Extracellular vesicles in cardiac regeneration and aging: mechanisms and translation
Abstract
Myocardial infarction (MI) and age-related cardiac remodeling remain major causes of morbidity and mortality worldwide. Because the adult heart has only limited regenerative capacity, extracellular vesicles (EVs) have emerged as promising acellular mediators of tissue repair, with the potential to mitigate both ischemic injury and age-associated decline. Preclinical studies demonstrate that EVs derived from stem and progenitor cells exert
Keywords
INTRODUCTION
Cardiovascular aging is a fundamental driver of morbidity and mortality worldwide, contributing to a gradual decline in cardiac function, increased susceptibility to ischemic events, and impaired reparative capacity[1]. Despite advances in acute cardiac care, long-term outcomes after myocardial infarction (MI) remain suboptimal-especially in older patients-due to the heart’s limited regenerative potential and the compounding effects of age-related remodeling[2,3]. Addressing these dual challenges requires innovative, multifaceted therapeutic strategies that go beyond traditional pharmacological interventions.
Extracellular vesicles (EVs)-membrane-bound nanoparticles released by nearly all cell types-have recently emerged as key players in intercellular communication and tissue regeneration[4-6]. Unlike cell-based therapies, EVs exert their therapeutic effects primarily through the delivery of functional RNAs, proteins, and lipids that modulate apoptosis, inflammation, angiogenesis, fibrosis, and oxidative stress[7,8]. These properties make them attractive candidates for cell-free cardiac regeneration.
However, while EVs have been extensively studied in models of acute ischemia and heart failure (HF)[9-11], their potential to reverse or attenuate age-related cardiac deterioration remains an underexplored frontier. Accumulating evidence suggests that EVs not only mitigate injury in the context of MI, but also possess the capacity to modulate pathways implicated in cardiac aging - such as cellular senescence, metabolic dysfunction, telomere attrition, and chronic inflammation[12-14].
Although numerous reviews have addressed the role of EVs in MI[9,15] or, separately, in cardiovascular aging[1,12], very few have systematically integrated these two domains. Existing literature typically focuses either on acute post-infarction repair mechanisms or on age-associated cardiac remodeling, without exploring how aging-specific pathophysiology influences the efficacy, mechanisms, and translational potential of EV-based therapies. This represents a critical knowledge gap, as older patients - who make up the majority of those affected by MI - may respond differently to regenerative interventions due to
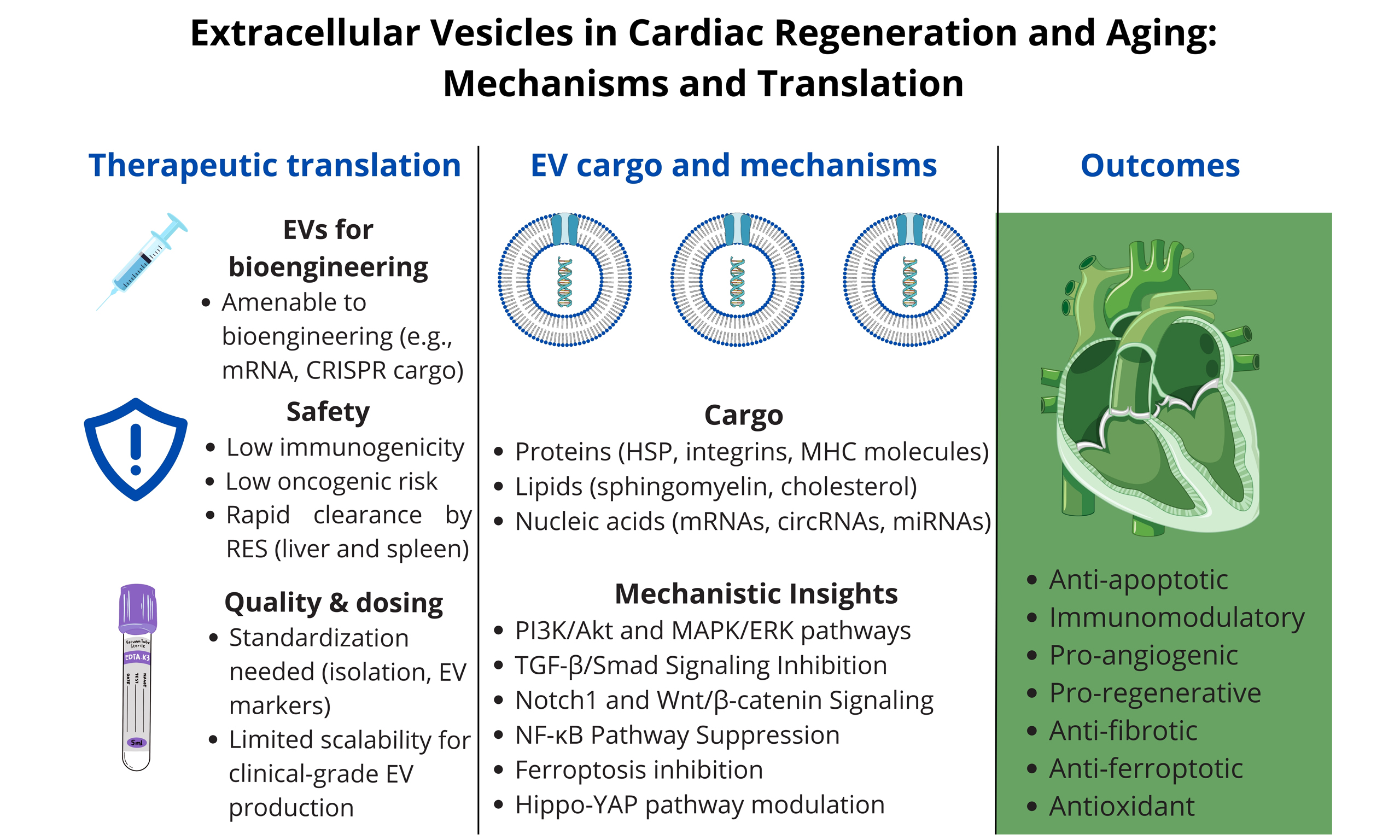
In this review, we aim to address this gap by synthesizing evidence across both preclinical and early clinical studies that investigate EV-mediated repair in the context of MI and cardiac aging. Specifically, we examine: (i) the mechanistic pathways by which EVs modulate inflammation, angiogenesis, fibrosis, oxidative stress, and ferroptosis; (ii) the influence of age-related alterations in the cardiac microenvironment on EV efficacy; and (iii) emerging bioengineering and delivery strategies to optimize EV-based interventions for older patients. Publications were identified through structured searches of PubMed, Scopus, and Web of Science, with inclusion criteria encompassing original research and clinical trials on EVs in myocardial ischemia, cardiac remodeling, and aging. By bridging the mechanistic and translational perspectives of MI and aging, this review provides a novel integrated framework for understanding how EVs function at the interface of cardiac injury and aging. This dual focus underscores their potential as both regenerative and rejuvenating tools, while highlighting translational challenges that are particularly relevant for elderly patients.
CLASSIFICATION AND BIOGENESIS OF EXTRACELLULAR VESICLES
Under both physiological and pathological conditions, diverse cell types release EVs, which are nanoscale, membrane-bound particles. EVs represent a conserved mechanism of intercellular communication that is increasingly recognized for its diagnostic and therapeutic potential[4].
EVs are generally classified into three major categories based on their size and biogenesis:
• Exosomes (30-150 nm)
Exosomes originate from the endosomal pathway. Intraluminal vesicles form within multivesicular bodies (MVBs), which subsequently fuse with the plasma membrane to release exosomes into the extracellular space. Their formation is regulated by the Endosomal Sorting Complex Required for Transport (ESCRT) machinery as well as ESCRT-independent mechanisms involving tetraspanins (e.g., CD63, CD81)[7].
