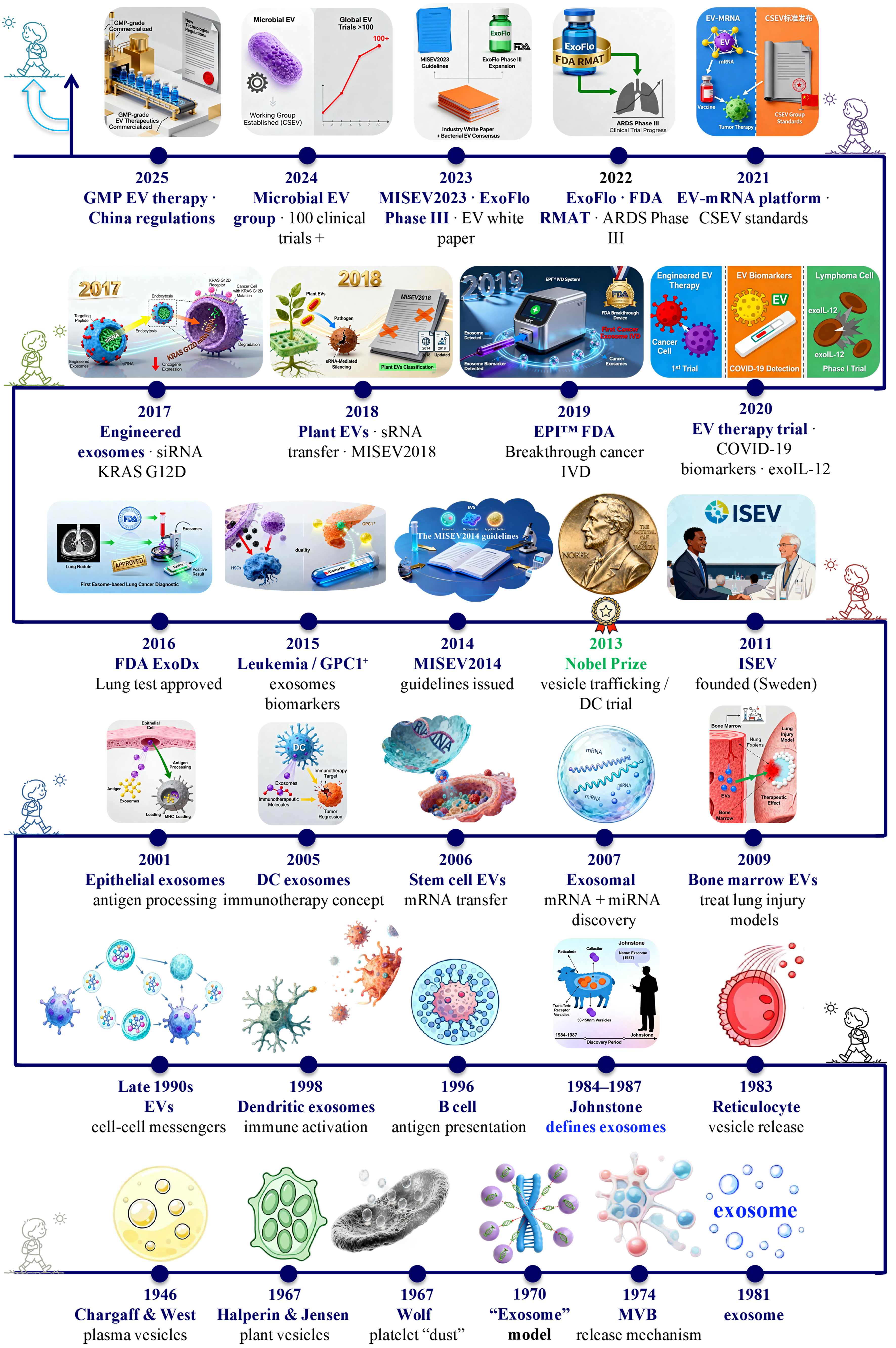
fig1
Figure 1. Milestones in the discovery, conceptual evolution, and clinical translation of EVs from 1946 to 2025. This timeline charts eight decades of progress, beginning with the early observations of vesicle-like particles in plasma (1946) and the identification of platelet “dust” (1967). Key advances include: the demonstration of exosome release from multivesicular bodies and the coining of the term “exosome” in the 1980s; the recognition of their role in intercellular communication in the 1990s; and the transformative discovery of their nucleic acid cargo in the 2000s. The field’s maturation is marked by the establishment of ISEV (2011) and the MISEV guidelines (2014, 2018, and 2023). Recent years have witnessed accelerated clinical translation, evidenced by FDA-approved diagnostics, engineered therapeutics, and scalable manufacturing platforms. EVs: Extracellular vesicles; GMP: good manufacturing practice; mRNA: messenger RNA; FDA: Food and Drug Administration; RMAT: regenerative medicine advanced therapy; ARDS: acute respiratory distress syndrome; IVD: in vitro diagnostic; GPC1: glypican-1; MISEV2014: Minimal Information for Studies of Extracellular Vesicles 2014; MISEV2018: Minimal Information for Studies of Extracellular Vesicles 2018; MISEV2023: Minimal Information for Studies of Extracellular Vesicles 2023; ExoDx: exosome diagnostics test; EPITM: ExoDx Prostate IntelliScore (a liquid biopsy test for prostate cancer); DC: dendritic cell; MVB: multivesicular body.