Fe3+-driven tunnel engineering for stabilizing metastable ramsdellite MnO2 in high-performance zinc-ion batteries
Abstract
Ramsdellite MnO2 (R-MnO2), with its expanded (1 × 2) tunnels, offers superior Zn2+ diffusion kinetics for aqueous zinc-ion batteries but suffers from metastability-induced phase collapse. Herein, Fe3+ doping is demonstrated as a critical strategy to thermodynamically stabilize R-MnO2 while optimizing its electrochemical functionality. Through a synergistic H+/Fe3+ hydrothermal process, spent ZnMn2O4 from alkaline batteries is converted into orthorhombic R-FexMn1-xO2 nanocrystals. Fe3+ incorporation enlarges the tunnel structure, reduces surface energy, and mitigates Jahn-Teller distortion by increasing the Mn4+/Mn3+ ratio. This yields a high specific surface area, enhanced ion diffusion kinetics, and exceptional cycling stability. The R-FexMn1-xO2 cathode achieves a 286.8 mAh g-1 capacity at 0.1 A g-1, outperforming β-MnO2 (30.9 mAh g-1 at 1.5 A g-1). This work establishes Fe3+ doping as an essential mechanism for stabilizing high-performance metastable cathodes, enabling sustainable upcycling of battery waste.
Keywords
INTRODUCTION
Aqueous zinc-ion batteries (AZIBs) have attracted significant attention due to their high safety, low cost, and environmentally friendly characteristics[1-3]. Among previously reported cathode materials, Mn-based oxides have become promising cathode materials for AZIBs due to their advantages in safety and high theoretical capacity. Mn-based materials from spent alkaline batteries (SABs) share chemical homology with AZIB cathodes, enabling resource regeneration from spent batteries into high-performance AZIBs[4,5]. On the other hand, the casual discarding of SABs inevitably leads to the leakage of heavy metals such as zinc (Zn) and manganese (Mn), posing potential threats to the ecological balance and human health, thereby making the recycling and safe disposal of SABs imperative[6].
Manganese dioxide (MnO2) exhibits various phases in aqueous environments, including β-MnO2, α-MnO2, R-MnO2 (ramsdellite-MnO2), δ-MnO2, λ-MnO2, and γ-MnO2. While β-MnO2 demonstrates phase stability, it is limited by its (1 × 1) narrow tunnel structure; conversely, R-MnO2 with (1 × 2) expanded tunnels significantly improves Zn2+ diffusion kinetics[7-9]. Recently, our group investigated the recycling and synthesis of β-MnO2 from SABs through hydrochloric acid hydrothermal treatment[10]. Previous studies show that highly reactive R-MnO2 forms initially during hydrothermal processing, but metastable R-MnO2 transforms rapidly into β-MnO2 with longer treatment or at high temperatures[11]. β-MnO2 hinders H+/Zn2+ extraction/insertion due to its narrow tunnels[12]. In contrast, (1 × 2) tunnels in R-MnO2 enhance ion insertion kinetics[13]. Huang et al. synthesized an R-MnO2-based composite material with supercapacitor characteristics and excellent electrochemical performance[14]. Chen et al. prepared R-MnO2 through the
Meanwhile, challenges remain; lattice collapse of metastable phases (e.g., R-MnO2) during ion insertion causes pulverization of cathode materials. Over the years, much research has employed multidimensional regulation strategies, including ionic doping, surface engineering, and defect engineering, to optimize the physicochemical properties of materials[18]. Pan et al. synthesized Fe-MnO/C via annealing to achieve an MnO crystal structure and demonstrated that Mn-O-Fe bond formation promotes charge transfer kinetics while mitigating structural collapse, thereby synergistically improving rate capability and cycling stability[19]. Zhong et al. showed that iron doping effectively mitigates the Jahn-Teller effect, stabilizes lattice water, and prevents lattice collapse, thus regulating the layered structure in AZIBs[20]. Although ionic doping is widely utilized to stabilize MnO2, the stabilization of metastable phases during synthesis remains underexplored[21,22].
