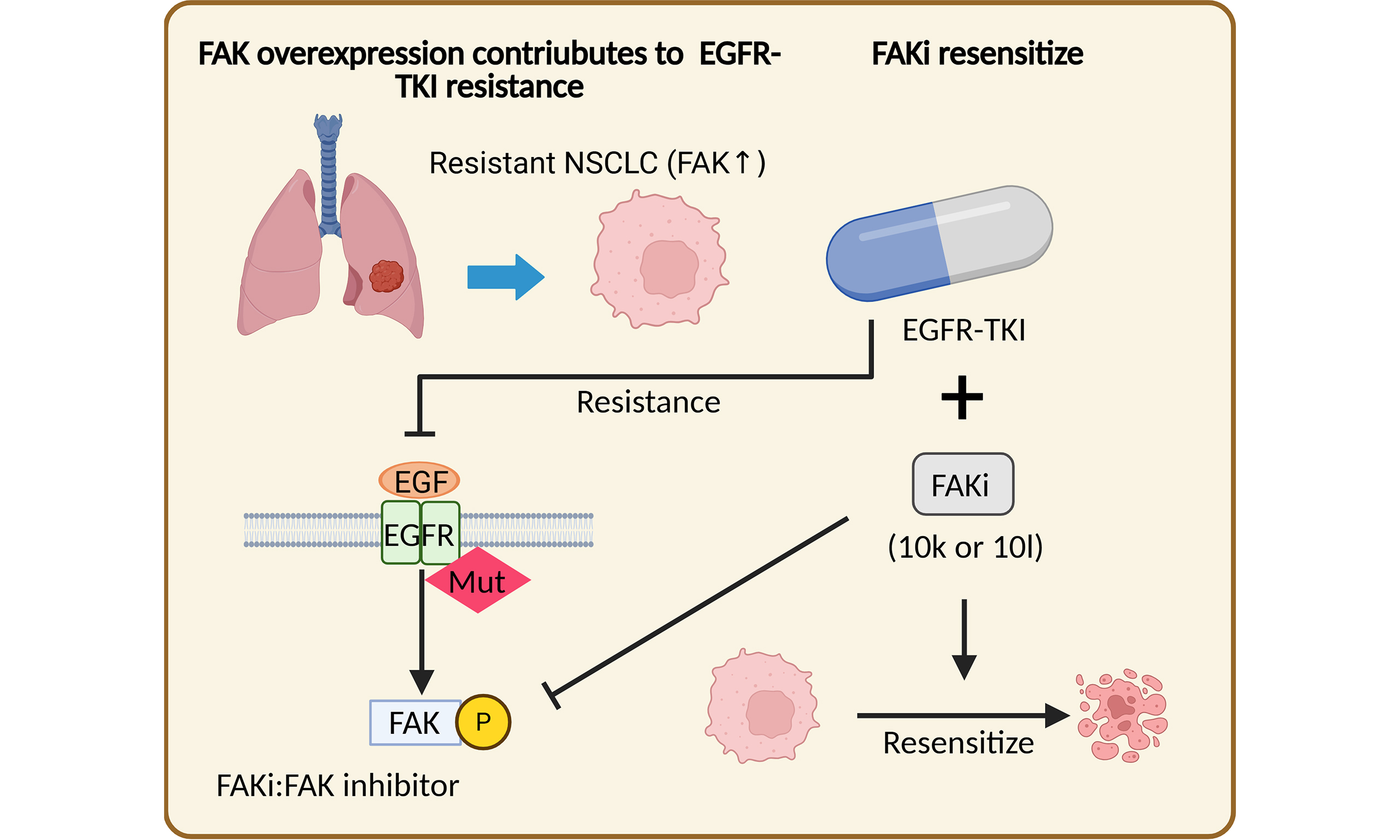
Novel FAK inhibitors suppress tumor growth and reverse EGFR-TKI resistance in non-small cell lung cancer
Abstract
Aim: The current study aims to investigate the critical role of the focal adhesion kinase (FAK) oncogenic signaling pathway in mediating drug resistance to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (EGFR-TKIs) and evaluate the potential of two novel FAK inhibitors, 10k and 10l, as therapeutic strategies for drug resistant non-small cell lung cancer (NSCLC).
