclin a-cyclin-dependent kinase 2 regulates its inhibition by eEF2 kinase. Mol Cell Biol. 2013;33:596-604.
50. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469-84.
51. Hanson AM, Harrison BC, Young MH, Stodieck LS, Ferguson VL. Longitudinal characterization of functional, morphologic, and biochemical adaptations in mouse skeletal muscle with hindlimb suspension. Muscle Nerve. 2013;48:393-402.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].