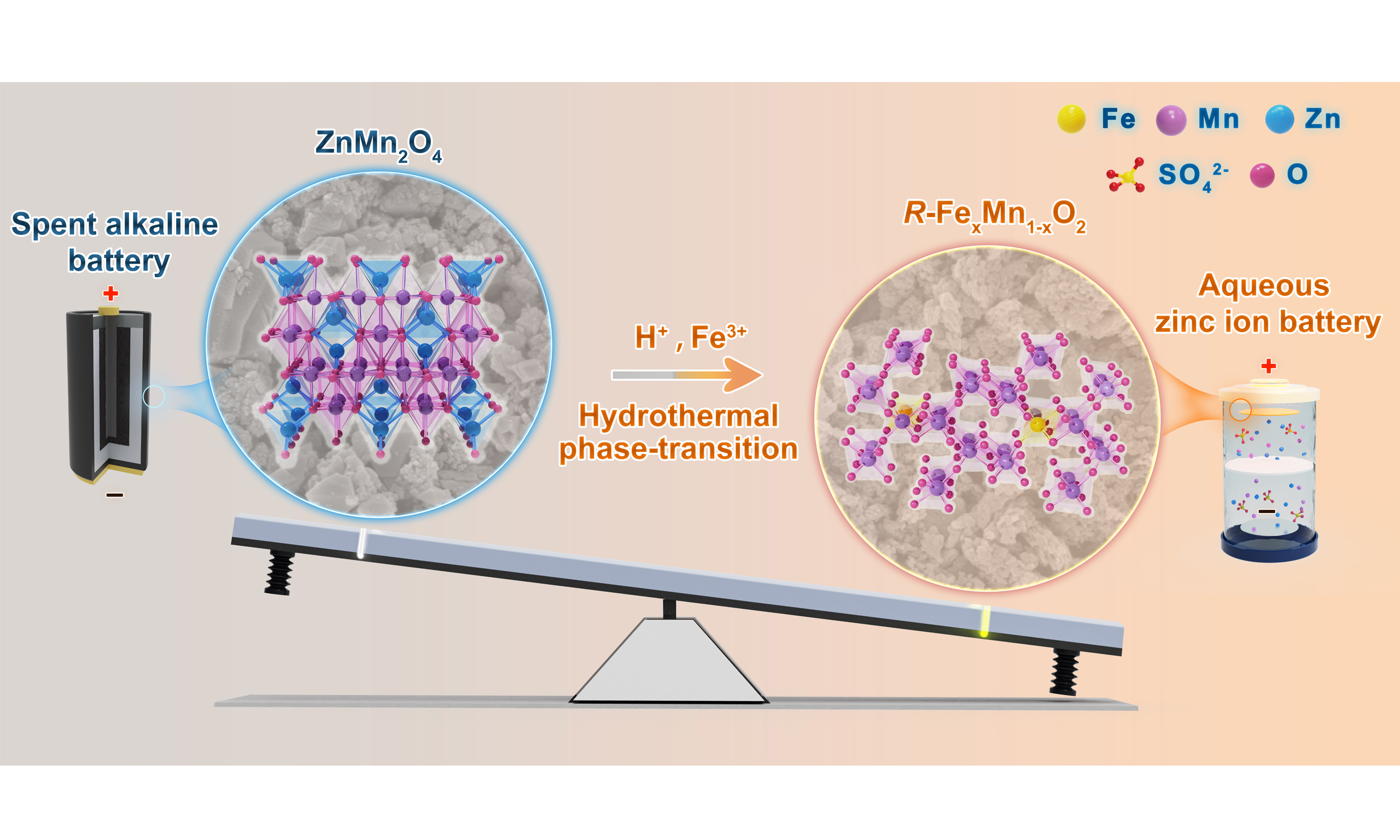
Herein, we demonstrate that Fe3+ doping acts as a dual-function regulator by thermodynamically stabilizing R-MnO2 through reduced surface energy and enlarged (1 × 2) tunnels, while kinetically enhancing Zn2+ diffusion via optimized Mn4+/Mn3+ ratios that suppress Jahn-Teller effects. Through a synergistic H+/Fe3+ hydrothermal process, spent ZnMn2O4 waste is successfully converted into stable R-FexMn1-xO2 nanocrystals, achieving retention of the metastable R-phase, a high surface area of 218 m2 g-1, and low charge-transfer resistance. Electrochemical performance reaches 286.8 mAh g-1 at 0.1 A g-1 with 88.9% capacity retention after 1000 cycles. This study establishes Fe3+ doping as a universal design principle for metastable functional materials, offering simultaneous advancements in AZIB cathode engineering and sustainable battery upcycling.
EXPERIMENTAL
Materials
The materials used in this study include Nanford alkaline batteries (spent battery, LR6-2B/1.5V), potassium hydroxide (KOH, AR), hydrochloric acid (HCl, AR), Sulfuric acid (H2SO4, AR, 98 wt.%), N-methyl pyrrolidone (NMP, AR), and polyvinylidene fluoride (PVDF, AR).
Preparation of materials from spent alkaline batteries
Used Nanford alkaline batteries were further discharged to 0.8 V using a Neware Battery Test System
Synthesis of β-MnO2 and R-FexMn1-xO2
Firstly, 0.9 g of SABs powder was dispersed in 66 mL of DI water. Then, 4 mL of concentrated hydrochloric acid (AR, 37 wt.%) was added dropwise. Subsequently, 0.2g Fe2(SO4)3 was added under continuous stirring at 20 °C for 30 min. The mixture was then transferred to a 100 mL hydrothermal reactor and reacted at
Characterizations
The materials were further characterized using an X-ray diffraction (XRD, Cu Kα, Bruker D8 Advanced, λ = 1.5481 Å) to obtain their structural information. The morphology and microstructure were examined using Scanning Electron Microscopy (SEM, FEI Quanta FEG 250) and Transmission Electron Microscopy (TEM, JEOL 2100). The selective area electron diffraction (SAED) patterns were employed to examine the polycrystalline nature of the materials. The surface elemental properties of the samples were analyzed using an X-ray Photoelectron Spectrometer (XPS, Thermo Fisher Scientific K-Alpha) with an
Electrochemical measurement
The active materials (β-MnO2 and R-FexMn1-xO2), carbon black, and PVDF were uniformly mixed at a 7:2:1 mass ratio. The resulting slurry was coated onto stainless steel foil with mass loading of 0.8~1.2 mg cm-2 and vacuum-dried. The 2032 button cells were assembled using zinc foil anode and glass fiber filter (Whatman GF/D grade) as a separator. The electrolyte was a mixed solution of ZnSO4 (3 M) and MnSO4 (0.05 M). The electrochemical performance was evaluated using a Neware Battery Test System (CT-3008). Cyclic voltammetry (CV) was conducted on a CHI 760E electrochemical station, while electrochemical impedance spectroscopy (EIS) was recorded on a DH7000D electrochemical station.
