Conjugated-engineering covalent organic framework for synergistic artificial photosynthesis of hydrogen peroxide and high-value chemicals
Abstract
Artificial photosynthesis holds great promise for the production of hydrogen peroxide (H2O2), an environmentally friendly oxidant and clean fuel. However, the synergistic photosynthesis of H2O2 and high-value chemicals in blue light-emitting diodes (LEDs) has not yet been realized. Herein, we develop a conjugated-engineering covalent organic framework (COF), designated as TANB-Py-COF, which serves as an efficient catalyst in the photosynthesis of H2O2 under blue LEDs. An apparent quantum yield of 7.87% and a H2O2 production rate of 18.32 mmol g-1 h-1 are achieved. In addition, the synergistic photosynthesis of imines via amine-coupling or photooxidation of thioethers is realized with a yield up to 99%. This work establishes a precedent for the development of COF-based photocatalytic strategies for the simultaneous artificial photosynthesis of hydrogen peroxide and high-value chemicals in blue LEDs.
Keywords
INTRODUCTION
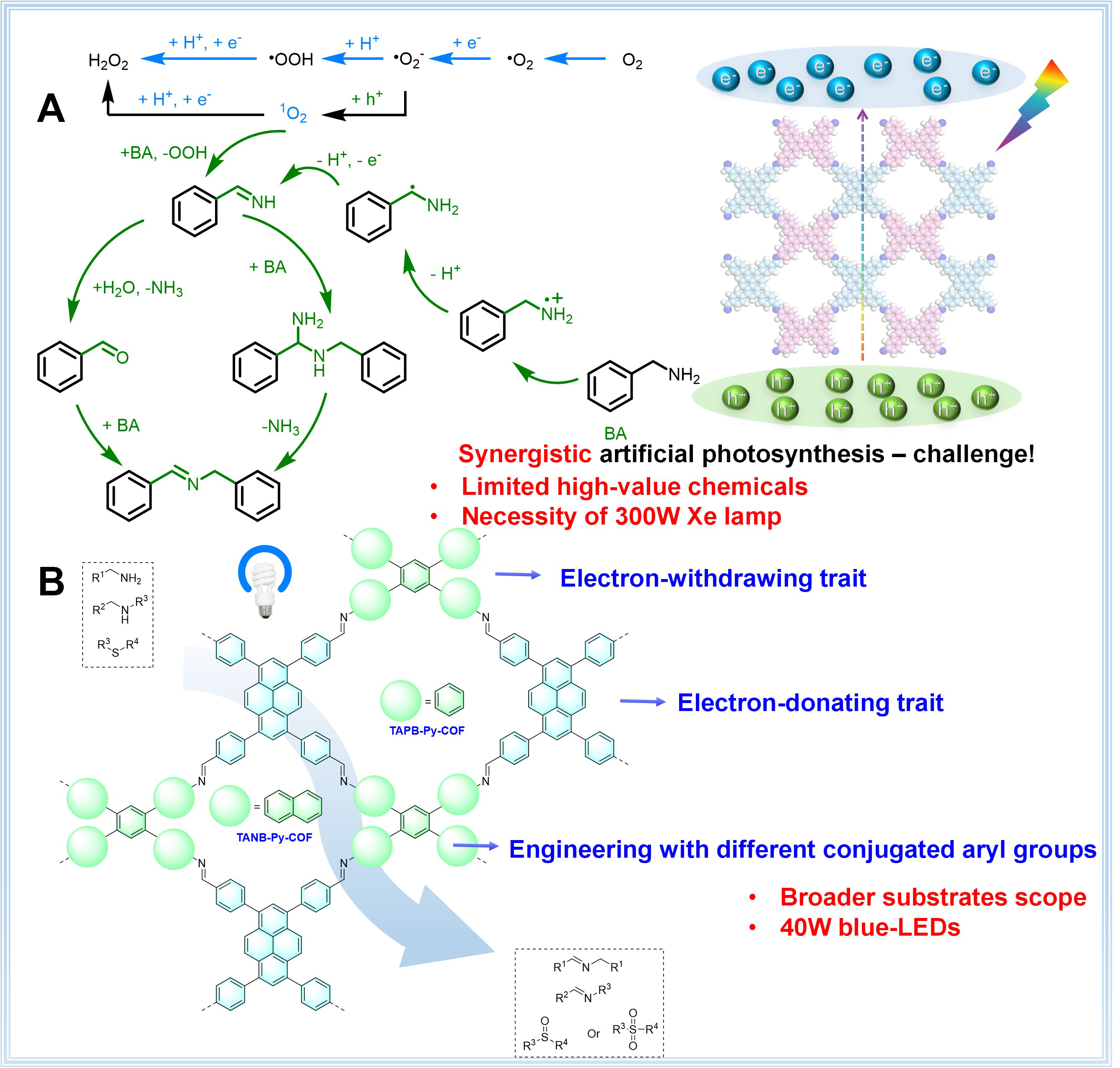
Since the discovery and initial reporting of H2O2 in 1818, it has garnered extensive attention worldwide[1-3]. Among the various strategies developed, photocatalytic H2O2 production is considered the most promising[4-15]. In this process, O2 captures a photogenerated electron from the conduction band (CB) to form a superoxide radical anion (O2•-), which then captures a proton to form hydroperoxyl radicals (•OOH). These intermediates subsequently undergo further proton- and electron-coupling steps to yield H2O2. Simultaneously, other electron donors, such as benzyl amine, can donate electrons to photogenerated holes in the valence band (VB). This process is followed by a series of steps that lead to the formation of imines
Figure 1. Conjugated-engineering COF for synergistic artificial photosynthesis of H2O2 and high-value chemicals. (A) Mechanism of synergistic artificial photosynthesis and challenge; (B) Structure and advantages of TAAB-Py-COF. COF: Covalent organic framework; LEDs: light-emitting diodes.
