Sea urchin-like La-doped MnO2 for electrocatalytic oxidation degradation of sulfonamide in water
Abstract
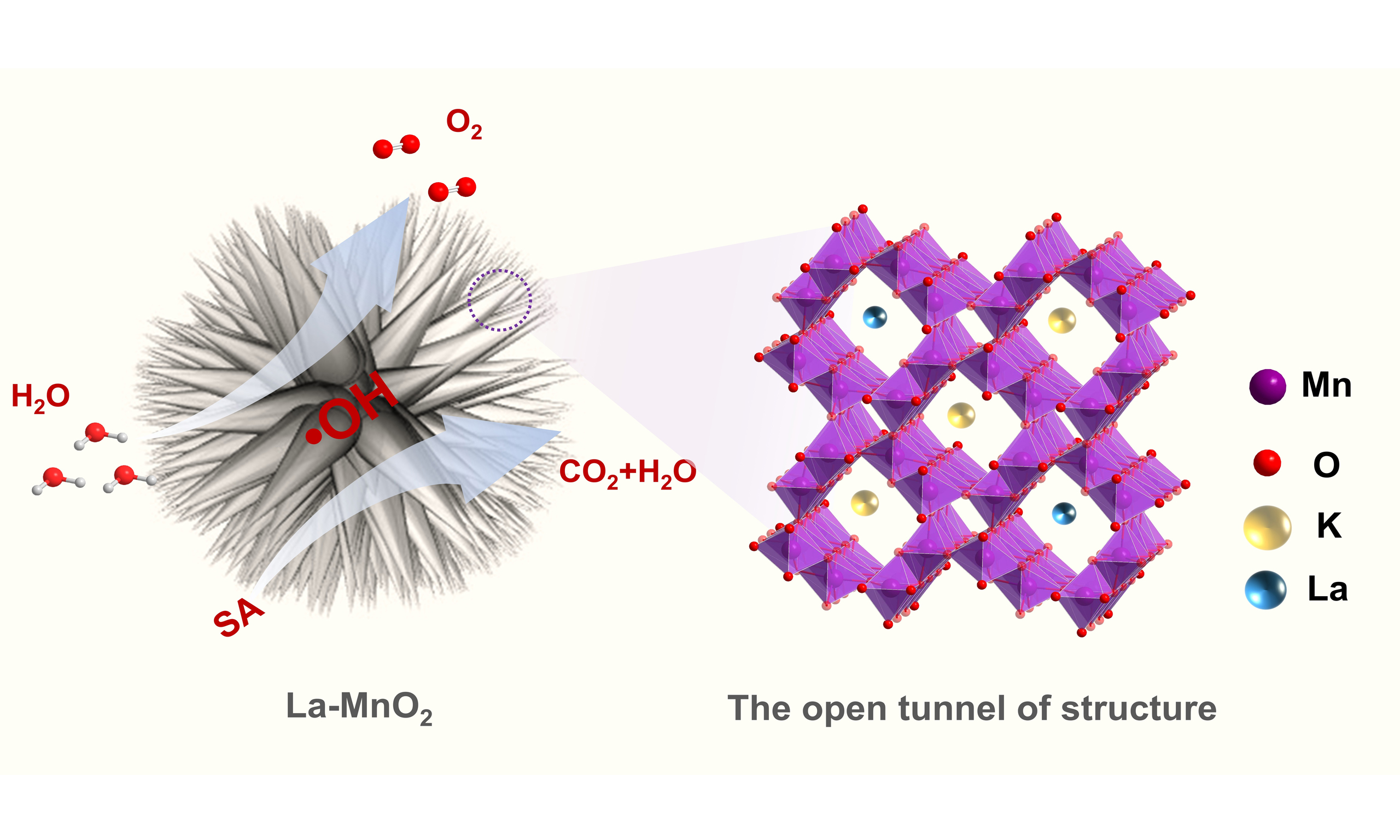
A sea urchin-like La-doped α-MnO2 catalyst was successfully synthesized by a one-step hydrothermal method. The as-prepared material exhibits electrocatalytic activity for water oxidation, achieving an overpotential of 450 mV to reach a catalytic current density of 10 mA/cm2 in 1.0 M KOH solution. The urchin morphology and open tunneling structure can fully expose the rich active sites and facilitate mass transfer during electrochemical oxidation process. Moreover, the catalyst is also applied for sulfonamide degradation due to the generation of strong oxidizing •OH species during the process of water oxidation. The indirect oxidation of sulfonamide by •OH species was confirmed through radical quenching and capture studies. The catalyst degraded sulfonamide antibiotics with up to 40% efficiency within 2 h. The introduction of the heteroatom La3+ into MnO2 led to a redistribution of electrons around Mn, which altered the electron density of the metal sites, lowered the average valence state of Mn, facilitated the production of reactive MnIII species, and optimized the exposure density of the active sites. Therefore, the sea urchin-like La-doped MnO2 material shows potential for applications in electrochemical sustainability research.
