Recent progress on tyrosine kinase inhibitors resistance in renal cell carcinoma: another brick in the wall?
Abstract
Renal cell carcinoma (RCC), the predominant form of kidney cancer, accounts for 90% of cases and poses significant clinical challenges due to frequent late-stage or metastatic presentation. Based on literature and surveillance data from 2020 to 2025, despite therapeutic advancements, metastatic RCC still exhibits a dismal 5-year survival rate. While tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor/platelet-derived growth factor pathways have been a cornerstone of RCC treatment, their efficacy is limited by acquired resistance, necessitating novel strategies to improve patient outcomes. This review synthesizes advancements from 2020 to 2025 in understanding and overcoming TKI resistance in RCC. We explored emerging mechanisms of resistance, including tumor microenvironment remodeling, metabolic reprogramming, and activation of alternative survival pathways. Furthermore, we evaluated innovative therapeutic approaches. By consolidating recent insights, this review highlights promising strategies to circumvent resistance and underscores the importance of personalized, mechanism-driven therapies. Our analysis aims to inform future research directions and clinical translation, ultimately advancing the management of TKI-resistant RCC.
Keywords
INTRODUCTION
According to the American Cancer Society, an estimated 80,980 new cases and 14,510 deaths from kidney cancer were expected in the United States in 2025[1]. Worldwide, kidney cancer represented the 14th most frequently diagnosed malignancy, with more than 400,000 new cases in 2020[2,3]. Among all subtypes, renal cell carcinoma (RCC) is the predominant form, accounting for approximately 90% of cases[4]. RCC is often asymptomatic in its early stages[5]; however, approximately 30%-60% of patients present with metastatic disease at initial diagnosis according to different studies[6,7]. Moreover, almost 20% of patients with complete surgical removal of the primary tumor will develop metastatic disease[8]. The prognosis of metastatic RCC (mRCC) remains poor despite advancements in targeted therapy and immunotherapy, with a 5-year survival rate of approximately 17%[9]. The most common histological subtype of RCC, clear cell RCC (ccRCC), comprises about 70%-80% of cases and is characterized by frequent genetic alterations in the von Hippel-Lindau (VHL) tumor suppressor gene, leading to dysregulated hypoxia-inducible factor (HIF) signaling and angiogenesis[10,11]. These characteristics provide potential therapeutic targets for RCC.
The treatment landscape for advanced and mRCC has evolved significantly over the past decade, shifting from monotherapy with tyrosine kinase inhibitors (TKIs) to combination regimens incorporating immune checkpoint inhibitors (ICIs)[12]. Currently, the standard first-line therapies for advanced RCC primarily include TKI-based regimens, ICI-based combinations, or a combination of both[13]. TKIs, such as sunitinib, pazopanib, cabozantinib, lenvatinib, and axitinib, target vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), and other tyrosine kinases involved in angiogenesis and tumor progression[14]. While TKIs remain the mainstay of RCC treatment, their efficacy is inevitably limited by the development of acquired resistance after modest benefits in terms of disease-free progression[15]. As a result, TKI resistance remains a major challenge for the survival of RCC patients.
In this review, we provide a comprehensive update on the latest advancements in overcoming acquired resistance to TKIs in RCC over the past five years (2020-2025). We summarize recent insights into the underlying mechanisms driving resistance, including the evolving understanding of tumor microenvironmental adaptations, metabolic reprogramming, and alternative survival pathways. More importantly, we highlighted the diverse strategies researchers have explored in recent years to counteract TKI resistance, ranging from novel drug combinations to emerging therapeutic approaches that targeted previously unrecognized vulnerabilities in RCC. By consolidating these findings, we aimed to provide a valuable resource for guiding future research in the fight against TKI resistance in RCC.
TYPES OF TKIS IN THE TREATMENT OF RCC AND THEIR MECHANISM
Although different TKIs share common targets, their resistance mechanisms can vary significantly. Marona et al. (2022) demonstrated that while both sunitinib- and sorafenib-resistant RCC cells activated cellular-mesenchymal epithelial transition factor (c-MET) and interleukin-1 receptor-associated kinase 1 (IRAK1), their downstream effects diverged: sunitinib resistance led to E-cadherin expression, IL-6/IL-8 secretion, tumor senescence, and angiogenesis, whereas sorafenib resistance promoted mesenchymal traits, MMP9 secretion, and endothelial disruption, enhancing invasion[16]. These findings highlighted the heterogeneity of TKI resistance and suggested that mesenchymal epithelial transition factor (MET)-targeting agents such as cabozantinib could serve as late-line therapies to counteract bypass activation. Thus, the targets of different drugs should be outlined. Here, we summarized the mechanisms of action of all TKI agents recommended for the treatment of mRCC based on the latest clinical guidelines [Table 1 and Figure 1][12,13].
Figure 1. TKIs used in the treatment of RCC and their mechanisms. Created in BioRender. Dong, Z. (2025) https://BioRender.com/34g6akl. TKIs: Tyrosine kinase inhibitors; RCC: renal cell carcinoma.
TKIs used in the treatment of RCC and their targets (A: active; U: unknown)
| Drug name | Target(s) | Ref. |
| Axitinib | VEGFR1(A), VEGFR2(A), VEGFR3(A), c-Abl(U) | [17,18] |
| Cabozantinib | VEGFR1(A), VEGFR2(A), VEGFR3(A), c-Kit(A), c-Met(A), c-Ret(A), TrkB(A), FLT3(A), TYRO3(A), c-Mer(A), TIE2(A) | [19,20] |
| Lenvatinib | VEGFR1(A), VEGFR2(A), VEGFR3(A), FGFR1(A), FGFR2(A), FGFR3(A), FGFR4(A), PDGFRα(A), c-Ret(A), c-Kit(A) | [21] |
| Pazopanib | VEGFR1(A), VEGFR2(A), VEGFR3(A), FGFR3(U), PDGFRα(A), PDGFRβ(A), c-Kit(A), FGFR1(U), SH2B3(U) | [22,23] |
| Sunitinib | VEGFR1(A), VEGFR2(A), VEGFR3(A), PDGFRα(A), PDGFRβ(A), FLT3(A), c-Met(U), c-Kit(A), CSF1R(A) | [18,24] |
THE MECHANISMS OF TKI RESISTANCE IN RCC: AN UPDATE (PRECLINICAL LEVEL: IN VITRO OR IN VIVO)
Many previous reviews have thoroughly summarized the mechanisms of TKI resistance in RCC, especially regarding sunitinib. Building on this foundation, we focused on studies from 2020-2025 that not only deepen our understanding of established resistance pathways but also introduce emerging mechanisms. Given the multifactorial nature of acquired resistance in RCC, some studies spanned multiple pathways; for clarity, we have categorized them according to their primary mechanism.
Bypass activation
Bypass activation is a crucial mechanism underlying acquired resistance to TKIs in RCC[25]. While TKIs primarily target VEGF/VEGFR signaling to inhibit tumor angiogenesis, prolonged drug exposure can lead to the activation of alternative pro-survival signaling cascades that allow tumor cells to evade VEGF inhibition. These compensatory pathways often involve receptor tyrosine kinases (RTKs) such as MET[26], AXL[27], and IGF-1R[28], which can sustain tumor growth and angiogenesis independently of VEGF signaling. Understanding these bypass mechanisms is essential for developing rational combination therapies to overcome resistance and improve patient outcomes. Below, we summarize and compile studies from 2020-2025 on bypass activation leading to reduced sensitivity to TKIs in RCC [Figure 2].
Figure 2. Recent progress of Bypass activation in TKI-resistant RCC. Created in BioRender. Dong, Z. (2025) https://BioRender.com/px2fkfx. TKI: Tyrosine kinase inhibitor; RCC: renal cell carcinoma.
Beyond MET signaling mentioned earlier[16], other bypass pathways have been implicated in TKI resistance. Fukumoto et al. (2023) identified SCG2 as a novel mediator of sunitinib resistance, as it interacted with HIF-1α to activate the VHL/HIF/VEGF axis, thereby sustaining angiogenesis despite VEGFR inhibition[29]. Similarly, He et al. (2023) discovered that SIRT7 deacetylated CHD1L, stabilizing CHD1L protein levels by attenuating its ubiquitination levels. Accumulated CHD1L amplified HIF-2α signaling by interacting with HIF-2α, resulting in sunitinib resistance[30].
The PI3K/AKT pathway has emerged as a central player in TKI resistance for a while[31]. New findings on the AKT pathway include: Xiong et al. (2021) reported that RRM2 stabilized ANXA1 to activate AKT signaling[32], while Liu et al. (2024) further demonstrated that IKBKE phosphorylates RRM2, leading to sustained AKT activation in sunitinib-resistant (SR) RCC cells[33]. These findings underscore the significance of targeting PI3K/AKT signaling as a potential strategy to overcome TKI resistance.
Epithelial-to-mesenchymal transition (EMT) has also been identified as a crucial contributor to bypass activation[34]. Bouchalova et al. (2021) conducted proteomic analyses comparing TKI responders and non-responders, revealing that EMT was one of the most prominent pathways distinguishing resistant tumors[35]. This aligned with the observed increase in mesenchymal markers in sorafenib-resistant cells, further supporting the role of EMT in mediating escape from antiangiogenic therapy.
Beyond examining individual signaling pathways, a more comprehensive systems-level analysis was conducted by Xie et al. (2021), who established a SR cell-derived xenograft (CDX) model and identified enrichment of multiple resistance-associated pathways, including the aforementioned PI3K-AKT, HIF-1, NF-κB, and MAPK signaling[36]. The involvement of these pathways suggested a complex adaptive response in RCC that extended beyond VEGF signaling. Further supporting this notion, Stokes et al. (2023) demonstrated that treatment with multiple VEGFR-TKIs, including axitinib, cabozantinib, lenvatinib, and sunitinib, could activate PERK in 786-O RCC xenografts, implicating the unfolded protein response (UPR) as another potential mechanism of resistance[37].
Together, these studies highlighted the intricate network of bypass mechanisms that sustained RCC progression despite VEGFR inhibition. The convergence of multiple signaling pathways - ranging from MET, PI3K/AKT to PERK and EMT - underscored the need for rational combination therapies targeting these alternative routes to effectively combat TKI resistance in RCC.
RNAs (long non-coding RNA, circular RNA)
Circular RNAs (circRNAs) have emerged as key regulators of TKI resistance in RCC, influencing post-transcriptional gene regulation, metabolic reprogramming, and tumor microenvironment (TME) adaptation[38]. These non-coding RNAs function primarily by acting as molecular sponges for microRNAs (miRNAs) or interacting with RNA-binding proteins (RBPs), ultimately modulating critical signaling pathways that drive drug resistance[39].
One of the interesting mechanistic insights into circRNA-mediated TKI resistance came from Huang et al. (2021), who identified circSNX6 as a molecular sponge for miR-1184. By sequestering miR-1184, circSNX6 alleviated its suppressive effect on GPCPD1, leading to an increase in intracellular lysophosphatidic acid (LPA) levels and enhanced sunitinib resistance[40]. In 2023, our study revealed that circPTPN12 promoted sunitinib resistance through the hnRNPM/IL-6/STAT3 signaling axis. Specifically, circPTPN12 enhanced hnRNPM binding to IL-6 mRNA, increasing its stability and sustaining STAT3 pathway activation, thereby highlighting the contribution of inflammatory cytokine signaling to TKI resistance[41]. In a 2023 study from our lab, circRARS was shown to bind the KH1-KH2 domains of IGF2BP3, enhancing its recognition of m6A-modified transcripts. This interaction promoted lipid accumulation in RCC cells and contributed to sunitinib resistance through downstream targets, underscoring the role of metabolic adaptation in therapeutic evasion[42].
Long non-coding RNAs (lncRNAs) have recently been identified as critical regulators in the development of resistance to TKIs in RCC[43]. These lncRNAs modulate various cellular processes such as autophagy, protein stability, and oxidative stress, contributing to TKI resistance by interacting with RBPs, key transcription factors, and cellular pathways[44].
In 2023, Pan et al. uncovered that IGFL2-AS1, a lncRNA, enhanced the expression of TP53INP2, which promoted autophagy, leading to sunitinib resistance[45]. In a separate study conducted by our lab in 2020, we identified lncRNA SNHG12 as a key player in sunitinib resistance. SNHG12 binds to SP1, preventing its ubiquitylation-dependent degradation. This stabilization of SP1 led to increased expression of CDCA3, a gene that played a key role in the cell cycle, and promoted resistance to sunitinib. The interaction between SNHG12 and SP1 underlined the importance of transcription factor regulation in mediating resistance in RCC[46]. Further investigation into the roles of lncRNAs in RCC TKI resistance revealed that lncRNA SNHG1 binds to PTBP1 and regulates the ATG7 axis, a crucial player in autophagy. This interaction not only enhanced autophagic flux but also contributed to RCC resistance to sunitinib by sustaining cellular survival under therapeutic pressure, as identified by Tian et al. in 2024[47]. In addition to autophagy, oxidative stress and ferroptosis have also been shown to be modulated by lncRNAs in RCC[43,44]. Pan et al. (2025) discovered that STX17-DT, a lncRNA upregulated in axitinib-resistant RCC cells, interacted with hnRNPA1, stabilizing IFI6 mRNA. This interaction led to increased ROS levels and suppression of ferroptosis, further contributing to drug resistance[48]. The study suggested that lncRNA-mediated modulation of oxidative stress pathways might represent a therapeutic target to overcome TKI resistance. Moreover, He et al. (2022) discovered that sunitinib could increase the expression of lncRNA-ECVSR, enhancing ERβmRNA and transcriptionally upregulating HIF-2α[49].
These studies collectively emphasized the growing importance of circRNAs and lncRNAs as pivotal regulators of TKI resistance in RCC. By influencing key cellular processes, circRNAs and lncRNAs represented promising targets for novel therapeutic strategies aimed at overcoming resistance and improving the efficacy of TKI therapies in RCC [Figure 3].
Figure 3. Recent updates on RNAs’ function in RCC TKI resistance. Created in BioRender. Dong, Z. (2025) https://BioRender.com/qmr14qn. RCC: Renal cell carcinoma; TKI: tyrosine kinase inhibitor.
Lysosome
Lysosomal pumping/sequestration has recently emerged as a key mechanism contributing to resistance against TKIs in RCC[50]. This process involves the enhanced activity of lysosomes, which actively pump therapeutic agents out of the cytoplasm, thereby reducing drug efficacy.
In 2021, Li et al. demonstrated that long-term exposure to sunitinib induced lysosomal biosynthesis and exocytosis, thereby promoting sunitinib resistance. Under sunitinib treatment, TFE3, a transcription factor, continuously translocated into the nucleus, where it drove the expression of E-Syt1, an endoplasmic reticulum (ER) protein. E-Syt1 induced fragmentation of the ER, which in turn promoted lysosomal exocytosis. This process facilitated the active export of sunitinib from the cytoplasm, effectively lowering its intracellular concentration and reducing its therapeutic effects on RCC cells[51]. This study underscored the significant role of lysosomal pumping in mediating resistance to sunitinib in RCC, highlighting the importance of lysosomal dynamics in the development of drug resistance.
Cell death (apoptosis, ferroptosis, cuproptosis, etc.)
Recent research has highlighted the critical role of various cell death pathways, including ferroptosis, apoptosis, and cuproptosis, in the development of resistance to TKIs in RCC.