Metal organic frameworks (MOFs) or COFs are porous crystalline materials built from organic units, which are prized in photocatalysis for their customizable porosity, extended π-conjugation, tunable bandgaps, high surface areas, and stability[29-36]. Recent studies indicated that 2D COFs could serve as heterogeneous photocatalysts, driving light-induced chemical reactions[37-40]. Among them, TAAB-Py-COFs, synthesized by condensing 1,2,4,5-tetrakis(4-aminoaryl)-benzene (TAAB) and Py-CHO, have shown great photocatalytic ability in various organic reactions[41-46]. One of the key advantages of TAAB-Py-COFs is their broad
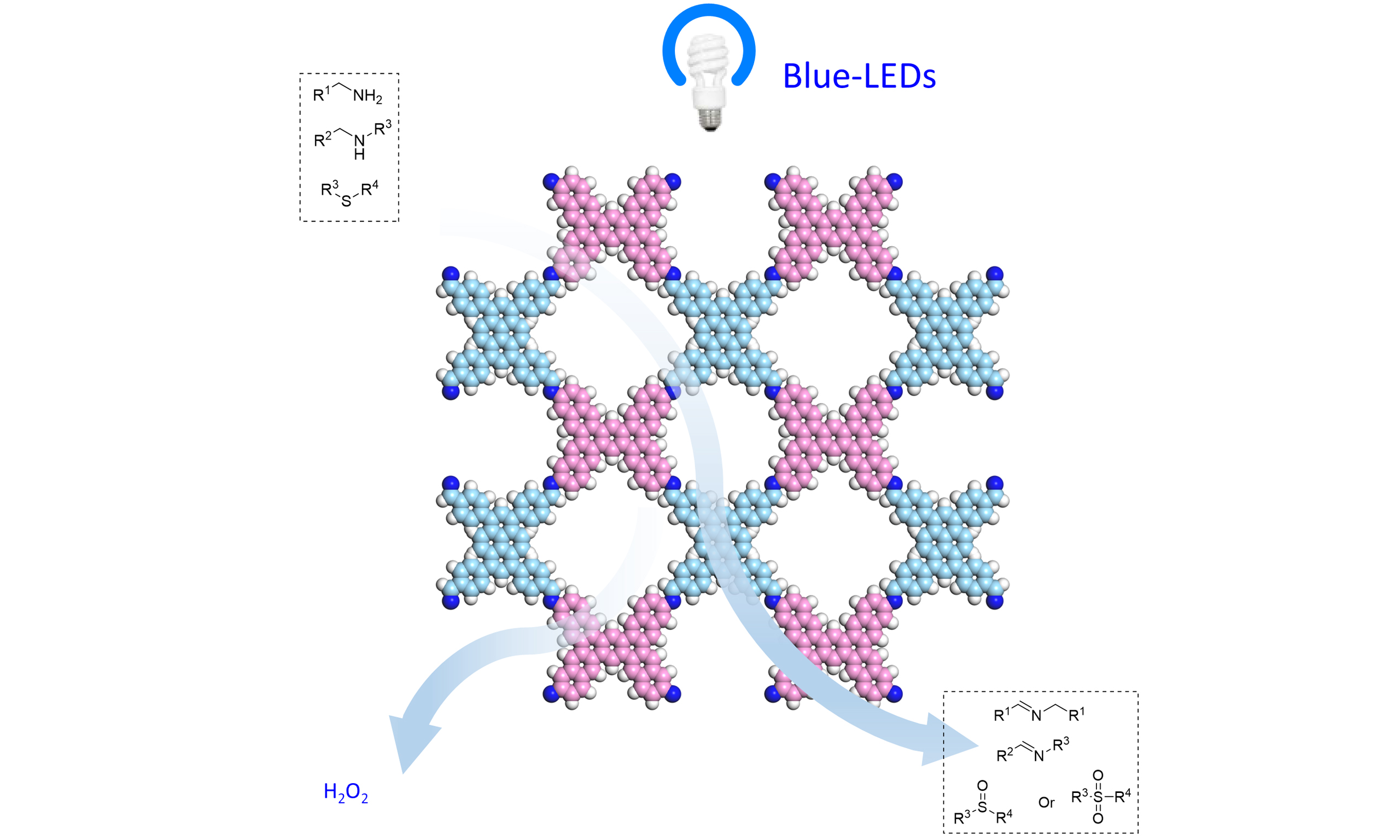
Based on the aforementioned considerations, we engineered naphthyl group instead of phenyl group at the 1,2,4,5 positions of central benzene in TAAB, yielding TANB-Py-COF. Naphthyl substituents enhance π-π stacking and electronic delocalization, often improving crystallinity, porosity, and optoelectronic properties compared to phenyl groups[51,52]. This material exhibited the expected photocatalytic performance in a heterogeneous visible-light catalytic system, which facilitated synergistic artificial photosynthesis of H2O2 and a series of high-value chemicals [Figure 1B]. Using TANB-Py-COF as the catalyst in CH3CN under an air atmosphere, an apparent quantum yield (AQY) of 7.87% was achieved. Moreover, the synergistic artificial photosynthesis of imines or sulfoxides, via amine couplings or the photooxidations of thioethers, was achieved with yields of up to 99%. This work provided a precedent synergistic artificial photosynthesis system of hydrogen peroxide and high-value chemicals via TAAB-Py-COF.
RESULTS AND DISCUSSION
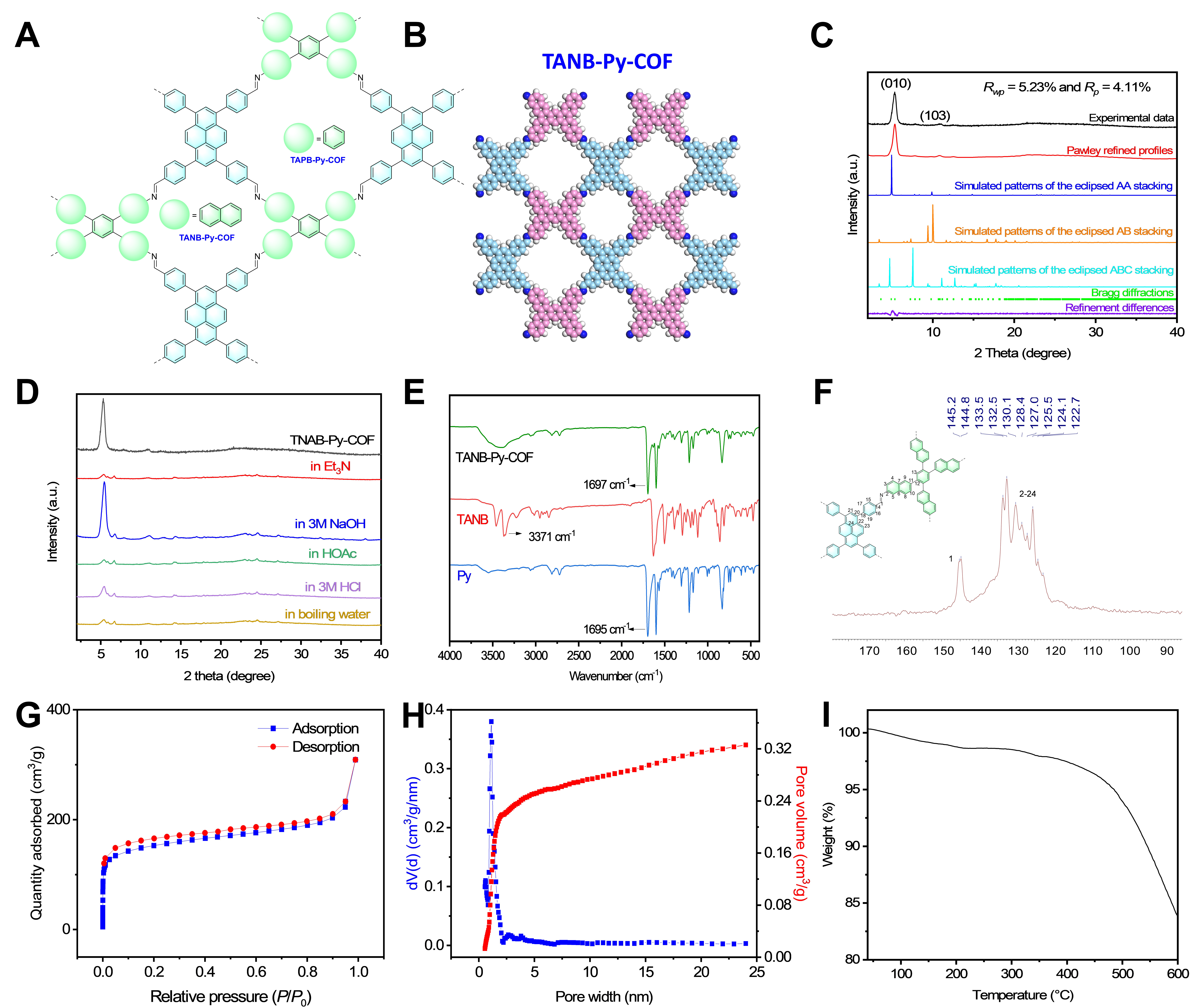
Initially, we synthesized the TANB-Py-COF by the condensation between 1,2,4,5-tetrakis(6-aminonaphthyl)-benzene (TANB) and 4,4’,4’’,4’’’-(pyrene-1,3,6,8-tetrayl)tetrabenzaldehyde (Py-CHO)
Figure 2. Structure and characterization of TANB-Py-COF. (A) Chemical structure of TANB-Py-COF and TAPB-Py-COF; (B) Graphic view of one layer of TANB-Py-COF simulated by Material Studio. (azure, pink, C; blue, N; white, H); (C) PXRD patterns of TANB-Py-COF; (D) PXRD patterns of TANB-Py-COF before and after treatment in triethylamine, 3M NaOH, HOAc, 3M HCl and boiling water for 3 days; (E) Fourier transform infrared (FT-IR) spectroscopy spectra of TANB-Py-COF, TANB and Py; (F)13C-CP/TOSS NMR spectrum of TANB-Py-COF; (G) Nitrogen adsorption and desorption isotherm at 77 K of TANB-Py-COF; (H) Pore size distribution of TANB-Py-COF; (I) Thermogravimetric analysis of TANB-Py-COF. COF: Covalent organic framework; PXRD: powder X-ray diffraction; 13C-CP/TOSS:
