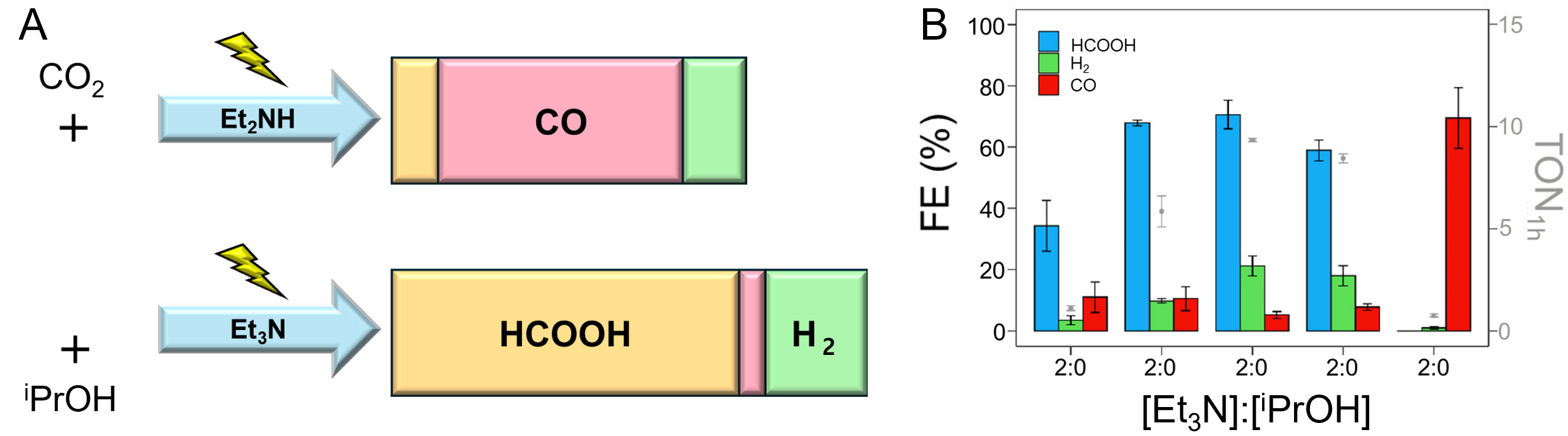
fig7
Figure 7. (A) Illustration for different methods for reducing CO2 using two types of additives such as Et2NH or Et3N, producing CO about 59% (above) or HCOOH about 73% (below), respectively; (B) Faraday Efficiency of formation of products such as HCOOH, H2, and CO using different ratios of Et3N and iPrOH[144]. Reprinted with permission from Ref.[144]. Copyright © 2021, American Chemical Society.