Overview of extracellular vesicles as biomarkers and therapeutic tools in pediatric diseases: focus on the gut-lung axis
Abstract
Although anatomically separate, the gut and lungs are interconnected through intricate pathways involving their respective microbiota, supporting the concept of a gut-lung axis. In the pediatric field, devastating intestinal pathologies such as necrotizing enterocolitis and inflammatory bowel diseases mostly affect preterm infants. In parallel, in the lung, bronchopulmonary dysplasia and chronic obstructive pulmonary disease represent pediatric unmet clinical needs. In this review, we discuss how the extracellular vesicles (EVs), nanoparticles secreted by all cell types, represent a common element in the gut-lung axis. Specifically, EVs play a dual role, serving both as novel disease biomarkers and as promising therapeutic agents.
Keywords
INTRODUCTION
In recent years, the concept of intercellular communication has undergone a profound transformation with the discovery and characterization of extracellular vesicles (EVs), which are small, membrane-bound particles actively secreted by nearly all cell types. While in the past they were considered cellular garbage, EVs are now recognized as functional cell-cell messengers that carry a wide array of bioactive molecules, including proteins, lipids, messenger RNAs (mRNAs), microRNAs (miRNAs) and even DNA fragments[1,2]. These vesicles modulate the behavior of recipient cells, becoming key players in local and systemic biological processes[3]. EVs are broadly categorized based on their size, surface markers and biogenesis into three main classes: exosomes, microvesicles, and apoptotic bodies[4].
Each class arises from distinct intracellular pathways and carries specific cargo reflective of its cellular origin and the physiological or pathological state of the parent cell. The selective packaging of this cargo is not random, but rather a tightly regulated process that enables targeted communication and functional specificity[5]. The ability of EVs to transfer complex information from one cell to another has established them as crucial mediators in a broad spectrum of physiological functions, including immune regulation, tissue development, cellular homeostasis, and inter-organ communication[5,6]. EVs in fluids, such as in peripheral blood, urine and saliva, are valuable as disease biomarkers and prognostic indicators of treatment response or disease progression. EVs hold significant potential in pediatric diseases, given that many childhood conditions involve intricate developmental processes and can have lasting impacts on growth and organ function. EVs, as a therapeutic agent, may provide a less invasive and potentially more effective alternative to existing treatment approaches.
Beyond their physiological functions, EVs are also involved in various pathological conditions, playing a paramount role in disease progression. Concerning inflammatory diseases, EVs can either propagate inflammation or act as regulatory agents that limit tissue damage[7,8]. The dual nature of EVs, which can serve as both disease vectors and potential therapeutic tools, has generated considerable interest in their application as biomarkers, therapeutic targets, and drug delivery systems[9,10].
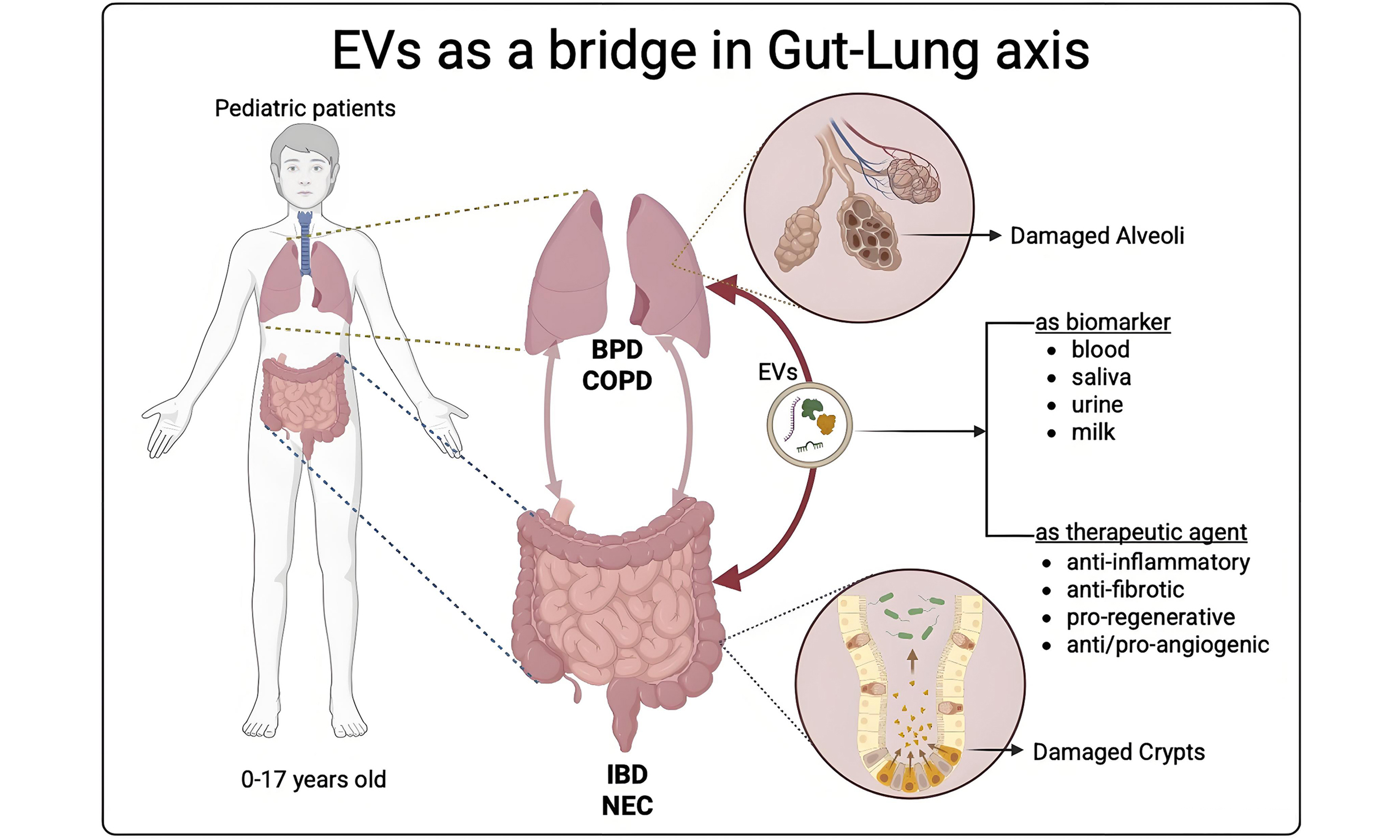
One particularly intriguing and emerging area of EV research is their role in mediating communication between distant organs. A notable example of this systemic interaction is the gut-lung axis, a bidirectional communication network between the gastrointestinal and respiratory systems. Although these organs are anatomically distinct and functionally specialized, research suggests that they share immune, neural, and microbial connections that allow them to influence each other’s physiological state[11,12]. EVs represent the natural connection among organs. Specifically, this review will focus on the communication between the gastrointestinal and respiratory systems. The crosstalk between the gut and the lungs, indeed, is mediated not only by circulating immune cells and microbial metabolites but also by EVs[13].
These nanoparticles carry signals capable of modulating inflammation, barrier integrity, and immune responses in patients with gastrointestinal inflammation and respiratory complications[14,15].
