Advanced proton exchange membranes for high-efficiency fuel cells: material innovations and durability optimization
Abstract
Proton exchange membranes (PEMs) are critical components that influence both the performance and potential of PEM fuel cells. Recent advancements in hybrid organic-inorganic and nanostructured fillers containing membranes have improved proton conductivity, chemical stability, and mechanical durability. The integration of advanced nanomaterials has enhanced dimensional stability and reduced fuel crossover, while emerging polymer chemistries offer superior electrochemical stability and conductivity. High-temperature PEMs have demonstrated excellent stability at elevated temperatures. System innovations, including optimized flow field designs, have further addressed mass transfer and water management challenges, enhancing overall fuel cell performance and longevity. Additionally, life cycle assessments and techno-economic analyses have provided insights into the environmental and economic impacts of PEM fabrication. While challenges remain in balancing performance, cost, and scalability, ongoing interdisciplinary research in material science and fuel cell engineering continues to drive improvements, supporting the broader adoption of fuel cells in sustainable energy systems.
Keywords
INTRODUCTION
There is an urgent need to develop green energy technologies that minimize environmental impact, enhance energy security, and support a sustainable future in response to increasing global energy demands[1-3]. Fuel cells have emerged as a promising technology for clean energy due to their high efficiency and minimal environmental impact. Among the various types of fuel cells, proton exchange membrane (PEMFC)[4,5] and direct methanol fuel cells (DMFC)[6,7] have garnered significant attention for their potential in portable, stationary, and automotive applications. The core component of these systems is the proton exchange membrane (PEM). PEM facilitates proton transfer from the anode to the cathode while acting as a barrier to electron flow and fuel crossover (hydrogen for PEMFC and methanol for DMFC). The performance, durability, and cost-effectiveness of PEMs are crucial in determining the overall viability of fuel cell systems[4,8].
The bibliometric analysis (shown in Figure 1A) of PEM research highlights a significant growth in publications, particularly after 2000. Fuel cells dominate the field of PEM research. Most research is published in journal articles (70.3%), with energy (21.4%), engineering (19.4%), and chemistry (16.1%) being the primary disciplines involved [Figure 1B and C]. Keyword mapping [Figure 1D] reveals strong research focus on proton conductivity, fuel cell performance, catalyst development, and nanomaterials. The increasing complexity and cross-field collaborations suggest that PEM advancements will continue to drive sustainable energy innovations, particularly in hydrogen fuel cells and next-generation energy storage systems.
Figure 1. Publications related to proton exchange membrane. (A) Annual publications. (B) Publications by type. (C) Publications by subjects. (D) Author keywords density (generated by using VOSviewer). Data source: Scopus; Data obtained on Feb 7th, 2025. Queries on Scopus search: TITL-ABS-KEY (“terms”).
Recent advancements in PEM development have focused on enhancing proton conductivity, dimensional stability, and chemical resistance to address the limitations of conventional materials. For instance, composite membranes integrating advanced materials such as carbon nanotubes (CNTs) and graphene oxide (GO) have shown significant promise. Sulfonated poly(arylene ether nitrile)-based composite membranes enhanced with Ca2+-bridged CNT-GO networks exhibit improved proton conductivity and mechanical properties, essential for high-performance fuel cells[9]. Similarly, chitosan-based composite membranes containing chitosan-coated CNTs offer high proton conductivity and stability under operational conditions[10]. These innovations address the dual challenges of enhancing conductivity while maintaining structural integrity.
Another notable approach involves the use of block copolymers, which have emerged as a significant trend in PEM development. Research on controlling microdomain orientation in block copolymer thin films illustrates how electric fields can be utilized to enhance the performance of proton-conducting membranes[11]. Ion-containing block copolymers self-assemble into ordered nanostructures that facilitate proton transport across varying conditions, enhancing the robustness of fuel cell performance[12]. These materials not only improve proton conductivity but also contribute to the mechanical stability of the membranes.
Durability optimization remains a critical focus in PEM research. The degradation of PEMs under operational stresses can significantly shorten their lifespan, hindering their adoption in large-scale applications. Carbon stability in catalyst layers (CLs) is crucial, as graphitized carbons provide better corrosion resistance in acidic environments[13]. Additionally, challenges associated with platinum-group-metal (PGM) catalysts have led to the development of low-PGM and PGM-free catalysts, improving both cost-effectiveness and stability[14].
Operational conditions also determine in PEM performance and longevity. Analysis of flow-field configurations demonstrates their impact on the uniform distribution of reactants and performance consistency across the membrane[15]. This understanding is essential for optimizing fuel cell efficiency, particularly in applications requiring consistent and reliable operation. By bridging the gap between material science and fuel cell engineering, this paper aims to provide a comprehensive review of the latest innovations in PEM technology. Key advancements in membrane materials and durability optimization are critically examined. Furthermore, the paper highlights strategies to address operational challenges, offering insights into the development of high-efficiency, durable PEMs that can support the widespread adoption of fuel cells in a sustainable energy future.
FUNDAMENTALS OF PROTON EXCHANGE MEMBRANES (PEMs)
PEM is the key component in PEMFC and DMFC. A typical PEMFC consists of a membrane electrode assembly (MEA) flanked by bipolar plates (BPs), with each component serving a specific electrochemical and structural function [Figure 2A][16]. The MEA comprises several critical layers: a gas diffusion layer (GDL) incorporating a microporous layer (MPL), a CL, and a PEM at the core. The CL, embedded with platinum-based catalysts, facilitates the hydrogen oxidation reaction at the anode and the oxygen reduction reaction (ORR) at the cathode. The GDL ensures uniform gas distribution, supports water management, and maintains both mechanical integrity and electrical continuity. The PEM functions as a selective ion conductor, enabling proton transport from anode to cathode while electronically insulating the electrodes, thereby compelling electron flow through an external circuit to generate electrical power. This dual role sustains the redox couple that drives external current.
The performance of PEMs is heavily influenced by their material properties, including proton conductivity, mechanical strength, thermal stability, and chemical resistance[17]. Effective proton conduction in PEMs relies on mechanisms such as the Grotthuss mechanism, where protons hop between water molecules, and the vehicle mechanism, where protons are transported alongside water molecules as hydronium ions[18]. These mechanisms are highly dependent on factors such as membrane hydration, the density of ionic groups, and the structural morphology of the membrane[19].
Perfluorinated sulfonic acid membranes, commonly used in PEMFCs, consist of three phases: a hydrophobic polymer backbone, a hydrophilic network of water and sulfonate groups, and an intermediate phase that facilitates proton transport through a combination of Grotthuss, vehicle, and surface hopping mechanisms[20,21] [Figure 2B]. The integration of multi-scale nanofiber/Nafion composite membranes has demonstrated enhanced proton conductivity, with values reaching 0.192 S cm-1 at 80 °C and 100% relative humidity (RH), significantly improving upon conventional Nafion membranes[22]. Similarly, nanofiber-reinforced MIL-53(Al)-NH2 nanofibers (MNFs)@sulfonated poly(ether sulfone) (SPES) hybrid membranes have shown outstanding proton conductivity, with values increasing from 0.113 to 0.248 S cm-1 at 80 °C and 100% RH, depending on the membrane composition, due to enhanced water retention and interconnected proton-hopping pathways[23].
For instance, the incorporation of sulfonated polystyrene fiber networks has been shown to significantly improve the proton conductivity of hybrid membranes[24]. Membranes with increased sulfonic acid group content exhibit higher ion exchange capacities, directly correlating with enhanced proton conductivity. Similarly, polybenzimidazole covalent organic frameworks (COFs) have demonstrated ultrafast proton conduction capabilities at elevated temperatures, achieving conductivities as high as 0.152 S cm-1 at 160 °C under anhydrous conditions, making them promising candidates for high-temperature fuel cell applications[25]. Composite membranes, which integrate organic and inorganic components, have also emerged as a significant area of development. Sulfonated polyimide-based membranes have been shown to optimize proton transport pathways while maintaining structural integrity under operational conditions[26].
Membrane hydration strongly modulates proton conductivity, making water management an important design parameter. Proton conductivity in membranes with low water content increases with temperature, emphasizing the importance of temperature-dependent hydration strategies[27]. Effective water management strategies are therefore essential to maintaining optimal hydration levels and ensuring consistent performance in PEMFCs[28]. To support future high-power-density PEMFCs, advanced designs focus on optimizing GDLs, CLs, and flow-field structures to facilitate better hydration and reactant transport[16].
The durability and stability of PEMs under operational stresses are also critical for the long-term viability of fuel cells. While increasing the sulfonic acid group content improves conductivity, it can also compromise mechanical stability, necessitating a balance to prevent membrane degradation[29]. Efforts to enhance durability have included the development of self-hydrating membranes, which address hydration challenges and improve the operational lifespan of PEMs in fuel cell systems[30]. Additionally, multifunctional hybrid membranes with long-range hydration networks and interfacial conduction pathways have been designed to sustain high proton conductivity while preventing degradation over extended use[22]. These advancements underscore the importance of integrating high proton conductivity, effective water management, and enhanced durability to develop next-generation PEMs capable of meeting the demands of sustainable energy applications.
CHALLENGES IN CURRENT PEMs
PEM is a key component of proton conduction while effectively preventing electron flow, gas crossover, and methanol leakage in PEMFCs and DMFCs. Despite their importance, PEMs face several significant challenges limiting their performance, durability, and widespread adoption. These issues encompass proton conductivity, thermal stability, water management, mechanical integrity, and high material costs, collectively imposing operational and economic constraints.
Among these challenges, insufficient proton conductivity, particularly at elevated temperatures, stands out prominently. Traditional membranes such as Nafion exhibit excellent conductivity under humidified conditions but experience substantial performance deterioration at higher temperatures due to dehydration[31]. This drawback severely restricts their operational temperature range and efficiency. Elevated operational temperatures are advantageous as they enhance reaction kinetics and mitigate catalyst poisoning. However, current state-of-the-art PEMs still struggle to sustain performance under such conditions[32]. Recent advancements in phosphoric acid (PA)-doped membranes have shown promising improvements in high-temperature proton conductivity. For instance, a 1.5% TPB-PBAP/263% PA membrane exhibited increased proton conductivity from 0.04 S cm-1 at 100 °C to 0.0745 S cm-1 at 180 °C, highlighting its potential as a high-temperature PEM candidate[33] [Figure 3A]. Similarly, another study demonstrated that P(TP-co-DMF)/194% PA membranes achieved conductivity values rising from
Figure 3. (A) Temperature effects on proton conductivity of phosphoric acid membranes (left) and PEMFC power density (right). Reprinted with permission from[33]. (B) Chemical degradation of PEM. Model (left) and framework (right). Reprinted with permission from[41]. (C) Doubly hydrated Nafion membrane model. The chemical formula (left) and detailed view of the ether linkage (right). Reprinted with permission from[42].
Water management further complicates PEM performance. Proper hydration is crucial for maintaining proton conductivity, but excessive water accumulation can cause flooding, especially in the cathode CL, impeding ORRs[35]. Studies have highlighted the intricate dynamics of water uptake within CLs, stressing the need for effective water management solutions to optimize fuel cell operations. Moreover, reliance on external humidification systems increases system complexity and cost, further hindering commercialization efforts[36]. Recent research efforts have explored self-hydrating membranes and reinforced nanostructures. PA-doped membranes, for example, maintain high proton conductivity even without external humidification. P(TP-co-DMF)/194% PA membranes notably retained high conductivities of 0.0745 S cm-1 at 180 °C under unhumidified conditions[34]. Such developments reduce dependency on humidification systems, simplifying design and enhancing cost-effectiveness.
Material costs and long-term stability also present considerable obstacles. High costs associated with perfluorosulfonic acid (PFSA) membranes such as Nafion represent a significant barrier to widespread adoption[31]. Additionally, oxidative degradation adversely affects these membranes over time, causing performance decline[37]. The degradation of PEMs in PEMFCs is a complex, multifactorial issue that critically impacts system efficiency, durability, and long-term performance. Among the primary degradation mechanisms is chemical attack, predominantly driven by reactive oxygen species such as hydroxyl radicals
Mechanical integrity remains another critical concern. Repeated hydration-dehydration cycles and operational stresses cause membrane swelling and potential mechanical failures. This phenomenon undermines structural stability, performance, and lifespan, increasing fuel crossover and reducing efficiency. Addressing these mechanical challenges demands a multidisciplinary approach to enhance durability and reliability under operational conditions[44].
Environmental and scalability considerations further constrain PEM adoption. Fluorinated membrane production and disposal raise environmental sustainability questions due to poor biodegradability. Moreover, expanding fuel cell applications, including transportation and portable devices, demand PEMs that are versatile in size, flexibility, and performance under diverse conditions. Addressing these issues requires innovative material and engineering strategies, paving the way for broader PEM utilization in sustainable energy technologies.
MATERIAL INNOVATIONS IN PEMs
Material innovations in PEM are crucial for enhancing the performance and durability of fuel cells. Recent advancements focus on improving proton conductivity, thermal stability, and mechanical properties, while addressing the limitations of traditional PEM materials such as Nafion. These developments aim to create membranes with higher efficiency, enhanced durability, and cost-effectiveness, enabling broader adoption in fuel cell technology.
Advanced polymer-based membranes have garnered considerable attention, with non-fluorinated polymers such as sulfonated polybenzimidazole (sPBI) demonstrating notable potential for high-temperature fuel cell applications. Studies have demonstrated that sPBI membranes can maintain proton conductivity in non-humidified conditions, utilizing PA as a proton carrier to facilitate conductivity at elevated temperatures[45].
Hybrid organic-inorganic membranes have emerged as another critical innovation[46-48]. The incorporation of metal-organic frameworks (MOFs) into PEMs has demonstrated significant performance improvements. Recent work has shown that doping Nafion with NH3-modified Zn-MOFs enhances proton conductivity by 1.3-fold, attributed to host-guest collaborative hydrogen bonds that facilitate proton transport[49] [Figure 4A]. Specifically, Zn-MOF/Nafion membranes with 5 wt% Zn-MOF filler reached a peak conductivity of 0.073 S cm-1 at 80 °C, 1.87 times higher than pure Nafion (0.0039 S cm-1). However, increasing the filler to 10-20 wt% led to a drop in conductivity due to excessive filler content obstructing proton pathways[49]. Another example is functionalizing sPBI with acid-functionalized GO fillers, which leads to enhanced proton conductivity of up to 0.098 S cm-1 at 180 °C[50]. Other hybrid or mixed matrix membrane, such as Me@GO/poly(2,5-benzimidazole) (ABPBI) composite membranes, exhibited 0.028 S cm-1 at
Figure 4. (A) Proton conductivity of Zn-MOF containing membranes [effect of temperature (left) and filler content (right)]. Reprinted with permission from[49]. (B) PEM with HPW/COF (P-COF). Synthesis procedure, structure, and proton transport in P-COF (left). Proton conductivities at various relative humidity. Reprinted with permission from[52]. (C) Proton conductivity of PPy@ZIF-67/SPI nanofiber composites membrane and possible proton transport. Reprinted with permission from[54].