Computational details
In order to study the mechanism of the reconstruction process, density functional theory (DFT) calculations were used to determine the surface energies of the exposed crystal surfaces R-FexMn1-xO2 (210),
RESULT AND DISCUSSION
R-FexMn1-xO2 was synthesized via a pretreatment of SABs (sequential cleaning with mixed alkali solutions and acid solutions) followed by a 12 h hydrothermal reaction at 140 °C co-induced by H+ and Fe3+, while
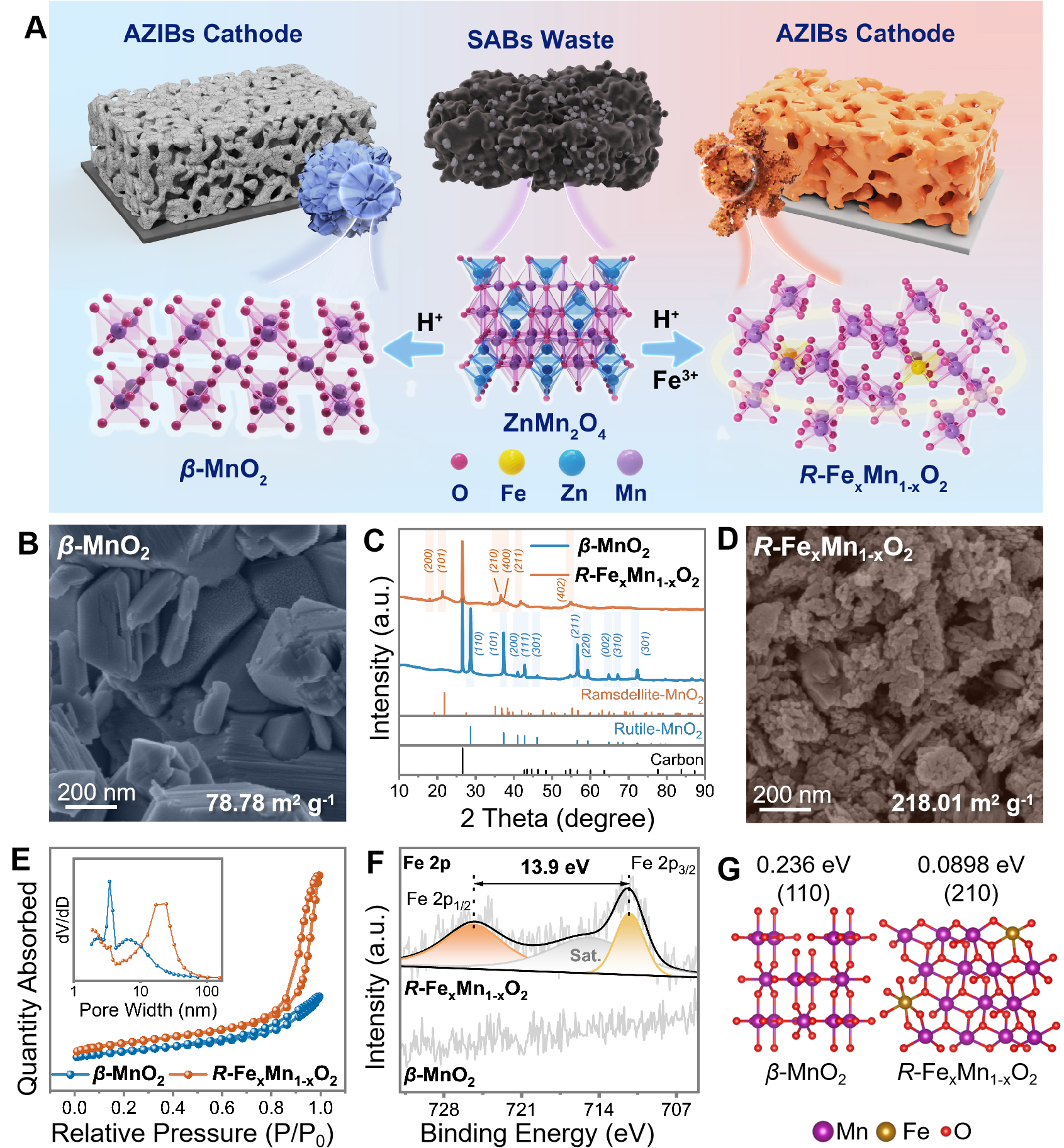
Figure 1. (A) Schematic illustration of the phase evolutions from SABs to β-MnO2 and R-FexMn1-xO2; SEM image and BET surface area of (B) β-MnO2 and (D) R-FexMn1-xO2 (x≈3 wt.%); (C) XRD patterns; (E) N2 adsorption/desorption isotherms and corresponding pore size distributions (inset); (F) Fe 2p XPS spectra; (G) Surface morphology and surface quantities of the pristine surface. SEM: Scanning electron microscopy; BET: brunauer-emmett-teller; XPS: X-ray photoelectron spectrometer; SABs: spent alkaline batteries; XRD: X-ray diffraction.
