MOFs-based single/dual-atom catalysts: atomically active sites engineering for efficient CO2 reduction
Abstract
The excessive emission of carbon dioxide (CO2) has seriously threatened the global climate environment and human health; the efficient CO2 reduction reaction (CO2RR) as an effective method to reduce CO2 emissions has become a hot spot in the current catalyst field. Metal-organic frameworks (MOFs) serve as ideal substrates for the preparation of single-atom catalysts (SACs) and dual-atom catalysts (DACs), attributed to their distinctive structure and superior pore characteristics. These catalysts address the issues of inadequate selectivity and activity in CO2RR by facilitating efficient electron transfer at the atomic level. This review provides an overview of the design strategies for active sites in MOFs-based SACs and DACs, focusing on the synergy between single-atom and multi-atom configurations. It comprehensively summarizes the mechanisms of both photocatalytic and electrocatalytic CO2RR and the relationship between active sites and catalytic efficiency. Additionally, the review provides an in-depth discussion of their applications in electrocatalysis and photocatalysis of CO2RR, highlighting specific examples and advancements in these fields. Finally, the challenges of the future directions for active sites design and the development of improving catalytic activity in CO2RR at industrial current density of MOFs-based SACs/DACs were looked forward.
Keywords
INTRODUCTION
In recent decades, the rapid increase of global population has brought about unprecedented energy crises, especially the excessive emission of carbon dioxide (CO2)[1-3]. This problem makes the utilization of energy gradually develop towards low-carbon renewable energy[4-6]. Therefore, the global community is focusing on and advancing the two prominent areas of “carbon peak” and “carbon neutrality” to facilitate the efficient transformation of renewable resources[7-10]. For this purpose, the development of CO2 reduction reaction (CO2RR) has been an effective approach for mitigating CO2 emissions, thereby addressing the aforementioned environmental challenges[11-13]. Catalysis is essential for enhancing both electrocatalytic and photocatalytic efficiency in CO2RR[14-16]. The development of high-efficiency catalysts represents a significant global challenge, because the utilization of highly efficient catalysts can effectively expedite the kinetics of chemical reactions[17-19]. The catalytic activity is determined by the number of active sites present on the catalyst[20-22]. Numerous studies have demonstrated that the atomic utilization efficiency of metal active sites is exceptionally high, which can be close to 100%, encouraging increased catalyst research to focus on the dispersion system with atomic level[23-26].
Single/dual-atom catalysts (SACs/DACs), with atomic-scale catalytic active sites, are the most emerging catalyst material in the field of efficient CO2RR[27-29]. The most typical advantages of SACs/DACs are that they have clear coordination structure, optimal atomic utilization and distinctive electronic configuration, which are crucial for the precise regulation of active catalytic sites[30-33]. However, when dispersed to the atomic scale, the high surface energy of metal atoms prevents them from achieving good dispersion and thus clumping together, greatly reducing the catalytic efficiency of SACs/DACs[34-37]. Previous research has demonstrated that the synthesized atomic-scale SACs/DACs exhibit low structural porosity and limited metal loading capacity[38-40]. Therefore, it is particularly critical to find suitable poriferous carrier materials to provide stable and effective support for metal atoms of SACs/DACs[41-43]. Among them, metal-organic frameworks (MOFs) are very suitable porous carrier materials for SACs/DACs[44,45].
MOFs are a kind of poriferous crystal framework that is formed by the combination of metal active center (ion or cluster) and organic macromolecule (N-containing or carboxylic acid ligands) through strong coordination[46-48]. The advantages of MOFs are also obvious, such as the controllable composition of metal and organic connections, large specific surface area, both metal-organic composite properties, controllable porous structure and precise active adsorption sites[49-51]. Coupled with high porosity and high metal loading rate, pristine MOFs are already one of the best carrier choices for atomic-level catalysts[52-54]. In addition, derivatives (porous carbon/metal matrix) obtained by carbonization, calcination and other pyrolysis treatments of MOFs have been widely used as carriers for fixing precisely active catalytic sites at the atomic dispersion scale[55-58]. With distinctive structure and superior pore characteristics, MOFs serve as ideal substrates for the preparation of SACs and DACs (MOFs-based SACs/DACs)[59-61].
So far, the MOFs-based SACs/DACs have shown a strong performance advantage in efficient electrocatalytic and photocatalytic of CO2RR and address the issues of inadequate selectivity and activity in CO2RR by facilitating efficient electron transfer at the atomic level[62-64]. The studies of the design strategies for active sites in MOFs-based SACs and DACs were systematically summarized herein in this review, focusing on the synergy between single-atom and multi-atom configurations. The review comprehensively summarizes the mechanisms of both photocatalytic and electrocatalytic CO2RR (ECRR) and the relationship between active sites and catalytic efficiency. Additionally, the review provides an in-depth discussion of their applications in photocatalysis and electrocatalysis of CO2RR, highlighting specific examples and advancements in these fields [Figure 1]. Finally, the prospects of the future directions of MOFs-based SACs/DACs in CO2RR were looked forward to further improving the catalytic activity at industrial current density.
Figure 1. Overview of MOFs-based SACs/DACs for efficient CO2RR: design and characterization of active sites and mechanism analysis. MOF: Metal-organic framework; SACs: single-atom catalysts; DACs: dual-atom catalysts; CO2RR: CO2 reduction reaction; HAADF-STEM: high-angle annular dark field scanning transmission electron; EDS: energy-dispersive X-ray spectroscopy; XANES: X-ray absorption near-edge structure; EXAFS: extended XAFS; DFT: density functional theory; DRIFT: diffuse reflectance infrared fourier transform ; NMR: nuclear magnetic resonance; EPR: electron paramagnetic resonance.
DESIGN STRATEGIES FOR ACTIVE SITES: FROM SINGLE-ATOM TO MULTI-ATOMS COORDINATION
For the catalyst, the most concerned performance is the level of catalytic activity[65-67]. In order to obtain MOFs-based SACs/DACs with high catalytic activity, the design strategies for active sites are of critical importance, as well as the characterization of active sites[68-70]. In this part, we systematically concluded the design and regulation of MOFs-based SACs/DACs active sites together with the characterization of active sites.
Design and regulation of MOFs-based SACs active sites
MOFs-based SACs literally stand for SACs obtained from MOFs, for example, original MOFs and materials derived from MOFs (carbonaceous material, metal oxides, or other substances)[71,72]. Unlike single-site heterogeneous catalysts, the active sites in MOFs-based SACs are formed by completely isolated metal atoms that do not interact with each other[46,73,74]. Therefore, the design and regulation of active sites directly affect the catalytic efficiency of MOFs-based SACs[75-77].
The single atomic active sites in MOFs-based SACs could be roughly categorized into three distinct types according to the location or connection mode of the active sites in MOFs [Figure 2]: (1) MOFs skeleton limited single-atom sites. With the three-dimensional frame structure, MOFs could incorporate metal-based substances to create final single-atom metal active sites by hosting a variety of functional guest substances within their pores[78-81]; (2) ligands regulated single-atom sites. The organic ligands in MOFs contain abundant chelating binding sites, providing sufficient binding locations for single metal atoms sites[82-85]; (3) defect engineering regulated single-atom sites. Defects in MOFs (mainly referred to as metal nodes), with higher energy state, could be fully combined with heterogeneous metal atoms to form single-atom active sites[86-90]. In this part, synthesis strategies and works of MOFs-based SACs would be discussed around spatial skeleton, ligand regulation and defect regulation of single-atom sites.
Figure 2. (A) Structure diagram of MOFs skeleton limited single-atom sites, quoted with permission from Leng et al.[81]; (B) Structure diagram of Ligands regulated single-atom sites, quoted with permission from Chen et al.[85]; (C) Structure diagram of defect engineering regulated single-atom sites, quoted with permission from Zhang et al.[90]. MOFs: Metal-organic frameworks; ZIF: zeolitic imidazolate framework; PCN: porous coordination network.
MOFs skeleton limited single-atom sites
Isolating metal atoms within the rigid structure of MOFs effectively restricts the mobility of single atoms, preventing their aggregation and ensuring their stability during catalytic processes[91-93]. The MOFs structure provides a tunable environment that could optimize the electronic features and coordination states of the single atomic sites[94-96]. This spatial confinement enhances the mutual influence of the active sites on reactants, thereby improving activation efficiency[97-99]. By leveraging the unique characteristics of MOFs, such as tunable porosity and functionalization, skeleton-limited single-atom sites are applicable to a variety of chemical reactions[100-102].
Zuo et al. prepared Co SACs supported on ultrathin MOFs nanosheets (Co-MNSs) according to a bottom-up synthetic strategy [Figure 3A][103]. Co single atoms were fixed on the ultra-thin MOFs nanosheets and Co-N4 moiety functioned as active sites. By lowering the energy barrier and enhancing charge separation, the ultrathin structure increased accessible sites and facilitated CO2 adsorption to form reactive radicals, thereby improving photocatalytic CO2RR. Xie et al. prepared a series of M-N-C-based SACs using a direct pyrolysis method [Figure 3B][104]. The strong coordination bonds between metal and nitrogen effectively anchored the metal single atoms onto the nitrogen-doped carbon support obtained from ZIF-8. In the process of synthesizing M-N-C-based SACs, the porous structure of ZIF-8 effectively restricted the dispersion of metal single atoms, preventing their agglomeration. Additionally, through the ion exchange strategy, the abundant terminal -OH groups on the Zr6(μ3-OH)8(OH)8(CO2)8 clusters in PCN-222 were deprotonated and utilized to anchor Cu on the Zr6-oxo nodes and stabilizing Cu in the nanopores of PCN-222 by Bao et al. [Figure 3C][105]. This pore confinement effect enabled Cu and Zr6-oxo clusters to join closely via the Zr4+-O2-(-Cu2+) and Zr4+-Cu2+ interface sites, which also led to the alteration of the electronic structure of Cu.
Figure 3. (A) Schematic diagram of fabrication of Co-MNSs for photocatalytic CO2RR, quoted with permission from Zuo et al.[103]; (B) Schematic diagram of preparation of M-N-C based SACs for electrocatalytic CO2RR, quoted with permission from Xie et al.[104]; (C) Schematic diagram of fabrication of Cu/PCN-222, quoted with permission from Bao et al.[105]. ZIF: Zeolitic imidazolate framework; SACs: single-atom catalysts; TCPP: tetrakis(carboxyphenyl)phthalocyanine cobalt; MNSs: metal-nitrogen supramolecular structure.
Ligands regulated single-atom sites
By coordinating with isolated metal atoms, ligands could adjust the associated electronic characteristics and coordination settings, influencing the catalytic efficacy[106-108]. The choice of ligands affects the oxidation condition, electron spreading, and steric hindrance around the metal centers, which could enhance reactivity and selectivity in catalytic processes[109-111]. Additionally, ligands could stabilize single-atom sites, prevent aggregation and ensure their availability for reactions[112-114]. This ligand regulation mechanism allows for the precise tuning of catalytic performance, making it a vital strategy in the programming of effective single atomic catalysts for various chemical applications[115-117].
Chen et al. designed a MOF (UiO-67) encapsulated N-heterocyclic carbene (NHC) linked copper single atom site (Cu SAS) catalyst (2Bn-Cu@UiO-67) using a multi-step method [Figure 4A][118]. The catalyst's porosity promoted CO2 diffusion to 2Bn-Cu sites. The NHC ligand was crucial in determining the catalyst’s property, influencing the electronic structure and interactions of Cu SAS with reactants, and enhancing CO2RR activity. To optimize catalytic performance for single atoms, modifying ligands to regulate the coordination condition around the catalytic sites was essential. Wang et al. developed a series of single cobalt (Co) site catalysts, UiO-Co-Nx (x = 2, 3, 4), using a mixed ligand and post-synthetic modification strategy [Figure 4B][119]. This was achieved by reacting hexahydrated cobalt chloride with ligands such as 2-phenylpyridine and 2,2′-bipyridine at presence of UiO-67-20% bipyridine, thereby anchoring the single Co sites within the UiO-type MOF. The underlying mechanism revealed that varying the nitrogen coordination number influenced the electronic density and charge transfer kinetics of the Co sites. Notably, UiO-Co-N3 exhibited optimal performance, demonstrating the fastest charge transfer rate and the minimum energy obstacles for both the rate-determining procedure in CO2 reduction and CO desorption. Additionally, Dong et al. utilized the Ni-MOF-74 synthesis approach to fabricate a mesoporous Ni-74-Am [Figure 4C][120]. Ni-74-Am, an amorphous MOF with ligand defects, had more unsaturated Ni active sites, enhancing photocatalytic CO2 reduction. Under visible light, it achieved a 1.38 mmol·g-1·h-1 CO generation rate and 94% CO selectivity, due to its suitable valence and band structure, high electron-hole separation efficiency, and fast carrier migration rate.
Figure 4. (A) Schematic diagram of fabrication of 2Bn-Cu@UiO-67, quoted with permission from Chen et al.[118]; (B) Preparation strategy of UiO-Co-NX (x = 2, 3, 4), quoted with permission from Wang et al.[119]; (C) Schematic diagram of fabrication of Ni-74-Am, quoted with permission from Dong et al.[120]. DMF: N,N-dimethylformamide; MOF: metal-organic framework.
Defect engineering regulated single-atom sites
Introducing defects such as vacancies or interstitials could alter the local environment around metal centers, thereby affecting their electronic structure and reactivity[121-123]. These defects could create additional active sites and contribute to the stabilization of isolated metal atoms, preventing their aggregation[124-126]. Furthermore, they could modify the adsorption characteristics of reactants, facilitating more efficient catalytic pathways[22,127,128]. This strategic manipulation of defects allows for the fine-tuning of single atomic catalysts, enhancing their performance for various catalytic processes[129-131].
Huang et al. synthesized a core-shell structured heterojunction catalyst based on Co and Zn atoms (Co3S4/NC@ZnS/NC) using a MOFs-on-MOFs strategy [Figure 5A][132]. Analysis confirmed a higher sulfur-vacancy concentration in Co3S4/NC@ZnS/NC than in Co3S4/NC. The interaction between ZIF-67 and ZIF-8, along with subsequent transformations, located the Co and Zn atoms at specific locations within the core-shell structure. This arrangement, along with the carbon-nitrogen-doped matrix and the formation of sulfides, maintained a relatively stable distribution, enabling effective immobilization. Shan et al. synthesized UiO-66-based Cu SACs via solvothermal method. Defect engineering retained the MOF framework integrity [Figure 5B], with Cu2+ anchored at UiO-66 defect sites (-OH/-OH2) to form Cu-O coordination structures [Figure 5C]. Energy-dispersive X-ray spectroscopy (EDS) elemental mapping confirmed uniform Cu distribution across the support [Figure 5D][133]. Additionally, Wen et al. developed a microwave-assisted rapid pyrolysis approach for the preparation of carbon-based SACs (Ni1NC), in which Ni was implanted in N-doped carbon [Figure 5E][134]. The aberration-corrected high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) results obviously demonstrated that Ni was dispersed in a single atomic state [Figure 5F].
Figure 5. (A) Schematic diagram of fabrication of Co3S4/NC@ZnS/NC, quoted with permission from Huang et al.[132]; (B) PXRD patterns of bare UiO-66-defect and UiO-66-M materials; (C) View of the structure of the cluster node in UiO-66-Cu incorporating a single Cu(II) center at the defect site. Color scheme: Cu, orange; O, red; C, grey; H, white; (D) The EDS elemental mapping images of UiO-66-Cu; (B-D) are quoted with permission from Shan et al.[133]; (E) Preparation strategy of Ni1NC; (F) Aberration-corrected HAADF-STEM image of Ni1NC; (E and F) are quoted with permission from Wen et al.[134]. ZIF: Zeolitic imidazolate framework; PXRD: powder X-ray diffraction; EDS: energy-dispersive X-ray spectroscopy; HAADF-STEM: high-angle annular dark field scanning transmission electron microscopy.
Design and synergistic effects of MOFs-based DACs active sites
Owing to the synergistic effect between the dual-atomic active sites, it has been demonstrated that the catalytic performance of MOFs-based DACs markedly outperforms that of MOFs-based SACs. MOFs-based DACs referred to the direct or indirect establishment of DACs with MOFs as the basic materials[135-137]. With the support of proper preparation methods and frame structure, the bimetallic atoms are uniformly dispersed in the MOFs[138-140]. The derivatives of the MOFs precursors obtained by pyrolysis such as carbonization or calcination could be loaded with various dual-atom metal catalytic sites[141-143]. In MOFs-based DACs, adjacent metal atoms are directly or indirectly linked together to form two catalytic sites. These metal atoms could be homologous or heterologous, usually connected with some non-metallic atoms featuring high electronegativity, including elements such as N, S, O, or C[144-146]. According to the type of metal atoms at the catalytic sites and the connection mode, MOFs-based DACs were usually divided into three types: homologous bimetallic atomic pairs, heterobimetallic isolated atom pairs and heterobimetallic atomic pairs [Figure 6][147-149]. Each of these types would be discussed in detail in different sections below.
Figure 6. (A) Structure diagram of homologous bimetallic atomic pairs, quoted with permission from Leng et al.[150]; (B) Structure diagram of heterobimetallic isolated atom pairs, quoted with permission from Chen et al.[135]; (C) Structure diagram of heterobimetallic atomic pairs, quoted with permission from Zhang et al.[151].
Homologous bimetallic atomic pairs
For homologous bimetallic atomic pairs, the active catalytic sites are formed by the cooperative interaction between the same metallic atoms with the non-metallic chelating elements (such as N) [Figure 6A][152-154]. CO2RR requires high selectivity and stability of catalyst, which brings difficulty to the design and application of MOFs-based DACs with homologous bimetallic atomic pairs[155-157].
Based on a two-step template-assisted method, Wang et al. prepared precursor Fe-ZIF-8 by a host-guest encapsulation strategy. Then, through 950 °C pyrolysis under a 95% Ar + 5% H2 mixed atmosphere for one hour, a dual-atom iron sites electrocatalyst with N-doped carbon matrix (Fe2-N6-C DAC) was obtained
Figure 7. (A and B) Schematic diagram of fabrication (A) and XRD spectrum(B) of Fe1-N4-C SAC and Fe2-N6-C DAC; (C and D) LSV curves (C) and FECO test (D) of different Fex-Ny-C catalysts; (A-D) are quoted with permission from Wang et al.[158]; (E) Structure diagram of Fe2NPC; (F and G) FECO (F) and JCO (G) of Fe2NPC and other reference catalysts; (E-G) are quoted with permission from Zhao et al.[159]. SACs: Single-atom catalysts; DACs: dual-atom catalysts; RHE: reversible hydrogen electrode; XRD: X-ray diffraction; LSV: linear sweep voltammetry; FECO: Faradaic efficiency for CO.
Heterobimetallic isolated atom pairs
Contrary to homologous bimetallic atomic pairs, the active sites in the catalytic center assembled with the coordination of heterogeneous metallic atoms with N atoms are called heterobimetallic isolated atom pairs
Figure 8. (A) Schematic diagram of fabrication of Fe1-Ni1-N-C; (B and C) LSV curves (B) and FECO test (C) of Fe1-Ni1-N-C and other catalysts; (A-C) are quoted with permission from Jiao et al.[163]; (D) Preparation strategy of NiN3@CoN3-NC; (E and F) HRTEM test (E) and HAADF-STEM elemental mapping images (F) of NiN3@CoN3-NC; (D-F) are quoted with permission from Zhang et al.[170]. ZIF: Zeolitic imidazolate framework; RHE: reversible hydrogen electrode; LSV: linear sweep voltammetry; FECO: Faradaic efficiency for CO; HAADF-STEM: high-angle annular dark field scanning transmission electron microscopy.
Heterobimetallic atomic pairs
For heterobimetallic atomic pairs, the active catalytic sites are formed by direct connections between different dual-metal atoms in addition to coordination connections by metal and N atoms, etc.
Additionally, Zhu et al. strategically engineered quasi-covalently linked Ni-Cu dual-atomic sites, optimizing the Ni 3d electronic structure to achieve high-efficiency CO2RR [Figure 9A][171]. Ni-ZIF-8 was used as the host, with Cu/1,10-phenanthroline as guest; the precursor Ni/Cu-ZIF-8 was generated by a host-guest construction approach. After Ni/Cu-ZIF-8 was pyrolyzed under a flowing Ar/H2 atmosphere, the final product Ni-Cu dual-atom sites catalyst was got and named Ni/Cu-N-C. CO2RR performance evaluation showed the Ni/Cu-N-C catalyst exhibited the highest FECO of 97.7% and turnover frequency (TOF) of
Figure 9. (A) Schematic diagram of fabrication of Ni/Cu-N-C; (B and C) FECO tests (B) and TOF (C) of Ni/Cu-N-C and other reference catalysts; (D) Current-time response and the corresponding FECO of Ni/Cu-N-C at -0.7 V; (A-D) are quoted with permission from Zhu et al.[171]; (E) Preparation strategy of Zn1Mn1-SNC, quoted with permission from Pei et al.[147]. ZIF: Zeolitic imidazolate framework; RHE: reversible hydrogen electrode; TOF: turnover frequency; FECO: Faradaic efficiency for CO.
Characterization of active sites
In MOFs with atomic catalysts, characterization techniques have been essential in identifying the locations of metal active sites, as their distribution and environment have directly influenced catalytic performance[172-174]. Identifying the position of metal active sites aids in understanding the mechanisms of catalytic reactions, thereby optimizing catalyst design[175-177]. Furthermore, different positions of metal sites may exhibit varying selectivity towards reactants, and understanding their locations could enhance the selectivity of the catalyst[178-180]. Additionally, the environment of the metal sites could affect their stability, and knowing their positions could help predict the catalyst’s behavior during the reduction reaction course[181]. Characterization of the architecture and composition of materials is often performed using various instruments, including HAADF-STEM, powder XRD (PXRD), EDS, synchrotron-based soft X-ray absorption near-edge structure (XANES), X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS), nuclear magnetic resonance (NMR), electron paramagnetic resonance (EPR), and PXRD[182]. Additionally, spin-polarized density functional theory (DFT) is used to forecast which sites may exhibit higher catalytic activity[183].
Electron microscopy techniques and EDS-mapping
HAADF-STEM, TEM, and SEM are used to observe the microstructure, morphology, and elemental distribution of catalysts[184]. Electron microscopy plays a vital part in examining the active sites of catalysts and their microstructures, studying the elemental distribution and interactions of materials, and revealing the characteristics and behavior of metal sites[185]. EDS provides qualitative and quantitative information about elements by analyzing the characteristic X-rays emitted from the sample. EDS could analyze the distribution of elements at the nanoscale, revealing the specific locations of metal sites within the MOFs structure[186].
Wang et al. used HAADF-STEM to observe the atomic-level configuration of catalyst (FeSe/NC) during their research, identifying the atomic positions of different elements based on differences in atomic brightness, thereby determining the distribution of metal sites[187]. A HAADF-STEM image of Fe/ZIF-8, which exhibited a uniform rhombic dodecahedron shape, indicated that Fe ions have been successfully encapsulated within the cage-like structure of ZIF-8, laying the foundation for the subsequent synthesis of FeSe/NC [Figure 10A]. The HRTEM image of FeSe/NC showed that it had a dodecahedron shape [Figure 10B]. From results of HAADF-STEM image of FeSe/NC, it could be seen that many voids appeared within FeSe/NC, which increased the disclosure of Fe sites and contributed to mass diversion during ORR process [Figure 10C]. The EDS mapping images of FeSe/NC further confirmed a homogeneous distribution of Fe, Se, N, and C throughout the catalyst, indicating that these elements work synergistically within the catalyst system, with Fe sites closely interacting with other elements to form the catalytic active center [Figure 10D]. Liu et al. analyzed the Fe-Fe bimetallic center catalyst (Fe2N6-S) within ZIF-8[188]. The HRTEM image of Fe2N6-S visually presented the micro-morphology and crystal structure characteristics of the material, indicating no significant aggregation of metal particles and an amorphous state [Figure 10E]. The HAADF-STEM images of Fe2N6-S clearly showed the distribution of Fe, with bright spots formed by higher molecular weight Fe species confirming their uniform dispersion within the graphite carbon and existence of bimetallic sites, revealing the distribution patterns of the metal sites at the microscopic level [Figure 10F and G]. Sun et al. researched Cu-Se DACs[189]. Characterization through EDS image of Cu-Se DACs clearly proved the equal interspersion of copper and selenium atoms in the Cu-Se DACs [Figure 10H]. This observation indicated a similarity in the copper content. Additionally, HAADF-STEM images of Cu-Se DACs proved that the Cu-Se DACs were evenly distributed throughout the carbon matrix
Figure 10. (A) HAADF-STEM of FeSe/NC; (B) HRTEM of FeSe/NC; (C) HAADF-STEM of FeSe/NC; (D) EDS mapping images of FeSe/NC; (A-D) are quoted with permission from Wang et al.[187]; (E) HRTEM image of Fe2N6-S (insert was SAED pattern with selective area); (F and G) HAADF-STEM images of Fe2N6-S; (E-G) are quoted with permission from Liu et al.[188]; (H) EDS image of Cu-Se DACs; (I and J) HAADF-STEM image of Cu-Se DACs (I) and Cu-Se SACs (J); (H-J) are quoted with permission from Sun et al.[189]. HAADF-STEM: High-angle annular dark field scanning transmission electron microscopy; EDS: energy-dispersive X-ray spectroscopy; HRTEM: high-resolution transmission electron microscopy; DACs: dual-atom catalysts; SACs: single-atom catalysts.
