In-situ polymerized and crosslinked electrolytes with interchangeable Li/Na transport for battery applications
Abstract
The next generation of batteries requires electrolytes with high conductivity, mechanical stability, good adhesion with electrodes, wide electrochemical windows, and scalability. The present study introduces a concept of doped quasi single-ion conducting copolymers based on methacrylate-(trifluoromethanesulfonyl)imide (TFSI) and vinyl ethylene carbonate which at room temperature are mechanically robust and display ionic conductivities of ~0.1 mS/cm. These electrolytes can be polymerized/crosslinked in-situ, making them easily implementable in current battery manufacturing technologies. They also allow for switching between Li+ and Na+ transport using simple chemistry procedures. To demonstrate their potential for battery applications, the newly developed Li conductors have been tested in symmetric cells, exhibiting overall impedance below 350 Ohm and plating/stripping stability up to 1 mA/cm2. Moreover, lithium metal batteries incorporating this electrolyte and high-voltage Lithium Nickel Manganese Cobalt Oxide (NMC) cathodes show good capacity retention (~79%) during charging and discharging for 80 cycles at C/10 rate and a Coulombic efficiency close to 100% in the entire measurement range. The compositional, mechanical and electrochemical versatility of these electrolytes opens new venues for the design of polymer-based batteries capable of fast charging and extended cycle life, aligning with the current global green energy storage strategies.
Keywords
INTRODUCTION
The surging demand for safe, high-energy density storage in household appliances, mobile electronics and electric vehicles calls for new strategies in mitigating current limitations of liquid electrolyte-based batteries[1,2]. Most ceramic electrolytes, despite their high Li+/Na+ conductivities and large mechanical stiffnesses[3], suffer from electrochemical instability, poor electrode contact, inability to suppress dendrite formation, and deficient large-area manufacturing[4]. While polymer electrolytes such as salt-doped poly(ethylene oxide) (PEO) and their ceramic composites provide a good alternative in terms of mechanical flexibility, electrode adhesion, and processability[5-7], they often display relatively low room temperature conductivity and limited electrochemical window[8-10].
Combining high charge density of ionic liquids with safety and operational stability of nonfluid electrolytes, single-ion conducting polymers (SICPs)[11-13] can overcome many technological drawbacks of ceramics and salt-doped polymers[14,15]. These polymers can be produced in a wide morphological diversity, allow
Despite these many advantages, the deployment of bulk SICPs for battery applications is hampered by their relatively low conductivity near ambient temperature[25] and by their faulty (solid-solid) adhesion to the porous cathode material[26]. Salt doping can be a solution to the first issue[27], as long as the cation transport number does not drop significantly below 0.5. Regarding the second issue, a uniform, low impedance electrolyte-electrode interface (EEI) is required for good battery performance[28]. In traditional batteries, liquid electrolytes penetrate porous electrodes, forming smooth EEI that facilitates cation transport from the electrolyte to the electrode[1,29]. In this regard, in-situ polymerization of SICPs has recently gained increased popularity[30-32]. The main idea of this approach is that the initial liquid monomer precursor fills in all the pores and forms good contact with electrodes in the assembled cell, and then the electrolyte progressively increases its mechanical strength in direct contact with the electrodes during polymerization upon heating. This technique provides homogeneous electric contact and strong adhesion between the SICP and the electrodes, preventing nucleation and growth of Li dendrites during cycling even at relatively high current densities[33].
Based on the above considerations, in our previous study we introduced an electrolyte concept based on a quasi-SICP obtained via in-situ copolymerization of lithium (4-styrenesulfonyl) (trifluoromethanesulfonyl) imide (LiSTFSI, a monomer of a “classical” Li+ SICP) and vinyl ethylene carbonate (VEC), in the presence of Li-TFSI salt and thermal initiators[32]. The proposed concept was built on several fundamental ideas: polymerized anions increase t+; in-situ polymerization enables homogeneous contact with electrodes; high polarity of VEC units decreases the strength of electrostatic interactions[34] for achieving high Li+ mobility; and small amounts of non-polymerized small-molecule precursors and salt enable high ambient ionic conductivity, while preserving relatively high t+. Several preliminary tests demonstrated the feasibility of this electrolyte concept in Li symmetric- and high-voltage cell configurations[32].
Despite these benefits, the above-mentioned prototypic material presented several technological drawbacks: (i) it was not crosslinked and contained a significant amount of unpolymerized monomers; its reduced viscosity posed substantial safety concerns; (ii) the polymerization of styrene units required relatively long time, which is a major drawback for energy efficient scalability, and (iii) it was not fully optimized for compliance with high-voltage electrodes [e.g., Lithium Nickel Manganese Cobalt Oxide (NMC)-based] as required for high energy batteries. The present work continues the development of this polymer electrolyte concept to include in-situ crosslinking in addition to polymerization for increasing the mechanical robustness of the material to the level of a solid-like, free-standing film at ambient conditions. In addition, to significantly shorten the polymerization process, we replaced the precursor solution styrene-TFSI- with methacrylate-TFSI- (MTFSI-)[35]. Moreover, we demonstrate that the same electrolyte can be used for
While the concept of in-situ polymerized polymer electrolytes has been explored in previous
EXPERIMENTAL
Materials and Synthesis
Materials
LiMTFSI and NaMTFSI were purchased from Specific Polymers. Lithium bis(trifluoromethanesu-lfonyl)imide (LiTFSI), Sodium bis(trifluoromethanesu-lfonyl)imide (NaTFSI), VEC, azodiisobutyronitrile (AIBN), and TTA and dimethyl carbonate (DMC) were purchased from Sigma Aldrich. TTA has been passed through an aluminum oxide/inhibitor remover-loaded column to remove the inhibitor. AIBN was recrystallized from methanol before use.
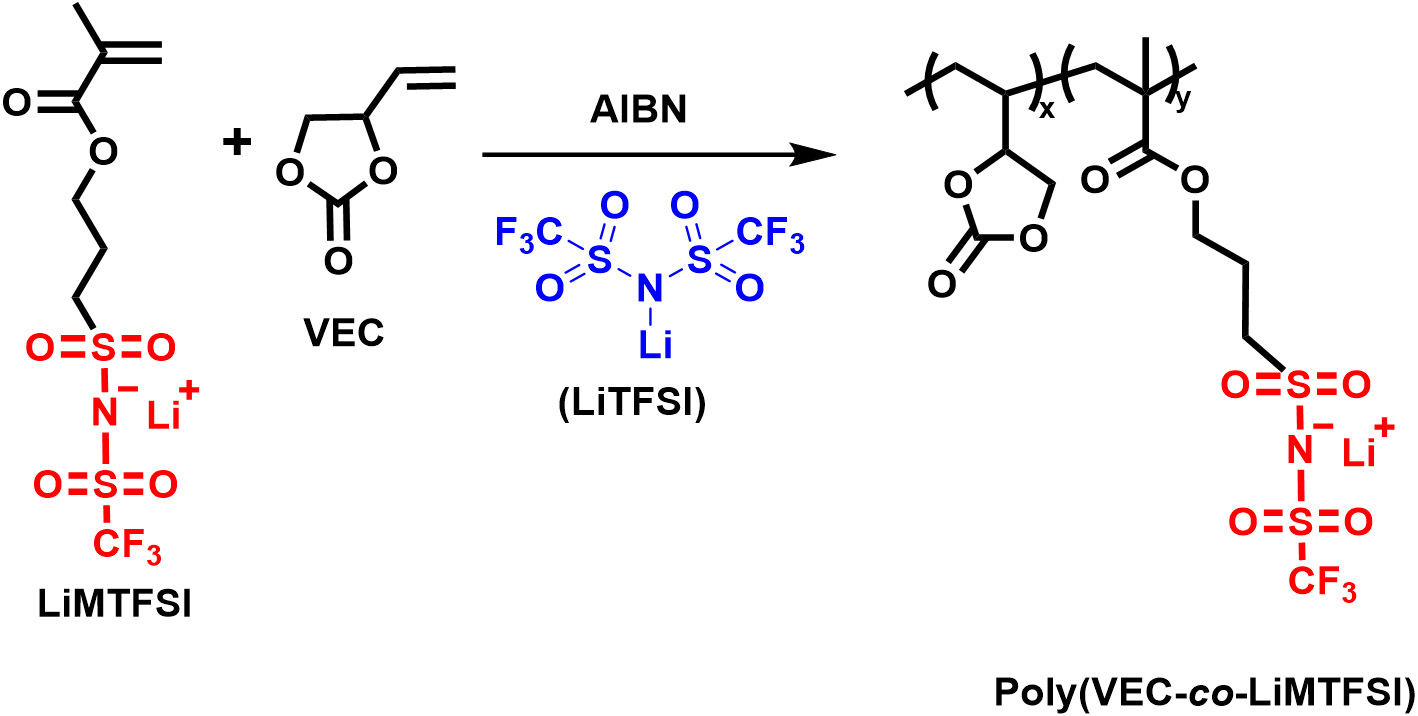
Synthesis of poly(VEC-r-LiMTFSI)
The VEC (2 g, 17.5 mmol), LiMTFSI (~0.604 g, 1.75 mmol), and LiTFSI (0.5 g) were taken in a glass bottle and stirred for 30 min to form a homogeneous solution. The AIBN (28 mg, 0.171 mmol) as the initiator was added to the solution and stirred for ~30 min, yielding a clear precursor solution. The resulting solution was polymerized under an inert atmosphere at 75 °C for 2-3 h to form a gel-type polyelectrolyte [Scheme 1].