After successfully synthesizing TANB-Py-COF, we investigated its potential in artificial photosynthesis for the production of hydrogen peroxide and N-benzyl imines. Using benzyl amine 1a as a model substrate, the reaction was conducted under a 40 W blue LED light source with a wavelength of 410 nm in an air atmosphere at room temperature [Supplementary Figure 2]. TANB-Py-COF catalyzed the reaction to yield N-benzyl imines 2a with an impressive 99% yield in CH3CN [Table 1, entry 1], while also generating hydrogen peroxide at a rate of 18.32 mmol g-1 h-1. In contrast, TAPB-Py-COF, synthesized via condensation between 1,2,4,5-tetrakis(4-aminophenyl)-benzene (TAPB) and Py-CHO, exhibited a lower hydrogen peroxide generation rate of 16.24 mmol g-1 h-1 [Table 1, entry 2]. One hour was insufficient for complete conversion [Table 1, entry 3], and extending the reaction time to two hours resulted in a disappointing hydrogen peroxide generation rate due to H2O2 decomposition [Table 1, entry 4]. The LED wavelength selection process indicated that the catalytic reactions were most effective in 410 nm [Table 1, entry 1 vs. entries 5-7]. As anticipated, no product was formed in the absence of light or under a nitrogen atmosphere
Optimizations of the reaction
 | |||||
| Entries | Catalysts | Wavelength/nm | Time/h | Conv./% | Generation rate of H2O2/mmol g-1 h-1 |
| 1 | TANB-Py-COF | 410 | 1.5 | 99 | 18.32 |
| 2 | TAPB-Py-COF | 410 | 1.5 | 99 | 16.24 |
| 3 | TANB-Py-COF | 410 | 1 | 78 | 16.83 |
| 4 | TANB-Py-COF | 410 | 2 | 99 | 11.33 |
| 5 | TANB-Py-COF | 390 | 1.5 | 64 | 5.65 |
| 6 | TANB-Py-COF | 420 | 1.5 | 57 | 5.45 |
| 7 | TANB-Py-COF | 450 | 1.5 | 74 | 7.56 |
| 8a | TANB-Py-COF | 410 | 1.5 | 0 | 0 |
| 9b | TANB-Py-COF | 410 | 1.5 | 0 | 0 |
| 10c | TANB-Py-COF | 410 | 1.5 | 99 | 22.18 |
| 11d | TANB-Py-COF | 410 | 1.5 | 86 | 13.22 |
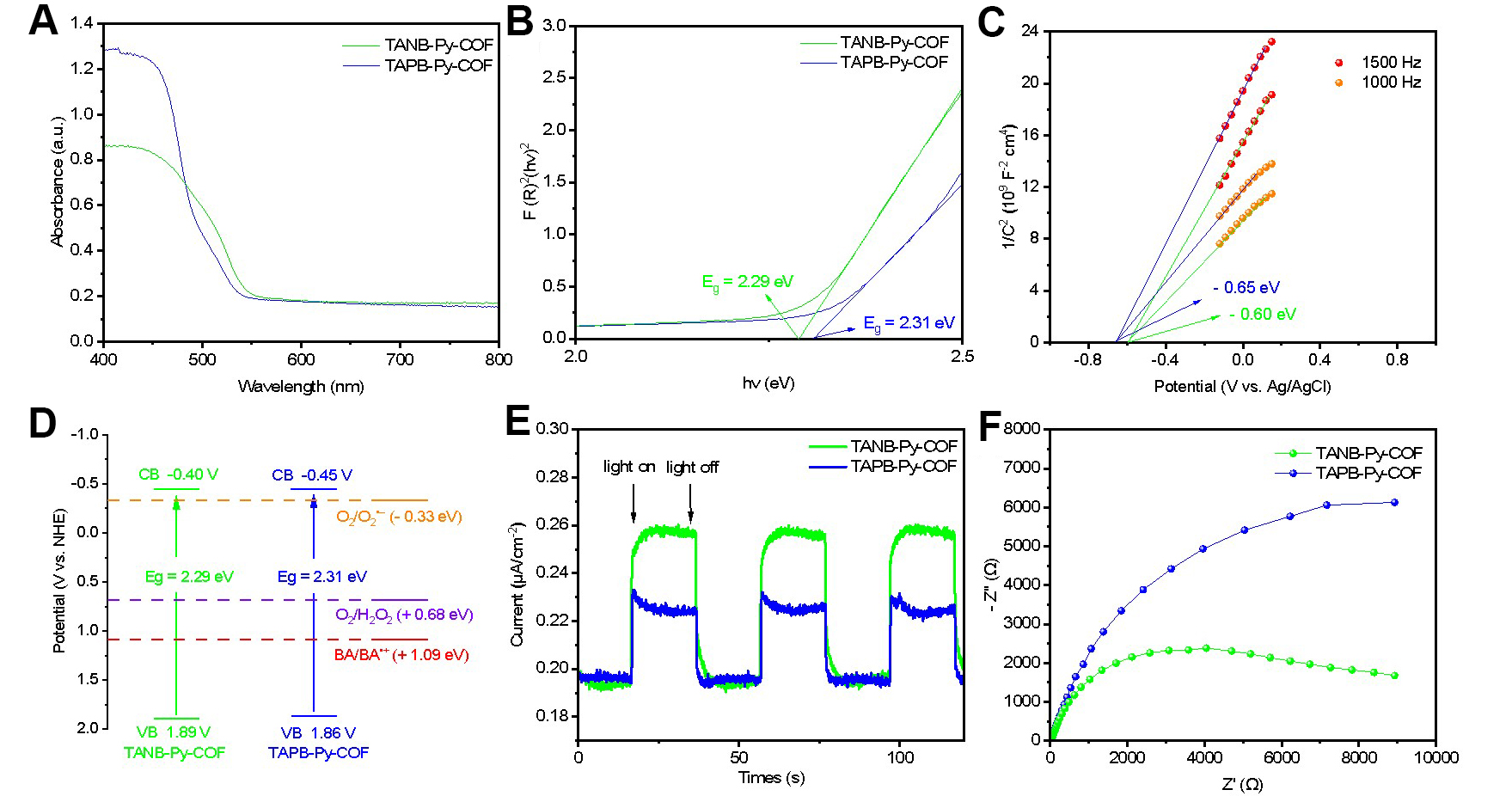
Both TANB-Py-COF and TAPB-Py-COF were evaluated for their optical band gaps and electronic structures using ultraviolet/visible (UV/Vis) diffuse reflectance spectroscopy (DRS) and Mott-Schottky measurements. As shown in Figure 3A, both COFs demonstrated similar optical absorption with an edge around 550 nm. The optical band gaps were calculated to be approximately 2.29 eV for TANB-Py-COF and 2.31 eV for TAPB-Py-COF from the Tauc plots [Figure 3B]. Mott-Schottky measurements further revealed that both COFs are n-type semiconductors, with flat band potentials (Efb) of -0.60 V and -0.65 V vs.
