fig1
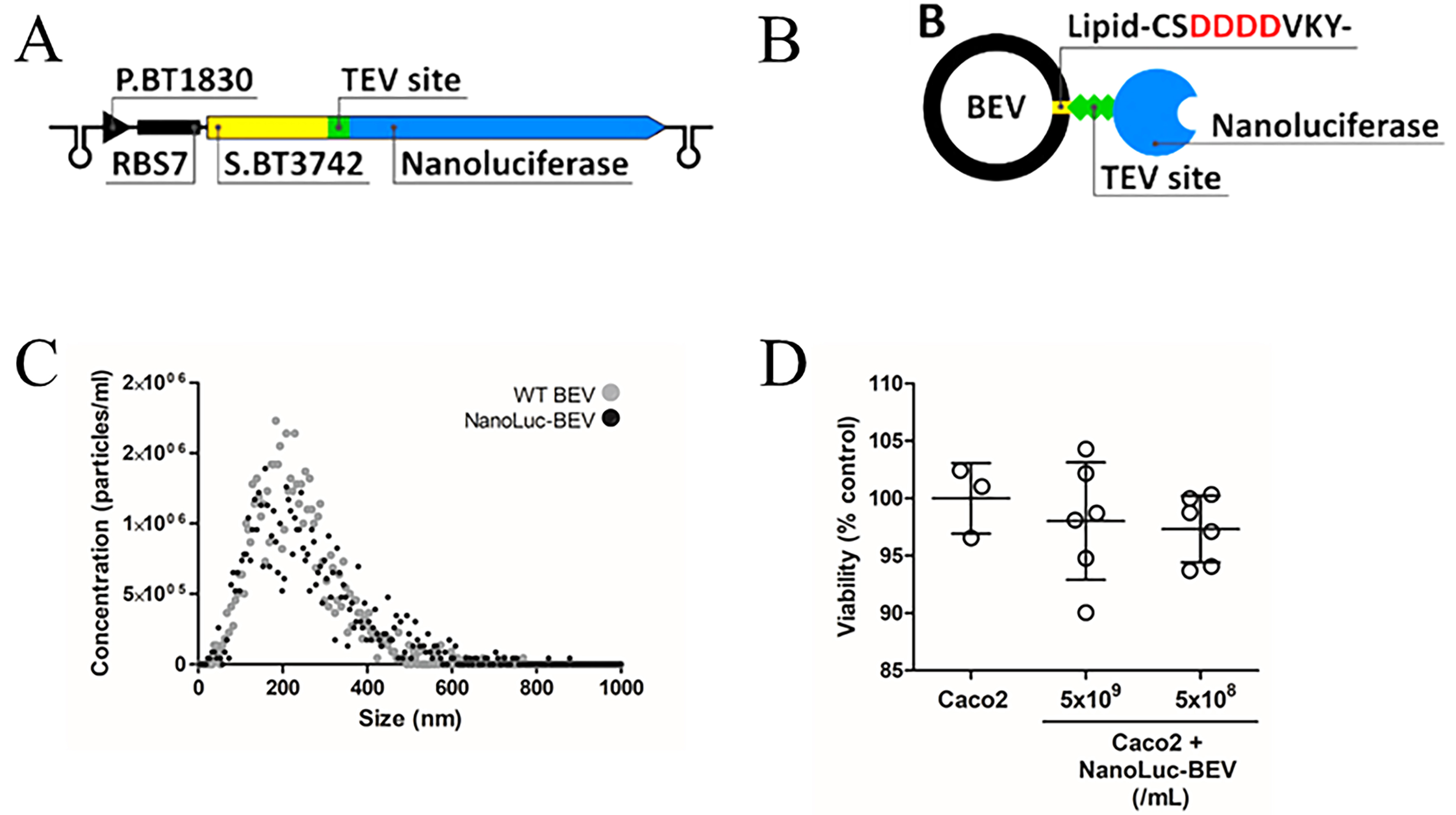
Figure 1. Design and characterisation of NanoLuc-BEVs. (A) BEV-targeted NanoLuc expression cassette cloned using the pBATH vector system. P.BT1830: promoter; RBS7: ribosome binding site; S.BT3742: signal peptide sequence; TEV site: TEV protease cleavage site. The cassette is flanked by bidirectional terminators; (B) Expected mature BEV-targeted NanoLuc structure. S.BT3742 directs the protein to the periplasm, where the signal peptide is cleaved and lipidated at Cys-24. The modified lipoprotein is inserted into the outer membrane, flipped to the outer leaflet, and incorporated into BEVs. Negatively charged aspartic acid residues upstream of the lipidation site facilitate this process (highlighted in the scheme); (C) Hydrodynamic diameter, distribution, and concentration of WT and NanoLuc-BEVs determined by nanoparticle tracking analysis; (D) Cytotoxicity of NanoLuc-BEVs to Caco-2 cells 24 h post-treatment, expressed as % viability relative to untreated control. Error bars represent mean ± SD. BEV: Bacterial extracellular vesicle; NanoLuc: Nanoluciferase; P.BT1830: promoter of BT1830; RBS7: ribosome binding site 7; S.BT3742: signal peptide of BT3742; TEV: tobacco etch virus; WT: wild type; SD: standard deviation.