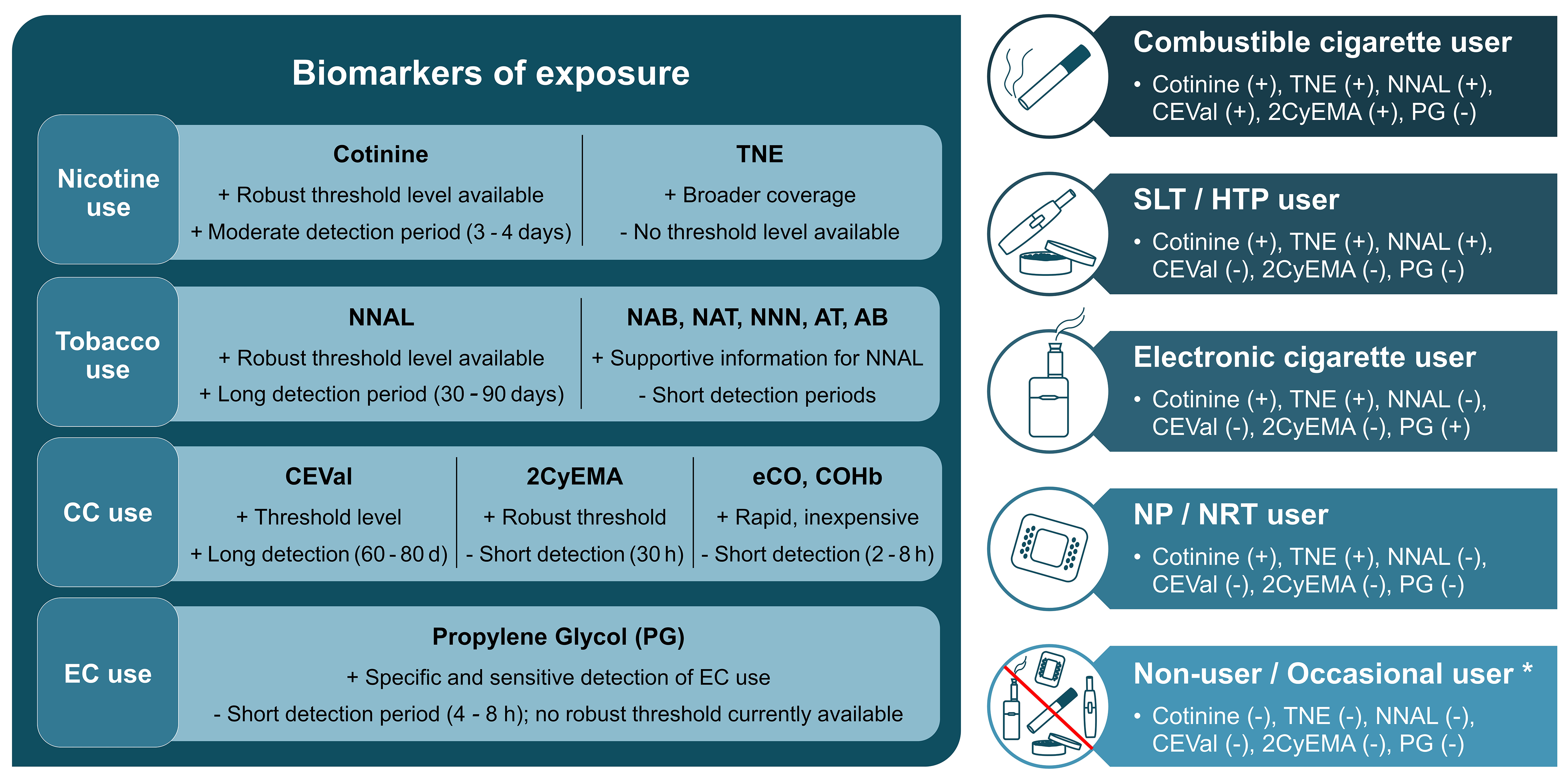
fig1
Figure 1. Left: BoEs for different use cases ranked by priority (left to right). Strengths and limitations illustrated by + and -. Right: Different product user groups and presence (+) or absence (-) of the biomarkers above a predefined threshold to discriminate between those groups, adapted from[8]. Occasional users might have borderline positive levels of some biomarkers depending on the use frequency and recency of exposure. Figure created in Microsoft PowerPoint. BoEs: Biomarkers of exposure; TNE: total nicotine equivalent, NNAL: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NAB: N-nitrosoanabasine; NAT: N-nitrosoanatabine; NNN: N-nitrosonornicotine; AT: anatabine; AB: anabasine; CC: combustible cigarette, CEVal: cyanoethylvaline; 2CyEMA: 2-cyanoethyl mercapturic acid; eCO: exhaled carbon monoxide; COHb: carboxyhemoglobin; EC: electronic cigarette, SLT: smokeless tobacco, HTP: heated tobacco product, NP: nicotine pouch, NRT: nicotine replacement therapy.