• Microvesicles (100-1,000 nm)
Microvesicles bud directly from the plasma membrane through calcium-dependent cytoskeletal remodeling. Their release is frequently upregulated under stress, inflammatory, or hypoxic conditions[5].
• Apoptotic bodies (500-2,000 nm)
These vesicles are generated during programmed cell death and contain nuclear fragments, organelles, and cytoplasmic components. Once considered biologically inert, apoptotic bodies are now recognized as active participants in immune signaling and intercellular transfer of genetic material[16].
EVs carry a wide array of bioactive molecules, including proteins [e.g., heat shock proteins, integrins, major histocompatibility complex (MHC) molecules], lipids (e.g., sphingomyelin, cholesterol), and nucleic acids [e.g., messenger RNAs (mRNAs), circular RNAs, microRNAs (miRNAs)]. Their molecular composition reflects the cell of origin and its physiological state, thereby offering potential as disease-specific biomarkers[8].
A major advantage of EVs is their stability in circulation. The lipid bilayer protects their cargo from enzymatic degradation, enabling systemic transport while preserving functional integrity[6]. Additionally, EVs exhibit intrinsic biodistribution patterns-often referred to as natural tropism-that enable them to cross biological barriers such as the blood-brain barrier, fibrotic tissues, and endothelial junctions. This capacity is mediated both by receptor-ligand interactions (e.g., integrins, tetraspanins) and by size-dependent passive transport mechanisms under conditions of increased vascular permeability[17].
Recent profiling studies have identified EV subtypes with enhanced therapeutic potential. For example, EVs derived from hypoxia-preconditioned mesenchymal stem cells (MSCs) are enriched with regenerative miRNAs such as miR-210 and miR-21, which augment their reparative capacity[7,9].
Furthermore, bioengineered EVs-modified with targeting ligands, reporter molecules, or synthetic cargo-are being developed as “smart delivery systems” for RNA-based therapeutics and proteins, with potential applications across regenerative medicine, oncology, and neurology[18,19].
Despite this growing interest, significant challenges persist in EV characterization, nomenclature, and isolation. Techniques such as ultracentrifugation and size-exclusion chromatography (SEC) vary in efficiency and yield. For this reason, the International Society for Extracellular Vesicles (ISEV) recommends the use of the umbrella term EVs unless their specific biogenesis can be conclusively determined[4]. An overview of EV subtypes is presented in Table 1.
Comparison of major types of extracellular vesicles
| Property | Exosomes | Microvesicles | Apoptotic bodies |
| Size | 30-150 nm[7] | 100-1,000 nm[5] | 500-2,000 nm[16] |
| Origin | MVB fusion[7] | Plasma membrane budding[5] | Apoptosis-mediated release[16] |
| Cargo | Membrane proteins, RNA, lipids[7] | Similar to parent cell[5] | Nuclear and organelle fragments[16] |
| Markers | CD9, CD63, CD81[7] | No exclusive markers[5] | No exclusive markers[16] |
FUNCTIONAL CARGO AND MECHANISMS OF CARDIOPROTECTION
EVs exert cardioprotective effects by transferring bioactive molecules that modulate key biological processes involved in MI, including apoptosis, inflammation, angiogenesis, oxidative stress, and fibrosis[7,20]. Owing to their ability to shuttle functional RNAs and proteins, EVs act as effective mediators of cardiac repair, particularly in models of ischemia/reperfusion (I/R) injury[20,21].
Anti-apoptotic effects
One of the earliest observed benefits of EV therapy is the reduction in cardiomyocyte apoptosis. EVs derived from MSCs (MSC-EVs) are enriched in anti-apoptotic miRNAs such as miR-21, miR-19a, and
In murine MI models, administration of miR-21-enriched EVs significantly reduced cardiomyocyte apoptosis, increased Akt phosphorylation, and improved ventricular function[23]. Similarly, miR-210 enhances hypoxia tolerance and inhibits apoptosis by targeting caspase 8-associated protein 2 (Casp8ap2)[24].
Immunomodulation
Post-infarction inflammation is necessary for debris clearance but can become detrimental if prolonged. EVs modulate immune responses by influencing macrophage polarization. MSC-derived EVs containing miR-181b, miR-182, and miR-146a have been shown to promote a shift toward the M2 (anti-inflammatory) macrophage phenotype, reducing the release of
Additionally, these EVs inhibit nuclear factor-κB (NF-κB) activation, a key transcription factor in inflammation, and enhance IL-10 expression while suppressing Toll-like receptor signaling - creating a tissue repair-favorable environment[28].
However, the extent and consistency of these immunomodulatory effects vary depending on the donor cell source, culture conditions, and timing of administration.
Pro-angiogenic properties
EVs contribute to post-MI neovascularization by delivering pro-angiogenic factors such as vascular endothelial growth factor (VEGF), angiopoietin-1, miR-126, miR-132, and miR-210. These mediators stimulate endothelial cell proliferation, migration, and tube formation. EVs from endothelial progenitor cells (EPCs) significantly enhance capillary density in infarcted myocardium and restore perfusion in ischemic zones[29]. MiR-126-rich EVs activate mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt pathways, thereby promoting vascular sprouting and endothelial survival[30]. Hypoxia-preconditioned EVs show even greater angiogenic potential due to upregulation of hypoxia-inducible miRNAs such as miR-210[9]. Large-animal studies confirm pro-angiogenic benefits but report variability in vessel maturity and functional integration, highlighting the need for standardized angiogenesis assessment.
Anti-fibrotic and pro-regenerative effects
Excessive extracellular matrix (ECM) deposition leads to cardiac fibrosis, reducing contractility and increasing arrhythmogenic risk. EVs help counter fibrosis by modulating the transforming growth factor-β (TGF-β)/Smad pathway and inhibiting myofibroblast differentiation.
Exosomal miR-29 targets ECM-related genes such as Collagen Type I Alpha 1 Chain (COL1A1), Collagen Type III Alpha 1 Chain (COL3A1), and fibrillin-1 (FBN1), reducing collagen synthesis[31]. EVs enriched in miR-133a and miR-30d also exhibit anti-fibrotic effects by limiting fibroblast proliferation and downregulating profibrotic transcription factors such as connective tissue growth factor (CTGF)[32].
Furthermore, cardiosphere-derived cell EVs (CDC-EVs) represent a heterogeneous population enriched in exosome-sized vesicles (30-150 nm) carrying Y RNA fragments, cardioprotective miRNAs, and other
Antioxidant and anti-ferroptotic actions
Oxidative stress is a major contributor to ischemic injury. EVs mitigate redox imbalance by delivering antioxidant enzymes (e.g., catalase, superoxide dismutase) and regulatory miRNAs that suppress reactive oxygen species (ROS) production.
Some EVs also prevent ferroptosis - an iron-dependent form of cell death - by restoring expression of glutathione peroxidase 4 (GPX4) and maintaining iron homeostasis[33]. The anti-ferroptotic role of EVs in cardiac tissue remains based mainly on small-animal models; translation to human pathology has not yet been demonstrated. Table 2 summarizes the major mechanisms by which EVs exert cardioprotective effects following MI.
Therapeutic mechanisms of EVs in ischemic heart injury
| Mechanism of Action | Key molecules/pathways | Therapeutic effect | References |
| Anti-apoptotic | miR-21, miR-19a, miR-210 | Reduced cardiomyocyte apoptosis, improved cardiac function | [19-21] |
| Immunomodulatory | miR-181b, miR-182, miR-146a, IL-10 | Promoted M2 macrophage polarization; reduced inflammatory cytokines | [22-25] |
| Pro-angiogenic | VEGF, angiopoietin-1, miR-126, miR-132, miR-210, PI3K/Akt, MAPK/ERK | Enhanced neovascularization and perfusion | [9,26,27] |
| Pro-regenerative and Anti-fibrotic | TGF-β/Smad, miR-29, miR-133a, miR-30d, CTGF | Inhibited fibroblast activation and ECM deposition | [9,10,28,29] |
| Anti-ferroptotic and Antioxidant | Catalase, SOD, GPX4 | Reduced oxidative damage and ferroptosis | [30] |
PRECLINICAL EVIDENCE AND EXPERIMENTAL MODELS
Extensive preclinical studies have highlighted the regenerative potential of EVs in MI and HF. Experimental models - ranging from rodents to large animals - consistently demonstrate that EVs derived from stem and progenitor cells can modulate injury responses and promote cardiac repair.