Chen et al. (2024) integrated single-cell RNA sequencing (scRNA-seq) data from both pre-treatment and post-treatment surgical samples with SR ccRCC cell lines. They found that ferroptosis, a form of regulated cell death characterized by iron-dependent lipid peroxidation, played a key role in mediating resistance to sunitinib. Specifically, the inflammatory cytokine IL-6 was shown to reverse ferroptosis, thereby promoting RCC resistance to sunitinib[52]. This suggested that targeting ferroptosis could be a promising strategy to overcome TKI resistance in RCC. Additionally, our lab (2023) discovered that AIM2, a pattern recognition receptor, promoted sunitinib resistance by enhancing the phosphorylation and proteasomal degradation of FOXO3a, a transcription factor involved in ferroptosis. This process inhibited the transcriptional activation of ACSL4, a key regulator of ferroptosis, thereby reducing ferroptosis and conferring resistance to sunitinib in RCC cells[53]. In addition, the lncRNA STX17-DT mediates sunitinib resistance in RCC by regulating ferroptosis, highlighting its role in cell death−related mechanisms of TKI resistance[48]. Furthermore, Wu et al. (2023), through multi-omics analysis, identified PDHB, a critical gene involved in cuproptosis (copper-induced cell death), as a key factor in overcoming sunitinib resistance in RCC. Activation of PDHB promoted copper-induced cell death, providing insight into targeting cell death pathways to overcome TKI resistance[54]. Moreover, Zeng et al. (2024) identified the regulatory role of O-GlcNAcylation on RIPK1, a key protein in cell death regulation. The modification of RIPK1 by O-GlcNAcylation influenced the assembly of the RIPK1/FADD/Caspase8 complex and activated the NF-κB pathway, suppressing RIPK1-dependent apoptosis induced by sunitinib treatment[55]. This mechanism may contribute to the resistance of RCC cells to sunitinib by preventing apoptosis, a form of programmed cell death.
Metabolic reprogramming
Recent studies have increasingly highlighted metabolic reprogramming as a crucial factor in the development of resistance to TKIs in RCC. Changes in metabolic pathways, such as glycolysis, amino acid metabolism, and fatty acid metabolism, have been shown to play pivotal roles in mediating resistance mechanisms in RCC[56] [Figure 4].
Figure 4. Recent updates on metabolic shifting during RCC TKI resistance. Created in BioRender. Dong, Z. (2025) https://BioRender.com/vs7bn3w. RCC: Renal cell carcinoma; TKI: tyrosine kinase inhibitor.
Di et al. (2024) performed scRNA-seq on 14 RCC patients and identified glycolysis as a central metabolic pathway in the development of TKI resistance in RCC. This was mediated through the activation of the AKT/mTOR/HIF-1α pathway, emphasizing the metabolic shift that RCC cells underwent during TKI resistance[57]. The study underlined how metabolic alterations contribute to the survival and proliferation of RCC cells in the presence of TKIs. In another systematic study, Amaro et al. (2024) explored the metabolic profiling of sunitinib- and pazopanib-resistant RCC cells, analyzing both intracellular and extracellular metabolomes[58]. While the study’s limitation lies in its in vitro approach, it revealed several metabolic pathways related to TKI resistance in RCC. These pathways included those involving amino acids, glycerophospholipids, fatty acids, and nicotinate/nicotinamide. Interestingly, the metabolic changes in fatty acid metabolism observed in this study aligned with findings from our lab[59], suggesting a common metabolic shift in TKI-resistant RCC. Moreover, these RNA-related studies further underscore the critical role of lipid metabolism in mediating TKI resistance in RCC[40,42].
In another study, Liu et al. (2025) identified MTHFD2 as a key player in the metabolic adaptation of RCC cells to sunitinib resistance. MTHFD2 drove the folate cycle, stimulating the upregulation of UDP-GlcNAc and promoting c-Myc O-GlcNAcylation, both of which contributed to resistance to sunitinib[60]. This mechanism highlighted the role of epigenetic regulation through metabolic intermediates in modulating TKI resistance.
Additionally, Teisseire et al. (2025) observed that sunitinib treatment induced a metabolic shift in RCC cells, leading to increased serine synthesis. The GCN2-eIF2α-ATF4 stress response pathway was identified as the key link between sunitinib treatment and elevated serine production, promoting nucleotide synthesis and supporting the metabolic needs of resistant RCC cells[61].
These findings underscore the critical role of metabolic reprogramming in RCC resistance to TKIs. The ability of RCC cells to adapt their metabolism in response to treatment not only enabled their survival but also enhanced their resistance to TKIs. Targeting these metabolic pathways may provide promising therapeutic strategies to overcome resistance in RCC patients treated with TKIs.
Angiogenic switch
The development of TKI resistance in RCC is often accompanied by an angiogenic switch, where tumors adapt to prolonged antiangiogenic therapy by modifying their vascular microenvironment[62]. Several recent studies have elucidated key mechanisms through which RCC cells reprogram angiogenesis to evade TKI treatment.
Xuan et al. (2021) reported that exosomal miR-549a was significantly reduced in TKI-resistant RCC, promoting angiogenesis and vascular permeability by upregulating HIF-1α in endothelial cells, thus contributing to resistance against antiangiogenic therapies[63]. The previously mentioned study has also shown that lncRNA-ECVSR modulates RCC sensitivity to sunitinib via transcriptional regulation of
Similarly, Gu et al. (2020) demonstrated that estrogen receptor beta (ERβ) transcriptionally upregulates ANGPT-2, leading to Tie-2 phosphorylation, a key event in promoting angiogenesis and reinforcing resistance to sunitinib treatment[64]. This study highlighted the intricate role of hormonal signaling in modulating angiogenic pathways and TKI resistance.
Moreover, in a clinical study, Zhao et al. (2021) analyzed tumor samples from sunitinib-responsive and non-responsive patients and identified CTCF as a crucial factor in promoting sunitinib resistance by enhancing angiogenesis[65]. This finding underscored the significance of epigenetic regulators in driving vascular adaptation in RCC under TKI therapy.
These studies collectively indicated that RCC cells undergoing TKI resistance reactivated pro-angiogenic pathways to sustain tumor growth despite antiangiogenic treatment. Targeting these alternative angiogenic mechanisms may offer new therapeutic strategies to counteract TKI resistance in RCC.
Epigenetic modifying
Epigenetic modifications play a crucial role in the development of TKI resistance in RCC by regulating gene expression without altering the DNA sequence. In 2022, Chen et al. found that TRAF1 is upregulated in SR cells and clinical samples due to elevated N6-methyladenosine (m6A) modification in a METTL14-dependent manner, underscoring the role of RNA methylation in TKI resistance[66]. In 2020, our lab discovered that methylation of the PCK2 promoter leads to ER stress in RCC cells, ultimately contributing to sunitinib resistance[67]. More recently, in 2024, by establishing an in vivo sunitinib resistance model, our lab identified that MIER2 facilitates p53 deacetylation by interacting with HDAC1, leading to resistance in RCC[68].
Together, these studies revealed that epigenetic regulation, including m6A RNA modification, DNA methylation, and histone deacetylation, constituted a key mechanism underlying TKI resistance in RCC, offering potential therapeutic targets for overcoming resistance.
Drug resistance transmission
The transmission of drug sensitivity was proposed as a concept in microbiome; however, studies have found that drug resistance could be transmitted between cancer cells and has been validated in other studies[69,70]. In recent years, a few studies have reported that the sensitivity of RCC to TKIs can be transmitted through extracellular vesicles (EVs)[27,71]. Notably, a 2021 study demonstrated that treatment with sunitinib and axitinib led to increased EV secretion in RCC cells, along with metabolic reprogramming of these vesicles, particularly toward glycolysis[72]. This finding strongly suggested the potential involvement of EVs in RCC’s adaptive response and the transmission of resistance to TKIs.
For instance, as discussed earlier, Pan et al. (2025) discovered that STX17-DT, beyond its intrinsic role in TKI resistance within RCC cells, could be packaged into EVs via hnRNPA1, enabling the transmission of axitinib resistance to other cells[48]. Similarly, in a 2023 study mentioned above, Pan et al. demonstrated that IGFL2-AS1, despite its intracellular function previously mentioned in this review, can also be encapsulated into EVs through hnRNPC, thereby facilitating the transfer of sunitinib resistance between RCC cells[45].
Cancer stem cell phenotype
Cancer stem-like cells (CSCs) possess self-renewal capabilities, plasticity, and the ability to survive under therapeutic stress, contributing to tumor recurrence and drug resistance[73,74]. In 2022, Guo et al. found that under hypoxic conditions, the androgen receptor regulated the stem cell phenotype of RCC through the lncTCFL5-2/YBX1/SOX2 signaling axis, thereby enhancing RCC cell resistance to sunitinib[75]. Similarly, as discussed above, sunitinib-triggered activation of lncRNA-ECVSR/ERβ/HIF-2α signaling could result in an enhanced cancer stem cell phenotype, which ultimately leads to resistance[49].
TME
As another malignancy treated with the combination of ICIs and TKIs, lung cancer has been extensively investigated, with numerous studies reporting that alterations in the TME profoundly modulate tumor responses to TKIs[76,77]. Recent studies have also highlighted that changes in TME components play a pivotal role in shaping drug responsiveness in RCC [Figure 5]. However, a greater number of influential studies on this topic were conducted earlier than 2020[78-81], whereas recent research has shown a decline in investigations of the interactions between TKIs and the TME[36,82]. In the following sections, we will discuss recent progress on how individual components of the TME influence the sensitivity of RCC to TKI therapy.
Figure 5. Recent updates on the relationship of TME with RCC TKI resistance. Created in BioRender. Dong, Z. (2025) https://BioRender.com/kqpg9zr. TME: Tumor microenvironment; RCC: renal cell carcinoma; TKI: tyrosine kinase inhibitor.
Myeloid cell
As the predominant component of the TME, myeloid cells are widely recognized to be closely associated with the response of RCC to TKI therapy[83]. Recent studies have shown that M0 macrophages within the TME are closely associated with tumor responsiveness to both immunotherapy and TKI treatment[84]. Aggen et al. demonstrated that IL-1β blockade reduced polymorphonuclear MDSC (PMN-MDSC) infiltration and, in combination with cabozantinib, enhanced antitumor efficacy. These effects were accompanied by decreased immunosuppressive MDSCs and increased M1-like TAMs within the TME. Collectively, these findings provide the first evidence of potential synergy between IL-1β inhibition and VEGF-targeted TKI therapy, although the precise mechanisms - particularly how MDSC reduction and M1-like TAM enrichment contribute to this synergy - remain to be elucidated[85].
Lymphocyte
Recent studies have also shown that TKI treatment in RCC can induce PD-L1 expression and the release of soluble PD-L1 from tumor cells, thereby impairing T cell activation, reducing cytokine production, and decreasing the proportion of activated T cells[86]. Specifically, Liu et al. demonstrated that in SR RCC, NFAT1 is stabilized via PI3K/AKT/GSK-3β signaling and FOXA1/SETD2-mediated downregulation of FBW7, leading to increased PD-L1 expression. This mechanism promotes immune evasion by impairing T cell activity, underscoring NFAT1 as a key link between TKI resistance and T cell suppression[87]. Similarly, Greenberg et al. discovered that exosomes from SR RCC cells impair T cell function, exhibiting cytotoxicity that correlates with elevated PD-L1 levels compared with sunitinib-sensitive (SS) exosomes[88]. Interestingly, the expression levels of soluble PD-L1 and soluble PD-1 have also been identified as independent prognostic factors for progression-free survival (PFS) in mRCC patients treated with sunitinib[89]. Moreover, studies also identified several hub genes that simultaneously regulate the responsiveness of RCC cells to both TKIs and ICIs, suggesting that tumor cells may employ convergent adaptive mechanisms in response to therapy-induced alterations within the microenvironment[32,54,90-92]. However, it is noteworthy that the regulatory trends of certain hub genes in RCC are not always concordant between these two therapeutic modalities. For instance, in 2025, Yang et al. reported that high expression of COL6A2 was associated with increased sensitivity to sunitinib but reduced responsiveness to ICIs, underscoring the complexity of the underlying mechanisms[93]. In addition to tumor cell–intrinsic alterations influencing lymphocyte function and therapeutic responsiveness, the nature of T cell infiltration has also been linked to TKI efficacy in RCC. A clinical multi-omics study in 2020 demonstrated that infiltration of CD39+CD8+ T cells was associated with poorer responses to TKI therapy, potentially due to reduced levels of TNF-α and IFN-γ[94]. This is particularly interesting, as another independent study demonstrated that TKI treatment alters the heterogeneity of T cell infiltration in RCC, especially by affecting the ratio of CD39+CD8+ T cells[95].
Vascular endothelial cells
As the principal targets of TKIs in RCC, vascular endothelial cells (VECs) play a pivotal role in shaping the TME, and endothelial cell responsiveness to TKIs represents a key mechanism underlying both TKI resistance and immunotherapy responsiveness[96]. In 2023, researchers discovered that treatment with VEGF-targeted agents such as sunitinib or bevacizumab restored ICAM-1 expression in endothelial cells, promoted leukocyte adhesion and infiltration, and generated a pro-inflammatory tumor milieu. These findings highlight the potential of combining antiangiogenic agents with immunotherapy to enhance antitumor immune responses[97].
Cancer-associated fibroblasts
Activated fibroblasts educated by cancer cells, known as cancer-associated fibroblasts (CAFs), exhibit sustained activation properties[98]. In RCC, CAFs have been reported to display pronounced heterogeneity and to contribute to tumor progression by promoting proliferation[99], enhancing stemness[100], suppressing immune cell functions[101], and - most relevant to this review - mediating resistance to TKI therapy[102]. In 2024, Wang et al. discovered that CAFs could secrete CXCL3 and activate its receptor CXCR2 on RCC, resulting in the activation of the downstream ERK1/2 signaling pathway, thus promoting RCC sunitinib resistance[103].
The infiltration of CAFs is not merely a unidirectional process leading to RCC resistance to TKI therapy[104]. A 2021 study analyzing three cohorts demonstrated that VEGFR-TKI treatment increases CAF infiltration within the TME, thereby mediating resistance. This finding highlights a more complex interplay between TKI therapy and CAFs[105].
Above all, a comprehensive study by Stupichev et al. developed an AI-driven multimodal algorithm based on transcriptomic data from 14 cohorts, which constructed a harmonized immune tumor microenvironment (HiTME) landscape to predict RCC responsiveness to both ICI and TKI therapies[106].
RECENT REPORTS ON OVERCOMING TKI RESISTANCE IN RCC (PRECLINICAL LEVEL: IN VITRO OR IN VIVO)
From a translational perspective, recent research has increasingly focused on strategies to overcome TKI resistance in RCC. Building on the summarized mechanisms, the following section reviews key therapeutic advances proposed from 2020 to 2025, with an emphasis on their clinical relevance. These strategies are summarized in Table 2.