Keywords
INTRODUCTION
Sulfonamides (SAs) are a common class of spectral antimicrobial drugs widely used in clinical medicine and veterinary health. However, their biotoxicity in aquatic environments has raised concerns[1-4]. Their antibacterial properties can inhibit biodegradation, leading to pollution and the accumulation of this emerging contaminant in soil, surface water, groundwater, and drinking water[5-7]. Moreover, sulfonamide antibiotics are readily transported in the environment due to their extensive use and strong hydrophilic and acid-base properties. As a result, even trace amounts in water bodies can be bioaccumulated through food chains, posing a serious threat to human health[8]. Therefore, there is an urgent need to develop environmentally friendly, efficient, and cost-effective methods for the degradation and removal of sulfonamide antibiotics, which have become a major focus of research. Current approaches to managing antibiotic contamination include physical adsorption, biodegradation, and chemical degradation[9,10]. Physical adsorption offers simple operation, low cost, and reproducibility. Common adsorbents include activated carbon, carbon nanotubes, graphene, and minerals and metal oxides such as montmorillonite and kaolinite. To enhance the performance of traditional adsorbents, modifications such as acidic, alkaline and oxidative treatment can be employed to optimize surface properties and pore structures, thereby improving adsorption capacity and selectivity[11,12]. However, this method typically only transfers the antibiotic to a different phase without achieving actual degradation. It also carries the risk of secondary pollution upon adsorption saturation and is susceptible to interference from coexisting substances. Biological degradation relies on microbial metabolism, offering advantages such as environmental friendliness, low operating costs, and the potential for complete harmless disposal[13,14]. Degrading bacteria are often sourced from contaminated environments or activated sludge in wastewater treatment systems. Degradation occurs either through direct metabolism of antibiotics or via co-metabolism during growth on substrates. However, microbial activity is limited by the antibiotics’ antimicrobial properties and low environmental concentrations, resulting in generally low degradation efficiency, slow processes, and potential induction of drug-resistant bacteria and resistance genes. Chemical degradation, in contrast, relies on strong oxidative reactions to break down antibiotic molecules, offering high efficiency, rapid processing, suitability for
Inspired by the structure of Mn4CaO5 clusters in natural photosystem II (PS II), manganese-based catalysts have attracted considerable research interest[26,27]. Manganese dioxide (MnO2) is a promising electrocatalyst with great potential in electrocatalytic and environmental purification applications due to its strong oxidizing properties, high abundance, good acid resistance, low toxicity, low cost, and environmental adaptability[28,29]. Strategies to optimize the activity of MnO2 electrocatalysts primarily include increasing intrinsic surface activity, improving mass diffusion efficiency, and enhancing charge transfer ability[30]. Considerable progress has been made in optimizing MnO2 electrocatalyst activity through morphological control. MnO2 nanostructures can be synthesized in diverse forms, including sea urchin-like, flower-like, tubular, plate-like, spherical, and wire-like architectures. Tailored morphologies significantly influence electrocatalytic performance by modulating surface area, charge transfer kinetics, and exposure of active sites. For example, Chen et al. synthesized a unique three-dimensional α-MnO2 nanowire network (NWN) via a mild, surfactant-free hydrothermal method. The structure consists of interconnected nanowires extending continuously in all directions, enhancing both hydrophilicity and electrical conductivity, which is highly favorable for electrocatalytic applications[31]. Further improvements have been achieved through heteroatom doping, a key strategy for enhancing oxygen evolution reaction (OER) activity. Doping modifies electronic structure, increases active site density, and optimizes reaction pathways[32,33]. Heteroatom regulation is an efficient and precise approach for material design, encompassing both non-metallic and metallic doping. Non-metallic doping primarily modifies the surface chemistry of carbon-based materials through charge polarization and defect engineering[34,35], whereas metallic doping introduces active metallic centers or induces lattice strain and oxygen vacancies, resulting in significant improvements in electronic properties[36]. Rare earth metals exhibit exceptional capabilities in doping modifications due to their unique 4f electron configurations, variable oxidation states, and abundant energy level transitions[37,38]. For instance, lanthanum, with its large ionic radius (~106.1 pm), can induce substantial lattice distortion and strain, while its high oxygen affinity contributes to modulation of surface oxygen chemistry.
Herein, sea urchin-like La-doped MnO2 was prepared via a one-step simple hydrothermal method. By controlling the addition of H2SO4, uniform sea urchin-like La-doped MnO2 was synthesized. The material exhibited electrocatalytic activity for water oxidation in 1.0 M KOH solution, achieving a catalytic current density of 10 mA/cm2 at an overpotential of 450 mV. Additionally, the synthesized catalyst was applied for the electrochemical oxidation degradation of SA. Leveraging its optimized interfacial charge transfer and the generation of hydroxyl radicals (•OH), the sea urchin-like La-doped MnO2 synergistically facilitates both direct and indirect oxidation processes, achieving a degradation efficiency of nearly 40% within 2 h. Therefore, the sea urchin-like La-doped MnO2 shows promising potential for applications in electrochemical sustainability research.
EXPERIMENTAL
General materials
MnSO4·H2O (95.0%, Alfa), La(NO3)3·6H2O (99.99%, Alfa), K2S2O8 (98.0%, Alfa), H2SO4 (98%, Energy Chemical), sulfanilamide (SA) (≥ 98%, Merck), Na2SO4 (98%, Energy Chemical), tert-butanol (TBA) (99.5%, Energy Chemical), methanol (MeOH) (≥ 99.5, Energy Chemical), p-Benzoquinone (p-BQ) (95%, Energy Chemical), 5,5-Dimethyl-1-pyrroline N-oxide (DMPO) (98%, Energy Chemical), and Nafion (5 wt%, DuPont) were used without further purification. Milli-Q water of 18 MΩ cm was used in all experiments unless otherwise stated.
Preparation of catalysts
The synthesis of the sea urchin-like MnO2 (α-MnO2): 2 mmol MnSO4·H2O, 2 mmol K2S2O8 and 2 mL concentrated H2SO4 were mixed in 38 mL of water and stirred for 10 min to form a homogeneous solution at room temperature. Then, the solution was transferred into a Teflon-lined stainless-steel autoclave
The synthesis of the sea urchin-like La-doped MnO2: The procedure was the same as above, except that an additional 0.1 mmol La(NO3)3·6H2O was added to the reactant solution prior to the hydrothermal treatment.