In vitro models
In vitro studies are crucial for dissecting how EVs influence cardiomyocytes and related processes. Most of these experiments rely on cell cultures, which offer reproducibility and experimental control, but their results must be interpreted with caution, as they cannot fully replicate the complexity of in vivo conditions. Nevertheless, they provide valuable mechanistic insights into angiogenesis, anti-apoptotic signaling, immunomodulation, and anti-fibrotic effects. Below, we summarize selected in vitro studies investigating the impact of EVs on cardiomyocytes and other cardiac-related cells under controlled culture conditions.
Exosomes derived from bone marrow MSCs (BMMSCs) overexpressing miRNA-126 stimulated proliferation, migration, and tube formation of human umbilical vein endothelial cells (HUVECs)[34]. This effect was attributed to the transfer of miRNA-126, which suppressed Phosphoinositide-3-Kinase Regulatory Subunit 2 (PIK3R2) and activated the PI3K/Akt pathway[34], suggesting a potential therapeutic strategy for enhancing angiogenesis.
Cheng et al. demonstrated that exosomes from hypoxia-damaged MSCs reduce apoptosis in ischemic cardiomyocytes[35]. Specifically, exosomal miRNA-210 activated the PI3K/Akt pathway in cardiomyocytes, thereby decreasing apoptosis[35]. Similarly, EVs promoted the growth of H9C2 cardiomyocytes through vesicle-derived miRNA-17-3p, which targets and inhibits Tissue Inhibitor of Metalloproteinase-3 (TIMP3)[36].
In another study, exosomes derived from H9C2 cardiomyoblasts exposed to hypoxia were enriched with cardioprotective miRNAs, including miRNA-21-5p, miRNA-378-3p, miRNA-152-3p, and let-7i-5p[37]. Functional gain- and loss-of-function analyses revealed that these exosomal miRNAs attenuate
Finally, small EV-like vesicles (ELVs) enriched with miRNA-126 were produced from c-kit+ progenitor
Rodent models
In murine and rat models of acute myocardial infarction (AMI), administration of EVs - via intramyocardial, intravenous, or intracoronary routes - shortly after I/R injury has consistently been shown to:
• Reduce infarct size;
• Improve left ventricular ejection fraction (LVEF);
• Decrease myocardial fibrosis and cardiomyocyte hypertrophy;
• Enhance angiogenesis and neovascularization;
• Increase cardiomyocyte survival and proliferation.
For example, intravenous injection of MSC-derived EVs within 30 min post-MI significantly reduced infarct size and improved LVEF in rats. These effects were linked to reduced cardiomyocyte apoptosis, increased capillary density, and attenuated inflammation[11].
EVs derived from embryonic stem cells (ESC-EVs) also demonstrated therapeutic activity by activating endogenous cardiac progenitor cells (CPCs), thereby promoting neovascularization and myocardial repair. One study reported that ESC-EV treatment increased Ki-67 expression and the number of c-kit+ cells in the infarct border zone, consistent with stimulated cell proliferation[39].
Similarly, CDC-EVs improved systolic function, reduced scar formation, and enhanced myocardial structure in both acute and chronic porcine MI models. Their beneficial effects have been attributed to complex RNA cargo, including Y RNA fragments and regulatory miRNAs[10].
EVs from induced pluripotent stem cells (iPSC-EVs) also displayed cardioprotective effects in murine I/R models. By delivering hypoxia-inducible factor 1-alpha (HIF-1α)-modulating miRNAs such as miR-210, they improved cell survival under hypoxic conditions and limited infarct expansion[40].
Large animal models
Translation of EV-based therapies into large animal models is a critical step toward clinical application. In porcine MI models, intracoronary infusion of CDC-EVs following coronary occlusion significantly reduced infarct size, preserved myocardial contractility, and prevented adverse left ventricular remodeling. Remarkably, these therapeutic effects persisted for up to three months post-treatment, suggesting durable benefits[10].
Likewise, EVs derived from human umbilical cord MSCs (hUC-MSC-EVs) administered to pigs after I/R injury reduced serum troponin levels, improved global longitudinal strain, and decreased collagen deposition in the peri-infarct region[41].
Route, timing, and dosage considerations
The route of EV delivery strongly influences biodistribution, myocardial retention, and therapeutic efficacy. Intramyocardial injection ensures direct targeting of the infarcted myocardium but is invasive and less feasible in clinical practice. Intravenous and intracoronary routes are more practical and have shown efficient homing to injured cardiac tissue, largely due to enhanced vascular permeability and inflammatory signaling in ischemic regions[42,43].
Timing of administration is equally critical. Early delivery - typically within hours of MI - maximizes cardioprotection by counteracting acute apoptotic and inflammatory responses[9]. Nevertheless, delayed administration, several days post-injury, may still confer regenerative benefits by reducing fibrosis and promoting angiogenesis during the chronic remodeling phase[10].
EV dosage varies widely across studies, with most preclinical protocols employing between 108 and 1011 particles per administration, depending on EV origin, delivery route, and animal model[44]. The absence of standardized quantification and normalization methods remains a major obstacle to cross-study comparisons and to defining optimal dosing for clinical translation.
Mechanistic insights
Elaborating on the general cardioprotective mechanisms described earlier (comprising anti-apoptotic,
• PI3K/Akt and MAPK/ERK pathways
Particularly activated by MSC-EVs, these pathways promote cardiomyocyte survival, proliferation, and angiogenesis via mechanisms such as Akt-mediated B-cell lymphoma-2 (Bcl-2) upregulation and endothelial tube formation[45,46].
• TGF-β/Smad signaling inhibition
EVs suppress cardiac fibrosis by downregulating Smad2/3 phosphorylation and delivering anti-fibrotic miRNAs (e.g., miR-29, miR-133a, miR-30d), leading to reduced expression of ECM-related genes such as COL1A1 and CTGF[47,48].
• Notch1 and Wnt/β-catenin signaling
These pathways regulate the self-renewal, differentiation, and survival of CPCs. CPCs are commonly identified by markers such as c-kit, Sca-1, and Isl1, although their precise phenotype remains under debate[49,50]. EVs may deliver ligands such as Jagged-1 or regulatory miRNAs (e.g., miR-199a, miR-17-92 cluster) to activate these developmental programs[51,52].
• NF-κB pathway suppression
EVs attenuate post-MI inflammation by inhibiting NF-κB nuclear translocation and downregulating inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β). This is facilitated by miRNAs such as miR-146a and
• Emerging pathways
○ Ferroptosis inhibition via GPX4 restoration and iron balance [e.g., miR-214, nuclear factor-erythroid
○ Mitochondrial transfer supporting oxidative phosphorylation in damaged cardiomyocytes[55];
○ Hippo-Yes-associated protein (YAP) pathway modulation promoting cardiomyocyte proliferation[56].
Taken together, these findings demonstrate that EVs engage multiple cardioprotective pathways-including PI3K/Akt, MAPK/ERK, Notch1, Wnt/β-catenin, TGF-β/Smad inhibition, and NF-κB suppression-while also influencing emerging processes such as Hippo-YAP signaling, ferroptosis, and mitochondrial transfer. Through these mechanisms, EVs orchestrate cell survival, angiogenesis, resolution of inflammation, and tissue repair. Recent evidence further suggests that EVs can directly reprogram cardiomyocyte metabolism, highlighting their multifaceted therapeutic potential[33].