High-resolution XPS analysis of the Fe 2p in R-FexMn1-xO2 revealed a characteristic spin-orbit splitting energy of 13.9 eV between Fe 2p1/2 and Fe 2p3/2 peaks, confirming iron predominantly exists as Fe3+
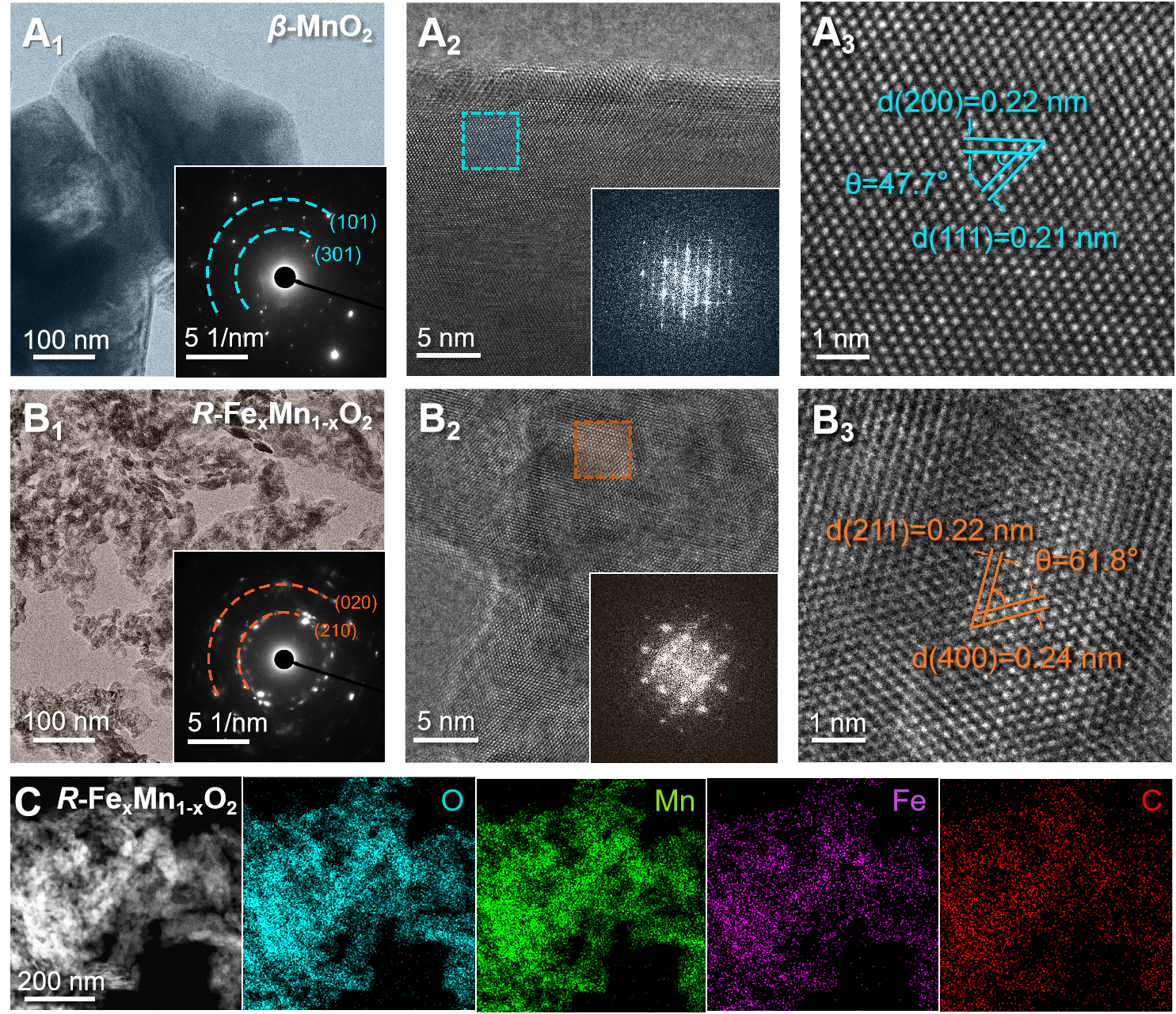
TEM analysis provided detailed elucidation of the material’s morphological characteristics. Figure 2A and B corroborated the morphological features observed by SEM. Compared with β-MnO2 [Supplementary Figure 8]; the particle dimensions of R-FexMn1-xO2 were significantly reduced, consistent with the BET surface area analysis results. SAED and High-Resolution Transmission Electron Microscopy (HRTEM) patterns verified the polycrystalline nature of
Figure 2. The TEM and HRTEM images with corresponding SAED images of (A) β-MnO2, (B) R-FexMn1-xO2. (C) corresponding elemental mapping images of R-FexMn1-xO2 powders. TEM: Transmission electron microscopy; HRTEM: high-resolution transmission electron microscopy; SAED: selective area electron diffraction.
To verify the role of Fe3+ doping in enhancing electrode stability and reaction kinetics, we assembled coin cells to evaluate the electrochemical performance of the materials.
