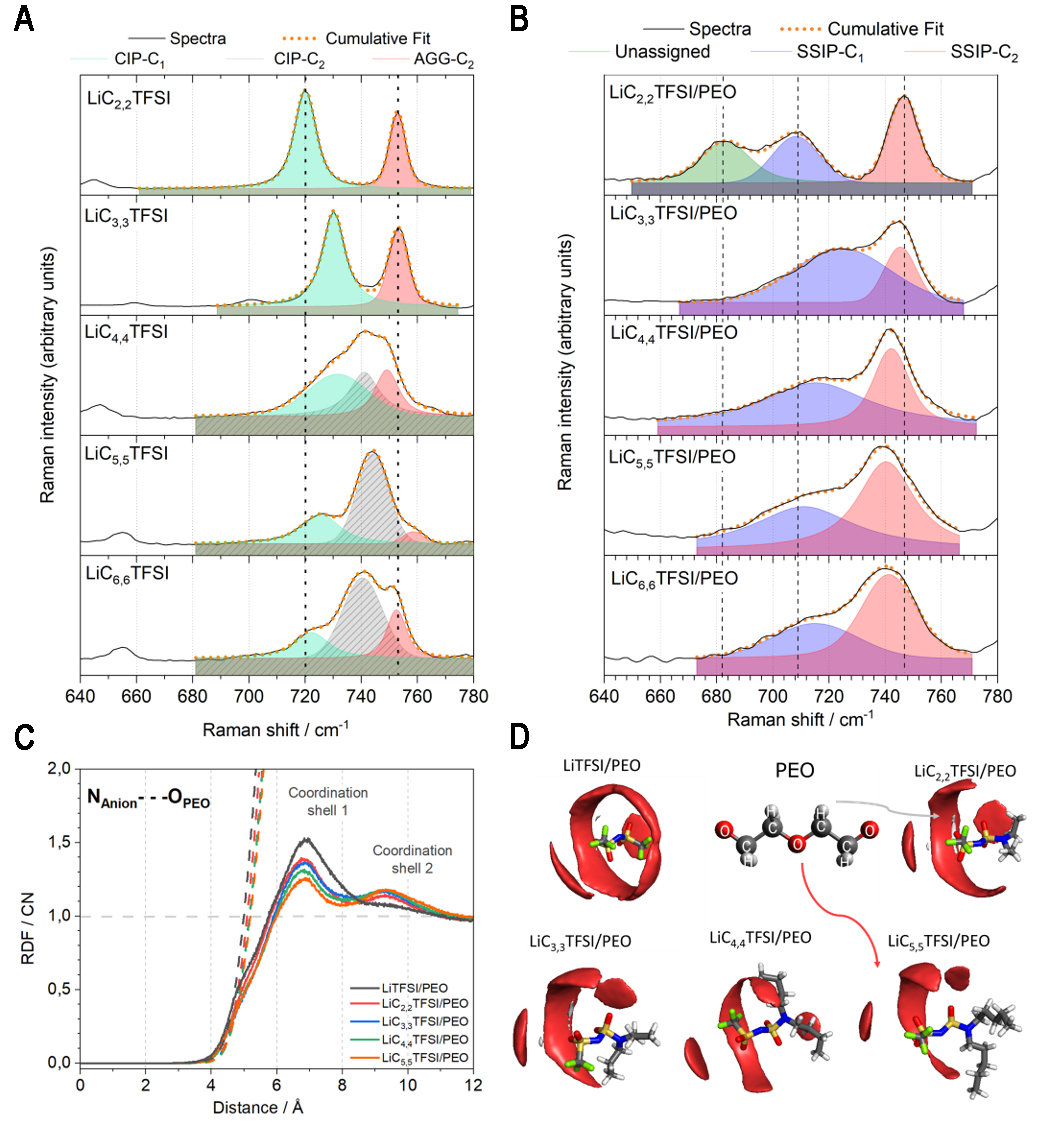
fig2
Figure 2. Coordination environment analysis: Raman spectra illustrating the S-N-S vibration for (A) neat LiCn,nTFSI salts at room temperature and (B) LiCn,nTFSI/PEO at 70 °C; (C) Radial Distribution Functions (RDFs) (solid lines) with the anion as the reference ion, and PEO as the observed species, via their N-atom and O-atoms, respectively. The coordination numbers (CNs) are shown as dashed lines; (D) Spatial Distribution Functions (SDFs) of LiCn,nTFSI/PEO. The red and grey iso-surfaces represent the distribution of O-atoms and H-atoms from PEO, respectively. RDFs and SDFs were computed from MD simulations at 70 °C. LiC2,2TFSI: Lithium(trifluoromethanesulfonyl)(N,N-diethylsulfamoyl)imide; LiC3,3TFSI: lithium(trifluoro-methanesulfonyl)(N,N-dipropylsulfamoyl)imide; LiC4,4TFSI: lithium(trifluoromethanesulfonyl) (N,N-dibutylsulfamoyl)imide; LiC5,5TFSI: lithium(trifluoromethanesulfonyl)(N,N-dipentyl-sulfamoyl)imide; LiC6,6TFSI: lithium(trifluoromethanesulfonyl)(N,N-dihexylsulfamoyl)-imide; PEO: poly(ethylene oxide); MD: molecular dynamics.