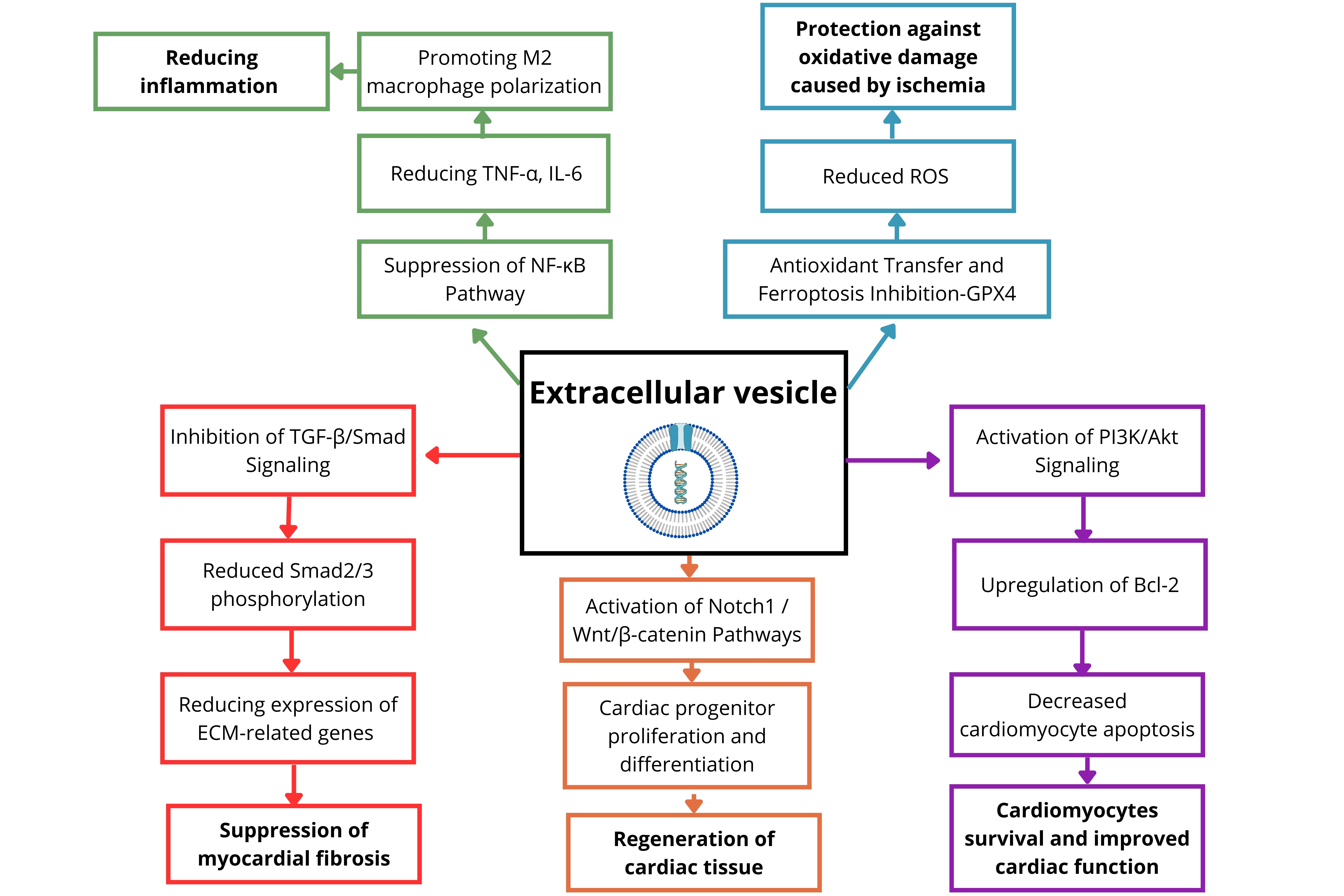
Figure 1 provides a schematic overview of signaling pathways modulated by EVs and their associated regenerative effects in the myocardium.
Figure 1. Key signaling pathways modulated by extracellular vesicles (EVs) in post-myocardial infarction repair. TNF-α: Tumor necrosis factor-alpha; IL-6: interleukin-6; NF-κB: nuclear factor-κB; TGF-β: transforming growth factor-β; ECM: excessive extracellular matrix; ROS: reactive oxygen species; GPX4: glutathione peroxidase 4; PI3K: phosphoinositide 3-kinase; Bcl-2: B-cell lymphoma-2.
EVs reduce inflammation by suppressing NF-κB, lowering TNF-α and IL-6, and promoting M2 macrophage polarization. They limit fibrosis via TGF-β/Smad inhibition, reduced Smad2/3 phosphorylation, and downregulation of ECM-related genes. Pro-regenerative effects include activation of Notch1 and
AGING-ASSOCIATED CARDIAC REMODELING AND THE EMERGING ROLE OF EXTRACELLULAR VESICLES
Cardiac aging is a progressive physiological process that profoundly affects cardiovascular disease. It is a multifactorial phenomenon driven by the interplay of genetic, environmental, and intracellular alterations. With advancing age, various cardiac cell types become less efficient and increasingly prone to promoting chronic low-grade inflammation, which significantly impairs myocardial function[1].
Even low-grade, persistent inflammation reduces cardiac resilience, while aging-related changes in calcium handling impair the contractile and relaxation capacity of cardiomyocytes[1]. Moreover, aging promotes myocardial hypertrophy, fibrosis, and valvular degeneration, ultimately leading to reduced cardiac output and diastolic dysfunction[2].
The effects of aging on the heart can be broadly described across four domains:
• Structural changes include atrial enlargement, myocardial fibrosis, vascular stiffening, and left ventricular wall thickening, all of which decrease myocardial compliance.
• Functional changes encompass diastolic dysfunction, reduced cardiac output, impaired relaxation, diminished β-adrenergic responsiveness, increased susceptibility to arrhythmias, and cardiac autonomic dysregulation.
• Cellular changes involve cardiomyocyte senescence, mitochondrial dysfunction, impaired autophagy, and heightened fibroblast activity that promotes extracellular matrix deposition. Endothelial cells also display reduced angiogenic capacity, further compromising tissue repair.
• Genetic and epigenetic changes include telomere attrition, accumulation of DNA damage, altered methylation patterns, and deposition of amyloid fibrils, all of which exacerbate myocardial decline.
Together, these multifactorial processes create an aged cardiac microenvironment that compromises endogenous repair and increases vulnerability to stressors. Contemporary studies emphasize the contribution of mitochondrial dysfunction and metabolic decline[2] as well as senescence-driven inflammatory signaling[57] in driving adverse remodeling.
Addressing cardiac aging requires a multifaceted therapeutic approach, encompassing pharmacological agents, gene modulation, and regenerative strategies[58]. While stem cell therapy has shown promise in cardiac regeneration, accumulating evidence suggests that its benefits are primarily mediated through paracrine signaling rather than direct cell engraftment[12]. Consequently, increasing attention is being directed toward EVs - key mediators of intercellular communication that transport functional proteins, nucleic acids, and lipids.
EVs are currently being explored not only as regenerative agents but also as modulators of aging-related processes, including oxidative stress, inflammation, and telomere attrition[12]. Their capacity to influence multiple cellular pathways positions them as a compelling tool in the development of cell-free strategies to counteract age-associated cardiac dysfunction.