In summary, EVs are increasingly recognized as promising tools for both therapeutic and diagnostic applications, marking a pivotal advancement in biomedical research. Since their relatively recent identification as regulators of both physiological and pathological processes, there has been a rapid expansion of interest in their clinical utility. Their intrinsic ability to transfer bioactive molecules between cells highlights their potential to influence disease progression and tissue homeostasis
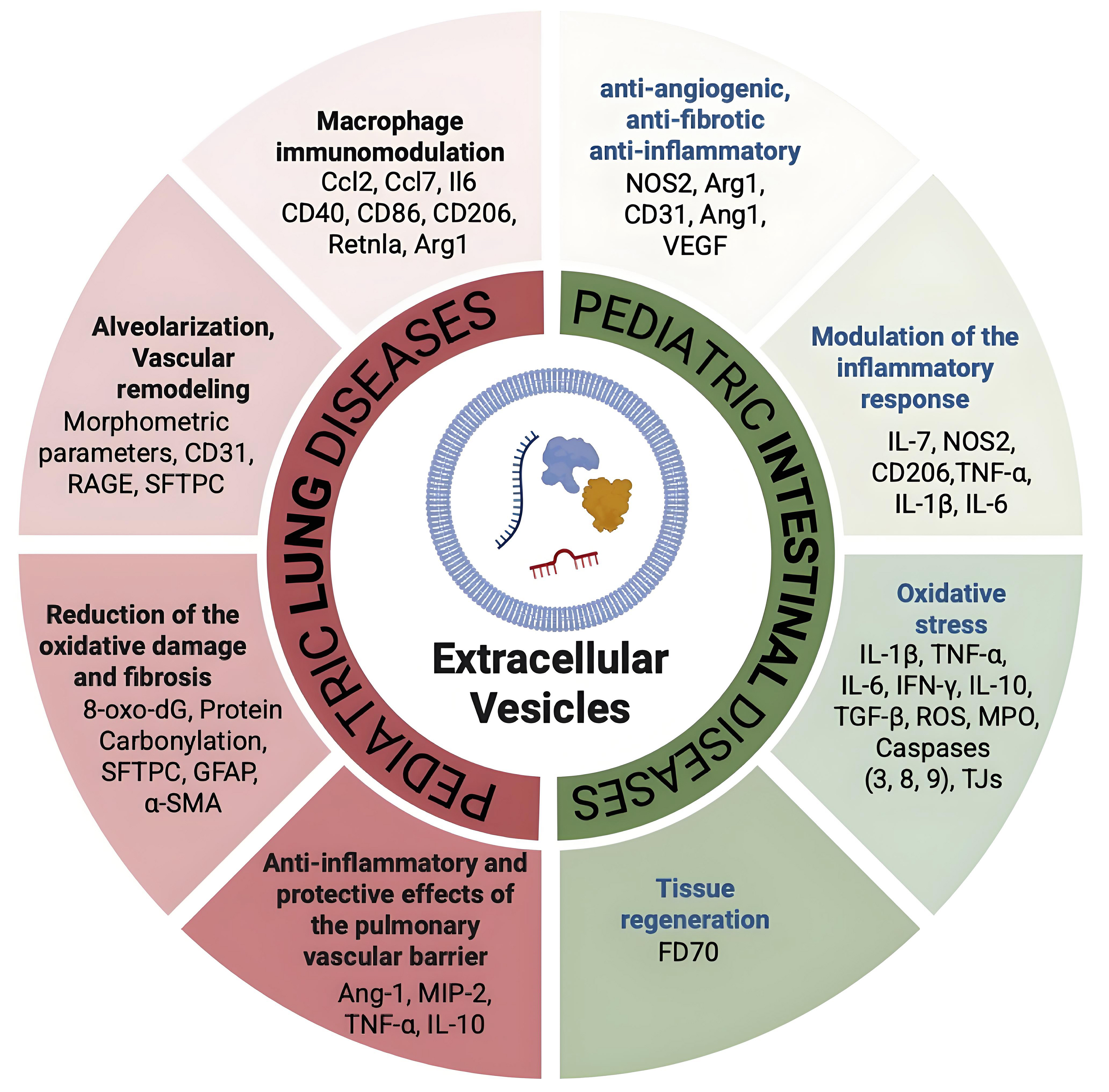
Figure 1. Summary of the EV molecular targets in diseased intestine and lung. The multifaceted target molecules of EVs are depicted in this cartoon: EVs, as one important natural bridge between intestine and lung, act by modulating compartment-specific molecules but also common molecules such as TNF-α and macrophage markers (CD80, CD86, Arg1, NOS2). [Created with BioRender. Dorigo Hochuli, A. (2025) https://BioRender.com/awjluhs]. IL-1β: Interleukin-1 beta; IL-6: interleukin-6; IL-7: interleukin-7; IL-10: interleukin-10; IFN-γ: interferon gamma; TNF-α: tumor necrosis factor alpha; TGF-β: transforming growth factor beta; NOS2: nitric oxide synthase 2; Arg1: arginase 1; Ang-1: angiopoietin-1; CD31: cluster of differentiation 31; CD40: cluster of differentiation 40; CD86: cluster of differentiation 86; CD206: mannose receptor CD206; CCL2: C-C motif chemokine ligand 2; CCL7: C-C motif chemokine ligand 7; MIP-2: macrophage inflammatory protein-2; RAGE: receptor for advanced glycation end products; SFTPC: surfactant protein C; GFAP: glial fibrillary acidic protein; α-SMA: alpha-smooth muscle actin; ROS: reactive oxygen species; MPO: myeloperoxidase; TJs: tight junctions; 8-oxo-dG: 8-oxo-2’-deoxyguanosine; EVs: extracellular vesicles; FD70: fluorescein isothiocyanate-dextran 70; RetnIa: resistin-like alpha.
Summary of preclinical studies evaluating the therapeutic effects of EVs derived from various stem cell sources in models of inflammatory and degenerative pediatric diseases
| Model | Cell source of EVs | Dose of EVs | Number of administration | Route of administration | Therapeutic mechanism | Molecular target as a biomarker | Reference |
| IBD | MSC from umbilical cord | 1.3 × 106 | 3 | IV (tail vein) | Modulation of the inflammatory response | IL-7, NOS2, CD206, TNF-α, IL-1β, IL-6 | [29] |
| IFN-γ-primed bone marrow- MSC | 200 µg | Single dose | IV | Inhibition of Stat3 via miR-125a/b | N.A. | [30] | |
| MSC (murine source) | nEV: 1.109 iEV: 1.109 | 5 | IP | iEV: immunomodulation, anti-angiogenic, anti-fibrotic effects Tissue regeneration | NOS2, Arg1, CD31, Ang1, VEGF | [31] | |
| Human placental MSC | 200 µg | Single dose | In situ administration in the site of injury (colon mesangial margin) | Inhibition of inflammation and oxidative stress | IL-1β, TNF-α, IL-6, IFN-γ, IL-10, TGF-β, ROS, MPO, Caspases (3, 8, 9), Tight junction proteins (Claudin-1, ZO-1, Occludin) | [32] | |
| NEC | Bovine milk-derived exosomes | 1 µg/µL | 3 doses/day (from P5 to P9) | Gavage | Modulation of NLRP3 inflammasome and NF-κB signaling in the lung during neonatal necrotizing enterocolitis | MPO, IL-1β, P-NF-κB/NF-κB ratio | [34] |
| Bone marrow- MSC | 2.5 × 109 | Single dose at 5 h after delivery | IP | Preservation of gut barrier function wound healing of IEC-6 cells promotion in vitro | FD70 | [41] | |
| BPD | Wharton’s Jelly and bone marrow MSC | Bolus dose | Single dose at P4 | IV | Macrophage immunomodulation | Ccl2, Ccl7, IL-6 CD40, CD86 CD206, Retnla, Arg1 | [72] |
| Wharton’s Jelly MSC | EV dose/g body wt: 8 × 108 at P3, 4.5 × 108 at P7, and 3 × 108 at P10 | 3 doses: P3, P7, P10 | IT | Promotion of the alveolarization and of the vascular remodelling Inhibition of the fibrotic process Immunomodulation | Morphometric parameters | [73] | |
| Wharton’s Jelly MSC | 6,4 × 109 | 3 doses: P3, P7, P10 | IT | Reduction of oxidative damage and fibrosis | 8-oxo-dG, protein carbonylation, SP-C, GFAP, α-SMA | [74] | |
| Human bone marrow MSC | 2 × 1011 | 2 doses: 6 and 72 h after delivery | IV | Promotion of alveolarization and vascularization, reduction of airway and vascular smooth muscle thickening, improvement of gas exchange VEGF-R2 | SP-B, PCNA, caspase-3 | [75] | |
| Wharton’s Jelly MSC | 2 × 108 EV/g body wt | Single dose at P3 | IT, IV | Reduction of inflammation, angiogenesis restoration, prevention of long-term cardiopulmonary damage | VEGF, CXCL12, CXCR4, IL-1β, IL-6, TNF-α | [76] | |
| Bone marrow MSC | 800 μg | Single dose | IV (tail vein) | Therapeutic tool miR-425 Delivered via exosomes to suppress PTEN and activate the PI3K/AKT pathway Therapeutic target: PTEN Target of miR-425, inhibited to reduce apoptosis | miR-425 Its levels indicate disease status and treatment effectiveness | [77] | |
| COPD | Human bone marrow-derived MSCs - MVs | 10 µL of MVs released by 1 × 106 cells | Single dose | IT | Anti-inflammatory and protective effects on the pulmonary vascular barrier Ang-1 | Ang-1, MIP-2, TNF-α, IL-10 | [78] |
| Human placenta MSC | 0.1 mL | 5 days per week for 3 weeks | IP | Restoration of lung function and inflammation reduction. Suppression of pro-inflammatory immune cells, promotion of anti-inflammatory response | N.A. | [80] |
Overview of selected clinical trials using EVs derived from stem cells for the treatment of pediatric diseases
| Disease | Source of EVs | Name of the preparation | Clinical trial ID | Route of administration | Status of the trial |
| BPD | Human bone marrow MSC | UNEX-42 | NCT03857841 | Intravenous | Terminated |
| Human Umbilical Cord MSC | EXOB-001 | NCT06279741 | Endotracheal | Recruiting | |
| IBD | Human bone marrow MSC | ExoFlo | NCT05130983 | Intravenous | Terminated |