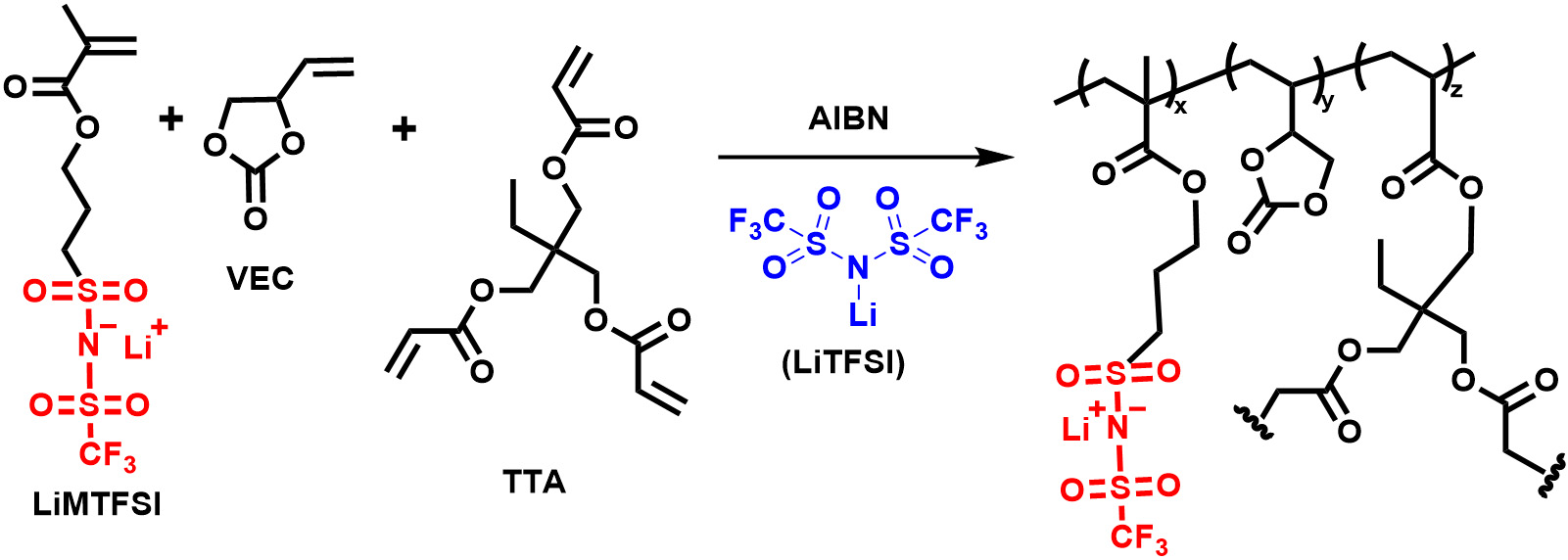
Synthesis of crosslinked Li- polyelectrolyte
A similar polymerization procedure was used to synthesize in-situ crosslinked poly-electrolyte gel [Scheme 2]. The VEC (2 g, 17.5 mmol), LiMTFSI (~0.604 g, 1.75 mmol), and LiTFSI (0.5 g) were taken in a glass bottle and stirred for 30 min to form a homogeneous solution. The crosslinker TTA (0.047 g,
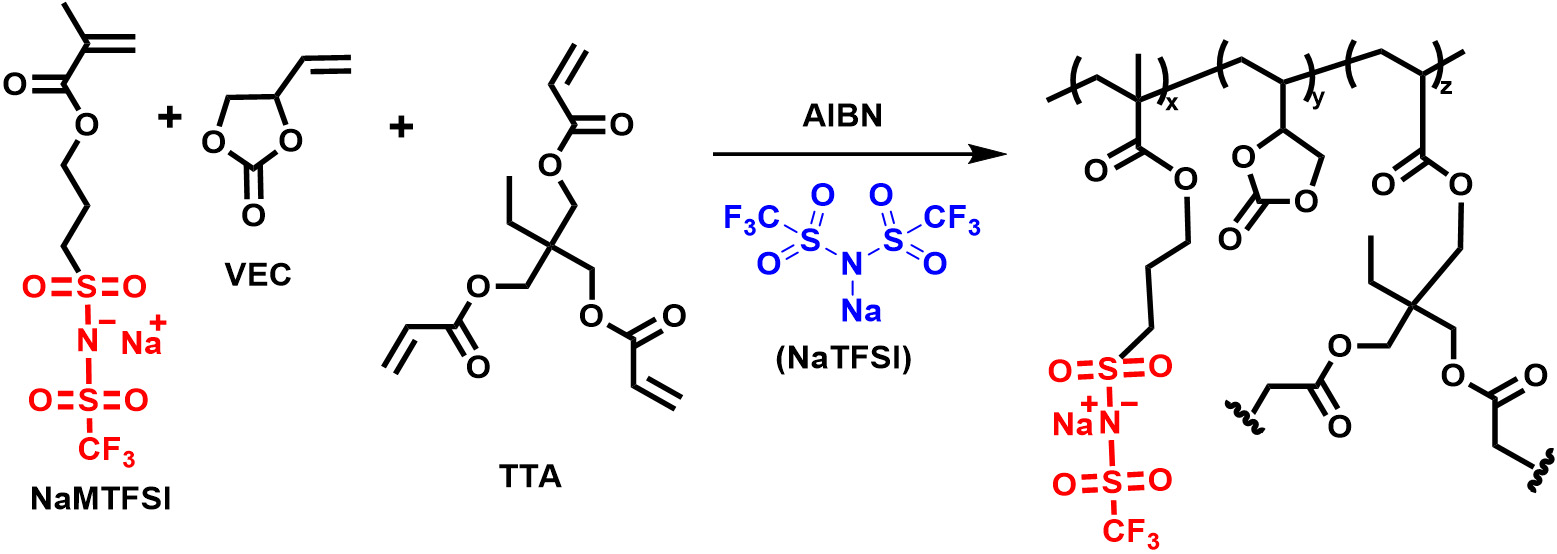
Synthesis of crosslinked Na-polyelectrolyte
A similar polymerization procedure was used to synthesize in-situ crosslinked sodium (Na)-based
Broadband dielectric spectroscopy
The conductivity response of the newly introduced electrolytes was probed in a frequency range covering 10-2-106 Hz with a 0.1 V voltage amplitude using an Alpha-A analyzer from Novocontrol. A Quattro temperature controller (also from Novocontrol) was used to achieve a temperature stabilization of the samples within ±0.2 K. The dielectric capacitor consisted of two electrodes with a diameter of 10.2 mm and separated by means of a sapphire disk at a fixed distance of 0.4 mm.
Pulse field gradient nuclear magnetic resonance
Pulse Field Gradient Nuclear Magnetic Resonance (PFG-NMR) experiments were performed on a 400 MHz Bruker NEO spectrometer equipped with a diff50 1H/X diffusion probe using a stimulated echo with bipolar gradient pulses ranging from 2-2.5 ms and a diffusion delay of 200 ms. Variable temperature experiments were performed using a BCU II and the samples were allowed to equilibrate for 5 min before each measurement.