Figure 3. The photoelectrochemical measurements of TANB-Py-COF (green line) and TAPB-Py-COF (blue line). (A) UV/Vis diffuse reflectance spectroscopy; (B) Tauc plots; (C) Mott-Schottky plots; (D) Band structure diagram as determined from Mott-Schottky plots and UV-DRS; (E) Transient photocurrent measurements; (F) EIS Nyquist plots. COF: Covalent organic framework; UV/Vis: ultraviolet/visible; DRS: diffuse reflectance spectroscopy; EIS: electrochemical impedance spectroscopy.
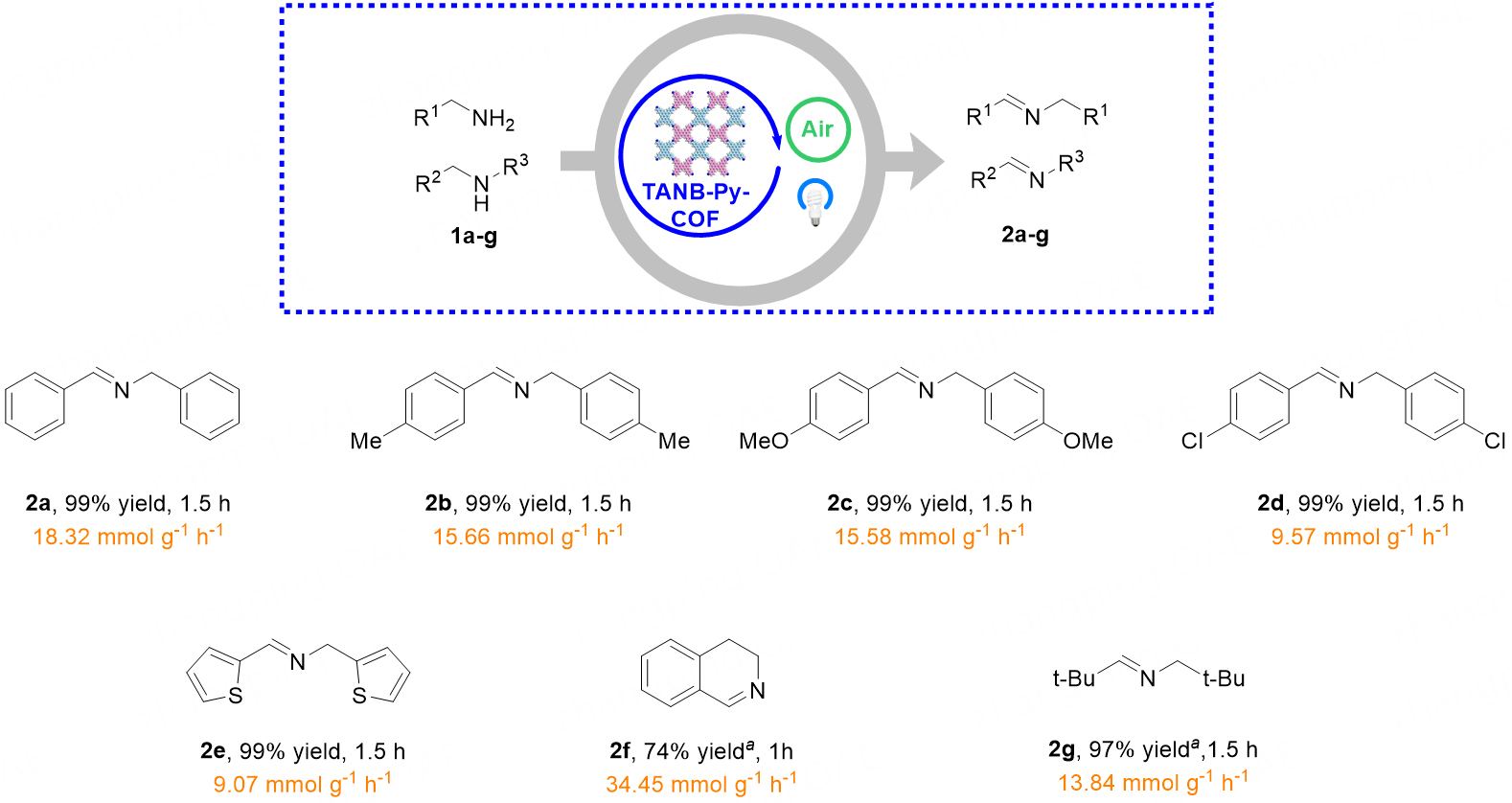
Under the optimized reaction conditions, we investigated the substrate scope using various benzyl amines to assess the generality of this artificial photosynthesis process [Figure 4]. The reaction proved to be versatile, accommodating a wide range of benzyl amines with different substituents, including methyl, halogens, and alkoxy groups (2a-d), and consistently delivering the corresponding imines with 99% yields while maintaining good to excellent H2O2 generation rates. Both electron-donating and
Figure 4. All reactions were carried out on a 0.1 mmol scale with 1.0 eq. amines 1a-g and 2 mol% TANB-Py-COF in 5.0 mL CH3CN under blue LED irradiation at room temperature in air. Isolated yield. The orange segments in the figure denote the generation rates of H2O2.
where Cc is the carbon moles of the model compound, Cis is the carbon moles of the internal standard (biphenyl), Ac is the area of the model compound, Ais is the area of the internal standard, Rf is the response factor of the compound, ECNis is the effective carbon number of the internal standard, and ECNc is the effective carbon number of the compound.