EVs as mediators of cardiac aging
Aging-associated transcriptional remodeling in cardiac fibroblasts and macrophages enhances
Macrophages in the aging heart also participate in EV-mediated signaling. Macrophage-derived EVs carrying miR-155 propagate inflammation and accelerate cardiac aging. During MI, these EVs transfer
Systemic therapies can modulate this process. Notably, macrophages pre-treated with PD-1 inhibitors release EVs that promote cardiomyocyte senescence by delivering miR-34a-5p, which suppresses phosphatase 1 nuclear targeting subunit (PNUTS) - a gene essential for DNA integrity and cell cycle regulation[59]. Loss of PNUTS leads to G0/G1 cell cycle arrest and accelerated cellular aging. In contrast, EVs from untreated macrophages did not display measurable pro-aging effects, underscoring the
Beyond cardiomyocytes, fibroblasts, and macrophages, endothelial cells also play a significant role in cardiac aging. Experimental data show that vascular relaxation responses to acetylcholine are markedly reduced in old mice, indicating impaired endothelial function[60]. Remarkably, this age-related endothelial dysfunction was almost completely reversed by EV treatment, restoring acetylcholine-mediated vasodilation. In contrast, endothelium-independent vasodilation to sodium nitroprusside (a nitric oxide donor) remained unaffected by aging or EVs, suggesting that vascular smooth muscle responsiveness to nitric oxide is preserved[60].
Molecular mechanisms of aging associated with extracellular vesicles
The aged cardiac microenvironment is characterized by elevated levels of pro-inflammatory cytokines such as IL-6 and IL-8, which are hallmark components of the senescence-associated secretory phenotype (SASP)[12]. Multiple aging-related stressors-including chemotherapy[61], oxidative stress[62], oncogene activation[63], and replicative exhaustion[64,65]-increase EV production. These vesicles constitute a critical element of the SASP, enabling the paracrine propagation of senescence.
Evidence from non-cardiac contexts further supports this concept. In osteoarthritis, Jeon et al. demonstrated that EVs derived from aged chondrocytes can induce paracrine senescence in healthy chondrocytes, thereby inhibiting cartilage formation[66]. Similarly, proteomic profiling of EVs released by triple-negative breast cancer cells undergoing therapy-induced senescence revealed alterations associated with cell proliferation, Adenosine Triphosphate (ATP) depletion, apoptosis, and SASP activity[61]. In primary sclerosing cholangitis (PSC), aged human biliary epithelial cells secrete higher levels of EVs compared with normal cells, and murine PSC models likewise show elevated plasma EV levels[67].
Within the heart, EVs actively regulate signaling pathways that control remodeling, survival, and regeneration. For instance, the mitogenic factor HIMF (hypoxia-induced mitogenic factor) stimulates IL-6 expression in fibroblast-cardiomyocyte communication, contributing to inflammatory remodeling. Wnt signaling also plays a dual role in post-infarction repair: global Wnt inhibition enhances cardiac regeneration, whereas cardiomyocyte-specific inhibition produces the opposite effect, emphasizing the context-dependent nature of this pathway[13].
At the molecular level, EVs deliver non-coding RNAs that modulate key intracellular responses. One example is long non-coding RNAs (lncRNA) urothelial carcinoma associated 1 (UCA1), enriched in EVs secreted by hypoxia-preconditioned bone marrow-derived MSCs. UCA1 functions as a molecular sponge for miR-873-5p, leading to upregulation of XIAP (X-linked inhibitor of apoptosis protein) and subsequent activation of activated protein kinase signaling (AMPK) signaling in cardiomyocytes, thereby promoting cell survival under stress[13,14].
Aging cardiomyocytes also exhibit metabolic dysregulation, including elevated acetyl-coenzyme A
Beyond metabolic reprogramming, ADSC-sEVs have demonstrated broader anti-aging effects in murine models, including improved cardiac function, reduced myocardial fibrosis, attenuation of oxidative and DNA damage, suppression of pro-inflammatory signaling, and increased expression of developmental and regenerative markers. Collectively, these findings underscore the potential of ADSC-sEVs as a cell-free therapeutic strategy to counteract age-related cardiac remodeling and enhance myocardial resilience[12].
EVs as rejuvenation tools
Cardiovascular aging is characterized by progressive degenerative changes, many of which may be delayed or even partially reversed through the application of EVs as rejuvenation agents.
Chen et al. demonstrated that lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1), enriched in exosomes secreted by human ADSCs stimulated with migration inhibitory factor (MIF), protects cardiomyocytes against H2O2-induced apoptosis[69]. Dual-luciferase reporter assays confirmed that these MIF-induced ADSC-derived exosomes alleviate oxidative stress by modulating the miR-142-3p/forkhead box protein O1 (FOXO1) signaling axis, thereby exerting cardioprotective effects[69]. Similarly, Zhuang et al. showed that systemic administration of MIF-treated MSC-derived exosomes, also enriched in NEAT1, reversed doxorubicin-induced cardiac aging in mice and significantly improved cardiac function[70].
The rejuvenating properties of EVs are further supported by preclinical models of myocardial injury. In a murine MI model, exosomes derived from embryonic stem cells (ESCs) promoted the survival and proliferation of CPCs via delivery of miR-294. Likewise, intramuscular injection of cardiosphere-derived cell (CDC) exosomes reduced both acute and chronic myocardial injury in porcine models, minimized infarct size, attenuated scar formation, and reversed adverse cardiac remodeling[10].
Additional evidence comes from studies by Ma et al., who reported that exosomes derived from mouse bone marrow-derived stem cells deliver miR-132, which promotes tube formation in HUVECs in vitro and stimulates angiogenesis in ischemic myocardium in vivo[71].
Together, these findings highlight the regenerative and rejuvenating potential of EVs in restoring cardiac function, not only by counteracting age-related decline but also by repairing ischemic damage.
CLINICAL TRANSLATION AND ONGOING TRIALS
Despite compelling preclinical data supporting the therapeutic potential of EVs in MI, their clinical translation remains in its early stages. Numerous scientific, technological, and regulatory hurdles must be overcome before EV-based therapies can be widely implemented in cardiovascular medicine.
Challenges to clinical translation
Major limitations include the lack of standardized protocols for EV isolation and characterization, variations in cargo composition depending on cell source and culture conditions, and the absence of consensus on optimal dosage and delivery routes. Issues such as long-term storage stability and the ability to manufacture EVs at scale under Good Manufacturing Practice (GMP) conditions also remain unresolved. Importantly, little is known about the biodistribution, half-life, and clearance kinetics of exogenously administered EVs in humans[4].
Compared to traditional cell-based therapies, EVs offer several advantages. They are non-replicating, carry minimal immunogenic risk, and have not been shown to induce tumorigenesis in preclinical cardiac studies, although long-term safety data are still needed. Their lipid bilayer preserves protein and nucleic acid cargo in circulation and enables passive or engineered targeting. These features make EVs attractive candidates for cell-free regenerative therapy in cardiology[15,72].
Early clinical trials and initial results
Although limited in number, early-phase clinical trials have begun to demonstrate the feasibility, safety, and potential clinical relevance of EV-based approaches in cardiovascular settings. These studies can be divided into biomarker-focused trials and interventional trials involving EV or EV-enriched products:
A. Biomarker and Mechanistic Studies (No EV Administration)
• NCT02931045 - AFFECT-EV study
This study evaluated the effect of dual antiplatelet therapy (ticagrelor vs. clopidogrel) on circulating levels of procoagulant EVs in patients with ST-segment (ST)-elevation MI. Results indicated that ticagrelor significantly reduced the release of platelet-derived EVs, suggesting an additional antithrombotic effect beyond platelet inhibition[73].
B. Interventional Studies (EV or EV-Enriched Product Administration)
• NCT04327635 - Safety Evaluation of Intracoronary Infusion of Extracellular Vesicles in Patients Following Coronary Stent Implantation (Phase I):
This trial investigated the feasibility and safety of intracoronary infusion of allogeneic platelet-derived extracellular particles (PEPs), not MSC-EVs, immediately after percutaneous coronary intervention (PCI) in MI patients. Preliminary results: safe, well-tolerated, no serious cardiovascular adverse events in early follow-up[74].