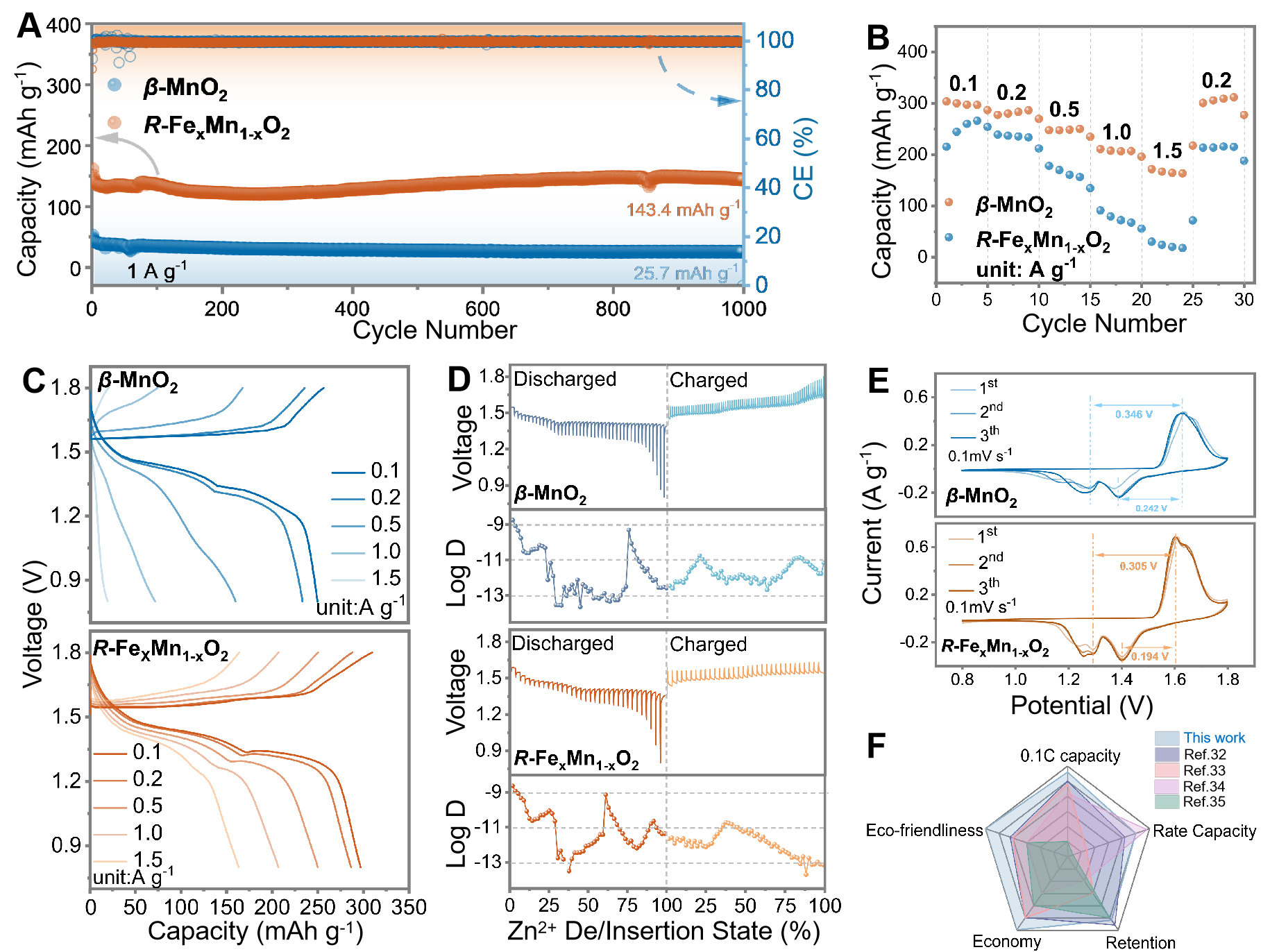
We systematically evaluated the effect of Fe3+ feeding amount on the electrode materials and found that the optimal electrochemical performance was achieved at a Mn:Fe feeding ratio of 100:5 [Supplementary Figures 9-11]. Figure 3A shows the cycling performance of the β-MnO2 and R-FexMn1-xO2 electrodes. The results demonstrate enhanced cycling stability for R-FexMn1-xO2 cathodes compared to β-MnO2 cathodes. At 1.0 A g-1, the R-FexMn1-xO2 cathode exhibited specific capacity of 161.9 mAh g-1 and achieved 88.9% capacity retention after 1000 cycles. In contrast, the β-MnO2 cathode showed a specific capacity of 52.58 mAh g-1 and retained only 48.8 % after cycling [Figure 3A]. The superior cycling capability of R-FexMn1-xO2 can be attributed to Fe3+ doping, which optimizes ion diffusion pathways and enhances the stability of Mn-O bonds (~1.91 Å) through substitution with shorter Fe-O bonds (~1.89 Å). Additionally, the R-FexMn1-xO2 cathodes demonstrated much better rate performance than β-MnO2 cathodes [Figure 3B and Supplementary Figure 11], delivering specific capacities of 286.8, 269.7, 248.5, 206.8, and 163.3mAh g-1 at current densities of 0.1, 0.2, 0.5, 1.0, and 1.5 A g-1, respectively. The β-MnO2 exhibited poor rate capability, achieving only
Figure 3. Electrochemical performance of β-MnO2 and R-FexMn1-xO2 cathodes of (A) cycling performance [Coulombic Efficiency (CE)]; (B) rate capabilities; (C) The GCD curves; (D) GITT profiles and corresponding ion diffusion coefficients of AZIBs; (E) CV curves at scan rate of 0.1 mV s-1. (F) radar chart comparing properties of Mn-based cathodes for AZIBs. GCD: Galvanostatic charge-discharge; GITT: galvanostatic intermittent titration technique; AZIBs: aqueous zinc-ion batteries; CV: cyclic voltammetry.
Furthermore, Galvanostatic Intermittent Titration Technique (GITT) was employed to evaluate Zn2+/H+ diffusion kinetics. The GITT curves for β-MnO2 and R-FexMn1-xO2 cathodes, along with the corresponding diffusion coefficients (D) were presented in Figure 3D. The D value was calculated
These findings suggest that Fe3+ doping stabilizes the R-MnO2 structure, forming the R-FexMn1-xO2 cathode, which not only alleviates lattice strain to enhance structural resilience against H+/Zn2+ extraction/insertion during cycling but also accelerates reaction kinetics. To figure out the structural transitions and reversibility, in situ XRD analysis was performed on the R-FexMn1-xO2 cathode [Supplementary Figure 12]. During discharge, new diffraction peaks emerged corresponding to Zn4SO4(OH)6·5H2O (JCPDS #039-0688), attributed to precipitation involving dissolved H+ ions from the electrolyte. These peaks disappeared during charging. Notably, the peak intensities of R-MnO2 remained basically unchanged throughout the cycling process, confirming the structural stability of R-FexMn1-xO2 under electrochemical operation.
