fig4
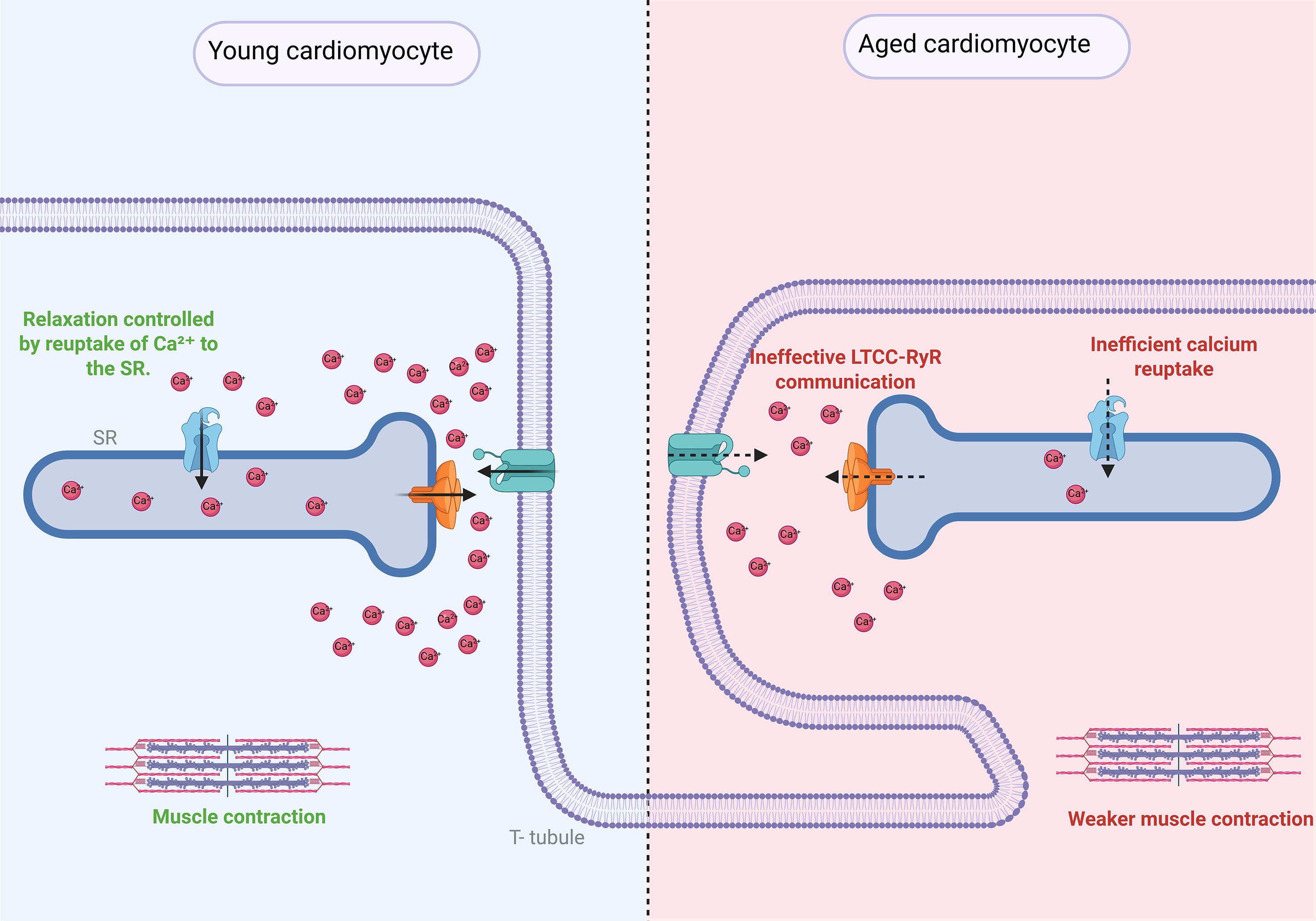
Figure 4. Structural and functional coupling between T-tubules and the sarcoplasmic reticulum (SR) in cardiomyocytes and its disruption during aging. In young cardiomyocytes, T-tubules invaginate from the plasma membrane and align precisely with the sarcoplasmic reticulum (SR), allowing for a rapid propagation of action potentials and synchronized Ca2+ release for excitation-contraction (E-C) coupling. L-type Ca2+ channels (LTCCs) located in T-tubules interact closely with ryanodine receptors (RyR) on the SR to trigger Ca2+-induced Ca2+ release. During aging, T-tubule architecture becomes disorganized and less dense, leading to misalignment with the SR, reduced LTCC-RyR coupling efficiency, delayed Ca2+ transients, and impaired contractile function. These structural and functional alterations contribute to the development of cardiac dysfunction, including heart failure with preserved ejection fraction (HFpEF), arrhythmias, and impaired relaxation.