Further advances have also been reported with the incorporation of porous COFs, which enhance membrane performance through improved water retention and the formation of continuous proton conduction pathways. Nafion/P-COF hybrid membranes demonstrated significantly enhanced water retention and proton conductivity at low RH levels. At 80 °C under 55% RH, Nafion/P-COF membranes achieved 0.13 S cm-1, a 225% increase over recast Nafion (0.040 S cm-1). Even at 25% RH, the hybrid membrane maintained 0.013 S cm-1, which is 983% higher than recast Nafion (1.2 × 10-3 S cm-1)[52] [Figure 4B]. This improvement is attributed to Keggin-type HPW units within the COF framework, which enhance proton retention and create continuous conduction pathways.
Nanocomposite PEMs incorporating silica, titanium dioxide, and zirconium phosphate enhance thermal stability and proton conductivity. Introducing triazole groups into high-temperature PEMs has been shown to improve conductivity to 0.085 S cm-1 at 150 °C, facilitating stable operation in harsh conditions[53]. Additionally, PPy@ZIF-67/SPI nanofiber composites exhibited a significant increase in conductivity from 20 to 80 °C due to their high surface area and continuous proton transfer pathways[54] [Figure 4C].
The exploration of ionically cross-linked membranes presents opportunities for improving mechanical properties, although challenges remain. While cross-linked PEMs exhibit enhanced proton conductivity of up to 0.075 S cm-1, studies have shown that they suffer from thermal instability above 120 °C, leading to swelling and performance degradation[55].
Plasma-induced grafting has emerged as a novel approach for modifying PEM surfaces. Styrene-grafted polytetrafluoroethylene (PTFE) membranes exhibited a 30% increase in proton conductivity compared to untreated PTFE[56]. Furthermore, HS(CH2)3Si(OCH3)3 (MPTS)-based silicate-modified membranes exhibited a 1.95-fold higher conductivity than Nafion at 90 °C due to stable proton transfer pathways[57].
Bio-inspired and biomimetic approaches further expand the possibilities for PEM innovation. Proton-selective permeation mechanisms observed in biological proton channels have inspired designs where protons undergo concurrent transport via structured hydrogen-bond networks[58]. A compromise mechanism combining Grotthuss and Vehicle pathways was also observed, where proton hopping alternates between structured hydrogen bonds and free diffusion channels[58].
Material innovations in PEMs are driving transformative progress in fuel cell technology. By combining advanced polymer chemistry, nanotechnology, and bio-inspired designs, researchers are addressing critical challenges in performance, durability, and scalability. These advancements pave the way for more sustainable and efficient energy systems, supporting the transition to clean energy solutions and a hydrogen-based economy.
STRATEGIES FOR DURABILITY OPTIMIZATION
Optimizing the durability of PEMs is essential for improving the performance and longevity of PEMFCs. The durability of PEMs directly affects fuel cell reliability and efficiency, making it a key focus in ongoing research. Various strategies have emerged to tackle the challenges associated with PEM durability, including the use of radical scavengers, advanced membrane architectures, optimization of operating conditions, and novel material innovations.
One effective approach for enhancing PEM durability involves incorporating radical scavengers. Free radicals, particularly hydroxyl radicals (·OH), can severely degrade PEM materials such as PFSA membranes[59]. Recent studies show that embedding cerium oxide (CeO2) within PEMs effectively neutralizes these harmful radicals, thereby improving membrane stability[37]. For instance, Huang et al. demonstrated that manganese-doped cerium-based MOFs significantly enhanced PEM stability, reducing fluoride emission rates by 43%, a clear indicator of suppressed radical-induced degradation [Figure 5A][37]. This method not only extends membrane life but also maintains proton conductivity, with only a 5.3% loss in conductivity after accelerated stress tests compared to a 15.6% loss in untreated membranes[37]. Furthermore, in situ OCV tests reinforced these results, showing that the
Developing advanced membrane architectures, such as double-layer reinforced membranes, is another promising strategy. Liu et al. reported that using expanded PTFE (ePTFE) as reinforcement in PEMs significantly boosts mechanical strength and durability[60]. These double-layer reinforced membrane electrode assemblies (MEAs) showed excellent durability, retaining 95% of their initial performance after 100 h of accelerated testing. This double-layer structure provides additional membrane support, reducing mechanical failures during operation. Moreover, these reinforced MEAs delivered high performance, achieving power densities of 1.02 W cm-2 at 0.6 V, making them suitable for long-term applications[60]. Further analysis revealed that MEAs fabricated via the novel dry membrane deposition process (DR-MEA) exhibited significantly better interfacial bonding between the PEM and CLs. This reduced interfacial resistance and improved power output compared to conventional MEAs (C-MEA). Accelerated durability tests showed that DR-MEA consistently outperformed C-MEA, underlining the importance of optimized membrane architectures for durability[60]. Recent developments in gel-state polybenzimidazole (PBI) membranes featuring double cross-linked, three-dimensional layered architectures have demonstrated PEMFC performance at temperatures exceeding 200 °C [Figure 5B][61]. These advanced membranes exhibit outstanding acid retention, effectively mitigating dehydration and condensation with a retention rate of 96%. Furthermore, they sustain high proton conductivity (0.348 S cm-1) and achieve peak power densities ranging from 1.20 to 1.48 W cm-2, while maintaining minimal voltage degradation (0.27 mV h-1 over
Optimizing operating conditions is equally important for enhancing PEM durability. A study demonstrated that cyclic exposure to wet and dry gas leads to a 45.7% performance drop in PEMFCs after 1,200 cycles, primarily due to increased resistances, hydrogen crossover, and reduced electrochemical active surface area[62]. This highlights how fluctuations in RH critically impair MEA structure and fuel cell durability. Moreover, carefully controlling parameters such as temperature and pressure reduces membrane stress, further enhancing durability[63]. Additional studies confirmed that reducing humidification from 100%/100% RH to 50%/70% RH significantly improved MEA durability, cutting voltage decay rates nearly in half compared to fully humidified conditions over 1,000 h of testing. These findings highlight the importance of precise humidity control for extending PEM lifespan[63].
Advanced material innovations also play a crucial role in durability optimization. Incorporating nanostructured fillers such as silica, titanium dioxide, or GO into PEMs enhances thermal and mechanical properties while preserving high proton conductivity[64,65]. Self-healing technologies, such as microencapsulated repair agents and dynamic bonding polymers, enable membranes to self-repair during operation, further extending lifespan. Surface engineering methods, including anti-fouling coatings and balanced hydrophilic-hydrophobic properties, enhance water management and reduce degradation from local drying or flooding.
Understanding PEM degradation mechanisms under varying operating conditions is vital for developing effective control strategies. Research by Zhang et al. emphasizes the importance of standardized accelerated durability testing methods to assess PEM performance under typical automotive scenarios[66]. Their study found that dynamic load conditions significantly degraded performance, with voltage losses of up to 18% after 500 h. However, implementing targeted control strategies, such as dynamic humidification and precise temperature management, reduced degradation, limiting performance losses to only 8%. This underscores the value of tailored operational strategies for improving PEM durability[66]. Additionally, voltage decay rates under high dynamic load rates (415.3 μV/h at 300 mA cm-2 s-1) were significantly greater than under slower dynamic load rates (66.7 μV/h at 60 mA cm-2 s-1). Thus, slower load transitions help preserve membrane integrity and reduce degradation[66].
By integrating material innovations, structural advancements, and optimized operational strategies, significant progress has been made in addressing PEM durability challenges. These advances pave the way for more reliable, efficient, and sustainable fuel cell technologies, supporting broader adoption in clean energy systems.
PERFORMANCE EVALUATION OF ADVANCED PEMs
Evaluating the performance of advanced PEMs is critical to ensuring their efficacy and reliability in PEMFC systems. The overall efficiency and durability of PEMFCs are intrinsically linked to the functional robustness of the membrane component. Key performance indicators for PEMs include proton conductivity, thermal and chemical stability, mechanical strength, water retention capacity, and resistance to hydrogen crossover.
Foremost among these is high proton conductivity, which underpins efficient ion transport and directly influences the overall energy conversion efficiency of the fuel cell. Materials such as PFSA and sulfonated poly(ether ether ketone) (SPEEK) are particularly valued for their ability to sustain proton conductivity under a range of temperature and humidity conditions[67]. A range of analytical techniques, including impedance spectroscopy and four-point probe measurements, are employed to evaluate the ionic conductivity of PEMs under diverse temperature and humidity conditions. Among these, impedance spectroscopy is particularly valuable for characterizing the membrane frequency-dependent response, offering detailed insights into ion transport dynamics and the associated resistance and capacitance behaviors within the membrane matrix[68].
Equally vital are chemical and thermal stability, which enable membranes to withstand the harsh oxidative and high-temperature conditions typical of fuel cell environments, thereby ensuring long-term operational durability[19,69]. Thermal stability of PEMs is commonly evaluated using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). These techniques provide critical information on thermal degradation temperatures and phase transition behavior, which are essential for understanding membrane performance under elevated operating conditions. High thermal stability is particularly important for high-temperature PEM applications, where membranes must maintain structural and functional integrity. Through these analyses, researchers can effectively identify materials that are well-suited for demanding thermal environments[45]. Chemical stability, particularly resistance to oxidative degradation, is a critical parameter in the evaluation of PEMs. This stability is commonly assessed by exposing PEMs to reactive oxygen species, such as hydroxyl radicals (•OH) and peroxyl radicals (•OOH), which simulate the harsh oxidative conditions encountered during PEMFC operation. The extent of degradation is typically monitored through measurements of weight loss, mechanical integrity, and ionic conductivity over time[70].
Mechanical robustness - including tensile strength and flexibility - is also essential, as it maintains membrane integrity during physical stress and thermal cycling, especially in applications subject to dynamic loads such as automotive and portable systems[71]. Mechanical properties, including tensile strength and elasticity, are typically assessed via tensile testing, which provides quantitative measures of a membrane’s structural resilience[72]. These characteristics are critical for evaluating the durability and robustness of PEMs under mechanical and thermal stresses encountered during fuel cell operation. To ensure long-term reliability, PEMs must retain their mechanical integrity over extended use, particularly in applications subject to frequent cycling and dynamic load conditions.
Low gas permeability coupled with high proton selectivity is indispensable for preventing fuel crossover and sustaining electrochemical efficiency; effective PEMs must facilitate selective proton conduction while impeding the passage of gases such as hydrogen and oxygen[73]. Gas permeability in PEMs is typically measured using permeation cells, which enable precise assessment of gas transmission under controlled pressure and temperature conditions. These systems facilitate the quantification of gas flux - commonly hydrogen or oxygen - across the membrane. From these measurements, gas permeability coefficients can be calculated, providing critical insights into the membrane selectivity and its ability to prevent fuel crossover[74].
To improve proton conductivity, researchers have developed composite membranes with functional additives. For example, a covalent triazine framework
Assessing thermal and chemical stability involves evaluating how membranes withstand high temperatures and oxidative conditions. Zwitterionic COFs have shown impressive water retention and stability at elevated temperatures, making them promising candidates for high-performance fuel cells[76]. Similarly, Nafion composite membranes modified with phosphotungstic acid and UiO-66-NH2 enhanced water retention and proton conductivity due to the porous structure of UiO-66-NH2, which improves water uptake essential for high conductivity[77].
Mechanical strength tests measure how PEMs handle stress and deformation during operation. Incorporating materials such as CNTs and GO into sulfonated poly(arylene ether nitrile) membranes has notably improved their mechanical strength and conductivity[9]. These composites exhibited better dimensional stability and proton conductivity, making them attractive for high-performance PEMFC applications. Similarly, graft poly(arylene ether sulfone)-based copolymers showed improved phase-separated morphology and proton conductivity thanks to their controlled structural design[78].
Water retention capability is critical for maintaining conductivity, especially in low-humidity environments. Efficient water transport mechanisms within the membrane are essential for sustaining hydration and proton conductivity[28]. Thus, designing membranes that effectively manage water under various conditions remains a priority.
Resistance to hydrogen or methanol crossover is vital for minimizing fuel losses and ensuring efficiency. Functionalized graphene materials, such as sulfanilic-functionalized holey graphene, effectively reduce methanol and formate crossover while maintaining proton conductivity. These membranes exhibit relatively high defect levels, indicated by a D-band to G-band (ID/IG) intensity ratio between 0.88 and 1.75, enabling efficient suppression of fuel crossover[79]. This dual functionality is crucial for improving DMFC efficiency, where fuel crossover significantly affects performance.
Hybrid membranes have also become prominent, employing innovative methods to enhance performance. For instance, aligning proton-conducting channels using magnetic fields optimized proton transport in electrolyte membranes, providing a novel approach for enhancing PEM conductive pathways[80].
Incorporating catalysts such as iron and zirconium-doped nitrogen-carbon (Fe/Zr-N-C) has improved chemical stability and proton conductivity. Detailed analyses, including Mössbauer spectroscopy and X-ray absorption spectroscopy, confirmed the atomic dispersion of Fe and Zr, primarily coordinated with nitrogen or oxygen atoms, significantly enhancing catalytic stability and performance[81] [Figure 6A-C].
Dimensional stability of PEMs under operational conditions is another critical factor. A phosphoric acid-doped quaternary ammonium ion-modified poly (vinylidene fluoride) nanofiber/sulfonated poly (ether ether ketone) composite PEM demonstrated significantly reduced dimensional changes (54%-73%) in methanol solutions at 80 °C, attributed to its 3D PVDF nanofiber structure. Moreover, these membranes exhibited excellent proton conductivity with activation energies ranging from 14.01 to 29.99 kJ mol-1, underscoring their potential for high-performance DMFC applications[82].
Figure 6D presents reconstructed μ-computed tomography images comparing pristine MEAs of conventional and SiC-reinforced HT-PEMFCs. The SiC-based MEA exhibits less distinct layer separation in the cross-sectional view due to reduced contrast between the membrane and CL, attributed to the presence of SiC particles. Additionally, the CLs in the SiC-based MEA appear more uneven, suggesting increased membrane stiffness and deformation of the softer CL during assembly and hot-pressing[83].
Despite these advances, standardizing performance evaluation protocols remains a challenge, complicating direct comparisons across studies. Balancing key properties such as conductivity, mechanical strength, and long-term stability remains critical[84]. Hence, recent developments in PEM materials have focused on enhancing these properties [Table 1]. Additionally, considering environmental impacts and recyclability alongside technical performance is increasingly important due to the growing emphasis on sustainable technologies. The evaluation of advanced PEMs underscores significant strides in addressing fuel cell technology challenges. Innovations such as composite membranes, zwitterionic frameworks, and hybrid structures have enhanced efficiency, durability, and scalability. However, ongoing research is necessary to optimize these materials for broader adoption, ultimately paving the way toward a sustainable energy future driven by high-performance PEMs.