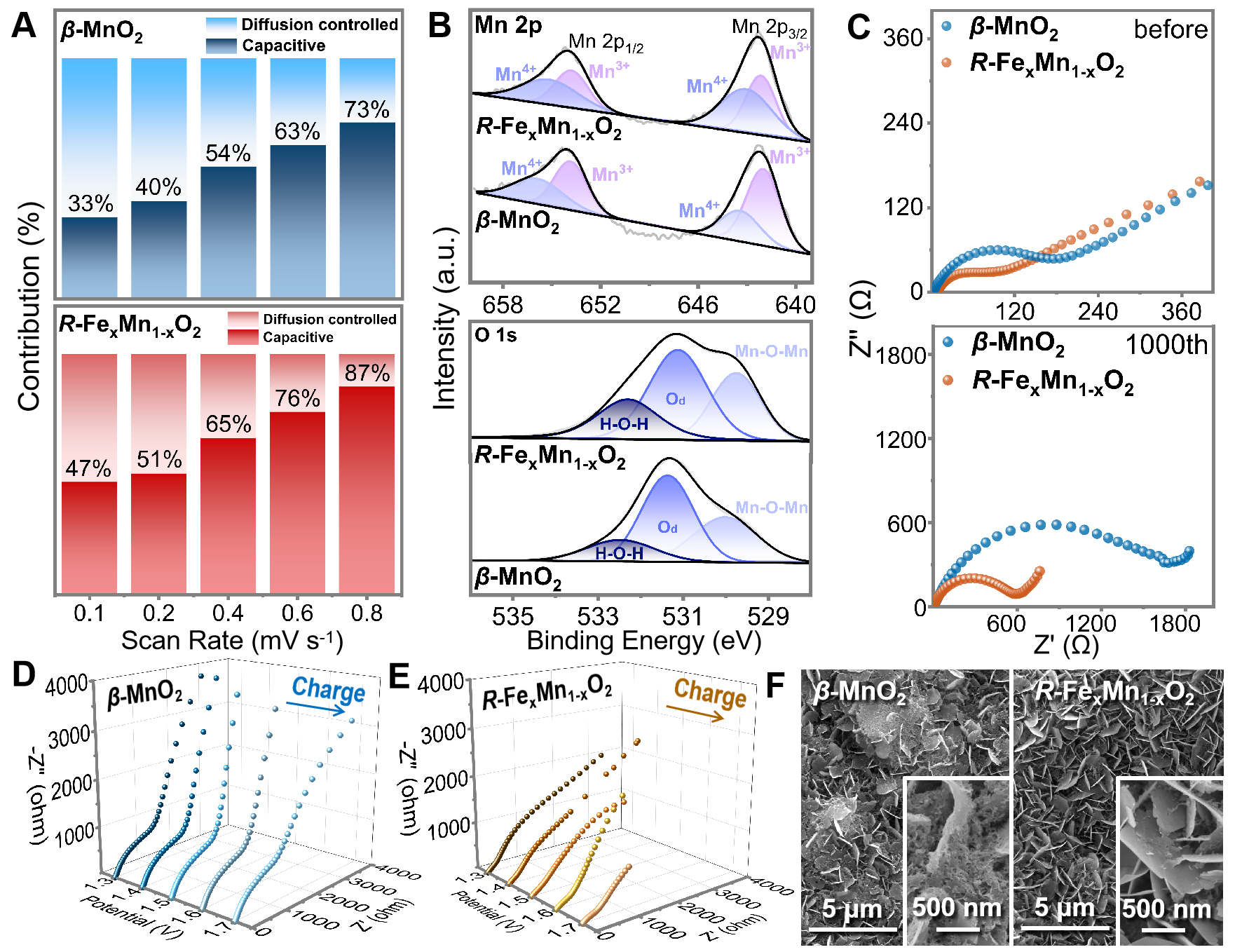
By plotting i/v1/2 versus v1/2 [i(V) = k1v1/2 + k2v] at different potentials, the values of k1 were obtained to calculate the percentage of capacitance contribution. The capacitive contribution for the R-FexMn1-xO2 electrode increased gradually from 47% to 87%, while ranging between 33% and 73% for the β-MnO2 electrode [Figure 4A and Supplementary Figure 13], demonstrating the relatively higher capacitance contribution of R-FexMn1-xO2 cathode[28]. Thus, the enhancement stems from the Fe3+-induced porous nanostructure of R-FexMn1-xO2, which increases the specific surface area and provides additional active sites for faradic reactions, thereby improving surface pseudo-capacitance. Post-cycling XPS analysis [Figure 4B and Supplementary Figure 14] revealed higher Mn4+ content in R-FexMn1-xO2 compared to β-MnO2. This effectively suppresses the Mn3+ proportion, mitigating Jahn-Teller distortion-induced manganese dissolution and enhancing capacity retention. The O 1s spectra showed significantly intensified H-O-H peaks in cycled R-FexMn1-xO2, indicating guest-bonded water that provides shielding by reducing positive charge density at vertex oxygen sites within the host frameworks[29]. Based on above, Fe3+ provided more active sites and expedited reaction kinetics.