Recent strategies proposed by researchers to overcome TKI resistance in RCC
| Mechanism | Ref. | |
| Natural products | ||
| ATL-I | Degradation of EPAS1 | [107] |
| Wogonin | Inhibits CDK4-RB | [108] |
| PTE, 6-S | Inhibit Ras/ERK and Akt/mTOR | [109] |
| Small molecules | ||
| Kinase inhibitors | ||
| Targeting PI3K | ||
| STS | Inhibits PI3K/Akt and MDR1 | [110] |
| TGX-221 | Inhibits PI3Kβ | [111] |
| Pictilisib | Pan-PI3K inhibitor | [112] |
| CYT387 | IKBKE inhibitor | [33] |
| Targeting mTOR | ||
| sapanisertib | Inhibits mTORC1/2 | [113] |
| Rapalink-1 | Inhibits mTOR, MAPK, ErbB, and ABC transporters | [114] |
| Targeting ERK | ||
| Calcium saccharate | Enhances DUSP6, intervening in ERK/AKT | [115] |
| Targeting drug-resistant mutations | ||
| 15e | Targets MET V1238I and Y1248H | [111] |
| Other kinase inhibitors | ||
| Nilotinib | Degradation of MCL-1 | [116] |
| AZD4547 | FGFR inhibitor | |
| Nuclear receptor-targeting inhibitors | ||
| PHTPP | ERβ antagonist | [49] |
| Faslodex | [64] | |
| XCT-790 | Inhibits ERRα | [117] |
| HSP and stress response inhibitors | ||
| AUY922 | Targets HSP90 | [118] |
| HC-5404 | Inhibits PERK | [37] |
| Metabolic and angiogenesis pathway inhibitors | ||
| Sitagliptin | Inhibits DPP4 | [119] |
| Disulfiram | Inhibits ALDH1A | [119] |
| Simvastatin | Inhibits SREBP-1 | [120] |
| Angiotensin-(1-7) | [121] | |
| Meletin | HOOK1 agonist | [122] |
| CHD1Li | Targets CHD1L, influences HIF-2α | [30] |
| EMφ-siMTHFD2-MnO2@Suni | Targets folate-nucleotide metabolism | [123] |
| Drug repurposing | ||
| Penfluridol | Induces apoptosis | [124] |
| KTZ | Inhibits ERK1/2 | [125] |
| RNA interference | ||
| ASOs | Silences IGFL2-AS1 | [45] |
| St/siVEGFR-2@PCN-224@HA | RNAi + photodynamic | [126] |
| Others | ||
| [177Lu]Lu-cG250 RIT | [127] | |
| Lm-LLO-CD105A | Targets CD105 | [128] |
Natural products
Natural products offer unique chemical diversity, high biological activity, and better target selectivity, making them valuable in drug discovery. Their favorable pharmacokinetics and safety profiles enhance their potential for developing novel therapeutics[129].
Several natural compounds have shown potential in reversing TKI resistance in RCC by targeting key oncogenic pathways. Recently, Atractylenolide I (ATL-I), derived from Atractylodes macrocephala, was found to reverse sunitinib resistance by inhibiting EPAS1/HIF-2α-mediated VEGFA production and promoting the autophagic degradation of EPAS1 via lysosomal activation[107]. Similarly, Wogonin, an active component from Scutellaria baicalensis, restored sunitinib sensitivity by inhibiting the CDK4-RB pathway and inducing apoptosis[108]. Likewise, Honokiol, a phenolic compound, was identified as a potent inhibitor of c-Met-induced signaling, providing a potential strategy to overcome cabozantinib resistance[130]. More recently, Pterostilbene (PTE) and 6-Shogaol (6-S), natural phytochemicals found in edible sources, were shown to counteract sunitinib resistance by suppressing RLIP76-initiated Ras/ERK and Akt/mTOR pathways[109].
Small molecule inhibitor
Kinase inhibitors
Targeting PI3K
In 2020, Kinoh et al. discovered that staurosporine (STS), a competitive ATP mimetic with broad kinase inhibition affinity, could overcome sunitinib resistance by inhibiting PI3K/Akt and MDR1. In addition, to overcome the severe off-targeting toxicities in vivo, they developed strategies for tumor-selective delivery by loading STS with polymeric micelles[110]. In the same year, Azad et al. discovered that the inhibition of phosphoinositide 3-kinase (PI3K)β, an isoform uniquely coupled to both RTK and GPCRs, combined with sunitinib could reduce microvessel turnover and decrease heterogeneity of the TME[111]. Moreover, using the validated Therapeutically Guided Multidrug Optimization (TGMO) method, Pictilisib (pan-phosphatidylinositol 3-kinase inhibitor) was selected as a promising low-dose drug for combination therapy, effective in both naïve and resistant tumors[112].
Targeting mTOR
Recently, Wu et al. tested ten drugs and selected combinations across six RCC PDX models, finding cabozantinib and mTORC1/2 sapanisertib to be the most effective combinational therapies in suppressing tumors from patients who had failed prior TKI and ICI treatments, by blocking the ERK pathway[113]. Similarly, in 2020, Kuroshima et al. tested Rapalink-1, a new generation of mTOR inhibitor, in the treatment of SR RCC. The results showed that rapalink-1 had greater effects than the current second-line treatment, temsirolimus, by not only suppressing the mTOR signaling pathway but also the MAPK signaling pathway, ErbB signaling pathway, and several drug-resistance-associated ABC transporters[114]. These findings provided valuable insights that the mTORC1/2 double inhibitor may be effective against TKI resistance, as the mTOR inhibitor is currently the second-line treatment of mRCC following TKI monotherapy or TKI + ICI treatment.
Targeting ERK
In 2024, Liu et al. discovered that calcium saccharate could overcome sunitinib resistance by enhancing the expression of DUSP6, thus intervening in the ERK-AKT pathway[115].
Targeting drug-resistant mutation
In 2021, Li et al. synthesized a novel derivative of APG compound 15e, which demonstrated satisfying anti-proliferation effectivity against proven cabozantinib-resistant mutant MET V1238I and Y1248H[131].
Other kinase inhibitors
Liu et al. screened synergistic reagents of sunitinib from a compound library containing 1,374 FDA-approved drugs by in vitro cell viability evaluation. As a result, nilotinib stood out as a potential synergistic killer, rendering MCL-1 degradation and RCC autophagy to overcome sunitinib resistance[116].
Another inhibitor screened as a promising drug to overcome sunitinib resistance in RCC is AZD4547, an FGFR inhibitor[112].
Nuclear receptor-targeting inhibitors
Emerging evidence suggests that nuclear receptors play crucial roles in sunitinib resistance in RCC. As aforementioned, He et al. identified a positive-feedback loop between ERβ and lncRNA-ECVSR, where sunitinib-induced ERβ overexpression further upregulated lncRNA-ECVSR, promoting vasculogenic mimicry (VM). Disrupting this loop with the ERβ antagonist PHTPP enhanced sunitinib sensitivity and reduced VM formation[49]. Similarly, targeting the ERβ/ANGPT-2/Tie-2P signaling pathway with the FDA-approved anti-estrogen drug Faslodex has been proposed as a potential combination therapy to overcome sunitinib resistance[64]. Beyond ERβ, Feng et al. recently demonstrated that inhibiting ERRα acetylation-mediated autophagy-lysosome pathways by XCT-790 sensitized RCC cells to sunitinib, further supporting the therapeutic potential of nuclear receptor modulation in overcoming drug resistance[117].
Heat shock protein and stress response inhibitors
Recently, Saito et al. discovered that heat shock transcription factor 4 (HSF4) is elevated in SR RCC cells and the combination of pazopanib with HSF4 knockdown reduced cell proliferation in SR cells[90]. Similarly, another inhibitor targeting heat shock protein HSP90, AUY922, demonstrated the ability to increase the sensitivity of ccRCC cells by targeting the HIF-1α/VEGFA/VEGFR pathway[118].
As discussed above, to encounter PERK activation in TKIs-treated RCC, Stokes et al. discovered that PERK inhibitor HC-5404 enhanced the antiangiogenic effects of axitinib and lenvatinib. Moreover, the results of the in vivo experiment further showed that HC-5404 induced greater effects on the axitinib-resistant model[37].
Metabolic and angiogenesis pathway inhibitors
Metabolic reprogramming plays a critical role in TKI resistance in RCC, influencing cancer stemness, lipid metabolism, and angiogenesis. Several recent studies have identified metabolic regulators as potential therapeutic targets to enhance sunitinib efficacy. For example, recently, Lv et al. developed an engineered CD276-CD133 dual-targeting biomimetic nanovesicle EMφ-siMTHFD2-MnO2@Suni to overcome drug resistance and terminate tumor progression of ccRCC by remodeling folate-nucleotide metabolism[123].
Retinoic acid signaling has been implicated in cancer stemness and acquired resistance to TKIs. Kamada et al. (2021) found that dipeptidyl peptidase IV (DPP4) and ALDH1A, key regulators in retinoic acid metabolism, contributed to RCC resistance. Pharmacological inhibition using sitagliptin (DPP4 inhibitor) or disulfiram (ALDH1A inhibitor) effectively restored sunitinib sensitivity, highlighting the therapeutic potential of targeting this pathway[119].
Similarly, SREBP-1-mediated lipid metabolism is another key metabolic driver of resistance. Chen et al. (2022) demonstrated that simvastatin, a widely used cholesterol-lowering drug, could inhibit SREBP-1 activity, thereby reversing sunitinib resistance in RCC cells[120]. This finding supported the potential repurposing of lipid-lowering agents as anti-cancer adjuvants.
In addition to metabolic rewiring, angiotensin signaling has also been linked to TKI resistance. Khanna et al. (2021) reported that angiotensin-(1-7), a heptapeptide generated by ACE2, could synergize with axitinib in VEGFR-resistant ccRCC, offering a novel angiogenesis-modulating strategy to combat resistance[121].
Furthermore, Yin et al. (2023) identified a role for HOOK1, a negative regulator of VEGF-1 via TGF-β signaling, in overcoming sunitinib resistance. Their study selected meletin as a HOOK1 agonist, providing a metabolism-related approach to reverse drug resistance through TGF-β pathway modulation[122].
Collectively, these findings underscore the significance of metabolic interventions in overcoming TKI resistance. Targeting retinoic acid metabolism, lipid metabolism, and alternative angiogenic pathways represents a promising strategy to enhance RCC treatment outcomes.
Drug repurposing
Drug repurposing, which involves identifying new therapeutic applications for existing FDA-approved drugs, has emerged as a promising strategy to overcome TKI resistance in RCC. Tung et al. (2022) identified that penfluridol, a dopamine receptor D2 (DRD2)-targeting antipsychotic, could suppress cancer stemness by inducing autophagy-mediated apoptosis in RCC. Notably, its combination with sunitinib exhibited a synergistic effect, suggesting its potential as an adjunct therapy to enhance TKIs sensitivity[124]. Similarly, Greenberg et al. (2021) highlighted the role of EVs, particularly exosomes, in promoting TKI resistance. They discovered that ketoconazole (KTZ), an FDA-approved antifungal agent, could inhibit exosome biogenesis and secretion via ERK1/2 suppression, thereby enhancing sunitinib therapeutic effect in resistant 786-O RCC cells[125].
RNA interference
Non-coding RNAs, including lncRNAs and siRNAs, play crucial roles in the regulation of drug resistance mechanisms in RCC as discussed above. Recent advancements in RNA-based therapeutic strategies have explored innovative nanoparticle delivery systems to counteract sunitinib resistance.
Pan et al. investigated the role of IGFL2-AS1, a lncRNA that promoted sunitinib resistance in RCC. To counteract this mechanism, they developed a chitosan-solid lipid nanoparticle system carrying antisense oligonucleotides (ASOs) targeting IGFL2-AS1. This delivery system successfully silenced IGFL2-AS1 expression, effectively restoring sunitinib sensitivity[45].
Similarly, Hua et al. developed a Zr-based porphyrinic nanoscale metal-organic framework (PCN-224) for the co-delivery of sunitinib and VEGFR-2-targeting siRNA (siVEGFR-2). To enhance tumor targeting and drug stability, the system was coated with hyaluronic acid (HA), promoting CD44-mediated uptake. This multifunctional platform (St/siVEGFR-2@PCN-224@HA) achieves triple inhibition of tumor growth by integrating targeted therapy, RNA interference, and photodynamic therapy, markedly improving the therapeutic efficacy of TKIs in RCC[126].
These studies highlighted the potential of RNA-based therapeutics in reversing sunitinib resistance. By leveraging lncRNA inhibition (IGFL2-AS1 ASOs) and siRNA-mediated VEGFR-2 suppression through nanoparticle-mediated delivery systems, researchers are developing novel approaches to enhance drug efficacy and tumor selectivity in RCC treatment.
Others
In 2022, Oosterwijk-Wakka et al. reported that SR SK-RC-52 cells expressed pAXL and pMET, unlike the SS NU12 cells. NGS analysis revealed higher expression of VEGFA, VEGFB, VEGFD, PGF, and VEGFR1/2/3 in NU12, while SK-RC-52 exhibited lower VEGFC and PDGFA levels. Notably, combining sunitinib with [177Lu]Lu-cG250 radioimmunotherapy (RIT) achieved the best response in SK-RC-52 tumor-bearing mice after two treatment cycles, suggesting a promising strategy for overcoming sunitinib resistance in RCC[127].
In 2022, Oladejo et al. reported that a Listeria-based vaccine encoding an antigenic fragment of CD105 (Lm-LLO-CD105A) showed promising therapeutic efficacy in RCC by targeting both tumor cells and tumor-associated vasculature. This effect was mediated by CD8+ T cells and relied on strong CD105 expression in RCC cells[128].
RECENT CLINICAL TRIALS ADDRESSING TKI RESISTANCE IN RCC (CLINICAL TRIAL LEVEL)
Finally, we reviewed the information on recent clinical trials of combination therapies with TKIs or strategies to overcome TKI resistance in RCC [Table 3]. From Table 3, it is evident that the most common clinical investigations involve the combination of recently FDA-approved HIF-2α inhibitors[132] with TKIs, which aligns with the previously described pivotal role of the HIF-2α pathway in renal cancer angiogenesis, particularly in TKI resistance[30,49,107,133]. In addition, combinations with second-line agents such as mTOR inhibitors are also frequently explored.