Performance of advanced PEMs
| PEMs | Performance Improvement | Refs. |
| SPEEK/s-pCTF (Sulfonated Piperazine Covalent Triazine Framework) | Maintains 85% energy efficiency over 900 cycles (400 h) at 0.120 A/cm2; Outperforms SPEEK and Nafion212 | [75] |
| Nafion/PWA-UiO-66-NH2 composite | Proton conductivity up to 0.092 S/cm at 25 °C | [77] |
| Sulfonated Poly(arylene ether nitrile) (SPEN)/GO-Ca2+-MWCNT composite | Proton conductivity enhanced to 0.193 S/cm at 90 °C (vs. < 0.1 S/cm typical for SPEN); Improved dimensional stability, oxidation resistance, and mechanical strength | [9] |
| Phosphoric acid-doped quaternary ammonium ion-modified poly (vinylidene fluoride) nanofiber composite SPEEK | Proton conductivity increased to 0.202 S/cm at 80 °C, which is 2.6 times that of SPEEK (0.078 S/cm) and 1.31 times that of Nafion 211 (0.154 S/cm); Peak power density (PPD) of 0.121 W/cm2, 1.36 times higher than Nafion 211 | [82] |
| SiC-doped polybenzimidazole (PBI) | Ohmic resistance reduced by 1/3; Degradation rate decreased to < 65 μV/h compared to > 100 μV/h for conventional HT-PEMFCs after 1,000 h | [83] |
| Sulfonated polyimide (SPI) composite films filled with ionic liquids @ molybdenum sulfide (ILs@MoS2/SPI) | Proton conductivity increased by 68%, from 0.0778 to 0.1308 S/cm at 80 °C under 100% RH | [85] |
| Sulfonated microblock copolymer with metal organic framework (MOF)-based superprotonic conductor UiO-66(SO3H)2 mixed matrix membrane (SPP-3/UiO-66(SO3H)2) | Proton conductivity increased by 34.38%, reaching 0.165 S/cm at 80 °C; PPD of 0.388 W/cm2 | [86] |
| Nafion/covalent organic framework (COF) nanosheets | Mass activity and PPD increased by 1.6 times compared to unmodified Nafion | [87] |
| PA-doped Tröger’s base polymer | Operational temperature range extended from -20 to 200 °C; Over three orders of magnitude higher proton conductivity retention under humidified conditions; 95% PPD retention after 150 cycles at 15 °C | [88] |
| Sulfonated polyimide (SPI)/nano carbon sulfonic acid (NCSA) | Proton conductivity increased to ~0.140 S/cm, 2.3 times higher than base SPI and ~50% higher than commercial PFSA membrane; PPD of 1.58 W/cm2 at ambient pressure with 0.1 mg/cm2 Pt loading | [89] |
| SPEEK with carbon dots@metal organic framework and phosphoric acid (SPEEK/40%CDs@MOF)3/PA | Proton conductivity of (0.05 S/cm at 160 °C; PPD of 0.37 W/cm2 at 120 °C | [90] |
| PVDF-COF/BmimPF₆/PA | Proton conductivity of 0.181 S/cm at 160 °C; PPD of 0.327 W/cm2 at 120 °C | [91] |
| SPEEK-PVA with 10% Ludox | Proton conductivity increased to 0.65 S/cm at 80 °C; Excellent hydrolytic stability with ~88% mass retention after 650 h | [92] |
| Oxalic Acid modified Ozone-treated Graphene Oxide | Conductivity increased to 0.024 S/cm at 50 °C; Power output increased to 0.317 W/cm2 at 50 °C; Enhanced thermal and mechanical stability due to carboxy group cross-linking | [93] |
INTEGRATION WITH FUEL CELL SYSTEMS
Integrating advanced PEMs into fuel cell systems is an important step toward improving fuel cell performance, efficiency, and durability. This process involves carefully matching the membrane materials with flow field designs and the overall system layout to meet the demands of real-world operation. For advanced PEMs to work effectively in a fuel cell stack, they must be compatible with other key components, such as electrodes, catalysts, and GDLs. Good interaction between the membrane and these components helps reduce interfacial losses, maintain strong proton conductivity, and ensure mechanical stability under operating conditions.
Effective water management is crucial for PEMFCs to maintain membrane hydration while preventing flooding or dehydration. Optimizing flow channel design and reducing excess water production can significantly improve fuel cell lifespan and durability[94]. Janus GDLs enhance water transport, increasing peak power density to 1.89 W cm-2 compared to 1.17 W cm-2 in commercial GDLs[95]. Advanced flow fields further improve water discharge. Hierarchical metal foam flow fields enhance drainage by 14.1% and increase net power density by 9.5%[96]. A partially narrowed channel (PNC) flow field achieves 9.88% ms-1 water drainage efficiency while maintaining a moderate pressure drop[97]. Nickel metal foam structures with optimized cathodes (75 pores per inch, 0.75 compression ratio) boost power density by 9.8% and energy efficiency by 4.6%[98]. Additionally, a four-way valve system improves air flow, reducing flooding and optimizing humidification by bypassing 20% of the supply air[99]. Computational fluid dynamics (CFD) simulations reveal an optimal GDL contact angle of 102.63° maximizes liquid removal[100]. New water fault diagnostics leverage deep learning, with 1DCNN-XGB achieving 98.10% accuracy in detecting and classifying PEMFC water management faults[101]. These advancements highlight the importance of water management for maximizing PEMFC performance and durability.
The selection of appropriate PEM materials is another critical factor in fuel cell integration. Material innovations, such as hybrid organic-inorganic membranes and functionalized polymers, have shown potential in improving proton conductivity, chemical resistance, and mechanical strength. Evaluating various PEM technologies for transportation applications is essential, emphasizing the need for continued research into novel materials and configurations to advance fuel cell development[102]. For instance, PEMs incorporating phosphotungstic acid or other functional additives can enhance conductivity and reduce fuel permeability, contributing to efficient fuel cell operation[103].
Optimizing flow field designs plays a crucial role in improving reactant distribution, water removal, and overall electrochemical efficiency in EMFCs[104]. Recent innovations have significantly enhanced performance. A curved flow field increased peak power by 55.2%[105], while a tapered design reduced pressure drop by 68.74% and boosted power density by 12.63%[106]. Metal foam cathode flow fields improved thermal management, lowering cell temperature by 8.4 °C and increasing net output by up to 55.1%[107]. Alternative flow structures have also demonstrated promising results. A traveling-wave flow field enhanced oxygen transport by 35.89%-118%, leading to a net power density increase of up to 11.17%[108]. The raccoon-shaped wavy flow field outperformed traditional straight channels, boosting output power by 20%[109]. Similarly, a plant-hopper gear-shaped channel improved peak power by 46%, achieving 1.04 W cm-2[110]. Artificial intelligence (AI)-driven optimization has further accelerated advancements in flow field design. A Generative Adversarial Network (GAN) model improved thermal and structural performance by over 20%, reducing computational time from 30.2 h to under 1 s[111]. Additionally, a wave interdigitated flow field, optimized via AI, achieved a 27.3% higher power density, reaching 3 W cm-2[112]. Nature-inspired designs are also making a significant impact. A nautilus-inspired flow channel increased power density by 21.53%[113], while a river-diversion-inspired design removed 50% more water, leading to a 13.3% boost in net power[114].
Ensuring long-term durability remains a major challenge in PEMFCs, especially under demanding operating conditions. Advances in modified PBI membranes have demonstrated their potential for high-temperature applications, enhancing both performance and lifespan[115,116]. Additionally, research into self-healing mechanisms and oxidative resistance is further extending membrane longevity[117]. Recent breakthroughs in membrane durability include the use of single-layer graphene coatings, which reduced hydrogen crossover by 78.5% and increased power density to 480 mW cm-2 even after accelerated stress testing[118]. Meanwhile, functionalized PTFE (ePTFE) membranes have improved proton conductivity and retained moisture at low RH, ensuring stable mechanical performance[119]. Advancements in catalyst development have also contributed to longer PEMFC lifespans. Pt-Co-ZrO2 catalysts exhibited 6.6 times higher ORR mass activity compared to conventional Pt/C catalysts, while retaining 88% of initial activity even after 50,000 cycles[120]. Additionally, Zr-doped Fe-N-C catalysts showed improved stability, retaining 40% of performance after 100 h, compared to a 70% loss in undoped samples[81]. Structural innovations have also helped mitigate degradation. Mesoporous carbon-supported catalysts demonstrated an 86% reduction in voltage decay over 1,000 h under real-world conditions[121]. Similarly, CNT-interlayer MEAs improved hydration, extending durability by 89.2% at 120 °C and 25% RH[122] Additionally, Cr7C3 ceramic-coated GDLs increased corrosion resistance, maintaining conductivity 1.5 times higher than untreated carbon paper[123]. Further improvements include the introduction of CeO2-based free radical scavengers, which reduced membrane degradation by 30%[124], and graphitized black pearl-supported Pt catalysts, which maintained voltage stability for 1,003 h, significantly outperforming conventional Pt/C catalysts[125]. These advancements emphasize the crucial role of material innovations in enhancing PEMFC durability, paving the way for more robust and commercially viable fuel cell technologies.
Hybrid systems that combine PEMFCs with other energy storage technologies, such as batteries or electrolyzers, are emerging as promising solutions for enhancing overall system performance. The integration of advanced alkaline electrolysis with PEMFC systems demonstrates the benefits of hybrid approaches for improving energy efficiency and power quality[126]. These integrated systems offer increased resilience and adaptability, particularly in applications requiring rapid power delivery or variable energy demands.
Catalysts are essential for optimizing PEMFC performance, directly influencing both efficiency and durability. Enhancing platinum-based catalysts and exploring alternative materials are key to extending fuel cell lifespan and reducing cost[127]. Recent innovations in Pt-alloy catalysts, non-precious metal alternatives, and advanced support structures are driving significant improvements[128,129]. Pt-Co alloy catalysts anchored on multiwalled CNTs (MWCNTs) with ZrO2 nanoparticles demonstrated a 6.6× higher mass activity than commercial Pt/C, retaining 88% of initial activity after 50,000 cycles[120]. Similarly, Pt-Fe alloys embedded in carbon boosted fuel cell performance by 115%, with 89% enhanced durability at 120 °C and 25% RH[122]. Zr-doped Fe-N-C catalysts enhanced ORR activity, maintaining 40% of performance after 100 h, while undoped Fe-N-C lost 70% of activity within 20 h[81]. S-doped Co-N-C catalysts outperformed Pt/C in both alkaline and acidic media, achieving a 0.91 V onset potential and -6.7 mA cm-2 limiting current density, making them strong candidates for PEMFCs[130]. To improve oxygen transport, a perfluoronated ionomer with dioxole segments replaced Nafion, increasing power density by 100 mW cm-2[131]. Additionally, gradient multi-layered CLs with optimized Pt and Nafion distribution enhanced electrochemical surface area (ECSA) and efficiency[132]. Catalyst degradation remains a challenge due to Pt agglomeration, carbon support corrosion, and proton connectivity loss. Over 650 h, catalyst dissolution increased from 23% to 46%, highlighting the need for improved support structures[133]. Pt-alloy catalysts (PtFe, PtCo, PtNi) embedded in carbon mitigated degradation by reducing oxygen adsorption strength, improving stability[134]. Enhancing mass transport and active site accessibility has led to nitrogen-modified carbon supports, which reduced oxygen transport resistance and improved Pt utilization, increasing current density to 1.11 A cm-2 at
Performances of PEMFCs employing several reported advanced PEMs are summarized in Table 2. The integration of advanced PEMs with fuel cell systems requires a holistic approach that encompasses material innovations, system design optimization, and operational strategies. By addressing these challenges, advanced PEMs can enable the widespread adoption of fuel cell technologies across diverse applications, from automotive and stationary power systems to portable energy solutions, contributing significantly to the transition toward a sustainable energy future.
Performances of several PEMs
| Membrane | Catalyst | Operating conditions | Power density (W cm-2) | Refs. |
| Poly(dibenzofuran isatin) (PBFI) with glycidyl Trimethyl ammonium chloride/PA | Pt/C | Ambient pressure within the temperature range 120-180 °C | 0.876 (180 °C) | [137] |
| Poly(terphenyl piperidine) membranes | Not specified | H2-O2 fuel cell at 180 °C | 0.655 | [138] |
| Sulfonyl imide-based poly(benzoyl diphenyl benzene) (SI-PBDPB) | Carbon-supported Pt | H2/air at 70 °C, stoichiometry 1.5/2.0, 100% RH | 0.63 | [139] |
| Sulfide-linked sulfonated poly(triphenylene pentafluorophenyl) | Pt/C | Single H2/air fuel cell, at 30% RH and 80 °C | 0.407 | [140] |
| PVP/cPIM-1 composite membrane | Pt/C ink | 160 °C unhumidified | 1.09 (160 °C) | [141] |
| HPW@MOF-808/ poly (terphenyl piperidine) (PTP)/PA | Pt/C | 160 °C | 0.862 | [142] |
| Triazine-rich COF (EB-COF-1) in poly [2,2’-(p-oxydiph-enylene)-5,5′-benzimidazole (OPBI) matrix | Pt | Anhydrous, 80 to 180 °C | 0.768 (180 °C) | [143] |
| 2-isocyanatopyridine-modified hydroxy-PBI with quaternary ammonium polymeric ionic liquid | Pt/C | 180 °C, 0% RH | 0.838 | [144] |
| Prussian Blue (PB) in sulfonated poly(ether ketone) (SPEEK) matrix | Pt/C | 100% RH | 2.078 (60 °C) | [145] |
| Monolayer graphene/Nafion | ~80 °C, ~150-250 kPa-abs with H2/air | ~0.57-0.63 | [146] | |
| Hyperbranched poly(benzyl-triptycene) PBT with SnP2O7 hydrogen bond networks | Pt/C | 220 °C, dry H2/O2 | 0.75 | [147] |
| Sulfonated PEEK with gadolinium zirconium oxide (Gd2Zr2O7) and carbon nitride (C3N4) | 110 °C, 15% RH | 0.315 | [48] | |
| Cross-linked carboxylated-acryl amido propane sulfonic acid (C-AMPSA) with PVA and MoS2 nanosheets | - | > 0.7 | [148] | |
| (3-mercaptopropyl) trimethoxysilane-graphene oxide and basic amino ligands | Pt/C carbon cloth | 60 °C, 100% RH | 0.172 | [57] |
| PVDF blended with PVP and 1 nm H3PW12O40 (PW12) clusters | Pt/C | 70 °C, 100% RH | 0.343 | [91] |
| Nafion/polynorepinephrine-regulated sulfonated montmorillonite | Pt/C | 80 °C, 100% RH | 1.121 | [149] |
ENVIRONMENTAL AND ECONOMIC CONSIDERATIONS
The environmental and economic aspects of advanced PEM fabrication are crucial for assessing the sustainability and viability of fuel cell technology [Table 3]. As the push for clean energy solutions accelerates, understanding the lifecycle impacts and cost-effectiveness of PEMs becomes increasingly important. These considerations extend beyond material synthesis and operation, encompassing durability, recyclability, and long-term environmental implications.