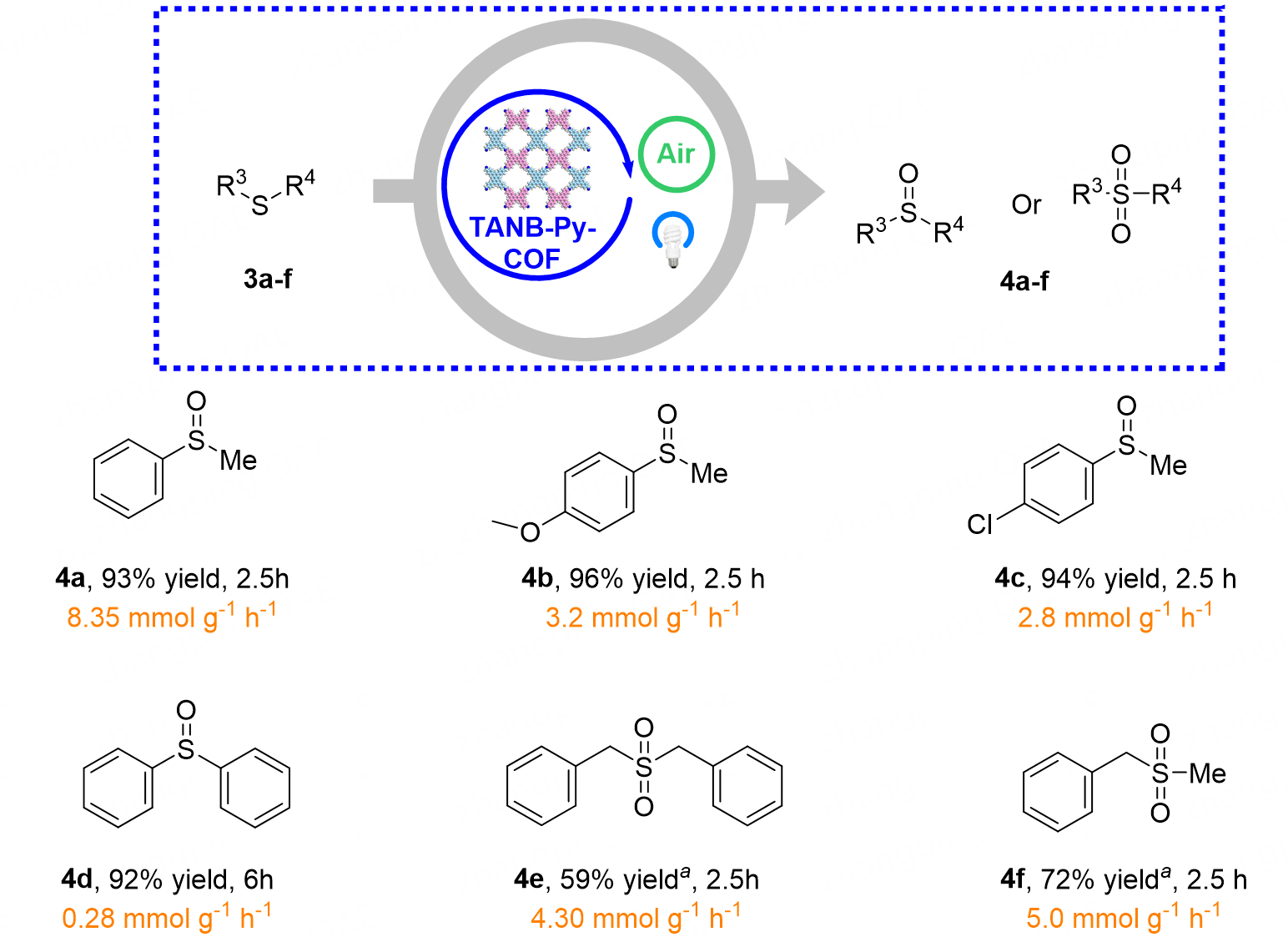
Beyond carbon-nitrogen bond construction, the versatility of this artificial photosynthetic platform extends to the selective oxygenation of thioethers [Figure 5]. Under the same photoredox conditions, a broad spectrum of thioethers 3a-f underwent controllable oxidation to deliver the corresponding sulfoxides or sulfones 4a-f in a single step. Alkyl thioethers proved particularly compatible and the conversion of simple dialkyl sulfides to sulfones 4e-f proceeded smoothly, furnishing isolated yields of 62%-85 % while simultaneously sustaining steady H2O2 evolution rates of 120-150 μmol g-1 h-1. These results underscore the catalysts’ capacity to orchestrate both photoredox transformations and selective oxygen-atom transfer, thereby expanding the synthetic utility of COF-based photocatalysis beyond amination to encompass sulfur functionalization.
Figure 5. All reactions were carried out on a 0.1 mmol scale with 1.0 eq. thioethers 3a-f and 2 mol% TANB-Py-COF in 5.0 mL CH3CN under irradiation of blue LEDs in air atmosphere at room temperature. Isolated yield. The orange segments in the figure denote the generation rates of H2O2. aYield detected by effective carbon number (ECN) concept. COF: Covalent organic framework; LEDs:
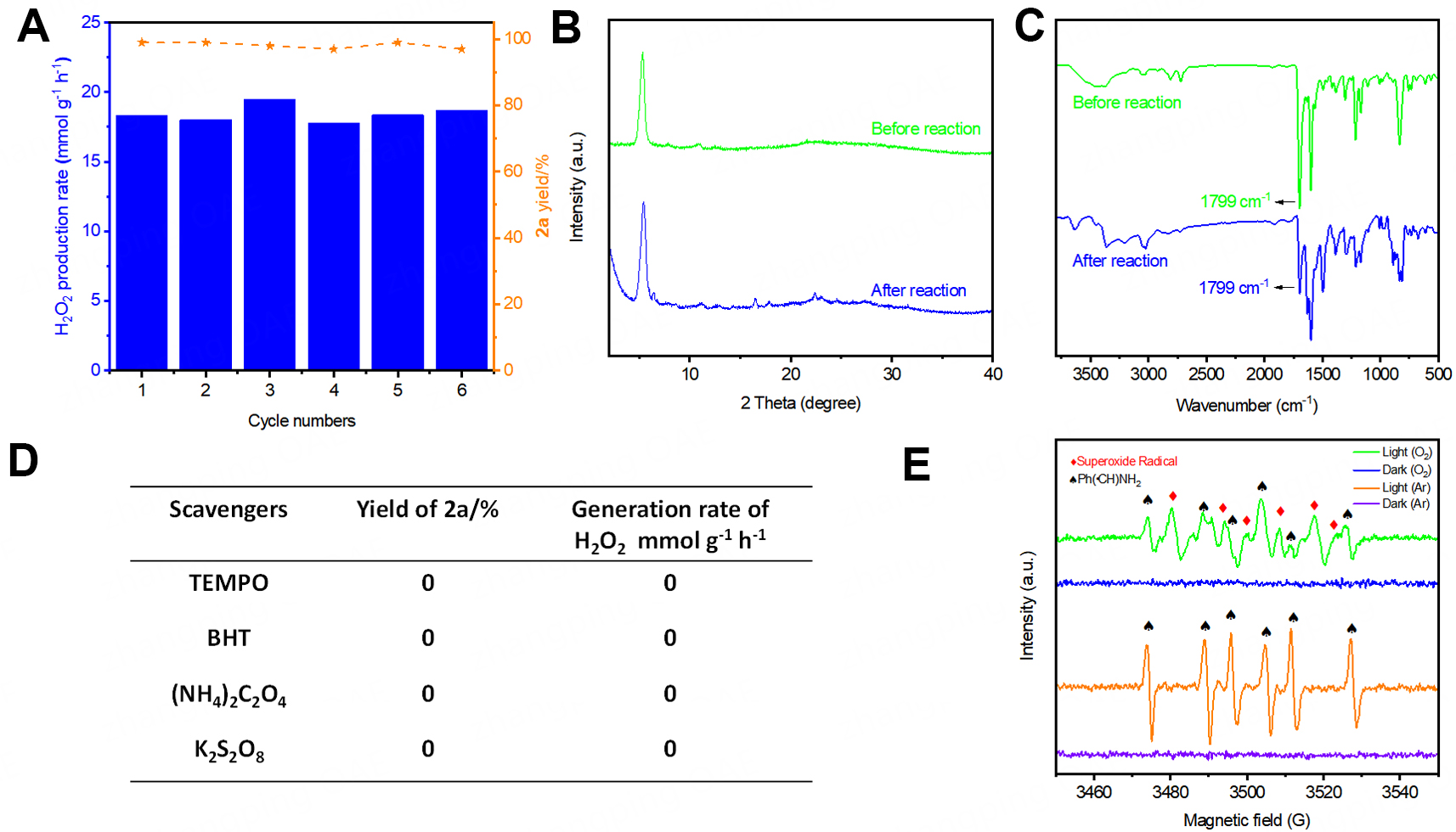
To further demonstrate the practicality of this reaction, we conducted a recycle experiment with
Figure 6. Practicality of the reactions and mechanistic studies. (A) Recycle experiments of TANB-Py-COF; (B) PXRD patterns of