Battery testing
The electrolyte precursor and the AIBN initiator were mixed inside an Argon-filled glove box. The solution was cast onto the cathodes and CR2032 coin cells were assembled using lithium metal for the anode. Prior to testing, the cells were annealed at 60 °C overnight to allow polymerization and crosslinking of the electrolyte monomers. The electrochemical long-term cycling testing was performed at 30 °C on a Maccor battery cycler (series 4000) at a C/10 rate. The cutoff voltage was 2.5 and 4.2 V for the Lithium Iron Phosphate (LFP)-based cell and 3 and 4.2 V for NMC622-based cells. Electrochemical impedance spectra (EIS) were conducted on a BioLogic VSP3 potentiostat at an amplitude of 10 mV. The frequency range was from 1 MHz to 10 mHz. The EIS and cyclic voltammetry (CV) measurements were performed on a full-cell configuration with a lithium metal anode. The cathode formulation was based on 90 wt% active material,
RESULTS AND DISCUSSION
Transport properties of the proposed electrolytes
The “final” electrolyte products (after thermally-induced free radical polymerization/crosslinking) are random copolymers including MTFSI- and VEC monomers, which incorporate unpolymerized VEC fragments and mobile ions. According to proton NMR data [Supplementary Figure 1], while all LiMTFSI monomers were fully polymerized, only 40% of the VEC monomers underwent polymerization. The remaining unreacted VEC monomers function as plasticizers, increasing the conductivity. The mechanism of conductivity in our gel-like electrolyte resembles that in liquid electrolytes - strongly coupled to structural relaxation of the matrix, i.e., similar to doped PEO[20]. However, due to the significant number of anions attached to the chain, the transport number is higher than in PEO electrolytes[21]. The relative amount of freely migrating cations is controlled by the relative concentrations of MTFSI- present in the matrix and of the doping salt (LiTFSI or NaTFSI). Considering this morphological complexity, our initial tests focused on the Li-based material for determining which [VEC]:[MTFSI-]:[LiTFSI] composition provides the highest conductivity near room temperature. To this end, we performed temperature-dependent
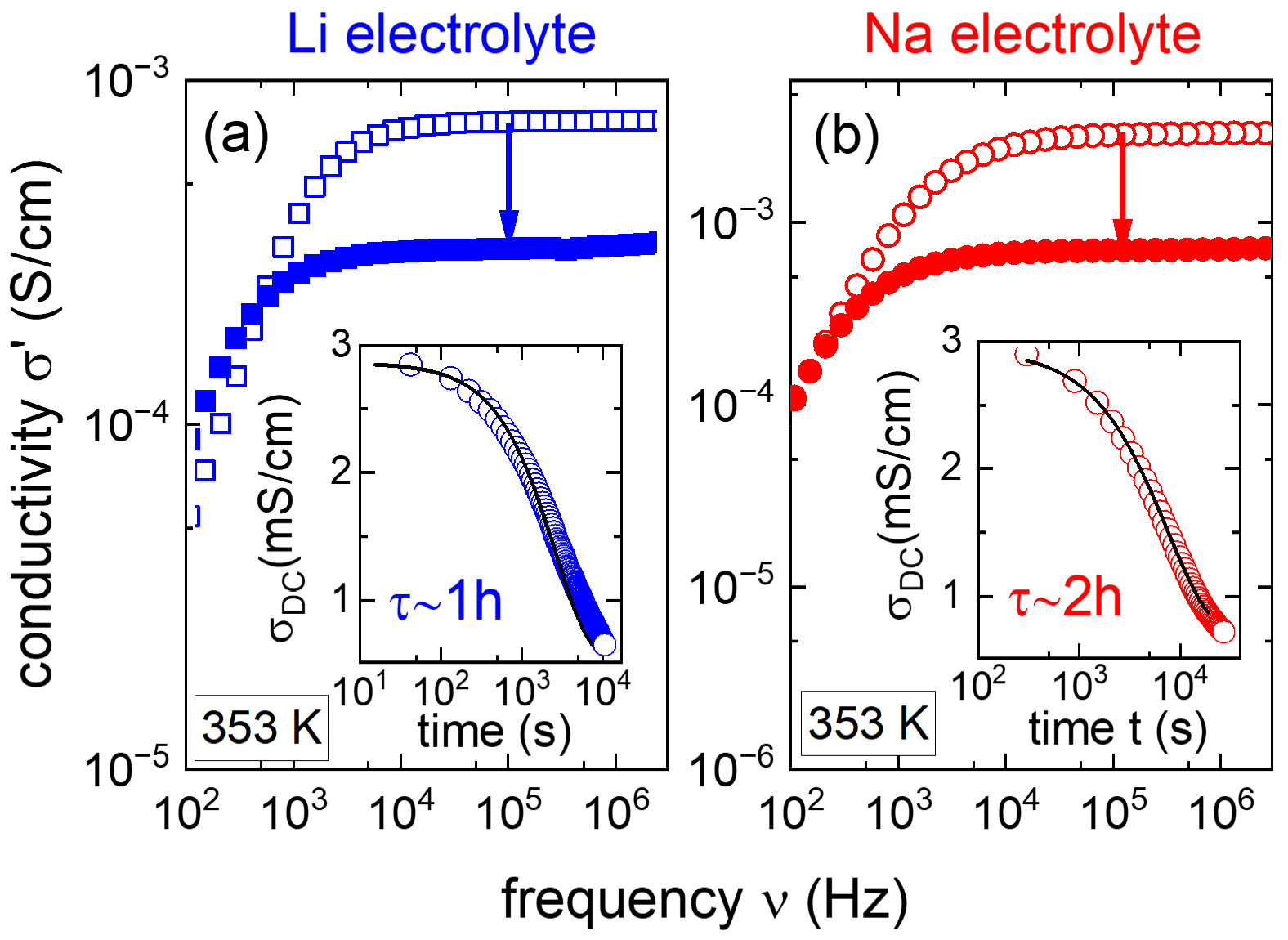
Figure 1 presents the time evolution of several selected conductivity responses recorded during the in-situ polymerization/crosslinking performed at 80 °C for the (100:10:10) Li and Na electrolytes. These spectra display at high frequencies the direct current (DC) conductivity plateaus with amplitudes σDC[44], while at low frequencies, they reflect polarization effects due to ion blocking at the electrodes[45]. As indicated by the two arrows, polymerization/crosslinking of electrolytes triggers a progressive decrease of their DC conductivities below the 3 × 10-3 S/cm value corresponding to both precursors at 80 °C (the short-time σ0 value in inset Figure 1).
Figure 1. Selected spectra illustrating the evolution of the conductivity response during the polymerization/ crosslinking process induced at 80 °C for (A) Li electrolyte and (B) Na electrolyte. The two insets present the corresponding time evolution of σ0 for the two materials. The solid lines represent fits using exponential decay functions.
To parameterize the time evolutions of σDC, we performed interpolations of the data presented in the two insets of Figure 1 with exponential decay functions F(t)∝exp[-(t/τ)] providing the characteristic times τ of polymerization/crosslinking kinetics. These functions provided relatively good interpolation of experimental data with τ close to one hour for the Li system and two hours for the Na counterpart. These time constants are logistically relevant, since they can guide the optimization of polymerization/crosslinking inside the batteries. Based on our results, for the presently introduced methacrylate-based Li electrolyte, at 80 °C, this process can be considered effectively completed after (4-5 × τ) ~ 4-5 h. However, the polymerization of methacrylate-based Na electrolytes requires eight to ten hours, and further optimization of its composition could reduce this time.
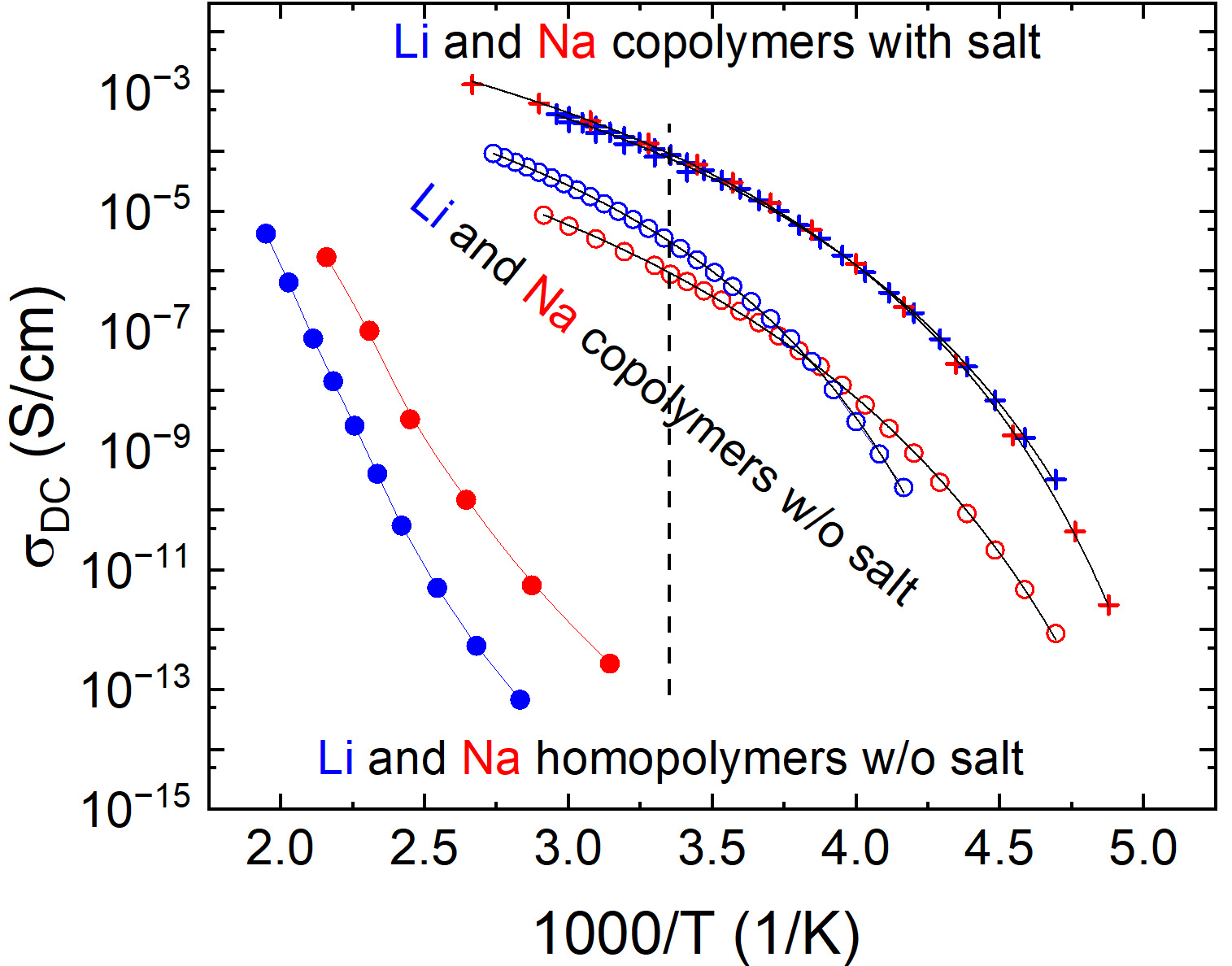
After the isothermal polymerization/crosslinking of the two electrolytes, their frequency-dependent conductivity response were probed over a temperature range from 80 °C down to -65 °C. These spectra are shown in Supplementary Figure 3 and have been used for extracting the DC conductivities plotted as a function of the inverse temperature in Figure 2. Here, the presently considered electrolytes are denoted as “copolymers with salt”.