Environmental and economic aspects of PEM fabrication
| Aspect | Key findings | Refs. |
| Material sustainability | PFSA-based membranes such as Nafion have high proton conductivity but significant environmental footprints. Alternatives such as sulfonated poly(arylene ether nitrile) offer lower impact | [9,150] |
| Lifecycle analysis (LCA) | HT-PEMFCs provide 40% more electricity output per unit environmental burden compared to Stirling engine-based μ-CHP plants | [151] |
| Energy system comparison | PEMFC-based systems reduce greenhouse gas emissions by 76% compared to gas condensing boilers, but contribute 117% more to abiotic depletion potential due to reliance on PGMs | [152] |
| Durability improvement | Increasing PEM lifespan can cut lifecycle greenhouse gas emissions by up to 13%. Switching from PTFE to FEP coatings in gas diffusion media can reduce emissions per driven km by 7%-32% | [153] |
| Fuel type comparison | Diesel and hydrogen-fueled PEMFCs emit similar CO2-eq (1,010.2 vs. 1,001.1 g/kWh), but hydrogen-based PEMFCs have zero direct emissions, making fuel transport a key concern | [154] |
| Economic feasibility | High material costs and fabrication complexity hinder commercial adoption. MWCNT-supported platinum catalysts offer cost reduction while maintaining performance | [155] |
| Recycling and end-of-life management | PGMs and carbon-based components contribute significantly to environmental impact. Recycling strategies can reduce the burden and improve sustainability | [151] |
Producing PEMs, particularly PFSA-based membranes such as Nafion, requires energy-intensive processes with notable environmental footprints. Despite their high proton conductivity and widespread use, their complex and costly production raises sustainability concerns[150]. To address this, alternative materials such as sulfonated poly(arylene ether nitrile) membranes offer comparable conductivity with potentially lower environmental impacts[9].
Lifecycle analysis (LCA) plays a critical role in evaluating the environmental footprint of PEMFCs. For instance, research has shown that high-temperature PEMFCs (HT-PEMFCs) can achieve environmental performance comparable to Stirling engine-based micro-combined heat and power (μ-CHP) plants while delivering 40% more electricity output per unit of environmental burden[151]. Additionally, integrating MWCNTs as a carbon substrate for platinum catalysts has shown promise, contributing only 1% of the total MEA mass, whereas bipolar flow plates remain the dominant component at 52.2%[151]
The benefits of PEMFCs extend to residential energy systems. A comparative LCA between a PEMFC-based energy system and a traditional gas condensing boiler (GCB) with grid electricity mix showed a 76% reduction in greenhouse gas emissions, lowering emissions from 65,607 kg CO2-eq to 15,510 kg CO2-eq[152]. However, PEMFCs contribute 117% more to abiotic depletion potential (ADP) due to the reliance on platinum-group metals (PGMs), emphasizing the need for material optimization.
Extending the durability of PEMFCs is crucial for reducing their environmental footprint. Studies have shown that increasing PEM longevity can cut lifecycle greenhouse gas emissions by up to 13%, as greater durability translates to reduced hydrogen consumption and longer vehicle lifespan[153]. Additionally, modifying gas diffusion medium (GDM) coatings - switching from PTFE to fluorinated ethylene propylene (FEP) - can decrease global warming potential per driven kilometer by 7% to 32%, depending on hydrogen production methods.
Fuel choice also plays a key role in sustainability. A comparison between diesel-fueled and hydrogen-fueled PEMFCs found that total greenhouse gas emissions were nearly identical, with diesel PEMFCs emitting 1,010.2 g CO2-eq/kWh and hydrogen PEMFCs emitting 1,001.1 g CO2-eq/kWh[154]. However, while hydrogen-based PEMFCs produce zero direct emissions during operation, diesel PEMFCs are burdened by combustion-related emissions[154]. Hydrogen transport also presents challenges, as long-distance transportation increases emissions significantly.
The economic feasibility of advanced PEMs remains a major consideration, as high material costs and complex fabrication processes hinder large-scale adoption. Reducing expenses while maintaining high performance is key to making fuel cell technology commercially viable. Studies highlight the importance of assessing social and economic impacts across the PEMFC lifecycle, including supply chain efficiency and scalability[155]. While platinum remains a cost barrier, MWCNT-supported platinum catalysts offer a pathway for cost reduction while maintaining electrochemical performance[151]. For example, cost issue related to PMFC fabrication has been discussed in ref.[156]. PGMs used in CLs contribute to 61% of total fuel cell stack costs[157-159]. The U.S. Department of Energy (DOE) has recommended reducing PGM loading to below 0.10 mgPGM/cm2[160]. Although membranes constitute only about 9% of the cost and GDLs 3%, their functional importance is far greater. A failure in the membrane can render the entire system inoperative, making membrane performance a crucial factor in cost efficiency. MEAs - which include the CL, membrane, GDL, and gaskets - represent nearly 79% of the fuel cell stack cost, emphasizing the importance of manufacturing quality and robustness[158,161]. Meanwhile, the MEA’s dominance in overall fuel cell cost is further evident at production scales, where it contributes approximately 49% of the system’s list price, primarily due to material costs - which make up 56% of MEA expenses, versus only 5% for manufacturing. Critical material inputs such as the catalyst, membrane, decal sheets, and GDL are added during key stages such as mixing and hot pressing, which are identified as cost-intensive steps[162].
Recycling and end-of-life management are essential for improving the sustainability of PEM technology. PGMs and carbon-based components, though a small fraction of the total PEMFC mass, account for a disproportionate share of the environmental impact. Efficient recycling strategies for these materials can significantly reduce the environmental burden of PEMFCs[151].
The sustainability of PEM technology depends on continuous advancements in material innovation, cost-effective manufacturing, and comprehensive lifecycle management. Developing low-cost, environmentally friendly production methods while incorporating circular economy principles will be crucial in driving PEM adoption. As fuel cells become integral to the hydrogen economy, ensuring a balance between technological progress, environmental responsibility, and economic feasibility will be key to their long-term impact. Enhancing membrane durability, optimizing hydrogen transport strategies, and improving recycling methods will be essential in minimizing the environmental footprint of PEMFCs while maintaining scalability and cost-effectiveness.
FUTURE DIRECTIONS IN PEM RESEARCH
The future of PEM research is focused on overcoming key challenges related to performance, durability, and cost-effectiveness [Figure 7]. As the demand for clean energy solutions grows, innovations in PEM materials and system integration will be instrumental in advancing fuel cell commercialization. Research efforts are concentrated on material enhancements, durability improvements, integration of advanced technologies, and optimizing operational parameters to ensure efficient and sustainable fuel cell applications.
Developing advanced materials is at the forefront of PEM research. Hydrocarbon-based polymers such as polybenzimidazoles (PBIs) and sulfonated aromatic polymers are emerging as alternatives to PFSA membranes due to their superior thermal and chemical stability. For instance, studies have shown that low-sulfonation aromatic polymer membranes improve performance in decentralized power generation[163]. Additionally, graft-type polymer electrolyte membranes with controlled grafting degrees exhibit promising thermal stability and conductivity[164]. These innovations could lead to robust PEMs capable of functioning under diverse conditions.
Graphene and MOFs are gaining attention for their ability to enhance proton conductivity and stability. Research indicates that graphene-based materials significantly improve electrode kinetics, leading to better overall fuel cell performance[165]. Similarly, flexible MOFs demonstrate superior proton conductivity and structural stability, making them attractive for advanced PEM applications[166]. Incorporating these materials into PEM designs could produce highly efficient and durable membranes suitable for various fuel cell systems.
Fine-tuning operational conditions is another crucial area of research. Studies highlight the importance of optimizing temperature and humidification strategies to enhance PEM performance and longevity[167]. While higher temperatures accelerate electrochemical reactions, they also increase the risk of membrane dehydration, requiring a delicate balance. Effective water management is also essential, as humidity levels significantly impact membrane efficiency, particularly in high-temperature applications[168].
Innovations in flow channel design are playing a critical role in improving PEM efficiency. Optimized flow channel geometries enhance water management and overall system performance[94]. Researchers suggest that modifying flow channel structures can further boost both durability and efficiency, supporting the long-term viability of fuel cells[169]. These design improvements are crucial for maximizing the potential of PEM technology.
Addressing membrane degradation is essential for extending PEM lifespan. Researchers are developing self-healing materials and oxidative-resistant membranes to mitigate mechanical and chemical wear. Computational modeling is also becoming a valuable tool, providing insights into degradation mechanisms and predicting performance under different conditions[170]. These efforts are critical for ensuring long-term fuel cell operation.
As PEM research advances, sustainability remains a key focus. Green manufacturing processes, recyclable materials, and cost-effective production methods are essential for reducing environmental impact and increasing accessibility. Researchers are also exploring non-hydrogen applications, such as ammonia-fed fuel cells and electrochemical carbon dioxide reduction, expanding PEM use beyond traditional energy systems.
Multidisciplinary research combining materials science, system optimization, and computational modeling is driving the development of high-performance, durable, and sustainable PEMFCs. By addressing material properties, system design, and operational efficiency, next-generation PEMs will play a significant role in the global transition to clean energy.
CONCLUSIONS AND OUTLOOK
PEMs are key components in the operation of fuel cells, directly influencing efficiency, durability, and overall system performance. Recent advances in PEM materials - particularly hydrocarbon-based polymers, inorganic-organic hybrids, and nanostructured composites - have significantly improved key membrane properties such as proton conductivity, thermal stability, and mechanical strength. High-temperature-operable membranes have shown great potential in overcoming the limitations of conventional Nafion-based membranes. The integration of functional fillers and surface-modified architectures has further enhanced conductivity and dimensional stability, enabling more reliable operation under diverse and demanding conditions.
Addressing PEMs degradation is essential for long-term fuel cell performance. Effective strategies such as the incorporation of radical scavengers, use of reinforced membrane architectures, and improvement of interfacial bonding within MEAs have contributed to notable gains in chemical and mechanical stability. System-level innovations, including optimized flow field designs and controlled humidification, are equally critical for mitigating dehydration and flooding, both of which accelerate membrane failure. These efforts are complemented by advances in catalyst design, where Pt-alloys and non-precious metal alternatives improve activity and reduce cost without compromising durability. Together, these developments highlight the importance of integrating materials engineering with operational optimization in PEMFC system design.
Looking ahead, the future of PEM technology depends on achieving a balance between performance, cost, and environmental impact. Reducing reliance on perfluorinated materials and PGMs, improving membrane recyclability, and minimizing production energy demands are crucial for sustainable scale-up. Research must continue to emphasize multifunctional membrane platforms capable of meeting the requirements of hybrid systems and variable operating environments. As fuel cell technologies advance, PEMs will play a central role in enabling clean, efficient, and economically viable energy systems. Continued progress will rely on interdisciplinary collaboration and innovation across various subjects or fields.
DECLARATIONS
Authors’ contributions
Writing original draft: Khoiruddin, K.; Wenten, I. G.
Writing, review & editing: Nandar, C. S. A.; Kawi, S.; Lim, T. M.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by Institut Teknologi Bandung.
Conflicts of interest
Kawi S. is an Editorial Board Member of the journal Energy Materials but is not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision-making, while the other authors have declared that they have no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Kong, F.; Ren, H. Advances in green energy, environment and carbon neutralization. Energies 2025, 18, 1016.
2. Zhang, Q.; Suresh, L.; Liang, Q.; et al. Emerging technologies for green energy conversion and storage. Adv. Sustain. Syst. 2021, 5, 2000152.
3. Wenten, I. G.; Khoiruddin, K.; Siagian, U. W. R. Green energy technologies: a key driver in carbon emission reduction. J. Eng. Technol. Sci. 2024, 56, 143-92.
4. Zhang, G.; Qu, Z.; Tao, W.; et al. Advancing next-generation proton-exchange membrane fuel cell development in multi-physics transfer. Joule 2024, 8, 45-63.
5. Cai, F.; Cai, S.; Tu, Z. Proton exchange membrane fuel cell (PEMFC) operation in high current density (HCD): problem, progress and perspective. Energy. Convers. Manag. 2024, 307, 118348.
6. Asghar, M. R.; Xu, Q. A review of advancements in commercial and non-commercial Nafion-based proton exchange membranes for direct methanol fuel cells. J. Polym. Res. 2024, 31, 3964.
7. Rehman Asghar, M.; Divya, K.; Su, H.; Xu, Q. Advancement of PVDF and its copolymer-based proton exchange membranes for direct methanol fuel cells: a review. Eur. Polym. J. 2024, 213, 113110.
8. Zunita, M.; Raizki, A.; Aditya, R.; Wenten, I. G. Proton exchange polyionic liquid-based membrane fuel cell applications. Results. Eng. 2022, 16, 100653.
9. Feng, M.; Ma, Y.; Chang, J.; et al. Sulfonated poly(arylene ether nitrile)-based composite membranes enhanced with Ca2+ bridged carbon nanotube-graphene oxide networks. J. Inorg. Organomet. Polym. 2022, 32, 2103-12.
10. Ou, Y.; Tsen, W.; Gong, C.; et al. Chitosan-based composite membranes containing chitosan-coated carbon nanotubes for polymer electrolyte membranes. Polym. Adv. Technol. 2018, 29, 612-22.
11. Bae, J. Control of microdomain orientation in block copolymer thin films by electric field for proton exchange membrane. Adv. Chem. Eng. Sci. 2014, 04, 95-102.
13. Borup, R.; Meyers, J.; Pivovar, B.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904-51.
14. Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-free catalysts for proton exchange membrane fuel cells: stability challenges and material solutions. Adv. Mater. 2021, 33, e1908232.
15. Li, Y.; Han, Y.; Zhan, J. Uniformity analysis in different flow-field configurations of proton exchange membrane fuel cell. J. Fuel. Cell. Sci. Technol. 2013, 10, 031003.
16. Jiao, K.; Xuan, J.; Du, Q.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361-9.
17. Amano, F.; Tsushiro, K. Proton exchange membrane photoelectrochemical cell for water splitting under vapor feeding. Energy. Mater. 2024, 4, 400006.
18. Pourzare, K.; Zargar, M.; Farhadi, S.; Hassani Sadrabadi, M. M.; Mansourpanah, Y. Aminosilica-functionalized Co3O4 nanostructures in proton exchange mixed matrix membranes for enhanced separation efficiency of direct methanol fuel cells. ACS. Appl. Nano. Mater. 2023, 6, 296-304.