X-ray absorption fine structure
X-ray absorption fine structure (XAFS) is a highly effective method to detect the local structure of single and dual atoms. XRD has been used to establish the crystalline construction of catalyst, while XPS detects the chemical component and electronic states of catalyst, including the oxidation number and coordination conditions of metallic elements[190]. Meanwhile, XAS, specifically XANES, has been used to analyze the oxidation number and coordination conditions of metallic elements within the catalyst, while extended XAFS (EXAFS) provides information on the local structure of metallic atoms, determining bond lengths and coordination numbers for metal-ligating atom interactions[191-193].
Sun et al. determined the changes in oxidation states of Ru and Zr elements in Ru1Zr1/Co DACs by analyzing their XANES spectra[194]. The near-edge absorption energy of Ru shifted to smaller values under H2 reduction and syngas atmosphere, approaching the absorption energy of Ru foil, indicating a reduction in the oxidation state of Ru atoms [Figure 11A]. In contrast, the near-edge absorption energy of Zr remained similar and close across different stages, suggesting that Zr atoms maintained a positive charge and their oxidation state remained relatively unchanged throughout the reaction process [Figure 11B]. Through EXAFS analysis, a fresh peak appears around 2.4 Å, attributed to Ru-Co or Ru-Ru coordination, suggesting Ru atoms undergoing bond reconstruction in the back of reductive reaction [Figure 11C]. For Zr atoms, a peak associated with Zr-O coordination was found at approximately 1.6 Å in samples at different stages, indicating that Zr atoms primarily maintained Zr-O coordination, being uniformly dispersed in Co nanoparticles and the support, and were difficult to reduce [Figure 11D]. Additionally, Liu et al. confirmed the presence of Fe-Cu coordination through the appearance of the Fe-Cu peak in the EXAFS spectra within K-edges of Fe and Cu [Figure 11E][195]. The analysis indicated that the metal atoms were in an atomically dispersed state on the FeCu-NC catalyst, with no evidence of Fe-Fe or Cu-Cu scattering. Meanwhile, Zhao et al. investigated Ni-Co bimetallic site relay electrocatalysts and found that the structures of the synthesized catalysts (such as Co-MOFs and NiCo-MOFs series) were consistent with the simulated MOFs structure (CCDC no. 1502849) through XRD analysis [Figure 11F][196]. Furthermore, XPS analysis revealed a survey spectrum confirming the presence of Ni, Co and N elements in Ni2Co1-MOFs, which was consistent with the EDS results [Figure 11G]. Post-fresh electrolysis testing showed the disappearance of the N 1s peak and changes in the oxidation states of Ni and Co, indicating that Ni2+/Ni3+ and Co2+/Co3+ participate as redox pairs in the reaction [Figure 11H and I].
Figure 11. (A and B) Ru K-edge (A) and Zr K-edge (B) XANES spectra of samples under different conditions; (C and D) Ru K-edge (C) and Zr K-edge (D) EXAFS spectra of samples under different conditions; (A-D) are quoted with permission from Sun et al.[194]; (E) Wavelet-transformed K3-weighted EXAFS spectra and corresponding metal foil reference of FeCu-NC, quoted with permission from Liu et al.[195]; (F) XRD patterns of synthesized MOFs catalysts; (G) XPS spectra of Ni2Co1-MOFs; (H and I) High-resolution XPS of Ni 2p (H) and Co 2p (I) of post and fresh Ni2Co1-MOFs; (F-I) are quoted with permission from Zhao et al.[196]. XANES: X-ray absorption near-edge structure; EXAFS: extended X-ray absorption fine structure; XRD: X-ray diffraction; MOFs: metal-organic frameworks; XPS: X-ray photoelectron spectroscopy; FT: Fourier transform.
DFT calculations
DFT plays a pivotal role in studying atomically dispersed catalysts within MOFs, particularly in characterizing metal active sites. It provides insights into electronic properties, revealing band structures and density of states crucial for understanding catalytic behavior. DFT enables the simulation of potential energy surfaces to identify reaction pathways and transition states, elucidating catalytic mechanisms[197]. Additionally, it calculates adsorption energies to evaluate how reactants and intermediates interact with metal sites, informing on selectivity and efficiency[198].
Qiao et al. methodically studied the property of a succession of two-dimensional MOFs (M3(HADQ)2) as single atomic catalysts used in oxygen reduction reaction (ORR) using DFT method and microkinetic simulations[199]. A significant linear correlation was found between ΔGOH* and either ΔGOOH* or ΔGO* [Figure 12A], which governed the ORR activity trends across different monolayers. The positioning of the volcano plot allowed for a clear assessment of the relative activity of different metal active sites in the ORR, enabling effective characterization of these sites [Figure 12B]. Additionally, Ming et al. conducted the catalytic cycle for CO2 reduction in Co2-MOFs(-NH2), where the catalyst accepted electrons to form Int-1, which subsequently led to the formation of Int-2 upon adsorption [Figure 12C][200]. The energy barrier resulting from the DFT calculations clearly demonstrated the free energy changes (ΔG) during various stages of reduction process, such as the constitution of Int-2 and the proton-coupled electron transfer (PCET) stage [Figure 12D]. Meanwhile, Hu et al. utilized DFT calculations to intuitively demonstrate the impact of amino groups on the water adsorption capability of the catalyst [Ru1/UiO-67-X, X represented -H, -m-(NH2)2, or -o-(NH2)2], as well as the differences introduced by amino groups positioned at various locations[201]. The computed adsorption energies of H2O adsorbed on Ru1/UiO-67-X showed that the -NH2 group located closer to the Ru1 site resulted in lower adsorption energy, as shown in Figure 12E. Additionally, the structural simulation results between H2O and Ru1/UiO-67-X showed that Ru1/UiO-67-o-(NH2)2 was connected to H2O by three short bonds O–H···O, O–H···N, and O–H···S, while for Ru1/UiO-67-H and Ru1/UiO-67-m-(NH2)2, there was only a long O–H···O [Figure 12F].
Figure 12. (A) The linear relationships between ΔGOOH*, ΔGO*, and ΔGOH* on different monolayers of 2D MOFs; (B) Changing of ORR catalytic activity with ΔGOOH* and ΔGOH* of the 2D MOFs monolayers; (A and B) are quoted with permission from Qiao et al.[199]; (C) Catalytic cycle for CO2 reduction in Co2-MOFs(-NH2); (D) DFT calculations of energy barriers for Co2-MOFs(-NH2) and Co-MOFs(-NH2); (C and D) are quoted with permission from Ming et al.[200]; (E) The computed adsorption energies of H2O adsorbed on Ru1/UiO-67-X; (F) The structural simulation between H2O and Ru1/UiO-67-X; (E and F) are quoted with permission from Hu et al.[201]. PCET: Proton-coupled electron transfer; Cat: catalyst; Int: intermediate; MOFs: metal-organic frameworks; ORR: oxygen reduction reaction; DFT: density functional theory.
Overall, for MOFs-based SACs, the main difference is the location and connection of the active metal atoms, such as skeleton-limited, ligand-linked and defect sites. For MOFs-based DACs, the main difference is the type and connection mode of the metal atoms at the two active sites, so there are three types of homologous bimetallic atomic pairs, heterobimetallic isolated atom pairs and heterobimetallic atomic pairs[202]. In order to have a better understanding of MOFs-based SACs/DACs, we summarized the relevant information of recent studies of them for CO2RR mentioned in this review [Table 1]. A clear understanding of these types of MOFs-based SACs/DACs can help us to better study the reaction mechanism of catalysts and regulate the activity of catalysts more efficiently[203]. In addition, HAADF-STEM emphasizes the identification of isolated single and diatomic pairs through multi-regional statistical analysis and atomic-level resolution images, and demonstrates local magnification. EXAFS and XANES can be used to judge the coordination environment. The coordination number of single atom and diatom may be different. Compare the coordination environment differences between SAC and DAC: single atoms usually show a single coordination peak (such as M-N/O), while diatoms may show a double coordination path (such as M-M coordination bonds). DFT can simulate the spectral characteristics of different structures, thus helping to interpret experimental results[204].
Summary of Recent Studies in MOFs-based SACs/DACs for CO2RR
| SACs/DACs | MOFs | Type | Metal sites | Synthetic strategy | Characterization | Ref. |
| SAC | MNSs | Skeleton limited | Co(Co-N4) | Surfactant-stabilized coordination strategy | HAADF-STEM, EXAFS, XANES, XPS | |
| SAC | ZIF-8 | Skeleton limited | Fe, Ni, Mn, Co, Cu(M-N) | Pyrolysis | HAADF-STEM, XPS | |
| SAC | PCN-222 | Skeleton limited | Cu (Zr4+-O2-(-Cu2+) and Zr4+-Cu2+) | Ion exchange | XPS, EXAFS, XANES, DRIFTS | |
| SAC | UiO-67 | Ligands regulated | Cu(Cu-C, Cu-O) | MOF encapsulation | HAADF-STEM, XAS, NMR, XPS | |
| SAC | UiO-67 | Ligands regulated | Co(Co-N3) | Post-synthetic modification | XPS, XANES, EXAFS, PXRD | |
| SAC | Ni-MOF-74 | Ligands regulated | Ni(Ni-O) | Solvothermal modulation | PXRD, XPS, HAADF-STEM | |
| SAC | ZIF-67@ZIF-8 | Defect engineering regulated | Co(Co-S) | MOF-on-MOF | XPS, HRTEM, XRD | |
| SAC | UiO-66 | Defect engineering regulated | Fe, Cu(M-OH,M-OH2) | Solvothermal method | PXRD, EXAFS, HAADF-STEM, XANES | |
| SAC | Ni-ZIF-8 | Defect engineering regulated | Ni(Ni-N4) | Microwave-Assisted | HAADF-STEM, XANES, EXAFS, XPS | |
| DAC | Fe-ZIF-8 | Homologous bimetallic | Fe-Fe(Fe2-N6) | Template-assisted protocol with H2 pyrolysis conditions | HAADF-STEM, EXAFS, XANES, XPS | |
| DAC | Fe-ZIF-8 | Homologous bimetallic | Fe-Fe(Fe2-N6) | Precursor encapsulation, pyrolysis | HAADF-STEM, XANES, EXAFS | |
| DAC | ZIF-8 | Heterobimetallic isolated | Fe, Ni(Fe-N4 Ni-N4) | Mechanochemical methods, pyrolyzed | HAADF-STEM, XANES, EXAFS, XPS | |
| DAC | Zn/Co-ZIFs | Heterobimetallic | Ni-Co(NiN3 CoN3) | Pyrolysis | HAADF-STEM, XANES, EXAFS, XRD, XPS | |
| DAC | Ni-ZIF-8 | Heterobimetallic | Ni, Cu(Ni-Cu-N6) | Pyrolysis | HAADF-STEM, XANES, EXAFS, XPS, XAS, EDS | |
| DAC | ZIF-8 | Heterobimetallic | Zn, Mn(S/N-Zn-Mn-S/N) | Two-step ligand etch-pyrolysis protocol | HAADF-STEM, EXAFS, XANES, XPS, EDS | |
| DAC | ZIF-8 | Heterobimetallic | Fe-Se(Fe-N, Fe-Se) | Grind,pyrolysis | HAADF-STEM, XANES, EXAFSXPS, EDS, | |
| DAC | ZIF-8 | Homologous bimetallic | Fe-Fe(Fe2N6) | Host-guest encapsulation strategy | HAADF-STEM, XANES, XPS | |
| DAC | ZIF-8 | Heterobimetallic | Cu-Se(Cu-N4,Se-C3) | Co-pyrolysis | XPS, XANES, EXAFS, HAADF-STEM, EDS | |
| DAC | ZIF-67 | Heterobimetallic | Ni, Co(Ni2+/Ni3+Co2+/Co3+) | Solvothermal method | XPS, TEM, HAADF-STEM, EDS, XRD, FTIR | |
| DAC | MOF-69C | Heterobimetallic isolated | Ru, Zr(surface of Co NPs) | Solvothermal method | HAADF-STEM, EDS, XPS, XANES, EXAFS | |
| DAC | IISERP-MOF27 | Heterobimetallic | Fe, Cu(FeN3O-CuN4) | Pyrolysis | HAADF-STEM, XANES, EXAFS, XPS | |
| SAC | 2D M3(HADQ)2 | Skeleton limited | Co, Cr, Cu, Fe, Mn, Ni, Pd, Rh and Ru(M-N4) | Solvothermal synthesis | DFT | |
| DAC | MOF-(NH2) | Homologous bimetallic | Co(Co-L1-Co) | Mechanochemical methods | PXRD, HRTEM, EDS, XPS, DFT | |
| SAC | UiO-67-X | Skeleton limited | Ru(Ru-O,Ru-S) | Microwave assisted methods | HAADF-STEM, XANES, EXAFS, DRIFTS |
CATALYTIC MECHANISM OF ACTIVE SITES IN CO2RR
Various strategies have been employed to reduce CO2 to usable chemicals, such as photocatalysis, electrocatalysis, thermal catalysis, biological reduction, and various co-catalytic methods[205-207]. Electrochemical and photochemical catalytic CO2RR are considered two very promising strategies because they are capable of converting CO2 under ambient temperature and pressure conditions[118,208,209]. Electrocatalytic and photocatalytic CO2RR use different energies: electrocatalysis reduces CO2 by stimulating electron movement through current and voltage, while photocatalysis absorbs light energy to stimulate highly active photoelectrons to realize CO2 under light irradiation[210-212]. Although the common goal of both is to activate CO2 molecules by catalyzing energy conversion at the active sites, the CO2RR of whether electrocatalyzed or photocatalyzed, is quite complex[213]. Therefore, to achieve efficient catalytic reduction of CO2, understanding the mechanism of electrocatalytic and photocatalytic CO2RR is a very basic and key problem. In addition, the relationship between active sites and catalytic efficiency should be deeply understood.
Mechanism of electrocatalytic CO2RR
The ECRR has the advantages of strong controllability, adjustable electrochemical parameters and high conversion efficiency under voltage drive[214-216]. However, this CO2RR process encompasses several electron and proton transfer stages and requires overcoming the high overpotential brought by the thermodynamic and kinetic obstacles caused by the stability of the CO2 molecule[217,218]. Therefore, a low overpotential means a low electrolytic potential, resulting in a high current density and Faraday efficiency, achieving a high catalytic rate[219,220]. ECRR is performed at the electrode/electrolyte interface, where MOFs could be used as both electrode and electrocatalyst, and the materials with similar structure to graphene derived from MOFs by pyrolysis have good conductivity[221,222]. Metal centers such as Fe, Co, Ni, etc. and their assembly serve as atomic-level catalytic sites within MOFs that could promote the efficiency of CO2 adsorption and activation[223-225]. Meanwhile, organic ligands could modify the electron atmosphere of the metallic ion and provide additional proton transfer sites, immobilizing the intermediate product of the reaction[226,227]. Moreover, the porous structure of MOFs offers an extensive surface area, enhancing the mass transfer of CO2 and intermediates. The pore dimensions and morphology of MOFs could be adjusted to optimize both adsorption and diffusion processes[228,229].
In MOFs-based SACs/DACs, the CO2RR typically encompasses several stages, during which protons and electrons are transferred simultaneously, resulting in the generation of a variety of products[70,230]. With the difference in the number of electrons transferred during CO2 reduction, these products could be divided into C1 (CO, HCHO, CH4, HCOOH and CH3OH) and C2 (C2H4, C2H5OH and CH3COOH) according to the number of carbon atoms. The products and their corresponding reaction equations are listed in
Products and corresponding reaction equations of electrocatalytic CO2RR
| C1 or C2 | Products | Reaction equations |
| C1 | Carbon monoxide (CO) | CO2 + 2H+ +2e- → CO + H2O |
| Formaldehyde (HCHO) | CO2 + 4H+ + 4e- → HCHO + H2O | |
| Methane (CH4) | CO2 + 8H+ + 8e- → CH4 + 2H2O | |
| Formic acid (HCOOH) | CO2 + 2H+ + 2e- → HCOOH | |
| C2 | Methanol (CH3OH) | CO2 + 6H+ + 6e- → CH3OH + H2O |
| Ethylene (C2H4) | CO2 + 12H+ + 12e- → CH4 + 4H2O | |
| Ethanol (C2H5OH) | CO2 + 12H+ + 12e- → C2H5OH + 3H2O | |
| Acetic acid (CH3COOH) | 2CO2 + 8H+ + 8e- → CH3COOH + 2H2O |
Mechanism of photocatalytic CO2RR
Generally, the process of CO2RR conversion is divided into three steps: (1) adsorption of CO2; (2) transportation of carriers and electrons; and (3) activation of CO2. For photocatalytic CO2RR, the third step is the key[235]. From the dynamic process of reduction reaction, the process of photocatalytic CO2RR is similar to electrocatalysis, involving multi-electron reactions that break multiple chemical bonds, and the reaction equations are the same[236,237]. However, the products of photocatalytic CO2RR are not as many as electrocatalysis, almost C1 products (CO, HCHO, CH4, HCOOH and CH3OH) [Figure 13][238,239]. As mentioned above, the activation barrier due to the stability of CO2 requires an energy of 750 kJ/mol to break the C–O bond, meaning that a catalyst needs to absorb enough light energy and convert it into electron-hole pairs which determines the photocatalytic activity[240-242]. Therefore, the activity of photocatalyst could be significantly enhanced by broadening the wavelength range and increasing, improving the light absorption efficiency, and stimulating the separation of electrons and holes from each other[243].
The conjugated pi-system of MOFs could absorb additional photons and promote photochemical reactions, while its porous structure is conducive to surface adsorption of the active sites with more CO2 molecules captured[244]. Therefore, MOFs materials could improve the activity of photocatalytic CO2RR to a certain extent. However, the conversion efficiency of MOFs-based photocatalytic CO2RR has not been as efficient as expected[245,246]. Combined with the atomic active sites and their diverse electronic and coordination structures, MOFs-based SACs/DACs have more selectivity and higher catalytic activity for photocatalytic CO2RR[247]. Combining the dual advantages of MOFs and atomic-level catalysts and overcoming the disadvantages of other homogeneous and heterogeneous catalysts, MOFs-based SACs/DACs have become one of the important members in photocatalytic CO2RR[248].
Relationship between active sites and catalytic efficiency
From the analysis of catalytic mechanism of active sites in CO2RR above, whether photocatalysis or electrocatalysis, the active sites are the key to determining the activation of CO2 molecules and the final catalytic performance[249]. Thus, it is particularly important to understand the relationship between active sites and catalytic efficiency. For MOFs-based SACs/DACs, the actual catalytic active sites are mainly determined by the coordination effect[81,250].
On the one hand, regulating the coordination N atom is the key factor that directly determines the synergies between metal active sites and carriers, and even the overall catalytic efficiency. On common N-doped carbon-based carriers, metal atom generally forms 4-coordinated active sites (M-NxCy)[251]. A decrease in the coordination saturation of the metallic active centers leads to a reduction in its coordination number, thereby enhancing the likelihood of interaction with the matrix, and resulting in better catalytic performance[252,253]. In addition, the unsaturated coordination of M-Nx (x < 4) site is more favorable to electron transfer in the catalytic process due to the vacancy defect[254,255]. Therefore, by establishing multi-vacancy, the unsaturation of the metallic active atoms could be improved with the reduction of coordination, thus enhancing the catalytic activity. On the other hand, the electronic structure of M-N4 could be changed through the incorporation of heterogeneous atoms (P, S, or B). Through the doping or substitution of heterologous atoms, the original coordination structure of M-N4 will produce a reconstruction of the local coordination environment, and the coordination atoms could transfer more dynamic electrons to the metal center, so enhancing the catalytic efficiency[256].
APPLICATIONS OF MOFS-BASED SACS/DACS DESIGNED BASED ON ACTIVE SITES IN CO2RR
MOFs-based materials and their derivatives provide more selectivity in creating highly active SACs and DACs[257,258]. Investigators could leverage their innovative thinking and creativity to build MOFs-based SACs/DACs featuring diverse structural configurations and performance for electrocatalytic and photocatalytic CO2RR[259-261].
Electrocatalytic CO2RR
ECRR offers greater controllability and flexibility, allowing for the adjustment of current density and potential to precisely control the reaction rate and product selectivity[262-264]. For MOFs-based SACs/DACs used in ECRR, synergistic effects and coordination conditions of single/dual-atom active sites, as well as fine-tuning of preparation conditions, will result in different catalytic activities[265,266].
Here in a MOFs-based SAC, the coordination environment of single-atom nickel significantly influenced its electrocatalytic performance in CO2 reduction. Gong et al. anchored Ni atoms within nitrogen-doped porous carbon, forming active sites with different Ni-N coordination structures and quantity, such as NiSA-N4-C (4.0), NiSA-N3-C (3.4), and NiSA-N2-C (2), respectively [Figure 14A][267]. Result of electrocatalytic CO2 reduction reaction showed that NiSA-N2-C exhibited a top FECO of 98% at 0.8 V, demonstrating a best catalytic efficiency of NiSA-N2-C in ECRR among all measured SACs [Figure 14B]. Furthermore, the TOF for CO production from NiSA-N2-C reached 1,622 h-1 at 0.8 V and increased to 3,467 h-1 at 1.0 V, significantly surpassing that of NiSA-N4-C and NiSA-N3-C, thus demonstrating enhanced catalytic activity [Figure 14C]. Peng et al. developed single-atom Ni catalysts (Ni1-N-C(X), where X = Cl, Br, I) based on ZIF-8 using a strategy for modifying metal halides post-synthesis[268]. EXAFS fitting revealed that the coordination environment of Ni atoms consisted of four nitrogen atoms in the plane and one chlorine atom in an axial position, successfully anchored onto the N-doped carbon support with a specific coordination structure [Figure 14D]. Regarding electrocatalytic CO2 reduction performance, Ni1-N-C(Cl) achieved a highest FE of 94.7% for CO production at -0.7 V, significantly surpassing that of Ni1-N-C(Br) and Ni1-N-C(I) [Figure 14E]. Additionally, the CO partial current density of Ni1-N-C(Cl) also outperformed that of two other samples [Figure 14F]. McCarver et al. employed first-principles calculations to investigate Fe-MOFs-74 for CO2 reduction[269]. Result of schematic diagram of structural change of Fe-MOFs-74 showed that Fe atoms were fixed at specific sites within the MOFs-74 framework as catalysts [Figure 14G]. At a potential of 0.32 eV, the transition from *OCHO to *HCOOH reached a state of equal energy, rendering the reaction feasible and ultimately favoring the selective production of CH4 without binding to the Fe sites.
Figure 14. (A) EXAFS fitting and structural model of NiSA-N2-C; (B and C) FECO (B) and corresponding TOFs of CO (C) of NiSA-NX-C; (A-C) are quoted with permission from Gong et al.[267]; (D) EXAFS fitting and structural model of Ni1-N-C (Cl); (E and F) FECO (E) and JCO (F) over Ni1-N-C(X); D-F are quoted with permission from Peng et al.[268]; (G) Schematic diagram of free energy change (top) and structural change (bottom) of Fe-MOFs-74 in CO2RR, quoted with permission from McCarver et al.[269]. TOF: Turnover frequency; RHE: reversible hydrogen electrode; FECO: Faradaic efficiency for CO; EXAFS: extended X-ray absorption fine structure; MOFs: metal-organic frameworks.
With fine-tuning of preparation conditions, Lim et al. synthesized and pyrolyzed Ni ZIF-8 via a solution method to prepare pyrrolic nitrogen-stabilized Ni SACs (Ni SAC-800 and Ni SAC-1000) with low-coordination Ni-NX sites [Figure 15A][270]. EXAFS analysis revealed the presence of a Ni-N peak without a Ni-Ni peak, indicating that the Ni atoms were distributed as isolated atoms throughout the carbon matrix within Ni SAC [Figure 15B]. The FECO of Ni SAC significantly changed with increasing pyrolysis temperature, showing a 40.76% FECO of Ni SAC-800 and a 98.24% FECO of Ni SAC-1000 at -0.8 V RHE, respectively [Figure 15C]. Meanwhile, Ni SAC-1000 demonstrated exceptional CO partial current density
Figure 15. (A) Preparation process and active site diagram of Ni SAC-800 and Ni SAC-1000; (B) FT-EXAFS spectra of the Ni K-edge for Ni-based catalysts; (C and D) FECO (C) and JCO (D) over Ni-based catalysts; (A-D) are quoted with permission from Lim et al.[270]; (E) Preparation process diagram of CuN3O/C and CuCO3/C; (F) FT-EXAFS fitting and structural model of CuN3O/C; (G-I) LSV curves (G), FECO (H) and TOFs of CO (I) over CuN3O/C and CuCO3/C; (E-I) are quoted with permission from Song et al.[271]. RHE: Reversible hydrogen electrode; ZIF: zeolitic imidazolate framework; FECO: Faradaic efficiency for CO; SAC: single-atom catalyst; TOF: turnover frequency; FT: Fourier transform; EXAFS: extended X-ray absorption fine structure; LSV: linear sweep voltammetry.