• NCT05774509 - SECRET-HF Study (Phase I):
Interventional trial delivering EV-enriched secretome from cardiovascular progenitor cells intravenously in patients with drug-refractory left ventricular dysfunction due to non-ischemic dilated cardiomyopathy. Still recruiting; early case reports indicate safety and possible symptomatic benefit[75].
These trials are crucial for bridging the gap between preclinical promise and clinical reality. They demonstrate the possibility of producing clinical-grade EVs and administering them safely in human subjects, thereby paving the way for more efficacy-focused studies. Given that the majority of patients affected by MI are older adults, the clinical relevance of EV-based therapies must also be considered in the context of age-related cardiac remodeling. Aging hearts exhibit increased fibrosis, chronic low-grade inflammation, and reduced angiogenic capacity, all of which may influence EV uptake, biodistribution, and therapeutic efficacy. To date, no cardiovascular EV trials have reported outcomes stratified by patient age or biological markers of aging, representing an important gap in clinical translation.
In Table 3, we present selected clinical trials that aim to evaluate the therapeutic application of EVs in cardiovascular disease. Most cardiovascular EV trials are phase I, focus on safety, and lack standardized efficacy endpoints. We are awaiting final results, which may prove as promising as those from other
Selected clinical trials evaluating EV-based therapies
| Number | Category | Phase | Study status | Population/Intervention | Key Finding(s) |
| NCT02931045 | Biomarker | IV | Completed | STEMI; ticagrelor vs clopidogrel effect on EVs | Ticagrelor ↓ platelet-derived EVs |
| NCT04327635 | Interventional | I | Active, not recruiting | STEMI; intracoronary allogeneic PEPs post-PCI | Safe; no early serious CV events |
| NCT05774509 | Interventional | I | Recruiting | Non-ischemic DCM; IV EV-enriched secretome | Safe; case reports suggest functional improvement |
Toward next-generation EV therapies
Current efforts are focused on enhancing the therapeutic precision and potency of EVs through strategies such as:
• Surface functionalization with cardiac-targeting ligands;
• Hypoxic preconditioning of donor cells to optimize cargo;
• Direct engineering of EV cargo [e.g., Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) components, cardioprotective miRNAs][77,78];
• Development of biomaterial carriers and synthetic EV mimetics for scalable manufacturing[78].
Although still in the early stages of translational readiness for MI, EV-based therapies remain a promising, safe, and versatile platform for cardiac regeneration.
CHALLENGES AND FUTURE PERSPECTIVES
Despite a growing body of preclinical and early clinical evidence demonstrating the cardioprotective potential of EVs, several key obstacles remain before these therapies can be broadly implemented in clinical practice. These challenges span biological variability, technological limitations, and translational gaps.
Standardization and characterization
One of the most significant hurdles in EV research is the lack of standardized methods for isolation, purification, and characterization. Common techniques such as SEC, polymer-based precipitation, differential ultracentrifugation, and microfluidic platforms yield EV subpopulations with varying purity, recovery rates, and bioactivity[79]. This variability complicates the interpretation and reproducibility of results.
Although the ISEV’s minimal information for studies of extracellular vesicles 2018 (MISEV2018) guidelines provide clear recommendations, discrepancies persist in naming and classifying EVs - particularly when attempting to distinguish exosomes from microvesicles based solely on size or surface markers[4]. These ambiguities present challenges not only for experimental reproducibility but also for regulatory approval, where product identity and consistency are critical.
Scalability and manufacturing
Large-scale EV production remains another major limitation. Current isolation methods are
However, GMP-grade EV production still requires further technological development. Storage and
Cargo heterogeneity and functional variability
EVs closely reflect the phenotype and physiological status of their parent cells, which introduces substantial variability in their cargo. This content - including miRNAs, non-coding RNAs, proteins, and lipids - is strongly influenced by the cell type (e.g., CDCs, MSCs, iPSCs), culture conditions (e.g., hypoxia, serum-free media), and environmental stimuli[82,83].
Such biological variability complicates the development of a standardized therapeutic product. Even minor changes in production protocols can markedly affect EV bioactivity and potency. Unlike monoclonal antibodies or small-molecule drugs, EVs are complex, heterogeneous biological entities with only partially defined mechanisms of action. Therefore, regulatory frameworks must incorporate rigorous pharmacodynamic and pharmacokinetic profiling to ensure product consistency and safety.
Another underexplored translational challenge is the lack of age-stratified analyses in both preclinical and clinical studies. Age-associated changes in the cardiac microenvironment - including altered extracellular matrix composition, SASP signaling, and reduced regenerative cell populations - may modulate the therapeutic response to EVs. Addressing this requires preclinical designs that model aging and clinical trials that incorporate age as a key variable in patient selection and outcome interpretation.
Targeted delivery to ischemic myocardium
A major translational challenge is the limited targeting efficiency of EVs after systemic administration. A large proportion of intravenously injected EVs are rapidly sequestered by the reticuloendothelial system (RES), primarily in the liver, spleen, and lungs[43].
Several strategies are under investigation to improve cardiac-specific delivery:
• Surface modification with cardiac-homing peptides [e.g., CSTSMLKAC (a polypeptide with the sequence Cys-Ser-Thr-Ser-Met-Leu-Lys-Ala-Cys)] that bind ischemia-induced molecules on cardiomyocytes or endothelial cells[84].
• Magnetic targeting, in which EVs are loaded with superparamagnetic nanoparticles and guided to the heart using external magnetic fields[85].
• Bioinspired systems, such as hybrid vesicles coated with cell membranes or platelet-derived proteins to enhance retention within injured cardiac tissue[86].
Although still at the experimental stage, these approaches have the potential to substantially improve the therapeutic index of EV-based therapies.
Contextualizing EV therapy in MI pathophysiology
The pathophysiology of MI is multifactorial, encompassing acute ischemia, inflammation, oxidative stress, and progressive fibrotic remodeling. Effective post-infarction therapy is challenged by rapid cellular turnover, limited intrinsic regenerative capacity, and narrow therapeutic windows for intervention.
EVs provide a uniquely multifaceted therapeutic approach capable of modulating inflammation, apoptosis, angiogenesis, and fibrosis simultaneously. To fully realize these benefits, delivery must be carefully timed to align with the evolving pathophysiological stages of MI, and targeting strategies should be optimized to enhance homing and retention within the ischemic myocardium.
Advantages, disadvantages and limitations of EV-based therapies
EVs possess several desirable characteristics that make them attractive candidates for regenerative medicine. They circulate stably in vivo while protecting their molecular cargo, can cross biological barriers, and display low immunogenicity with a favorable safety profile regarding oncogenic risk[15,17,72]. In addition, EVs
Despite these advantages, several challenges limit the clinical translation of EV-based therapies. EV cargo is heterogeneous and varies considerably depending on the source cell type and culture conditions[82,83]. After systemic administration, EVs exhibit low targeting efficiency toward infarcted myocardium and are rapidly cleared by the RES, particularly the liver and spleen[18,19,43,44]. Storage and stability also remain problematic, as repeated freeze-thaw cycles or lyophilization can compromise vesicle integrity[80,81].
Key limitations include the absence of standardized protocols for EV isolation and quantification, as well as the lack of universally accepted markers for EV subtype identification[7,9,79]. Large-scale production suitable for clinical application remains technically challenging, and pharmacokinetic and pharmacodynamic properties are still poorly defined[68,80,81]. Importantly, EVs also demonstrate limited homing and retention within infarcted myocardium following systemic administration, which significantly constrains therapeutic efficacy[42,43].
Future directions and research priorities
Despite substantial preclinical and emerging clinical evidence for the regenerative potential of EVs, several challenges must be addressed to achieve clinical translation. Standardization of EV isolation, purification, and characterization protocols is essential to ensure reproducibility and comparability across studies. The inherent heterogeneity of EV populations, variability in donor cell sources, and batch-to-batch differences necessitate rigorous quality control and potency assays.