19. Bai, X.; Cao, L.; Ji, C.; et al. Ultra-high proton conductivity iHOF based on guanidinium arylphosphonate for proton exchange membrane fuel cells. Chem. Mater. 2023, 35, 3172-80.
20. Tsushima, S.; Teranishi, K.; Hirai, S. Experimental elucidation of proton conducting mechanism in a polymer electrolyte membrane of fuel cell by nuclei labeling MRI. ECS. Trans. 2006, 3, 91-6.
21. Ogawa, T.; Kamiguchi, K.; Tamaki, T.; Imai, H.; Yamaguchi, T. Differentiating Grotthuss proton conduction mechanisms by nuclear magnetic resonance spectroscopic analysis of frozen samples. Anal. Chem. 2014, 86, 9362-6.
22. Wang, M.; Wang, L.; Deng, N.; et al. Electrospun multi-scale nanofiber network: hierarchical proton-conducting channels in Nafion composite proton exchange membranes. Cellulose 2021, 28, 6567-85.
23. Wang, L.; Deng, N.; Wang, G.; Ju, J.; Cheng, B.; Kang, W. Constructing amino-functionalized flower-like metal-organic framework nanofibers in sulfonated poly(ether sulfone) proton exchange membrane for simultaneously enhancing interface compatibility and proton conduction. ACS. Appl. Mater. Interfaces. 2019, 11, 39979-90.
24. Yao, Y.; Ji, L.; Lin, Z.; et al. Sulfonated polystyrene fiber network-induced hybrid proton exchange membranes. ACS. Appl. Mater. Interfaces. 2011, 3, 3732-7.
25. Li, J.; Wang, J.; Wu, Z.; Tao, S.; Jiang, D. Ultrafast and stable proton conduction in polybenzimidazole covalent organic frameworks via confinement and activation. Angew. Chem. Int. Ed. 2021, 60, 12918-23.
26. Zhang, Y. P.; Yue, M. Z.; Chen, Y. Proton exchange membrane based on sulfonated polyimide for fuel cells: state-of-the-art and recent developments. Adv. Mat. Res. 2011, 239-42, 3032-8.
27. Chen, X.; Wang, T.; Shi, C.; et al. Preparation and characterization of phosphoric acid doped polyacrylamide/β-cyclodextrin high-temperature proton exchange membrane. Macro. Chem. Phys. 2022, 223, 2200006.
28. Wang, H.; Yang, G.; Li, S.; Shen, Q.; Li, Y.; Wang, R. Pore-scale modeling of liquid water transport in compressed gas diffusion layer of proton exchange membrane fuel cells considering fiber anisotropy. Membranes 2023, 13, 559.
29. Jiang, Z.; Meng, Y.; Jiang, Z.; Shi, Y. Preparation of highly sulfonated ultra-thin proton-exchange polymer membranes for proton exchange membrane fuel cells. Surf. Rev. Lett. 2009, 16, 297-302.
30. Zheng, W.; Wang, L.; Deng, F.; et al. Durable and self-hydrating tungsten carbide-based composite polymer electrolyte membrane fuel cells. Nat. Commun. 2017, 8, 418.
31. Lin, H. D.; Yang, X. Y.; Sun, C. X. Characterization of multiblock sulfonated poly(arylene ether sulfone) as proton exchange membranes. Adv. Mat. Res. 2013, 805-6, 1321-4.
32. Lu, S.; Wang, D.; Jiang, S. P.; Xiang, Y.; Lu, J.; Zeng, J. HPW/MCM-41 phosphotungstic acid/mesoporous silica composites as novel proton-exchange membranes for elevated-temperature fuel cells. Adv. Mater. 2010, 22, 971-6.
33. Shi, N.; Wang, G.; Wang, Q.; Wang, L.; Li, Q.; Yang, J. Acid doped branched poly(biphenyl pyridine) membranes for high temperature proton exchange membrane fuel cells and vanadium redox flow batteries. Chem. Eng. J. 2024, 489, 151121.
34. Lv, R.; Jin, S.; Li, L.; et al. The influence of comonomer structure on properties of poly(aromatic pyridine) copolymer membranes for HT-PEMFCs. J. Membr. Sci. 2024, 701, 122703.
35. Gunterman, H. P.; Kwong, A.; Gostick, J. T.; Kusoglu, A.; Weber, A. Z. Water uptake in PEMFC catalyst layers. ECS. Trans. 2011, 41, 647-50.
36. Ortiz Sainz De Aja, A.; Díaz Vejo, M.; Ortiz Uribe, I. Proton exchange membranes based on polymeric ionic liquids for fuel cell applications. ECS. Trans. 2016, 75, 589-96.
37. Huang, H.; Zhong, Z.; Li, J.; Li, H. A manganese-doped cerium-based metal-organic framework as a radical scavenger for proton exchange membrane fuel cells with superior stability. ACS. Appl. Energy. Mater. 2024, 7, 10804-14.
38. Pahon, E.; Jemei, S.; Steiner, N. Y.; Hissel, D. Effect of load cycling on the performance of fuel cell stacks; In 2019 IEEE Vehicle Power and Propulsion Conference (VPPC); 2019, pp. 1-4.
39. Zhang, X.; Yang, Y.; Zhang, X.; Guo, L.; Liu, H. Performance degradation of proton exchange membrane fuel cell caused by an accelerated stress test. Fuel. Cells. 2019, 19, 160-8.
40. Tsushima, S.; Kaneko, K.; Hirai, S. Two-stage degradation of PEMFC performance due to sulfur dioxide contamination. ECS. Trans. 2010, 33, 1645-52.
41. Chen, S.; Hao, M.; Hu, Y.; Liu, K.; Li, Y. Insight into the evolution of membrane chemical degradation in proton exchange membrane fuel cells: from theoretical analysis to model developing. J. Power. Sources. 2024, 599, 234238.
42. Tsuneda, T.; Singh, R. K.; Iiyama, A.; Miyatake, K. Theoretical investigation of the H2O2-induced degradation mechanism of hydrated nafion membrane via ether-linkage dissociation. ACS. Omega. 2017, 2, 4053-64.
43. Ghelichi, M.; Melchy, PÉ.; Eikerling, M. H. Radically coarse-grained approach to the modeling of chemical degradation in fuel cell ionomers. J. Phys. Chem. B. 2014, 118, 11375-86.
44. Liu, Y.; Du, M.; Li, Z.; Cheng, Y.; Shi, L. Molecular dynamics study on swelling and exfoliation properties of montmorillonite nanosheets for application as proton exchange membranes. ACS. Appl. Nano. Mater. 2023, 6, 2133-40.
45. Mader, J. A.; Benicewicz, B. C. Sulfonated polybenzimidazoles for high temperature PEM fuel cells. Macromolecules 2010, 43, 6706-15.
46. Khoiruddin, K.; Kadja, G.; Wenten, I. Sustainable membranes with FNMs for energy generation and fuel cells. In: Membranes with functionalized nanomaterials; 2022. pp. 245-74.
47. Akli, K.; Khoiruddin, K.; Wenten, I. G. Preparation and characterization of heterogeneous PVC-silica proton exchange membrane. J. Membr. Sci. Res. 2016, 2, 141-6.
48. Vinothkannan, M.; Gikunoo, E. K.; Shanmugam, S. Toward extended durability and power output of high temperature proton exchange membrane fuel cells with Gd2Zr2O7-C3N4 composite membrane. Ionics 2025.
49. Wang, H.; Zhao, Y.; Shao, Z.; et al. Proton conduction of nafion hybrid membranes promoted by NH3-modified Zn-MOF with host-guest collaborative hydrogen bonds for H2/O2 fuel cell applications. ACS. Appl. Mater. Interfaces. 2021, 13, 7485-97.
50. Sulaiman, R. R. R.; Walvekar, R.; Wong, W. Y.; Khalid, M.; Pang, M. M. Proton conductivity enhancement at high temperature on polybenzimidazole membrane electrolyte with acid-functionalized graphene oxide fillers. Membranes 2022, 12, 344.
51. Ling, Z.; Wang, B.; Liu, Q.; et al. In-situ strategies for melamine-functionalized graphene oxide nanosheets-based nanocomposite proton exchange membranes in wide-temperature range applications. J. Colloid. Interface. Sci. 2025, 678, 388-99.
52. Zhai, S.; Lu, Z.; Ai, Y.; et al. High performance nanocomposite proton exchange membranes based on the nanohybrids formed by chemically bonding phosphotungstic acid with covalent organic frameworks. J. Power. Sources. 2023, 554, 232332.
53. Liu, J.; Wang, S.; Wang, L. Constructing high-performance proton transport channels in high-temperature proton exchange membranes by introducing triazole groups. ACS. Appl. Energy. Mater. 2021, 4, 10263-72.
54. Yang, J.; Lin, J.; Sun, S.; Li, X.; Liu, L.; Wang, C. Multidimensional network of polypyrrole nanotubes loaded with ZIF-67 to construct multiple proton transport channels in composite proton exchange membranes for fuel cells. J. Mater. Sci. Technol. 2023, 152, 75-85.
55. Wu, L.; Zhou, D.; Wang, H.; Pan, Q.; Ran, J.; Xu, T. Ionically cross-linked proton conducting membranes for fuel cells. Fuel. Cells. 2015, 15, 189-95.
56. Lan, Y.; Cheng, C.; Zhang, S.; et al. Plasma-induced styrene grafting onto the surface of polytetrafluoroethylene powder for proton exchange membrane application. Plasma. Sci. Technol. 2011, 13, 604-7.
57. Chowdury, M. S. K.; Park, Y. J.; Jeong, S. M.; Park, S. B.; Park, Y. Enhanced proton transfer in proton exchange membrane fuel cells via novel nanocomposite membrane incorporating (3-Mercaptopropyl)trimethoxysilane-graphene oxide and basic amino ligands: a synergistic acid-base approach. Electrochim. Acta. 2024, 507, 145197.
58. Zhao, Y.; Gao, Q.; Xu, X.; et al. Compromise mechanism of proton transfer in crown ether-based biomimetic proton exchange membranes: Insights from molecular dynamics simulations. J. Membr. Sci. 2025, 715, 123456.
59. Nasim, F.; Ali, H.; Nadeem, M. A. CoOx-CoP/nitrogen-doped tubular-carbon nanostructures supported over ceria nanorods as an efficient scavenger in the electrocatalytic oxygen reduction reaction. ACS. Appl. Eng. Mater. 2023, 1, 3379-88.
60. Liu, L.; Fu, Z.; Xing, Y.; et al. Double-layer ePTFE-reinforced membrane electrode assemblies prepared by a reverse membrane deposition process for high-performance and durable proton exchange membrane fuel cells. ACS. Appl. Mater. Interfaces. 2023, 15, 30281-93.
61. Zhang, L.; Liu, M.; Zhu, D.; et al. Double cross-linked 3D layered PBI proton exchange membranes for stable fuel cell performance above 200 °C. Nat. Commun. 2024, 15, 3409.
62. Kang, J.; Kim, J. Membrane electrode assembly degradation by dry/wet gas on a PEM fuel cell. Int. J. Hydrogen. Energy. 2010, 35, 13125-30.
63. Hong, K.; Li, S.; Zhu, K.; et al. Effects of relative humidification on durability of membrane electrode assembly of proton exchange membrane fuel cells. J. Electrochem. Soc. 2021, 168, 064507.
64. Farooqui, U.; Ahmad, A.; Hamid, N. Graphene oxide: a promising membrane material for fuel cells. Renew. Sustain. Energy. Rev. 2018, 82, 714-33.
65. Zhao, Z.; Liu, Z.; Zhang, A.; et al. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 2022, 17, 968-75.
66. Zhang, Z.; Yang, D.; Yao, H.; Chu, T.; Li, B. Investigation of performance degradation and control strategies of PEMFC under three typical operating conditions. J. Electrochem. Soc. 2024, 171, 054510.
67. Pang, X.; Shi, B.; Liu, Y.; et al. Confining phosphoric acid in quaternized COF channels for ultra-stable and fast anhydrous proton transport. Angew. Chem. Int. Ed. 2025, 64, e202423458.
68. Park, M. J.; Kim, S. Y. Ion transport in sulfonated polymers. J. Polym. Sci. B. Polym. Phys. 2013, 51, 481-93.
69. Wu, B.; Choo, H. L.; Ng, W. K.; Pang, M. M.; Yoon, L. W.; Wong, W. Y. Phosphoric acid electrolyte uptake and retention analysis on UiO-66-NH2 polybenzimidazole nanocomposite membranes. Fuel. Cells. 2025, 25, e202400045.
70. Liu, F.; Wang, S.; Chen, H.; et al. Cross-linkable polymeric ionic liquid improve phosphoric acid retention and long-term conductivity stability in polybenzimidazole based PEMs. ACS. Sustain. Chem. Eng. 2018, 6, 16352-62.
71. Xu, X. Q.; Cao, L. H.; Yang, Y.; Zhao, F.; Bai, X. T.; Zang, S. Q. Hybrid Nafion membranes of ionic hydrogen-bonded organic framework materials for proton conduction and PEMFC applications. ACS. Appl. Mater. Interfaces. 2021, 13, 56566-74.
72. Wang, R.; Li, M. Experiment and simulation studies on SPEEK PEM with different sulfonation degrees. IOP. Conf. Ser. Earth. Environ. Sci. 2017, 61, 012026.
73. Yang, S.; Liu, W.; Zhang, Y.; et al. A post-modified donor-acceptor covalent organic framework for enhanced photocatalytic H2 production and high proton transport. J. Mater. Chem. A. 2024, 12, 28161-9.
74. Tao, H.; Yang, K.; Wang, B.; et al. Numerical study of gas crossover effect on hydrogen-oxygen proton exchange membrane fuel cell. Int. J. Heat. Mass. Transfer. 2024, 234, 126060.
75. Xu, W.; Wang, Y.; Wu, Y.; et al. Sub-2-nm channels within covalent triazine framework enable fast proton-selective transport in flow battery membrane. Adv. Funct. Mater. 2023, 33, 2300138.
76. Liu, L.; Ma, Y.; Li, B.; et al. Continuous ultrathin zwitterionic covalent organic framework membrane via surface-initiated polymerization toward superior water retention. Small 2024, 20, e2308499.
77. Yang, X.; Zhao, L.; Goh, K.; Sui, X.; Meng, L.; Wang, Z. Ultra-high ion selectivity of a modified nafion composite membrane for vanadium redox flow battery by incorporation of phosphotungstic acid coupled UiO-66-NH2. ChemistrySelect 2019, 4, 4633-41.
78. Zhao, Y.; Li, X.; Wang, Z.; Xie, X.; Qian, W. Preparation of graft poly(arylene ether sulfone)s-based copolymer with enhanced phase-separated morphology as proton exchange membranes via atom transfer radical polymerization. Polymers 2019, 11, 1297.
