fig2
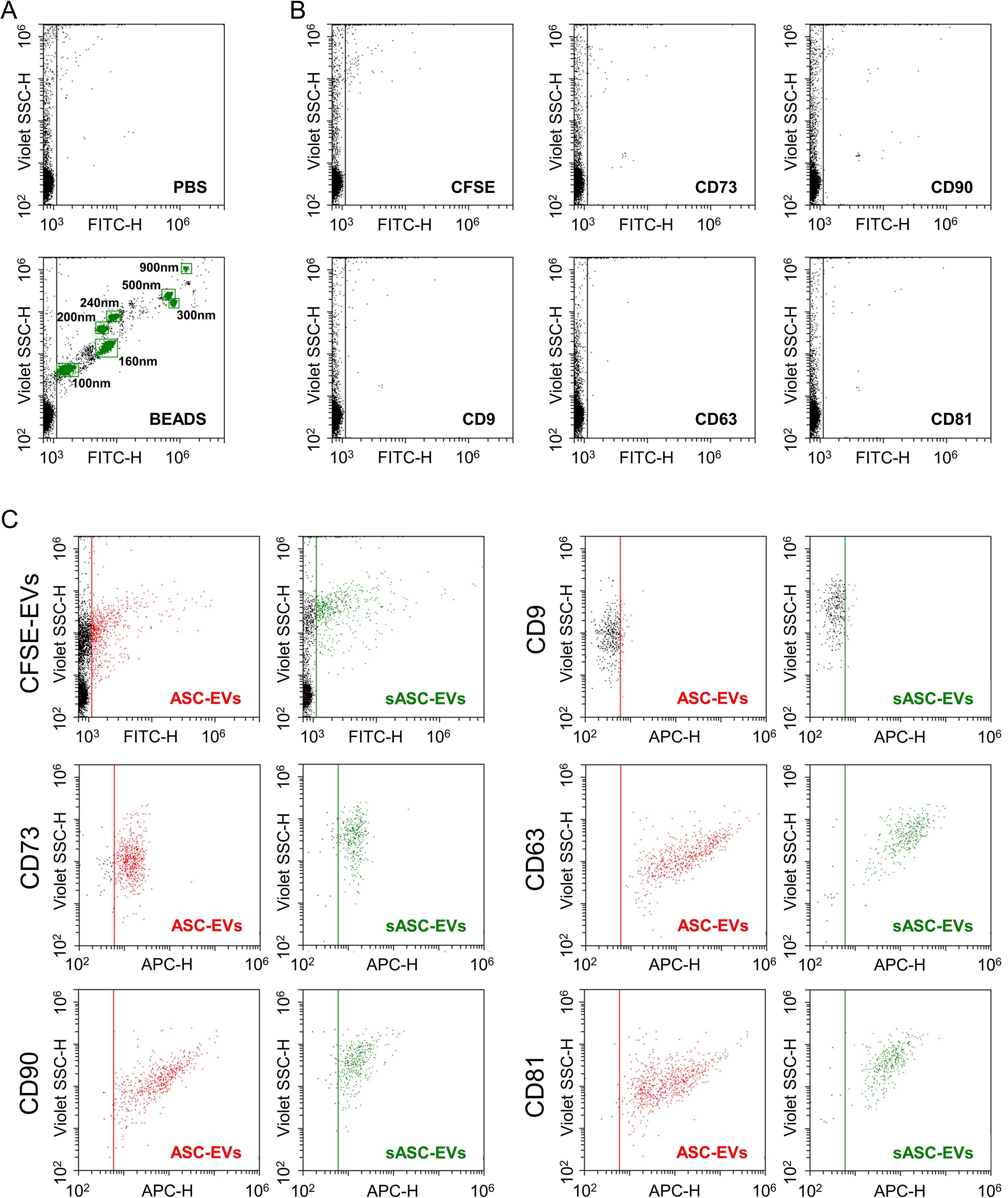
Figure 2. Immunophenotypic characterization of ASC-derived EVs and sASC-EVs by flow cytometry. (A) Calibration of the flow cytometer using FITC-labeled reference nanobeads with nominal diameters ranging from 100 to 900 nm, enabling reliable detection of fluorescent nanoparticles down to 100 nm and potentially below; (B) Assessment of background fluorescence in the 488 nm (FITC) channel using CFSE alone and single-antibody staining controls (anti-CD9, CD63, CD81, CD73, and CD90), demonstrating negligible background signal under the acquisition settings used for EV analysis; (C) Representative flow cytometry plots of CFSE-positive ASC-EVs and CFSE-positive sASC-EVs gated in the 488 nm (FITC) channel. Both EV preparations displayed strong expression of the canonical EV markers CD63 and CD81 and of the mesenchymal stromal cell-associated markers CD73 and CD90, whereas CD9 was only weakly detectable. Quantitatively, ASC-EVs showed low CD9 positivity (8% ± 2%) and high positivity for CD63 (98% ± 1%), CD81 (98% ± 1%), CD73 (93% ± 1%), and CD90 (100% ± 0). A highly comparable immunophenotypic profile was observed for sASC-EVs (CD9, 8% ± 1%; CD63, 93% ± 5%; CD81, 93% ± 5%; CD73, 89% ± 3%; CD90, 89% ± 8%), with no statistically significant differences between pre-sorted and sorted EVs. Representative cytograms are shown. ASC: Adipose-derived stromal cell; EVs: extracellular vesicles; sASC: sorted adipose-derived stromal cell; FITC: fluorescein isothiocyanate; CFSE: carboxyfluorescein succinimidyl ester; CD: cluster of differentiation.