Recent clinical trials of combination therapies with TKIs or strategies to overcome TKI resistance in RCC
| NCT number/target | Study title | Study status | Study design | Start date | Primary completion date | Completion Date | Locations |
| HIF-2α | |||||||
| NCT07097935 | Study of HS-10516 Combination Therapy in Patients With Advanced Renal Cell Carcinoma | RECRUITING | Allocation: NA Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2025/7/10 | 2027/7/10 | 2028/7/10 | Beijing Cancer Hospital |
| NCT07011719 | Study of Casdatifan and Cabozantinib Versus Placebo and Cabozantinib in Patients With Advanced Clear Cell Renal Cell Carcinoma | NOT_YET_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: QUADRUPLE (PARTICIPANT, CARE_PROVIDER, INVESTIGATOR, OUTCOMES_ASSESSOR) Primary purpose: TREATMENT | 2025/9/1 | 2028/4/1 | 2030/12/1 | NA |
| NCT05899049 | A Study of Pembrolizumab (MK-3475) in Combination With Belzutifan (MK-6482) and Lenvatinib (MK-7902), or Pembrolizumab/Quavonlimab (MK-1308A) in Combination With Lenvatinib, vs. Pembrolizumab and Lenvatinib, for Treatment of Advanced Clear Cell Renal Cell Carcinoma (MK-6482-012)-China Extension Study | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2022/7/27 | 2026/12/1 | 2026/12/1 | Beijing Cancer Hospital-Renal carcinoma and melanoma (Site 6000) |
| NCT05536141 | A Phase 1 Study of AB521 Monotherapy and Combination Therapies in Renal Cell Carcinoma and Other Solid Tumors | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2022/10/26 | 2027/7/1 | 2027/7/1 | University of Alabama at Birmingham |
| NCT05030506 | A Study of Belzutifan (MK-6482) as Monotherapy and in Combination With Lenvatinib (E7080/MK-7902) With or Without Pembrolizumab (MK-3475) in China Participants With Advanced Renal Cell Carcinoma (MK-6482-010) | ACTIVE_NOT_RECRUITING | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2021/10/13 | 2026/10/21 | 2026/10/21 | Beijing Cancer Hospital-Digestive Oncology (Site 0001) |
| NCT04736706 | A Study of Pembrolizumab (MK-3475) in Combination With Belzutifan (MK-6482) and Lenvatinib (MK-7902), or Pembrolizumab/Quavonlimab (MK-1308A) in Combination With Lenvatinib, Versus Pembrolizumab and Lenvatinib, for Treatment of Advanced Clear Cell Renal Cell Carcinoma (MK-6482-012) | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2021/4/14 | 2026/10/29 | 2026/10/29 | The University of Alabama at Birmingham (Site 0010) |
| NCT04626479 | Substudy 03A: A Study of Immune and Targeted Combination Therapies in Participants With First Line (1L) Renal Cell Carcinoma (MK-3475-03A) | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2020/12/16 | 2026/5/31 | 2026/5/31 | University of California at San Francisco (Site 1008) |
| NCT04586231 | A Study of Belzutifan (MK-6482) in Combination With Lenvatinib Versus Cabozantinib for Treatment of Renal Cell Carcinoma (MK-6482-011) | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2021/2/25 | 2026/2/11 | 2027/2/11 | Ironwood Cancer & Research Centers (Site 0077) |
| NCT03634540 | A Trial of Belzutifan (PT2977, MK-6482) in Combination With Cabozantinib in Patients With Clear Cell Renal Cell Carcinoma (ccRCC) (MK-6482-003) | ACTIVE_NOT_RECRUITING | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2018/9/27 | 2027/2/26 | 2027/2/26 | USC Norris Comprehensive Cancer Center (Site 0060) |
| NCT07049926 | Substudy 03C: A Study of Combination Therapies in Participants With Renal Cell Carcinoma With Recurrent Disease During or After Anti-PD-(L)1 Therapy (MK-3475-03C/KEYMAKER-U03) | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: SINGLE (OUTCOMES_ASSESSOR) Primary purpose: TREATMENT | 2025/7/20 | 2031/10/26 | 2031/10/26 | UCSF Medical Center at Mission Bay (Site 5008) |
| mTOR | |||||||
| NCT05012371 | Lenvatinib With Everolimus Versus Cabozantinib for Second-Line or Third-Line Treatment of Metastatic Renal Cell Cancer | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2022/2/16 | 2025/10/25 | 2025/10/25 | Moffitt Cancer Center |
| NCT03324373 | Lenvatinib and Everolimus in Renal Cell Carcinoma (RCC) | COMPLETED | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2019/3/20 | 2023/9/27 | 2024/8/28 | University of Iowa Hospitals and Clinics |
| NCT03173560 | Trial to Assess Safety and Efficacy of Lenvatinib (18 mg vs. 14 mg) in Combination With Everolimus in Participants With Renal Cell Carcinoma | COMPLETED | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2017/8/17 | 2020/2/14 | 2024/6/20 | City of Hope National Medical Center |
| NCT02915783 | A Trial to Evaluate Efficacy and Safety of Lenvatinib in Combination With Everolimus in Subjects With Unresectable Advanced or Metastatic Non Clear Cell Renal Cell Carcinoma (nccRCC) Who Have Not Received Any Chemotherapy for Advanced Disease | COMPLETED | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2017/2/20 | 2021/11/2 | 2021/11/2 | H. Lee Moffitt Cancer Center and Research Institute |
| NCT02811861 | Lenvatinib/Everolimus or Lenvatinib/Pembrolizumab Versus Sunitinib Alone as Treatment of Advanced Renal Cell Carcinoma | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2016/10/13 | 2020/8/28 | 2026/3/31 | Stanford School of Medicine |
| NCT06317298 | Fruquintinib Plus Everolimus as 2nd Line Therapy of ccRCC Patients Progressed Post IO and TKI Therapy | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2024/2/21 | 2025/8/1 | 2025/12/1 | Peking University First Hospital |
| NCT02479490 | Prednisone Plus Everolimus in Patients With Metastatic Renal Cell Cancer After Failure of VEGFR-TKI | TERMINATED | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2015/9/22 | 2017/5/5 | 2017/9/1 | U.O Oncologia Medica |
| PARP | |||||||
| NCT04337970 | Talazoparib and Axitinib for People With Previously Treated Advanced Kidney Cancer | COMPLETED | Allocation: RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2020/4/6 | 2025/5/23 | 2025/5/23 | Memorial Sloan Kettering Basking Ridge (Limited Protocol Activities) |
| FTase | |||||||
| NCT06026410 | KO-2806 Monotherapy and Combination Therapies in Advanced Solid Tumors | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2023/10/18 | 2027/1/1 | 2027/4/1 | Mayo Clinic Comprehensive Cancer Center |
| HDAC | |||||||
| NCT03592472 | A Study of Pazopanib With or Without Abexinostat in Patients With Locally Advanced or Metastatic Renal Cell Carcinoma (RENAVIV) | RECRUITING | Allocation: RANDOMIZED Intervention model: CROSSOVER Masking: DOUBLE (PARTICIPANT, INVESTIGATOR) Primary purpose: TREATMENT | 2018/7/17 | 2026/12/30 | 2028/6/30 | University Of UA Cancer Center(UACC)/DH-SJHMC |
| NCT02795819 | Study of the Pan-DAC Inhibitor AR-42 and Pazopanib in Advanced Sarcoma and Kidney Cancer | TERMINATED | Allocation: NON_RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2016/7/8 | 2016/11/24 | 2019/3/14 | Virginia Commonwealth University/Massey Cancer Center |
| Serine/threonine kinase | |||||||
| NCT03571438 | Evaluation of a Promising New Combination of Protein Kinase Inhibitors on Organotypic Cultures of Human Renal Tumors | UNKNOWN | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2017/10/16 | 2022/9/30 | 2024/9/30 | Grenoble Alps Hospital |
| NCT00448721 | A Phase II Trial of Perifosine Following Tyrosine Kinase Inhibitor (TKI) - Failure in Patients With Renal Cancer | COMPLETED | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2007/3/1 | 2010/10/1 | 2011/10/1 | Investigative Site |
| GLS1 | |||||||
| NCT03428217 | CANTATA: CB-839 With Cabozantinib vs. Cabozantinib With Placebo in Patients With Metastatic Renal Cell Carcinoma | COMPLETED | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: QUADRUPLE (PARTICIPANT, CARE_PROVIDER, INVESTIGATOR, OUTCOMES_ASSESSOR) Primary purpose: TREATMENT | 2018/4/24 | 2020/8/31 | 2021/7/16 | University of Alabama at Birmingham |
| NCT02771626 | Study CB-839 in Combination With Nivolumab in Patients With Melanoma, Clear Cell Renal Cell Carcinoma (ccRCC) and Non-Small Cell Lung Cancer (NSCLC) | TERMINATED | Allocation: NON_RANDOMIZED Intervention model: FACTORIAL Masking: NONE Primary purpose: TREATMENT | 2016/8/1 | 2020/4/24 | 2020/4/24 | Honor Health |
| β-Adrenoceptor blocker | |||||||
| NCT03323710 | Study of Propranolol Plus Sunitinib in First-line Treatment of Metastatic Renal Cell Carcinoma | WITHDRAWN | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2025/9/18 | 2025/12/19 | 2025/12/19 | Military Institute of Medicine |
| CXCR4 | |||||||
| NCT02667886 | Trial of X4P-001 in Participants With Advanced Renal Cell Carcinoma | COMPLETED | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2016/4/27 | 2022/4/14 | 2022/4/14 | Scottsdale |
| ENPP3 and tubulin | |||||||
| NCT02639182 | A Study of AGS-16C3F vs. Axitinib in Metastatic Renal Cell Carcinoma | COMPLETED | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2016/5/3 | 2020/10/2 | 2020/10/2 | Site US01026 |
| CDK4/6 | |||||||
| NCT06835972 | A Study of Abemaciclib and Cabozantinib in People With Clear Cell Renal Cell Carcinoma (ccRCC) | RECRUITING | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2025/2/14 | 2027/2/1 | 2027/2/1 | Johns Hopkins University (Data Collection Only) |
| NCT05176288 | Avelumab, Palbociclib and Axitinib in Advanced RCC | WITHDRAWN | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2024/5/31 | 2026/8/31 | 2027/8/31 | |
| NCT03905889 | A Study of Abemaciclib in Combination With Sunitinib in Metastatic Renal Cell Carcinoma | TERMINATED | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2019/6/5 | 2022/3/8 | 2022/3/8 | Rhode Island Hospital |
| Cell therapy | |||||||
| NCT07087158 | A Study of IBR854 Combined With Pazopanib Versus Pazopanib in Advanced Renal Cell Carcinoma | NOT_YET_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2025/8/10 | 2026/12/31 | 2026/12/31 | The Cancer Hospital of Fudan University |
| NCT06716853 | A Clinical Gene Therapy Study with Hematopoietic Stem Cells for the Treatment, with Single Dose of Temferon, of Patients Suffering from Metastatic Renal Cell Carcinoma | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2024/10/22 | 2026/9/30 | 2026/9/30 | Ospedale San Raffaele |
| NCT05127824 | Autologous Dendritic Cell Vaccine in Kidney Cancer | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2023/7/6 | 2026/12/1 | 2026/12/1 | UPMC Department of Urology |
| NCT04203901 | Dendritic Cell Immunotherapy Plus Standard Treatment of Advanced Renal Cell Carcinoma | TERMINATED | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2020/7/22 | 2023/9/28 | 2023/9/28 | Moffitt Cancer Center |
| NCT03736330 | A Study of Anti-PD-1 Combinations of D-CIK Immunotherapy and Axitinib in Advanced Renal Carcinoma | UNKNOWN | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2018/9/8 | 2020/10/8 | 2021/11/8 | Cancer Center |
| ADC | |||||||
| NCT05620134 | Study of JK08 in Patients with Unresectable Locally Advanced or Metastatic Cancer | ACTIVE_NOT_RECRUITING | Allocation: NON_RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2022/10/17 | 2025/10/17 | 2026/2/20 | Institut Jules Bordet |
| NCT06962787 | A Study of BL-B01D1 + Axitinib Without or With Pembrolizumab in Patients With Locally Advanced or Metastatic Renal Cancer | RECRUITING | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2025/7/28 | 2025/5/27 | 2025/12/27 | Fudan University Shanghai Cancer Center |
| Radiotherapy | |||||||
| NCT02956798 | SAbR For Oligometastatic Renal Cell Carcinoma | ACTIVE_NOT_RECRUITING | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2018/7/19 | 2025/12/30 | 2025/12/31 | University of Texas Southwestern Medical Center |
| NCT06889649 | SABR Combined with Axitinib and Toripalimab in Recurrent or Metastatic RCC | RECRUITING | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2019/1/1 | 2027/2/28 | 2028/2/20 | Peking University First Hospital |
| NCT06726421 | Systemic Therapy Alone or with Stereotactic Body Radiotherapy for Oligometastatic Kidney Cancer (STROKER Study) | RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: SINGLE (OUTCOMES_ASSESSOR) Primary purpose: TREATMENT | 2024/9/18 | 2030/9/30 | 2033/9/30 | Sun Yat-sen University Cancer Center |
| NCT02307474 | A Pilot Study of SBRT With Adjuvant Pazopanib for Renal Cell Cancer | WITHDRAWN | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2025/9/15 | 2025/9/15 | 2025/9/15 | Case Medical Center |
| NCT02599779 | A Proof of Principle Study of Pembrolizumab With SBRT in TKI mRCC Patients | COMPLETED | Allocation: NON_RANDOMIZED Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2016/12/1 | 2021/8/1 | 2021/8/5 | Tom Baker Cancer Centre |
| Hormone therapy | |||||||
| NCT06222593 | Study to Evaluate the Safety and Efficacy of Bicalutamide in Combination with Sunitinib in Patients with TKIs-resistant RCC | RECRUITING | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2024/10/1 | 2026/7/1 | 2027/7/1 | UB/Great Lakes Cancer Care |
| NCT03379012 | Testosterone in Metastatic Renal Cell Carcinoma Patients | COMPLETED | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2016/2/8 | 2018/1/30 | 2019/7/30 | A.I. Kryzhanovsky Krasnoyarsk Cancer Center |
| Radiation therapy | |||||||
| NCT06132945 | A Study of Cabozantinib and Nivolumab With Radiation Therapy for People With Renal Cell Carcinoma That Has Spread to the Brain | RECRUITING | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2023/11/10 | 2027/11/1 | 2027/11/1 | Memorial Sloan Kettering Basking Ridge (All Protocol Activities) |
| NCT04071223 | Testing the Addition of a New Anti-cancer Drug, Radium-223 Dichloride, to the Usual Treatment (Cabozantinib) for Advanced Renal Cell Cancer That Has Spread to the Bone, RadiCaL Study | RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2020/7/29 | 2026/10/1 | 2026/10/1 | University of Alabama at Birmingham Cancer Center |
| NCT02406521 | Exploratory Study of Radium-223 and VEGF-Targeted Therapy in Patients With Metastatic Renal Cell Carcinoma and Bone Mets | COMPLETED | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2025/4/15 | 2025/12/17 | 2019/12/31 | Massachusetts General Hospital |
| NCT05663710 | Phase 1b/2 Study of Combination 177Lu Girentuximab Plus Cabozantinib and Nivolumab in Treatment naïve Patients With Advanced Clear Cell RCC | RECRUITING | Allocation: RANDOMIZED Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2023/6/30 | 2025/10/30 | 2027/10/30 | MD Anderson Cancer Center |
| Natural products | |||||||
| NCT05363631 | Seleno-L Methionine (SLM)-Axitinib-Pembrolizumab | RECRUITING | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2022/9/19 | 2026/12/31 | 2026/12/31 | University of Iowa Hospitals & Clinics |
| NCT02535533 | SLM + Axitinib for Clear Cell RCC | COMPLETED | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2025/1/16 | 2023/8/22 | 2025/4/4 | University of Iowa Hospitals and Clinics |
| NCT05122546 | CBM588 in Combination With Nivolumab and Cabozantinib for the Treatment of Advanced or Metastatic Kidney Cancer | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2021/11/1 | 2025/10/25 | 2025/10/25 | City of Hope Medical Center |
| NCT03334409 | Pazopanib Hydrochloride With or Without Ascorbic Acid in Treating Patients With Kidney Cancer That Is Metastatic or Cannot Be Removed by Surgery | TERMINATED | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2018/2/16 | 2021/3/13 | 2021/3/13 | Illinois CancerCare-Peoria |
| NCT02446795 | Isoquercetin as an Adjunct Therapy in Patients With Kidney Cancer Receiving First-line Sunitinib: a Phase I/II Trial | UNKNOWN | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: TRIPLE (PARTICIPANT, CARE_PROVIDER, INVESTIGATOR) Primary purpose: TREATMENT | 2025/11/16 | 2025/12/17 | 2025/12/17 | Azienda Ospedaliera Cardarelli Divisione Di Oncologia |
| Switch TKIs or with a higher dose | |||||||
| NCT05931393 | Sequential Treatment of Cabozantinib for Advanced Renal Cell Carcinoma (RCC) | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2023/12/20 | 2025/9/1 | 2027/12/31 | UT Southwestern Medical Center |
| NCT05678673 | Study of XL092 + Nivolumab vs. Sunitinib in Subjects With Advanced or Metastatic Non-Clear Cell Renal Cell Carcinoma | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2023/1/1 | 2025/7/25 | 2025/6/28 | Exelixis Clinical Site #1 |