Physical characterizations
The X-ray diffraction (XRD) patterns were collected using a Rigaku D/Max2550VB+/PC X-ray diffractometer operated with Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 100 mA. The X-ray photoelectron spectroscopy (XPS) spectra were obtained on a Kratos AXIS ULTRA XPS analyzer using monochromatic
Electrocatalytic water oxidation
The electrochemistry was tested on CHI 660E electrochemical workstation with a three-electrode system using 1.0 M KOH as electrolyte under constant temperature of 25 °C. A standard three-electrode system, in which the Glass Carbon (GC, 0.07 cm2) loaded with catalyst was the working electrode, Ag/AgCl (3 M KCl) electrode was the reference electrode and the graphite rod was the counter electrode. Typically, the catalyst link was prepared with 2 mg sample, 20 μL of Nafion, and 0.5 mL of water-ethanol solution at a volume ratio of 2:1. The mixed solution was ultrasonically treated for 30 min. Then, 6 μL of the catalyst ink was dropped on the effective working area of the GC and dried at room temperature. The potentials were determined with respect to the reversible hydrogen electrode (RHE) using ERHE = EAg/AgCl + (0.197 + 0.059
Electrochemical degradation of sulfonamide
The electrochemical degradation of SA was conducted using a CHI 660E electrochemical workstation at room temperature with a standard three-electrode configuration. The working electrode was a
RESULTS AND DISCUSSION
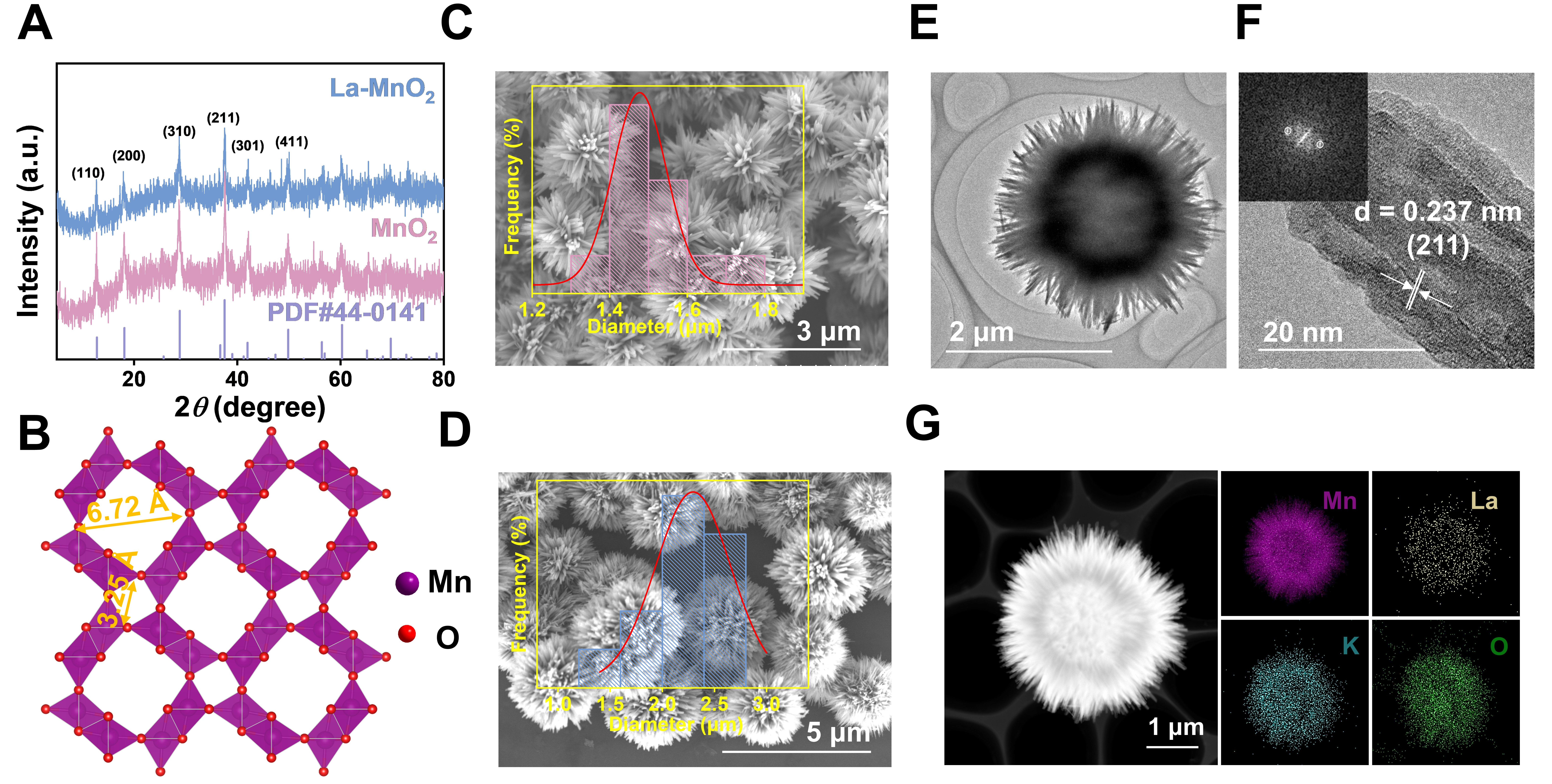
The XRD patterns show the phase information of the material. The XRD patterns of synthesized samples in Figure 1A indicate a pure phase of α-MnO2 (JCPDS 44-0141), and the introduction of trace La ions did not change the physical phase structure of the catalysts. MnO6 octahedral units of α-MnO2 structure are connected in an edge-shared and vertex-shared forms, resulting in 2 × 2 and 1 × 1 tunnel structures at a space of 6.72 and 3.25 Å, respectively [Figure 1B]. During synthesis, K+ ions may be present in these tunnels as anti-balance cations[40]. These open pore structures can provide abundant reaction sites and a large number of pathways for molecules, which enhance catalytic efficiency. The pore structure can be clearly seen from the high-resolution transmission electron microscopy (HR-TEM) image shown in Supplementary Figure 1A[41]. The SEM images show that the MnO2 exhibits a sea urchin spherical structure with a uniform scale and a diameter of about 1.4~1.5 μm from the particle size distribution graph [Figure 1C]. Sea urchin-like MnO2 was synthesized by fine control of the amount of H2SO4 used in the hydrothermal process, where H2SO4 offers the acidic environment. Supplementary Figure 1B-E shows the morphologies of MnO2 synthesized by adding different concentrations of H2SO4. An imperfect sea
Figure 1. (A) The XRD patterns of La-doped MnO2 and MnO2; (B) The crystal structure of α-MnO2; The SEM images of (C) the sea urchin-like MnO2 and (D) La-doped MnO2 (The inset shows the particle size distributions); (E) The TEM and (F) HR-TEM images of
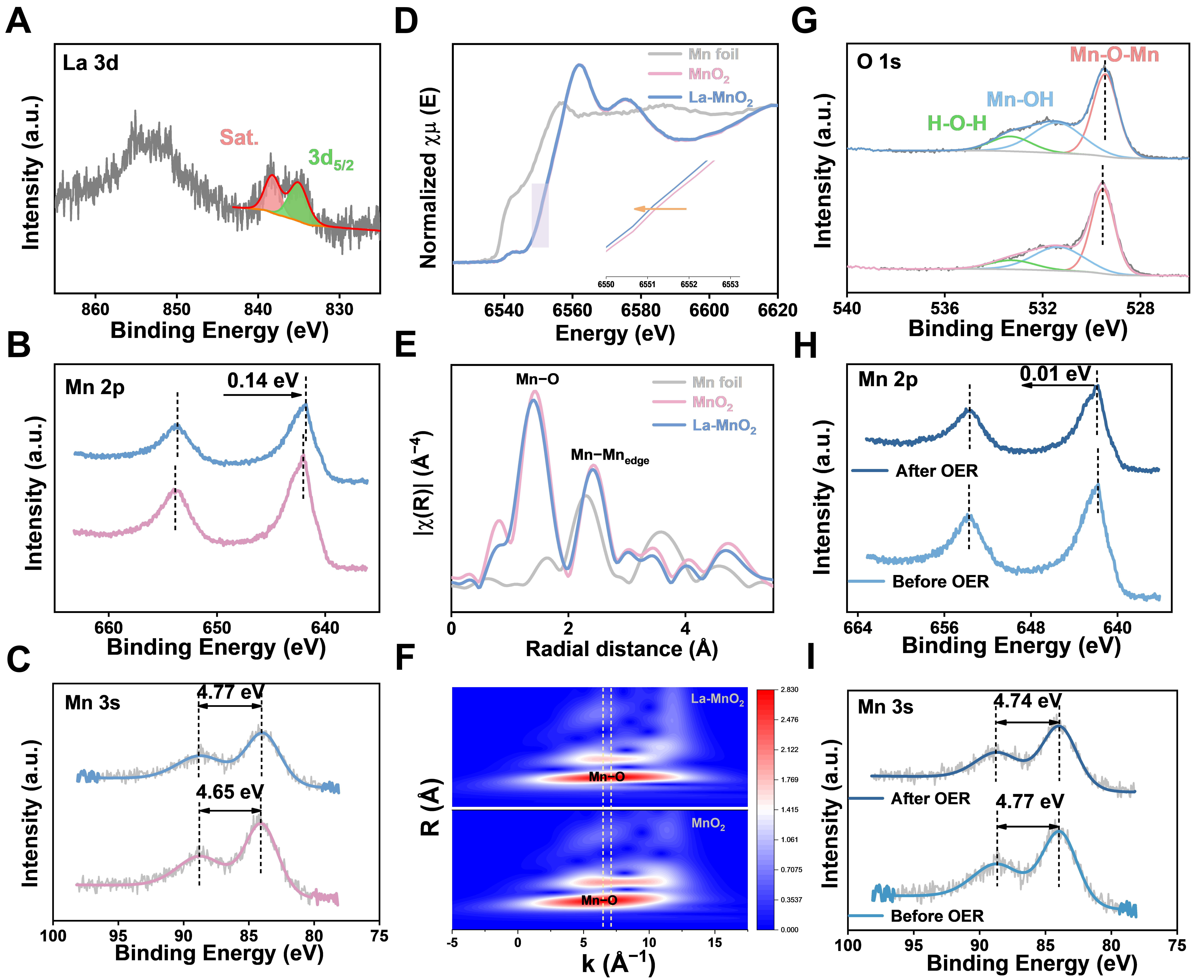