Table 1 provides an overview of experimental studies exploring the therapeutic potential of EVs isolated from different mesenchymal stromal/stem cell (MSC) sources—including human umbilical cord, placenta, bone marrow (BM), Wharton’s Jelly (WJ), and bovine milk—in multiple preclinical models of pediatric diseases such as IBD, necrotizing enterocolitis (NEC), BPD, COPD. Reported parameters include the disease model, EV source, dosage, number and timing of administrations, and route of injection [intravenous (IV), intraperitoneal (IP), intratracheal (IT), oral gavage, or local in situ delivery at the site of injury]. The column “Therapeutic mechanism” outlines the key mechanisms by which EVs exert their therapeutic effects, including immune modulation, inhibition of inflammation, oxidative stress and fibrosis, and promotion of tissue repair and barrier integrity. The “Molecular Target as a Biomarker” summarizes molecular indicators used to evaluate efficacy, such as markers of inflammation, oxidative stress, apoptosis, epithelial integrity, and angiogenesis. Overall, EVs demonstrated multiple benefits across models, supporting their translational potential as a cell-free therapeutic strategy for pediatric inflammatory and degenerative diseases.
GUT-LUNG AXIS: THE ROLE OF EVs
Pediatric gastrointestinal diseases
The disruption of intestinal homeostasis caused by impairment of the intestinal barrier is common to two pediatric gastrointestinal disorders: NEC and IBD[16]. The gut-lung axis describes the bidirectional communication between the gastrointestinal and respiratory systems, mediated by the immune system, microbial metabolites, and soluble mediators[17,18]. Dysregulation of this axis contributes to extra-intestinal manifestations in IBD and systemic complications in NEC, such as lung injury and impaired alveolar development[19]. NEC is the most prevalent severe gastrointestinal condition seen in neonatal intensive care units. It is a significant cause of morbidity and mortality among newborns, especially in premature infants[20].
Studies in neonatal mice have shown that at the cellular level, a key event preceding widespread systemic inflammation is the activation of Toll-like receptor 4 (TLR4) on the intestinal epithelium by bacterial lipopolysaccharides (LPS). TLR4 activation triggers enterocyte apoptosis and the release of High Mobility Group Box 1 (HMGB1), which amplifies inflammation by perpetuating TLR4 signaling in distant organs. As HMGB1 enters systemic circulation, it reaches the lungs, where TLR4 activation on pulmonary epithelial cells induces a pro-inflammatory response. This leads to nuclear translocation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and the upregulation of cytokines such as pro-interleukin (IL)-1β (pro-IL-1β), priming the Nucleotide-binding oligomerization domain-containing protein 2 (NOD)-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome. The resulting cytokine storm contributes to lung injury, a process implicated in the development of chronic lung disease in NEC survivors[16,17]. Interestingly, the open field of engineered EVs demonstrated that EVs modified with Heme Oxygenase-1 (HO-1) can convey microRNA-200b to intestinal epithelial cells, thereby suppressing HMGB3-mediated inflammatory responses[18].
IBD represents a global healthcare challenge with rising incidence, characterized by chronically relapsing intestinal inflammation. The two main types are Crohn’s disease (CD) and ulcerative colitis (UC), which differ in the extent and localization of inflammation[19]. This disruption not only affects intestinal homeostasis but also has systemic effects on the patient’s overall health. Patients with IBD often exhibit pulmonary manifestations, including bronchitis, interstitial lung disease, and airway inflammation. The shared embryonic origin of the gut and lung, alongside systemic immune priming and microbial translocation, contributes to these effects. Animal models of colitis demonstrate altered pulmonary cytokine profiles[20], underlining the crosstalk between the gut and lung (gut-lung axis). Briefly, gut dysbiosis - an imbalance in the composition or function of the gut microbiota - can significantly alter gut anatomy, physiology, and immune responses. Intestinal inflammation resulting from this dysbiosis can compromise the epithelial barrier, allowing the translocation of gut contents, including microbes, immune cells, and pro-inflammatory molecules, into distant organs such as the lungs, where they can trigger or worsen inflammatory responses. Finally, immune cell mis-homing refers to the phenomenon where immune cells that are activated in the gut and commonly express gut-specific homing receptors, such as alpha 4 beta 7
EVs as biomarkers in inflammatory bowel disease and NEC
IBD and NEC represent significant challenges in pediatric gastroenterology, primarily characterized by intestinal inflammation and epithelial injury. Different studies suggest that EVs may play a pivotal role not only in the pathogenesis but also as promising non-invasive biomarkers for diagnosis. In 2015, Leoni et al. observed the presence of annexin A1 (ANXA1) in EVs derived from intestinal epithelial cells and their ability to activate wound repair pathways[24]. ANXA1 is recognized for its anti-inflammatory properties in the gut, limiting leukocyte recruitment by inhibiting neutrophil migration and promoting apoptosis, acting downstream of glucocorticoids and mimicking many of their effects. Elevated serum levels of ANXA1-containing EVs were also observed when comparing healthy controls and patients with active inflammation due to IBD, indicating that ANXA1-containing EVs are systemically distributed in response to the inflammatory process. Therefore, a high level of ANXA1 could potentially serve as a biomarker of intestinal mucosal inflammation.