In the setting of MI, EVs have demonstrated the capacity to reduce apoptosis, promote angiogenesis, and modulate post-infarction remodeling in preclinical models. However, optimizing delivery routes, targeting strategies, and dosing regimens is critical to improve myocardial retention, bioavailability, and therapeutic index. Integration of EVs with biomaterials such as hydrogels or scaffolds may enhance localization and sustain bioactivity at the site of injury.
Recent findings also extend the potential applications of EVs beyond acute injury to encompass cardiac aging and age-associated remodeling. Future investigations should therefore integrate the biology of aging into EV therapy development. Key priorities include identifying EV cargos that specifically target
Such approaches are particularly relevant given that aging hearts are characterized by chronic inflammation, oxidative stress, and cellular senescence, all of which compromise regenerative capacity and drive progressive dysfunction. Preclinical studies indicate that EVs derived from stem or progenitor cells can restore metabolic homeostasis, reduce fibrosis, suppress pro-senescent signaling, and reverse certain features of cardiac aging[87]. A crucial next step will be to perform age-stratified evaluations of EV efficacy, considering both donor age and recipient biological age. Identifying cargos specifically linked to rejuvenation - such as lncRNAs, miRNAs (e.g., let-7, miR-132), and anti-apoptotic proteins (e.g., Hsp20) - will help to develop tailored interventions. Moreover, engineering EV mimetics, targeted delivery systems, and hybrid platforms may further enhance regenerative outcomes in aged and diseased hearts.
In summary, EV-based therapies remain at the forefront of innovation in both ischemic and aging-related cardiac regeneration. Bridging the gap between mechanistic understanding and clinical application will require interdisciplinary collaboration, regulatory alignment, and well-designed clinical trials with robust, age-relevant endpoints. Emerging computational approaches such as machine learning and artificial intelligence may further accelerate the development of EV-based therapeutics. Algorithms trained on
Final conclusions
EV-based therapies represent a promising frontier for both ischemic and aging-related cardiac regeneration. Evidence from diverse preclinical models demonstrates that EVs reduce apoptosis, enhance angiogenesis, modulate inflammation, and limit fibrosis. In aged hearts, they additionally restore metabolic balance, suppress pro-senescent signaling, and reverse structural deterioration.
Despite these encouraging results, translation into clinical practice is still limited by variability in EV production, incomplete pharmacokinetic and biodistribution data, suboptimal targeting efficiency, and the lack of standardized potency assays. Current safety profiles appear favorable in both cardiovascular and non-cardiovascular trials, yet long-term monitoring remains essential.
Closing the gap between mechanistic insights and tangible patient benefit will require rigorous standardization, innovative delivery platforms, age-stratified clinical evaluations, and integration with advanced biomaterials. Ultimately, progress will depend on close interdisciplinary collaboration-bringing together cardiology, molecular biology, materials science, and regulatory science-to enable the safe and effective transition of EV-based cardiac therapies from bench to bedside. EV-based interventions represent the convergence of regenerative and anti-aging cardiology, yet successful translation will depend on
DECLARATIONS
Authors’ contributions
Conceptualized the review and prepared the figures: Porada M
Wrote the manuscript and prepared the final version: Porada M, Janas A, Bajdak-Rusinek K
Prepared the tables: Porada M, Bajdak-Rusinek K
Guided the writing and edited the manuscript, supervision: Bajdak-Rusinek K
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Wood C, Salter WZ, Garcia I, et al. Age-associated changes in the heart: implications for COVID-19 therapies. Aging. 2025;17:1340-67.
2. Marín-Aguilar F, Lechuga-Vieco AV, Alcocer-Gómez E, et al. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell. 2020;19:e13050.
3. Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29-49.
4. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
5. Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
6. Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-57.
7. Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63-78.
8. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977.
9. Ibrahim AG, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606-19.
10. Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201-11.
11. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-22.
12. Sanz-Ros J, Huete-Acevedo J, Mas-Bargues C, et al. Small extracellular vesicles from young adipose-derived stem cells ameliorate age-related changes in the heart of old mice. Stem Cell Res Ther. 2025;16:138.
13. Nicin L, Wagner JUG, Luxán G, Dimmeler S. Fibroblast-mediated intercellular crosstalk in the healthy and diseased heart. FEBS Lett. 2022;596:638-54.
14. Yuan Z, Huang W. New developments in exosomal lncRNAs in cardiovascular diseases. Front Cardiovasc Med. 2021;8:709169.
15. Sluijter JPG, Davidson SM, Boulanger CM, et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2018;114:19-34.
16. Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol. 2018;9:1486.
17. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-5.
18. Wang L, Wang D, Ye Z, Xu J. Engineering extracellular vesicles as delivery systems in therapeutic applications. Adv Sci. 2023;10:2300552.
19. Nieland L, Mahjoum S, Grandell E, Breyne K, Breakefield XO. Engineered EVs designed to target diseases of the CNS. J Control Release. 2023;356:493-506.
20. Shao L, Zhang Y, Lan B, et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Res Int. 2017;2017:4150705.
21. Jansen F, Li Q. Exosomes as diagnostic biomarkers in cardiovascular diseases. In: Xiao J, Cretoiu S, editors. Exosomes in cardiovascular diseases. Singapore: Springer; 2017. pp. 61-70.
22. Zhao Y, Sun X, Cao W, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. 2015;2015:761643.
23. Song Y, Zhang C, Zhang J, et al. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics. 2019;9:2346-60.
24. Li K, Pan J, Li Q, et al. The MicroRNA-210/Casp8ap2 pathway alleviates hypoxia-induced injury in myocardial cells by regulating apoptosis and autophagy. Heart Surg Forum. 2020;23:E797-802.
25. Zhao DZ, Yang RL, Wei HX, et al. Advances in the research of immunomodulatory mechanism of mesenchymal stromal/stem cells on periodontal tissue regeneration. Front Immunol. 2024;15:1449411.
26. Zhao J, Li X, Hu J, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205-16.
27. Song Y, Dou H, Li X, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208-21.
28. Liu J, Chen T, Lei P, Tang X, Huang P. Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. Int J Med Sci. 2019;16:1238-44.
29. Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2014;92:387-97.
30. Liu W, Li L, Rong Y, et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212.
31. Zhao Y, Du D, Chen S, Chen Z, Zhao J. New insights into the functions of micrornas in cardiac fibrosis: from mechanisms to therapeutic strategies. Genes. 2022;13:1390.
32. Duisters RF, Tijsen AJ, Schroen B, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170-8.
33. Nashtahosseini Z, Nejatollahi M, Fazilat A, et al. The crosstalk between exosomal miRNA and ferroptosis: a narrative review. Biol Cell. 2025;117:e2400077.
34. Zhang L, Ouyang P, He G, et al. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J Cell Mol Med. 2021;25:2148-62.
35. Cheng H, Chang S, Xu R, et al. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 2020;11:224.
36. Liu Z, Zhang Z, Yao J, et al. Serum extracellular vesicles promote proliferation of H9C2 cardiomyocytes by increasing miR-17-3p. Biochem Biophys Res Commun. 2018;499:441-6.
37. Zhang J, Ma J, Long K, et al. Overexpression of exosomal cardioprotective miRNAs mitigates hypoxia-induced H9c2 cells apoptosis. Int J Mol Sci. 2017;18:711.
38. Bheri S, Brown ME, Park HJ, Brazhkina O, Takaesu F, Davis ME. Customized loading of microRNA-126 to small extracellular vesicle-derived vehicles improves cardiac function after myocardial infarction. ACS Nano. 2023;17:19613-24.
39. Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52-64.