After the OER test, the K 2s signal further weakens in MnO2, while the La 3d signal in La-doped MnO2 gradually disappears [Supplementary Figure 6B-D], suggesting that La3+ may occupy sites similar to those of K+. This inference is further supported by the ICP-OES results, which show a reduction in K+ content following La3+ incorporation. In summary, the La3+ likely occupies tunnel sites analogous to K+.
In order to further characterize the surface states of samples, the synthesized α-MnO2 samples were studied by XPS. The peaks at 834.8 eV correspond to La 3d5/2, along with one satellite feature at 838.1 eV[42]. However, the splitting peak of La 3d3/2 around 850 eV is affected by the Mn LMM peak (852.69 eV), which prevents an accurate fit. This suggests the oxidation state of La3+ in La-doped MnO2 [Figure 2A]. The
Figure 2. (A) The La 3d XPS spectrum of La-doped MnO2; The XPS spectra comparison of Mn 2p (B), Mn 3s (C) for La-doped MnO2 (blue) and pure MnO2 (pink); (D) The XANES of Mn K-edge for La-doped MnO2, MnO2 and Mn foil reference; (E) FT spectra of
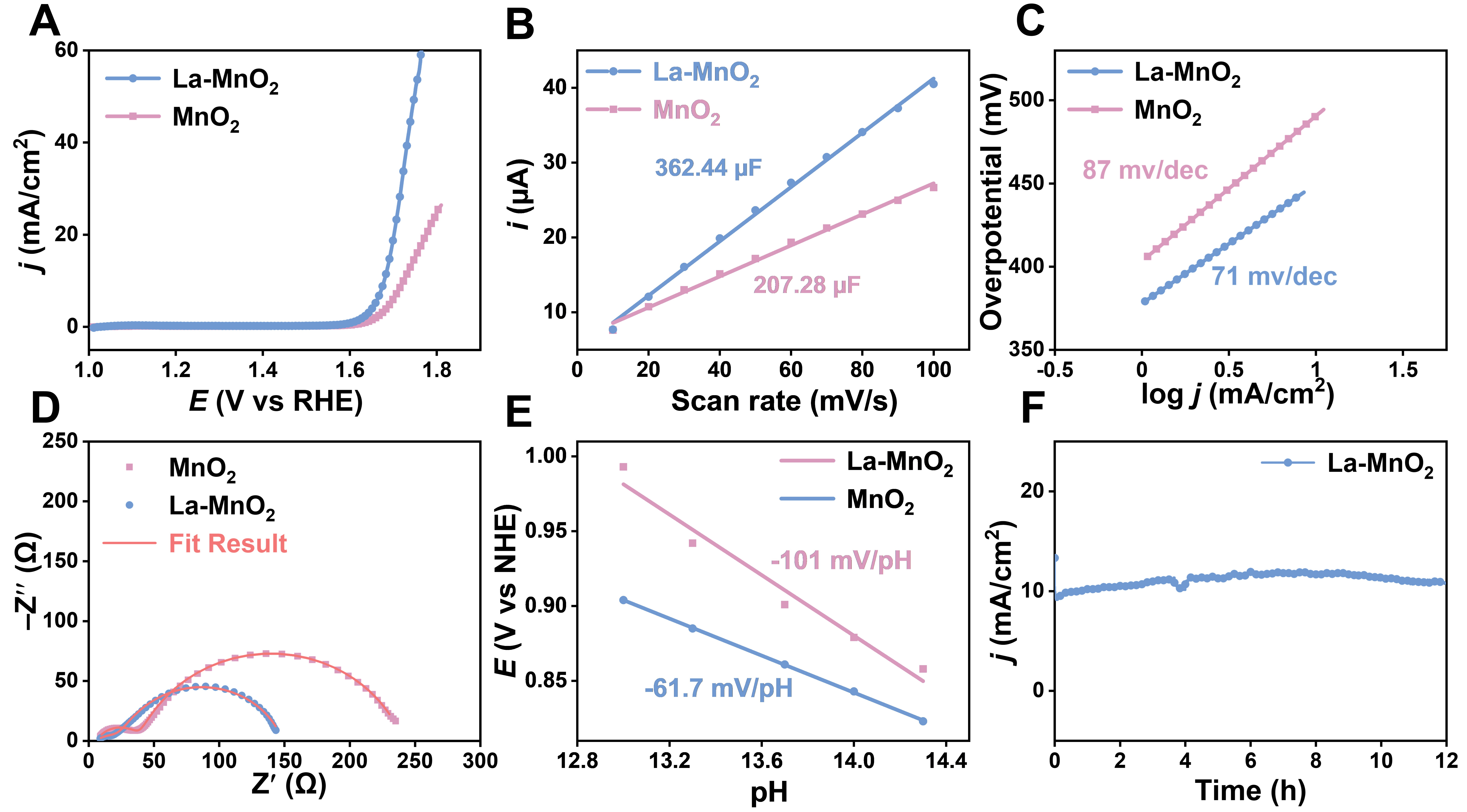
Electrocatalytic water decomposition and electrochemical oxidative degradation of SAs are two important electrochemical processes. The former is for hydrogen generation with clean energy and the latter is used to degrade organic pollutants to remediate the environment. Despite having different objectives, they both involve electron transfer and redox reactions that depend on the performance of the electrocatalyst. The catalysts are required to have high electrical conductivity, high specific surface area, abundant active sites, and good stability. The electrocatalytic water decomposition process also produces strongly oxidizing species, and the generation of such intermediates is equally applicable to the electrochemical oxidative degradation of SAs. Therefore, a typical three-electrode system was used to test OER activity of the sample with a scan rate of 5 mV s-1 in 1.0 M KOH, first. The overpotential of sea urchin-like La-doped MnO2 is
Figure 3. (A) OER LSV polarization curves, (B) the anodic charging currents at 1.34 V (vs. RHE) plotted against the scan rates, (C) the Tafel plots, (D) the Nyquist plots with fitting, and (E) the potentials against the pH values of the electrolytes at 4 mA/cm2 of La-doped MnO2 and MnO2; (F) The CPE of the La-doped MnO2 electrocatalyst at 1.75 V (vs. RHE) without iR compensation. OER: Oxygen evolution reaction; RHE: reversible hydrogen electrode; CPE: controlled potential electrolysis.
Long-term stability testing was conducted on La-doped MnO2 at a potential of 1.75 V (without iR compensation) [Figure 3F]. The results of the CPE tests show that the La-doped MnO2 maintains excellent catalytic activity with a current density of around 10 mA/cm2 over a period of 12 h, which highlights its remarkable electrochemical stability in alkaline environments. The CPE of the pure MnO2 is also shown in Supplementary Figure 13. Initial fluctuations are a natural manifestation of the system’s transition from a non-equilibrium state to a quasi-steady/steady state. Initial fluctuations in electrocatalytic stability tests typically stem from transient processes: (1) electrode surface activation, involving reconstruction, dissolution/redeposition, or chemical state evolution of metastable fresh surfaces toward electrochemical equilibrium; (2) interfacial