Besides MOFs-based SACs, MOFs-based DACs were used a lot for ECRR. Feng et al. in situ doped Cu2+ and Fe(acac)3 molecules into ZIF-8 and prepared a Fe/Cu-N-C catalyst through pyrolysis under Ar gas flow[272]. The coordination structure of Fe/Cu-N-C was identified as N4Fe-CuN3, reflecting the existence of coordination between Fe atoms and N atoms, along with a specific bonding relationship with Cu atoms, featuring an average bond distance of approximately 2.01 Å [Figure 16A]. The FECO values for Fe/Cu-N-C were above 95% in the potential range of 0.4-1.1 V and peaked at nearly 99% when the potential was 0.8 V, far higher than those of other reference catalysts [Figure 16B]. Meanwhile, the TOF of Fe/Cu-N-C was
Figure 16. (A) EXAFS fitting and structural model of Fe/Cu-N-C catalyst; (B and C) FECO (B) and TOFs of CO (C) over different N-C catalysts; (A-C) are quoted with permission from Feng et al.[272]; (D) EDS mapping of Ru1Zr1/Co catalyst; (E) TOF and C5+ selectivity of four Co-containing catalysts; (D and E) are quoted with permission from Sun et al.[194]; (F-H) EFCO of Cu/Ni-NC, Ni-NC, and Cu-NC in different electrolytes: acidic (F), alkaline (G), and neutral (H); (I) CO2 utilization efficiency of Cu/Ni-NC catalyst in acidic and alkaline electrolyte; (F-I) are quoted with permission from Zhang et al.[151]. FT: Fourier transform; TOF: turnover frequency; FECO: Faradaic efficiency for CO; EXAFS: extended X-ray absorption fine structure; EDS: energy-dispersive X-ray spectroscopy.
Photocatalytic CO2RR
Photocatalysis is a light-driven chemical reaction that exhibits green, environmentally friendly, and efficient characteristics in CO2RR. MOFs-based SACs/DACs have more selectivity and higher catalytic activity for photocatalytic CO2RR with controllable active sites and coordination environment[150,273].
For MOFs-based SACs, Liu et al. prepared Ni single-atom fixed ZIF-8 catalysts (Ni/SOM-ZIF-8) featuring an organized hierarchical structure of macro and micro pores via pyrolysis for photocatalytic CO2RR[274]. EXAFS spectrum of Ni/SOM-ZIF-8 exhibited no characteristic peak for Ni–Ni bonding in R space, and the main peak corresponded to Ni–N bonding (1.43 Å) [Figure 17A]. In terms of photocatalytic CO2RR performance, Ni/SOM-ZIF-8 exhibited the best performance, achieving a CO yield of 11.44 m·molg-1 after four hours, which was 44 times and 22 times that of ZIF-8 and SOM-ZIF-8, respectively, and showing high selectivity for CO [Figure 17B]. The cumulative production yield of Ni/SOM-ZIF-8 indicated a progressive rise in CO yield while unchanging for H2, demonstrating good stability [Figure 17C]. Zhao et al. synthesized NTU-9 (bulky MOFs) via solvothermal methods and subsequently coupled Ni@MOFs with BiVO4 (BVO) nanosheets through induced assembly to obtain a single atomic Ni@6MOFs/BVO heterojunction catalyst for photocatalysis[275]. FT-EXAFS spectra of the Ni K-edge for Ni@6MOFs/BVO showed that no peaks of Ni–Ni bond in Ni@6MOFs/BVO were found at 2.15 Å [Figure 17D]. The photocatalytic CO2 reduction activities of various materials were compared [Figure 17E]. The prepared Ni@6MOFs/BVO exhibited the best catalytic property, with a CO generation of 178 μmol·g-1 and a CO selectivity of 99.2%, representing a 66-fold increase in CO2 photoconversion efficiency compared to BVO nanoparticles, while effectively suppressing H2 production and nearly 100% CO selectivity. Li et al. synthesized materials such as UiO-66-NH2, MIL-88B (Fe), and MIL-on-xUiO-NH2 (x = 1, 2, 3) via solvothermal reactions[276]. The Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectrum indicated a blue shift of the Zr-oxo cluster and carboxylate stretching bands, suggesting coordination between the Zr-oxo clusters of UiO-66-NH2 and the benzene rings of MIL-88B (Fe), establishing a chemical link between the two [Figure 17F]. The HRTEM image, TEM images and structural schematics revealed that MIL-on-2UiO-NH2 exhibited a specific rod-octahedral structure, formed through an epitaxial growth method [Figure 17G-I]. In the photocatalytic CO2 reaction using triethanolamine as a sacrificial agent, MIL-on-2UiO-NH2 maintained high CO generation activity, with CO selectivity approaching 100% [Figure 17J]. Specifically, without employing a sacrificial agent, the CO generation rate of MIL-on-2UiO-NH2 was approximately 2.26 μmol·g-1·h-1. In the photocatalytic process for capturing OH radicals to form 7-hydroxycoumarin, photogenerated holes accumulated on the UiO-66-NH2 side [Figure 17K].
Figure 17. (A) FT-EXAFS spectra of the Ni K-edge for Ni/SOM-ZIF-8 and other catalysts; (B) Photocatalytic CO2RR performance of 6 different ZIF-8 based catalysts; (C) Cumulative production of CO, CH4 and H2 by Ni/SOM-ZIF-8; (A-C) are quoted with permission from Liu et al.[274]; (D) FT-EXAFS spectra of the Ni K-edge for Ni@6MOFs/BVO; (E) Photoactivities of CO2RR over Ni@6MOFs/BVO and other reference samples; (D and E) are quoted with permission from Zhao et al.[275]; (F) ATR-FTIR spectra of MIL-on-2UiO-NH2 and other reference samples; (G-I) TEM image (G), HRTEM image (H), and Schematic diagram (I) of MIL-on-2UiO-NH2; (J) Production of CO over MIL-on-2UiO-NH2 and other reference samples; (K) Detection of ·OH radical trapping efficiency with 7-hydroxycoumarin at 457 nm over MIL-on-2UiO-NH2 and other reference samples by PL spectrometer; (F-K) are quoted with permission from Li et al.[276]. SOM: Surfactant-assisted ordered mesoporous; ZIF: zeolitic imidazolate framework; MOF: metal-organic framework; BVO: BiVO4; BVNP: bismuth vanadate nanoparticles; MIL: materials of Institut Lavoisier; FT: Fourier transform; EXAFS: extended X-ray absorption fine structure; CO2RR: CO2 reduction reaction; TEM: transmission electron microscope.
In addition to the single product reduction (CO) mentioned above, MOFs-based SACs could also be used for multi-product reduction in photocatalytic CO2RR[277]. Pan et al. prepared CA/Ni1CuNP N-C using an ion exchange strategy followed by calcination[278]. Based on the EXAFS fitting results, it was found that Ni atoms in CA/Ni1CuNP N-C predominantly existed in a form coordinated with four N atoms, with a coordination number determined to be approximately 3.6 and a bond length of about 1.86 Å [Figure 18A]. Photocatalytic experiments showed that with the increasing proportion of CA/Ni1CuNP N-C in the composite material, the yields of CH4, CO, and C2H6 exhibited a trend of first increasing and then decreasing, achieving optimal catalytic performance at CA/Ni1CuNP N-C (1:2) [Figure 18B]. The transfer of electrons generated by light from CoAl-LDH to N-C ultimately accumulated at dispersed single atom sites of Ni to drive the reaction [Figure 18C]. Zeng et al. employed an in situ synthesis method by immersing Mil-125 in CoPy4 solution followed by calcination to prepare CoNX cluster-modified TiO2 (CoNX/TiO2) catalysts[279]. The AC-HADDF-STEM image of CoNX/TiO2 indicated a uniform dispersion of CoNX clusters in TiO2, where the bright contrast spots represented the positions of Co atoms within the TiO2 lattice [Figure 18D]. Under irradiation with a 300 W Xe lamp, the photocatalytic activity of CoNX/TiO2 varied with the amount of CoPy4 added, with CoNX/TiO2-4.0 achieving the highest CO/CH4 yield (24.4/119.9 μmol·g-1·h-1) and top photocatalytic activity (1,007.6 μmol·g-1·h-1), which were 10.6 times and 4.6 times that of TiO2 and CoOX/TiO2, respectively [Figure 18E and F]. Wang et al. synthesized Cu isolated-atom modified UiO-66-NH2 catalyst (Cu SAs/UiO-66-NH2) using a photoinduction method[280]. The XANES spectrum indicated that the absorption edge of Cu SAs/UiO-66-NH2 was positioned between the edges observed for Cu and CuO [Figure 18G]. In presence of Cu SAs/UiO-66-NH2, the liquid-phase products of photocatalytic CO2 reduction were detected to be only CH3OH and CH3CH2OH, indicating that this catalyst was selective for these two liquid fuels [Figure 18H]. The rate of CH3OH production by Cu SAs/UiO-66-NH2 was approximately 5.33 μmol·g-1·h-1 while that of CH3CH2OH was 4.22 μmol·g-1·h-1, significantly far more than those of other samples, demonstrating that the addition of isolated Cu atoms obviously boosted the catalytic efficiency [Figure 18I].
Figure 18. (A) FT-EXAFS fitting and structural model of CA/Ni1CuNP N-C; (B) Photocatalytic CO2RR product efficiency of CH4, CO, and C2H6 with different percentage of Ni1CuNP N-C; (C) Diagram of photocatalytic CO2RR mechanism over Ni1CuNP N-C; (A-C) are quoted with permission from Pan et al.[278]; (D) AC-HADDF-STEM image of CoNX/TiO2; (E and F) CO/CH4 yields (E) and the corresponding TCEN (F) of CoNX/TiO2 and other reference samples; (D-F) are quoted with permission from Zeng et al.[279]; (G) Normalized XANES spectra of the Cu K-edge for Cu SAs/UiO-66-NH2 and other samples; (H and I) CH3OH/CH3CH2OH yields (H) and comparative rates of formation (I) by Cu SAs/UiO-66-NH2; (G-I) are quoted with permission from Wang et al.[280]. FT: Fourier transform; EXAFS: extended X-ray absorption fine structure; CO2RR: CO2 reduction reaction; XANES: X-ray absorption near-edge structure; TCEN: total carbon-based product.
For MOFs-based DACs in photocatalytic CO2RR, Yuan et al. prepared one Cu-In double atomic MOFs-based catalyst (CuIn-DACs) through pyrolysis and etching[281]. As shown in EDS-mapping images of CuIn-DACs, Cu, In, C, and N were homogeneously dispersed in the CuIn-DACs [Figure 19A]. The Cu-In double atoms were distributed within CuIn-DACs at atomic level [Figure 19B], fully exerting the collaborative catalytic action of dual atoms. The FE for methanol of CuIn-DACs reached 55.63% at -0.62 V vs. RHE, significantly far more Cu-SACs, indicating a higher selectivity for methanol and effectively promoting the conversion of CO2 to methanol while suppressing other side reactions [Figure 19C]. Tan et al. synthesized MnS/In2S3 p-n heterojunctions using MIL-68 (In) sub-microrods as templates followed by impregnation and sulfidation[282]. In photocatalytic CO2RR, 30 wt.% MnS/In2S3 showed optimal CO generation rate, reaching 58 μmol·g-1·h-1, while the CO generation rates for In2S3 and MnS were only about 14 μmol·g-1·h-1 and 16 μmol·g-1·h-1, respectively [Figure 19D]. This indicated that the formation of the MnS/In2S3 significantly enhanced photocatalytic activity. After the formation of the heterojunction between In2S3 and MnS, the photocurrent density was significantly increased [Figure 19E]. The p-n heterojunction effectively promoted charge separation and improved the utilization efficiency of photogenerated carriers. After a 10-hour photocatalytic CO2 reduction experiment with the 30 wt.% MnS/In2S3 catalyst, XRD analyses showed no significant changes in structure, indicating structural stability under photocatalytic conditions [Figure 19F]. Hu et al. prepared CuNi SAs/UiO-66(Hf) using one combined method of hydrothermal fixation and radiation reduction[283]. In a saturated aqueous solution of CO2 irradiated with 60Co γ-rays, the blank experiment yielded no carbonaceous products. UiO-66(Hf) caused a slight increase in CO production, while CuNi SAs/UiO-66(Hf) could generate CH3OH but with poor selectivity. The addition of Na2SO3 allowed a small amount of CuNi SAs/UiO-66(Hf) to achieve a CH3OH selectivity of 97.8%, significantly enhancing the G value [Figure 19G]. As the CuNi SAs/UiO-66(Hf) weight ratio increased from 0.05% to 0.2%, the G value increased from 0.9 × 10-7 mol·J-1 to 1.5 × 10-7 mol·J-1, indicating that enhancing the weight ratio of catalyst could effectively promote CH3OH generation [Figure 19H].
Figure 19. (A and B) EDS-mapping images (A) and HAADF-STEM image (B) of CuIn-DACs; (C) FEmethanol of CuIn-DACs and Cu-SACs; (A-C) are quoted with permission from Yuan et al.[281]; (D) CO product rate of different catalysts; (E) Transient photocurrents of different catalysts; (F) XRD patterns of MnS/In2S3 catalyst before and after photocatalytic CO2RR experiment (10 h); (D-F) are quoted with permission from Tan et al.[282]; (G) G-valueMeOH and G-valueCO of 0.05 wt.% with CuNi SAs/UiO-66(Hf) and other catalysts in H2O or 0.01 M Na2SO3 solution; (H) The amplified of G-valueMeOH and G-valueCO by the increase of CuNi SAs/UiO-66(Hf) content; (G and H) are quoted with permission from Hu et al.[283]. RHE: Reversible hydrogen electrode; EDS: energy-dispersive X-ray spectroscopy; HRTEM: high-resolution transmission electron microscopy; DACs: dual-atom catalysts; SACs: single-atom catalysts; XRD: X-ray diffraction;
CONCLUSION AND OUTLOOK
Attributable to the excellent activity of active sites in SACs and DACs, combined with the superior mass transfer and photoelectric performance of MOFs, MOFs-based SACs/DACs could be used as an ideal bridge connecting heterogeneous catalysis and homogeneous catalysis, with high selectivity and high activity, as well as strong durability, and excellent performance in electrocatalytic and photocatalytic CO2RR. This review systematically introduces the design and regulation of MOFs-based SACs/DACs active sites together with the characterization of active sites. Moreover, the mechanism of electrocatalytic and photocatalytic CO2RR is analyzed carefully, especially emphasizing the relationship between the active sites and catalytic efficiency. Finally, recent processes of MOFs-based SACs/DACs in electrocatalytic and photocatalytic CO2RR are summarized.
Although the significant progress of MOFs-based SACs/DACs in electrocatalytic and photocatalytic CO2RR has been achieved, the challenges of the future directions for active sites design and the development of improving catalytic activity in CO2RR at industrial current density of MOFs-based SACs/DACs still exist and need to be addressed in future studies.
(1) The coordination conditions of the active sites seriously influence the activity of MOFs-based SACs/DACs; however, the basis of the formation of different coordination environments is not clear, especially the physical basis. Although in many previous works, different active sites were prepared by changing the pyrolysis temperature or the ratio of coordination atoms to feed, these methods were still in the experimental stage of small-scale production, and did not form a regular and certain scale. Furthermore, the limited number of active sites in MOFs restricts the provision of high-density metal sites. Therefore, the mechanism of active site formation and the mature method of large-scale production of high-density MOFs-based SACs/DACs need to be explored further. For example, in the preparation process of MOFs-based SACs/DACs, the structure of the intermediate product is qualitatively or quantitatively analyzed, and the formation process of the active site is gradually explored.
(2) From various studies and analyses, it could be seen that MOFs-based SACs/DACs with many advantages are superior to other catalysts, especially the synergistic impact of the active sites of polymetallic atoms, which could not be compared with a single metal atom. For MOFs-based SACs/DACs over CO2RR, the reduction products are also diverse; there is a single product CO, which could also be complex products, such as CO and methane. These are all C1 products. There are very few studies on the C2 products of MOFs-based SACs/DACs in CO2RR, because the generation of C2 products requires higher requirements on the active site, which requires the generation of C–C bonding. Therefore, the research progress of MOFs-based SACs/DACs in multi-carbon products of CO2RR needs to be further explored.
(3) Many catalysts lack selectivity for CO2 in CO2RR. For MOFs-based SACs/DACs, selectivity and activation of CO2 could be improved by selecting characteristic high metal active sites and regulating and optimizing the coordination environment between active sites and organic ligands. The catalyst activity could be improved by adding metal nanoparticles or clusters, adjusting the valence state of the metal center to achieve low coordination, and selecting organic ligands with oxygen bridge structure. Furthermore, the selectivity of CO2 in MOF-based SACs/DACs can also be enhanced by modifying the MOF structure. This includes constructing core-shell or multi-layer nanosheet architectures using the MOFs-on-MOFs approach.
(4) In the evaluation of various performances of MOFs-based SACs/DACs, stability is an essential item. Although through various characterization methods, we could observe that the active sites are distributed in atomic states of MOFs-based SACs/DACs, this is only the preparation conditions in the laboratory. Industrial catalysis demands that catalysts exhibit long-term stability and reliability, alongside high activity. For large-scale industrial production, especially under high-temperature conditions and special reducing atmospheres, the active sites in MOFs-based SACs/DACs are very likely to be lost, aggregated or redistributed. Therefore, first, under laboratory conditions, we need to ensure that the synthesized MOFs-based SACs/DACs have long-term thermodynamic stability through a series of explorations at different times, while maintaining a high activity state. By modifying the coordination number of central metallic atoms in MOFs-based SACs/DACs, too low coordination could indeed bring about high activity, but it could seriously affect stability. Therefore, balancing the stability and activity of the MOFs-based SACs/DACs is an important challenge. In addition, for large-scale production of catalysts, from the perspective of the environmental cost of preparation, we still have to follow the principle of green chemical preparation.
DECLARATIONS
Authors’ contributions
Writing-original draft: Li, J.; Zhang, W.
Investigation: Peng, Y.; Wu, Q.; Zhu, W.
Data curation, validation: Zhang, Y.; Wang, S.
Conceptualization, methodology, resources: Liu, S.; Wang, D.; Dai, Z.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (22234005 and 22494632) and the Natural Science Foundation of Jiangsu Province (BK20222015), Young Academic Leaders of the Qing Lan Project of Jiangsu Province (SUJIAOSHIHAN [2022] No.29), Industry-University-Research Cooperation Program of Jiangsu Province (BY20230054), and Jiangsu Provincial University Key Laboratory of Intelligent Medical Sensing Materials and Devices.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Jiao, K.; Xuan, J.; Du, Q.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361-9.
2. Yasin, G.; Kumar, A.; Ajmal, S.; et al. Advances and perspectives on heteronuclear dual-atomic catalysts for prevailing the linear scaling relationship in electrocatalytic CO2 reduction. Coord. Chem. Rev. 2024, 501, 215589.
3. Chen, Y.; Mao, J.; Zhou, H.; et al. Coordination shell dependent activity of CuCo diatomic catalysts for oxygen reduction, oxygen evolution, and hydrogen evolution reaction. Adv. Funct. Materials. 2024, 34, 2311664.
4. Zhuang, J.; Wang, D. Recent advances of single-atom alloy catalyst: properties, synthetic methods and electrocatalytic applications. Mater. Today. Catal. 2023, 2, 100009.
5. Pei, S.; Wang, S.; Lu, Y.; Li, X.; Wang, B. Application of metal-based catalysts for Fenton reaction: from homogeneous to heterogeneous, from nanocrystals to single atom. Nano. Res. 2024, 17, 9446-71.
6. Li, X.; Duan, M.; Ou, P. High-throughput screening of single-atom catalysts confined in monolayer black phosphorus for efficient nitrogen reduction reaction. Nano. Res. 2024, 17, 2360-7.
7. Yu, J.; Wang, J.; Ma, Y.; et al. Recent progresses in electrochemical carbon dioxide reduction on copper-based catalysts toward multicarbon products. Adv. Funct. Materials. 2021, 31, 2102151.
8. Sun, R. M.; Zhang, L.; Feng, J. J.; Fang, K. M.; Wang, A. J.
9. Tan, X.; Yu, C.; Ren, Y.; Cui, S.; Li, W.; Qiu, J. Recent advances in innovative strategies for the CO2 electroreduction reaction. Energy. Environ. Sci. 2021, 14, 765-80.
10. Shu, X.; Yang, M.; Liu, M.; Pan, W.; Zhang, J. The regulation of coordination structure between cobalt and nitrogen on graphene for efficient bifunctional electrocatalysis in Zn-air batteries. J. Energy. Chem. 2022, 68, 213-21.
11. Lai, W.; Qiao, Y.; Zhang, J.; Lin, Z.; Huang, H. Design strategies for markedly enhancing energy efficiency in the electrocatalytic CO2 reduction reaction. Energy. Environ. Sci. 2022, 15, 3603-29.
12. Yang, C. L.; Wang, L. N.; Yin, P.; et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459-64.
13. Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and challenges for the electrochemical reduction of CO2 to CO: from fundamentals to industrialization. Angew. Chem. Int. Ed. Engl. 2021, 60, 20627-48.
14. Guo, B.; Wang, Z.; Chen, J.; et al. Cryo-EM revealing the origin of excessive capacity of the Se cathode in sulfide-based all-solid-state Li-Se batteries. ACS. Nano. 2022, 16, 17414-23.
15. Zhang, W.; Liu, D.; Liu, T.; et al. Coordinately unsaturated nickel single atom electrocatalyst for efficient CO2 conversion. Nano. Res. 2023, 16, 10873-80.
16. Chen, Y.; Jiang, B.; Hao, H.; et al. Atomic-level regulation of cobalt single-atom nanozymes: engineering high-efficiency catalase mimics. Angew. Chem. Int. Ed. Engl. 2023, 62, e202301879.
17. Liu, G.; Liu, S.; Lai, C.; et al. Strategies for enhancing the photocatalytic and electrocatalytic efficiency of covalent triazine frameworks for CO2 reduction. Small 2024, 20, e2307853.
18. Lv, Q.; Zhu, Z.; Zhao, S.; et al. Semiconducting metal-organic polymer nanosheets for a photoinvolved Li-O2 battery under visible light. J. Am. Chem. Soc. 2021, 143, 1941-7.
19. Sun, Y.; Jiang, Y.; Wei, H.; et al. Nano-enabled strategies for greenhouse gases emission mitigation: a comprehensive review. Nano. Today. 2024, 57, 102378.
20. Liu, Y.; Cai, J.; Zhou, J.; et al. Tailoring the adsorption behavior of superoxide intermediates on nickel carbide enables high-rate Li-O2 batteries. eScience 2022, 2, 389-98.
21. Guo, L.; Zhou, J.; Liu, F.; et al. Electronic structure design of transition metal-based catalysts for electrochemical carbon dioxide reduction. ACS. Nano. 2024, 18, 9823-51.
22. Gan, T.; Wang, D. Atomically dispersed materials: ideal catalysts in atomic era. Nano. Res. 2024, 17, 18-38.
23. Zhang, T.; Zhang, L.; Hou, Y. MXenes: synthesis strategies and lithium-sulfur battery applications. eScience 2022, 2, 164-82.
24. Jiang, M.; Wang, H.; Zhu, M.; et al. Review on strategies for improving the added value and expanding the scope of CO2 electroreduction products. Chem. Soc. Rev. 2024, 53, 5149-89.
25. Hu, P.; Xiao, F.; Wu, Y.; et al. Covalent encapsulation of sulfur in a graphene/N-doped carbon host for enhanced sodium-sulfur batteries. Chem. Eng. J. 2022, 443, 136257.
26. Wang, F.; Lu, Z.; Guo, H.; Hao, G.; Jiang, W.; Liu, G. Copper-based catalysts for CO2 electroreduction to C2/2+ products: advance and perspective. Coord. Chem. Rev. 2024, 515, 215962.
27. Li, H.; Yan, G.; Zhao, H.; Howlett, P. C.; Wang, X.; Fang, J. Earthworm-inspired Co/Co3O4/CoF2@NSC nanofibrous electrocatalyst with confined channels for enhanced ORR/OER performance. Adv. Mater. 2024, 36, e2311272.
28. Geng, M.; Han, D.; Huang, Z.; et al. A stable anode-free Na-S full cell at room temperature. Energy. Storage. Mater. 2022, 52, 230-7.
29. Yang, G.; Zhu, J.; Yuan, P.; et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat. Commun. 2021, 12, 1734.
30. Xiao, F.; Yang, X.; Yao, T.; Wang, H.; Rogach, A. L. Encapsulation of selenium in MOF-derived N,O-codoped porous flower-like carbon host for Na-Se batteries. Chem. Eng. J. 2022, 430, 132737.
31. Zhu, P.; Xiong, X.; Wang, D.; Li, Y. Advances and regulation strategies of the active moiety in dual-atom site catalysts for efficient electrocatalysis. Adv. Energy. Mater. 2023, 13, 2300884.