Figure 2. Comparison of DC conductivities corresponding to Li (blue symbols) and Na (red symbols) homopolymers (dots), copolymers without salt (open circles), and copolymers with salt (crosses); see text for details. The vertical dashed line corresponds to room temperature. The black lines represent VFT fits of the data for copolymers; see text for details. DC: Direct-current; VFT: vogel-fulcher-tammann.
Figure 2 shows that both Li and Na polymer-based electrolytes display similar conductivities in their common investigated T range. Accordingly, replacing MTFSI-Li with MTFSI-Na monomers and LiTFSI with NaTFSI salt is not detrimental in terms of conductivity. These results demonstrate the versatility of the present concept for enabling fast transport for different types of cations. According to Figure 2, the conductivity of the two doped copolymers near room temperature is about 0.1 mS/cm, which is quite high for mechanically robust electrolytes. To put these results in a broader perspective, we included in Figure 2 the σDC results corresponding to the same copolymers without salt (from this work, composition 100:10:0 according to the present nomenclature) and for MTFSI-Li/MTFSI-Na homopolymers, with no VEC and no salt (literature data[46] composition 0:100:0). The latter materials are the SICPs with the largest concentration of free Li+/ Na+ ions, however, due to their high glass transition temperatures (Tg > 450 K)[46], their ambient conductivities are very low. With corresponding σDC below 10-13 S/cm near room temperature, these homopolymers have no practical relevance for battery applications.
However, this situation changes when a large amount of mTFSI monomers are “replaced” with the VEC ones forming a copolymer without salt. As recognized in Figure 2, despite having a significantly lower number density of cations, these materials - which are still SICPs - display room-temperature σDC values close to 10-5 S/cm (for Li). This strong increase in conductivity occurs due to VEC acting both as a good plasticizer, strongly reducing the glass transition of the polymer, and as a highly polar group screening the Columbic interaction. To further increase σDC to about 10-4 S/cm, these copolymers are doped with a certain amount of salt [Figure 2], leading to the currently proposed electrolyte concept with both mechanical robustness and conductivity levels relevant for battery technologies. To quantify the conductivity changes induced by the salt addition, the temperature dependences of σDC of Na and Li copolymers included in Figure 2 have been interpolated with Vogel-Fulcher-Tammann (VFT) functions σDC = σDC,0·exp[-D/(T-T0)], and the corresponding results of σDC,0, D, and T0 parameters are included in Table 1. These data revealed that only copolymers with Li without salt have significantly higher T0 and probably higher glass transition temperatures. Adding salt strongly increases conductivity essentially without change of T0 in the case of
VFT parameters characterizing the temperature dependence of conductivity for the Na and Li copolymers with no salt and with salt
| Material | σDC,0 (S/cm) | D (K) | T0 (K) |
| Na no salt | 0.010 | 1302 | 157 |
| Li no salt | 0.028 | 1035 | 185 |
| Na with salt | 0.188 | 1025 | 164 |
| Li with salt | 0.129 | 1012 | 162 |
By dissolving salt in the system, one expects that the added mobile anions will show some impact on the cation transport number, t+. To access this parameter, two procedures are usually employed for
Employing this method to our Li electrolyte [Supplementary Figures 4 and 5] we obtained a t+,I value of 0.4.
The second approach relies on knowledge of number densities and diffusion coefficients of cations and anions[48], which is expressed as
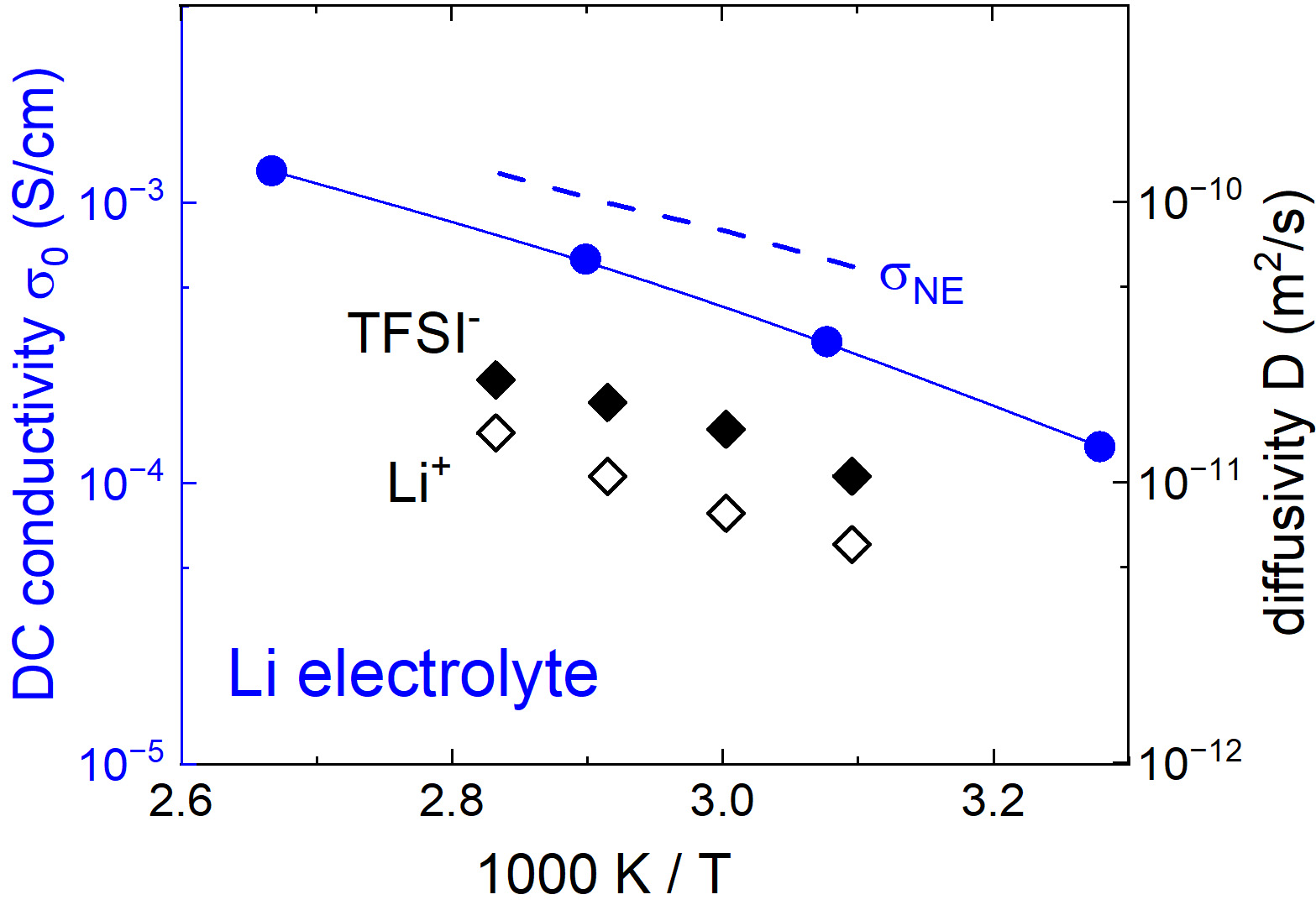
For employing this second procedure we measured D+ and D- corresponding to Li and TFSI ions, respectively, using NMR and their corresponding values are included in Figure 3. Interestingly, despite its significantly larger ion size, the diffusion coefficient of TFSI- is approximately twice larger than that of Li+. A possible reason for this behavior is that polymerized anionic groups slow down cations more strongly than anions. Using the experimentally probed diffusivities and the total number densities of mobile (excluding covalently bonded) ions, Eq. (2) yields a t+,D value of 0.5.