establishment, characterized by incomplete electrolyte wetting and evolving diffusion layers that affect the reaction area and mass transport; and (3) adsorbate accumulation, where dynamic coverage equilibria of intermediates or impurities influence catalytic activity and surface properties. Further observation of SEM images after OER testing showcased no significant change in the overall morphology of La-doped MnO2 and MnO2 catalysts, which retained their fine sea urchin-like morphology with a uniform particle size distribution, thus affirming their structural stability
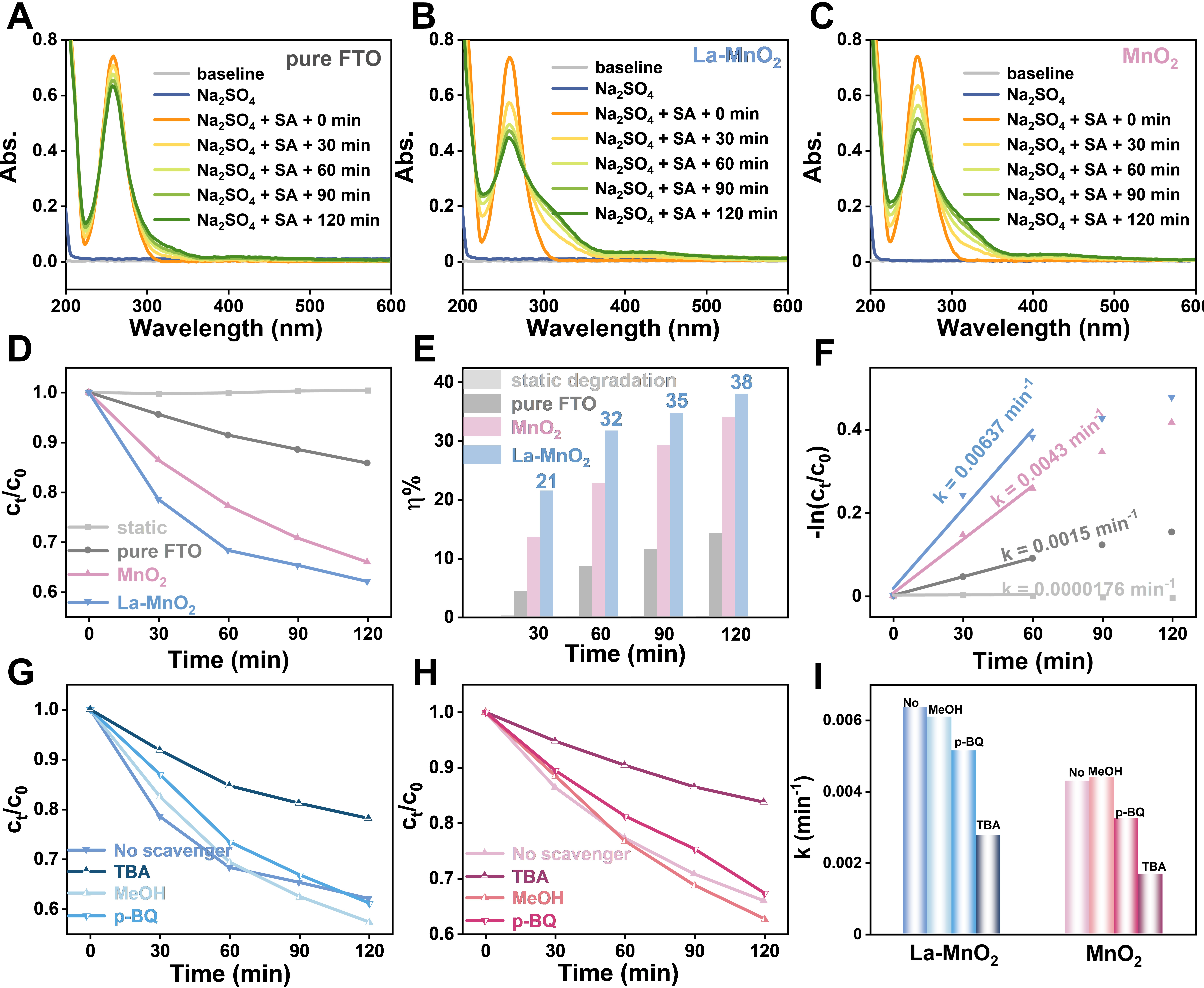
After clarifying the electrochemical activity of the α-MnO2 material, it was applied to SA degradation experiments. SAs are a class of medicines with p-aminobenzene sulfonamide structure, as shown in Supplementary Figure 16. Electrocatalytic oxidation of SA occurs simultaneously in two ways: direct and indirect oxidation processes. Direct oxidation uses electron transfer at the electrode to protonate sulfanilamide; and indirect oxidation uses electrocatalytic water oxidation to produce a strongly oxidizing intermediate product to oxidize SA to produce CO2, H2O, and small molecule acid [see Supplementary Equations 4 and 5][50]. It has the advantages of simple operation, wide application, high oxidation ability, environmental friendliness, low secondary waste generation and oxidant-free feature compared with other traditional technologies[51]. Thus, in this work, the prepared sea urchin-like La-doped MnO2 and pure MnO2 were used for electrochemical degradation of SA, respectively. The catalyst was loaded on FTO conductive glass and the degradation experiment was carried out with 0.05 M Na2SO4 electrolyte. The UV-vis absorption increased with increasing sulfanilamide concentration and was plotted against the standard curve [Supplementary Figure 17A and B]. The initial concentration was 0.034 mmol/L for SA and the electrolytic voltage was 2.1 V. Then, SA degradation occurs at different times of electrolysis. The primary reaction of SA degradation by electrocatalytic oxidation is a process in which direct and indirect oxidation work together. A part of SA is directly oxidized on the anode surface by electron transfer to generate primary products, while the other part of SA is oxidized indirectly with •OH produced by the electrolysis system. The two primary products continue to be oxidized by •OH to produce maleic acid and fumaric acid. Thus, the same potential applied to the blank FTO electrode was used to observe the degradation of SA at first to clarify the degradation of the electrode surface [Figure 4A]. The blank FTO electrode also showed degradation of SA, with 15% degradation efficiency of SA within two h. Then, the La-doped MnO2 and pure MnO2 catalysts were drop-coated on the surface of the FTO electrode, and the degradation of SA was dramatically enhanced. The change of UV-vis absorption of SA after 2 h electrocatalytic oxidation degradation showed marked difference compared with the starting solution [Figure 4B and C]. The results proved that sea urchin-like La-doped MnO2 has excellent ability to electrochemically degrade SA. At the same time, the catalyst was removed, and a control study was conducted