As previously mentioned, EVs were found in many fluids, including urine and saliva samples. The involvement of the oral cavity in IBD has already been documented[25]. Saliva from IBD patients contains characteristic EVs that may reflect the presence and development of IBD, potentially serving as biomarkers. Indeed, the isolation of EVs from the salivary samples of IBD patients and healthy controls revealed a distinct protein profile. Specifically, in 2017, Zheng et al. observed that eight proteins were uniquely present in patients with IBD. Among the eight, the proteasome subunit alpha type 7 (PSMA7) was found to be highly expressed in both patients with UC and CD[26]. This study also investigated the presence of this protein in an animal model. Animal experiments revealed that the expression level of PSMA7 in oral epithelial tissue was comparable to that in intestinal inflammation, suggesting its potential as a non-invasive diagnostic tool for identifying IBD patients.
Interestingly, a recent study detected EVs in the urine of premature neonates and revealed significantly altered miRNA profiles in those with NEC compared to healthy, age-matched controls. Notably, the differentially expressed miRNAs included miRNA-5703, miRNA-604, miRNA-5186, and miRNA-139-3p[27]. However, so far, no early biomarkers have been identified for these premature infants, and analyses are still ongoing[28].
EVs as therapeutic tool in inflammatory bowel disease and NEC
In preclinical models of IBD, particularly those induced by dextran sulfate sodium (DSS) and trinitrobenzene sulfonic acid (TNBS), mesenchymal stromal cell-derived EVs (MSC-EVs) have been shown to be potent immunomodulatory and regenerative agents. Their beneficial effects are multifaceted, involving the modulation of key inflammatory signaling pathways, the regulation of immune cells, and mucosal repair. Mao et al. demonstrated that human umbilical cord MSC-EVs reduced NF-κB p65 expression and nuclear translocation in DSS-induced colitis in mice, resulting in a marked decrease in IL-6 and TNF-α levels[29]. Yang et al. reported that BM MSC-EVs inhibited the phosphorylation of signal transducer and activator of transcription 3 (STAT3) in colonic epithelial cells and immune cells, leading to a reduced expression of downstream cytokines, such as IL-17A and IL-22[30]. Tolomeo et al. demonstrated the higher efficacy of naïve EVs (nEVs) and induced EVs (iEVs) from BM MSCs compared to MSCs alone in downregulating inflammation and modulating macrophage polarization toward alternatively activated (M2) macrophages[31].
Under the anti-inflammatory properties, in the TNBS-induced colitis model, placenta tissue-derived MSC-EVs significantly reduced levels of IL-6 and TNF-α, contributing to the amelioration of disease severity and improved histological architecture[32]. These EVs also downregulated the expression of IL-17A, a key cytokine in T helper 17 cells (Th17)-mediated colitis, indicating a role in rebalancing adaptive immune responses[33]. Moreover, bovine milk-derived EVs can attenuate intestinal inflammation by modulating key inflammatory pathways (e.g., TLR4-NF-κB-NLRP3), promoting the polarization of macrophages towards an anti-inflammatory M2 phenotype, and stimulating epithelial repair mechanisms[34].
miRNA cargo within EVs, such as miR-146a and miR-155, plays a pivotal role in immune modulation by targeting NF-κB signaling pathways and other key inflammatory mediators[35]. Additionally, proteomic analyses of EVs reveal enrichment of anti-inflammatory and tissue repair-associated proteins, including transforming growth factor beta (TGF-β) and IL-10, further supporting their immunosuppressive and regenerative capacities[35]. Although the therapeutic potential of EVs in preclinical models of NEC and IBD is promising, several challenges remain for their clinical use. These include standardizing EV isolation methods, scaling up production, and characterizing their bioactive contents. However, the growing evidence suggests that EVs provide a feasible and potentially effective alternative to cell-based therapies for IBD.
Multiple studies have demonstrated the efficacy of EVs in experimental NEC models. IP or IV administration of amniotic fluid-, BM-, or umbilical cord, breast milk-derived-EVs has been shown to reduce intestinal necrosis and histological injury, suppress pro-inflammatory cytokine production (e.g., IL-6, TNF-α, IL-1β), inhibit apoptosis and oxidative stress, restore epithelial integrity and tight junction (TJ) proteins (e.g., zonuline, occludin)[36-39]. Good et al. demonstrated that BM-derived EVs from amniotic fluid stem cells significantly decreased NEC severity and improved survival in a mouse model. The beneficial effects were linked to modulation of TLR4 signaling, a key inflammatory pathway implicated in NEC pathogenesis[40]. Similarly, studies by Rager et al. reported that EVs modulate tissue repair and the resolution of inflammation, playing a protective role in NEC onset[41,42]. As previously outlined, NEC is linked to systemic inflammation that can hinder lung development. Bovine-milk-derived EVs added to formula can decrease inflammasome activation, NF-κB pathway activity, and damage in the NEC lung, demonstrating their potential to restore the balance of the gut-lung axis[39].
Pediatric lung diseases
BPD, a complication of preterm birth, and COPD are both characterized by chronic pulmonary inflammation, epithelial dysfunction, and increased susceptibility to infection. Growing evidence suggests that the gut-lung axis, a system of immunological and microbial crosstalk between the gastrointestinal and respiratory tracts, plays a pivotal role in shaping pulmonary immunity in both diseases. Furthermore, EVs have emerged as key mediators of systemic communication, transporting cytokines, miRNAs and microbial components between the gut and lung compartments[43]. Understanding how the gut-lung axis and EVs intersect in BPD and COPD may unlock novel strategies for early intervention.
BPD is a common respiratory disorder affecting extremely preterm infants, associated with high morbidity and mortality. Newborns suffering from this chronic lung disease of multifactorial etiology present an undeveloped antioxidant system and very immature lungs, which lead to further complications that compromise their lives[42]. Since BPD may contribute to the development of COPD, it is crucial to reduce its burden to prevent long-term respiratory complications in patients suffering from the disease. There is evidence supporting various strategies to alleviate this burden. Notable approaches include surfactant therapy, vitamin A supplementation, protective non-invasive ventilation, and fluid restriction; however, these do not appear to have a significant impact on reducing the burden of BPD[42,44]. BPD can increase the risk of developing asthma, a chronic inflammatory airway disorder involving reversible airflow obstruction, airway hyperresponsiveness, and structural remodelling[42].
COPD is a progressive respiratory condition marked by persistent airflow limitation, chronic inflammation, and structural changes in the lung parenchyma and airways. In addition to adult smokers, infants born prematurely represent a distinct high-risk population who can develop COPD later in life[45]. Indeed, significant epidemiological evidence, such as maternal smoking, the effects of viral infections, nutrition, and pollution, supports classifying COPD as a pediatric disease[45]. The pathogenesis of COPD involves a complex interplay of oxidative stress, protease-antiprotease imbalance, and immune dysregulation[46]. Concurrently, elevated protease activity, especially from matrix metalloproteinases (MMPs), leads to degradation of the extracellular matrix (ECM), compromising the structural integrity of the lungs[47]. Immune dysregulation further intensifies the disease process by promoting infiltration of inflammatory cells, thereby sustaining and worsening airway inflammation[48]. Together, these interconnected mechanisms drive the hallmark features of COPD: emphysema, chronic bronchitis, and remodeling of the small airways. Dysbiosis in the gut microbiota has been observed in patients with COPD and is associated with increased systemic inflammation[49]. Dysbiosis, as described above, can lead to increased gut permeability (“leaky gut”), allowing microbial products to enter systemic circulation and trigger systemic and pulmonary inflammation[50]. Preterm infants are at high risk of gut microbial dysbiosis due to immature immune systems and antibiotic use[51]. Microbial products such as short-chain fatty acids (SCFAs) produced in the gut can influence pulmonary immune responses, and conversely, lung inflammation can alter gut permeability and microbiota composition[52].