Figure 4. Capacitive behaviors and both electrochemical and morphological analysis after 1000 cycles of β-MnO2 and R-FexMn1-xO2 cathodes: (A) Diffusion-controlled and surface-controlled contributions to capacity at different scan rates; (B) Mn 2p and O 1s spectra; (C) Nyquist plots at the open circuit voltage state (Supplementary Figure 15 illustrates the electrical equivalent circuit used for fitting EIS spectra); (D and E) EIS spectra of β-MnO2 and R-FexMn1-xO2 cathodes during charge process; (F) SEM images of β-MnO2 and
EIS before and after 1000 cycles (at 1 A g-1) further elucidated the role of Fe doping in enhancing interfacial stability, as shown in Figure 4C-E. Due to abundant electrochemically active sites and accelerated ion diffusion kinetics, the charge transfer resistance (Rct) value after cycling was significantly lower for
CONCLUSIONS
Fe3+ doping fundamentally transforms the structural stability and electrochemical behavior of metastable ramsdellite MnO2 for zinc-ion batteries. By introducing Fe3+ during the hydrothermal conversion of spent ZnMn2O4, we achieve R-FexMn1-xO2 with engineered (1 × 2) tunnels, reduced surface energy, and optimized Mn4+/Mn3+ ratios. The Fe-mediated mechanism suppresses phase transition to β-MnO2, alleviates lattice strain, and enhances Zn2+/H+ diffusion kinetics, validated by DFT calculations showing lowered surface energy and EIS revealing reduced charge-transfer resistance. Consequently, R-FexMn1-xO2 delivers a high capacity of 286.8 mAh g-1 at 0.1 A g-1, exceptional rate capability (163.3 mAh g-1 at 1.5 A g-1), and long-term stability (88.9% capacity retention after 1000 cycles). Post-cycling analyses confirm Fe3+ mitigates Mn dissolution and structural degradation. This study highlights Fe3+ doping as a universal tunnel-engineering strategy to unlock the potential of metastable MnO2 phases, while offering a sustainable route to repurpose battery waste into high-performance cathodes.
DECLARATIONS
Authors’ contributions
Data curation, methodology, software, writing - original draft, writing - review and editing, funding acquisition: Meng, Y.
Investigation, software, writing - review and editing: Li, Y.
Investigation, software: Xiao, H.; Wang, Z.
Investigation, writing-review and editing: Wang, X.
Software, resources, writing - review and editing: Zhang, F.
Investigation, software, writing - review and editing: Ma, W.
Resources: Xiong, D.; Zhou, T.; Yin, J.
Writing-review and editing: Xiao, Z.
Resources, funding acquisition: Yuan, Z.
Conceptualization, formal analysis, funding acquisition, methodology, resources, supervision, writing-review and editing: Yang, L.
Data analysis, resources: Liu, C.
Supervision, resources: Wu, X.
Availability of data and materials
The linked data has been added to the manuscript. The raw/ processed data required to reproduce these findings can be obtained upon request from the first author.
Financial support and sponsorship
This work is supported by Natural Science Foundation of China (52234001), Science and Technology Planning Project of Hunan Province (2018TP1017), and National Students’ Platform for Innovation and Entrepreneurship Training Program (S202410542060).
Conflicts of interest
Wu, X. is Editorial Board Member of the journal Energy Materials. Wu, X. was not involved in any steps of editorial processing, notably including reviewers’ selection, manuscript handling, and decision making. Yuan, Z. and Zhou, T. are affiliated with Xiangtan Electrochemical Technology Co., Ltd. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Chao, D.; Zhou, W.; Xie, F.; et al. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098.
2. Lai, G.; Zhao, Z.; Zhang, H.; et al. In-situ positive electrode-electrolyte interphase construction enables stable Ah-level Zn-MnO2 batteries. Nat. Commun. 2025, 16, 2194.
3. Luo, C.; Lei, H.; Xiao, Y.; et al. Recent development in addressing challenges and implementing strategies for manganese dioxide cathodes in aqueous zinc ion batteries. Energy. Mater. 2024, 4, 400036.
4. Hu, X.; Robles, A.; Vikström, T.; Väänänen, P.; Zackrisson, M.; Ye, G. A novel process on the recovery of zinc and manganese from spent alkaline and zinc-carbon batteries. J. Hazard. Mater. 2021, 411, 124928.
5. Xu, Z.; Zhang, N.; Wang, X. Upcycling spent alkaline batteries into rechargeable zinc metal batteries. Nano. Energy. 2022, 102, 107724.
6. Zhu, J.; Tie, Z.; Bi, S.; Niu, Z. Towards more sustainable aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2024, 63, e202403712.
7. Kitchaev, D. A.; Dacek, S. T.; Sun, W.; Ceder, G. Thermodynamics of phase selection in MnO2 framework structures through alkali intercalation and hydration. J. Am. Chem. Soc. 2017, 139, 2672-81.
8. Peng, H.; Wang, C.; Wang, D.; Song, X.; Zhang, C.; Yang, J. Dynamic Zn/electrolyte interphase and enhanced cation transfer of sol electrolyte for all-climate aqueous zinc metal batteries. Angew. Chem. Int. Ed. 2023, 62, e202308068.
9. Abdalla, K. K.; Wang, Y.; Abdalla, K. K.; et al. Rational design and prospects for advanced aqueous Zn-organic batteries enabled by multielectron redox reactions. Sci. China. Mater. 2024, 67, 1367-78.
10. Xiao, H.; Xiong, D.; Lu, B.; et al. Ramsdellite-MnO2 regeneration via acid-mediated redox tuning toward rechargeable aqueous zinc-ion batteries. Inorg. Chem. 2025, 64, 8322-33.
11. Cao, J. M.; Liu, Y.; Li, K.; et al. Coulombic-hinderance regulation on pyrovanadates for practicable calcium-ion batteries: a solid-solution strategy. Natl. Sci. Rev. 2025, 12, nwaf074.