79. Jeong, S.; Ohto, T.; Nishiuchi, T.; Nagata, Y.; Fujita, J. I.; Ito, Y. Suppression of methanol and formate crossover through sulfanilic-functionalized holey graphene as proton exchange membranes. Adv. Sci. 2023, 10, e2304082.
80. Liu, X.; Li, Y.; Xue, J.; et al. Magnetic field alignment of stable proton-conducting channels in an electrolyte membrane. Nat. Commun. 2019, 10, 842.
81. Chi, B.; Zhang, L.; Yang, X.; et al. Promoting ZIF-8-derived Fe-N-C oxygen reduction catalysts via Zr doping in proton exchange membrane fuel cells: durability and activity enhancements. ACS. Catal. 2023, 13, 4221-30.
82. Liu, N.; Bi, S.; Liu, J.; et al. PA-doped nanofiber composite proton exchange membranes with ultrahigh proton conductivity and methanol barrier performance for direct methanol fuel cells. Sep. Purif. Technol. 2025, 358, 130313.
83. Schonvogel, D.; Belack, J.; Vidakovic, J.; et al. Performance and durability of high temperature proton exchange membrane fuel cells with silicon carbide filled polybenzimidazole composite membranes. J. Power. Sources. 2024, 591, 233835.
84. Miyake, J.; Miyatake, K. Fluorine-free sulfonated aromatic polymers as proton exchange membranes. Polym. J. 2017, 49, 487-95.
85. Wang, Y.; Wang, Y.; Su, B.; et al. Preparation and performance analysis of ILs@MoS2 modified polyimide proton exchange membrane. Mater. Sci. Eng. B. 2025, 317, 118163.
86. Lv, S.; Zhang, B.; Wang, L.; et al. Preparation and properties of superprotonic conductor-based mixed matrix proton exchange membranes for energy conversion and storage. J. Membr. Sci. 2025, 730, 124189.
87. Zhang, Q.; Dong, S.; Shao, P.; et al. Covalent organic framework-based porous ionomers for high-performance fuel cells. Science 2022, 378, 181-6.
88. Tang, H.; Geng, K.; Wu, L.; et al. Fuel cells with an operational range of -20 °C to 200 °C enabled by phosphoric acid-doped intrinsically ultramicroporous membranes. Nat. Energy. 2022, 7, 153-62.
89. Wang, X.; Zhao, S.; Wang, S.; et al. Facile preparation of high-performance sulfonated polyimide proton exchange membrane by doping nano carbon sulfonic acid. J. Membr. Sci. 2025, 717, 123605.
90. Li, Q.; Gao, W.; Zhang, N.; Gao, X.; Wu, D.; Che, Q. Preparation of high temperature proton exchange membranes with multilayered structures through alternate deposition of carbon dots@Metal organic framework and Sulfonated Poly(Ether Ketone). J. Membr. Sci. 2025, 713, 123306.
91. Gao, W.; Li, Q.; Gao, X.; Zhang, N.; Wu, D.; Che, Q. Preparation of high temperature proton exchange membrane through covalent organic framework doped polyvinylidene fluoride nanofibers. Int. J. Hydrogen. Energy. 2024, 91, 625-35.
92. Yagizatli, Y.; Ulas, B.; Sahin, A.; Ar, I. Preparation and characterization of SPEEK-PVA blend membrane additives with colloidal silica for proton exchange membrane fuel cell. J. Polym. Environ. 2024, 32, 4699-715.
93. Liu, J.; Ishitobi, H.; Nakagawa, N. Different functional groups cross-linked graphene oxide membranes for proton exchange membrane fuel cell. Int. J. Hydrogen. Energy. 2024, 85, 586-97.
94. Taner, T. A flow channel with Nafion membrane material design of PEM fuel cell. J. Therm. Eng. 2019, 5, 456-68.
95. Wen, Q.; Pan, S.; Li, Y.; et al. Janus gas diffusion layer for enhanced water management in proton exchange membrane fuel cells (PEMFCs). ACS. Energy. Lett. 2022, 7, 3900-9.
96. Sun, Y.; Lin, Y.; Wan, Z.; et al. Water management and performance enhancement in proton exchange membrane fuel cell through metal foam flow field with hierarchical pore structure. Chem. Eng. J. 2024, 494, 152944.
97. Liu, Y.; Bao, Z.; Chen, J.; Lv, F.; Jiao, K. Design of a partially narrowed flow channel with a sub-distribution zone for the water management of large-size proton exchange membrane fuel cells. Energy 2024, 310, 133292.
98. Chen, X.; Yang, C.; Sun, Y.; et al. Water management and structure optimization study of nickel metal foam as flow distributors in proton exchange membrane fuel cell. Appl. Energy. 2022, 309, 118448.
99. Vu, H. N.; Truong Le Tri, D.; Nguyen, H. L.; Kim, Y.; Yu, S. Multifunctional bypass valve for water management and surge protection in a proton-exchange membrane fuel cell supply-air system. Energy 2023, 278, 127696.
100. Pourrahmani, H.; Van Herle, J. The impacts of the gas diffusion layer contact angle on the water management of the proton exchange membrane fuel cells: three-dimensional simulation and optimization. Int. J. Energy. Res. 2022, 46, 16027-40.
101. Xiao, F.; Chen, T.; Zhang, J.; Zhang, S. Water management fault diagnosis for proton-exchange membrane fuel cells based on deep learning methods. Int. J. Hydrogen. Energy. 2023, 48, 28163-73.
102. Ogungbemi, E.; Wilberforce, T.; Ijaodola, O.; Thompson, J.; Olabi, A. Selection of proton exchange membrane fuel cell for transportation. Int. J. Hydrogen. Energy. 2021, 46, 30625-40.
103. Zhang, Y.; Song, Y.; Chen, D.; Jin, Q.; Chen, J.; Cao, Y. Preparation of phosphotungstic acid hybrid proton exchange membranes by constructing proton transport channels for direct methanol fuel cells. Polymer 2023, 265, 125589.
104. Tolj, I.; Penga, Z.; Bosnic, P.; Radica, G. Proton exchange membrane fuel cell flow field configuration: modelling and experimental verification. ECS. Trans. 2022, 108, 143-51.
105. Jin, H.; Zou, S.; Wen, Q.; et al. Performance improvement of air-breathing proton exchange membrane fuel cell (PEMFC) with a condensing-tower-like curved flow field. Chinese. Chem. Lett. 2023, 34, 107441.
106. Binyamin Lim, O. Numerical investigation of tapered flow field configuration to improve mass transport and performance of proton exchange membrane fuel cell. Int. J. Hydrogen. Energy. 2024, 50, 470-91.
107. Wan, Z.; Yan, H.; Sun, Y.; et al. Thermal management improvement of air-cooled proton exchange membrane fuel cell by using metal foam flow field. Appl. Energy. 2023, 333, 120642.
108. Zhu, X.; Zhou, W.; Zhu, Z.; et al. Performance analysis of proton exchange membrane fuel cells with traveling-wave flow fields based on Grey-relational theory. Int. J. Hydrogen. Energy. 2023, 48, 740-56.
109. Rahmani, E.; Moradi, T.; Ghandehariun, S.; Naterer, G. F.; Ranjbar, A. Enhanced mass transfer and water discharge in a proton exchange membrane fuel cell with a raccoon channel flow field. Energy 2023, 264, 126115.
110. Ke, Y.; Zhang, B.; Yuan, W.; et al. Performance enhancement of proton exchange membrane fuel cells with bio-inspired gear-shaped flow channels. Chem. Eng. J. 2023, 474, 145870.
111. Wang, H.; Wang, Z.; Qu, Z.; Zhang, J. Deep-learning accelerating topology optimization of three-dimensional coolant channels for flow and heat transfer in a proton exchange membrane fuel cell. Appl. Energy. 2023, 352, 121889.
112. Asadi, M. R.; Ghasabehi, M.; Ghanbari, S.; Shams, M. The optimization of an innovative interdigitated flow field proton exchange membrane fuel cell by using artificial intelligence. Energy 2024, 290, 130131.
113. Li, N.; Wang, W.; Xu, R.; Zhang, J.; Xu, H. Design of a novel nautilus bionic flow field for proton exchange membrane fuel cell by analyzing performance. Int. J. Heat. Mass. Transfer. 2023, 200, 123517.
114. Chen, C.; Wang, C.; Zhang, Z. Numerical investigation of the water transport and performance of proton exchange membrane fuel cell with an imitating river flow field. Energy. Convers. Manag. 2023, 276, 116532.
115. Zuo, J.; Cadet, C.; Li, Z.; Bérenguer, C.; Outbib, R. A load allocation strategy for stochastically deteriorating multi-stack PEM fuel cells. In: Book of extended abstracts for the 32nd European safety and reliability conference. Research Publishing Services: Singapore; 2022, pp. 1204-11.
116. Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; Del Castillo, L. F.; Compañ, V. Recent progress in the development of composite membranes based on polybenzimidazole for high temperature proton exchange membrane (PEM) fuel cell applications. Polymers 2020, 12, 1861.
117. Mo, S.; Li, Z.; Chen, J.; et al. Hydrogen bond and dipole-dipole interaction enabling ultrastable, quick responding, and self-healing proton exchange membranes for fuel cells. ACS. Omega. 2024, 9, 26316-24.
118. Chen, J.; Bailey, J. J.; Britnell, L.; et al. The performance and durability of high-temperature proton exchange membrane fuel cells enhanced by single-layer graphene. Nano. Energy. 2022, 93, 106829.
119. Huang, H.; Yao, Q.; Zhang, X.; Wang, H. Microporous expanded polytetrafluoroethylene layer functionalized hydrophilic groups for excellent mechanical durability and superior performance in proton exchange membrane fuel cell. J. Power. Sources. 2022, 526, 231130.
120. Huang, M.; Liu, T.; Hou, K.; Sun, F.; Wu, C.; Guan, L. ZrO2 anchored core-shell Pt-Co alloy particles through direct pyrolysis of mixed Pt-Co-Zr salts for improving activity and durability in proton exchange membrane fuel cells. Int. J. Hydrogen. Energy. 2022, 47, 6679-90.
121. Xie, M.; Chu, T.; Wang, X.; et al. Study on durability of proton exchange membrane fuel cell stack based on mesoporous carbon supported platinum catalysts under dynamic cycles conditions. J. Power. Sources. 2023, 553, 232277.
122. Kwon, O.; Kim, J.; Yoo, H.; et al. Cathodic nanoporous CNT functional interlayer as a performance and durability boosting agent for proton exchange membrane fuel cells to operable at 120 °C. Carbon 2022, 199, 51-62.
123. Zhang, W.; Wang, Y. Modification and durability of carbon paper gas diffusion layer in proton exchange membrane fuel cell. Ceram. Int. 2023, 49, 9371-81.
124. Tu, Z.; He, X.; Gao, W.; et al. Enhanced radical scavenging performance of Pt@CeO2 via strong metal-support interaction effect for improving durability of proton exchange membrane fuel cell. Int. J. Hydrogen. Energy. 2024, 50, 41-51.
125. Li, B.; Xie, M.; Wan, K.; et al. A high-durability graphitic black pearl supported Pt catalyst for a proton exchange membrane fuel cell stack. Membranes 2022, 12, 301.
126. Ahmed, H.; Musa, A. Performance investigation of direct oupling advanced alkaline electrolysis and PEMFC system. Solar. Energy. Sustain. Dev. J. 2021, 9.
127. Li, J.; Xi, Z.; Pan, Y. T.; et al. Fe stabilization by intermetallic L10-FePt and Pt catalysis enhancement in L10-FePt/Pt nanoparticles for efficient oxygen reduction reaction in fuel cells. J. Am. Chem. Soc. 2018, 140, 2926-32.
128. Wu, B.; Yang, H.; Li, L.; et al. Integrating PtCo intermetallic with highly graphitized carbon toward durable oxygen electroreduction in proton exchange membrane fuel cells. Adv. Mater. 2025, 37, e2500096.
129. Wu, B.; Xiao, J.; Li, L.; et al. Arranging electronic localization of PtCu nanoalloys to stimulate improved oxygen electroreduction for high-performance fuel cells. CCS. Chem. 2023, 5, 2545-56.
130. Baek, J.; Son, H.; Joo, S. W.; Kim, M.; Lee, G. Bimetallic zeolitic imidazole framework-derived sulfur-doped porous carbon as highly efficient catalysts for oxygen reduction reaction in proton exchange membrane fuel cells. Appl. Surf. Sci. 2024, 642, 158609.
131. Fang, S.; Liu, G.; Li, M.; et al. Tailoring ionomer chemistry for improved oxygen transport in the cathode catalyst layer of proton exchange membrane fuel cells. ACS. Appl. Energy. Mater. 2023, 6, 3590-8.
132. Shin, J.; Son, M.; Kim, S.; Song, S. A.; Lee, D. H. Design of multi-layered gradient catalysts for efficient proton exchange membrane fuel cells. J. Power. Sources. 2023, 582, 233546.
133. He, W.; Tang, F.; Li, X.; Zhang, C.; Ming, P. Quantification and evolution on degradation mechanisms of proton exchange membrane fuel cell catalyst layer under dynamic testing conditions. Int. J. Hydrogen. Energy. 2023, 48, 18032-40.
134. Shin, S.; Lee, E.; Nam, J.; et al. Carbon-embedded Pt alloy cluster catalysts for proton exchange membrane fuel cells. Adv. Energy. Mater. 2024, 14, 2400599.
135. Li, Y.; Wu, Z.; Wang, C.; et al. Engineering triple-phase boundary in Pt Catalyst layers for proton exchange membrane fuel cells. Adv. Funct. Mater. 2024, 34, 2310428.
136. Yang, B.; Yu, H.; Jia, X.; et al. Atomically dispersed isolated Fe-Ce dual-metal-site catalysts for proton-exchange membrane fuel cells. ACS. Appl. Mater. Interfaces. 2023, 15, 23316-27.
137. Wang, Q.; Wang, L.; Zhang, M.; et al. Preparation of novel membranes with multiple hydrogen bonding sites and π-conjugated structure for high temperature proton exchange membrane fuel cells. Chem. Commun. 2024, 60, 5318-21.
138. Shen, Y.; Liu, M.; Mao, Z.; et al. Triazine containing additional N sites to prepare branched poly(terphenyl piperidine) membranes for high-temperature proton exchange membrane fuel cells. J. Membr. Sci. 2025, 731, 124238.
139. Sutradhar, S. C.; Bae, W.; Song, S.; et al. Synthesis and characterization of fluoro sulfonyl imide-based poly(benzoyl diphenyl benzene) membranes for proton exchange membrane fuel cells. Fuel 2025, 390, 134741.
140. Gao, Y.; Mu, T.; Hu, X.; Pang, Y.; Zhao, C. Facile synthesis of all-carbon fluorinated backbone polymers containing sulfide linkage as proton exchange membranes for fuel cells. Chinese. Chem. Lett. 2025, 36, 110763.