Methods: EGFR-TKI resistance in NSCLC cells was developed via stepwise drug selection. Kinases/polymerase chain reaction (PCR) arrays identified key resistance determinants, while reverse transcription quantitative polymerase chain reaction (RT-qPCR), enzyme-linked immunosorbent assay (ELISA), and immunohistochemistry evaluated FAK messenger RNA and phosphorylation levels. Antitumor activities were assessed using sulforhodamine-B, clonogenic, wound-healing, and apoptosis assays, spheroids and xenografts.
Results: FAK was identified as a key driver of acquired resistance to EGFR-TKIs. High FAK expression predicted poor prognosis in patients treated with EGFR-TKIs. Kinase and PCR profiling confirmed elevated FAK levels as a resistance mechanism. Compounds 10k and 10l reduced phosphorylated FAK and showed strong anti-proliferative, anti-migratory, and pro-apoptotic effects in both EGFR-TKI-sensitive and -resistant cells. Notably, these compounds were shown to resensitize resistant NSCLC cells to EGFR-TKIs, with 10k inhibiting tumor growth and enhancing Osimertinib efficacy in resistant xenografts.
Conclusion: These findings not only uncover a pivotal mechanism of EGFR-TKI drug resistance but also highlight innovative, promising therapeutic options for patients with therapy-refractory disease.
Keywords
INTRODUCTION
Lung cancer is one of the most common malignant neoplasms worldwide and the leading cause of cancer-related mortality[1]. Non-small cell lung cancer (NSCLC) is the main pathological type of lung cancer, with approximately 30% of cases diagnosed at advanced stages with a dismal 5-year survival rate < 10%[2]. The discovery of oncogenic drivers in advanced NSCLC, particularly the epidermal growth factor receptor (EGFR), has paved the way for the development of tyrosine kinase inhibitors (TKIs), which significantly improve progression-free survival in NSCLC patients[3].
Several EGFR-TKIs have been approved for the treatment of NSCLC patients harboring EGFR mutations[3]. Unlike the reversible first generation (gefitinib/erlotinib), the second-generation EGFR-TKI Afatinib is an irreversible inhibitor; however, acquired resistance still commonly occurs after initial efficacy[4]. In approximately 50% of patients, drug resistance emerges due to the acquisition of the “gatekeeper” mutation T790M, leading to the spatial blockade of first or second-generation TKI binding. Although the third-generation Osimertinib can inhibit T790M, resistance and relapse still occur, with mechanisms including EGFR-dependent (e.g., C797S) and EGFR-independent [e.g., MET proto-oncogene, receptor tyrosine kinase (MET) amplification][5,6]. After resistance, the available options are limited and the eligible population is small[5,7]. Therefore, there is an urgent need to develop new therapies to overcome EGFR-TKI resistance.
Focal adhesion kinase (FAK), encoded by the protein tyrosine kinase 2 (PTK2) gene, is a cytoplasmic non-receptor tyrosine kinase that plays a crucial role in cancer cell adhesion, survival, proliferation, DNA repair and metastasis through its cross-linked processes with Src, integrin and growth factor receptor signaling pathways[8,9]. FAK is often overexpressed and activated in some cancers and elevated levels of FAK are associated with poor prognosis in several cancers[9,10]. Notably, EGFR activates FAK through interactions with signaling molecules such as Src kinase. Activation of Src kinase leads to phosphorylation of FAK to promote its binding with other signaling molecules [such as growth factor receptor-bound protein 2 (Grb2), phosphatidylinositol 3-kinase (PI3K), etc.], further activating several downstream signaling pathways[11-13]. Therefore, targeting FAK can not only inhibit the progression of NSCLC, but may also reverse the acquired resistance of EGFR-TKI[13,14]. There is, however, a divergence in findings regarding FAK expression in EGFR-TKI resistant cell lines; some studies[15] have reported high expression, whereas surprisingly, others[16] have found low expression of FAK in EGFR-TKI resistant cell lines. Although FAK expression does not generally correlate with the overall survival (OS) of lung cancer patients[17-19], no research has specifically investigated its association with OS in patients receiving EGFR-TKI treatment. Therefore, the relationship between FAK and EGFR-TKI resistance warrants further exploration.