12. Xiao, J.; Gao, H.; Xiao, Y.; et al. A hydro-stable and phase-transition-free P2-type cathode with superior cycling stability for high-voltage sodium-ion batteries. Chem. Eng. J. 2025, 506, 160010.
13. Zhang, A.; Zhao, R.; Wang, Y.; et al. Hybrid superlattice-triggered selective proton grotthuss intercalation in δ-MnO2 for high-performance zinc-ion battery. Angew. Chem. Int. Ed. 2023, 62, e202313163.
14. Huang, X.; Li, Y.; Yan, X.; et al. Microstructure optimization and electrochemical behavior of in-situ growth Ramsdellite-MnO2@NCA-LDH@CC for supercapacitors and oxygen evolution reaction catalysts. J. Materiomics. 2024, 10, 552-65.
15. Chen, C.; Xu, K.; Ji, X.; Zhang, B.; Miao, L.; Jiang, J. Enhanced electrochemical performance by facile oxygen vacancies from lower valence-state doping for ramsdellite-MnO2. J. Mater. Chem. A. 2015, 3, 12461-7.
16. Li, S.; Zhong, Y.; Huang, J.; et al. Regulating interfacial kinetics boosts the durable A h-level zinc-ion batteries. Energy. Environ. Sci. 2025, 18, 2599-609.
17. Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A. 2015, 3, 2485-534.
18. Xu, Y.; Wang, Y.; Dong, N.; et al. Novel polyimide binder for achieving high-rate capability and long-term cycling stability of LiNi0.8Co0.1Mn0.1O2 cathode via constructing polar and micro-branched crosslinking network structure. J. Energy. Chem. 2023, 76, 19-31.
19. Pan, G.; Wang, Z.; Zhang, J.; Cao, M.; Zhang, L.; Zhang, J. Porous carbon-coated Fe-doped MnO as high-performance cathode for aqueous zinc ion batteries. Energy. Tech. 2025, 13, 2401690.
20. Zhong, Z.; Li, J.; Li, L.; et al. Improving performance of zinc-manganese battery via efficient deposition/dissolution chemistry. Energy. Storage. Mater. 2022, 46, 165-74.
21. Yang, Z.; Pan, X.; Shen, Y.; et al. New insights into phase-mechanism relationship of MgxMnO2 nanowires in aqueous zinc-ion batteries. Small 2022, 18, 2107743.
22. Li, X.; Sun, Y.; Zhou, L.; et al. Suppressing Jahn-Teller distortion and locking lattice water with doped Fe(III) in birnessite toward fast and stable zinc-ion batteries. Mater. Horiz. 2024, 11, 4133-43.
23. Chen, X.; Han, L.; Zhao, G.; et al. Construction of MnO2-Mn3O4 heterostructures to facilitate high-performance aqueous magnesium ion energy storage. Chem. Commun. 2024, 60, 3067-70.
24. Li, Z.; Huang, Y.; Zhang, J.; Jin, S.; Zhang, S.; Zhou, H. One-step synthesis of MnOx/PPy nanocomposite as a high-performance cathode for a rechargeable zinc-ion battery and insight into its energy storage mechanism. Nanoscale 2020, 12, 4150-8.
25. Zhao, Y.; Zhang, S.; Zhang, Y.; et al. Vacancy-rich Al-doped MnO2 cathodes break the trade-off between kinetics and stability for high-performance aqueous Zn-ion batteries. Energy. Environ. Sci. 2024, 17, 1279-90.
26. Ding, S.; Zhang, M.; Qin, R.; et al. Oxygen-deficient β-MnO2@ graphene oxide cathode for high-rate and long-life aqueous zinc ion batteries. Nano-Micro. Lett. 2021, 13, 173.
27. Yang, X.; Qiao, Z.; Liu, F.; et al. In-depth study of electrochemical capacitor performance of MnO2 during phase transition from Ramsdellite-MnO2 to birnessite-MnO2. Electrochim. Acta. 2018, 280, 77-85.
28. Huang, H.; Feng, H.; He, Z.; et al. Enhancing Zn2+/H+ joint charge storage in MnO2: concurrently tailoring Mn’s local electron density and O’s p-band center. ACS. Nano. 2024, 18, 25601-13.
29. Yao, H.; Yu, H.; Zheng, Y.; et al. Pre-intercalation of ammonium ions in layered δ-MnO2 nanosheets for high-performance aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2023, 62, e202315257.
30. Xu, Z.; Wang, J.; Zhang, W.; et al. Hydrogen-bond chemistry inhibits Jahn-Teller distortion caused by Mn 3d orbitals for long-lifespan aqueous Zn//MnO2 batteries. J. Mater. Chem. A. 2024, 12, 25491-503.
31. Long, J.; Yang, F.; Cuan, J.; et al. BoosteD CHARGE TRANSFER IN TWINBORn α-(Mn2O3-MnO2) heterostructures: toward high-rate and ultralong-life zinc-ion batteries. ACS. Appl. Mater. Interfaces. 2020, 12, 32526-35.
32. Jiang, S.; Han, W.; Fang, L.; et al. Achieving non-interfacial blocking zinc ion transport based on MOF derived manganese oxides and amorphous carbon hybrid materials. Chem. Eur. J. 2024, 30, e202401802.
33. Ren, Y.; Meng, F.; Zhang, S.; et al. CNT@MnO2 composite ink toward a flexible 3D printed micro-zinc-ion battery. Carbon. Energy. 2022, 4, 446-57.
34. Ruo, H.; Chen, L.; Huang, J.; et al. Constructing low-cost stable zinc-ion batteries with sodium-rich monoclinic manganese hexacyanoferrate cathode. Surf. Interfaces. 2024, 51, 104594.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].