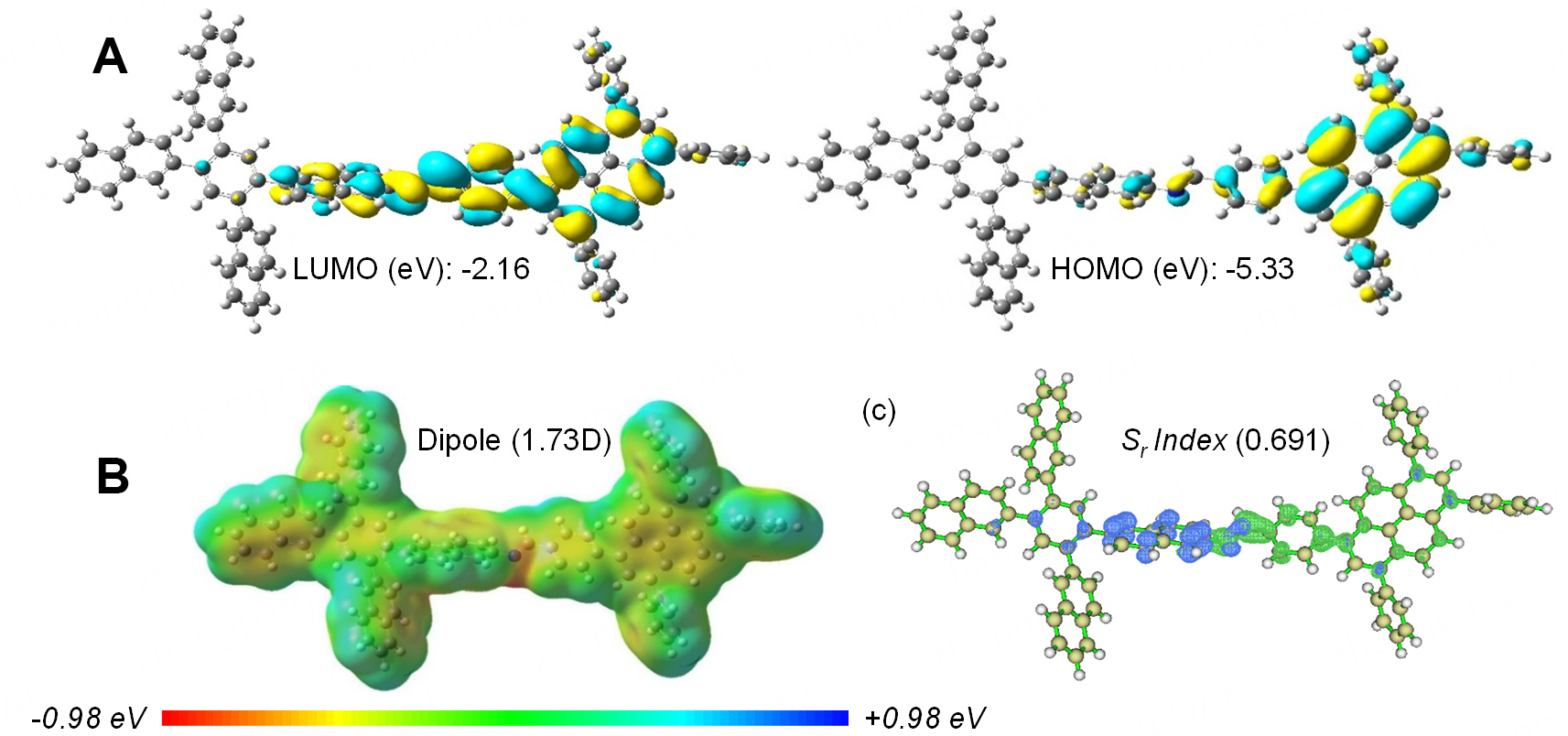
The local polarization and charge separation behavior of the TANB-Py-COF fragment were studied using density functional theory (DFT) calculations at the B3LYP/6-31G* level of theory using Gaussian 16. The highest occupied molecular orbital (HOMO, -5.33 eV) is primarily localized on the Py core, while the lowest unoccupied molecular orbital (LUMO, -2.16 eV) is delocalized along the imine bond linker into the naphthyl substituent of the TANB moiety [Figure 7A], resulting in a HOMO-LUMO gap of 3.17 eV. Electrostatic potential (ESP) mapping highlights the intrinsic polarization of the fragment; the Py unit shows pronounced negative potential, whereas the imine linker and naphthyl-substituted TANB moiety show alternating electron-rich and electron-deficient regions [Figure 7B]. The optimized fragment possesses a dipole moment of 1.73 D, consistent with a polarized conjugated network. The naphthyl group extends conjugation, enhancing LUMO delocalization while preserving the polarized network, ensuring the
Figure 7. DFT calculations (A) HOMO and LUMO orbitals and energy levels of the TANB-Py-COF fragment; (B) ESP map with dipole moment of the TANB-Py-COF fragment; (C) Electron-hole distribution of the TANB-Py-COF fragment. DFT: Density functional theory; HOMO: highest occupied molecular orbital; LUMO: lowest unoccupied molecular orbital; ESP: electrostatic potential; COF: covalent organic framework.