EVs as biomarkers in BPD and COPD
The potential of circulating EVs as diagnostic biomarkers in BPD and COPD is particularly promising[53-56]. EVs found in peripheral blood, bronchoalveolar lavage fluid (BALF), and even exhaled breath condensate reflect the molecular signature of diseased lung tissue, enabling early and less invasive detection, stratification, and monitoring of therapeutic responses[2]. In BPD, EVs enriched in pro-inflammatory miRNAs (e.g., miR-21, miR-155) and cytokines that can circulate systemically are produced through the activation of epithelial and immune cells. Interestingly, cord blood EVs in infants who develop BPD exhibit altered miRNA expression profiles linked to inflammation[57]. Interestingly, comparing EVs from neonates with and without BPD may help elucidate their contribution to disease pathogenesis and identify potential diagnostic biomarkers. For instance, EVs isolated from human umbilical cord neonates who later developed BPD exhibited significantly reduced cell proliferation, impaired capillary tube formation, and a more pronounced inhibition of endothelial cell migration in cultured human umbilical vein endothelial cells, compared to EVs from the non-BPD group[58]. miRNA profiling revealed significant differential expression of miR-103a-3p, miR-17-5p, miR-185-5p, miR-200a-3p, miR-20b-5p, and miR-765 between BPD- and non-BPD-derived EVs. Moreover, researchers have demonstrated the presence of microbiome-derived EVs in the urine of children with asthma, a disease that can be a consequence of BPD. These EVs were consistently altered, aligning with previous studies demonstrating changes in the lung and gut microbiomes. Urine may reflect specific patterns of EVs in the microbiome of children with asthma. Moreover, in these pediatric patients, EVs isolated from BALF present a unique phospholipid composition[59]. An interesting study displayed that umbilical cord blood EVs of patients with BPD exacerbate lung injury and disrupt lung angiogenesis in a hyperoxic mouse model. This negative impact can extend into adulthood. The authors proposed that EVs from umbilical cord cells of BPD patients may play a significant role in the progression of BPD and related lung diseases, suggesting that these EVs, along with their downstream genes and pathways, could serve as valuable predictive biomarkers and potential therapeutic targets for ventilator-induced pulmonary injury associated with BPD[58].
Ransom et al. highlighted that EVs were enriched with the epithelial marker cluster of differentiation 24 (CD24) in preterm infants born in the late canalicular stage, indicating a possible role of CD24+ EVs in lung development. Furthermore, this study observed an increased level of CD14+ EVs, an emerging biomarker of severe disease, suggesting that CD14 may be a predictive marker of BPD[60]. In COPD, elevated levels of EVs have been detected in biological fluids such as sputum, BALF, and plasma. For instance, serological levels of endothelial-derived EVs (CD31+ or CD62E+) are increased in COPD patients and correlate with disease severity and vascular dysfunction[61]. Furthermore, Nieri et al. demonstrated that circulating EVs isolated from COPD patients exerted prothrombotic and pro-inflammatory activity in vitro[62]. Additionally, EV-associated miRNAs such as miR-21, miR-146a, and miR-223 have been implicated in regulating inflammatory pathways central to COPD pathogenesis. They may serve as accessible, non-invasive biomarkers for disease progression and response to therapy[63]. Notably, a recent study found elevated expression of EV-associated miR-21 in in vitro cultured COPD cells, suggesting its potential role in promoting cellular ageing in neighbouring cells[64]. Furthermore, miR-21 levels in EVs were found to be significantly upregulated in patients with COPD[65].
EVs as therapeutic tool in BPD and COPD
In recent years, the potential role of EVs in treating lung diseases has been rapidly growing[66-68]. In the context of pediatric diseases, the BPD preclinical model analyses the therapeutic efficiency of EVs[69-71]. The Kourembanas team assessed the efficacy of BM MSC-derived EVs as a treatment for a BPD animal model generated by exposing newborn mice to hyperoxia. Umbilical cord MSC-derived EVs demonstrated improvements in lung function, a decrease in fibrosis and pulmonary vascular remodelling, and a reduction in pulmonary hypertension. They also showed that the MSC-EVs’ mechanism of action is associated with the modulation of lung macrophage phenotype. Specifically, the macrophage M1-like inflammatory state was suppressed, and an increase in the M2-anti-inflammatory state was observed both in vitro and in vivo on alveolar macrophages from the neonatal murine hypoxia model[72]. On a different model of BPD, Porzionato et al. demonstrated that IT administration of clinical-grade MSC-EVs improved alveolarization parameters, pulmonary vascular remodelling, and inflammation in a rat model of BPD. They compared results between MSCs and MSC-EVs, showing that both treatments aimed to reduce hyperoxia-induced damage, with better results achieved using the EVs[73]. The studies mentioned above underlined that EV administration leads to better outcomes in alveolarization and lung vascularization compared to the use of mesenchymal stromal cells. This suggests that intratracheally administered EVs could be an effective approach to treat BPD, improving the morphometric parameters of alveolarization, pulmonary vascular remodelling, and inflammation, paving the way for clinical use in humans[74]. An elegant study in preterm lambs subjected to mechanical ventilation demonstrated that IV MSC-sEVs improved respiratory indices (oxygenation and ventilation), alveolar formation (radial alveolar count), capillary density, and vascular endothelial growth factor (VEGF)-R2 expression[75]. In newborn rats exposed to hyperoxia, both BM- and WJ-MSC EVs, delivered either intratracheally or intravenously, preserved alveolar and vascular structures, attenuated pulmonary hypertension, and exerted durable cardiopulmonary protective effects lasting up to 3 months post-treatment. Transcriptomic analysis revealed the upregulation of angiogenesis-associated gene sets, in addition to VEGF and hepatocyte growth factor (HGF)[76]. Mechanistic studies point to enhanced angiogenesis, modulation of macrophage phenotype (favoring anti-inflammatory M2), reduced oxidative stress, and preserved thymic and systemic immune architecture via cargo such as TNF-stimulated gene 6 protein (TSG 6), VEGF, and miRNAs, e.g., miR-425 activating Phosphoinositide 3-kinase/Protein kinase B (PI3K/AKT) by targeting Phosphatase and Tensin homolog (PTEN)[77].
Along with BPD, EVs could offer a new therapeutic option for COPD patients, although so far, most efforts have focused on adults and not on preventing COPD in infants. For this reason, we selected the more relevant studies for the present review. For instance, in preclinical models of lung injury, MSC-EVs have shown immunomodulatory effects by reducing neutrophilic inflammation, suppressing cytokine production (TNF-α), and promoting epithelial repair[78]. Their cargo, particularly anti-inflammatory miRNAs (e.g., miR-126 and miR-30b), may suppress NF-κB signaling and modulate macrophage polarization toward a reparative phenotype[79]. In a chronic-induced murine model of COPD, administration of MSC-EVs led to significant improvements in lung function, including increased oxygen saturation, arterial pH, PaO2, and elevated IL-10 levels. Concurrently, treatment reduced the expression of pro-inflammatory cytokines
Furthermore, MSC-exosomes were shown to downregulate the activation of both CD4+ and CD8+ T lymphocytes. Notably, inhalation of MSC-exosomes was associated with an improvement in exercise capacity, as reflected by increased walking distance, and an overall enhancement in quality-of-life indicators in the COPD model[80]. It is essential to underline that the use of EVs developed for BPD patients suggests that COPD development can be at least partially prevented. Notably, EVs from umbilical cord-derived or BM-derived MSCs have been shown to reduce alveolar simplification, preserve capillary density, and suppress inflammatory cytokine expression in neonatal rodent models exposed to hyperoxia. These findings suggest that EV-based therapies may replicate the beneficial effects of MSCs while minimizing the risks associated with cell-based treatments, such as immunogenicity and tumorigenicity. Early-phase clinical studies are underway to evaluate the safety and efficacy of EVs in neonatal lung disease, although challenges in standardization and large-scale production remain. EVs have shown therapeutic promise in preclinical models of COPD by modulating key pathogenic mechanisms, including oxidative stress, inflammation, and ECM degradation. EVs derived from MSCs have demonstrated the capacity to restore alveolar architecture, reduce neutrophilic inflammation, and suppress matrix MMP activity for tissue remodeling. Moreover, specific cargo molecules, such as anti-inflammatory miRNAs (e.g., miR-126, miR-146a) and growth factors (e.g., VEGF, HGF), are believed to contribute to these protective effects by reprogramming immune cells and enhancing epithelial regeneration.