Figure 3. The temperature-dependent DC conductivity (blue dots) and the diffusivities of Li+ (black open diamonds) and TFSI- (black filled diamonds) ions for the Li electrolyte. The blue dashed line presents the theoretically expected DC conductivity according to the Nernst-Einstein relation. DC: Direct-current; TFSI: trifluoromethanesulfonyl)imide.
We note that both definitions (for t+,I and t+,D) are considered to be equivalent and rely on the validity of Nernst-Einstein relation; i.e., all ion-ion correlations are neglected[44,49]. However, in highly concentrated electrolytes in general, the Nernst-Einstein relation does not hold[50]. As demonstrated in Figure 3, for the present case, the estimations are based on this theoretical relation:
which provides conductivity values larger than those of the experimentally probed ones. Such deviations between experimental and theoretical conductivities are usually quantified in terms of inverse Haven ratio (sometimes also called ionicity), H-1≡σDC/σNE[14]. This parameter is a measure of the strength of ion
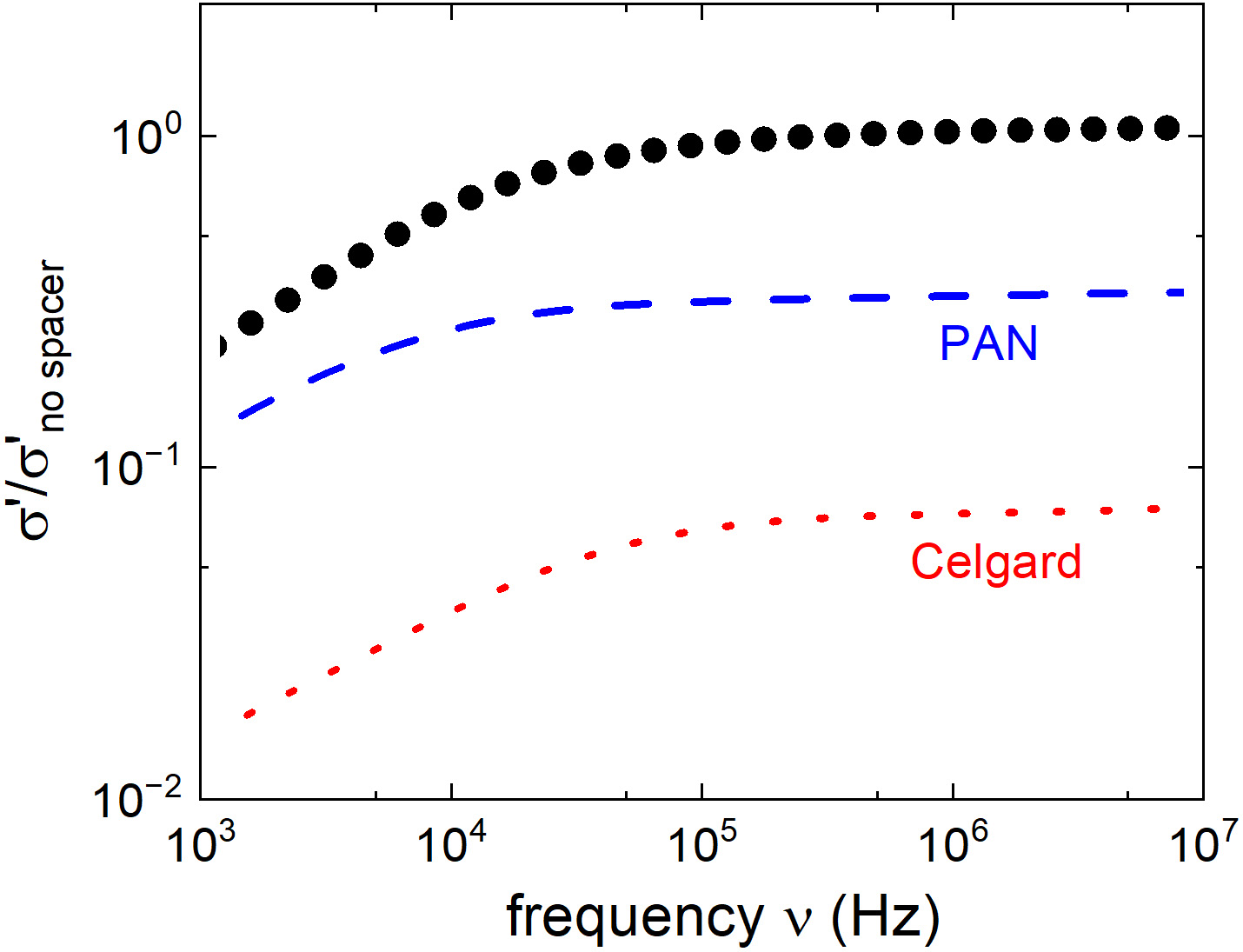
Before proceeding with battery testing, we also analyzed the role of the separator for the effective conductivity of the electrolyte. For this, we used a commercial Celgard separator [made of polypropylene (PP) and polyethylene (PE)] traditionally used for liquid-electrolyte batteries[56] and a PAN separator[57] (produced by electrospinning in our group). These have been impregnated with the Li-based precursor electrolyte, and their corresponding room-temperature conductivity spectra are included in Figure 4. These results reveal that Celgard reduces the conductivity of the electrolyte by about 15 times. Considering the nominal Celgard porosity of ~40%, this result demonstrates that this separator is poorly wetted by our electrolyte. In contrast, the PAN separator reduces the conductivity by a factor of 3 [Figure 4]. These results call for caution when choosing the battery separator, and prior analysis of their wettability by the chosen (liquid) electrolyte may be helpful in this regard.
Figure 4. The room-temperature conductivity spectra of in situ polymerized and crosslinked polymer electrolytes in cell with Celgard separator (red dotted line) and PAN separator (blue dashed line) compared with the conductivity of the electrolyte without separators (black dots). PAN: Polyacrylonitrile.