141. Yang, X.; Feng, Z.; Alshurafa, M.; et al. Durable proton exchange membrane based on polymers of intrinsic microporosity for fuel cells. Adv. Mater. 2025, 37, e2419534.
142. Liu, M.; Zhou, T.; Han, X.; et al. Polyoxometalates loaded metal-organic frameworks as functional proton conductors for high-temperature proton exchange membrane fuel cells with high performance. J. Membr. Sci. 2025, 724, 123981.
143. Ji, J.; Yang, T.; Zhang, W.; et al. Triazine-rich covalent organic framework composited proton exchange membranes for flexible operating temperature and enhanced long-term stability fuel cells. J. Power. Sources. 2025, 632, 236351.
144. Guan, X.; Wu, W.; Zhang, S.; et al. High hydrogen-bond density polymeric ionic liquid composited high temperature proton exchange membrane with exceptional long-term fuel cell performance. J. Membr. Sci. 2025, 717, 123523.
145. Yu, W.; Cui, Z.; Wang, Q.; et al. Ultra-high Prussian blue loading in SPEEK matrix fabricated by one-step electrostatic spraying as proton exchange membranes for fuel cell. J. Membr. Sci. 2025, 717, 123561.
146. Moehring, N. K.; Mansoor, Basha. A. B.; Chaturvedi, P.; et al. Overcoming the conductance versus crossover trade-off in state-of-the-art proton exchange fuel-cell membranes by incorporating atomically thin chemical vapor deposition graphene. Nano. Lett. 2025, 25, 1165-76.
147. Zeng, L.; Dong, D.; Lu, J.; et al. Hyperbranched interpenetrating hydrogen bond network (HIHBN) proton exchange membrane for fuel cells above 220 °C. Adv. Funct. Mater. 2025.
148. Ganesan, D.; Theerthagiri, S.; Yu, B.; et al. MoS2 nanosheet assisted poly vinyl alcohol cross-linked with carboxylated acryl-amido-2-methyl-1-propanesulfonic acid in medium temperature proton exchange membrane fuel cell application. Compos. Interfaces. 2025, 32, 251-72.
149. Huang, T.; Liu, X.; Ma, R.; et al. Polynorepinephrine-regulated filler sulfonation toward interfacial reformation and conductive promotion of nanocomposite proton exchange membrane. Chem. Eng. J. 2024, 484, 149582.
150. Zhang, X.; Ma, H.; Pei, T.; Zhang, R.; Liu, Y. Anchoring HPW by amino-modified MIL-101(Cr) to improve the properties of SPEEK in proton exchange membranes. J. Appl. Polym. Sci. 2023, 140, e53978.
151. Notter, D. A.; Kouravelou, K.; Karachalios, T.; Daletou, M. K.; Haberland, N. T. Life cycle assessment of PEM FC applications: electric mobility and μ-CHP. Energy. Environ. Sci. 2015, 8, 1969-85.
152. Slotyuk, L.; Part, F.; Schlegel, M.; Akkerman, F. Life cycle assessment of the domestic micro heat and power generation proton exchange membrane fuel cell in comparison with the gas condensing boiler plus electricity from the grid. Sustainability 2024, 16, 2348.
153. Arrigoni, A.; Arosio, V.; Basso, Peressut. A.; Latorrata, S.; Dotelli, G. Greenhouse gas implications of extending the service life of PEM fuel cells for automotive applications: a life cycle assessment. Clean. Technol. 2022, 4, 132-48.
154. Hwang, H.; Yoon, J.; Choi, W.; Barik, D. Mobile proton-exchange membrane fuel cell powered by diesel fuel: system simulation and life cycle analysis. Int. J. Energy. Res. 2023, 2023, 1-22.