32. Li, X. Y.; Feng, S.; Zhao, C. X.; et al. Regulating lithium salt to inhibit surface gelation on an electrocatalyst for high-energy-density lithium-sulfur batteries. J. Am. Chem. Soc. 2022, 144, 14638-46.
33. Song, Z.; Zhang, L.; Doyle-davis, K.; Fu, X.; Luo, J.; Sun, X. Recent advances in MOF-derived single atom catalysts for electrochemical applications. Adv. Energy. Mater. 2020, 10, 2001561.
34. Jiang, H.; Gu, J.; Zheng, X.; et al. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy. Environ. Sci. 2019, 12, 322-33.
35. Dai, T. Y.; Wang, T. H.; Wen, Z.; Jiang, Q. Recent progress on computation-guided catalyst design for highly efficient nitrogen reduction reaction. Adv. Funct. Materials. 2024, 34, 2400773.
36. Xu, J.; Ouyang, R.; Chen, N.; Wan, X. Financing constraints, risk of stock price crash and impact of new crown pneumonia. BCP. Bus. Manag. 2021, 13, 233-41.
37. Han, J.; Tan, H.; Guo, K.; et al. The “pull effect” of a hanging ZnII on improving the four-electron oxygen reduction selectivity with Co porphyrin. Angew. Chem. Int. Ed. Engl. 2024, 63, e202409793.
38. Li, W.; Lu, Y.; Tang, Y.; Sun, H. Carbon-carbon triple bond-containing materials for photo(electro)catalytic solar hydrogen production. Carbon. Energ. 2024, 6, e527.
39. Wang, Y.; Ma, F.; Zhang, G.; et al. Precise synthesis of dual atom sites for electrocatalysis. Nano. Res. 2024, 17, 9397-427.
40. Luo, E.; Yang, T.; Liang, J.; et al. Selective oxygen electroreduction to hydrogen peroxide in acidic media: the superiority of single-atom catalysts. Nano. Res. 2024, 17, 4668-81.
41. Zhang, H.; Cheng, W.; Luan, D.; Lou, X. W. Atomically dispersed reactive centers for electrocatalytic CO2 reduction and water splitting. Angew. Chem. Int. Ed. Engl. 2021, 60, 13177-96.
42. Feng, J.; Lv, F.; Zhang, W.; et al. Iridium-based multimetallic porous hollow nanocrystals for efficient overall-water-splitting catalysis. Adv. Mater. 2017, 29, 1703798.
43. Huang, B.; Wu, Z.; Zhou, H.; et al. Recent advances in single-atom catalysts for advanced oxidation processes in water purification. J. Hazard. Mater. 2021, 412, 125253.
44. Li, S.; Guan, A.; Yang, C.; et al. Dual-atomic Cu sites for electrocatalytic CO reduction to C2+ products. ACS. Materials. Lett. 2021, 3, 1729-37.
45. Hannagan, R. T.; Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E. C. H. Single-atom alloy catalysis. Chem. Rev. 2020, 120, 12044-88.
46. Wang, Y.; Cui, X.; Zhang, J.; et al. Advances of atomically dispersed catalysts from single-atom to clusters in energy storage and conversion applications. Prog. Mater. Sci. 2022, 128, 100964.
47. Kang, J.; Xue, Y.; Yang, J.; et al. Realizing two-electron transfer in Ni(OH)2 nanosheets for energy storage. J. Am. Chem. Soc. 2022, 144, 8969-76.
48. Ballesteros-soberanas, J.; Martín, N.; Bacic, M.; et al. A MOF-supported Pd1-Au1 dimer catalyses the semihydrogenation reaction of acetylene in ethylene with a nearly barrierless activation energy. Nat. Catal. 2024, 7, 452-63.
49. Liu, L.; Wang, S.; Huang, H.; Zhang, Y.; Ma, T. Surface sites engineering on semiconductors to boost photocatalytic CO2 reduction. Nano. Energy. 2020, 75, 104959.
50. Wang, W.; Chen, D.; Li, F.; Xiao, X.; Xu, Q. Metal-organic-framework-based materials as platforms for energy applications. Chem 2024, 10, 86-133.
51. Li, Y.; Peng, C. K.; Hu, H.; et al. Interstitial boron-triggered electron-deficient Os aerogels for enhanced pH-universal hydrogen evolution. Nat. Commun. 2022, 13, 1143.
52. Chang, H.; Shi, L. N.; Chen, Y. H.; Wang, P. F.; Yi, T. F. Advanced MOF-derived carbon-based non-noble metal oxygen electrocatalyst for next-generation rechargeable Zn-air batteries. Coord. Chem. Rev. 2022, 473, 214839.
53. Qu, J.; Cao, X.; Gao, L.; et al. Electrochemical carbon dioxide reduction to ethylene: from mechanistic understanding to catalyst surface engineering. Nanomicro. Lett. 2023, 15, 178.
54. Yan, Y.; Cheng, H.; Qu, Z.; et al. Recent progress on the synthesis and oxygen reduction applications of Fe-based single-atom and double-atom catalysts. J. Mater. Chem. A. 2021, 9, 19489-507.
55. Lu, C.; Fang, R.; Chen, X. Single-atom catalytic materials for advanced battery systems. Adv. Mater. 2020, 32, e1906548.
56. Lei, Y.; Wang, Z.; Bao, A.; et al. Recent advances on electrocatalytic CO2 reduction to resources: target products, reaction pathways and typical catalysts. Chem. Eng. J. 2023, 453, 139663.
57. Han, B.; Guo, Y.; Huang, Y.; et al. Strong metal-support interactions between Pt single atoms and TiO2. Angew. Chem. Int. Ed. Engl. 2020, 59, 11824-9.
58. Yang, D.; Wang, J.; Wang, Q.; et al. Electrocatalytic CO2 reduction over atomically precise metal nanoclusters protected by organic ligands. ACS. Nano. 2022, 16, 15681-704.
59. Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical synthesis of single atomic site catalysts. Chem. Rev. 2020, 120, 11900-55.
60. Hao, L.; Guo, C.; Hu, Z.; et al. Single-atom catalysts based on Fenton-like/peroxymonosulfate system for water purification: design and synthesis principle, performance regulation and catalytic mechanism. Nanoscale 2022, 14, 13861-89.
61. Yang, J.; Chen, Z.; Zhang, L.; Zhang, Q. Covalent organic frameworks for photocatalytic reduction of carbon dioxide: a review. ACS. Nano. 2024, 18, 21804-35.
62. Tian, L. C.; Hu, J. N.; Meng, Y.; Liang, J. X.; Zhu, C.; Li, J. Ultrastable nickel single-atom catalysts with high activity and selectivity for electrocatalytic CO2 methanation. Nano. Res. 2023, 16, 8987-95.
63. Yun, R.; Xu, R.; Shi, C.; et al. Post-modification of MOF to fabricate single-atom dispersed hollow nanocages catalysts for enhancing CO2 conversion. Nano. Res. 2023, 16, 8970-6.
64. Dai, F.; Zhang, M.; Han, J.; et al. Bifunctional core-shell co-catalyst for boosting photocatalytic CO2 reduction to CH4. Nano. Res. 2024, 17, 1259-66.
65. Fan, Y.; Yi, Y.; Rong, H.; Zhang, J. Silicon dioxide-protection boosting the peroxidase-like activity of Fe single-atom catalyst for combining chemo-photothermal therapy. Nano. Res. 2024, 17, 4924-33.
66. Hu, J.; Huang, H.; Yu, M.; Wang, S.; Li, J. Electron engineering of nickel phosphide for Niδ+ in electrochemical nitrate reduction to ammonia. Nano. Res. 2024, 17, 4864-71.
67. Chen, R.; Wang, X.; Dang, J.; et al. Shedding light on the reversible deactivation of carbon-supported single-atom catalysts in hydrogenation reaction. Nano. Res. 2024, 17, 4807-14.
68. Pedersen, A.; Barrio, J.; Li, A.; et al. Dual-metal atom electrocatalysts: theory, synthesis, characterization, and applications. Adv. Energy. Mater. 2022, 12, 2102715.
69. Chen, Z.; Li, N.; Zhang, Q. Covalent organic frameworks as promising platforms for efficient electrochemical reduction of carbon dioxide: a review. Small. Struct. 2024, 5, 2300495.
70. Gao, C.; Low, J.; Long, R.; Kong, T.; Zhu, J.; Xiong, Y. Heterogeneous single-atom photocatalysts: fundamentals and applications. Chem. Rev. 2020, 120, 12175-216.
71. Li, W. H.; Yang, J.; Wang, D. Long-range interactions in diatomic catalysts boosting electrocatalysis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202213318.
72. Sun, M.; Wong, H. H.; Wu, T.; et al. Double-dependence correlations in graphdiyne-supported atomic catalysts to promote CO2RR toward the generation of C2 products. Adv. Energy. Mater. 2023, 13, 2203858.
73. Yu, X.; Ng, S. F.; Putri, L. K.; Tan, L. L.; Mohamed, A. R.; Ong, W. J. Point-defect engineering: leveraging imperfections in graphitic carbon nitride (g-C3N4) photocatalysts toward artificial photosynthesis. Small 2021, 17, e2006851.
74. Xu, D.; Li, K.; Jia, B.; et al. Electrocatalytic CO2 reduction towards industrial applications. Carbon. Energ. 2023, 5, e230.
75. Sun, B.; Xu, M.; Li, X.; et al. Unlocking single-atom catalysts via amorphous substrates. Nano. Res. 2024, 17, 3533-46.
76. Li, R.; Yan, S.; Xue, T.; et al. A MOF/poly(thioctic acid) composite for enhanced gold extraction from water matrices. Nano. Res. 2024, 17, 382-9.
77. Liu, K.; Wu, J.; Li, Q.; et al. Molten metal-organic complex to synthesize versatile ultrathin non-layered oxides. Nano. Res. 2024, 17, 3147-55.
78. Li, X.; Cao, C.; Hung, S.; et al. Identification of the electronic and structural dynamics of catalytic centers in single-Fe-atom material. Chem 2020, 6, 3440-54.
79. Zou, L.; Wei, Y. S.; Hou, C. C.; Li, C.; Xu, Q. Single-atom catalysts derived from metal-organic frameworks for electrochemical applications. Small 2021, 17, e2004809.
80. Roth-Zawadzki, A. M.; Nielsen, A. J.; Tankard, R. E.; Kibsgaard, J. Dual and triple atom electrocatalysts for energy conversion (CO2RR, NRR, ORR, OER, and HER): synthesis, characterization, and activity evaluation. ACS. Catal. 2024, 14, 1121-45.
81. Ma, X.; Liu, H.; Yang, W.; Mao, G.; Zheng, L.; Jiang, H. L. Modulating coordination environment of single-atom catalysts and their proximity to photosensitive units for boosting MOF photocatalysis. J. Am. Chem. Soc. 2021, 143, 12220-9.
82. Wagner, A.; Sahm, C. D.; Reisner, E. Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction. Nat. Catal. 2020, 3, 775-86.
83. Huang, Y.; Chen, Y.; Xu, M.; et al. Catalysts by pyrolysis: transforming metal-organic frameworks (MOFs) precursors into metal-nitrogen-carbon (M-N-C) materials. Mater. Today. 2023, 69, 66-78.
84. Yang, P. P.; Gao, M. R. Enrichment of reactants and intermediates for electrocatalytic CO2 reduction. Chem. Soc. Rev. 2023, 52, 4343-80.
85. Li, X.; Zhang, F.; Han, X.; et al. Single atom Pd1/ZIF-8 catalyst via partial ligand exchange. Nano. Res. 2023, 16, 8003-11.
86. Zhang, Y. X.; Zhang, S.; Huang, H.; et al. General synthesis of a diatomic catalyst library via a macrocyclic precursor-mediated approach. J. Am. Chem. Soc. 2023, 145, 4819-27.
87. Tian, S.; Fu, Q.; Chen, W.; et al. Carbon nitride supported Fe2 cluster catalysts with superior performance for alkene epoxidation. Nat. Commun. 2018, 9, 2353.
88. Tang, T.; Wang, Z.; Guan, J. Structural optimization of carbon-based diatomic catalysts towards advanced electrocatalysis. Coord. Chem. Rev. 2023, 492, 215288.
89. Zhao, Q. P.; Shi, W. X.; Zhang, J.; et al. Photo-induced synthesis of heteronuclear dual-atom catalysts. Nat. Synth. 2024, 3, 497-506.
90. Mo, Q.; Zhang, L.; Li, S.; Song, H.; Fan, Y.; Su, C. Y. Engineering single-atom sites into pore-confined nanospaces of porphyrinic metal-organic frameworks for the highly efficient photocatalytic hydrogen evolution reaction. J. Am. Chem. Soc. 2022, 144, 22747-58.
91. Liu, X.; Zhang, L.; Wang, J. Design strategies for MOF-derived porous functional materials: preserving surfaces and nurturing pores. J. Materiomics. 2021, 7, 440-59.
92. Li, X.; Wang, B. Atomic regulations of single atom from metal-organic framework derived carbon for advanced water treatment. Nano. Res. 2023, 16, 10326-41.
93. Wang, Z.; Cheng, Y.; Wang, S.; et al. Promoting polysulfide conversions via cobalt single-atom catalyst for fast and durable lithium-sulfur batteries. Nano. Res. 2023, 16, 9335-43.
94. Johnson, D.; Pranada, E.; Yoo, R.; Uwadiunor, E.; Ngozichukwu, B.; Djire, A. Review and perspective on transition metal electrocatalysts toward carbon-neutral energy. Energy. Fuels. 2023, 37, 1545-76.
95. Hwang, C. K.; Kim, S.; Yoon, K. R.; et al. Arc plasma-deposited Co single-atom catalysts supported on an aligned carbon nanofiber for hydrogen peroxide electrosynthesis and an electro-Fenton process. Carbon. Energ. 2024, 6, e582.
96. Guo, B.; Wang, Z.; Zheng, L.; Mo, G.; Zhou, H.; Luo, D. Confined cobalt single-atom catalysts with strong electronic metal-support interactions based on a biomimetic self-assembly strategy. Carbon. Energ. 2024, 6, e554.
97. Hou, C.; Wang, H.; Li, C.; Xu, Q. From metal-organic frameworks to single/dual-atom and cluster metal catalysts for energy applications. Energy. Environ. Sci. 2020, 13, 1658-93.
98. Yang, J.; Zhu, C.; Wang, D. A simple organo-electrocatalysis system for the chlor-related industry. Angew. Chem. Int. Ed. Engl. 2024, 63, e202406883.
99. Wang, X. Y.; Pan, Y. Z.; Yang, J.; et al. Single-atom iron catalyst as an advanced redox mediator for anodic oxidation of organic electrosynthesis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202404295.
100. Wang, L.; Li, J.; Ji, S.; Xiong, Y.; Wang, D. Microenvironment engineering of covalent organic framework based single/dual-atom catalysts toward sustainable energy conversion and storage. Energy. Environ. Sci. 2024, 17, 8482-528.
101. Qiu, W.; Qin, S.; Li, Y.; et al. Overcoming electrostatic interaction via pulsed electroreduction for boosting the electrocatalytic urea synthesis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202402684.
102. Lv, L.; Tan, H.; Kong, Y.; et al. Breaking the scaling relationship in C-N coupling via the doping effects for efficient urea electrosynthesis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401943.
103. Zuo, Q.; Cui, R.; Wang, L.; et al. High-loading single cobalt atoms on ultrathin MOF nanosheets for efficient photocatalytic CO2 reduction. Sci. China. Chem. 2023, 66, 570-7.
104. Xie, M.; Wang, J.; Du, X. L.; et al. Metal-organic framework derived single-atom catalysts for electrochemical CO2 reduction. RSC. Adv. 2022, 12, 32518-25.
105. Bao, C.; Huo, Y. F.; Li, Y. T.; Yang, S. Q.; Li, W.; Hu, T. L. Anchoring Cu on Zirconium-oxo nodes in a pore-confined metal-organic framework for CO2 hydrogenation to methanol. Chem. Eng. J. 2025, 503, 158610.
106. Huang, J. M.; Zhang, X. D.; Huang, J. Y.; Zheng, D. S.; Xu, M.; Gu, Z. Y. MOF-based materials for electrochemical reduction of carbon dioxide. Coord. Chem. Rev. 2023, 494, 215333.
107. Uekert, T.; Pichler, C. M.; Schubert, T.; Reisner, E. Solar-driven reforming of solid waste for a sustainable future. Nat. Sustain. 2021, 4, 383-91.
108. Ma, F.; Zhang, P.; Zheng, X.; et al. Steering the site distance of atomic Cu-Cu pairs by first-shell halogen coordination boosts CO2-to-C2 selectivity. Angew. Chem. Int. Ed. Engl. 2024, 63, e202412785.
109. He, Y.; Wang, Z.; Wang, H.; et al. Metal-organic framework-derived nanomaterials in environment related fields: fundamentals, properties and applications. Coord. Chem. Rev. 2021, 429, 213618.
110. Wang, Y.; Liu, Y.; Liu, W.; et al. Regulating the coordination structure of metal single atoms for efficient electrocatalytic CO2 reduction. Energy. Environ. Sci. 2020, 13, 4609-24.
111. Zhong, G.; Zou, L.; Chi, X.; et al. Atomically dispersed Mn-Nx catalysts derived from Mn-hexamine coordination frameworks for oxygen reduction reaction. Carbon. Energ. 2024, 6, e484.
112. Peng, Y.; Xu, J.; Xu, J.; et al. Metal-organic framework (MOF) composites as promising materials for energy storage applications. Adv. Colloid. Interface. Sci. 2022, 307, 102732.
113. Zhou, Y.; Sheng, L.; Chen, L.; Zhao, W.; Zhang, W.; Yang, J. Metal and ligand modification modulates the electrocatalytic HER, OER, and ORR activity of 2D conductive metal-organic frameworks. Nano. Res. 2024, 17, 7984-90.
114. Fu, W.; Yun, Y.; Sheng, H.; et al. Design of bifunctional single-atom catalysts NiSA/ZIF-300 for CO2 conversion by ligand regulation strategy. Nano. Res. 2024, 17, 3827-34.
115. Mushtaq, N.; Ahmad, A.; Wang, X.; Khan, U.; Gao, J. MOFs/COFs hybrids as next-generation materials for electrocatalytic CO2 reduction reaction. Chem. Eng. J. 2024, 486, 150098.
116. Li, J.; Du, Z.; Xiong, L.; Fu, L.; Bo, W. Supramolecular isomerism in two nickel(II) coordination polymers constructed with the flexible 2-carboxyphenoxyacetate linker: syntheses, structure analyses and magnetic properties. J. Solid. State. Chem. 2021, 293, 121799.
117. Peng, Y.; Sanati, S.; Morsali, A.; García, H. Metal-organic frameworks as electrocatalysts. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214707.
118. Chen, S.; Li, W. H.; Jiang, W.; et al. MOF encapsulating N-heterocyclic carbene-ligated copper single-atom site catalyst towards efficient methane electrosynthesis. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114450.
119. Wang, J.; Sun, K.; Wang, D.; et al. Precise regulation of the coordination environment of single Co(II) sites in a metal-organic framework for boosting CO2 photoreduction. ACS. Catal. 2023, 13, 8760-9.
120. Dong, Y. L.; Jiang, Y.; Ni, S.; et al. Ligand defect-induced active sites in Ni-MOF-74 for efficient photocatalytic CO2 reduction to CO. Small 2024, 20, 2308005.
121. Adegoke, K. A.; Maxakato, N. W. Porous metal-organic framework (MOF)-based and MOF-derived electrocatalytic materials for energy conversion. Mater. Today. Energy. 2021, 21, 100816.
122. Woldu, A. R.; Huang, Z.; Zhao, P.; Hu, L.; Astruc, D. Electrochemical CO2 reduction (CO2RR) to multi-carbon products over copper-based catalysts. Coord. Chem. Rev. 2022, 454, 214340.
123. Wang, L.; Luo, Z.; Feng, S.; et al. Synthesis of MOF-derived hybrids for efficient electrocatalytic reduction of CO2 to syngas. Catal. Lett. 2023, 153, 1527-35.
124. Chen, X.; Zhang, M.; Zhu, J.; Wang, J.; Jiao, Z.; Li, Y. Boosting electrochemical performance of Li-S batteries by cerium-based MOFs coated with polypyrrole. J. Alloy. Compd. 2022, 901, 163649.
125. Li, R.; Ban, T.; Zhao, D.; et al. Defect engineering and Ni promoter synergistically accelerating electron transfer to Ru0 sites in UiO-66(Ce) for dicyclopentadiene hydrogenation under mild condition. Nano. Res. 2024, 17, 9550-63.
126. Tang, B.; Ji, Q.; Zhang, X.; et al. Symmetry breaking of FeN4 moiety via edge defects for acidic oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2025, 64, e202424135.
127. Deng, M.; Mukherjee, S.; Liang, Y. J.; Fang, X. D.; Zhu, A. X.; Zaworotko, M. J. Water vapour induced reversible switching between a 1-D coordination polymer and a 0-D aqua complex. Chem. Commun. 2022, 58, 8218-21.
128. Xiao, L.; Cheng, C.; Li, Z.; et al. Dynamically modulated synthesis of hollow metal-organic frameworks for selective hydrogenation reactions. Nano. Res. 2023, 16, 11334-41.
129. Sun, J. K.; Pan, Y. W.; Xu, M. Q.; et al. Heteroatom doping regulates the catalytic performance of single-atom catalyst supported on graphene for ORR. Nano. Res. 2024, 17, 1086-93.
130. Xu, H.; Wei, X.; Zeng, H.; et al. Recent progress of two-dimensional metal-organic-frameworks: from synthesis to electrocatalytic oxygen evolution. Nano. Res. 2023, 16, 8614-37.
131. Zhu, C.; Yang, J.; Zhang, J.; et al. Single-atom materials: the application in energy conversion. Interdiscip. Mater. 2024, 3, 74-86.
132. Huang, L.; Mo, S.; Zhao, X.; et al. Constructing Co and Zn atomic pairs in core-shell Co3S4/NC@ZnS/NC derived from MOF-on-MOF nanostructures for enhanced photocatalytic CO2 reduction to C2H4. Appl. Catal. B-Environ. Energy. 2024, 352, 124019.
133. Shan, L.; Ma, Y.; Xu, S.; et al. Efficient electrochemical reduction of nitrate to ammonia over metal-organic framework single-atom catalysts. Commun. Mater. 2024, 5, 104.
134. Wen, M.; Sun, N.; Jiao, L.; Zang, S. Q.; Jiang, H. L. Microwave-assisted rapid synthesis of MOF-based single-atom Ni catalyst for CO2 electroreduction at ampere-level current. Angew. Chem. Int. Ed. Engl. 2024, 63, e202318338.
135. Chen, S.; Zhu, Z.; Li, G.; et al. Ionic liquid-assisted synthesis of higher loaded Ni/Fe dual-atom catalysts in N, F, B codoped carbon matrix for accelerated sulfur reduction reaction. Small 2024, 20, 2406731.
136. Srinivas, K.; Yu, H.; Chen, Z.; et al. Densely accessible Fe/Co-Nx dual-atom site coupled core-shell Co3Fe7@C as an efficient bifunctional oxygen electrocatalyst for rechargeable zinc-air batteries. J. Mater. Chem. A. 2024, 12, 16863-76.
137. Ren, F.; Xu, J.; Feng, L. An effective bimetallic oxide catalyst of RuO2-Co3O4 for alkaline overall water splitting. Nano. Res. 2024, 17, 3785-93.
138. Chebrolu, V. T.; Jang, D.; Rani, G. M.; Lim, C.; Yong, K.; Kim, W. B. Overview of emerging catalytic materials for electrochemical green ammonia synthesis and process. Carbon. Energ. 2023, 5, e361.
139. Hu, J.; Xu, Q.; Wang, X.; et al. Charge-transfer-regulated bimetal ferrocene-based organic frameworks for promoting electrocatalytic oxygen evolution. Carbon. Energ. 2023, 5, e315.
140. Liu, S.; Liu, M.; Li, X.; et al. Metal organic polymers with dual catalytic sites for oxygen reduction and oxygen evolution reactions. Carbon. Energ. 2023, 5, e303.
141. Wang, M.; Gao, P.; Li, D.; et al. Cu/Fe dual atoms catalysts derived from Cu-MOF for Zn-air batteries. Mater. Today. Energy. 2022, 28, 101086.
142. Wang, L.; Wu, J.; Wang, S.; Liu, H.; Wang, Y.; Wang, D. The reformation of catalyst: from a trial-and-error synthesis to rational design. Nano. Res. 2024, 17, 3261-301.
143. Zheng, F.; Lin, T.; Wang, K.; Wang, Y.; Li, G. Recent advances in bimetallic metal-organic frameworks and their derivatives for thermal catalysis. Nano. Res. 2023, 16, 12919-35.
144. Cheng, C. C.; Lin, T. Y.; Ting, Y. C.; Lin, S. H.; Choi, Y.; Lu, S. Y. Metal-organic frameworks stabilized Mo and W binary single-atom catalysts as high performance bifunctional electrocatalysts for water electrolysis. Nano. Energy. 2023, 112, 108450.
