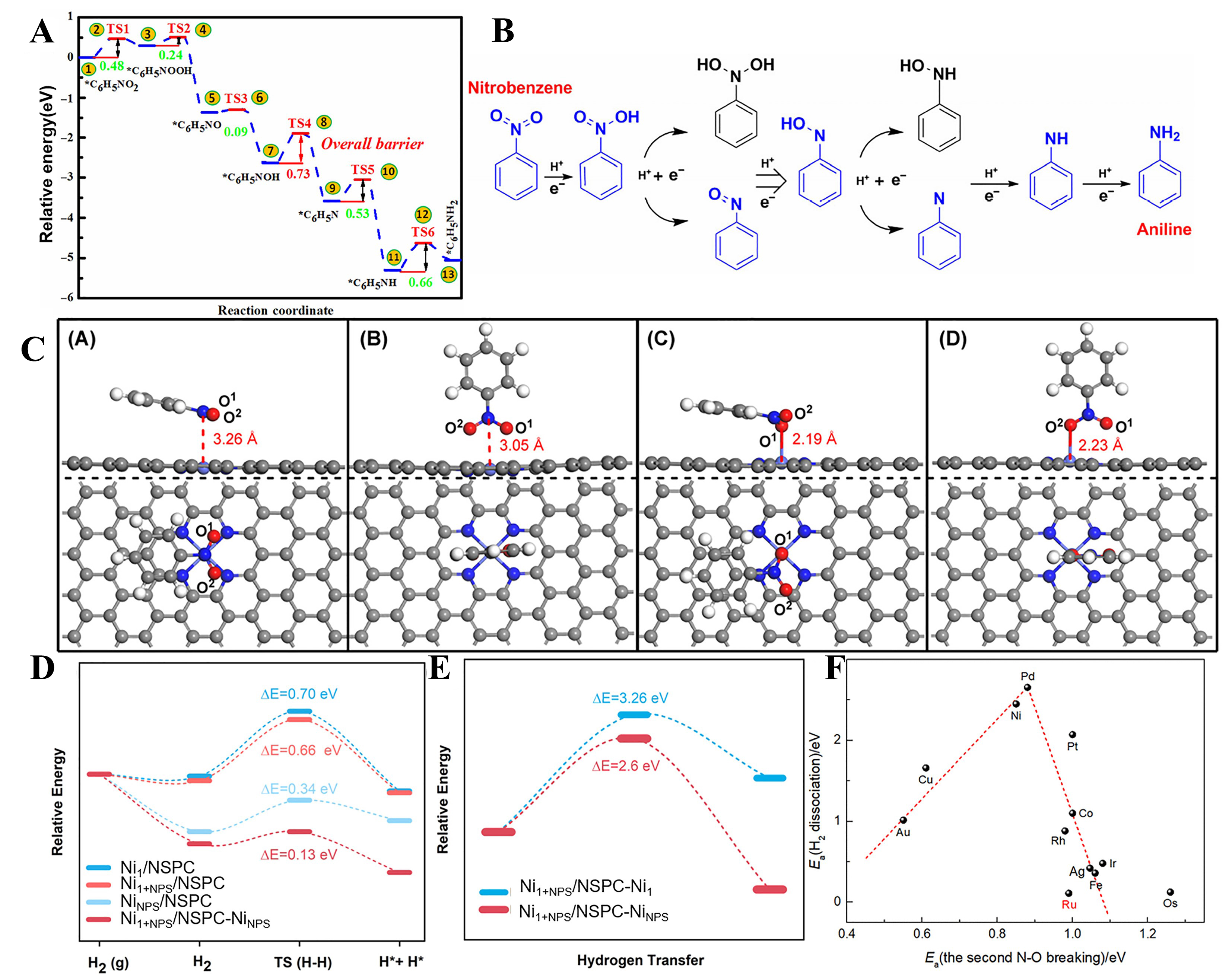
fig10
Figure 10. (A) Energy profiles of the optimal reaction pathway for the hydrogenation of nitrobenzene into aniline on single atom Pt@g-C3N4 catalyst[182]; (B) Proposed possible reaction pathways for the reduction of nitrobenzene into aniline through direct routes (the reaction pathway in blue is the optimal one)[182]; (C) Top and side views of (A) parallel adsorption via nitro N, (B) vertical adsorption via nitro N, (C) parallel adsorption via nitro O, and (D) vertical adsorption via nitro O of nitrobenzene on the Co-N4/C surface[5]; (D) The adsorption and dissociation energies of H2 on different active sites on Ni1/NSPC, NiNPs/NSPC, and Ni1+NPs/NSPC[5]; (E) Energy barriers for H-transfer at different positions on Ni1+NPs/NSPC[5]; (F) Relationship between the Ea of the dominant H2 dissociation and the second N-O bond breaking step of PhNOH* + H* → PhN* + H2O in nitrobenzene hydrogenation[13]. NP: Nanoparticle.