Several FAK inhibitors have entered clinical trials as monotherapies or in combination with chemotherapy and other targeted therapies; however, progress has been slow, with some trials ending due to suboptimal outcomes[20,21]. Although the most promising FAK inhibitor, Defactinib, has been approved for combination therapy with Avutometinib in low-grade serous ovarian cancer, it has not shown clear efficacy in clinical trials for other cancers, including NSCLC[20,22,23]. Therefore, exploring new FAK inhibitors is of significant preclinical and clinical interest.
This study integrated data from patients treated with EGFR-TKI and kinase/polymerase chain reaction (PCR) arrays of two drug-resistant cell lines [PC9 Afatinib resistant (PC9AR), H1975 Osimertinib resistant (H1975OR)]. The results showed that in The Cancer Genome Atlas (TCGA)-NSCLC database, FAK was not related to OS, but in our NSCLC patients receiving EGFR-TKI cohort, high FAK expression indicated poor prognosis; resistant cells likewise showed FAK upregulation, and FAK was identified as a resistance “hub” gene. Our newly synthesized FAK inhibitors 10k/10l [10k: 3-(6-Furan-2-yl-imidazo[2,1-b][1, 3, 4]thiadiazol-2-yl)-1H-indole; 10l: 3-(6-Furan-2-yl-imidazo[2,1-b][1, 3, 4]thiadiazol-2-yl)-1-methyl-1H-indole] significantly inhibited proliferation across multiple models[24], showed synergy with EGFR-TKI in resistant cells, and 10k suppressed tumors and restored sensitivity to Osimertinib in a drug-resistant xenograft model.
METHODS
Generation of drug-resistant tumor cells
PC9 Afatinib-resistant (AR) and H1975 Osimertinib-resistant (OR) sublines were generated through stepwise drug selection over 6-8 months. Drug concentrations were gradually increased until stable resistance developed. Half-maximal inhibitory concentration (IC50) values were periodically assessed, and cells were drug-free for at least 2 weeks before experiments.
Further information on Cell cultures, drugs and reagents, as well as on Cell mutations and copy number analyses is reported in the Supplementary Methods.
Evaluation of cell growth proliferation using the sulforhodamine B assay and pharmacological interaction
Sulforhodamine B (SRB) assay was performed as previously described[25]. Cells (5,000/well in 100 μL medium) were seeded in a 96-well plate and incubated overnight. After 72 h drug treatment, cells were fixed with 50% trichloroacetic acid (25 μL/well, 4 °C, 1 h), washed, and air-dried. SRB dye (0.4%, 50 μL/well) was added to each well and incubated for 30 min, and then removed with 1% acetic acid wash. After air-drying, SRB was dissolved in
Studies on multicellular spheroids, migration and apoptosis
Multicellular spheroids were formed by seeding 3,000 cells/well in ultra-low attachment (ULA) plates, followed by treatment and imaging. After 4 days, spheroids were dissociated, and cell proliferation was assessed using a resazurin assay. For migration, we used a wound healing assay. Additional details are provided in the Supplementary Methods.
Enzyme-linked immunosorbent assay for phosphorylated FAK, Akt and apoptosis
Phosphorylated FAK (p-FAK) levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Thermo Fisher)[27]. Samples from in vitro and in vivo studies were incubated with detection antibody, followed by horseradish peroxidase (HRP)-conjugated secondary antibody, and absorbance was measured at 450 nm using a BioTek plate reader. Similarly, phosphorylated Akt levels were measured using a specific ELISA assay (Invitrogen), while apoptosis induction was evaluated by Annexin V and Caspase-3 assays, as described in the Supplementary Methods.
PCR and kinase activity profiling arrays, bioinformatics, docking and in vivo studies
Gene expression was evaluated using reverse transcription quantitative polymerase chain reaction (RT-qPCR), PCR arrays, while kinase activity profiling was conducted with PamChip® arrays. Bioinformatics analyses, molecular docking studies and in vivo antitumor activity assessments in mouse models were also performed. Detailed descriptions of these methodologies are provided in the Supplementary Methods.