155. Bargiacchi, E.; Campos-carriedo, F.; Iribarren, D.; Dufour, J.; Cigolotti, V. Social life cycle assessment of a proton exchange membrane fuel cell stack. E3S. Web. Conf. 2022, 334, 09001.
156. Wang, M.; Peng, F.; Zou, J.; et al. Manufacturing-induced irregularities of membrane electrode assemblies: Impacts on proton exchange membrane fuel cells’ performance and diagnosis. Renew. Sustain. Energy. Rev. 2025, 216, 115707.
157. Aminudin, M.; Kamarudin, S.; Lim, B.; Majilan, E.; Masdar, M.; Shaari, N. An overview: current progress on hydrogen fuel cell vehicles. Int. J. Hydrogen. Energy. 2023, 48, 4371-88.
158. Silverman, T. J.; Huang, H. Solar energy technologies office multi-year program plan. Washington, DC: EERE Publication and Product Library; 2021.
159. Mohideen, M. M.; Liu, Y.; Ramakrishna, S. Recent progress of carbon dots and carbon nanotubes applied in oxygen reduction reaction of fuel cell for transportation. Appl. Energy. 2020, 257, 114027.
161. Fan, L.; Tu, Z.; Chan, S. H. Recent development of hydrogen and fuel cell technologies: a review. Energy. Rep. 2021, 7, 8421-46.
162. Kampker, A.; Heimes, H.; Kehrer, M.; Hagedorn, S.; Reims, P.; Kaul, O. Fuel cell system production cost modeling and analysis. Energy. Rep. 2023, 9, 248-55.
163. Gao, Y.; Zhang, Z.; Zhong, S.; Daneshfar, R.; Ahmadi, M. H. Preparation and application of aromatic polymer proton exchange membrane with low-sulfonation degree. Int. J. Chem. Eng. 2020, 2020, 1-9.
164. Yen, V. T. K.; Hieu, D. T. T.; Hao, L. H.; et al. Characterization of graft-type polymer electrolyte membranes at low grafting degrees for fuel cells. Sci. Technol. Dev. J. 2023, 26, 2799-807.
165. Yadav, R.; Subhash, A.; Chemmenchery, N.; Kandasubramanian, B. Graphene and graphene oxide for fuel cell technology. Ind. Eng. Chem. Res. 2018, 57, 9333-50.
166. Li, Z. H.; Zeng, H.; Zeng, G.; et al. Multivariate synergistic flexible metal-organic frameworks with superproton conductivity for direct methanol fuel cells. Angew. Chem. Int. Ed. 2021, 60, 26577-81.
167. San FG, Dursun S, Yazici MS. Optimization of the PEMFC operating parameters for cathode in the presence of PtCo/CVD graphene using factorial design. Int. J. Energy. Res. 2019, 43, 4506-19.
168. Zhou, F.; Singdeo, D.; Kær, S. K. Investigation of the effect of humidity level of H2 on cell performance of a HT-PEM fuel cell. Fuel. Cells. 2019, 19, 2-9.
169. Kannan, V. M.; Karthikeyan, M.; Karthikeyan, A. P.; Naresh, S. Flow channel modification of PEM fuel cells: a review of approaches to enhance performance and durability. J. Eng. App. Sci. Technol. 2023, 5, 1-3.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data






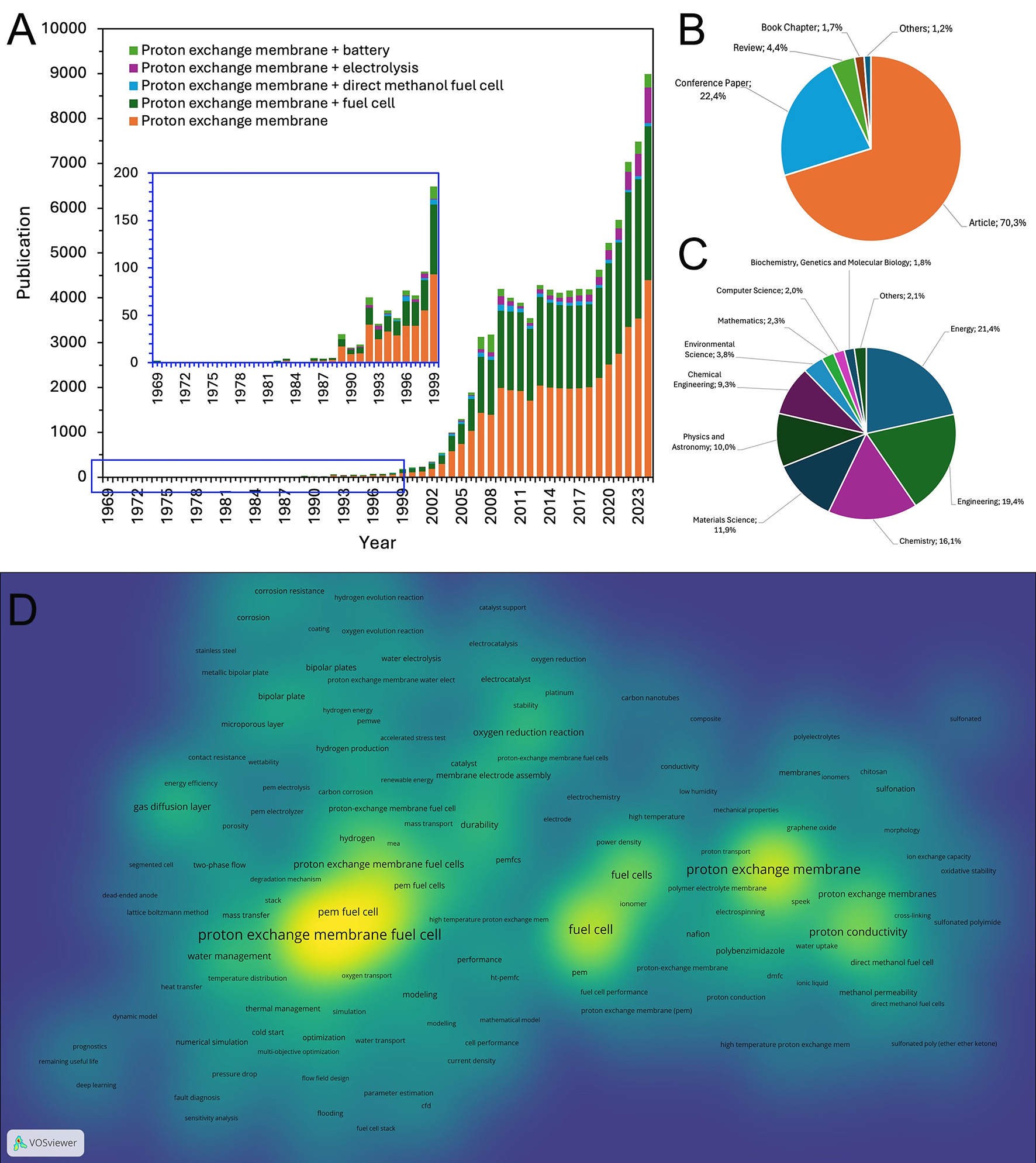
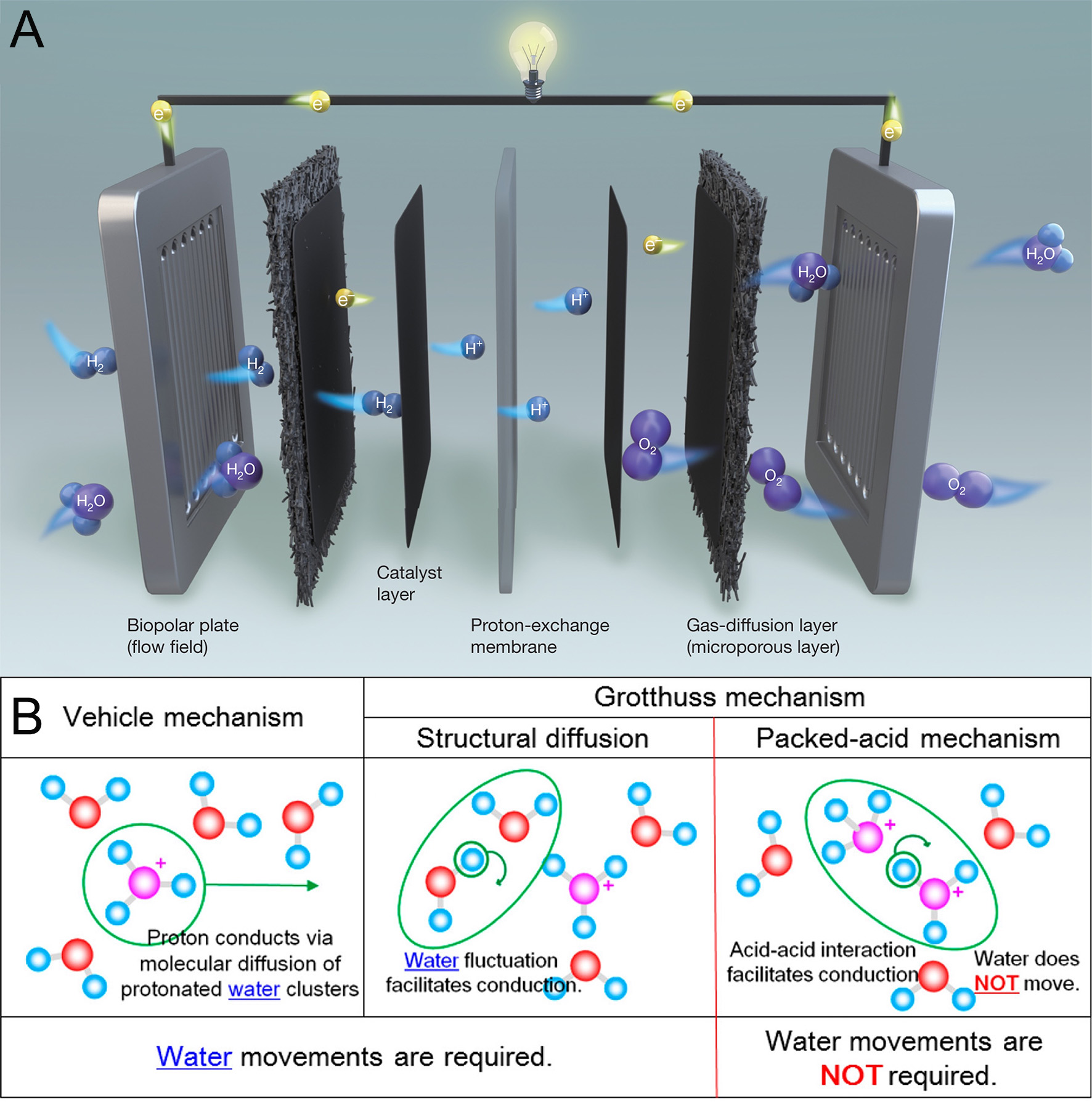
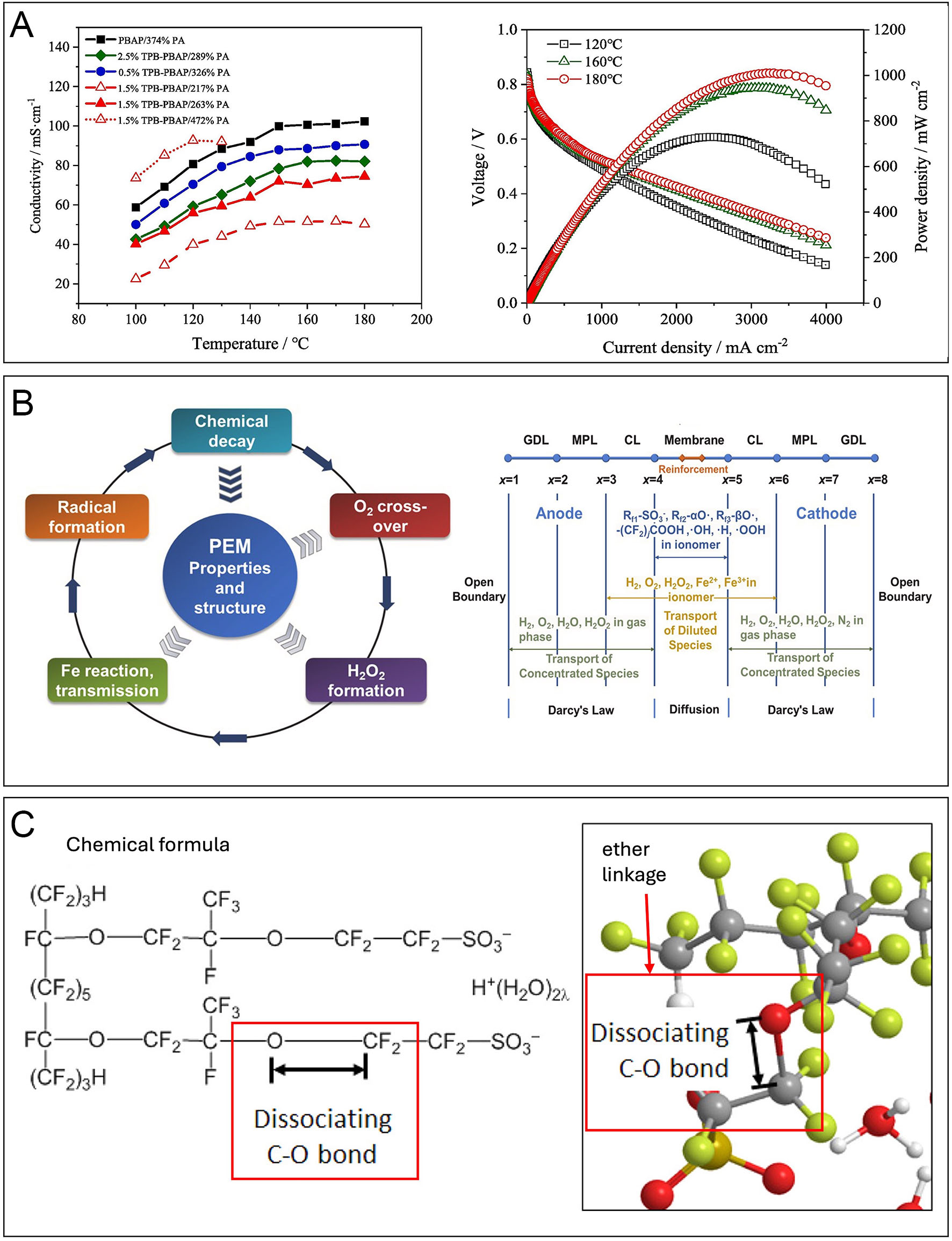
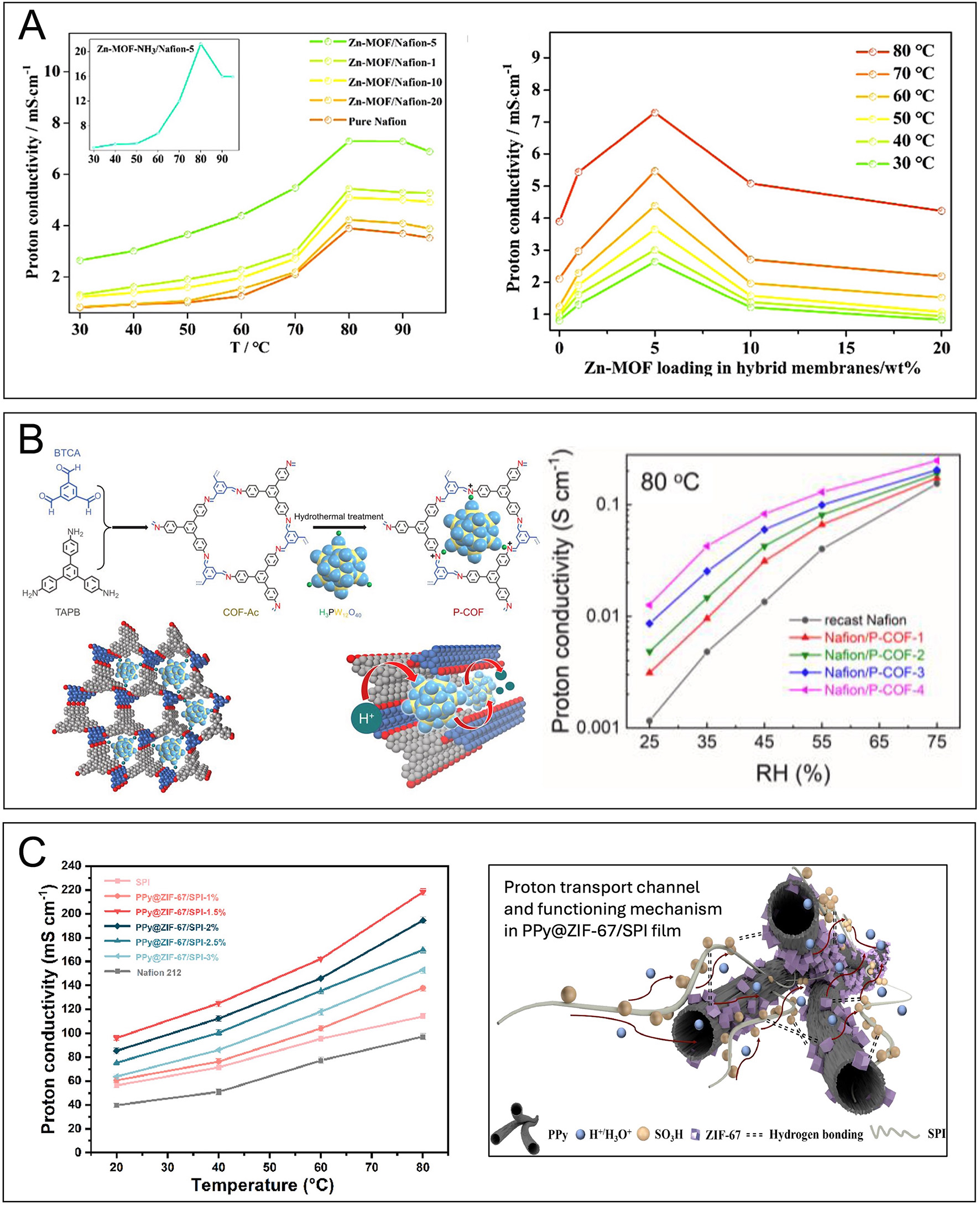
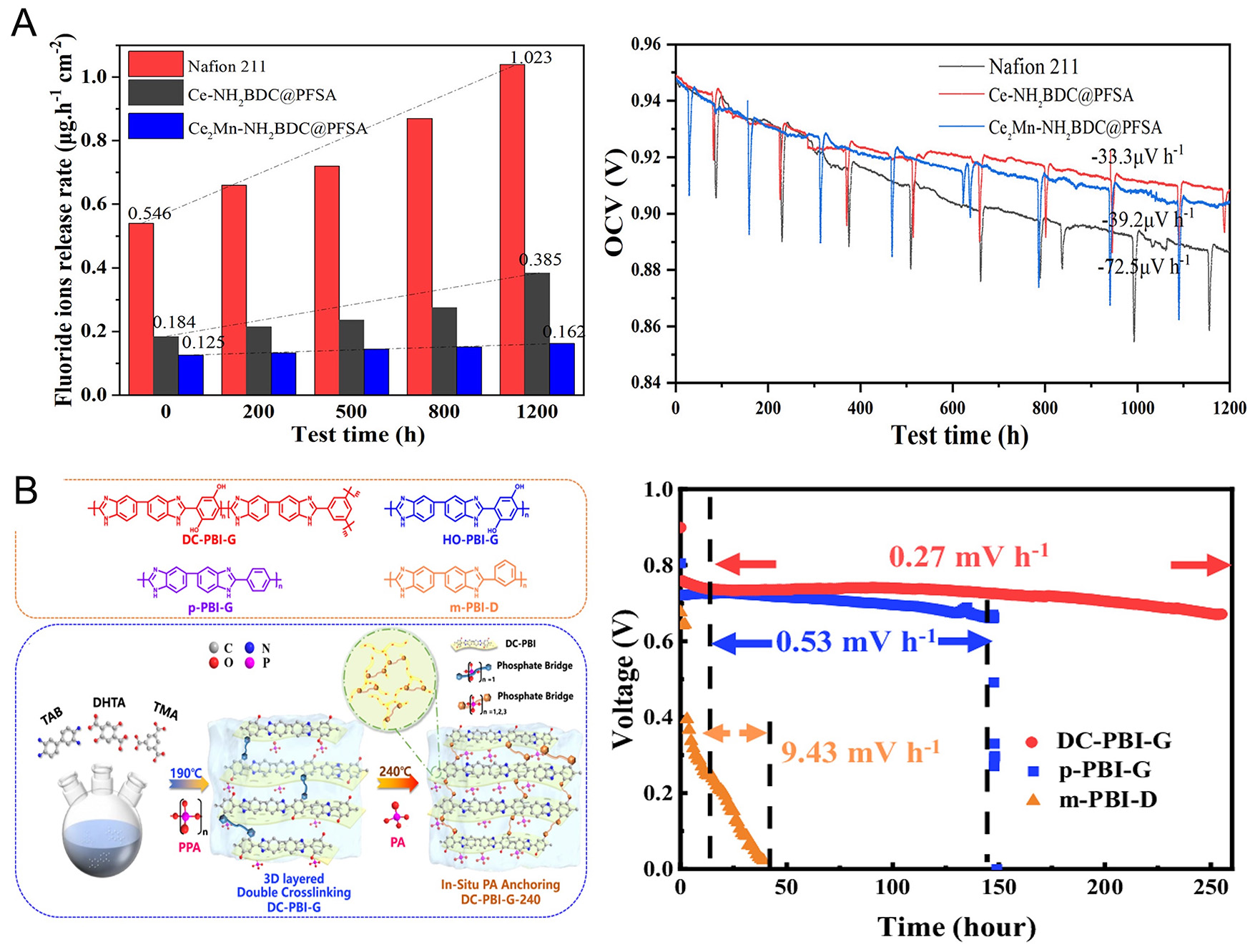
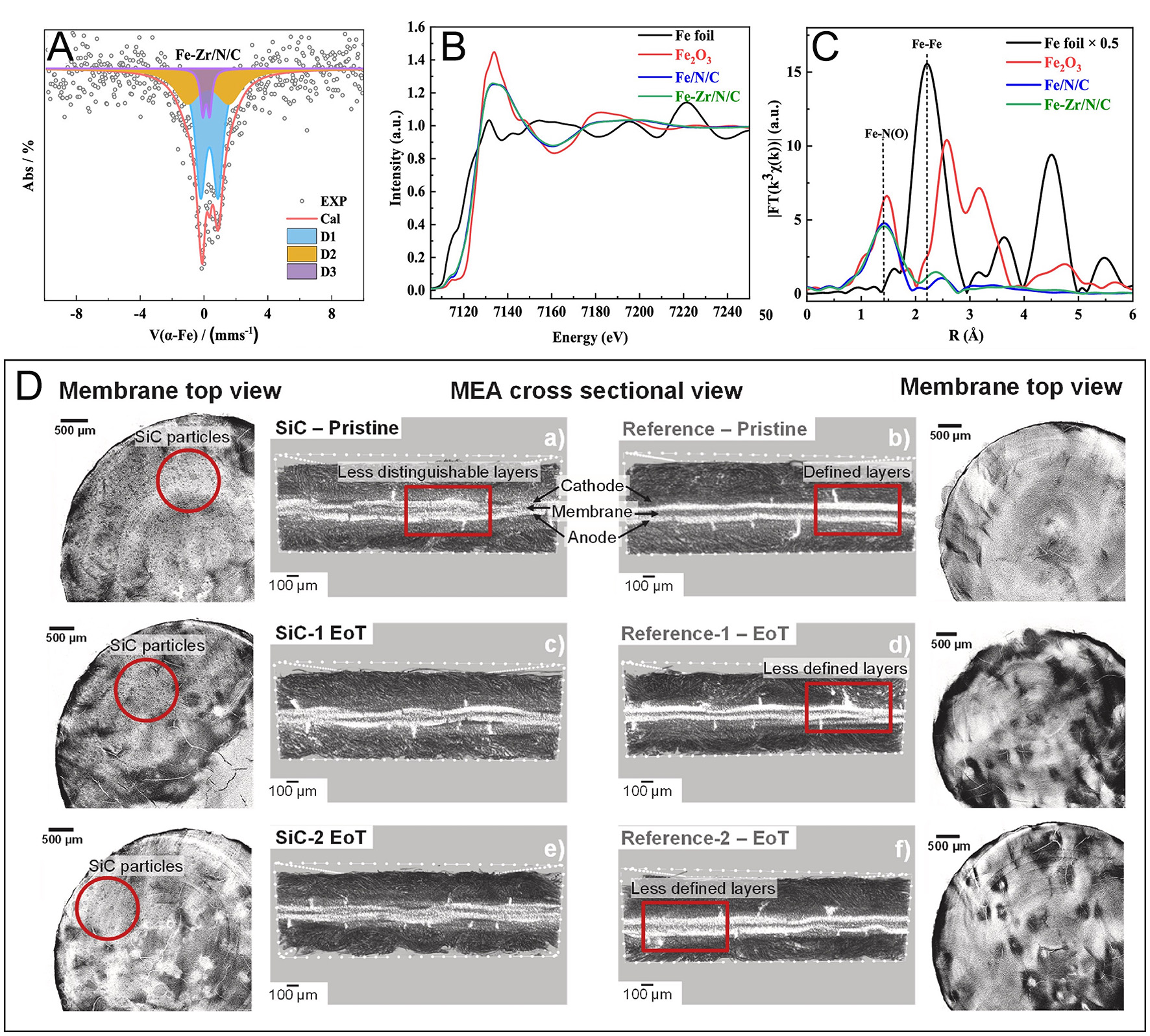












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].