40. Wang Y, Zhang L, Li Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61-9.
41. Loyer X, Zlatanova I, Devue C, et al. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction. Circ Res. 2018;123:100-6.
42. Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481-92.
43. Wiklander OP, Nordin JZ, O’Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316.
44. Mendt M, Kamerkar S, Sugimoto H, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3:e99263.
45. Barile L, Moccetti T, Marbán E, Vassalli G. Roles of exosomes in cardioprotection. Eur Heart J. 2017;38:1372-9.
46. Wu HY, Zhang XC, Jia BB, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acetaminophen-induced acute liver failure through activating ERK and IGF-1R/PI3K/AKT signaling pathway. J Pharmacol Sci. 2021;147:143-55.
47. Wang X, Zhu Y, Wu C, Liu W, He Y, Yang Q. Adipose-derived mesenchymal stem cells-derived exosomes carry MicroRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation. 2021;44:1815-30.
48. Huang J, Shi L, Yang Y, et al. Mesenchymal cell-derived exosomes and miR-29a-3p mitigate renal fibrosis and vascular rarefaction after renal ischemia reperfusion injury. Stem Cell Res Ther. 2025;16:135.
49. Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763-76.
50. Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647-53.
51. Xuan W, Khan M, Ashraf M. Extracellular vesicles from notch activated cardiac mesenchymal stem cells promote myocyte proliferation and neovasculogenesis. Front Cell Dev Biol. 2020;8:11.
52. Gross JC, Zelarayán LC. The mingle-mangle of wnt signaling and extracellular vesicles: functional implications for heart research. Front Cardiovasc Med. 2018;5:10.
53. Meng WT, Zhu J, Wang YC, et al. Targeting delivery of miR-146a via IMTP modified milk exosomes exerted cardioprotective effects by inhibiting NF-κB signaling pathway after myocardial ischemia-reperfusion injury. J Nanobiotechnology. 2024;22:382.
54. Li H, Ding J, Liu W, et al. Plasma exosomes from patients with acute myocardial infarction alleviate myocardial injury by inhibiting ferroptosis through miR-26b-5p/SLC7A11 axis. Life Sci. 2023;322:121649.
55. Chen J, Zhong J, Wang LL, Chen YY. Mitochondrial transfer in cardiovascular disease: from mechanisms to therapeutic implications. Front Cardiovasc Med. 2021;8:771298.
56. Ceja L, Escopete SS, Hughes L, et al. Neonatal cardiovascular-progenitor-cell-derived extracellular vesicles activate yap1 in adult cardiac progenitor cells. Int J Mol Sci. 2023;24:8088.
57. Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22:75-95.
58. Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2012;16:1492-526.
59. Xia W, Chen H, Chen D, Ye Y, Xie C, Hou M. PD-1 inhibitor inducing exosomal miR-34a-5p expression mediates the cross talk between cardiomyocyte and macrophage in immune checkpoint inhibitor-related cardiac dysfunction. J Immunother Cancer. 2020;8:e001293.
60. Feng R, Ullah M, Chen K, Ali Q, Lin Y, Sun Z. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. J Extracell Vesicles. 2020;9:1783869.
61. Estévez-Souto V, Da Silva-Álvarez S, Collado M. The role of extracellular vesicles in cellular senescence. FEBS J. 2023;290:1203-11.
62. Terlecki-Zaniewicz L, Lämmermann I, Latreille J, et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging. 2018;10:1103-32.
63. Borghesan M, Fafián-Labora J, Eleftheriadou O, et al. Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 2019;27:3956-3971.e6.
64. Riquelme JA, Takov K, Santiago-Fernández C, et al. Increased production of functional small extracellular vesicles in senescent endothelial cells. J Cell Mol Med. 2020;24:4871-6.
65. Mensà E, Guescini M, Giuliani A, et al. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J Extracell Vesicles. 2020;9:1725285.
66. Jeon OH, Wilson DR, Clement CC, et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. 2019;4:e125019.
67. Suraih MS, Trussoni CE, Splinter PL, LaRusso NF, O’Hara SP. Senescent cholangiocytes release extracellular vesicles that alter target cell phenotype via the epidermal growth factor receptor. Liver Int. 2020;40:2455-68.
68. Gimona M, Brizzi MF, Choo ABH, et al. Critical considerations for the development of potency tests for therapeutic applications of mesenchymal stromal cell-derived small extracellular vesicles. Cytotherapy. 2021;23:373-80.
69. Chen H, Xia W, Hou M. LncRNA-NEAT1 from the competing endogenous RNA network promotes cardioprotective efficacy of mesenchymal stem cell-derived exosomes induced by macrophage migration inhibitory factor via the miR-142-3p/FOXO1 signaling pathway. Stem Cell Res Ther. 2020;11:31.
70. Zhuang L, Xia W, Chen D, et al. Exosomal LncRNA-NEAT1 derived from MIF-treated mesenchymal stem cells protected against doxorubicin-induced cardiac senescence through sponging miR-221-3p. J Nanobiotechnology. 2020;18:157.
71. Ma T, Chen Y, Chen Y, et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int. 2018;2018:3290372.
72. Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259-72.
73. Gasecka A, Nieuwland R, Budnik M, et al. Randomized controlled trial protocol to investigate the antiplatelet therapy effect on extracellular vesicles (AFFECT EV) in acute myocardial infarction. Platelets. 2020;31:26-32.
74. ClinicalTrials.gov. Safety evaluation of intracoronary infusion of extracellular vesicles in patients following coronary stent implantation. U.S. National Library of Medicine; 2025 Mar. Report No.: NCT04327635. Available from: https://clinicaltrials.gov/study/NCT04327635 [Last accessed on 27 Oct 2025].
75. ClinicalTrials.gov. Treatment of non-ischemic cardiomyopathies by intravenous extracellular vesicles of cardiovascular progenitor cells (SECRET-HF). U.S. National Library of Medicine; 2023 Sep. Report No.: NCT05774509. Available from: https://clinicaltrials.gov/study/NCT05774509 [Last accessed on 27 Oct 2025].
76. Lightner AL, Sengupta V, Qian S, et al. Bone marrow mesenchymal stem cell-derived extracellular vesicle infusion for the treatment of respiratory failure from COVID-19: a randomized, placebo-controlled dosing clinical trial. CHEST. 2023;164:1444-53.
77. Lu Y, Godbout K, Lamothe G, Tremblay JP. CRISPR-Cas9 delivery strategies with engineered extracellular vesicles. Mol Ther Nucleic Acids. 2023;34:102040.
78. García-Manrique P, Matos M, Gutiérrez G, Pazos C, Blanco-López MC. Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles. 2018;7:1422676.
79. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789-804.
80. Ahmadian S, Jafari N, Tamadon A, Ghaffarzadeh A, Rahbarghazi R, Mahdipour M. Different storage and freezing protocols for extracellular vesicles: a systematic review. Stem Cell Res Ther. 2024;15:453.
81. Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017;20:1.
82. Williams T, Salmanian G, Burns M, et al. Versatility of mesenchymal stem cell-derived extracellular vesicles in tissue repair and regenerative applications. Biochimie. 2023;207:33-48.
83. Almeria C, Kreß S, Weber V, Egger D, Kasper C. Heterogeneity of mesenchymal stem cell-derived extracellular vesicles is highly impacted by the tissue/cell source and culture conditions. Cell Biosci. 2022;12:51.
84. Vandergriff A, Huang K, Shen D, et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8:1869-78.
85. Qi H, Liu C, Long L, et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano. 2016;10:3323-33.
86. Li Q, Song Y, Wang Q, et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics. 2021;11:3916-31.
87. Li YJ, Wu JY, Liu J, et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. 2021;19:242.
88. Greenberg ZF, Graim KS, He M. Towards artificial intelligence-enabled extracellular vesicle precision drug delivery. Adv Drug Deliv Rev. 2023;199:114974.
89. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].