145. Tang, M.; Shen, J.; Zhang, F.; et al. Upcycling of polyamide wastes to tertiary amines using Mo single atoms and Rh nanoparticles. Angew. Chem. Int. Ed. Engl. 2025, 64, e202416436.
146. Guan, S.; Yuan, Z.; Zhao, S.; et al. Efficient hydrogen generation from ammonia borane hydrolysis on a tandem ruthenium-platinum-titanium catalyst. Angew. Chem. Int. Ed. Engl. 2024, 63, e202408193.
147. Pei, J.; Yang, L.; Lin, J.; et al. Integrating host design and tailored electronic effects of yolk-shell Zn-Mn diatomic sites for efficient CO2 electroreduction. Angew. Chem. Int. Ed. Engl. 2024, 63, e202316123.
148. Liu, M.; Li, N.; Cao, S.; et al. A “pre-constrained metal twins” strategy to prepare efficient dual-metal-atom catalysts for cooperative oxygen electrocatalysis. Adv. Mater. 2022, 34, 2107421.
149. Yang, B.; Huang, J.; Tong, J.; et al. Microwave synthesis of Fe-Cu diatomic active center MOF: synergistic cyclic catalysis of persulfate for degrading norfloxacin. Environ. Sci: Nano. 2023, 10, 2778-89.
150. Leng, K.; Zhang, J.; Wang, Y.; et al. Interfacial cladding engineering suppresses atomic thermal migration to fabricate well-defined dual-atom electrocatalysts. Adv. Funct. Materials. 2022, 32, 2205637.
151. Zhang, L.; Feng, J.; Liu, S.; et al. Atomically dispersed Ni-Cu catalysts for pH-universal CO2 electroreduction. Adv. Mater. 2023, 35, 2209590.
152. Lv, Z.; Zhang, H.; Liu, C.; Li, S.; Song, J.; He, J. Oxygen-bridged cobalt-chromium atomic pair in MOF-derived cobalt phosphide networks as efficient active sites enabling synergistic electrocatalytic water splitting in alkaline media. Adv. Sci. 2024, 11, e2306678.
153. Li, Y.; Niu, S.; Liu, P.; et al. Ruthenium Nanoclusters and single atoms on α-MoC/N-doped carbon achieves low-input/input-free hydrogen evolution via decoupled/coupled hydrazine oxidation. Angew. Chem. Int. Ed. Engl. 2024, 63, e202316755.
154. Han, A.; Sun, W.; Wan, X.; et al. Construction of Co4 atomic clusters to enable Fe-N4 motifs with highly active and durable oxygen reduction performance. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303185.
155. Yang, X.; Qin, J.; Dai, Z.; et al. MOF-derived Fe based catalysts for efficiently advanced oxidation processes: from single atoms to diatomic and nanoparticles. Prog. Nat. Sci-Mater. 2023, 33, 534-43.
156. Zhao, J.; Zhang, Y.; Zhuang, Z.; et al. Tailoring d-p orbital hybridization to decipher the essential effects of heteroatom substitution on redox kinetics. Angew. Chem. Int. Ed. Engl. 2024, 63, e202404968.
157. Mu, X. Q.; Liu, S. L.; Zhang, M. Y.; et al. Symmetry-broken Ru nanoparticles with parasitic Ru-Co dual-single atoms overcome the volmer step of alkaline hydrogen oxidation. Angew. Chem. Int. Ed. Engl. 2024, 63, e202319618.
158. Wang, Y.; Park, B. J.; Paidi, V. K.; et al. Precisely constructing orbital coupling-modulated dual-atom fe pair sites for synergistic CO2 electroreduction. ACS. Energy. Lett. 2022, 7, 640-9.
159. Zhao, X.; Zhao, K.; Liu, Y.; et al. Highly efficient electrochemical CO2 reduction on a precise homonuclear diatomic Fe-Fe catalyst. ACS. Catal. 2022, 12, 11412-20.
160. Li, Q.; Zhang, P.; Li, H.; et al. Maximizing hydrogen evolution via Co-Ni dual atoms and nanoclusters on hierarchically ordered porous carbon framework. Sci. China. Mater. 2024, 67, 3197-205.
161. Ning, S.; Ou, H.; Li, Y.; et al. Co0-Coδ+ interface double-site-mediated C-C coupling for the photothermal conversion of CO2 into light olefins. Angew. Chem. Int. Ed. Engl. 2023, 62, e202302253.
162. Liu, M.; Liu, S.; Xu, Q.; et al. Dual atomic catalysts from COF-derived carbon for CO2RR by suppressing HER through synergistic effects. Carbon. Energy. 2023, 5, e300.
163. Jiao, L.; Zhu, J.; Zhang, Y.; et al. Non-bonding interaction of neighboring Fe and Ni single-atom pairs on MOF-derived N-doped carbon for enhanced CO2 electroreduction. J. Am. Chem. Soc. 2021, 143, 19417-24.
164. Wang, B.; Huang, J.; Wu, H.; Yan, X.; Liao, Y.; Li, H. Synergy of heterogeneous Co/Ni dual atoms enabling selective C–O bond scission of lignin coupling with in-situ N-functionalization. J. Energy. Chem. 2024, 92, 16-25.
165. Xia, J.; Xu, J.; Yu, B.; et al. A metal-sulfur-carbon catalyst mimicking the two-component architecture of nitrogenase. Angew. Chem. Int. Ed. Engl. 2024, 63, e202412740.
166. Qin, Y.; Yu, K.; Wang, G.; et al. Adjacent-ligand tuning of atomically precise Cu-Pd sites enables efficient methanol electrooxidation with a CO-free pathway. Angew. Chem. Int. Ed. Engl. 2025, 64, e202420817.
167. Pang, M.; Yang, M.; Zhang, H.; et al. Synthesis techniques, mechanism, and prospects of high-loading single-atom catalysts for oxygen reduction reactions. Nano. Res. 2024, 17, 9371-96.
168. Gao, Y.; Yang, C.; Sun, F.; et al. Ligand-tuning metallic sites in molecular complexes for efficient water oxidation. Angew. Chem. Int. Ed. Engl. 2025, 64, e202415755.
169. Hu, Y.; Chao, T.; Li, Y.; et al. Cooperative Ni(Co)-Ru-P sites activate dehydrogenation for hydrazine oxidation assisting self-powered H2 production. Angew. Chem. Int. Ed. Engl. 2023, 62, e202308800.
170. Zhang, Q.; Liu, D.; Zhang, Y.; et al. Insight into coupled Ni-Co dual-metal atom catalysts for efficient synergistic electrochemical CO2 reduction. J. Energy. Chem. 2023, 87, 509-17.
171. Zhu, J.; Xiao, M.; Ren, D.; et al. Quasi-covalently coupled Ni-Cu atomic pair for synergistic electroreduction of CO2. J. Am. Chem. Soc. 2022, 144, 9661-71.
172. Ahmad, M.; Chen, J.; Liu, J.; et al. Metal-organic framework-based single-atom electro-/photocatalysts: synthesis, energy applications, and opportunities. Carbon. Energy. 2024, 6, e382.
173. Song, Z.; Li, J.; Zhang, Q.; et al. Progress and perspective of single-atom catalysts for membrane electrode assembly of fuel cells. Carbon. Energy. 2023, 5, e342.
174. Yang, J.; Zhu, C.; Li, W.; Zheng, X.; Wang, D. Organocatalyst supported by a single-atom support accelerates both electrodes used in the chlor-alkali industry via modification of non-covalent interactions. Angew. Chem. Int. Ed. Engl. 2024, 63, e202314382.
175. Hu, Y.; Li, Z.; Li, B.; Yu, C. Recent progress of diatomic catalysts: general design fundamentals and diversified catalytic applications. Small 2022, 18, 2203589.
176. Tang, H. T.; Zhou, H. Y.; Pan, Y. M.; et al. Single-atom manganese-catalyzed oxygen evolution drives the electrochemical oxidation of silane to silanol. Angew. Chem. Int. Ed. Engl. 2024, 63, e202315032.
177. Wang, M.; Yao, Y.; Yang, F.; et al. Double spatial confinement on ruthenium nanoparticles inside carbon frameworks as durable catalysts for a quasi-solid-state Li-O2 battery. Carbon. Energy. 2023, 5, e334.
178. Xu, C.; Han, W.; Xue, W.; et al. An in-situ TEM characterization of electron beam induced dislocation motion in a single-crystalline gold thin film. Mater. Charact. 2022, 184, 111697.
179. Tao, Y.; Guan, J.; Zhang, J.; et al. Ruthenium single atomic sites surrounding the support pit with exceptional photocatalytic activity. Angew. Chem. Int. Ed. Engl. 2024, 63, e202400625.
180. Liu, Y.; Zhuang, Z.; Liu, Y.; et al. Shear-strained Pd single-atom electrocatalysts for nitrate reduction to ammonia. Angew. Chem. Int. Ed. Engl. 2024, 63, e202411396.
181. Sun, B.; Zhang, S.; Yang, H.; et al. Revealing the active sites in atomically dispersed multi-metal-nitrogen-carbon catalysts. Adv. Funct. Materials. 2024, 34, 2315862.
182. Wei, J.; Xiao, K.; Chen, Y.; Guo, X. P.; Huang, B.; Liu, Z. Q.
183. Sun, Z.; Li, C.; Wei, Z.; et al. Sulfur-bridged asymmetric CuNi bimetallic atom sites for CO2 reduction with high efficiency. Adv. Mater. 2024, 36, 2404665.
184. Su, Y.; Yuan, G.; Hu, J.; et al. Recent progress in strategies for preparation of metal-organic frameworks and their hybrids with different dimensions. Chem. Synth. 2023, 3, 1.
185. Abdel-mageed, A. M.; Rungtaweevoranit, B.; Impeng, S.; et al. Unveiling the CO oxidation mechanism over a molecularly defined copper single-atom catalyst supported on a metal-organic framework. Angew. Chem. Int. Ed. Engl. 2023, 62, e202301920.
186. Wang, X.; Guo, Y.; Cui, X.; et al. Graphene oxide membranes using MOF@Chitosan core-shell nanoparticles as dual modulators for dye separation. Chem. Synth. 2024, 4, 27.
187. Wang, Y.; Wu, J.; Tang, S.; et al. Synergistic Fe-Se atom pairs as bifunctional oxygen electrocatalysts boost low-temperature rechargeable Zn-Air battery. Angew. Chem. Int. Ed. Engl. 2023, 62, e202219191.
188. Liu, M.; Wang, X.; Cao, S.; et al. Ferredoxin-inspired design of S-synergized Fe-Fe dual-metal center catalysts for enhanced electrocatalytic oxygen reduction reaction. Adv. Mater. 2024, 36, 2309231.
189. Sun, Z.; Zhang, H.; Cao, L.; et al. Understanding synergistic catalysis on Cu-Se dual atom sites via operando X-ray absorption spectroscopy in oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217719.
190. Zhang, Y.; Yao, Z.; Yang, Y.; et al. Breaking the scaling relations of effective CO2 electrochemical reduction in diatomic catalysts by adjusting the flow direction of intermediate structures. Chem. Sci. 2024, 15, 13160-72.
191. Liu, J.; Xu, H.; Zhu, J.; Cheng, D. Understanding the pathway switch of the oxygen reduction reaction from single- to double-/triple-atom catalysts: a dual channel for electron acceptance-backdonation. JACS. Au. 2023, 3, 3031-44.
192. Zhang, S.; Hou, M.; Zhai, Y.; et al. Dual-active-sites single-atom catalysts for advanced applications. Small 2023, 19, e2302739.
193. Huang, H.; Sun, M.; Li, S.; et al. Enhancing H2O2 electrosynthesis at industrial-relevant current in acidic media on diatomic cobalt sites. J. Am. Chem. Soc. 2024, 146, 9434-43.
194. Sun, J.; Tao, L.; Ye, C.; et al. MOF-derived Ru1Zr1/Co dual-atomic-site catalyst with promoted performance for fischer-tropsch synthesis. J. Am. Chem. Soc. 2023, 145, 7113-22.
195. Liu, Y.; Yuan, S.; Sun, C.; et al. Optimizing Fe-3d electron delocalization by asymmetric Fe-Cu diatomic configurations for efficient anion exchange membrane fuel cells. Adv. Energy. Mater. 2023, 13, 2302719.
196. Zhao, Z.; Zhu, M.; Qu, M.; et al. Relay electrocatalysis with bimetallic sites for highly efficient oxidation in multiple cascade reaction. Chem. Eng. J. 2024, 484, 149768.
197. Li, B.; Du, W.; Wu, Q.; Dai, Y.; Huang, B.; Ma, Y. Coronene-based 2D metal-organic frameworks: a new family of promising single-atom catalysts for nitrogen reduction reaction. J. Phys. Chem. C. 2021, 125, 20870-6.
198. Zhang, R.; Jiao, L.; Yang, W.; Wan, G.; Jiang, H. L. Single-atom catalysts templated by metal-organic frameworks for electrochemical nitrogen reduction. J. Mater. Chem. A. 2019, 7, 26371-7.
199. Qiao, M.; Xie, J.; Zhu, D. Computational screening of two-dimensional metal-organic frameworks as efficient single-atom catalysts for oxygen reduction reaction. Chem-Eur. J. 2023, 29, e202300686.
200. Ming, M. T.; Wang, Y. C.; Tao, W. X.; Shi, W. J.; Zhong, D. C.; Lu, T. B. Designing dual-atom cobalt catalysts anchored on amino-functionalized MOFs for efficient CO2 photoreduction. Green. Chem. 2023, 25, 6207-11.
201. Hu, S.; Gao, M. L.; Huang, J.; et al. Introducing hydrogen-bonding microenvironment in close proximity to single-atom sites for boosting photocatalytic hydrogen production. J. Am. Chem. Soc. 2024, 146, 20391-400.
202. Chen, Y.; Lin, J. Design of efficient dual-atom catalysts for energy conversion. Chem Synth 2025;5:[Accept]. Available online: https://www.oaepublish.com/pre_onlines/cs.2024.104 (accessed 9 May 2025).
203. Zhao, B.; Han, J.; Liu, B.; Zhang, S. L.; Guan, B. Hierarchical metal-organic framework nanoarchitectures for catalysis. Chem. Synth. 2024, 4, 41.
204. Cui, X.; Bai, H.; Zhang, J.; et al. A cluster-nanozyme-coenzyme system mimicking natural photosynthesis for CO2 reduction under intermittent light irradiation. Nat. Commun. 2024, 15, 9048.
205. Qiu, B.; Du, M.; Ma, Y.; Zhu, Q.; Xing, M.; Zhang, J. Integration of redox cocatalysts for artificial photosynthesis. Energy. Environ. Sci. 2021, 14, 5260-88.
206. Zhu, Q.; Xu, Q.; Du, M.; et al. Recent progress of metal sulfide photocatalysts for solar energy conversion. Adv. Mater. 2022, 34, e2202929.
207. Zhuo, L. L.; Chen, P.; Zheng, K.; et al. Flexible cuprous triazolate frameworks as highly stable and efficient electrocatalysts for CO2 reduction with tunable C2H4/CH4 selectivity. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204967.
208. Angulo-Ibáñez, A.; Goitandia, A. M.; Albo, J.; et al. Porous TiO2 thin film-based photocatalytic windows for an enhanced operation of optofluidic microreactors in CO2 conversion. iScience 2021, 24, 102654.
209. Chen, Z.; Zhang, X.; Liu, W.; et al. Amination strategy to boost the CO2 electroreduction current density of M-N/C single-atom catalysts to the industrial application level. Energy. Environ. Sci. 2021, 14, 2349-56.
210. Cheng, H.; Wu, X.; Li, X.; et al. Construction of atomically dispersed Cu-N4 sites via engineered coordination environment for high-efficient CO2 electroreduction. Chem. Eng. J. 2021, 407, 126842.
211. Choi, S.; Jung, W. J.; Park, K.; et al. Rapid exciton migration and amplified funneling effects of multi-porphyrin arrays in a Re(I)/porphyrinic MOF hybrid for photocatalytic CO2 reduction. ACS. Appl. Mater. Interfaces. 2021, 13, 2710-22.
212. Gao, J.; Hu, Y.; Wang, Y.; et al. MOF structure engineering to synthesize Co-N-C catalyst with richer accessible active sites for enhanced oxygen reduction. Small 2021, 17, 2104684.
213. Cho, J. H.; Ma, J.; Lee, C.; et al. Crystallographically vacancy-induced MOF nanosheet as rational single-atom support for accelerating CO2 electroreduction to CO. Carbon. Energ. 2024, 6, e510.
214. Wang, G.; Wu, Y.; Li, Z.; et al. Engineering a copper single-atom electron bridge to achieve efficient photocatalytic CO2 conversion. Angew. Chem. Int. Ed. Engl. 2023, 62, e202218460.
215. Zhang, X.; Xue, D.; Jiang, S.; et al. Rational confinement engineering of MOF-derived carbon-based electrocatalysts toward CO2 reduction and O2 reduction reactions. InfoMat 2022, 4, e12257.
216. Li, J. X.; Zhang, Y. H.; Du, Z. X.; Feng, X. One-pot solvothermal synthesis of mononuclear and oxalate-bridged binuclear nickel compounds: structural analyses, conformation alteration and magnetic properties. Inorganica. Chimica. Acta. 2022, 530, 120697.
217. Zhao, Y.; Zeng, H.; Zhu, X. W.; Lu, W.; Li, D. Metal-organic frameworks as photoluminescent biosensing platforms: mechanisms and applications. Chem. Soc. Rev. 2021, 50, 4484-513.
218. Draper, B.; Yee, W. L.; Pedrana, A.; et al. Reducing liver disease-related deaths in the Asia-Pacific: the important role of decentralised and non-specialist led hepatitis C treatment for cirrhotic patients. Lancet. Reg. Health. West. Pac. 2022, 20, 100359.
219. Geng, W.; Chen, W.; Li, G.; et al. Induced CO2 electroreduction to formic acid on metal-organic frameworks via node doping. ChemSusChem 2020, 13, 4035-40.
220. Huo, J.; Cao, X.; Tian, Y.; et al. Atomically dispersed Mn atoms coordinated with N and O within an N-doped porous carbon framework for boosted oxygen reduction catalysis. Nanoscale 2023, 15, 5448-57.
221. Wei, Y. S.; Zhang, M.; Zou, R.; Xu, Q. Metal-organic framework-based catalysts with single metal sites. Chem. Rev. 2020, 120, 12089-174.
222. Hou, C. C.; Zou, L.; Sun, L.; et al. Single-atom iron catalysts on overhang-eave carbon cages for high-performance oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 2020, 59, 7454-9.
223. Li, C.; Ni, T.; Yue, M.; Li, S.; Zhang, Q. Pristine metal-organic frameworks for sodium-ion batteries: past, present, and future. Batteries. Supercaps. 2024, 7, e202400138.
224. Zhang, Y.; Wang, Q.; Wu, L.; et al. Manipulating photogenerated electron flow in nickel single-atom catalysts for photocatalytic CO2 reduction into tunable syngas. Carbon. Energ. 2024, 6, e533.
225. Peng, J. Z.; Li, Y. L.; Cheng, Y. T.; et al. Metal-N4 model single-atom catalyst with electroneutral quadri-pyridine macrocyclic ligand for CO2 electroreduction. Carbon. Energ. 2024, 6, e506.
226. Chen, Q.; Yao, M.; Zhou, Y.; Sun, Y.; Zhang, G.; Pang, H. Etching MOF nanomaterials: precise synthesis and electrochemical applications. Coord. Chem. Rev. 2024, 517, 216016.
227. Zou, Y.; Wang, S. An investigation of active sites for electrochemical CO2 reduction reactions: from in situ characterization to rational design. Adv. Sci. 2021, 8, 2003579.
228. Aiyappa, H. B.; Masa, J.; Andronescu, C.; Muhler, M.; Fischer, R. A.; Schuhmann, W. MOFs for electrocatalysis: from serendipity to design strategies. Small. Methods. 2019, 3, 1800415.
229. Liu, Y.; Tang, C.; Cheng, M.; et al. Polyoxometalate@metal-organic framework composites as effective photocatalysts. ACS. Catal. 2021, 11, 13374-96.
230. Zhao, Y.; Zheng, L.; Jiang, D.; et al. Nanoengineering metal-organic framework-based materials for use in electrochemical CO2 reduction reactions. Small 2021, 17, 2006590.
231. Yang, Y.; Yang, Y.; Liu, Y.; Zhao, S.; Tang, Z. Metal−organic frameworks for electrocatalysis: beyond their derivatives. Small. Sci. 2021, 1, 2100015.
232. Li, J. X.; Xiong, L. Y.; Fu, L. L.; Bo, W. B.; Du, Z. X.; Feng, X. Structural diversity of Mn(II) and Cu(II) complexes based on 2-carboxyphenoxyacetate linker: syntheses, conformation comparison and magnetic properties. J. Solid. State. Chem. 2022, 305, 122636.
233. Li, S.; Gao, Y.; Li, N.; Ge, L.; Bu, X.; Feng, P. Transition metal-based bimetallic MOFs and MOF-derived catalysts for electrochemical oxygen evolution reaction. Energy. Environ. Sci. 2021, 14, 1897-927.
234. Zhou, P.; Lv, J.; Huang, X.; Lu, Y.; Wang, G. Strategies for enhancing the catalytic activity and electronic conductivity of MOFs-based electrocatalysts. Coord. Chem. Rev. 2023, 478, 214969.
235. He, X.; Deng, Y.; Zhang, Y.; et al. Mechanochemical kilogram-scale synthesis of noble metal single-atom catalysts. Cell. Rep. Phys. Sci. 2020, 1, 100004.
236. Sui, J.; Liu, H.; Hu, S.; et al. A general strategy to immobilize single-atom catalysts in metal-organic frameworks for enhanced photocatalysis. Adv. Mater. 2022, 34, 2109203.
237. He, H.; Wang, H. H.; Liu, J.; Liu, X.; Li, W.; Wang, Y. Research progress and application of single-atom catalysts: a review. Molecules 2021, 26, 6501.
238. Chiromo, H.; Nyakuchena, J.; Streater, D.; et al. Metal-organic framework as a dual support for organic photosensitizers and single-atom catalysts. J. Phys. Chem. C. 2023, 127, 20354-9.
239. Xiao, M.; Zhang, L.; Luo, B.; et al. Molten-salt-mediated synthesis of an atomic nickel Co-catalyst on TiO2 for improved photocatalytic H2 evolution. Angew. Chem. Int. Ed. Engl. Engl. 2020, 59, 7297-301.
240. He, J.; Li, N.; Li, Z. G.; et al. Strategic defect engineering of metal-organic frameworks for optimizing the fabrication of single-atom catalysts. Adv. Funct. Materials. 2021, 31, 2103597.
241. Thompson, W. A.; Sanchez, Fernandez. E.; Maroto-valer, M. M. Probability Langmuir-Hinshelwood based CO2 photoreduction kinetic models. Chem. Eng. J. 2020, 384, 123356.
242. Kovačič, Ž.; Likozar, B.; Huš, M. Photocatalytic CO2 reduction: a review of Ab initio mechanism, kinetics, and multiscale modeling simulations. ACS. Catal. 2020, 10, 14984-5007.
243. Qureshi, W. A.; Najeeb-Uz-Zaman, Haider. S.; He, P.; Ali, R. N.; Liu, Q. Q.; Yang, J. Pt quantum dots-coupled AgVO3/g-C3N4 Z-scheme photocatalyst for efficient sunlight-driven hydrogen production. Mater. Today. Sustain. 2023, 23, 100416.
244. Lee, S. Y.; Jang, H. W.; Lee, H. R.; Joh, H. I. Size effect of metal-organic frameworks with iron single-atom catalysts on oxygen-reduction reactions. Carbon. Lett. 2021, 31, 1349-55.
245. Ma, S.; Han, Z.; Leng, K.; et al. Ionic exchange of metal-organic frameworks for constructing unsaturated copper single-atom catalysts for boosting oxygen reduction reaction. Small 2020, 16, e2001384.
246. Wang, K.; Chu, Y.; Zhang, X.; Zhao, R.; Tan, X. Facile method to synthesize a high-activity S-doped Fe/SNC single-atom catalyst by metal-organic frameworks for oxygen reduction reaction in acidic medium. Energy. Fuels. 2021, 35, 20243-9.
247. Qiu, Z.; Li, Y.; Gao, Y.; et al. 2D MOF-assisted pyrolysis-displacement-alloying synthesis of high-entropy alloy nanoparticles library for efficient electrocatalytic hydrogen oxidation. Angew. Chem. Int. Ed. Engl. 2023, 62, e202306881.
248. Yang, Y.; Sun, Q.; Xue, J.; et al. MOF-derived N-doped carbon nanosticks coupled with Fe phthalocyanines for efficient oxygen reduction. Chem. Eng. J. 2023, 464, 142668.
249. Chellasamy, G.; Arumugasamy, S. K.; Kuppusamy, S.; et al. MXene-MOF architectural hybrid-supported nickel single-atom catalysts for hydrogen evolution reactions. J. Mater. Chem. A. 2024, 12, 1115-27.
250. Li, J.; Li, H.; Xie, W.; et al. Flame-assisted synthesis of O-coordinated single-atom catalysts for efficient electrocatalytic oxygen reduction and hydrogen evolution reaction. Small. Methods. 2022, 6, e2101324.