| NCT05522231 | Efficacy and Safety of Fruquintinib in Combination With Sintilimab in Advanced Renal Cell Carcinoma (FRUSICA-2) | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2022/10/27 | 2025/1/25 | 2025/3/25 | Beijing Chao-Yang Hospital |
| NCT04609800 | Study on the Utilization of Cabozantinib in Adult Patients With Advanced or Metastatic Renal Cell Carcinoma (RCC) in 2nd Line Treatment Following Prior Vascular Endothelial Growth Factor (VEGF)-Targeted Therapy Under Real-real Life Clinical Setting in France | WITHDRAWN | Observational model: Cohort Time perspective: Prospective | 2020/11/6 | 2023/5/10 | 2023/5/10 | |
| NCT04458259 | Study of PF-07265807 in Participants With Metastatic Solid Tumors | TERMINATED | Allocation: NON_RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2020/9/24 | 2024/11/15 | 2024/11/15 | Henry Eye Clinic |
| NCT04300140 | Safety and Efficacy Study of AVB-S6-500 (Batiraxcept) in Patients With Advanced or Metastatic Clear Cell Renal Cell Carcinoma | TERMINATED | Allocation: RANDOMIZED Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2021/2/26 | 2023/8/14 | 2023/8/14 | University of Maryland Greenebaum Comprehensive Cancer Center |
| NCT02867592 | Cabozantinib-S-Malate in Treating Younger Patients With Recurrent, Refractory, or Newly Diagnosed Sarcomas, Wilms Tumor, or Other Rare Tumors | ACTIVE_NOT_RECRUITING | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2017/5/18 | 2021/6/30 | 2026/6/27 | Children’s Hospital of Alabama |
| NCT02761057 | Testing Cabozantinib, Crizotinib, Savolitinib and Sunitinib in Kidney Cancer Which Has Progressed | COMPLETED | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2016/7/25 | 2023/10/19 | 2023/10/19 | Anchorage Associates in Radiation Medicine |
| NCT02122003 | Second Line Sorafenib After Pazopanib in Patients With RCC | TERMINATED | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2025/9/16 | 2017/11/8 | 2017/11/8 | Istituto Tumori |
| Oncologic physical therapy | |||||||
| NCT05092373 | Phase I Study of Tumor Treating Fields (TTF) in Combination With Cabozantinib or With Pembrolizumab and Nab-Paclitaxel in Patients With Advanced Solid Tumors Involving the Abdomen or Thorax | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2022/4/29 | 2026/9/1 | 2026/9/1 | M D Anderson Cancer Center |
| Immune related | |||||||
| CD80 | |||||||
| NCT04977453 | GI-101 as a Single Agent or in Combination With Pembrolizumab, Lenvatinib or Local Radiotherapy in Advanced Solid Tumors | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2021/8/2 | 2025/10/26 | 2025/12/26 | Tisch Cancer Institute (TCI) |
| IL2 | |||||||
| NCT04540705 | A Study to Compare Bempegaldesleukin (BEMPEG: NKTR-214) Combined With Nivolumab and Tyrosine Kinase Inhibitor (TKI) to Nivolumab and TKI Alone in Participants With Previously Untreated Kidney Cancer That is Advanced or Has Spread | COMPLETED | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2020/9/11 | 2024/1/18 | 2024/1/18 | Local Institution - 0005 |
| LILRB2 | |||||||
| NCT04626518 | Substudy 03B: A Study of Immune and Targeted Combination Therapies in Participants With Second Line Plus (2L+) Renal Cell Carcinoma (MK-3475-03B/KEYMAKER-U03) | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2020/12/17 | 2026/5/31 | 2026/5/31 | University of California at San Francisco (Site 3008) |
| IL-1β | |||||||
| NCT03798626 | Gevokizumab With Standard of Care Anti-cancer Therapies for Metastatic Colorectal, Gastroesophageal, and Renal Cancers | COMPLETED | Allocation: NON_RANDOMIZED Intervention model: PARALLEL Masking: NONE Primary purpose: TREATMENT | 2019/5/22 | 2023/3/1 | 2025/2/5 | University of California LA |
| OX40 | |||||||
| NCT03092856 | Axitinib With or Without Anti-OX40 Antibody PF-04518600 in Treating Patients With Metastatic Kidney Cancer | ACTIVE_NOT_RECRUITING | Allocation: RANDOMIZED Intervention model: PARALLEL Masking: TRIPLE (PARTICIPANT, CARE_PROVIDER, INVESTIGATOR) Primary purpose: TREATMENT | 2017/7/19 | 2025/12/31 | 2026/12/31 | Los Angeles County-USC Medical Center |
| PTPN2/PTPN1 | |||||||
| NCT04777994 | Study With ABBV-CLS-484 in Participants With Locally Advanced or Metastatic Tumors | RECRUITING | Allocation: NON_RANDOMIZED Intervention model: SEQUENTIAL Masking: NONE Primary purpose: TREATMENT | 2021/3/9 | 2026/10/1 | 2026/10/1 | University of Arizona Cancer Center - Tucson / ID# 262698 |
| Dietary intervention | |||||||
| NCT02729194 | Pilot Study of Pazopanib With Low Fat Meal (PALM) in Advanced Renal Cell Carcinoma | COMPLETED | Allocation: NA Intervention model: SINGLE_GROUP Masking: NONE Primary purpose: TREATMENT | 2025/6/16 | 2025/9/17 | 2025/9/17 | University of Michigan Comprehensive Cancer Center |
Another interesting trend is that researchers are beginning to explore the combination of TKIs with immunomodulatory agents beyond the conventional first-line ICIs (PD-1/PD-L1/CTLA-4), aiming for enhanced efficacy. Even various cell therapies are now being tested in combination with TKIs, providing potential guidance for the future direction of pharmacological treatment in renal cancer.
Notably, some natural agents, including vitamin C and the gut microbiota modulator CBM588, are FDA-approved nutraceuticals or probiotics. This regulatory status suggests that such combination strategies may be more readily translatable into clinical application once supportive preclinical or early clinical evidence is obtained[134,135].
However, some clinical trials are still primarily focused on either using the next-generation TKIs or simply increasing drug dosages. However, based on previous experience, the pace of drug development and clinical testing for agents with similar mechanisms generally may not keep pace with the rate of resistance development in patients. Therefore, combination strategies involving drugs with distinct mechanisms should be the primary focus of future investigations.
DISCUSSION AND CONCLUSION
The role of TKIs in RCC and the challenge of resistance
TKIs have been a cornerstone in the treatment of RCC, particularly for advanced cases. These drugs primarily target tumor angiogenesis by inhibiting VEGFR, PDGFR, and other pathways that drive neovascularization. However, despite their clinical efficacy, the emergence of resistance remains an inevitable challenge. Over time, RCC tumors adapt to TKI therapy through a variety of mechanisms, rendering these treatments less effective and leading to disease progression[2,4,12,136].
Current landscape of TKI resistance mechanisms in RCC
A review of studies from 2020 to 2025 indicates that research on TKI resistance in RCC has largely focused on bypass pathway activation[32,33], the roles of lncRNAs[45,47] and circRNAs[41,42], and forms of regulated cell death, such as apoptosis[108] and ferroptosis[53]. However, a crucial aspect of RCC biology - metabolic reprogramming[137] - remains relatively underexplored in the context of TKI resistance. Given that metabolic adaptations play a significant role in RCC progression and therapy resistance, more research is needed to uncover metabolic vulnerabilities that could be exploited to overcome TKI resistance.
Additionally, while significant efforts have been made to investigate resistance mechanisms across various TKIs, most studies still primarily focus on sunitinib. This focus may be influenced by the increasing use of ICIs and other novel therapies, which have shifted research priorities. However, as TKIs remain a cornerstone of RCC treatment, resistance studies should extend to newer-generation TKIs and explore common resistance mechanisms across different TKIs. Furthermore, current strategies to overcome resistance mostly involve TKI combination therapies; nevertheless, systematic comparisons of their efficacy and toxicity have been limited. Future research should prioritize optimizing these combination strategies while ensuring their safety and clinical applicability.
Limitations of current research and the need for large-scale studies
While significant progress has been made in understanding traditional resistance mechanisms, most studies have remained limited in scale and scope. Many investigations focused on a single drug within a single RCC model, making it challenging to generalize findings across different tumor subtypes or treatment regimens. There is an urgent need for large-scale, multi-model studies conducted by research groups with the necessary resources to systematically examine TKI resistance across various settings. A notable example of such an approach is the highly systematic study by Zhang et al. in 2023, which closely reflects real-world clinical scenarios[138]. Expanding such efforts would provide invaluable foundational data to guide future research, particularly for groups with more constrained experimental capabilities. At present, studies on TKI resistance in RCC remain relatively isolated, with investigations of the immune microenvironment largely focused on mechanisms of ICI responsiveness while neglecting its interplay with TKI therapy. In real-world clinical settings, however, acquired resistance arising from combined TKI and ICI treatment should be regarded as an integrated and complex process rather than two independent phenomena. Future exploration of resistance mechanisms - whether to TKIs alone or to TKI–ICI combinations - should place greater emphasis on the intricate regulatory role of the immune microenvironment.
Unique aspects of TKI resistance in RCC: tumor-angiogenesis dependency
Unlike TKIs in cancers such as lung cancer, which target oncogenic driver mutations (e.g., EGFR, ALK, ROS1 alterations)[139], TKIs in RCC predominantly function by disrupting tumor angiogenesis rather than directly targeting tumor cells. ccRCC, the most common subtype, is characterized by frequent inactivation of the VHL tumor suppressor gene, leading to constitutive stabilization of HIFs and subsequent upregulation of VEGF and PDGF[10,11]. Consequently, RCC tumors exhibit strong dependence on neovascularization for sustained growth and survival. TKIs such as sunitinib, pazopanib, cabozantinib, and lenvatinib exert their effects by targeting VEGFR, PDGFR, and c-KIT - receptors primarily expressed on endothelial cells rather than the tumor cells themselves.
This fundamental difference in mechanism alters the patterns of resistance observed in RCC. While many cancers develop resistance through secondary kinase domain mutations[140-142], RCC tumors more commonly adapt by modifying their TME, increasing HIF-2α signaling, and undergoing metabolic reprogramming. Unfortunately, most studies on RCC TKI resistance focus on direct tumor cell responses, neglecting the role of endothelial cells and the broader TME. This presents a major gap in current research: instead of solely examining the direct effects of TKIs direct effects on RCC cells, it may be more insightful to investigate how RCC tumors adapt to vascular disruption and reestablish tumor progression. Just as RCC-immune cell interactions have been extensively studied in the context of immunotherapy resistance, there is a pressing need to explore RCC-endothelial cell interactions in the context of TKI resistance.
Future directions: leveraging advanced technologies to address knowledge gaps
Addressing the complexities discussed above requires advancements in both methodology and model systems. While technological and experimental limitations have constrained past research, emerging techniques such as single-cell sequencing, spatial transcriptomics and metabolomics, hemodynamic and vascularized organoids modeling offer promising new avenues[143-146]. These technologies enable deeper insights into the interplay between TKIs, RCC cells, and the surrounding endothelial network. Future studies should leverage these tools to explore resistance mechanisms at a higher resolution, ultimately guiding the development of more effective therapeutic strategies that consider both tumor-intrinsic and microenvironmental adaptations.
In summary, while significant progress has been made in understanding RCC TKI resistance, critical gaps remain. Future research should expand beyond sunitinib to newer TKIs, explore metabolic reprogramming as a key resistance mechanism, and incorporate large-scale, systematic studies. Shifting focus from tumor-centric to microenvironment-centric approaches may provide novel insights into overcoming resistance. With ongoing advancements in technology and research methodologies, future studies have the potential to refine therapeutic strategies and improve patient outcomes in RCC.
DECLARATIONS
Acknowledgments
The graphical abstract was created with BioRender.com [Created in BioRender. Dong, Z. (2025) https://BioRender.com/0qqs82z].
Authors’ contributions
Conceived the present study: Zhang X, Wang K, Li A
Drafted the manuscript: Dong Z, Li S, Li M
Revised the manuscript: Chen K, Fan M
All authors read and approved the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was supported by the National Natural Scientific Foundation of China (Grant No. 82303033), Science and Technology Department of Hubei Province (2023AFB416), and Natural Science Foundation of Wuhan (2024040801020349).
Conflict of interest
Wang K is a Chair of the Early-Career Editorial Board of the journal Cancer Drug Resistance. Wang K was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
3. Bukavina L, Bensalah K, Bray F, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82:529-42.
5. Usher-Smith J, Simmons RK, Rossi SH, Stewart GD. Current evidence on screening for renal cancer. Nat Rev Urol. 2020;17:637-42.
6. Rizzo M, Pezzicoli G, Tibollo V, Premoli A, Quaglini S. Clinical outcome predictors for metastatic renal cell carcinoma: a retrospective multicenter real-life case series.
7. Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193-205.
8. Ali O, Fishman EK, Kawamoto S. Recurrent renal cell carcinoma following nephrectomy and ablation therapy: radiology perspective. Eur J Radiol. 2018;107:134-42.
10. Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. 2021;17:245-61.
11. Cotta BH, Choueiri TK, Cieslik M, et al. Current landscape of genomic biomarkers in clear cell renal cell carcinoma. Eur Urol. 2023;84:166-75.
12. Rathmell WK, Rumble RB, Van Veldhuizen PJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol. 2022;40:2957-95.
13. Barragan-Carrillo R, Saad E, Saliby RM, et al. First and second-line treatments in metastatic renal cell carcinoma. Eur Urol. 2025;87:143-54.
14. Zhao Y, Bilal M, Raza A, et al. Tyrosine kinase inhibitors and their unique therapeutic potentialities to combat cancer. Int J Biol Macromol. 2021;168:22-37.
15. Sharma R, Kadife E, Myers M, Kannourakis G, Prithviraj P, Ahmed N. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J Exp Clin Cancer Res. 2021;40:186.
16. Marona P, Górka J, Kwapisz O, et al. Resistance to tyrosine kinase inhibitors promotes renal cancer progression through MCPIP1 tumor-suppressor downregulation and c-Met activation. Cell Death Dis. 2022;13:814.
17. Davis MI, Hunt JP, Herrgard S, et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046-51.
18. Liu T, Hwang L, Burley SK, et al. BindingDB in 2024: a FAIR knowledgebase of protein-small molecule binding data. Nucleic Acids Res. 2025;53:D1633-44.
19. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298-308.
20. Saha D, Ryan KR, Lakkaniga NR, et al. Targeting rearranged during transfection in cancer: a perspective on small-molecule inhibitors and their clinical development. J Med Chem. 2021;64:11747-73.
21. Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-71.
22. Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127-32.
24. Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327-37.
25. Wang Y, Liu X, Gong L, et al. Mechanisms of sunitinib resistance in renal cell carcinoma and associated opportunities for therapeutics. Br J Pharmacol. 2023;180:2937-55.
26. Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35:2687-97.