Additionally, EVs may serve as precision delivery vehicles for therapeutic agents in COPD, offering targeted modulation of diseased tissues with minimal systemic toxicity. The engineering of EVs to carry anti-fibrotic or anti-inflammatory drugs is an area of active investigation and holds potential for personalized medicine approaches in COPD management. It is worth underlining that some important studies on adults could be applied to pediatric patients. Engineered MSC EVs are modified to enhance targeted delivery, traverse physiological barriers, and more effectively reach diseased lung tissue, thereby amplifying their therapeutic potential[81]. Additionally, engineered EVs demonstrate superior immunomodulatory functions; they can inhibit pro-inflammatory M1 macrophage polarization via modulation of miRNA content (e.g., reduced miR-21-3p levels), resulting in decreased secretion of TNF α, IL-6, and IL-12, key mediators in COPD pathogenesis[82]. These advances in EV engineering, including surface functionalization, gene or cargo loading, and parental-cell modifications, offer a versatile platform for the development of next-generation, targeted treatments for COPD and related chronic lung diseases.
Clinical translation of stem cell-derived EVs in pediatrics - from bench to bedside
In recent years, EVs derived from stem cells have emerged as a promising therapeutic strategy for pediatric inflammatory and respiratory conditions. Several clinical trials are currently underway to evaluate their efficacy and safety. Table 2 provides a summary of selected trials, highlighting their key characteristics and current status. The information was sourced from the National Institutes of Health (NIH) Clinical Trials Database.
Conclusion: translational relevance of extracellular vesicle applications and future perspectives
The intestine-lung axis constitutes a critical bidirectional communication network between the gastrointestinal and respiratory systems, with significant implications for host immunity, mucosal homeostasis, and disease pathogenesis (graphical abstract). The role of EVs is paramount under two different aspects: on the one hand, taking advantage of their anti-inflammatory properties, they can help gut microbiota-driven activation of innate and adaptive immune cells, such as T helper 17 cells, T regulatory cells, and dendritic cells, migrating systemically to play protective or regulatory roles in the lung[83] [Table 1 and Figure 1]. On the other hand, EVs isolated in fluids, such as blood and saliva, help us understand the gut-lung axis also in early life, when microbial colonization and immune development co-evolve, shaping lifelong susceptibility to respiratory diseases. As a therapeutic tool, EVs may be delivered via inhalation (for lung inflammation), oral capsules (for gut dysbiosis), or intravenously, depending on the target compartment[84]. The preclinical data consistently show that EVs enhance intestinal stem cell survival, reducing pro-inflammatory signaling (e.g., NF-κB), and preserving epithelial barrier integrity. Innovations in EV engineering (e.g., cargo loading, surface modifications) may enhance specificity and efficacy for gut-lung axis restoration. Emerging evidence implicates gut microbiota-derived EVs as significant mediators of the gut-lung axis, potentially influencing the pathogenesis and clinical trajectory of both BPD and COPD[85]. Engineered EVs are rising as a promising therapeutic strategy due to their ability to be modified for targeted delivery to diseased tissues, while minimizing side effects on healthy cells. These modifications do not affect the size or morphology of EVs, enhance their accumulation at the target site, allow the loading of specific therapeutic molecules and improve their stability in the bloodstream, thereby extending their half-life. However, further research is needed to understand the pharmacodynamics, the in vivo metabolism and the interactions with the immune system (the mononuclear phagocyte system), before developing new therapeutics. To address the challenges of low yield and high production costs, alternative EV sources, such as plants, milk, and bacteria, are being explored. These naturally derived EVs have demonstrated therapeutic effects in preclinical models, including the control of inflammation, tissue regeneration, and immune modulation, offering cost-effective and scalable options for future clinical applications[86,87].
In conclusion, EV-based therapies hold great promise within the field of advanced therapy medicinal products, as recognized by the European Medicines Agency (EMA). As these therapies progress toward clinical translation, particular emphasis is being placed on addressing key challenges such as production scalability, standardization of isolation and characterization [Figure 2]. These efforts are particularly critical for ensuring the safety, quality, and efficacy of exosome-based treatments in neonates and pediatric patients, people who present unique physiological vulnerabilities and therapeutic needs. Adherence to Good Manufacturing Practices (GMP) and ongoing technological advancements will be crucial in supporting the safe and effective use of these products in pediatric clinical settings.
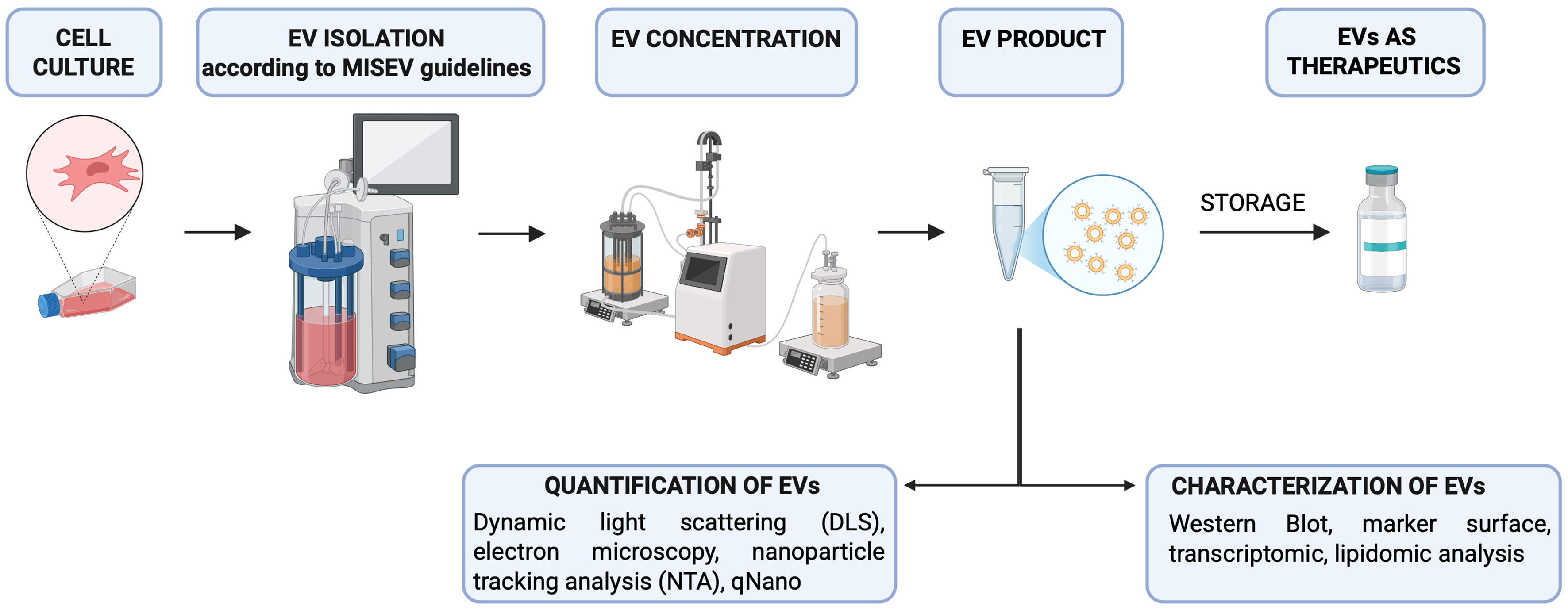
Figure 2. Schematic representation of the main steps for standardization of EV production as therapeutic tool. MISEV guidelines are highlighted in reference[87]. [Created with BioRender. Dorigo Hochuli, A. (2025) https://BioRender.com/37dge0q]. EVs: Extracellular vesicles; MISEV: Minimal Information for Studies of Extracellular Vesicles; DLS: dynamic light scattering; NTA: nanoparticle tracking analysis.