251. Liang, R. R.; Liu, Z.; Han, Z.; Yang, Y.; Rushlow, J.; Zhou, H. C. Anchoring catalytic metal nodes within a single-crystalline pyrazolate metal-organic framework for efficient heterogeneous catalysis. Angew. Chem. Int. Ed. Engl. 2025, 64, e202414271.
252. Tian, S.; Peng, C.; Dong, J.; et al. High-loading single-atomic-site silver catalysts with an Ag1-C2N1 structure showing superior performance for epoxidation of styrene. ACS. Catal. 2021, 11, 4946-54.
253. Ren, G.; Zhao, J.; Zhao, Z.; et al. Defects-induced single-atom anchoring on metal-organic frameworks for high-efficiency photocatalytic nitrogen reduction. Angew. Chem. Int. Ed. Engl. 2024, 63, e202314408.
254. Zhang, N.; Ye, C.; Yan, H.; et al. Single-atom site catalysts for environmental catalysis. Nano. Res. 2020, 13, 3165-82.
255. Li, L.; Li, N.; Xia, J.; et al. Metal-organic framework-derived Co single atoms anchored on N-doped hierarchically porous carbon as a pH-universal ORR electrocatalyst for Zn-air batteries. J. Mater. Chem. A. 2023, 11, 2291-301.
256. Wang, H.; Sun, C.; Zhu, E.; Shi, C.; Yu, J.; Xu, M. Core-shell MOF-derived Fe3C-Co-NC as high-performance ORR/OER bifunctional catalyst. J. Alloy. Compd. 2023, 948, 169728.
257. Wu, Y.; Tong, Y.; Luo, Y.; Xu, J.; Gu, X. K.; Ding, M. Chelation and stabilization of dynamic single- atom Cu in metal-organic frameworks for selective hydrogenation reactions. ACS. Catal. 2024, 14, 15869-78.
258. Xue, Q.; Wun, C. K. T.; Chen, T.; et al. Controlled synthesis of Cu,Fe dual-atom catalysts restrained on metal-organic frameworks for efficient O2 activation. J. Mater. Chem. A. 2023, 11, 14204-12.
259. Zhang, Y.; Sun, Y.; Wang, Q.; et al. Synergy of photogenerated electrons and holes toward efficient photocatalytic urea synthesis from CO2 and N2. Angew. Chem. Int. Ed. Engl. 2024, 63, e202405637.
260. Chen, S.; Zheng, X.; Zhu, P.; et al. Copper atom pairs stabilize *OCCO dipole toward highly selective CO2 electroreduction to C2H4. Angew. Chem. Int. Ed. Engl. 2024, 63, e202411591.
261. Gandionco, K. A.; Kim, J.; Bekaert, L.; Hubin, A.; Lim, J. Single-atom catalysts for the electrochemical reduction of carbon dioxide into hydrocarbons and oxygenates. Carbon. Energ. 2024, 6, e410.
262. Sun, Z.; Zhang, S.; Zheng, B.; et al. Metal-organic framework-derived Mn/Ni dual-metal single-atom catalyst for efficient oxygen reduction reaction. Inorganics 2023, 11, 101.