27. Qu L, Ding J, Chen C, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653-68.
28. Rosenzweig SA. Acquired resistance to drugs targeting receptor tyrosine kinases. Biochem Pharmacol. 2012;83:1041-8.
29. Fukumoto W, Yoshino H, Horike SI, et al. Potential therapeutic target secretogranin II might cooperate with hypoxia-inducible factor 1α in sunitinib-resistant renal cell carcinoma. Cancer Sci. 2023;114:3946-56.
30. He H, Li J, Wang W, et al. The SIRT7-mediated deacetylation of CHD1L amplifies HIF-2α-dependent signal that drives renal cell carcinoma progression and sunitinib resistance. Cell Biosci. 2023;13:166.
31. Ge X, Li M, Yin J, et al. Fumarate inhibits PTEN to promote tumorigenesis and therapeutic resistance of type2 papillary renal cell carcinoma. Mol Cell. 2022;82:1249-60.e7.
32. Xiong W, Zhang B, Yu H, Zhu L, Yi L, Jin X. RRM2 regulates sensitivity to sunitinib and PD-1 blockade in renal cancer by stabilizing ANXA1 and activating the AKT pathway. Adv Sci. 2021;8:e2100881.
33. Liu S, Li J, Zhang J, et al. IKBKE regulates renal cell carcinoma progression and sunitinib resistance through the RRM2-AKT pathway. Int J Biol Sci. 2024;20:6146-61.
34. Fang L, Zhang Y, Zang Y, et al. HP-1 inhibits the progression of ccRCC and enhances sunitinib therapeutic effects by suppressing EMT. Carbohydr Polym. 2019;223:115109.
35. Bouchalova P, Beranek J, Lapcik P, et al. Transgelin contributes to a poor response of metastatic renal cell carcinoma to sunitinib treatment. Biomedicines. 2021;9:1145.
36. Xie Y, Shangguan W, Chen Z, et al. Establishment of sunitinib-resistant xenograft model of renal cell carcinoma and the identification of drug-resistant hub genes and pathways. Drug Des Devel Ther. 2021;15:5061-74.
37. Stokes ME, Calvo V, Fujisawa S, et al. PERK inhibition by HC-5404 sensitizes renal cell carcinoma tumor models to antiangiogenic tyrosine kinase inhibitors. Clin Cancer Res. 2023;29:4870-82.
38. Wang Y, Zhang Y, Wang P, Fu X, Lin W. Circular RNAs in renal cell carcinoma: implications for tumorigenesis, diagnosis, and therapy. Mol Cancer. 2020;19:149.
39. Qin S, Wang Y, Wang P, Lv Q. Molecular mechanism of circRNAs in drug resistance in renal cell carcinoma. Cancer Cell Int. 2022;22:369.
40. Huang KB, Pan YH, Shu GN, et al. Circular RNA circSNX6 promotes sunitinib resistance in renal cell carcinoma through the miR-1184/GPCPD1/lysophosphatidic acid axis. Cancer Lett. 2021;523:121-34.
41. Shou Y, Yue C, Wang Q, et al. circPTPN12 promotes the progression and sunitinib resistance of renal cancer via hnRNPM/IL-6/STAT3 pathway. Cell Death Dis. 2023;14:232.
42. Liu Y, Chen K, Shou Y, et al. circRARS synergises with IGF2BP3 to regulate RNA methylation recognition to promote tumour progression in renal cell carcinoma. Clin Transl Med. 2023;13:e1512.
43. Liu Y, Zhang H, Fang Y, Tang D, Luo Z. Non-coding RNAs in renal cell carcinoma: implications for drug resistance. Biomed Pharmacother. 2023;164:115001.
44. Gao X, Zhang H, Zhang C, et al. The emerging role of long non-coding RNAs in renal cell carcinoma progression and clinical therapy via targeting metabolic regulation. Front Pharmacol. 2023;14:1122065.
45. Pan Y, Lu X, Shu G, et al. Extracellular vesicle-mediated transfer of lncRNA IGFL2-AS1 confers sunitinib resistance in renal cell carcinoma. Cancer Res. 2023;83:103-16.
46. Liu Y, Cheng G, Huang Z, et al. Long noncoding RNA SNHG12 promotes tumour progression and sunitinib resistance by upregulating CDCA3 in renal cell carcinoma. Cell Death Dis. 2020;11:515.
47. Tian P, Wei J, Li J, Ren J, He C. An oncogenic role of lncRNA SNHG1 promotes ATG7 expression and autophagy involving tumor progression and sunitinib resistance of renal cell carcinoma. Cell Death Discov. 2024;10:273.
48. Pan Y, Liu S, Shu G, et al. STX17-DT facilitates axitinib resistance in renal cell carcinoma by inhibiting mitochondrial ROS accumulation and ferroptosis. Cell Death Dis. 2025;16:125.
49. He M, Yang H, Shi H, et al. Sunitinib increases the cancer stem cells and vasculogenic mimicry formation via modulating the lncRNA-ECVSR/ERβ/Hif2-α signaling. Cancer Lett. 2022;524:15-28.
50. Azijli K, Gotink KJ, Verheul HM. The potential role of lysosomal sequestration in sunitinib resistance of renal cell cancer. J Kidney Cancer VHL. 2015;2:195-203.
51. Li L, Zhao S, Liu Z, et al. Sunitinib treatment promotes metastasis of drug-resistant renal cell carcinoma via TFE3 signaling pathway. Cell Death Dis. 2021;12:220.
52. Chen WJ, Pan XW, Song X, et al. Preoperative neoadjuvant targeted therapy remodels intra-tumoral heterogeneity of clear-cell renal cell carcinoma and ferroptosis inhibition induces resistance progression. Cancer Lett. 2024;593:216963.
53. Wang Q, Gao S, Shou Y, et al. AIM2 promotes renal cell carcinoma progression and sunitinib resistance through FOXO3a-ACSL4 axis-regulated ferroptosis. Int J Biol Sci. 2023;19:1266-83.
54. Wu J, Wang S, Liu Y, Zhang T, Wang X, Miao C. Integrated single-cell and bulk characterization of cuproptosis key regulator PDHB and association with tumor microenvironment infiltration in clear cell renal cell carcinoma. Front Immunol. 2023;14:1132661.
55. Zeng X, Chen Z, Zhu Y, et al. O-GlcNAcylation regulation of RIPK1-dependent apoptosis dictates sensitivity to sunitinib in renal cell carcinoma. Drug Resist Updat. 2024;77:101150.
56. Sato T, Kawasaki Y, Maekawa M, et al. Metabolomic analysis to elucidate mechanisms of sunitinib resistance in renal cell carcinoma. Metabolites. 2020;11:1.
57. Di SC, Chen WJ, Yang W, et al. DEPDC1 as a metabolic target regulates glycolysis in renal cell carcinoma through AKT/mTOR/HIF1α pathway. Cell Death Dis. 2024;15:533.
58. Amaro F, Carvalho M, Bastos ML, Guedes de Pinho P, Pinto J. Metabolomics reveals tyrosine kinase inhibitor resistance-associated metabolic events in human metastatic renal cancer cells. Int J Mol Sci. 2024;25:6328.
59. Wei Z, Cheng G, Ye Y, et al. A fatty acid metabolism signature associated with clinical therapy in clear cell renal cell carcinoma. Front Genet. 2022;13:894736.
60. Liu J, Huang G, Lin H, et al. MTHFD2 enhances cMYC O-GlcNAcylation to promote sunitinib resistance in renal cell carcinoma. Cancer Res. 2025;85:1113-29.
61. Teisseire M, Sahu U, Parola J, et al. De novo serine synthesis is a metabolic vulnerability that can be exploited to overcome sunitinib resistance in advanced renal cell carcinoma. Cancer Res. 2025;85:1857-73.
62. Longo R, D’Andrea MR, Sarmiento R, Salerno F, Gasparini G. Integrated therapy of kidney cancer. Ann Oncol. 2007;18 Suppl 6:vi141-8.
63. Xuan Z, Chen C, Tang W, et al. TKI-resistant renal cancer secretes low-level exosomal miR-549a to induce vascular permeability and angiogenesis to promote tumor metastasis. Front Cell Dev Biol. 2021;9:689947.
64. Gu J, Zhang Y, Han Z, et al. Targeting the ERβ/Angiopoietin-2/Tie-2 signaling-mediated angiogenesis with the FDA-approved anti-estrogen Faslodex to increase the Sunitinib sensitivity in RCC. Cell Death Dis. 2020;11:367.
65. Zhao T, Zhou Y, Wang Q, et al. QPCT regulation by CTCF leads to sunitinib resistance in renal cell carcinoma by promoting angiogenesis. Int J Oncol. 2021;59:48.
66. Chen Y, Lu Z, Qi C, et al. N6-methyladenosine-modified TRAF1 promotes sunitinib resistance by regulating apoptosis and angiogenesis in a METTL14-dependent manner in renal cell carcinoma. Mol Cancer. 2022;21:111.
67. Xiong Z, Yuan C, Shi J, et al. Restoring the epigenetically silenced PCK2 suppresses renal cell carcinoma progression and increases sensitivity to sunitinib by promoting endoplasmic reticulum stress. Theranostics. 2020;10:11444-61.
68. Wei Z, Ye Y, Liu C, et al. MIER2/PGC1A elicits sunitinib resistance via lipid metabolism in renal cell carcinoma. J Adv Res. 2025;70:287-305.
69. Liu J, Ren L, Li S, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783-97.
70. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75.
71. He J, He J, Min L, et al. Extracellular vesicles transmitted miR-31-5p promotes sorafenib resistance by targeting MLH1 in renal cell carcinoma. Int J Cancer. 2020;146:1052-63.
72. Lim AR, Vincent BG, Weaver AM, Rathmell WK. Sunitinib and Axitinib increase secretion and glycolytic activity of small extracellular vesicles in renal cell carcinoma. Cancer Gene Ther. 2022;29:683-96.
73. Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611-29.
74. Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat Rev Cancer. 2018;18:669-80.
75. Guo C, Sun Y, Zhai W, et al. Hypoxia increases RCC stem cell phenotype via altering the androgen receptor (AR)-lncTCFL5-2-YBX1-SOX2 signaling axis. Cell Biosci. 2022;12:185.
76. Lu J, Li J, Lin Z, et al. Reprogramming of TAMs via the STAT3/CD47-SIRPα axis promotes acquired resistance to EGFR-TKIs in lung cancer. Cancer Lett. 2023;564:216205.
77. Wang S, Rong R, Yang DM, et al. Features of tumor-microenvironment images predict targeted therapy survival benefit in patients with EGFR-mutant lung cancer. J Clin Invest. 2023;133:e160330.
78. Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171-80.
79. Puig-Kröger A, Relloso M, Fernández-Capetillo O, et al. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175-82.
80. Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506-13.
81. Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514-22.
82. Bilen MA, Rini BI, Voss MH, et al. Association of neutrophil-to-lymphocyte ratio with efficacy of first-line avelumab plus axitinib vs. sunitinib in patients with advanced renal cell carcinoma enrolled in the phase 3 JAVELIN renal 101 trial. Clin Cancer Res. 2022;28:738-47.
83. Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243-8.
84. Xin S, Su J, Li R, et al. Identification of a risk model for prognostic and therapeutic prediction in renal cell carcinoma based on infiltrating M0 cells. Sci Rep. 2024;14:13390.
85. Aggen DH, Ager CR, Obradovic AZ, et al. Blocking IL1 beta promotes tumor regression and remodeling of the myeloid compartment in a renal cell carcinoma model: multidimensional analyses. Clin Cancer Res. 2021;27:608-21.
86. Fumarola C, La Monica S, Bonelli M, et al. Immunomodulatory effects of antiangiogenic tyrosine kinase inhibitors in renal cell carcinoma models: impact on following anti-PD-1 treatments. Biochem Pharmacol. 2024;226:116397.
87. Liu W, Ren D, Xiong W, Jin X, Zhu L. A novel FBW7/NFAT1 axis regulates cancer immunity in sunitinib-resistant renal cancer by inducing PD-L1 expression. J Exp Clin Cancer Res. 2022;41:38.
88. Greenberg JW, Kim H, Ahn M, et al. Combination of tipifarnib and sunitinib overcomes renal cell carcinoma resistance to tyrosine kinase inhibitors via tumor-derived exosome and T cell modulation. Cancers. 2022;14:903.
89. Montemagno C, Hagege A, Borchiellini D, et al. Soluble forms of PD-L1 and PD-1 as prognostic and predictive markers of sunitinib efficacy in patients with metastatic clear cell renal cell carcinoma. Oncoimmunology. 2020;9:1846901.
90. Saito S, Yoshino H, Yokoyama S, et al. Targeting heat shock transcription factor 4 enhances the efficacy of cabozantinib and immune checkpoint inhibitors in renal cell carcinoma. Int J Mol Sci. 2025;26:1776.
91. Emanuelli A, Souleyreau W, Chouleur T, et al. Targeting the IL34-CSF1R axis improves metastatic renal cell carcinoma therapy outcome via immune-vascular crosstalk regulation. iScience. 2025;28:112752.
92. Lai Y, Wu W, Liang X, et al. Connexin43 is associated with the progression of clear cell renal carcinoma and is regulated by tangeretin to sygergize with tyrosine kinase inhibitors. Transl Oncol. 2023;35:101712.
93. Yang Y, Fan R, Zhang B, Liu K. COL6A2 in clear cell renal cell carcinoma: a multifaceted driver of tumor progression, immune evasion, and drug sensitivity. J Transl Med. 2025;23:875.
94. Qi Y, Xia Y, Lin Z, et al. Tumor-infiltrating CD39+CD8+ T cells determine poor prognosis and immune evasion in clear cell renal cell carcinoma patients. Cancer Immunol Immunother. 2020;69:1565-76.
95. Murakami T, Tanaka N, Takamatsu K, et al. Multiplexed single-cell pathology reveals the association of CD8 T-cell heterogeneity with prognostic outcomes in renal cell carcinoma. Cancer Immunol Immunother. 2021;70:3001-13.
96. Huinen ZR, Huijbers EJM, van Beijnum JR, Nowak-Sliwinska P, Griffioen AW. Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol. 2021;18:527-40.
97. Nowak-Sliwinska P, van Beijnum JR, Griffioen CJ, et al. Proinflammatory activity of VEGF-targeted treatment through reversal of tumor endothelial cell anergy. Angiogenesis. 2023;26:279-93.
98. Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792-804.
99. Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430-9.
100. Su S, Chen J, Yao H, et al. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841-56.e16.
101. Peng YL, Xiong LB, Zhou ZH, et al. Single-cell transcriptomics reveals a low CD8+ T cell infiltrating state mediated by fibroblasts in recurrent renal cell carcinoma. J Immunother Cancer. 2022;10:e004206.
102. Errarte P, Larrinaga G, López JI. The role of cancer-associated fibroblasts in renal cell carcinoma. An example of tumor modulation through tumor/non-tumor cell interactions. J Adv Res. 2020;21:103-8.
103. Wang Y, Ding W, Hao W, et al. CXCL3/TGF-β-mediated crosstalk between CAFs and tumor cells augments RCC progression and sunitinib resistance. iScience. 2024;27:110224.
104. Chakiryan NH, Kimmel GJ, Kim Y, et al. Geospatial cellular distribution of cancer-associated fibroblasts significantly impacts clinical outcomes in metastatic clear cell renal cell carcinoma. Cancers. 2021;13:3743.