Note. These are the criteria that we followed for writing this review: We selected from PubMed all the articles that demonstrate a connection between the use of vesicles for pulmonary and intestinal diseases, with a particular focus on the pediatric field (keywords: extracellular vesicles in COPD, IBD, ARDS and pediatrics, extracellular vesicles in IBD and pediatrics, in NEC). Since studies in the pediatric field are limited, we included studies currently available on adults with the possibility of being translated into pediatrics.
DECLARATIONS
Authors’ contributions
Wrote the manuscript: Bisaccia P, Zaramella A, Diaz RM, Duci M
Made the figure, and read the manuscript: Hochuli AHD
The “conclusion” paragraph of the manuscript: Muraca M, Baraldi E
Wrote and revised the manuscript: Pozzobon M
Availability of data and materials
Not applicable.
AI and AI-assisted tools statement
Not applicable.
Financial support and sponsorship
Zaramella A is supported by the PRIN 2022 project (POZZ_prin2022DM104.23_02) and the EVIDA project (POZZ_AT_UNIMPRESA22_01; Principal Investigator: Pozzobon M). Diaz RM is supported by the project Area Thematica: Spoke 9 - From Target to Therapy: Pharmacology, Safety and Regulatory Competence Center (MUR CN00000041; task responsible: Pozzobon M).
Conflicts of interest
Pozzobon M is a Guest Editor of the Special Issue Topic: GISM Annual Meeting 2025: From MSCs to Extracellular Vesicles of the journal Extracellular Vesicles and Circulating Nucleic Acids. Pozzobon M was not involved in any steps of the editorial process, notably including reviewers’ selection, manuscript handling and decision making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977.
2. Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
3. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17.
4. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-83.
5. Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116-25.
6. Buzás EI, Tóth EÁ, Sódar BW, Szabó-Taylor KÉ. Molecular interactions at the surface of extracellular vesicles. Semin Immunopathol. 2018;40:453-64.
7. Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16:24-43.
8. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195-208.
9. Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-57.
10. Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148-56.
11. Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12:S150-6.
12. Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843-50.
13. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237-55.
14. Budden KF, Gellatly SL, Wood DL, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55-63.
15. Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018;20:e12966.
16. Jia H, Sodhi CP, Yamaguchi Y, et al. Toll like receptor 4 mediated lymphocyte imbalance induces nec-induced lung injury. Shock. 2019;52:215-23.
17. Jia H, Sodhi CP, Yamaguchi Y, et al. Pulmonary epithelial TLR4 activation leads to lung injury in neonatal necrotizing enterocolitis. J Immunol. 2016;197:859-71.
18. Zeng B, Li Y, Khan N, et al. Yin-Yang: two sides of extracellular vesicles in inflammatory diseases. J Nanobiotechnology. 2024;22:514.
19. Lee M, Chang EB. Inflammatory bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. 2021;160:524-37.
20. Metwali A, Thorne PS, Ince MN, et al. Recirculating immunocompetent cells in colitic mice intensify their lung response to bacterial endotoxin. Dig Dis Sci. 2018;63:2930-9.
21. Eladham MW, Sharif-Askari NS, Sekar P, et al. The role of gut leakage and immune cell miss-homing on gut dysbiosis-induced lung inflammation in a DSS mice model. PLoS One. 2025;20:e0324230.
22. Shen Q, Huang Z, Yao J, Jin Y. Extracellular vesicles-mediated interaction within intestinal microenvironment in inflammatory bowel disease. J Adv Res. 2022;37:221-33.
23. Hou JJ, Li WW, Wang XL, Ma AH, Qin YH. Efficacy of extracellular vesicles as a cell-free therapy in colitis: a systematic review and meta-analysis of animal studies. Front Pharmacol. 2023;14:1260134.
24. Leoni G, Neumann PA, Kamaly N, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125:1215-27.
25. Park YE, Moon HS, Yong D, et al. Microbial changes in stool, saliva, serum, and urine before and after anti-TNF-α therapy in patients with inflammatory bowel diseases. Sci Rep. 2022;12:6359.
26. Zheng X, Chen F, Zhang Q, et al. Salivary exosomal PSMA7: a promising biomarker of inflammatory bowel disease. Protein Cell. 2017;8:686-95.
27. Galley JD, Mar P, Wang Y, Han R, Rajab A, Besner GE. Urine-derived extracellular vesicle miRNAs as possible biomarkers for and mediators of necrotizing enterocolitis: A proof of concept study. J Pediatr Surg. 2021;56:1966-75.
28. Gunasekaran A, Devette C, Levin S, Chaaban H. Biomarkers of necrotizing enterocolitis: the search continues. Clin Perinatol. 2022;49:181-94.
29. Mao F, Wu Y, Tang X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017;2017:5356760.
30. Yang R, Huang H, Cui S, Zhou Y, Zhang T, Zhou Y. IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis. 2020;11:603.
31. Tolomeo AM, Castagliuolo I, Piccoli M, et al. Extracellular vesicles secreted by mesenchymal stromal cells exert opposite effects to their cells of origin in murine sodium dextran sulfate-induced colitis. Front Immunol. 2021;12:627605.
32. Duan L, Huang H, Zhao X, et al. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int J Mol Med. 2020;46:1551-61.
33. Appiah MG, Park EJ, Darkwah S, et al. Intestinal epithelium-derived luminally released extracellular vesicles in sepsis exhibit the ability to suppress TNF-α and IL-17A expression in mucosal inflammation. Int J Mol Sci. 2020;21:8445.
34. Filler R, Yeganeh M, Li B, et al. Bovine milk-derived exosomes attenuate NLRP3 inflammasome and NF-κB signaling in the lung during neonatal necrotizing enterocolitis. Pediatr Surg Int. 2023;39:211.
35. Gupta S, Krishnakumar V, Soni N, Rao EP, Banerjee A, Mohanty S. Comparative proteomic profiling of small extracellular vesicles derived from iPSCs and tissue specific mesenchymal stem cells. Exp Cell Res. 2022;420:113354.
36. Zeng R, Wang J, Zhuo Z, Luo Y, Sha W, Chen H. Stem cells and exosomes: promising candidates for necrotizing enterocolitis therapy. Stem Cell Res Ther. 2021;12:323.
37. Matei AC, Antounians L, Zani A. Extracellular vesicles as a potential therapy for neonatal conditions: state of the art and challenges in clinical translation. Pharmaceutics. 2019;11:404.
38. Filip R. An update on the role of extracellular vesicles in the pathogenesis of necrotizing enterocolitis and inflammatory bowel diseases. Cells. 2021;10:3202.
39. Maghraby MK, Li B, Chi L, et al. Extracellular vesicles isolated from milk can improve gut barrier dysfunction induced by malnutrition. Sci Rep. 2021;11:7635.
40. Good M, Siggers RH, Sodhi CP, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci U S A. 2012;109:11330-5.
41. Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016;51:942-7.
42. Bonadies L, Zaramella P, Porzionato A, Perilongo G, Muraca M, Baraldi E. Present and future of bronchopulmonary dysplasia. J Clin Med. 2020;9:1539.