by observing the UV-vis absorption without potential applied under static conditions, which showed that the SA concentration was essentially unchanged to exclude the effect of SA volatilization [Supplementary Figure 18]. The kinetic curves of electrocatalytic degradation and the degradation efficiency of SA under the catalyst were shown in Figure 4D and E. The efficiency of the catalytic degradation of SA was calculated according to Supplementary Equation 6 in the Supplementary Materials. The SA degradation efficiency was nearly 40% after 2 h for sea urchin-like
Figure 4. Electrochemical degradation of SA by the sea urchin-like La-doped MnO2 and MnO2 catalysts. The UV-vis absorption spectra of mixed solution of Na2SO4 and SA during electrolysis by (A) pure FTO, (B) La-doped MnO2 and (C) MnO2 catalysts at 2.1 V for different times; (D) The comparison of electrochemical degradation efficiency and static degradation efficiency of SA; (E) Degradation efficiency of SA by La-doped MnO2 and MnO2 catalysts at different times; (F) The electrocatalytic degradation kinetics of SA. The comparison of the electrochemical degradation efficiency of SA using (G) La-doped MnO2 and (H) MnO2 in the presence of TBA, MeOH, or p-BQ; (I) The comparison of the electrocatalytic degradation kinetics of SA with TBA, MeOH, and p-BQ. SAs: Sulfonamides; FTO: fluorine-doped tin oxide; UV-vis: ultraviolet-visible; TBA: tert-butanol; p-BQ: p-Benzoquinone; MeOH: methanol.
Scavenger quenching tests were conducted to evaluate the contribution of reactive oxygen species (ROS) to SA degradation[52]. To quench the •OH, SO4•-, and O2•- radicals, 1 mL of 100 mM TBA, methanol (MeOH), or p-BQ was added, respectively [Supplementary Figure 19A-F]. When MeOH and p-BQ were introduced as scavengers for SO4•- and O2•-, respectively, the SA degradation efficiencies in both cases were comparable to those observed without the introduction of scavengers. Furthermore, the electrocatalytic degradation rate constants of La-doped MnO2 and MnO2 were 0.0061 and 0.00441 min-1, respectively, in the presence of MeOH, and were 0.00515 and 0.00346 min-1, respectively, in the presence of p-BQ [Supplementary Figure 19G and H]. These results rule out the possibility that ROS such as SO4•- and O2•- are produced during SA degradation. In contrast, after adding TBA, a scavenger of •OH radicals, the degradation efficiency of
CONCLUSIONS
In summary, the La-doped MnO2 catalyst was successfully synthesized by a one-step hydrothermal method. It has sea urchin-like morphology with rich, open tunnel structures, providing abundant reaction sites and a large number of pathways for molecules to enhance catalytic efficiency. The introduction of La3+ not only enhances the electrical conductivity of the catalyst, and promotes charge transfer during the OER process, but also positively influences the structural stability of the catalyst. Therefore, the prepared sea urchin-like La-doped MnO2 catalyst exhibits good electrocatalytic activity in OER, requiring an overpotential of
DECLARATIONS
Authors’ contributions
Supervised and designed the research: Xu, X.; Wang, W.; Zhang, W.; Cao, R.
Participated in catalyst synthesis and characterization: Liu, X.
Co-wrote the paper: Wu, X.; Chen, M.; Li, S.