Patient tissues and immunohistochemistry
Between 2006 and 2020, NSCLC tissue samples were collected from patients at multiple institutions under approved protocols. All cases were confirmed as NSCLC, and patients had received EGFR-TKI treatment. Immunohistochemistry (IHC) was performed to assess p-FAK expression using anti-p-FAK antibodies, followed by staining evaluation based on intensity and positive staining percentage. Samples were classified as “high” or “low” p-FAK based on the median staining score. Detailed methods are provided in the Supplementary Methods.
Statistical analysis
Experiments were performed in triplicate and repeated twice. Cell migration percentages were calculated from at least six scratches. Data analysis used Excel, SPSS (v19.0), R Studio, and GraphPad Prism (v9.0). Statistical significance was determined by Student’s t-test or one-way Analysis of Variance, with P < 0.05 considered significant. Normality was assessed through Shapiro-Wilk testing. Survival analysis was conducted using the Kaplan-Meier method with log-rank testing, and FAK expression correlations were assessed by Spearman’s rank test.
RESULTS
Establishment of EGFR-TKI resistant cells
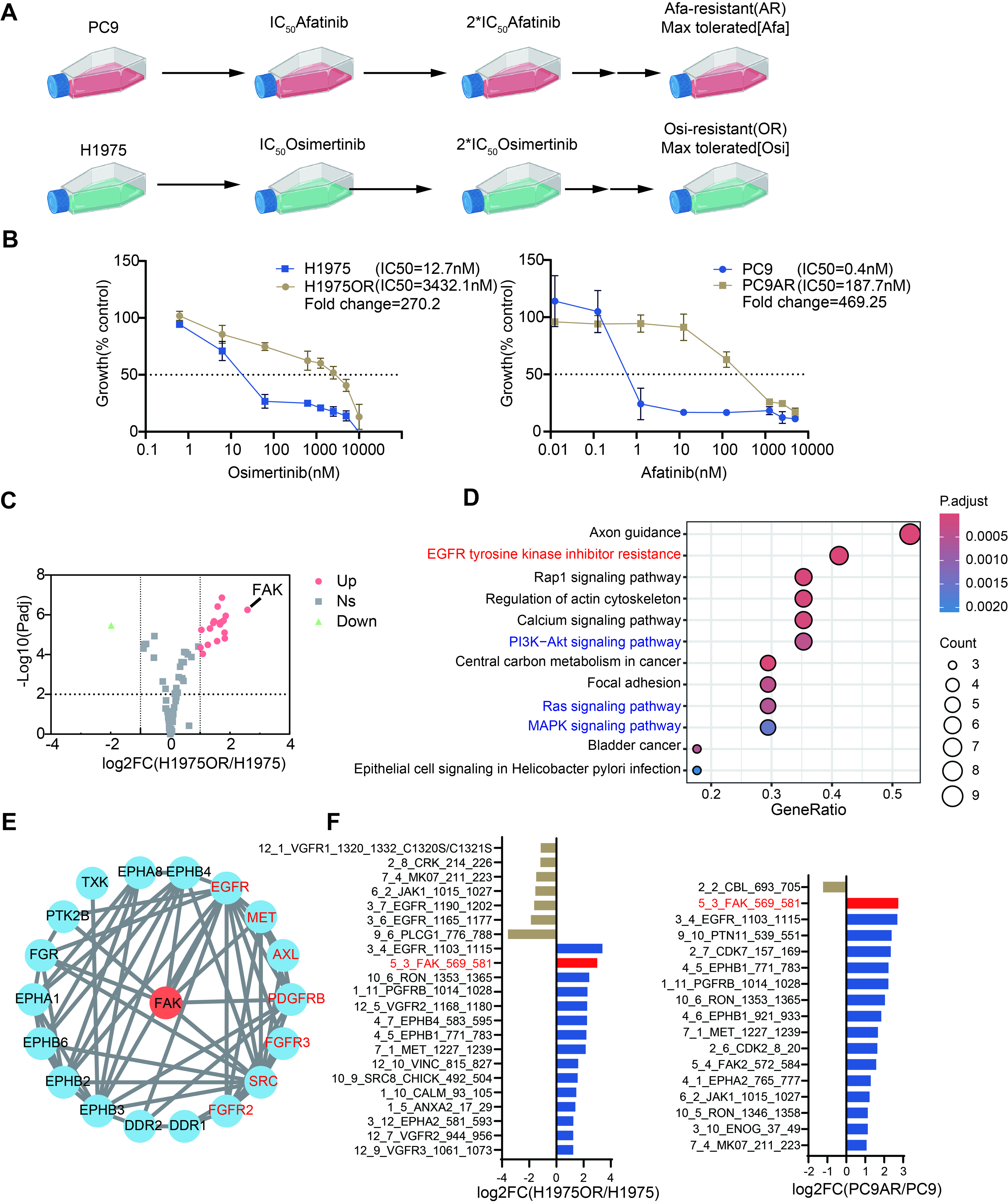
To investigate the relationships between FAK and EGFR-TKI resistance, we used two commonly used EGFR mutant NSCLC cell lines, PC9 and H1975[28]. By exposing these cells to gradually increasing concentrations of Afatinib or Osimertinib for over 6 months, we established EGFR-TKI resistant sublines PC9AR and H1975OR [Figure 1A]. The SRB assay showed that the IC50 values of EGFR-TKI resistant cells were hundreds-fold higher than their parental EGFR-TKI sensitive counterpart cells [Figure 1B]. However, we found that PC9AR cells were not resistant to Osimertinib, whereas H1975OR cells were highly resistant to both Osimertinib and Afatinib [Supplementary Figure 1A and B].