105. Ambrosetti D, Coutts M, Paoli C, et al. Cancer-associated fibroblasts in renal cell carcinoma: implication in prognosis and resistance to anti-angiogenic therapy. BJU Int. 2022;129:80-92.
106. Stupichev D, Miheecheva N, Postovalova E, et al. AI-driven multimodal algorithm predicts immunotherapy and targeted therapy outcomes in clear cell renal cell carcinoma. Cell Rep Med. 2025;6:102299.
107. Li Q, Zeng K, Chen Q, et al. Atractylenolide I inhibits angiogenesis and reverses sunitinib resistance in clear cell renal cell carcinoma through ATP6V0D2-mediated autophagic degradation of EPAS1/HIF2α. Autophagy. 2025;21:619-38.
108. Wang Y, Chen S, Sun S, et al. Wogonin induces apoptosis and reverses sunitinib resistance of renal cell carcinoma cells via inhibiting CDK4-RB pathway. Front Pharmacol. 2020;11:1152.
109. Chiang IC, Chen SY, Hsu YH, Shahidi F, Yen GC. Pterostilbene and 6-shogaol exhibit inhibitory effects on sunitinib resistance and motility by suppressing the RLIP76-initiated Ras/ERK and Akt/mTOR pathways in renal cancer cells. Eur J Pharmacol. 2024;967:176393.
110. Kinoh H, Shibasaki H, Liu X, Yamasoba T, Cabral H, Kataoka K. Nanomedicines blocking adaptive signals in cancer cells overcome tumor TKI resistance. J Control Release. 2020;321:132-44.
111. Azad AK, Zhabyeyev P, Vanhaesebroeck B, et al. Inactivation of endothelial cell phosphoinositide 3-kinase β inhibits tumor angiogenesis and tumor growth. Oncogene. 2020;39:6480-92.
112. Rausch M, Weiss A, Achkhanian J, Rotari A, Nowak-Sliwinska P. Identification of low-dose multidrug combinations for sunitinib-naive and pre-treated renal cell carcinoma. Br J Cancer. 2020;123:556-67.
113. Wu Y, Chen S, Yang X, et al. Combining the tyrosine kinase inhibitor cabozantinib and the mTORC1/2 inhibitor sapanisertib blocks ERK pathway activity and suppresses tumor growth in renal cell carcinoma. Cancer Res. 2023;83:4161-78.
114. Kuroshima K, Yoshino H, Okamura S, et al. Potential new therapy of Rapalink-1, a new generation mammalian target of rapamycin inhibitor, against sunitinib-resistant renal cell carcinoma. Cancer Sci. 2020;111:1607-18.
115. Liu H, Wang L, Shi X, et al. Calcium saccharate/DUSP6 suppresses renal cell carcinoma glycolytic metabolism and boosts sunitinib efficacy via the ERK-AKT pathway. Biochem Pharmacol. 2024;224:116247.
116. Liu T, Yue X, Chen X, et al. Nilotinib in combination with sunitinib renders MCL-1 for degradation and activates autophagy that overcomes sunitinib resistance in renal cell carcinoma. Cell Oncol. 2024;47:1277-94.
117. Feng C, Kong D, Tong B, et al. Hypoxia-triggered ERRα acetylation enhanced its oncogenic role and promoted progression of renal cell carcinoma by coordinating autophagosome-lysosome fusion. Cell Death Dis. 2025;16:23.
118. Chen Z, Jia X, Cai Y, et al. AUY922 improves sensitivity to sunitinib in clear cell renal cell carcinoma based on network pharmacology and in vitro experiments. Heliyon. 2024;10:e34834.
119. Kamada S, Namekawa T, Ikeda K, et al. Functional inhibition of cancer stemness-related protein DPP4 rescues tyrosine kinase inhibitor resistance in renal cell carcinoma. Oncogene. 2021;40:3899-913.
120. Chen TC, Huang CW, Lo CY, Chen CN, Chang SF, Chen YY. Suppression of SREBP-1 expression by simvastatin decreases visfatin-induced chemoresistance to sunitinib in human renal carcinoma 786-O cells. Life. 2022;12:1890.
121. Khanna P, Soh HJ, Chen CH, et al. ACE2 abrogates tumor resistance to VEGFR inhibitors suggesting angiotensin-(1-7) as a therapy for clear cell renal cell carcinoma. Sci Transl Med. 2021;13:eabc0170.
122. Yin L, Li W, Chen X, et al. HOOK1 inhibits the progression of renal cell carcinoma via TGF-β and TNFSF13B/VEGF-A axis. Adv Sci. 2023;10:e2206955.
123. Lv M, Liu B, Duan Y, et al. Engineered biomimetic nanovesicles synergistically remodel folate-nucleotide and γ-aminobutyric acid metabolism to overcome sunitinib-resistant renal cell carcinoma. ACS Nano. 2024;18:27487-502.