263. Liu, X.; Jia, C.; Jiang, G.; et al. Single-atom Pd anchored in the porphyrin-center of ultrathin 2D-MOFs as the active center to enhance photocatalytic hydrogen-evolution and NO-removal. Chinese. Chem. Lett. 2024, 35, 109455.
264. Liu, X.; Song, X.; Jiang, G.; et al. Pt single-atom collaborate with Pt atom-clusters by an in-situ confined strategy for accelerating electrocatalytic hydrogen evolution. Chem. Eng. J. 2024, 481, 148430.
265. Guan, G. W.; Zheng, S. T.; Xia, M.; et al. Incorporating CdS and anchoring Pt single atoms into porphyrinic metal-organic frameworks for superior visible-light and sunlight-driven H2 evolution. Chem. Eng. J. 2023, 464, 142530.
266. Wang, W.; Deng, X.; Tian, Z.; Li, D. C.; Wang, G. H. Mesopore-confined synthesis of cobalt nanoparticles with single atoms in close psroximity for efficient oxygen reduction. Chem. Mater. 2023, 35, 6070-82.
267. Gong, Y. N.; Jiao, L.; Qian, Y.; et al. Regulating the coordination environment of MOF-templated single-atom nickel electrocatalysts for boosting CO2 reduction. Angew. Chem. Int. Ed. Engl. 2020, 59, 2727-31.
268. Peng, J. X.; Yang, W.; Jia, Z.; Jiao, L.; Jiang, H. L. Axial coordination regulation of MOF-based single-atom Ni catalysts by halogen atoms for enhanced CO2 electroreduction. Nano. Res. 2022, 15, 10063-9.
269. McCarver, G. A.; Yildirim, T.; Zhou, W. Catalyst engineering for the selective reduction of CO2 to CH4: a first-principles study on X-MOF-74 (X = Mg, Mn, Fe, Co, Ni, Cu, Zn). Chemphyschem 2023, 24, e202300645.
270. Lim, J. W.; Choo, D. H.; Cho, J. H.; et al. A MOF-derived pyrrolic N-stabilized Ni single atom catalyst for selective electrochemical reduction of CO2 to CO at high current density. J. Mater. Chem. A. 2024, 12, 11090-100.
271. Song, P.; Hu, B.; Zhao, D.; et al. Modulating the asymmetric atomic interface of copper single atoms for efficient CO2 electroreduction. ACS. Nano. 2023, 17, 4619-28.
272. Feng, M.; Wu, X.; Cheng, H.; et al. Well-defined Fe-Cu diatomic sites for efficient catalysis of CO2 electroreduction. J. Mater. Chem. A. 2021, 9, 23817-27.
273. Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated bilayer MOF enables high-selectivity photocatalytic reduction of CO2 to CO. Adv. Mater. 2023, 35, e2209814.
274. Liu, Z.; Chen, Z.; Li, M.; et al. Construction of single Ni atom-immobilized ZIF-8 with ordered hierarchical pore structures for selective CO2 photoreduction. ACS. Catal. 2023, 13, 6630-40.