Battery implementation and testing
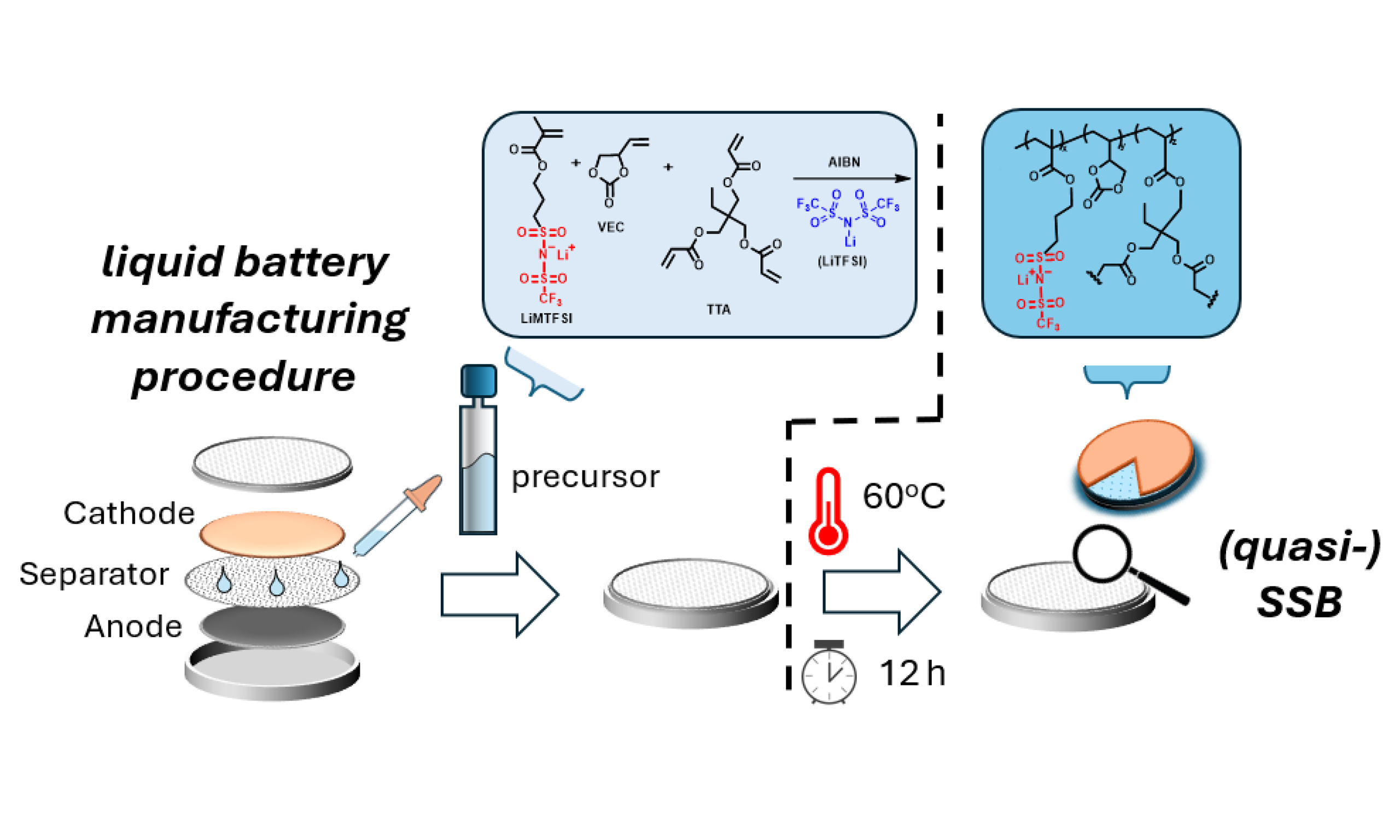
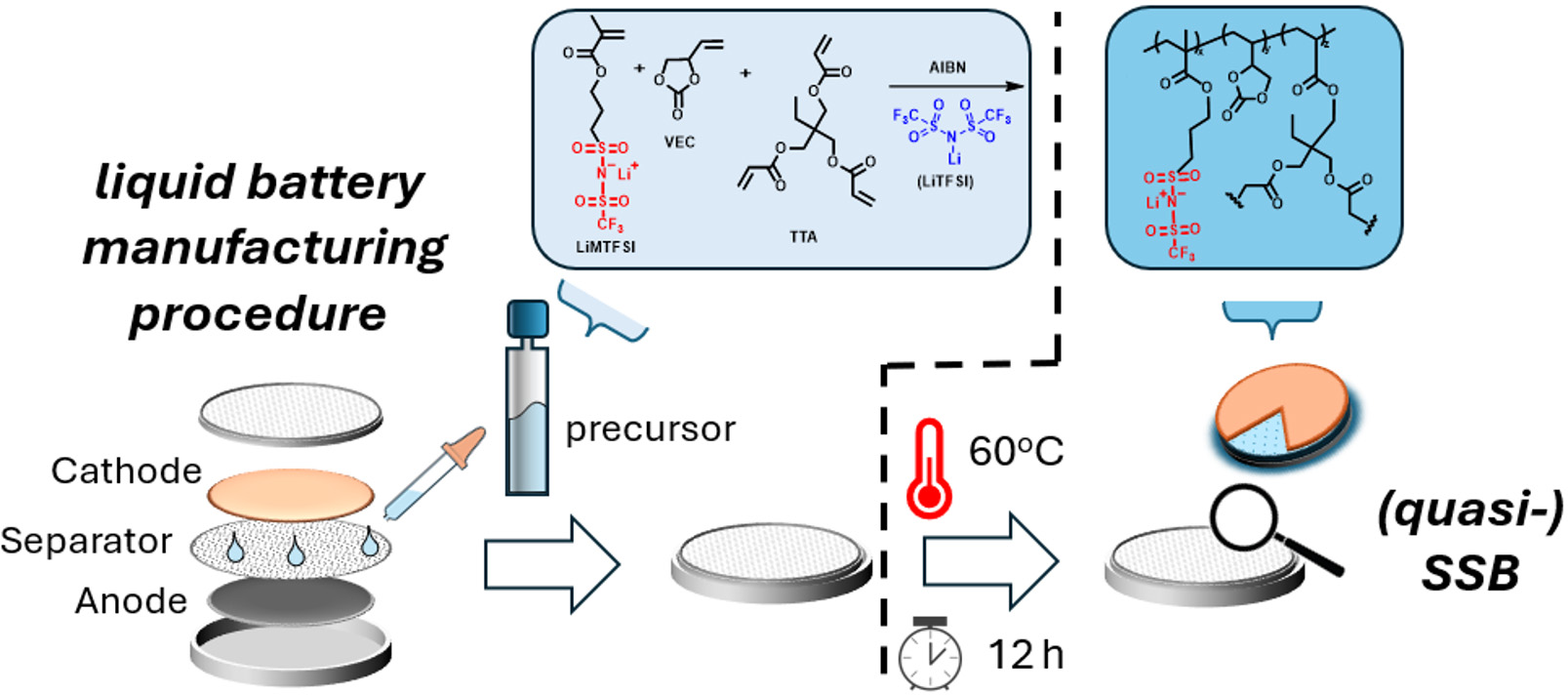
As schematically illustrated in Scheme 4, for preparing the battery cells, the precursor solution containing the two monomers, salt, initiator and crosslinkers is soaked into the separator and placed in direct contact with the electrodes. After the cell is sealed, the in-situ polymerization/crosslinking process is enabled within the battery by simply heating the latter at a given temperature for a given time. In this way, employing already existing technology for liquid electrolyte batteries[58], (quasi) solid-state batteries (SSBs) can be developed with one additional heating step. No solvent is used in this process, just a mixture of monomers, initiators, crosslinkers and salt. Non-polymerized VEC monomers remain well-trapped in the formed crosslinked gel and will not leak.
Scheme 4. Depiction of the employed procedure for producing polymer-based quasi solid-state batteries by heating liquid-state manufactured batteries.
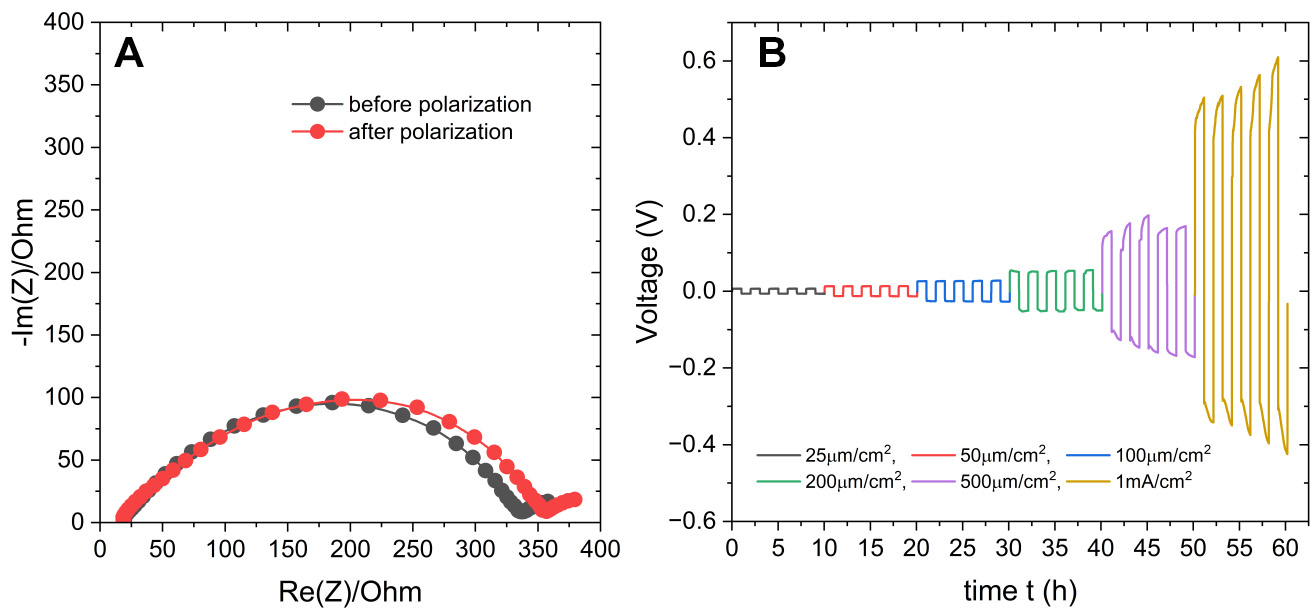
The Li+-based copolymer was initially tested in Li+/Li symmetric cells using a 25-micron thick Celgard separator. The corresponding Nyquist plots recorded before and after the polarization of such a cell used for the previously mentioned Bruce-Vincent tests are shown in Figure 5A. They reveal a relatively small overall impedance of the Li electrolyte-electrodes system, below 350 , demonstrating the formation of a low impedance EEI upon in-situ polymerization/crosslinking. These symmetric cells also display a stable voltage profile and low overpotential values of up to 1 mA/cm2 current density [Figure 5B].
Figure 5. (A) Nyquist plots obtained before and after polarization for the calculation of the Li+ transport number; (B) Plating/stripping experiment at 30 °C shows stability of the cell up to 1mA/cm2. The half-cycle time is 1 h.