124. Tung MC, Lin YW, Lee WJ, et al. Targeting DRD2 by the antipsychotic drug, penfluridol, retards growth of renal cell carcinoma via inducing stemness inhibition and autophagy-mediated apoptosis. Cell Death Dis. 2022;13:400.
125. Greenberg JW, Kim H, Moustafa AA, et al. Repurposing ketoconazole as an exosome directed adjunct to sunitinib in treating renal cell carcinoma. Sci Rep. 2021;11:10200.
126. Hua Y, Qin M, Lu S, et al. Hyaluronic acid-functionalized MOFs for combined sunitinib and siRNA therapy in renal cell carcinoma. Int J Biol Macromol. 2024;283:137317.
127. Oosterwijk-Wakka JC, de Weijert MCA, Franssen GM, et al. Combination of sunitinib and 177Lu-labeled antibody cG250 targeted radioimmunotherapy: a promising new therapeutic strategy for patients with advanced renal cell cancer. Neoplasia. 2022;32:100826.
128. Oladejo M, Nguyen HM, Silwal A, et al. Listeria-based immunotherapy directed against CD105 exerts anti-angiogenic and anti-tumor efficacy in renal cell carcinoma. Front Immunol. 2022;13:1038807.
129. Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111-29.
130. Rawat L, Balan M, Sasamoto Y, Sabarwal A, Pal S. A novel combination therapy with Cabozantinib and Honokiol effectively inhibits c-Met-Nrf2-induced renal tumor growth through increased oxidative stress. Redox Biol. 2023;68:102945.
131. Li J, Tan G, Cai Y, et al. A novel Apigenin derivative suppresses renal cell carcinoma via directly inhibiting wild-type and mutant MET. Biochem Pharmacol. 2021;190:114620.
133. Qian K, Li W, Ren S, et al. HDAC8 enhances the function of HIF-2α by deacetylating ETS1 to decrease the sensitivity of TKIs in ccRCC. Adv Sci. 2024;11:e2401142.
134. Zhang J, Zhang Q, Lin G, et al. Single-cell analysis reveals that vitamin C inhibits bone metastasis of renal cancer via cell cycle arrest and microenvironment remodeling. Adv Sci. 2025;12:e01011.
135. Paz Del Socorro T, Oka K, Boulard O, et al. The biotherapeutic Clostridium butyricum MIYAIRI 588 strain potentiates enterotropism of Rorγt+Treg and PD-1 blockade efficacy. Gut Microbes. 2024;16:2315631.
136. Barragan-Carrillo R, Saad E, Saliby RM, et al. First and second-line treatments in metastatic renal cell carcinoma. Eur Urol. 2025;87:143-54.
137. Chakraborty S, Balan M, Sabarwal A, Choueiri TK, Pal S. Metabolic reprogramming in renal cancer: events of a metabolic disease. Biochim Biophys Acta Rev Cancer. 2021;1876:188559.
138. Zhang H, Bai L, Wu XQ, et al. Proteogenomics of clear cell renal cell carcinoma response to tyrosine kinase inhibitor. Nat Commun. 2023;14:4274.
139. Meyer ML, Fitzgerald BG, Paz-Ares L, et al. New promises and challenges in the treatment of advanced non-small-cell lung cancer. Lancet. 2024;404:803-22.
140. Zhao Y, He Y, Wang W, et al. Efficacy and safety of immune checkpoint inhibitors for individuals with advanced EGFR-mutated non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitors: a systematic review, meta-analysis, and network meta-analysis. Lancet Oncol. 2024;25:1347-56.
141. Fu K, Xie F, Wang F, Fu L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J Hematol Oncol. 2022;15:173.
142. Passaro A, Jänne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2021;2:377-91.
143. Nagase K, Akutagawa T, Rikitake-Yamamoto M, et al. Cellular and physical microenvironments regulate the aggressiveness and sunitinib chemosensitivity of clear cell renal cell carcinoma. J Pathol. 2021;254:46-56.
144. Bi K, He MX, Bakouny Z, et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell. 2021;39:649-61.e5.
145. Quintard C, Tubbs E, Jonsson G, et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat Commun. 2024;15:1452.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data







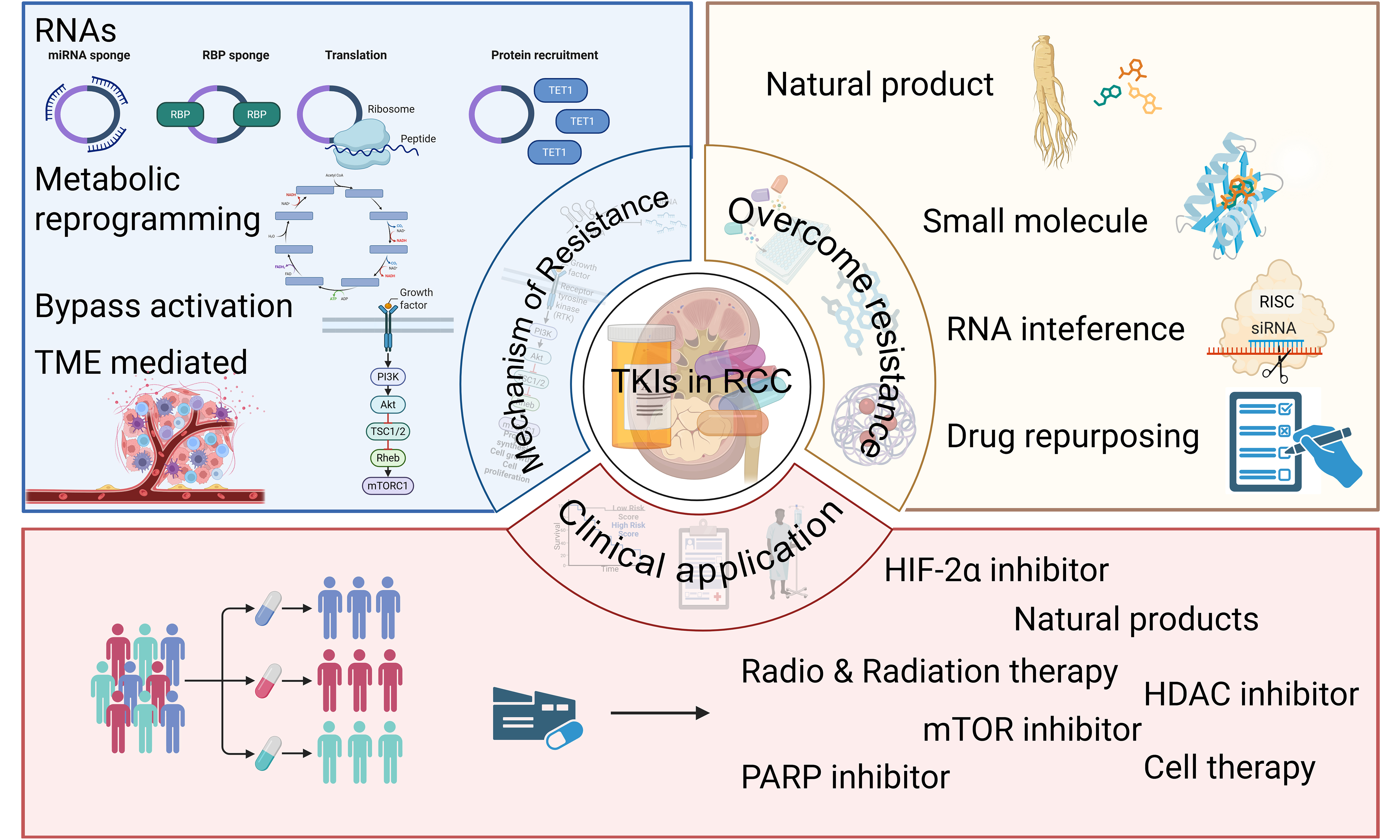
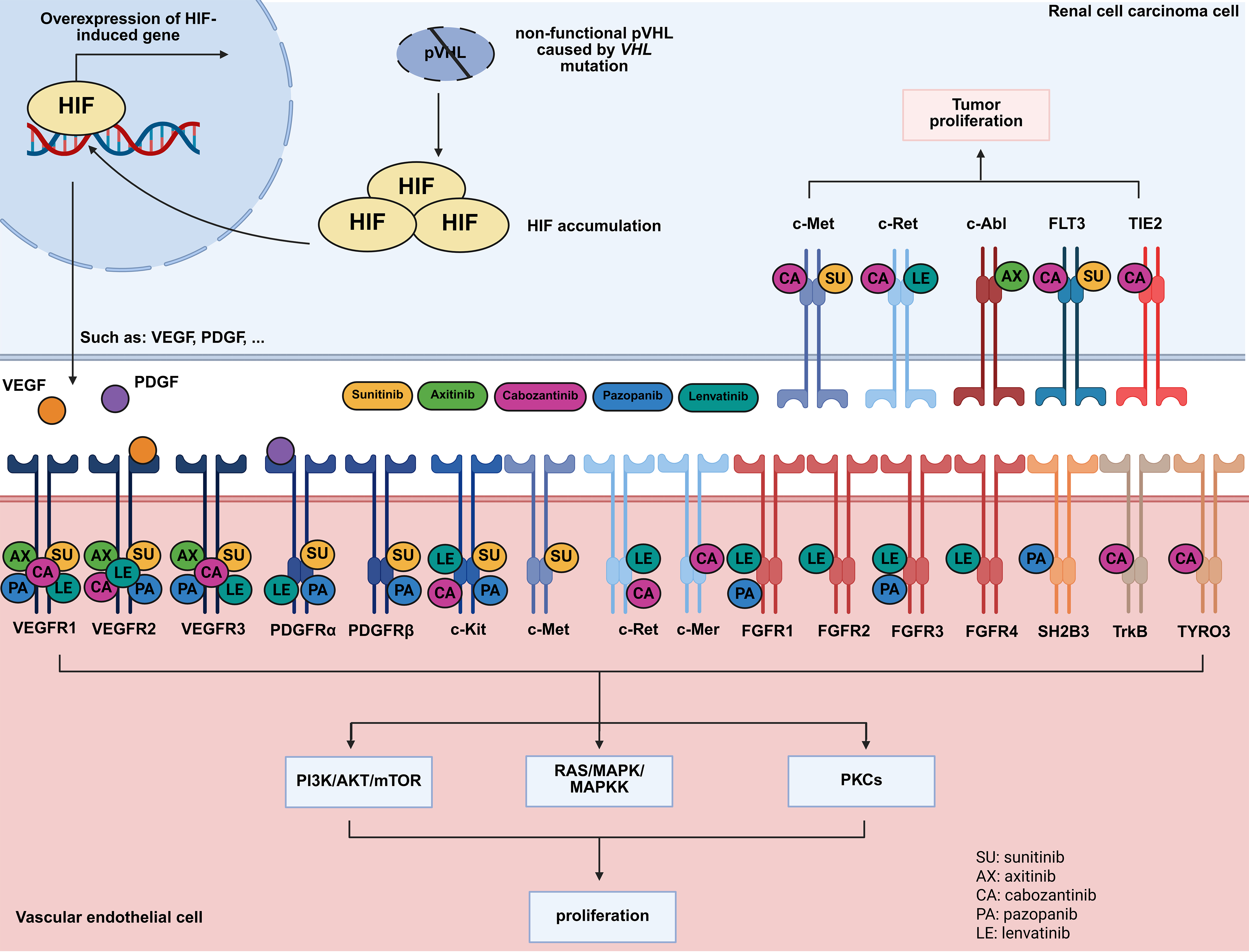
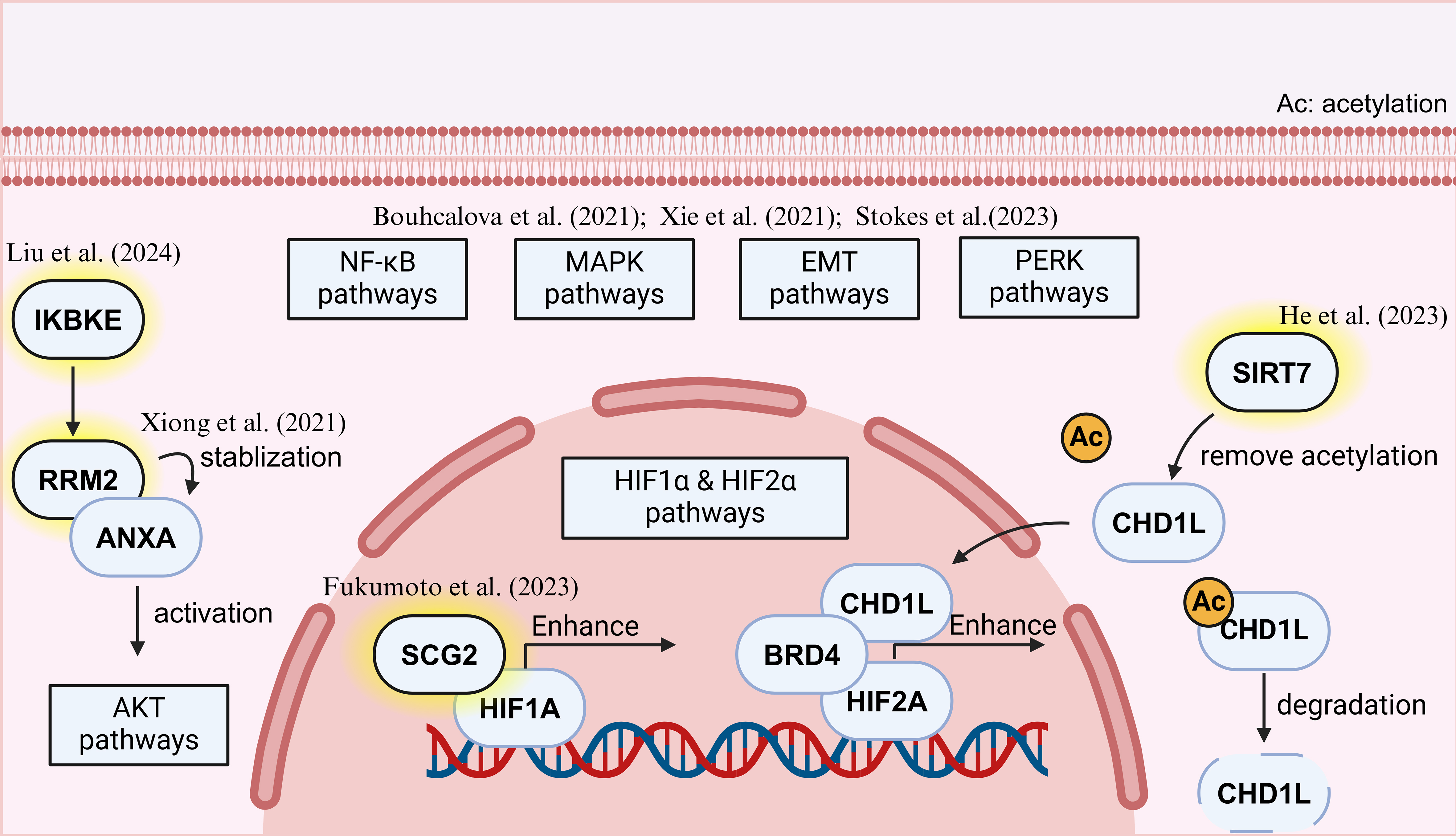
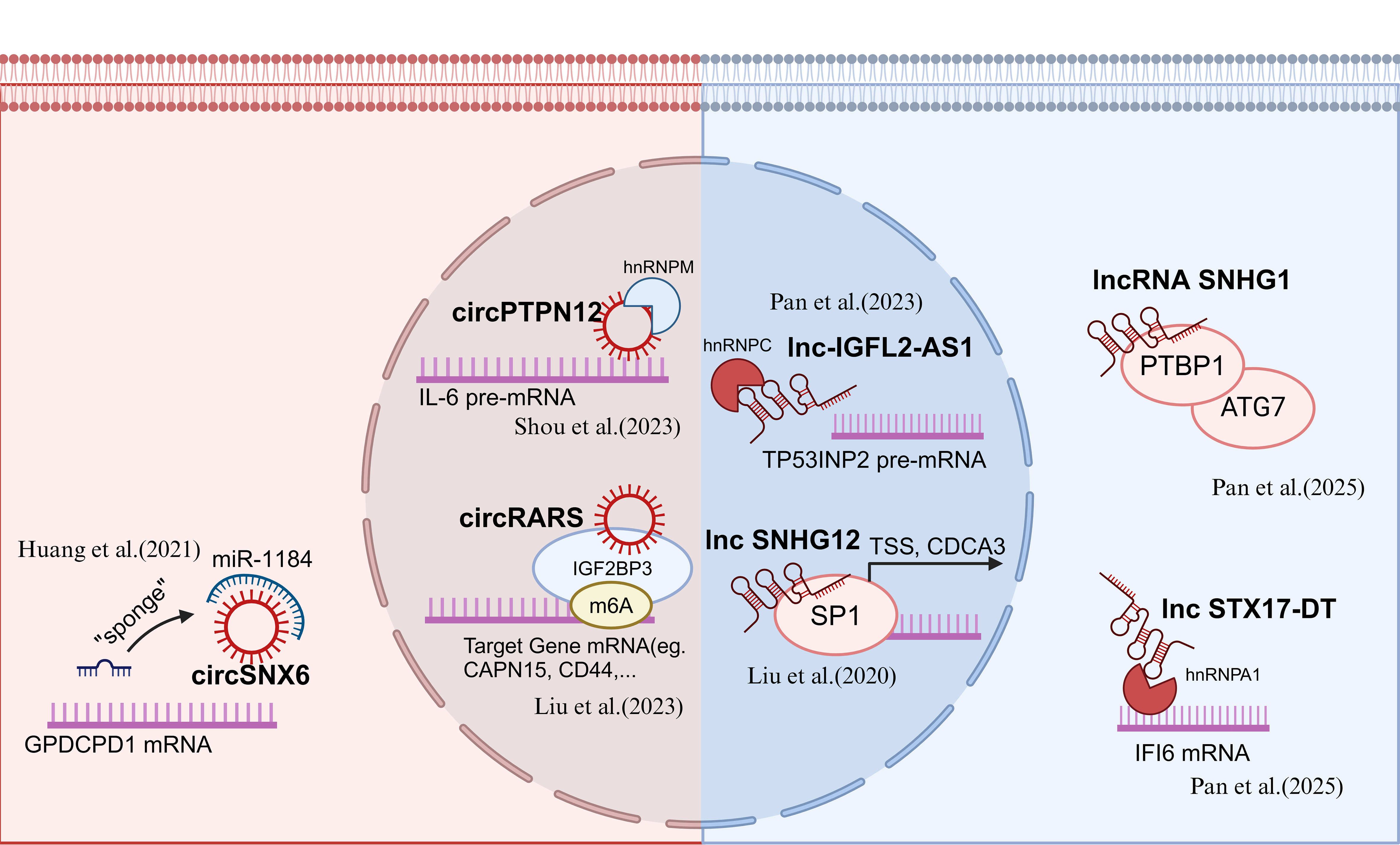
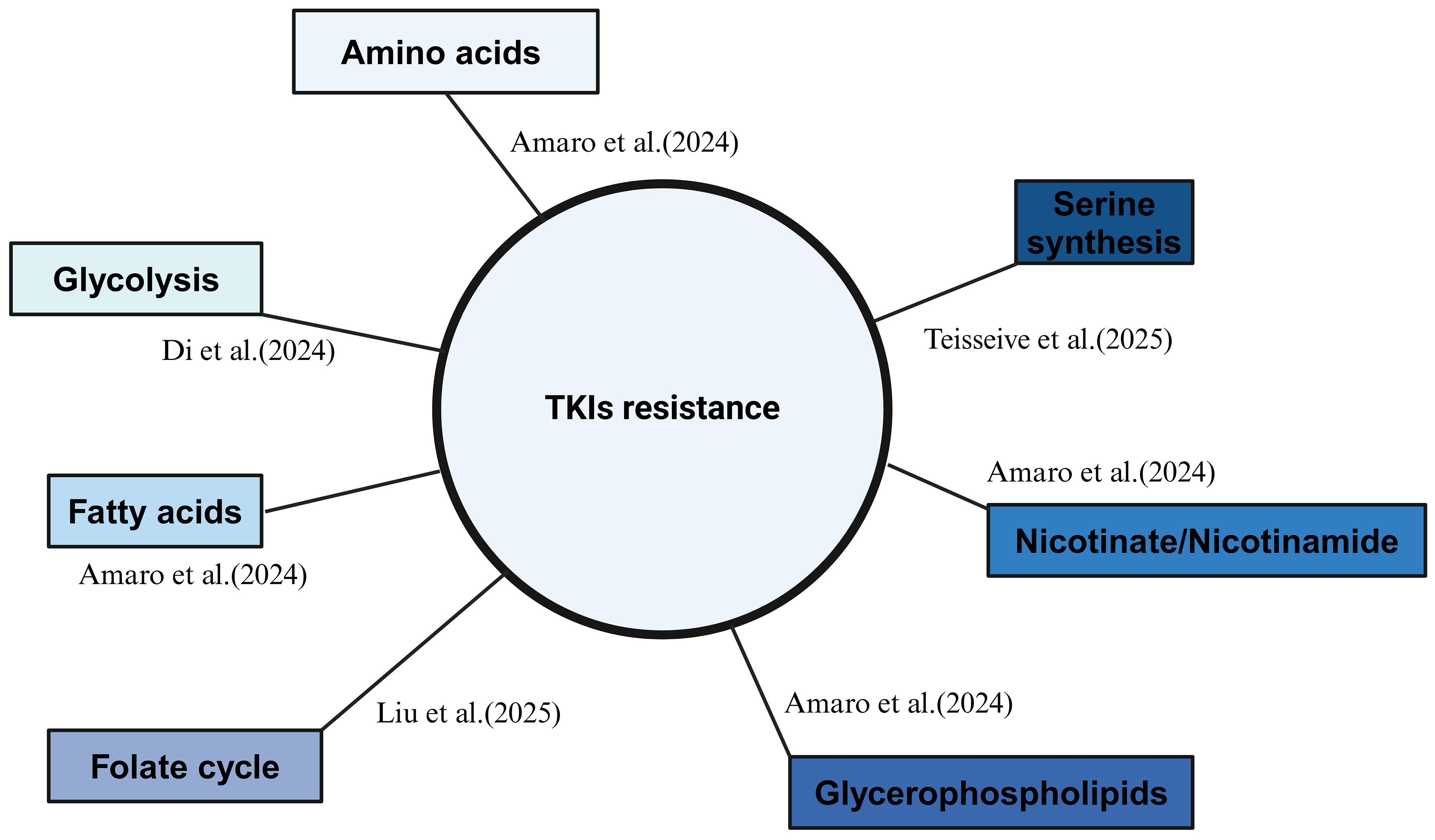
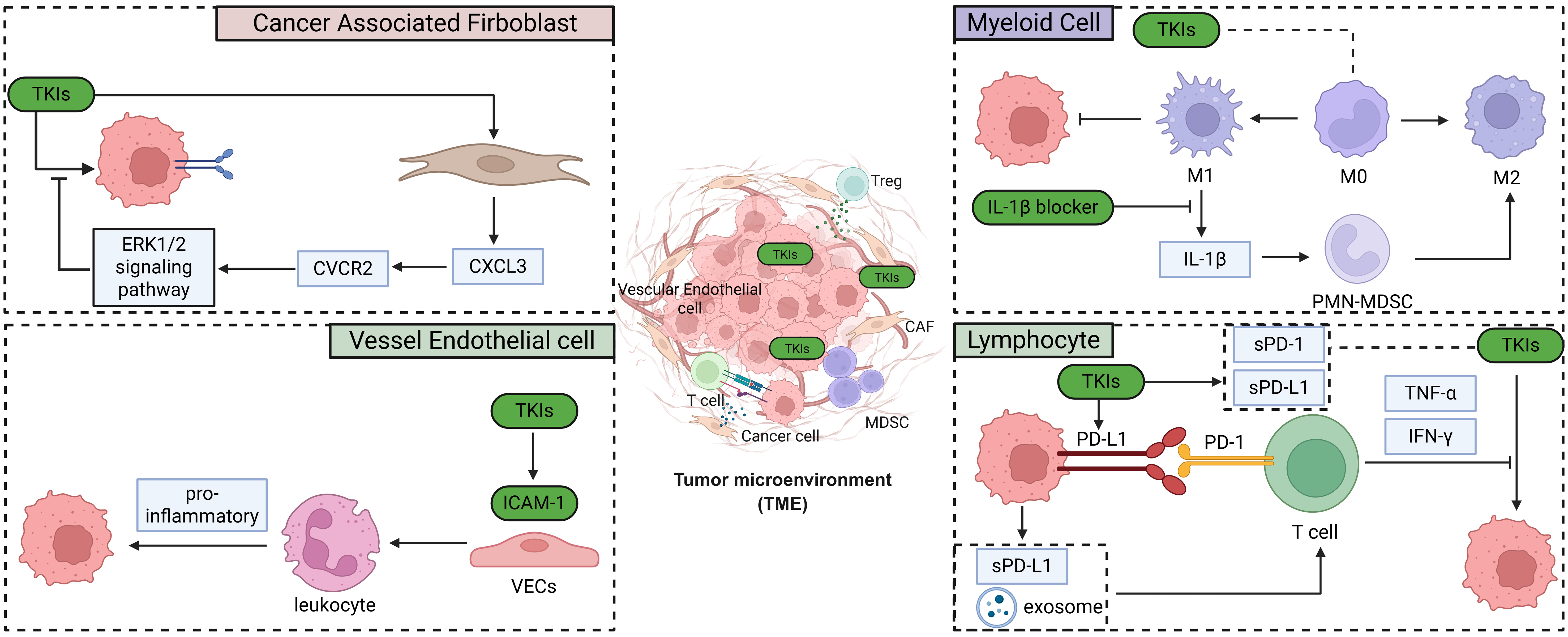












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].