All authors revised the manuscript and have given approval to the final version of the manuscript.
Availability of data and materials
All detailed materials and methods supporting the results of this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Grant No. 82271181) and Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund Project CXPY2022092. . We are grateful for the support of International Joint Research Center on Advanced Characterizations of Xi’an City.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Rana, S.; Kumar, A.; Dhiman, P.; Mola, G.; Sharma, G.; Lai, C. Recent advances in photocatalytic removal of sulfonamide pollutants from waste water by semiconductor heterojunctions: a review. Mater. Today. Chem. 2023, 30, 101603.
2. Du, Y.; Cheng, Q.; Qian, M.; et al. Biodegradation of sulfametoxydiazine by Alcaligenes aquatillis FA: performance, degradation pathways, and mechanisms. J. Hazard. Mater. 2023, 452, 131186.
3. Zhang, M.; Ruan, J.; Wang, X.; et al. Selective oxidation of organic pollutants based on reactive oxygen species and the molecular structure: degradation behavior and mechanism analysis. Water. Res. 2023, 246, 120697.
4. Chow, T. G.; Khan, D. A. Sulfonamide hypersensitivity. Clin. Rev. Allergy. Immunol. 2022, 62, 400-12.
5. Ottosen, C. F.; Bjerg, P. L.; Kümmel, S.; et al. Natural attenuation of sulfonamides and metabolites in contaminated groundwater - review, advantages and challenges of current documentation techniques. Water. Res. 2024, 254, 121416.
6. Robles-Jimenez, L. E.; Aranda-Aguirre, E.; Castelan-Ortega, O. A.; et al. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: a systematic review. Animals 2021, 12, 60.
7. Liu, X.; Guo, X.; Liu, Y.; et al. A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: performance and microbial response. Environ. Pollut. 2019, 254, 112996.
8. Pan, M.; Chu, L. M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total. Environ. 2017, 599-600, 500-12.
9. Tian, S.; Zhang, C.; Huang, D.; et al. Recent progress in sustainable technologies for adsorptive and reactive removal of sulfonamides. Chem. Eng. J. 2020, 389, 123423.
10. Anjali, R.; Shanthakumar, S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J. Environ. Manage. 2019, 246, 51-62.
11. Yang, J.; Li, Z.; Zhu, H. Adsorption and photocatalytic degradation of sulfamethoxazole by a novel composite hydrogel with visible light irradiation. Appl. Catal. B. Environ. 2017, 217, 603-14.
12. Wang, H.; Wang, S.; Jiang, J.; Shu, J. Removal of sulfadiazine by ferrate(VI) oxidation and montmorillonite adsorption-synergistic effect and degradation pathways. J. Environ. Chem. Eng. 2019, 7, 103225.
13. Hu, S.; Hu, H.; Li, W.; Ke, Y.; Li, M.; Zhao, Y. Enhanced sulfamethoxazole degradation in soil by immobilized sulfamethoxazole-degrading microbes on bagasse. RSC. Adv. 2017, 7, 55240-8.
14. Wang, J.; Wang, S. Microbial degradation of sulfamethoxazole in the environment. Appl. Microbiol. Biotechnol. 2018, 102, 3573-82.
15. Rivas-ortiz, I. B.; Cruz-gonzález, G.; Lastre-acosta, A. M.; et al. Optimization of radiolytic degradation of sulfadiazine by combining Fenton and gamma irradiation processes. J. Radioanal. Nucl. Chem. 2017, 314, 2597-607.
16. Deng, F.; Xie, J.; Garcia-rodriguez, O.; et al. A dynamic anode boosting sulfamerazine mineralization via electrochemical oxidation. J. Mater. Chem. A. 2021, 10, 192-208.
17. Xie, J.; Chen, W.; Lv, Y.; Chen, H.; Li, X.; Li, L. Synthesis of CeOx@SiO2 with tandem effect of mass transfer and activation for enhancing sulfanilamide degradation with ozone. Sep. Purif. Technol. 2021, 256, 117823.
18. Sun, M.; Zhang, Y.; Kong, S. Y.; Zhai, L. F.; Wang, S. Excellent performance of electro-assisted catalytic wet air oxidation of refractory organic pollutants. Water. Res. 2019, 158, 313-21.
19. Younis, M. A.; Lyu, S.; Lei, C.; et al. Efficient mineralization of sulfanilamide over oxygen vacancy-rich NiFe-LDH nanosheets array during electro-fenton process. Chemosphere 2021, 268, 129272.
20. Zeng, W.; Zhang, H.; Wu, R.; Liu, L.; Li, G.; Liang, H. Environment-friendly and efficient electrochemical degradation of sulfamethoxazole using reduced TiO2 nanotube arrays-based Ti membrane coated with Sb-SnO2. J. Hazard. Mater. 2023, 446, 130642.
21. Peng, Y.; Xiao, W.; Wang, H.; Bian, Z. Carbon defect induced electron transfer promotes electrocatalytic activation of molecular oxygen to selectively generate singlet oxygen for pollutants removal. Appl. Catal. B. Environ. Energy. 2025, 363, 124820.
22. Zhang, J.; Xie, J.; Chen, J.; et al. Mimicking the protein: hierarchically hydrophilic Co(OH)2 boosted peroxymonosulfate activation for ultrafast antibiotics degradation. Adv. Funct. Mater. , 2025, e10022.
23. Gu, Q.; Li, M.; Huo, Y.; et al. Theoretical evidence for a pH-dependent effect of carbonate on the degradation of sulfonamide antibiotics. Environ. Pollut. 2024, 361, 124710.
24. Tamilarasi, B.; Jithul, K.; Pandey, J. Non-noble metal-based electro-catalyst for the oxygen evolution reaction (OER): towards an active & stable electro-catalyst for PEM water electrolysis. Int. J. Hydrog. Energy. 2024, 58, 556-82.
25. Yang, S.; Liu, X.; Li, S.; et al. The mechanism of water oxidation using transition metal-based heterogeneous electrocatalysts. Chem. Soc. Rev. 2024, 53, 5593-625.
26. Li, H.; Nakajima, Y.; Nango, E.; et al. Oxygen-evolving photosystem II structures during S1-S2-S3 transitions. Nature 2024, 626, 670-7.
27. Kern, J.; Chatterjee, R.; Young, I. D.; et al. Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 2018, 563, 421-5.
28. Wang, P.; Zhang, S.; Wang, Z.; et al. Manganese-based oxide electrocatalysts for the oxygen evolution reaction: a review. J. Mater. Chem. A. 2023, 11, 5476-94.