CONCLUSIONS
In this work, we successfully synthesized a conjugated-engineered COF, designated as TANB-Py-COF, and explored its potential as an efficient catalyst for the photosynthesis of H2O2 under blue-LED irradiation. This innovative system demonstrated an impressive AQY of 7.87% at 420 nm, along with a robust H2O2 production rate of 18.32 mmol g-1 h-1. Moreover, the TANB-Py-COF enabled the synergistic photosynthesis of imines through both amine couplings and photooxidations of thioethers, achieving yields as high as 99%. These results highlight the versatility and efficiency of the TANB-Py-COF in facilitating multiple chemical transformations under visible-light irradiation. Future research will focus on further optimizing the COF structure and exploring its applications in other photocatalytic reactions.
DECLARATIONS
Authors’ contributions
Conceived the idea, carried out part of the experiments, and wrote the manuscript: Lin, X.
Performed additional experiments: Cai, X.
Conducted the substrate scope studies: Zheng, H.
Performed the DFT calculations: Umar, A. B.
Supervised the project: Zheng, J.; Yuan, Z.
All authors discussed the results and contributed to the revision and finalization of the manuscript.
Availability of data and materials
Some results of supporting the study are presented in the Supplementary Materials. Other raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This research is supported by the team building funding of Fujian Agriculture and Forestry University (No. 118360021) and the Hundred Talents Program of Fujian Province (No. 118360010). We acknowledge JST-ERATO Yamauchi Materials Space- Tectonics Project (JPMJER2003) and the Queensland Node of the Australian National Fabrication Facility (ANFF-Q).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Perry, S. C.; Pangotra, D.; Vieira, L.; et al. Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat. Rev. Chem. 2019, 3, 442-58.
2. Sun, Y.; Han, L.; Strasser, P. A comparative perspective of electrochemical and photochemical approaches for catalytic H2O2 production. Chem. Soc. Rev. 2020, 49, 6605-31.
3. Freese, T.; Meijer, J. T.; Feringa, B. L.; Beil, S. B. An organic perspective on photocatalytic production of hydrogen peroxide. Nat. Catal. 2023, 6, 553-8.
4. Baur, E.; Neuweiler, C. Über photolytische bildung von hydroperoxyd. Helv. Chim. Acta. 1927, 10, 901-7.
5. Hao, F.; Yang, C.; Lv, X.; et al. Photo-driven quasi-topological transformation exposing highly active nitrogen cation sites for enhanced photocatalytic H2O2 production. Angew. Chem. Int. Ed. 2023, 62, e202315456.
6. Liao, Q.; Sun, Q.; Xu, H.; et al. Regulating relative nitrogen locations of diazine functionalized covalent organic frameworks for overall H2O2 photosynthesis. Angew. Chem. Int. Ed. 2023, 62, e202310556.
7. Das, P.; Roeser, J.; Thomas, A. Solar light driven H2O2 production and selective oxidations using a covalent organic framework photocatalyst prepared by a multicomponent reaction. Angew. Chem. Int. Ed. 2023, 62, e202304349.
8. Liu, R.; Chen, Y.; Yu, H.; et al. Linkage-engineered donor-acceptor covalent organic frameworks for optimal photosynthesis of hydrogen peroxide from water and air. Nat. Catal. 2024, 7, 195-206.
9. Cao, L.; Wang, C.; Wang, H.; et al. Rationally Designed cyclooctatetrathiophene-based porous aromatic frameworks (COTh-PAFs) for efficient photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 2024, 63, e202402095.
10. Zhou, E.; Wang, F.; Zhang, X.; Hui, Y.; Wang, Y. Cyanide-based covalent organic frameworks for enhanced overall photocatalytic hydrogen peroxide production. Angew. Chem. Int. Ed. 2024, 63, e202400999.
11. Yue, J. Y.; Luo, J. X.; Pan, Z. X.; et al. Regulating the topology of covalent organic frameworks for boosting overall H2O2 photogeneration. Angew. Chem. Int. Ed. 2024, 63, e202405763.
12. Dong, P.; Xu, X.; Wu, T.; et al. Stepwise protonation of three-dimensional covalent organic frameworks for enhancing hydrogen peroxide photosynthesis. Angew. Chem. Int. Ed. 2024, 63, e202405313.
13. Wu, W.; Li, Z.; Liu, S.; et al. Pyridine-based covalent organic frameworks with pyridyl-imine structures for boosting photocatalytic H2O2 production via one-step 2e- oxygen reduction. Angew. Chem. Int. Ed. 2024, 63, e202404563.
14. Li, L.; Lv, X.; Xue, Y.; Shao, H.; Zheng, G.; Han, Q. Custom-design of strong electron/proton extractor on COFs for efficient photocatalytic H2O2 production. Angew. Chem. Int. Ed. 2024, 63, e202320218.
15. Zhang, X.; Cheng, S.; Chen, C.; et al. Keto-anthraquinone covalent organic framework for H2O2 photosynthesis with oxygen and alkaline water. Nat. Commun. 2024, 15, 2649.
16. Liu, W.; Su, Q.; Ju, P.; et al. A Hydrazone-based covalent organic framework as an efficient and reusable photocatalyst for the cross-dehydrogenative coupling reaction of N-aryltetrahydroisoquinolines. ChemSusChem 2017, 10, 664-9.
17. Zhi, Y.; Li, Z.; Feng, X.; et al. Covalent organic frameworks as metal-free heterogeneous photocatalysts for organic transformations. J. Mater. Chem. A. 2017, 5, 22933-8.
18. Chen, R.; Shi, J. L.; Ma, Y.; Lin, G.; Lang, X.; Wang, C. Designed synthesis of a 2D porphyrin-Based sp2 carbon-conjugated covalent organic framework for heterogeneous photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 6430-4.
19. Wei, H.; Guo, Z.; Liang, X.; Chen, P.; Liu, H.; Xing, H. Selective photooxidation of amines and sulfides triggered by a superoxide radical using a novel visible-light-responsive metal-organic framework. ACS. Appl. Mater. Interfaces. 2019, 11, 3016-23.
20. Li, S.; Li, L.; Li, Y.; et al. Fully Conjugated donor-acceptor covalent organic frameworks for photocatalytic oxidative amine coupling and thioamide cyclization. ACS. Catal. 2020, 10, 8717-26.
21. He, H.; Fang, X.; Zhai, D.; et al. A Porphyrin-based covalent organic framework for metal-free photocatalytic aerobic oxidative coupling of amines. Chem. Eur. J. 2021, 27, 14390-5.
22. Yang, F.; Li, C. C.; Xu, C. C.; et al. A covalent organic framework as a photocatalyst for window ledge cross-dehydrogenative coupling reactions. Chem. Commun. 2022, 58, 1530-3.
23. Cheng, Y.; Ji, W.; Wu, X.; Ding, X.; Liu, X.; Han, B. Persistent radical cation sp2 carbon-covalent organic framework for photocatalytic oxidative organic transformations. Appl. Catal. B. Environ. 2022, 306, 121110.
24. Jiménez-Almarza, A.; López-Magano, A.; Mas-Ballesté, R.; Alemán, J. Tuning the activity-stability balance of photocatalytic organic materials for oxidative coupling reactions. ACS. Appl. Mater. Interfaces. 2022, 14, 16258-68.
25. Liu, M.; Liu, J.; Li, J.; et al. Blending aryl ketone in covalent organic frameworks to promote photoinduced electron transfer. J. Am. Chem. Soc. 2023, 145, 9198-206.