275. Zhao, L.; Bian, J.; Zhang, X.; et al. Construction of ultrathin S-scheme heterojunctions of single Ni atom immobilized Ti-MOF and BiVO4 for CO2 photoconversion of nearly 100% to CO by pure water. Adv. Mater. 2022, 34, 2205303.
276. Li, J.; Yu, X.; Xue, W.; Nie, L.; Huang, H.; Zhong, C. Engineering the direct Z-scheme systems over lattice intergrown of MOF-on-MOF for selective CO2 photoreduction to CO. AIChE. J. 2023, 69, e17906.
277. Gao, C.; Li, L.; Yan, X.; et al. Triethylenediamine cobalt complex encapsulated in a metal-organic framework cage to prepare a cobalt single-atom catalyst with a high Co-N4 density for an efficient oxygen reduction reaction. J. Colloid. Interface. Sci. 2024, 653, 296-307.
278. Pan, W. G.; Li, C. F.; Zhang, Z. R.; Wu, T.; Guo, R. T. Efficient CO2 reduction under visible light: synergistic effects of Cu nanoparticles and Ni single atoms. Appl. Catal. B-Environ. 2024, 343, 123492.
279. Zeng, P.; Liu, H.; Jia, H.; Cai, J.; Deng, X.; Peng, T.
280. Wang, G.; He, C. T.; Huang, R.; Mao, J.; Wang, D.; Li, Y. Photoinduction of Cu single atoms decorated on UiO-66-NH2 for enhanced photocatalytic reduction of CO2 to liquid fuels. J. Am. Chem. Soc. 2020, 142, 19339-45.
281. Yuan, J.; Chen, Y.; Liu, F.; Su, Y. Fabrication of dual atomic copper-indium (CuIn) catalysts for electrochemical CO2 reduction to methanol. Catal. Commun. 2023, 177, 106640.
282. Tan, J.; Yu, M.; Cai, Z.; Lou, X.; Wang, J.; Li, Z. MOF-derived synthesis of MnS/In2S3 p-n heterojunctions with hierarchical structures for efficient photocatalytic CO2 reduction. J. Colloid. Interface. Sci. 2021, 588, 547-56.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data




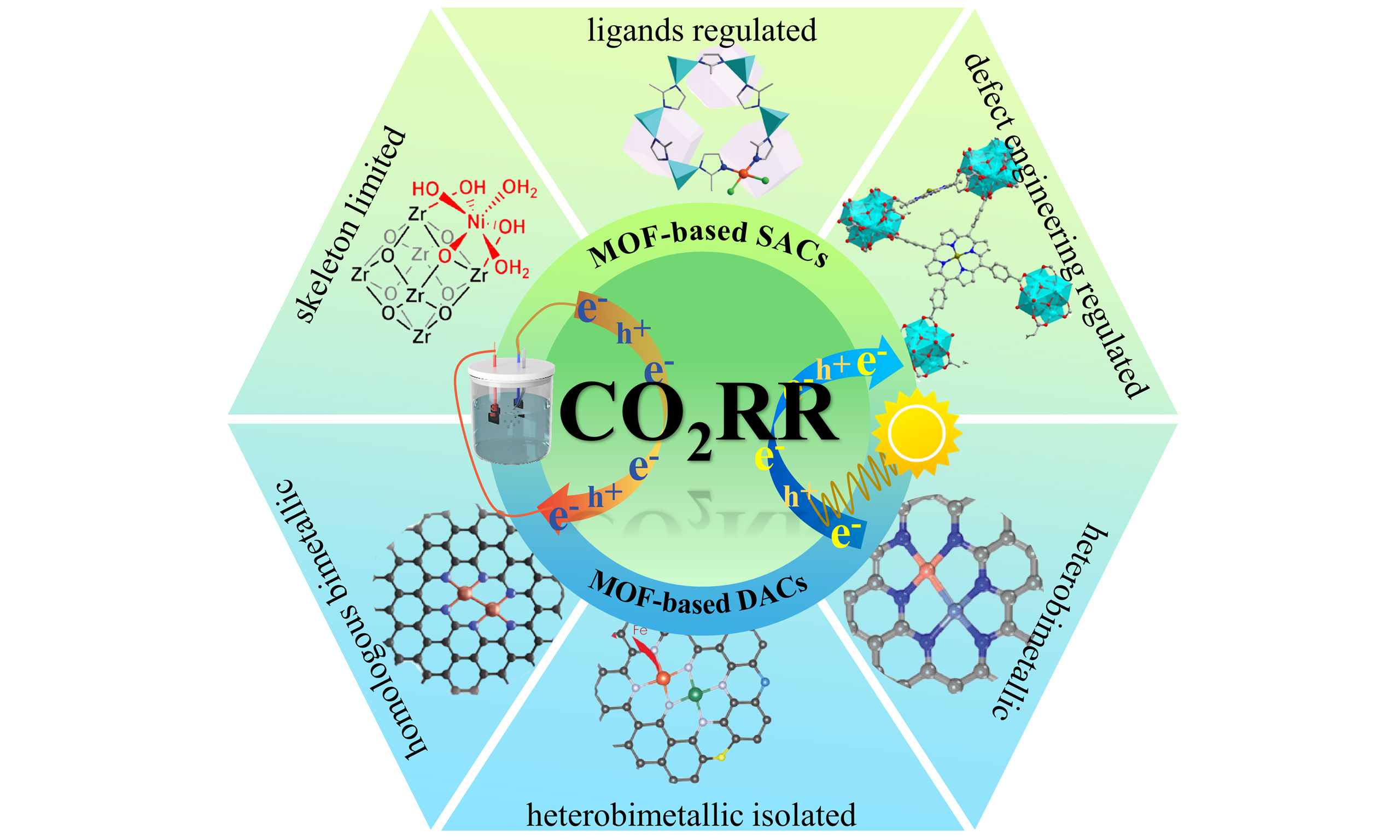
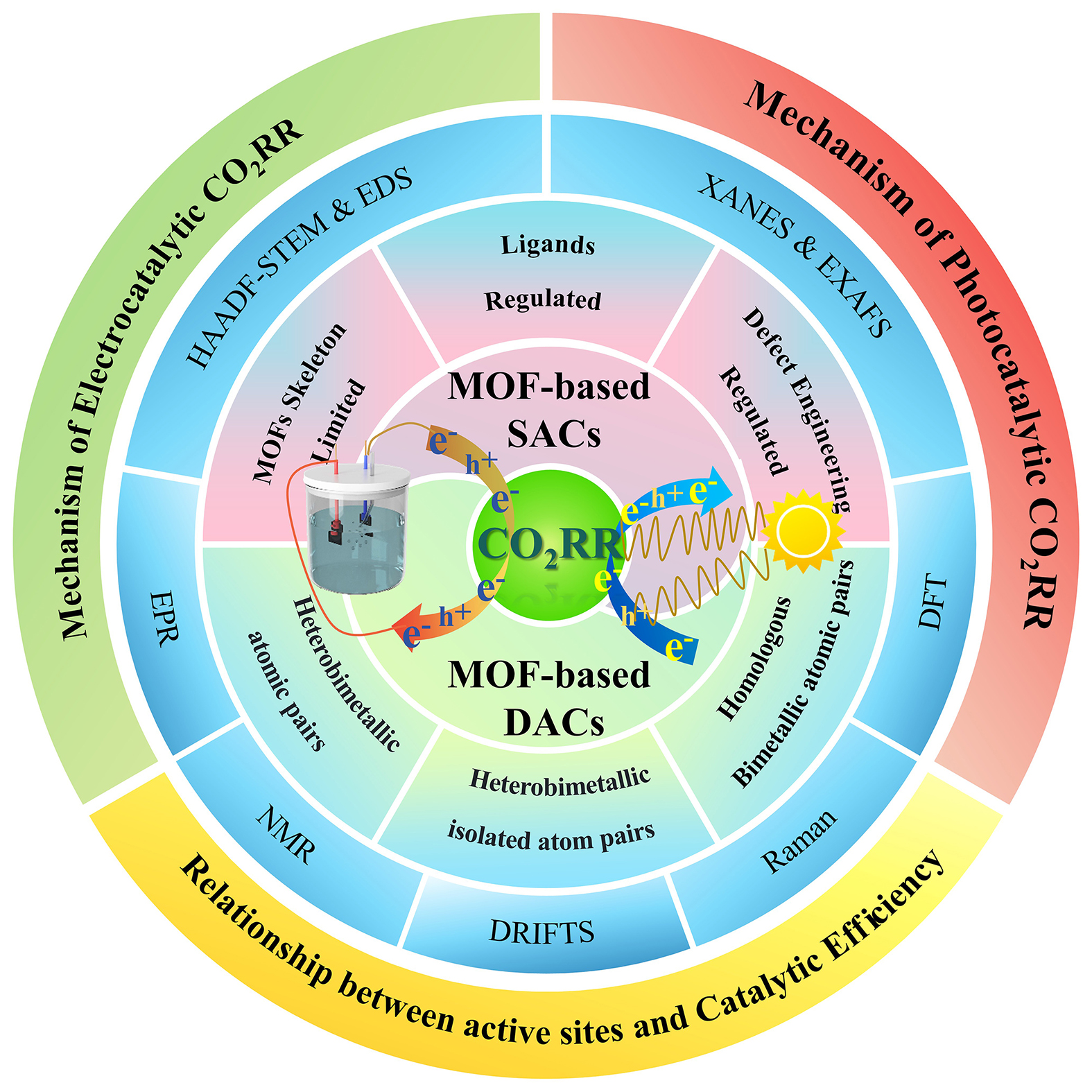
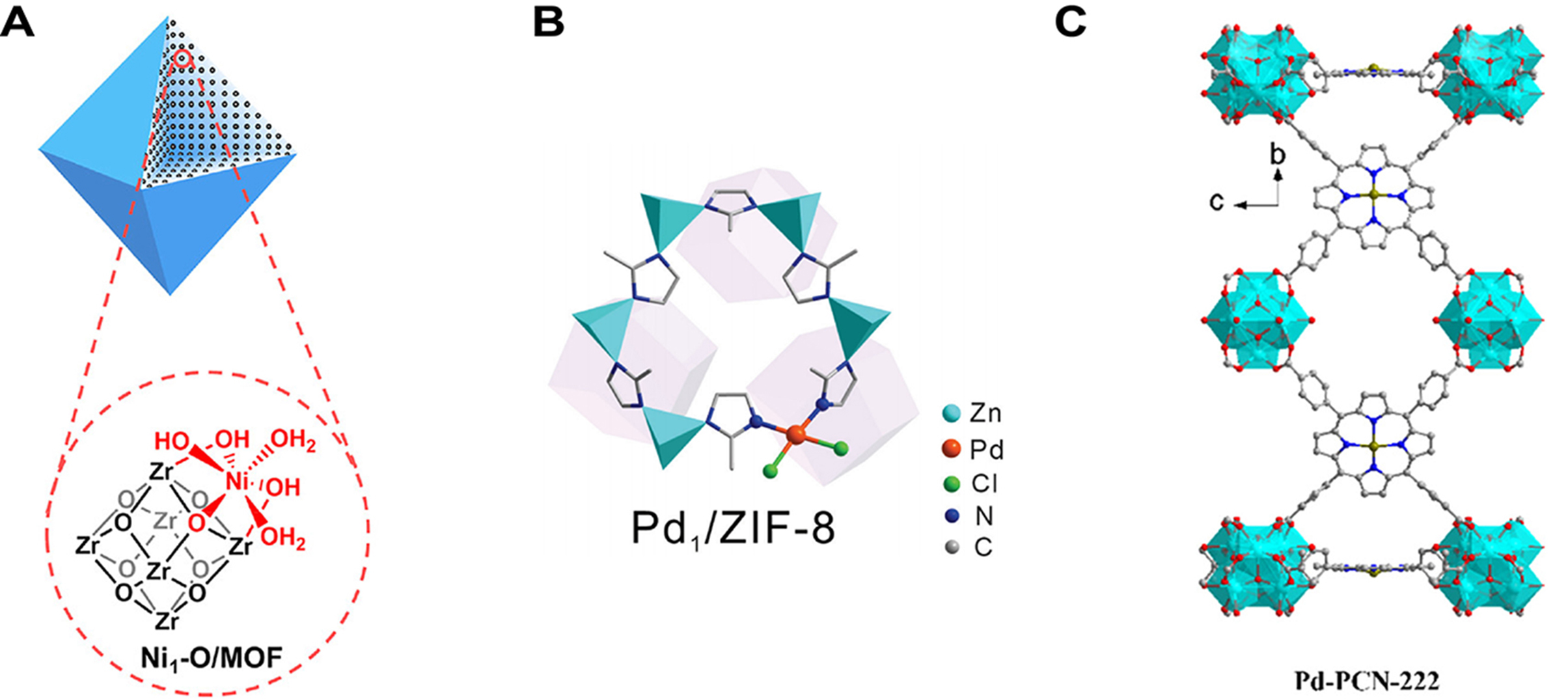
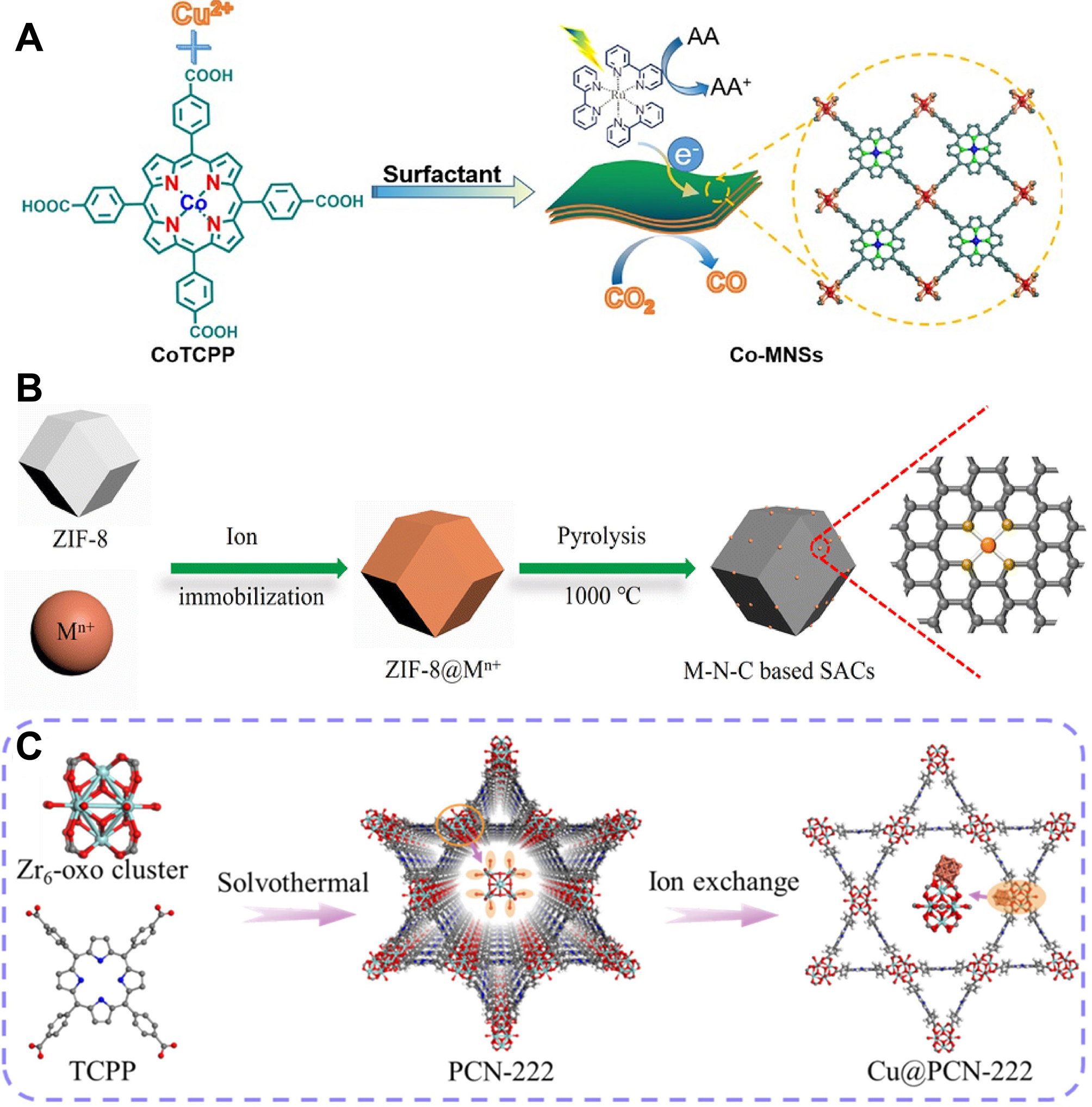
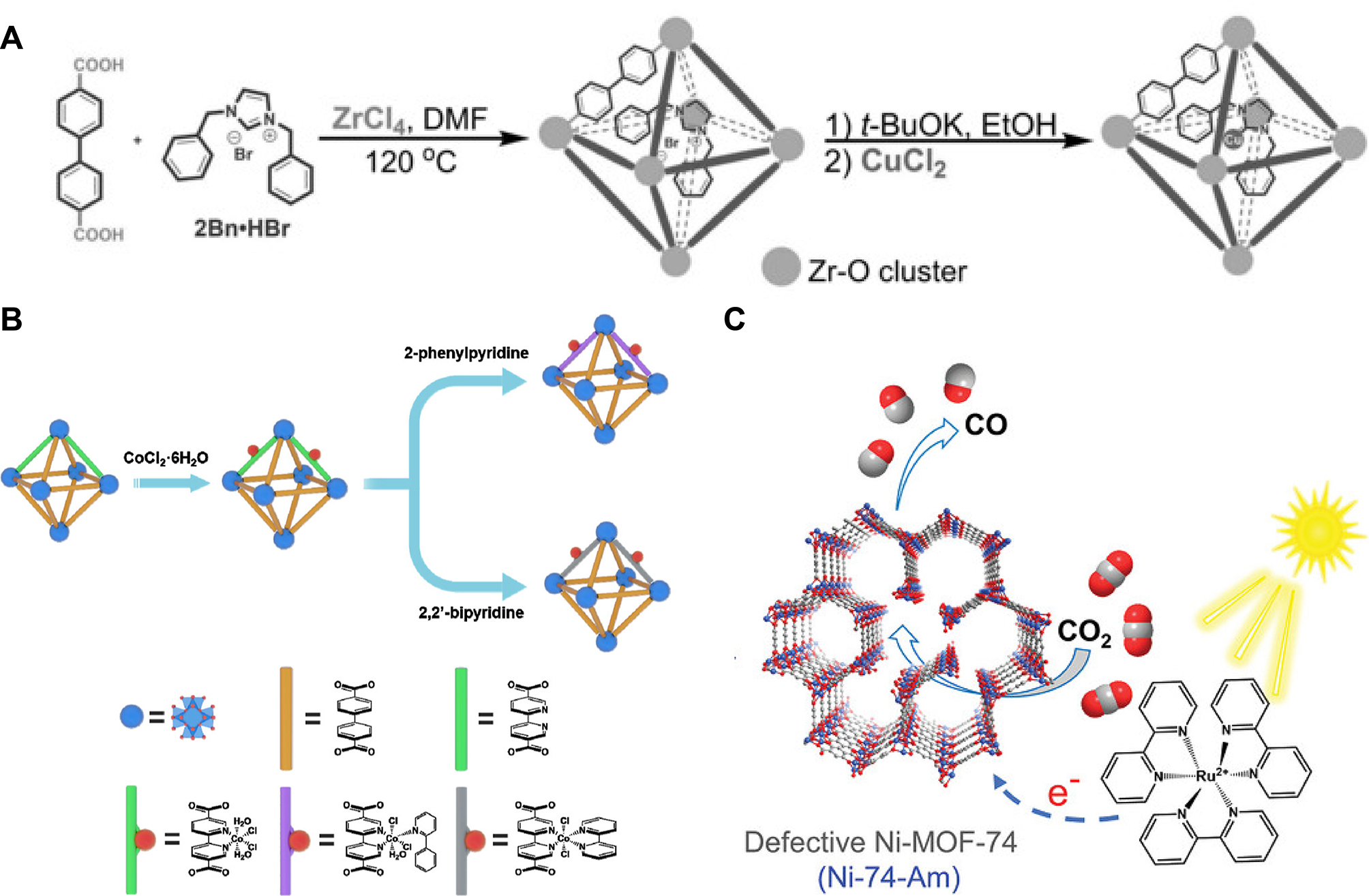
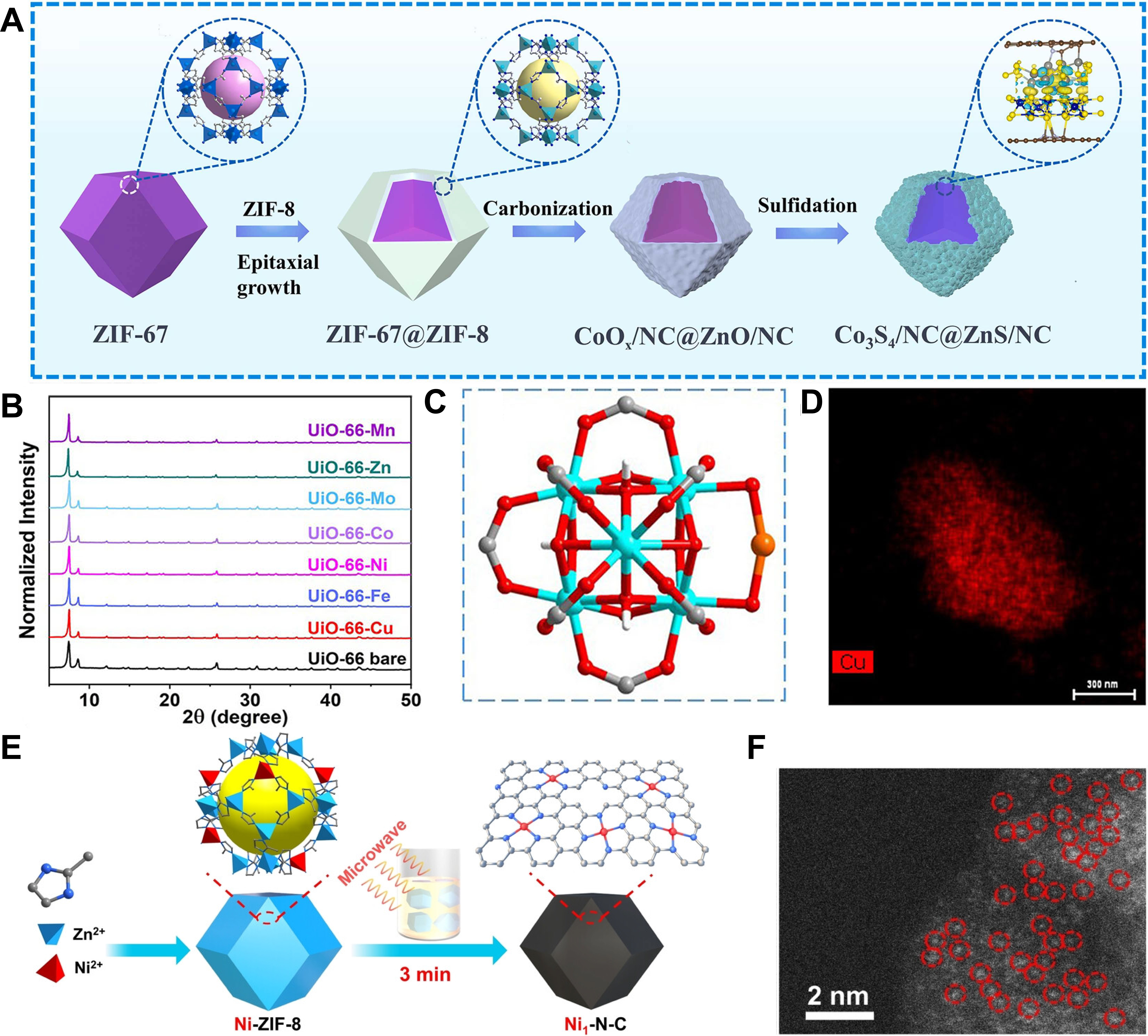
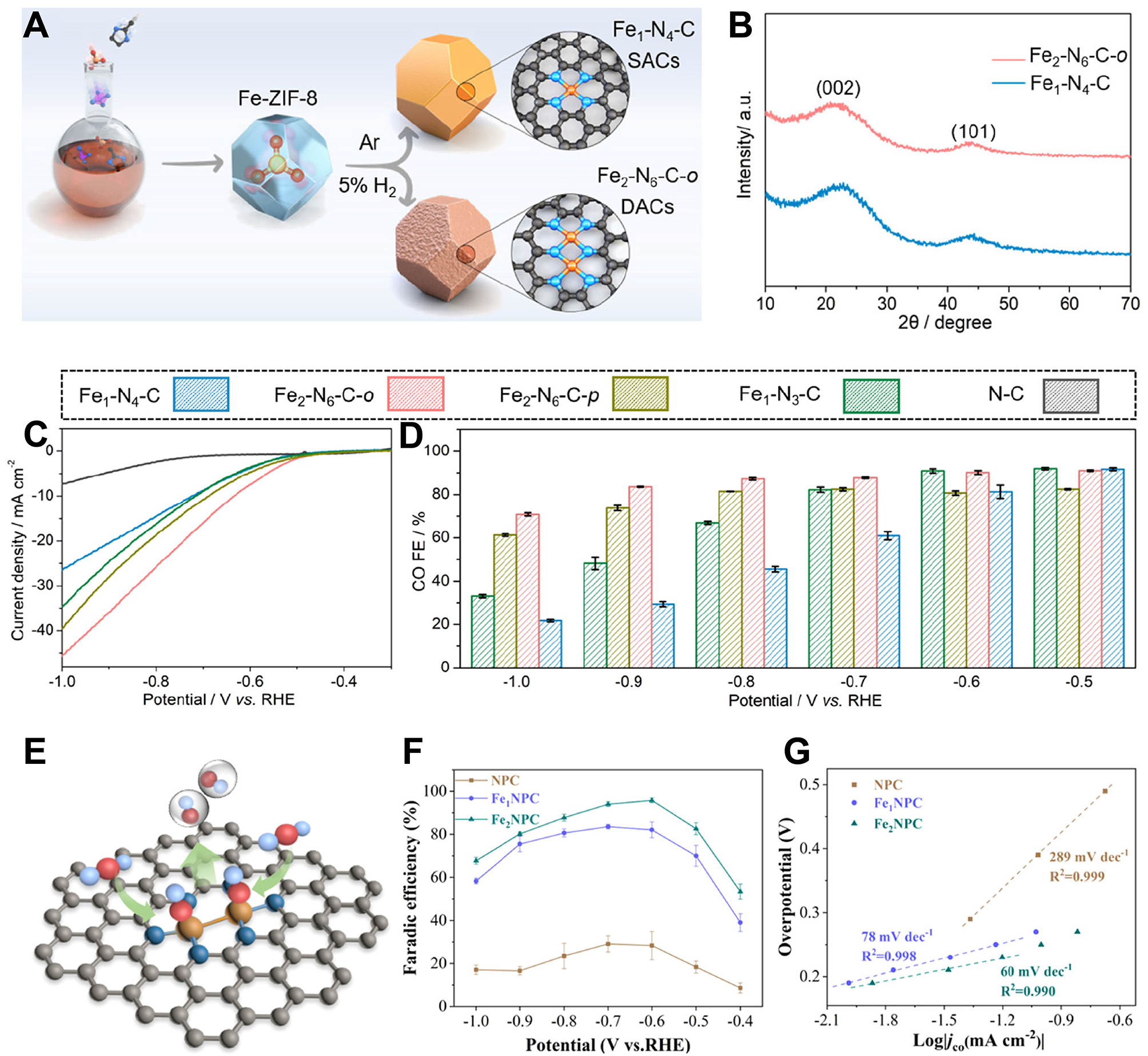
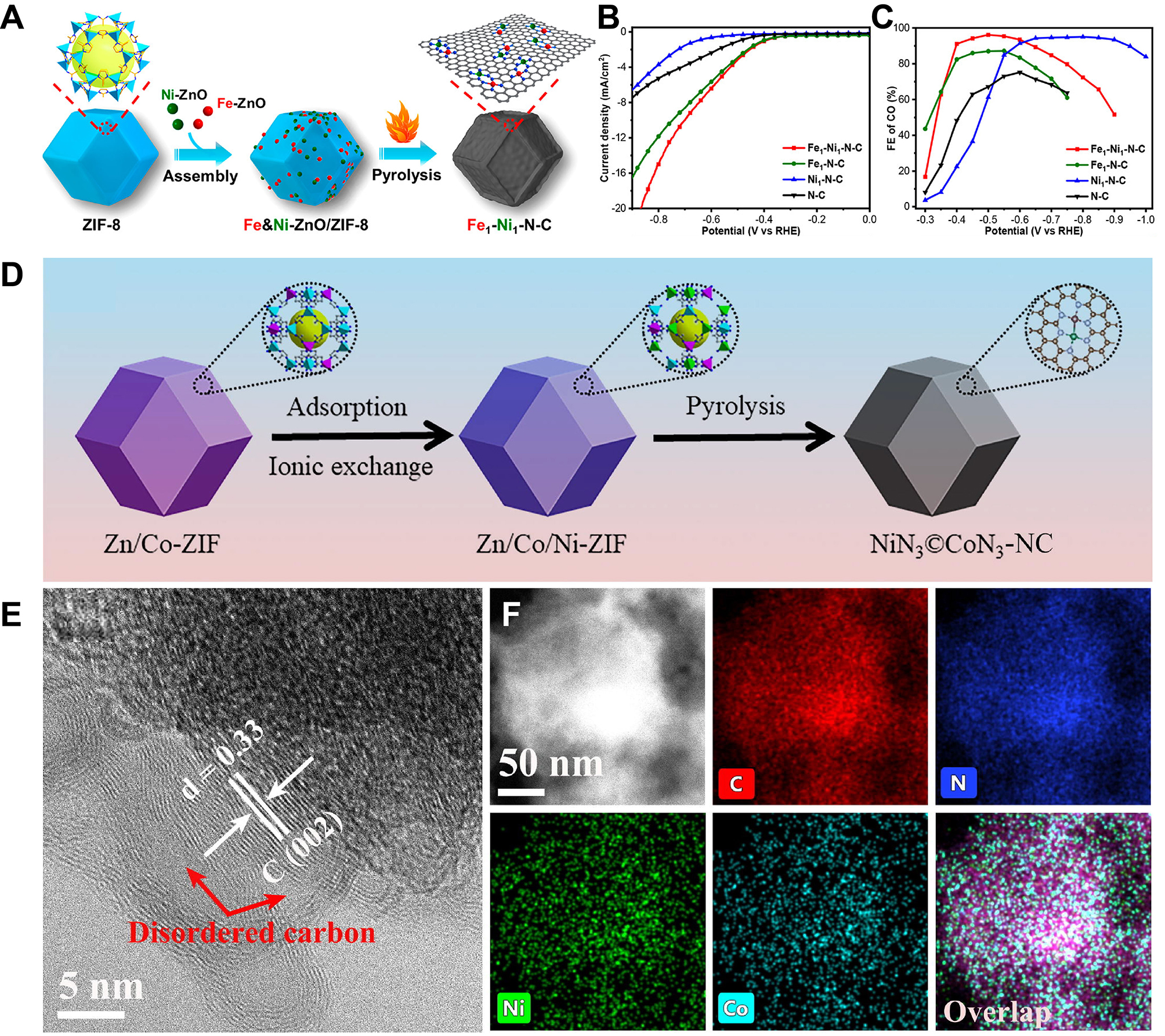
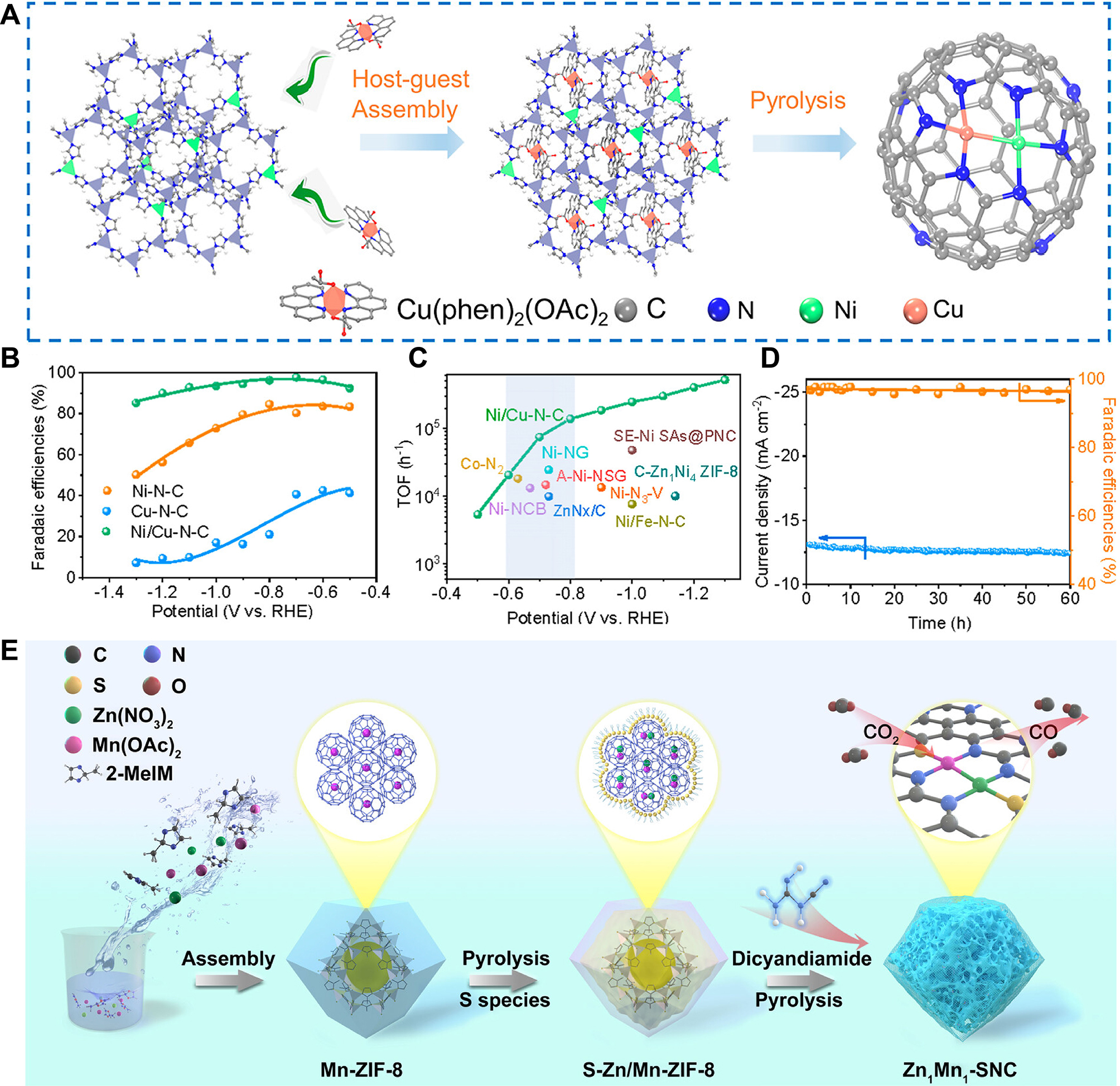

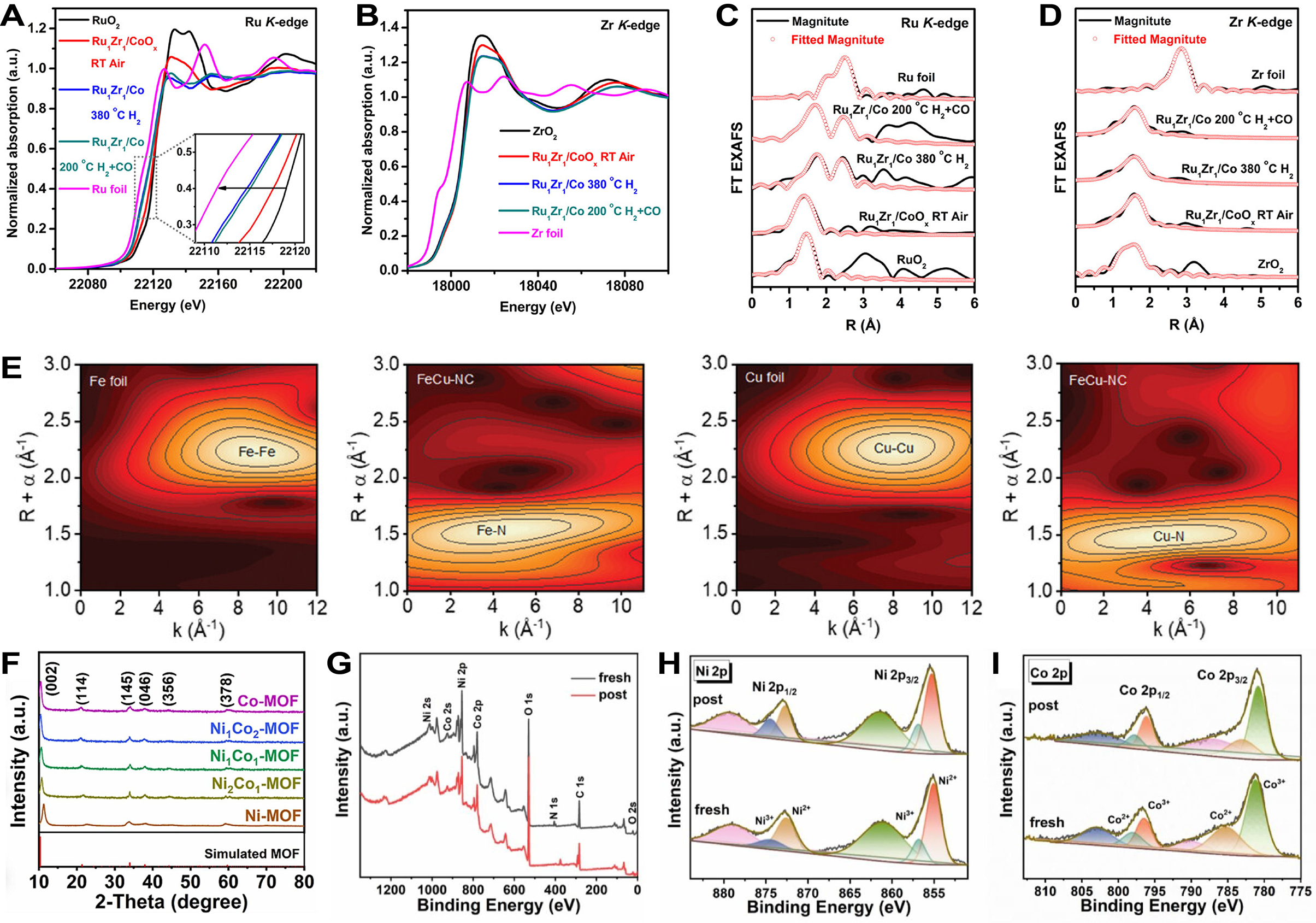
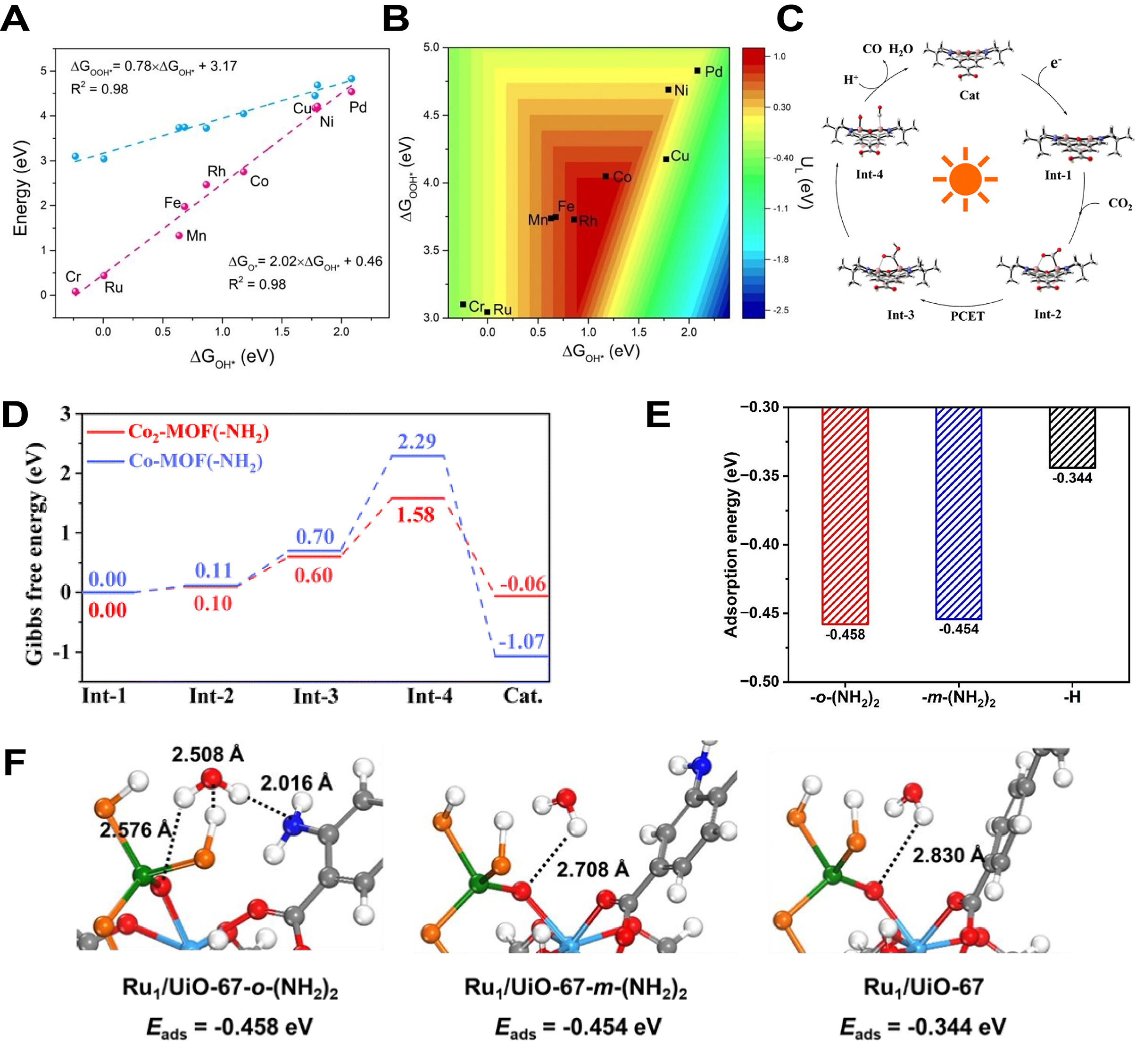
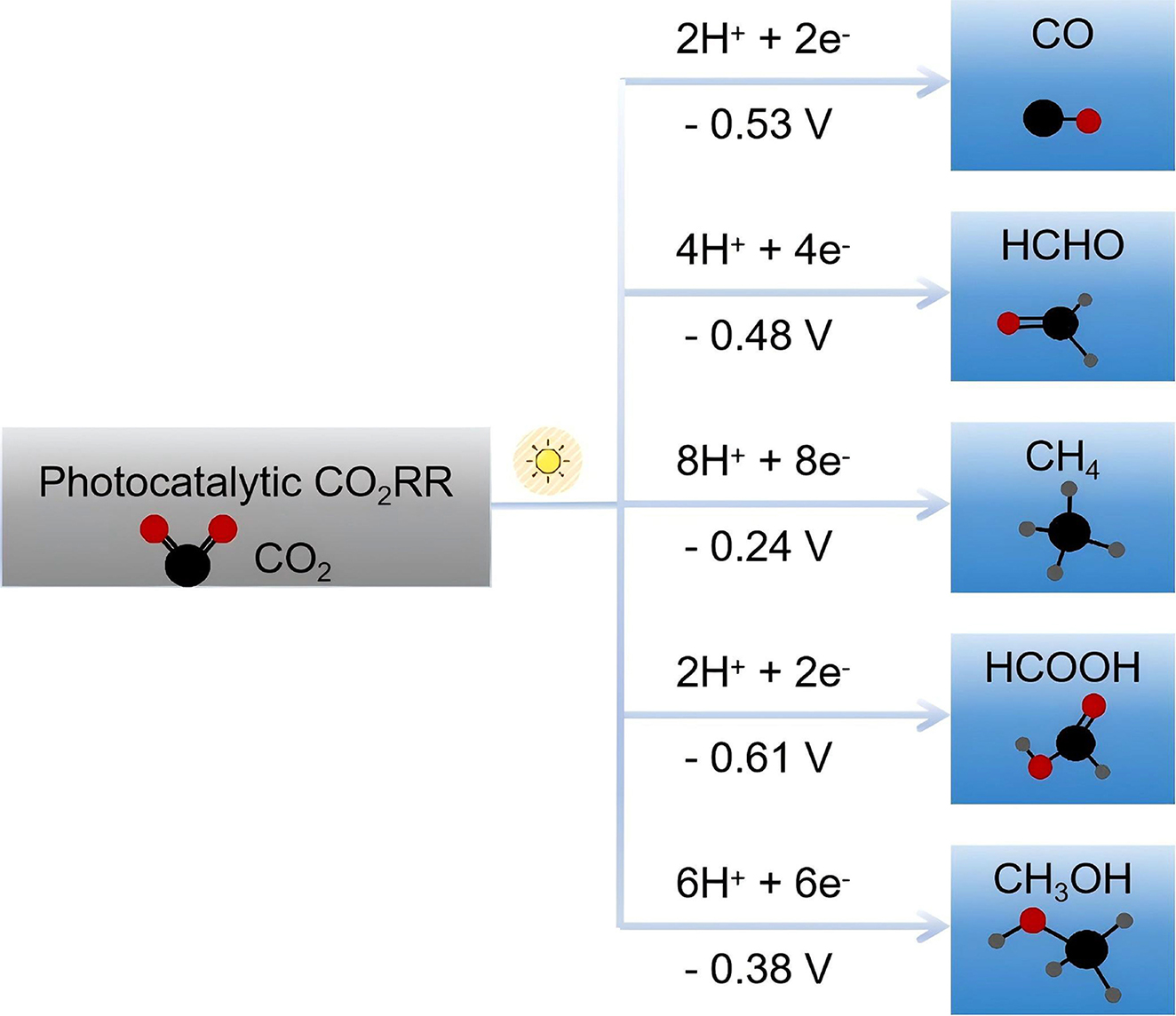
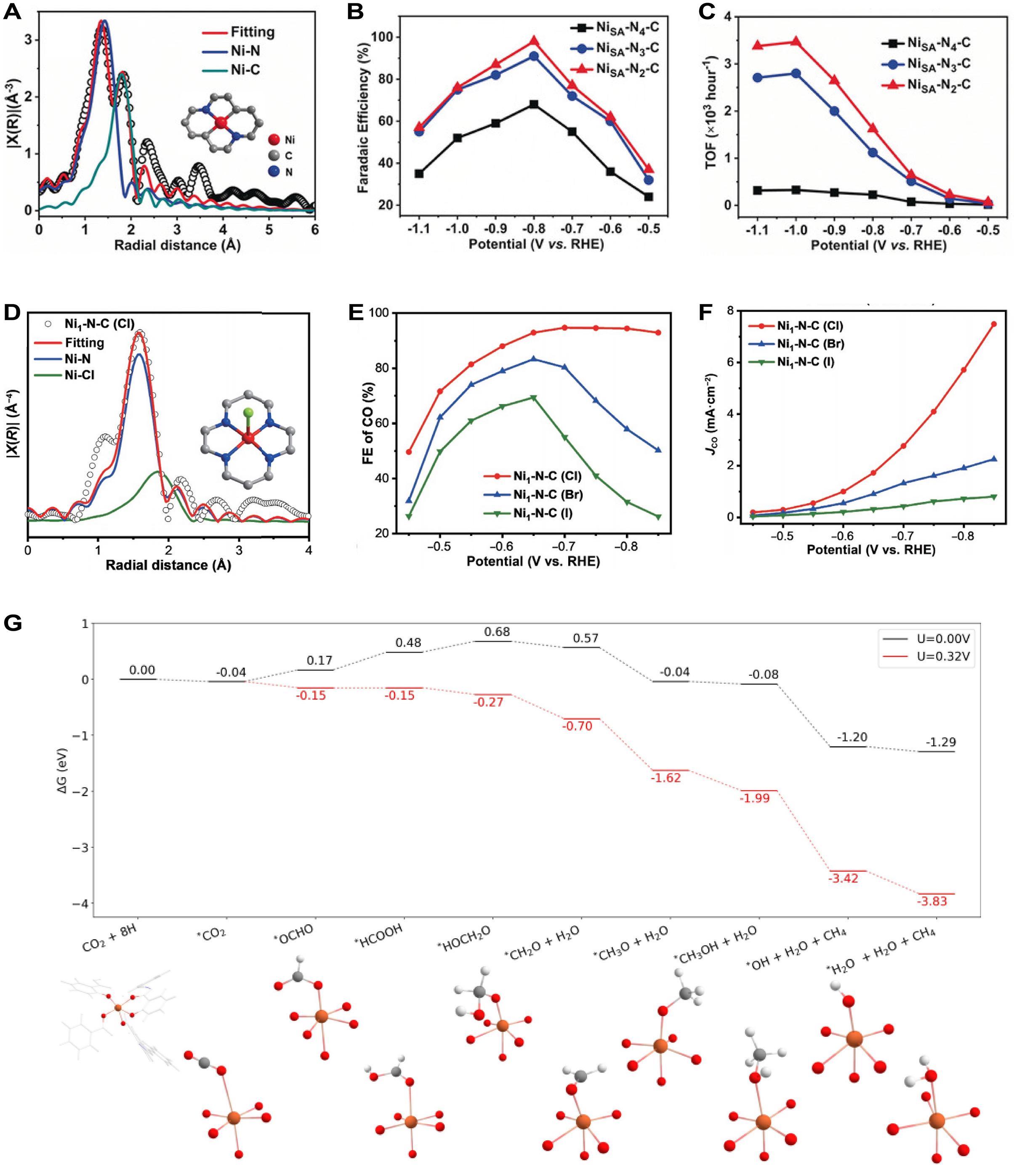
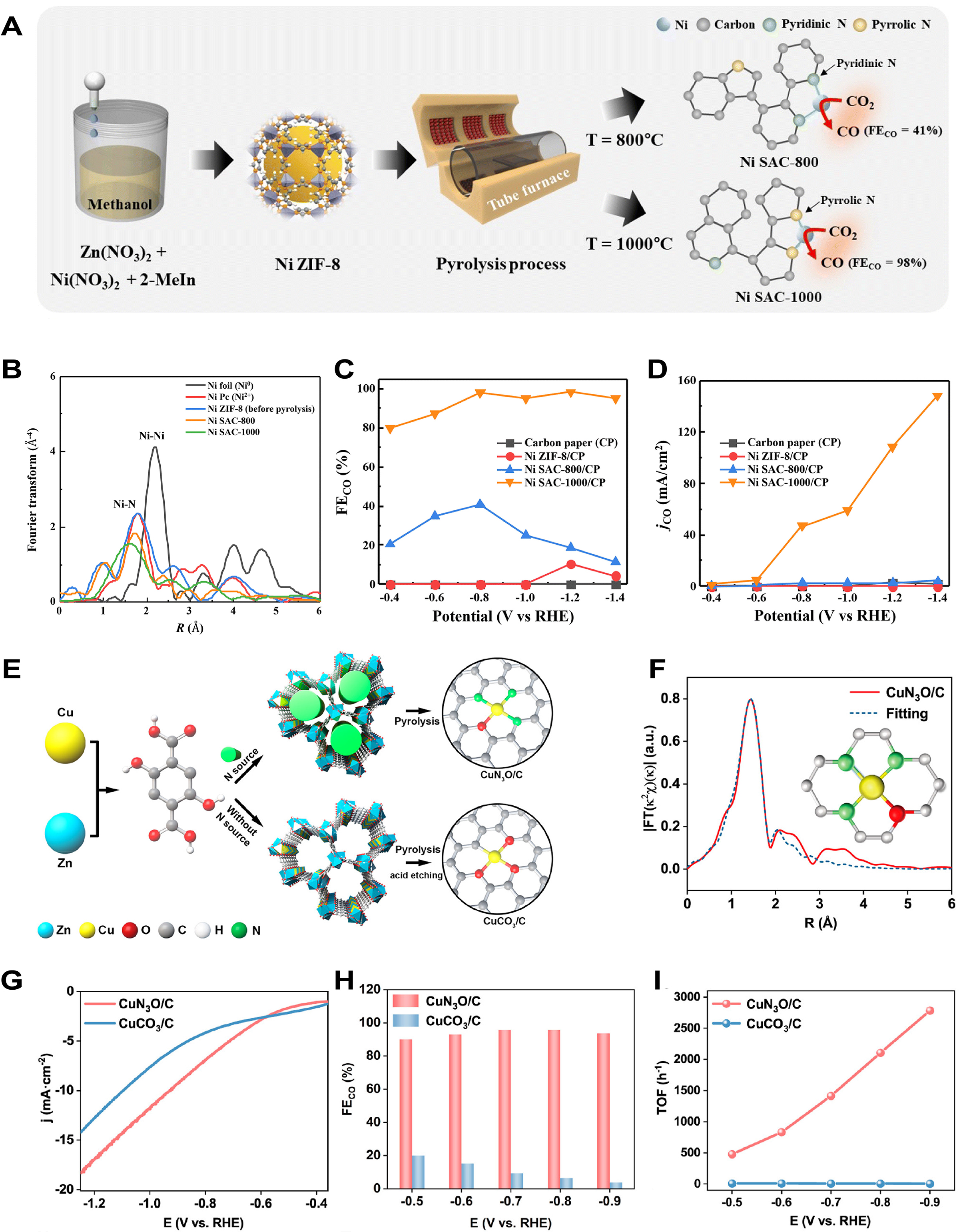
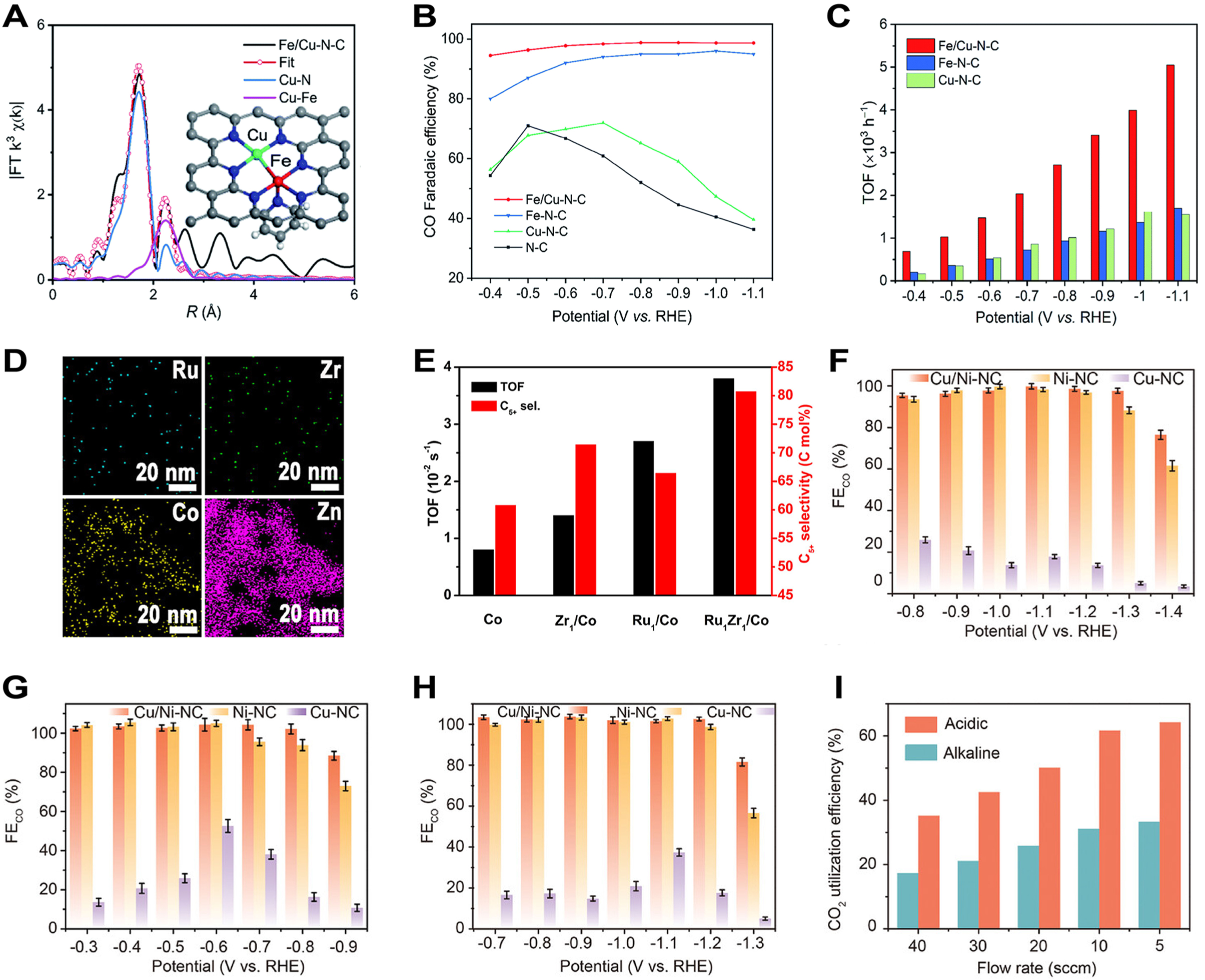
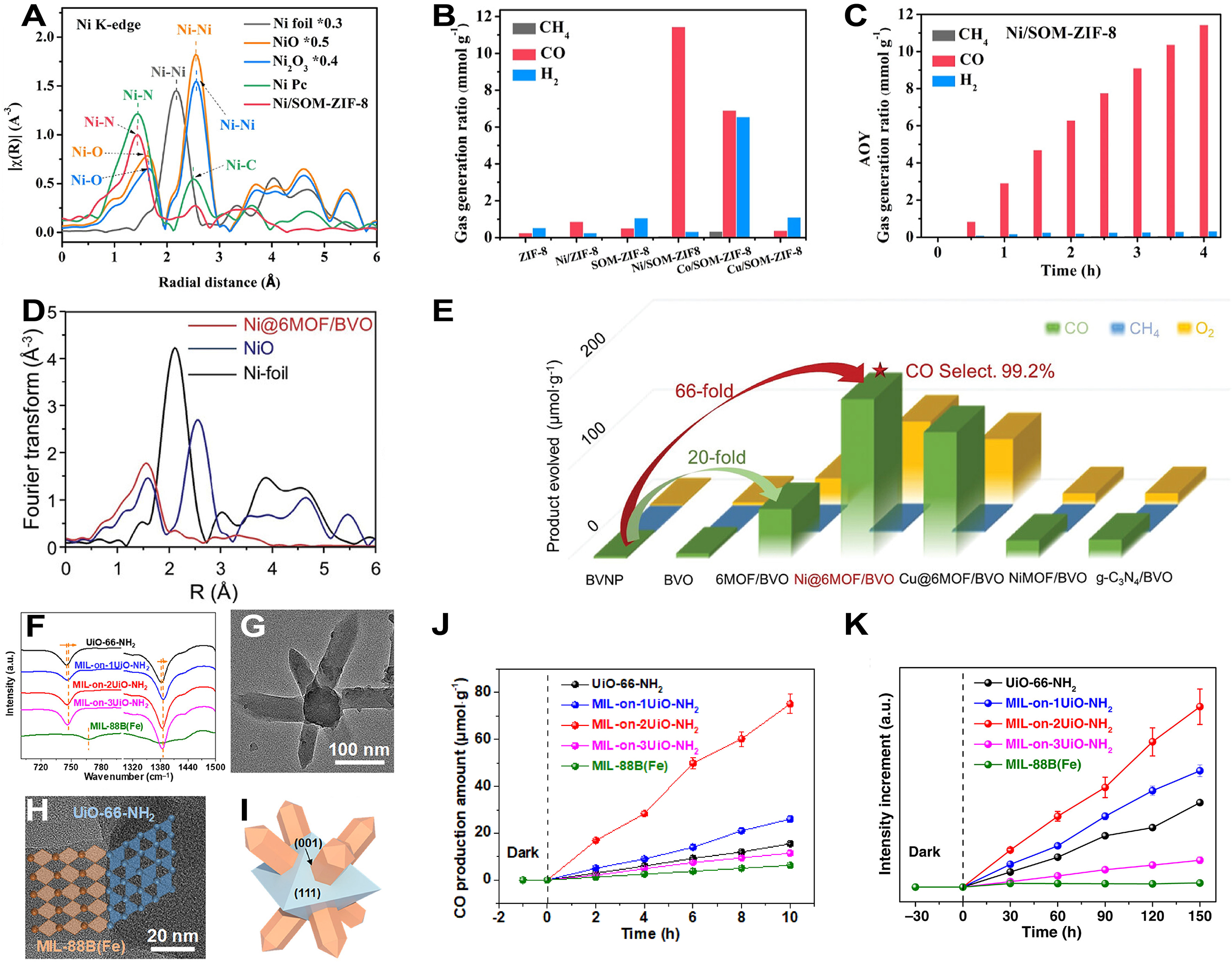
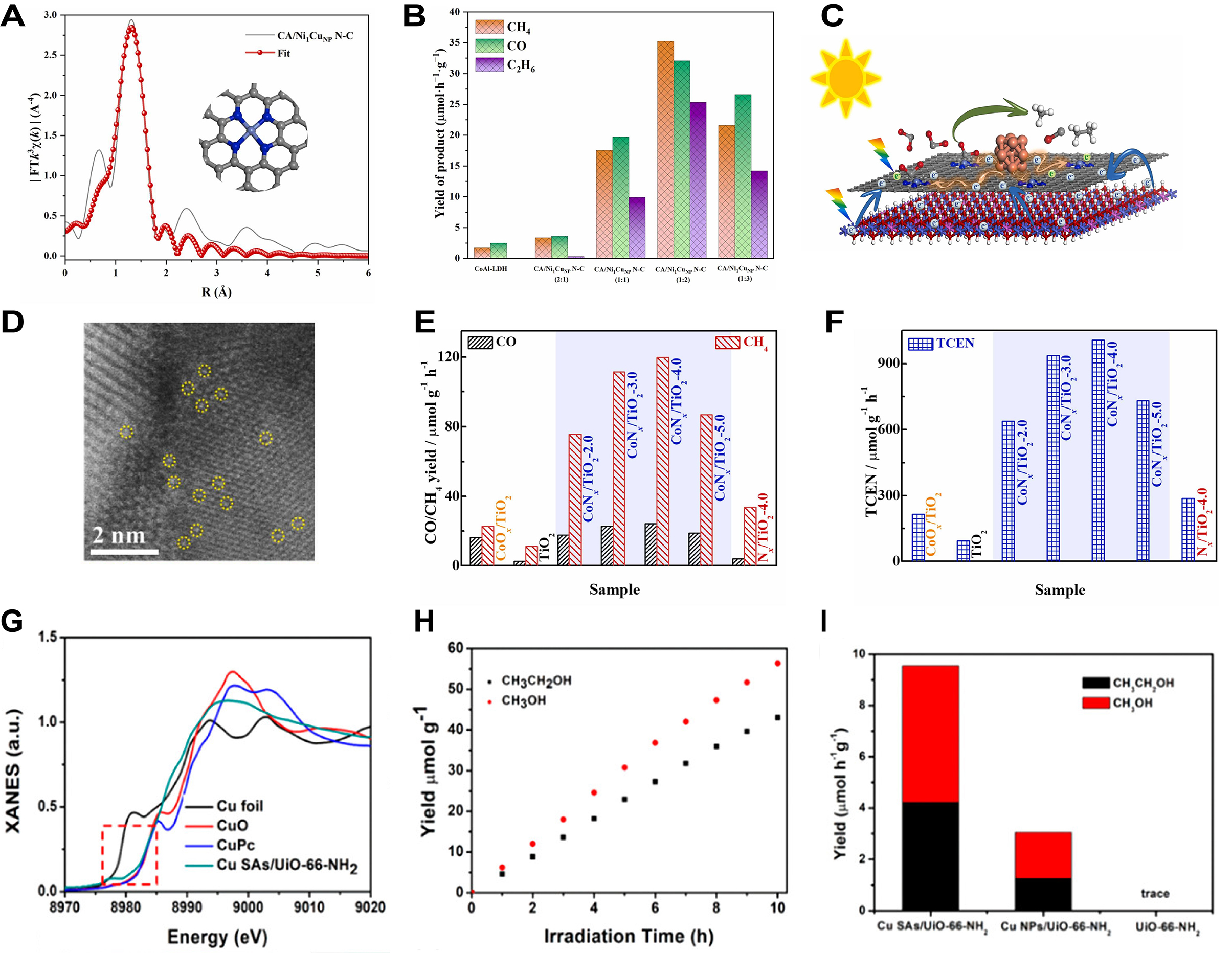
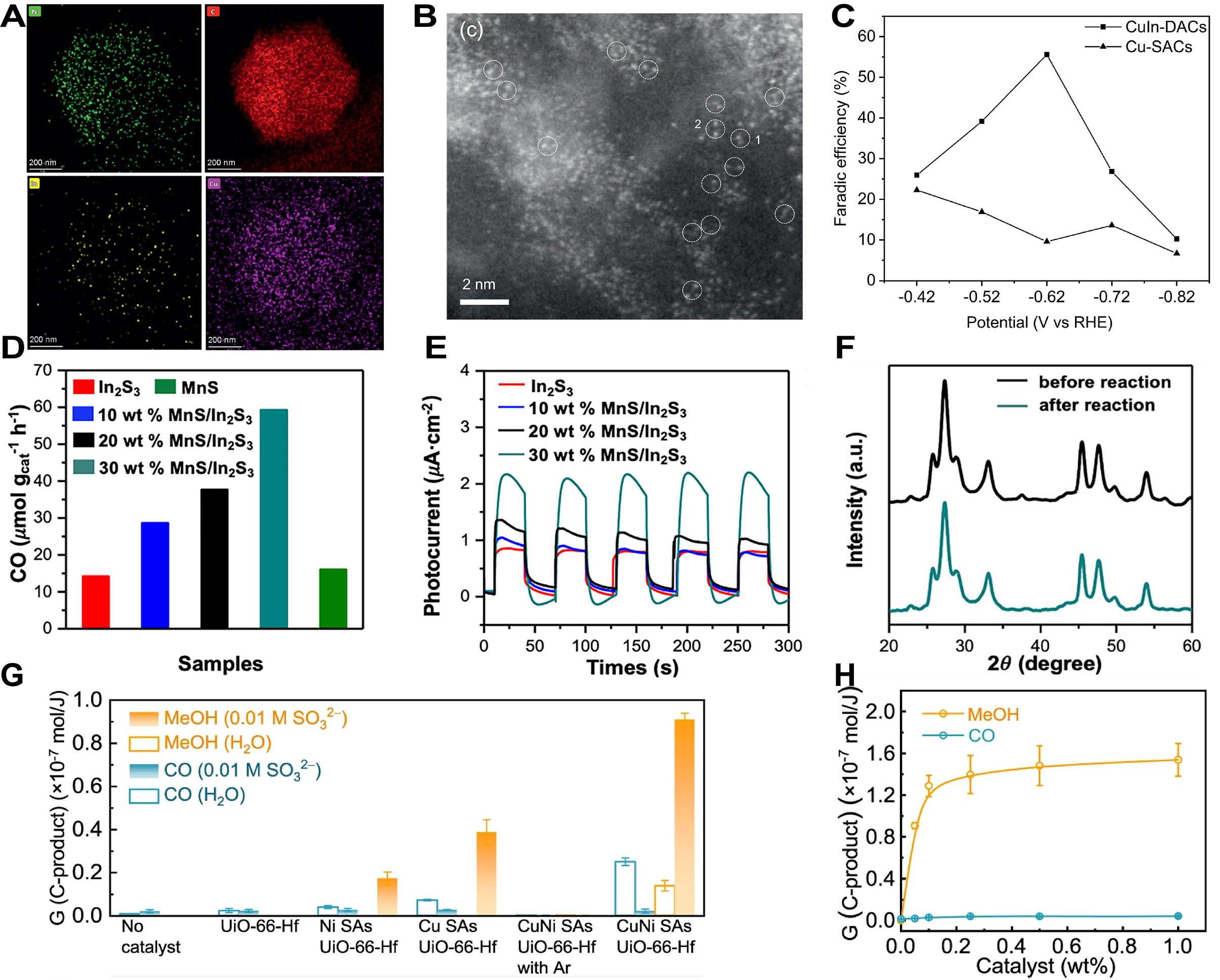












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].