29. Ham, K.; Kang, S.; Kim, Y.; Lee, Y.; Kim, Y.; Lee, J. Participation of the unstable lattice oxygen of cation-exchanged δ-MnO2 in the water oxidation reaction. J. Mater. Chem. A. 2023, 11, 21686-93.
30. He, Y.; Kang, Z.; Li, J.; Li, Y.; Tian, X. Recent progress of manganese dioxide based electrocatalysts for the oxygen evolution reaction. Ind. Chem. Mater. 2023, 1, 312-31.
31. Chen, Y.; Yang, S.; Liu, H.; Zhang, W.; Cao, R. An unusual network of α-MnO2 nanowires with structure-induced hydrophilicity and conductivity for improved electrocatalysis. Chinese. J. Catal. 2021, 42, 1724-31.
32. Huo, L.; Lv, M.; Li, M.; et al. Amorphous MnO2 lamellae encapsulated covalent triazine polymer-derived multi-heteroatoms-doped carbon for ORR/OER bifunctional electrocatalysis. Adv. Mater. 2024, 36, 2312868.
33. Sun, Y.; Chen, J.; Liu, L.; Chi, H.; Han, H. The mechanism of OER activity and stability enhancement in acid by atomically doped iridium in γ-MnO2. Chinese. J. Catal. 2025, 69, 99-110.
34. Chang, H.; Liu, X.; Zhao, S.; et al. Self-assembled 3D N/P/S-tridoped carbon nanoflower with highly branched carbon nanotubes as efficient bifunctional oxygen electrocatalyst toward high-performance rechargeable Zn-Air batteries. Adv. Funct. Mater. 2024, 34, 2313491.
35. Jiang, H.; Gu, J.; Zheng, X.; et al. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy. Environ. Sci. 2019, 12, 322-33.
36. Sari, F. N. I.; Lai, Y.; Huang, Y.; et al. Electronic structure engineering in NiFe sulfide via a third metal doping as efficient bifunctional OER/ORR electrocatalyst for rechargeable zinc-air battery. Adv. Funct. Mater. 2024, 34, 2310181.
37. Park, Y. S.; Liu, F.; Diercks, D.; Braaten, D.; Liu, B.; Duan, C. High-performance anion exchange membrane water electrolyzer enabled by highly active oxygen evolution reaction electrocatalysts: Synergistic effect of doping and heterostructure. Appl. Catal. B. Environ. 2022, 318, 121824.
38. Zhou, B.; Liu, X.; Li, L.; et al. Hydroxylation of IrO2 via La doping enhances oxygen evolution reaction performance for PEM water electrolysis. Chem. Eng. J. 2025, 521, 166886.
39. Yang, S.; Chen, D.; Cui, X.; Zhang, J.; Zhang, W.; Cao, R. Spherical Ni/Ni3Se2 heterostructure for efficient electrochemical oxidation reactions. ChemNanoMat 2023, 9, e202200509.
40. Cui, S.; Zhang, D.; Zhang, G.; Gan, Y. Reaction mechanism for the α-MnO2 cathode in aqueous Zn ion batteries revisited: elucidating the irreversible transformation of α-MnO2 into Zn-vernadite. J. Mater. Chem. A. 2022, 10, 25620-32.
41. Yuan, Y.; Liu, C.; Byles, B. W.; et al. Ordering heterogeneity of [MnO6] octahedra in tunnel-structured MnO2 and its influence on ion storage. Joule 2019, 3, 471-84.
42. Wu, Y.; Yao, R.; Zhao, Q.; Li, J.; Liu, G. La-RuO2 nanocrystals with efficient electrocatalytic activity for overall water splitting in acidic media: synergistic effect of La doping and oxygen vacancy. Chem. Eng. J. 2022, 439, 135699.
43. Alharbi, S. M.; Alkhalifah, M. A.; Howchen, B.; Rahmah, A. N. A.; Celorrio, V.; Fermin, D. J. Activating Mn sites by Ni replacement in α-MnO2. ACS. Mater. Au. 2024, 4, 74-81.
44. Xie, J.; Chen, Y.; He, Z.; et al. Single-atom Ni anchored on α-MnO2 nanorods as an electrocatalyst for the oxygen evolution and oxygen reduction reactions. ACS. Appl. Nano. Mater. 2024, 7, 18027-35.
45. Wang, Y.; Yang, R.; Ding, Y.; et al. Unraveling oxygen vacancy site mechanism of Rh-doped RuO2 catalyst for long-lasting acidic water oxidation. Nat. Commun. 2023, 14, 1412.
46. Lyons, M. E.; Brandon, M. P. The significance of electrochemical impedance spectra recorded during active oxygen evolution for oxide covered Ni, Co and Fe electrodes in alkaline solution. J. Electroanal. Chem. 2009, 631, 62-70.
47. Wang, H. Y.; Hung, S. F.; Chen, H. Y.; Chan, T. S.; Chen, H. M.; Liu, B. In operando identification of geometrical-site-dependent water oxidation activity of spinel Co3O4. J. Am. Chem. Soc. 2016, 138, 36-9.
48. Bai, L.; Lee, S.; Hu, X. Spectroscopic and electrokinetic evidence for a bifunctional mechanism of the oxygen evolution reaction. Angew. Chem. Int. Ed. 2021, 60, 3095-103.
49. Zhang, X.; Chen, Q. F.; Deng, J.; et al. Identifying Metal-oxo/peroxo intermediates in catalytic water oxidation by in situ electrochemical mass spectrometry. J. Am. Chem. Soc. 2022, 144, 17748-52.
50. Hu, J.; Li, X.; Liu, F.; Fu, W.; Lin, L.; Li, B. Comparison of chemical and biological degradation of sulfonamides: Solving the mystery of sulfonamide transformation. J. Hazard. Mater. 2022, 424, 127661.
51. Bu, J.; Deng, Z.; Liu, H.; Li, T.; Yang, Y.; Zhong, S. The degradation of sulfamilamide wastewater by three-dimensional electrocatalytic oxidation system composed of activated carbon bimetallic particle electrode. J. Clean. Prod. 2021, 324, 129256.
52. Yang, X.; Duan, J.; Qi, J.; et al. Modulating the electron structure of Co-3d in Co3O4-x/WO2.72 for boosting peroxymonosulfate activation and degradation of sulfamerazine: Roles of high-valence W and rich oxygen vacancies. J. Hazard. Mater. 2023, 445, 130576.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].