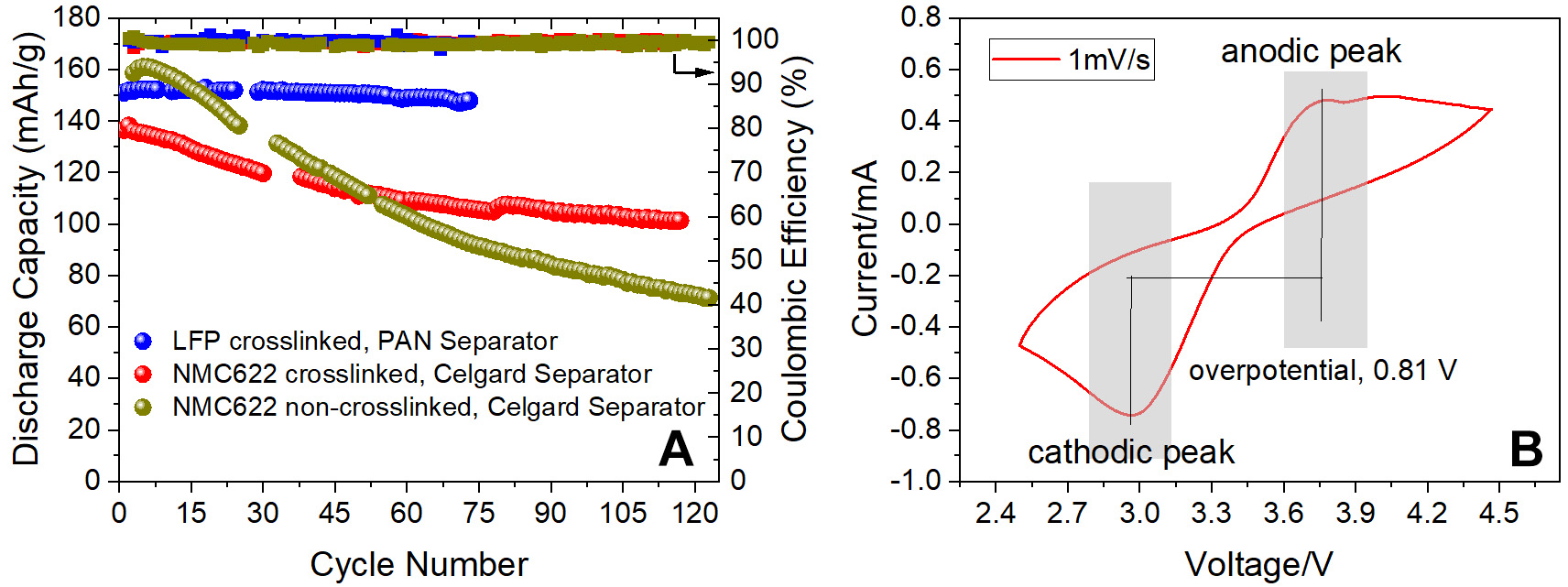
Long-term cycling of Li+ electrolyte was also performed at 30 °C in real batteries incorporating a
Figure 6. (A) Discharge capacity and Coulombic efficiency at 30 °C and C/10 rate for a high-voltage (4.2 V) NMC622 cathode with Celgard (2325) separator and an LFP cathode (3.7 V) with an electrospun PAN separator; A Li metal was used for the anode (B) CV plot for the electrolyte at 1 mV/s scan rate obtained for a cell with LFP cathode and Li metal anode. LFP: Lithium iron phosphate; PAN: polyacrylonitrile; CV: cyclic voltammetry.
The good performance of the proposed polymer electrolyte in both symmetric cells and NMC/LFP batteries can be attributed to homogenous charge distribution during cycling due to in-situ polymerization and crosslinking. As the precursor can penetrate the electrode pores, after the polymerization it forms a uniformly charged network which helps Li to distribute homogenously on the electrode. No signs of dendrite formation have been noted during these tests. Moreover, the decent transport numbers reduce cell polarization and enhance battery life by avoiding over-potential issues.
CONCLUSIONS
The present work introduces a concept of doped quasi single-ion conducting copolymers that can be polymerized and crosslinked in-situ and enabling (i) good adhesion with the electrodes, mitigating delamination and interfacial resistances; (ii) homogeneous distribution of charge carriers near the electrode surfaces to prevent dendrite formation; (iii) interchangeable Li and Na (and possibly other cations) transport using simple chemistry procedures, at variance with superionic ceramics where switching cations is obstructed by structural stability; (iv) relatively large Li and Na conductivities (~0.1 mS/cm at ambient conditions) and decent transport number (~0.5) which hampers polarization effects; and (v) mechanical robustness, with safety benefits in preventing electrolyte spilling upon battery damage. The proposed Li electrolyte demonstrated good performance in Li/Li symmetric cells, as well as in lithium metal cells with NMC or LFP cathode. Additionally, the proposed electrolyte concept is easy to implement with current battery technologies and provides a viable route for the design of high energy density safe polymer-based batteries capable of long duty cycles, in line with current global demands for energy storage.
DECLARATIONS
Authors’ contributions
Provided research direction and funding support: Rahman, M. A.; Polizos, G.; Sokolov, A. P.; Gainaru, C.
Performed the dielectric measurements: Singh, H.; Beard, R.; Eberhard, R.
Analyzed the conductivity data: Singh, H.; Popov, I.
Performed the NMR investigations: Damron, J. T.
Performed chemical syntheses and characterizations: Danielson, M.; Rahman, M. A.
Performed battery cell assembly, electrochemical testing and data analysis: Polizos, G.; Shahriar, M.
All authors contributed to discussion of the results and manuscript writing.
Availability of data and materials
Data are available from the corresponding authors upon reasonable request.
Financial support and sponsorship
The authors acknowledge the support from Laboratory Directed Research and Development Fund, Oak Ridge National Laboratory, USA. Electrochemical measurements were supported by the DOE Vehicle Technologies Office. Singh, H. acknowledges partial support from “Fast and Cooperative Ion Transport in Polymer-Based Materials (FaCT)”, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences for its contribution to conductivity measurements, data analysis and interpretation.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Li, Y.; Wang, X.; Dong, S.; Chen, X.; Cui, G. Recent advances in non-aqueous electrolyte for rechargeable Li-O2 batteries. Adv. Energy. Mater. 2016, 6, 1600751.
2. Yang, Y.; Zhao, L.; Zhang, Y.; et al. Challenges and prospects of low-temperature rechargeable batteries: electrolytes, interfaces, and electrodes. Adv. Sci. 2024, 11, 2410318.
3. Angell, C. A. Fast ion motion in glassy and amorphous materials. Solid. State. Ionics. 1983, 9-10, 3-16.
4. Ma, M.; Zhang, M.; Jiang, B.; Du, Y.; Hu, B.; Sun, C. A review of all-solid-state electrolytes for lithium batteries: high-voltage cathode materials, solid-state electrolytes and electrode-electrolyte interfaces. Mater. Chem. Front. 2023, 7, 1268-97.
5. Muldoon, J.; Bucur, C. B.; Boaretto, N.; Gregory, T.; di, Noto. V. Polymers: opening doors to future batteries. Polymer. Reviews. 2015, 55, 208-46.
6. Yi, J.; Guo, S.; He, P.; Zhou, H. Status and prospects of polymer electrolytes for solid-state Li-O2 (air) batteries. Energy. Environ. Sci. 2017, 10, 860-84.
7. Lee, M. J.; Han, J.; Lee, K.; et al. Elastomeric electrolytes for high-energy solid-state lithium batteries. Nature 2022, 601, 217-22.
8. Hu, P.; Chai, J.; Duan, Y.; Liu, Z.; Cui, G.; Chen, L. Progress in nitrile-based polymer electrolytes for high performance lithium batteries. J. Mater. Chem. A. 2016, 4, 10070-83.
9. Stacy, E. W.; Gainaru, C. P.; Gobet, M.; et al. Fundamental limitations of ionic conductivity in polymerized Ionic Liquids. Macromolecules 2018, 51, 8637-45.
10. Edman, L.; Doeff, M. M.; Ferry, A.; Kerr, J.; De, Jonghe. L. C. Transport properties of the solid polymer electrolyte system P(EO)n LiTFSI. J. Phys. Chem. B. 2000, 104, 3476-80.
11. Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629-48.
12. Eftekhari, A.; Saito, T. Synthesis and properties of polymerized ionic liquids. Eur. Polym. J. 2017, 90, 245-72.
13. Zhu, J.; Zhang, Z.; Zhao, S.; Westover, A. S.; Belharouak, I.; Cao, P. Single-ion conducting polymer electrolytes for solid-state lithium-metal batteries: design, performance, and challenges. Adv. Energy. Mater. 2021, 11, 2003836.
14. Gainaru, C.; Stacy, E. W.; Bocharova, V.; et al. Mechanism of conductivity relaxation in liquid and polymeric electrolytes: direct link between conductivity and diffusivity. J. Phys. Chem. B. 2016, 120, 11074-83.