26. Liu, Y.; Jiang, X.; Chen, L.; et al. Rational design of a phenothiazine-based donor-acceptor covalent organic framework for enhanced photocatalytic oxidative coupling of amines and cyclization of thioamides. J. Mater. Chem. A. 2023, 11, 1208-15.
27. Kong, K.; Zhong, H.; Chen, D.; Zhang, F.; Li, X.; Wang, R. Carbon nitride quantum dots decorated with cyano groups for boosting photocatalytic hydrogen peroxide production. Green. Energy. Environ. 2025, 10, 1551-8.
28. Liu, J.; Tuo, C.; Xiao, W. Y.; et al. Constructing donor-acceptor covalent organic frameworks for highly efficient H2O2 photosynthesis coupled with oxidative organic transformations. Angew. Chem. Int. Ed. 2025, 64, e202416240.
29. Côté, A. P.; Benin, A. I.; Ockwig, N. W.; O’Keeffe, M.; Matzger, A. J.; Yaghi, O. M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166-70.
31. Ding, S. Y.; Wang, W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 2013, 42, 548-68.
32. Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: strategic design and structure-function correlation. Chem. Rev. 2017, 117, 1515-63.
33. Jin, Y.; Hu, Y.; Zhang, W. Tessellated multiporous two-dimensional covalent organic frameworks. Nat. Rev. Chem. 2017, 1, 0056.
34. Diercks, C. S.; Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585.
35. Zhao, W.; Xia, L.; Liu, X. Covalent organic frameworks (COFs): perspectives of industrialization. CrystEngComm 2018, 20, 1613-34.
36. Prabhakar Vattikuti, S. V.; To Hoai, N.; Zeng, J.; et al. Pouch-type asymmetric supercapacitor based on nickel-cobalt metal-organic framework. Materials 2023, 16, 2423.
37. Wei, P. F.; Qi, M. Z.; Wang, Z. P.; et al. Benzoxazole-linked ultrastable covalent organic frameworks for photocatalysis. J. Am. Chem. Soc. 2018, 140, 4623-31.
38. Kang, X.; Wu, X.; Han, X.; Yuan, C.; Liu, Y.; Cui, Y. Rational synthesis of interpenetrated 3D covalent organic frameworks for asymmetric photocatalysis. Chem. Sci. 2019, 11, 1494-502.
39. Wang, K.; Kang, X.; Yuan, C.; Han, X.; Liu, Y.; Cui, Y. Porous 2D and 3D covalent organic frameworks with dimensionality-dependent photocatalytic activity in promoting radical ring-opening polymerization. Angew. Chem. Int. Ed. 2021, 60, 19466-76.
40. Wu, C. J.; Li, X. Y.; Li, T. R.; et al. Natural sunlight photocatalytic synthesis of benzoxazole-bridged covalent organic framework for photocatalysis. J. Am. Chem. Soc. 2022, 144, 18750-5.
41. Nguyen, H. L.; Gropp, C.; Ma, Y.; Zhu, C.; Yaghi, O. M. 3D covalent organic frameworks selectively crystallized through conformational design. J. Am. Chem. Soc. 2020, 142, 20335-9.
42. Han, W. K.; Liu, Y.; Yan, X.; Jiang, Y.; Zhang, J.; Gu, Z. G. Integrating light-harvesting ruthenium(II)-based units into three-dimensional metal covalent organic frameworks for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2022, 61, e202208791.
43. Yan, X.; Wang, F.; Su, X.; et al. A redox-active covalent organic framework with highly accessible aniline-fused quinonoid units affords efficient proton charge storage. Adv. Mater. 2023, 35, e2305037.
44. Davies, J. A.; Ronson, T. K.; Nitschke, J. R. Triamine and tetramine edge-length matching drives heteroleptic triangular and tetragonal prism assembly. J. Am. Chem. Soc. 2024, 146, 5215-23.
45. Yang, M. Y.; Zhang, S. B.; Zhang, M.; et al. Three-motif molecular junction type covalent organic frameworks for efficient photocatalytic aerobic oxidation. J. Am. Chem. Soc. 2024, 146, 3396-404.
46. Fang, L.; Xu, H.; Qiu, S.; et al. Autocatalytic interfacial synthesis of self-standing amide-linked covalent organic framework membranes. Angew. Chem. Int. Ed. 2025, 64, e202423220.
47. Guo, M.; Jayakumar, S.; Luo, M.; et al. The promotion effect of π-π interactions in Pd NPs catalysed selective hydrogenation. Nat. Commun. 2022, 13, 1770.
48. Gutiérrez, L.; Martin-diaconescu, V.; Casadevall, C.; et al. Low oxidation state cobalt center stabilized by a covalent organic framework to promote hydroboration of olefins. ACS. Catal. 2023, 13, 3044-54.
49. Fang, Y.; Liu, Y.; Huang, H.; et al. Design and synthesis of broadband absorption covalent organic framework for efficient artificial photocatalytic amine coupling. Nat. Commun. 2024, 15, 4856.
50. Zhu, Y.; Huang, D.; Wang, W.; Liu, G.; Ding, C.; Xiang, Y. Sequential oxidation/cyclization of readily available imine linkages to access benzoxazole-linked covalent organic frameworks. Angew. Chem. Int. Ed. 2024, 63, e202319909.
51. Wang, Z.; Song, Q.; He, C.; Feng, P.; Zhao, L.; Duan, C. Naphthalene-based donor-acceptor covalent organic frameworks as an electron distribution regulator for boosting photocatalysis. Chem. Commun. 2024, 60, 4793-6.
52. Zhang, G.; Tsujimoto, M.; Packwood, D.; et al. Construction of a hierarchical architecture of covalent organic frameworks via a postsynthetic approach. J. Am. Chem. Soc. 2018, 140, 2602-9.
53. Zhang, S.; Cheng, G.; Guo, L.; Wang, N.; Tan, B.; Jin, S. Strong-base-assisted synthesis of a crystalline covalent triazine framework with high hydrophilicity via benzylamine monomer for photocatalytic water splitting. Angew. Chem. Int. Ed. 2020, 59, 6007-14.
54. Wang, S.; Guan, B. Y.; Wang, X.; Lou, X. W. D. Formation of hierarchical Co9S8@ZnIn2S4 heterostructured cages as an efficient photocatalyst for hydrogen evolution. J. Am. Chem. Soc. 2018, 140, 15145-8.
55. Narani, A.; Gao, Y.; Zhang, J.; Beach, C. A.; Foston, M. Lignin monomer quantification without standards: using gas chromatography with dual quantitative carbon detection and mass spectrometry. Anal. Chem. 2025, 97, 8286-92.
56. Nutting, J. E.; Rafiee, M.; Stahl, S. S. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 2018, 118, 4834-85.
57. Beejapur, H. A.; Zhang, Q.; Hu, K.; Zhu, L.; Wang, J.; Ye, Z. TEMPO in chemical transformations: from homogeneous to heterogeneous. ACS. Catal. 2019, 9, 2777-830.
58. Chen, Z.; Wang, J.; Hao, M.; et al. Tuning excited state electronic structure and charge transport in covalent organic frameworks for enhanced photocatalytic performance. Nat. Commun. 2023, 14, 1106.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].