43. Kumar MA, Baba SK, Sadida HQ, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9:27.
44. Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med. 2018;16:36.
46. Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413-21.
47. Lomas DA. Does protease-antiprotease imbalance explain chronic obstructive pulmonary disease? Ann Am Thorac Soc. 2016;13:S130-7.
48. Lin S, Ma Z, Huang Y, Sun Y, Yi H. Chronic obstructive pulmonary disease is characterized by reduced levels and defective suppressive function of regulatory B cells in peripheral blood. Mol Immunol. 2022;141:87-93.
49. Toraldo DM, Conte L. Influence of the lung microbiota dysbiosis in chronic obstructive pulmonary disease exacerbations: the controversial use of corticosteroid and antibiotic treatments and the role of eosinophils as a disease marker. J Clin Med Res. 2019;11:667-75.
50. Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516-26.
51. Li Y, He L, Zhao Q, Bo T. Microbial and metabolic profiles of bronchopulmonary dysplasia and therapeutic effects of potential probiotics Limosilactobacillus reuteri and Bifidobacterium bifidum. J Appl Microbiol. 2022;133:908-21.
52. Kotlyarov S. Role of short-chain fatty acids produced by gut microbiota in innate lung immunity and pathogenesis of the heterogeneous course of chronic obstructive pulmonary disease. Int J Mol Sci. 2022;23:4768.
53. Wu J, Ma Y, Chen Y. Extracellular vesicles and COPD: foe or friend? J Nanobiotechnology. 2023;21:147.
54. Di Gioia S, Daniello V, Conese M. Extracellular vesicles’ role in the pathophysiology and as biomarkers in cystic fibrosis and COPD. Int J Mol Sci. 2023;24:228.
55. Nieri D, Daniele M, Lombardi S, et al. Circulating extracellular vesicles are associated with disease severity and interleukin-6 levels in COPD: a pilot study. J Clin Med. 2021;10:5014.
56. Guo Y, Pan JJ, Zhu W, et al. Hsa_circ_0001359 in serum exosomes: a promising marker to predict bronchopulmonary dysplasia in premature infants. J Inflamm Res. 2024;17:5025-37.
57. Chen W, Kongsomros S, Thorman A, et al. Extracellular vesicles and preterm infant diseases. Front Pediatr. 2025;13:1550115.
58. Zhong X qi, Hao T fang, Zhu Q jiong, et al. Umbilical cord blood exosomes from very preterm infants with bronchopulmonary dysplasia aggravate lung injury in mice. Sci Rep. 2023;13:8648.
59. Al-Humiari MA, Yu L, Liu LP, et al. Extracellular vesicles from BALF of pediatric cystic fibrosis and asthma patients increase epithelial sodium channel activity in small airway epithelial cells. Biochim Biophys Acta Biomembr. 2024;1866:184219.
60. Ransom MA, Bunn KE, Negretti NM, et al. Developmental trajectory of extracellular vesicle characteristics from the lungs of preterm infants. Am J Physiol Lung Cell Mol Physiol. 2023;324:L385-92.
61. Lacedonia D, Carpagnano GE, Trotta T, et al. Microparticles in sputum of COPD patients: a potential biomarker of the disease? Int J Chron Obstruct Pulmon Dis. 2016;11:527-33.
62. Nieri D, Morani C, De Francesco M, et al. Enhanced prothrombotic and proinflammatory activity of circulating extracellular vesicles in acute exacerbations of chronic obstructive pulmonary disease. Respir Med. 2024;223:107563.
63. Burke H, Cellura D, Freeman A, et al. Pulmonary EV miRNA profiles identify disease and distinct inflammatory endotypes in COPD. Front Med. 2022;9:1039702.
64. Dellago H, Preschitz-Kammerhofer B, Terlecki-Zaniewicz L, et al. High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging Cell. 2013;12:446-58.
65. Xu H, Ling M, Xue J, et al. Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium-fibroblast cross-talk in COPD induced by cigarette smoking. Theranostics. 2018;8:5419-33.
66. Worthington EN, Hagood JS. Therapeutic use of extracellular vesicles for acute and chronic lung disease. Int J Mol Sci. 2020;21:2318.
67. Abreu SC, Weiss DJ, Rocco PR. Extracellular vesicles derived from mesenchymal stromal cells: a therapeutic option in respiratory diseases? Stem Cell Res Ther. 2016;7:53.
68. Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J Clin Med. 2018;7:355.
69. Bellio MA, Young KC, Milberg J, et al. Amniotic fluid-derived extracellular vesicles: characterization and therapeutic efficacy in an experimental model of bronchopulmonary dysplasia. Cytotherapy. 2021;23:1097-107.
70. Tieu A, Hu K, Gnyra C, et al. Mesenchymal stromal cell extracellular vesicles as therapy for acute and chronic respiratory diseases: a meta-analysis. J Extracell Vesicles. 2021;10:e12141.
71. Lesage F, Thébaud B. Mesenchymal stromal cell-derived extracellular vesicles for neonatal lung disease: tiny particles, major promise, rigorous requirements for clinical translation. Cells. 2022;11:1176.
72. Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104-16.
73. Porzionato A, Zaramella P, Dedja A, et al. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2019;316:L6-19.
74. Bisaccia P, Magarotto F, D’Agostino S, et al. Extracellular vesicles from mesenchymal umbilical cord cells exert protection against oxidative stress and fibrosis in a rat model of bronchopulmonary dysplasia. Stem Cells Transl Med. 2024;13:43-59.
75. Albertine KH, Rebentisch A, Dawson E, et al. Mesenchymal stromal cell extracellular vesicles improve lung development in mechanically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2024;326:L770-85.
76. Sharma M, Bellio MA, Benny M, et al. Mesenchymal stem cell-derived extracellular vesicles prevent experimental bronchopulmonary dysplasia complicated by pulmonary hypertension. Stem Cells Transl Med. 2022;11:828-40.
77. Wu Y, Li J, Yuan R, Deng Z, Wu X. Bone marrow mesenchymal stem cell-derived exosomes alleviate hyperoxia-induced lung injury via the manipulation of microRNA-425. Arch Biochem Biophys. 2021;697:108712.
78. Tang XD, Shi L, Monsel A, et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem Cells. 2017;35:1849-59.
79. Serban KA, Rezania S, Petrusca DN, et al. Structural and functional characterization of endothelial microparticles released by cigarette smoke. Sci Rep. 2016;6:31596.
80. Harrell CR, Miloradovic D, Sadikot R, et al. Molecular and cellular mechanisms responsible for beneficial effects of mesenchymal stem cell-derived product “Exo-d-MAPPS” in attenuation of chronic airway inflammation. Anal Cell Pathol. 2020;2020:3153891.
81. Zhai Z, Cui T, Chen J, Mao X, Zhang T. Advancements in engineered mesenchymal stem cell exosomes for chronic lung disease treatment. J Transl Med. 2023;21:895.
82. Kang J, Hua P, Wu X, Wang B. Exosomes: efficient macrophage-related immunomodulators in chronic lung diseases. Front Cell Dev Biol. 2024;12:1271684.
83. Pu Q, Lin P, Gao P, et al. Gut microbiota regulate gut-lung axis inflammatory responses by mediating ILC2 compartmental migration. J Immunol. 2021;207:257-67.
84. Hou K, Wu ZX, Chen XY, et al. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7:135.
85. Kim HJ, Kim YS, Kim KH, et al. The microbiome of the lung and its extracellular vesicles in nonsmokers, healthy smokers and COPD patients. Exp Mol Med. 2017;49:e316.
86. Zhao S, Di Y, Fan H, et al. Targeted delivery of extracellular vesicles: the mechanisms, techniques and therapeutic applications. Mol Biomed. 2024;5:60.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].