15. Fan, F.; Wang, W.; Holt, A. P.; et al. Effect of molecular weight on the ion transport mechanism in polymerized ionic liquids. Macromolecules 2016, 49, 4557-70.
16. Bae, J.; Oh, S.; Lee, B.; et al. High-performance, printable quasi-solid-state electrolytes toward all 3D direct ink writing of shape-versatile Li-ion batteries. Energy. Storage. Mater. 2023, 57, 277-88.
17. Ahmed, F.; Choi, I.; Rahman, M. M.; et al. Remarkable conductivity of a self-healing single-ion conducting polymer electrolyte, poly(ethylene-co-acrylic lithium (fluoro sulfonyl)imide), for all-solid-state Li-ion batteries. ACS. Appl. Mater. Interfaces. 2019, 11, 34930-8.
18. Luo, Y.; Gao, L.; Kang, W. A new review of single-ion conducting polymer electrolytes in the light of ion transport mechanisms. J. Energy. Chem. 2024, 89, 543-56.
19. Pożyczka, K.; Marzantowicz, M.; Dygas, J.; Krok, F. Ionic conductivity and lithium transference number of poly(ethylene oxide):LiTFSI system. Electrochim. Acta. 2017, 227, 127-35.
20. Song, J.; Wang, Y.; Wan, C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power. Sources. 1999, 77, 183-97.
21. Lehmann, M. L.; Yang, G.; Gilmer, D.; et al. Tailored crosslinking of Poly(ethylene oxide) enables mechanical robustness and improved sodium-ion conductivity. Energy. Storage. Mater. 2019, 21, 85-96.
22. Li, L.; Li, S.; Lu, Y. Suppression of dendritic lithium growth in lithium metal-based batteries. Chem. Commun. 2018, 54, 6648-61.
23. Lee, H. G.; Kim, S. Y.; Lee, J. S. Dynamic observation of dendrite growth on lithium metal anode during battery charging/discharging cycles. npj. Comput. Mater. 2022, 8, 103.
24. Maity, A.; Svirinovsky-Arbeli, A.; Buganim, Y.; Oppenheim, C.; Leskes, M. Tracking dendrites and solid electrolyte interphase formation with dynamic nuclear polarization-NMR spectroscopy. Nat. Commun. 2024, 15, 9956.
25. Bocharova, V.; Sokolov, A. P. Perspectives for polymer electrolytes: a view from fundamentals of ionic conductivity. Macromolecules 2020, 53, 4141-57.
26. Shan, C.; Wang, Y.; Liang, M.; et al. A comprehensive review of single ion-conducting polymer electrolytes as a key component of lithium metal batteries: From structural design to applications. Energy. Storage. Mater. 2023, 63, 102955.
27. Ghorbanzade, P.; Loaiza, L. C.; Johansson, P. Plasticized and salt-doped single-ion conducting polymer electrolytes for lithium batteries. RSC. Adv. 2022, 12, 18164-7.
28. Swift, M. W.; Jagad, H.; Park, J.; Qie, Y.; Wu, Y.; Qi, Y. Predicting low-impedance interfaces for solid-state batteries. Curr. Opin. Solid. State. Mater. Sci. 2022, 26, 100990.
29. Wang, H.; Yu, Z.; Kong, X.; et al. Liquid electrolyte: the nexus of practical lithium metal batteries. Joule 2022, 6, 588-616.
30. Guan, X.; Wu, Q.; Zhang, X.; Guo, X.; Li, C.; Xu, J. In-situ crosslinked single ion gel polymer electrolyte with superior performances for lithium metal batteries. Chem. Eng. J. 2020, 382, 122935.
31. He, P.; Chen, S.; Choi, Y. Y.; Myung, N. V.; Nykaza, J. R.; Schaefer, J. L. In-situ crosslinked gel polymer electrolytes based on ionic monomers as charge carriers for lithium-ion batteries. ECS. Adv. 2024, 3, 010504.
32. Shan, X.; Morey, M.; Li, Z.; et al. A polymer electrolyte with high cationic transport number for safe and stable solid Li-metal batteries. ACS. Energy. Lett. 2022, 7, 4342-51.
33. Xiao, G.; Xu, H.; Bai, C.; Liu, M.; He, Y. Progress and perspectives of in situ polymerization method for lithium-based batteries. Interdiscip. Mater. 2023, 2, 609-34.
34. Zhang, Q.; Liu, S.; Lin, Z.; et al. Highly safe and cyclable Li-metal batteries with vinylethylene carbonate electrolyte. Nano. Energy. 2020, 74, 104860.
35. Devaux, D.; Liénafa, L.; Beaudoin, E.; et al. Comparison of single-ion-conductor block-copolymer electrolytes with polystyrene-TFSI and polymethacrylate-TFSI structural blocks. Electrochim. Acta. 2018, 269, 250-61.
36. Au, H.; Crespo-ribadeneyra, M.; Titirici, M. Beyond Li-ion batteries: performance, materials diversification, and sustainability. One. Earth. 2022, 5, 207-11.
37. Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-energy batteries: beyond lithium-ion and their long road to commercialisation. Nano-Micro. Lett. 2022, 14, 94.
38. Tian, Y.; Zeng, G.; Rutt, A.; et al. Promises and challenges of next-generation “beyond li-ion” batteries for electric vehicles and grid decarbonization. Chem. Rev. 2021, 121, 1623-69.
39. Vedhanarayanan, B.; Seetha, Lakshmi. K. C. Beyond lithium-ion: emerging frontiers in next-generation battery technologies. Front. Batter. Electrochem. 2024, 3, 1377192.
40. Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer electrolytes for lithium-based batteries: advances and prospects. Chem 2019, 5, 2326-52.
41. Barbosa, J. C.; Gonçalves, R.; Costa, C. M.; Lanceros-Méndez, S. Toward sustainable solid polymer electrolytes for lithium-ion batteries. ACS. Omega. 2022, 7, 14457-64.
42. Mu, J.; Liao, S.; Shi, L.; et al. Solid-state polymer electrolytes in lithium batteries: latest progress and perspective. Polym. Chem. 2024, 15, 473-99.
43. Song, Z.; Chen, F.; Martinez-Ibañez, M.; et al. A reflection on polymer electrolytes for solid-state lithium metal batteries. Nat. Commun. 2023, 14, 4884.
44. Dyre, J. C.; Maass, P.; Roling, B.; Sidebottom, D. L. Fundamental questions relating to ion conduction in disordered solids. Rep. Prog. Phys. 2009, 72, 046501.
45. Ishai, P. B.; Talary, M. S.; Caduff, A.; Levy, E.; Feldman, Y. Electrode polarization in dielectric measurements: a review. Meas. Sci. Technol. 2013, 24, 102001.
46. Gainaru, C.; Kumar, R.; Popov, I.; et al. Mechanisms controlling the energy barrier for ion hopping in polymer electrolytes. Macromolecules 2023, 56, 6051-9.
47. Evans, J.; Vincent, C. A.; Bruce, P. G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324-8.
48. Fong, K. D.; Self, J.; Diederichsen, K. M.; Wood, B. M.; McCloskey, B. D.; Persson, K. A. Ion transport and the true transference number in nonaqueous polyelectrolyte solutions for lithium ion batteries. ACS. Cent. Sci. 2019, 5, 1250-60.
49. Maass, P.; Meyer, M.; Bunde, A. Nonstandard relaxation behavior in ionically conducting materials. Phys. Rev. B. 1995, 51, 8164.
51. Sangoro, J. R.; Kremer, F. Charge transport and glassy dynamics in ionic liquids. Acc. Chem. Res. 2012, 45, 525-32.
52. Vargas-barbosa, N. M.; Roling, B. Dynamic ion correlations in solid and liquid electrolytes: how do they affect charge and mass transport? ChemElectroChem 2020, 7, 367-85.
53. Pothmann, T.; Middendorf, M.; Gerken, C.; Nürnberg, P.; Schönhoff, M.; Roling, B. Overdetermination method for accurate dynamic ion correlations in highly concentrated electrolytes. Faraday. Discuss. 2024, 253, 100-17.
54. Ahmed, M. D.; Zhu, Z.; Khamzin, A.; Paddison, S. J.; Sokolov, A. P.; Popov, I. Effect of ion mass on dynamic correlations in ionic liquids. J. Phys. Chem. B. 2023, 127, 10411-21.
55. Lorenz, M.; Kilchert, F.; Nürnberg, P.; et al. Local volume conservation in concentrated electrolytes is governing charge transport in electric fields. J. Phys. Chem. Lett. 2022, 13, 8761-7.
57. Sheng, J.; Zhang, Q.; Sun, C.; et al. Crosslinked nanofiber-reinforced solid-state electrolytes with polysulfide fixation effect towards high safety flexible lithium-sulfur batteries. Adv. Funct. Mater. 2022, 32, 2203272.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].