Figure 1. Establishment of NSCLC cell lines resistant to Osimertinib and Afatinib and studies on the key role of FAK in the acquired resistance to EGFR-TKIs in NSCLC. (A) H1975OR and PC9AR cells were established using a gradual stepwise selection protocol as illustrated in the figure [Created in BioRender. Xu, G. (2025)]; (B) H1975/H1975OR and PC9/PC9AR cells were treated with increasing concentrations of indicated drugs (Osimertinib or Afatinib) for 72 h. The growth rate was measured by the SRB assay. (IC50 values were calculated by GraphPad Prism and represented as mean ± standard deviation, n = 3); (C) H1975 and H1975OR cells were cultured in 6-well plates and harvested for preparation of total RNA and subsequent gene expression analyses. The Volcano plot shows the differential expression of protein kinase genes between H1975 and H1975OR cells. Log2Foldchange > 1 or < -1 and Log10 (Padj) > 2 indicate significant differences; (D) KEGG pathway enrichment analysis of significantly DEGs and (E) PPI analysis for DEGs. The genes in EGFR-TKI resistance pathway are depicted in Red; (F) NSCLC cell lines were cultured in 6-well plates and harvested for protein and subsequent PAMchip analyses. The left figure panel shows the difference in phosphorylated protein kinases between H1975 and H1975OR cells. The right panel includes data from PC9 and PC9AR cells. A log2 fold change > 2 was selected as the cutoff value. Kinases with reduced activity are depicted in yellow, while kinases with enhanced activity are in blue; p-FAK is shown in Red. NSCLC: Non-small cell lung cancer; FAK: focal adhesion kinase; EGFR: epidermal growth factor receptor; TKIs: tyrosine kinase inhibitors; H1975OR: H1975 Osimertinib resistant; PC9AR: PC9 Afatinib resistant; SRB: sulforhodamine B; IC50: half-maximal inhibitory concentration; KEGG: Kyoto Encyclopedia of Genes and Genomes; DEGs: differentially expressed genes; PPI: protein-protein interaction; p-FAK: phosphorylated focal adhesion kinase; Afa: Afatinib; Osi: Osimertinib; PI3K: phosphatidylinositol 3-kinase; MAPK: mitogen-activated protein kinase.
Batches of resistant cells used in experiments were maintained in drug-free medium for 2 months and resistance to the maximal tolerated concentration was analyzed by SRB assay at regular intervals. H1975 cells harbor the double EGFR exon 21 L858R mutation in cis with the T790M mutation in exon 20, whereas PC9 cells carry an E746-A750 deletion in exon 19 of the EGFR gene (c.2235_2249del15). These resistant variants retained their original mutations without acquiring additional EGFR mutations, such as the C797S mutation in exon 20 (observed in 10%-26% of cases with second-line Osimertinib resistance cases[5]), nor MET gene amplification. These results indicate that these drug resistant model tumor cell lines were genetically stable and constitute a valid model to uncover novel molecular mechanisms of EGFR-TKI resistance.
FAK is a key driver gene of EGFR-TKI resistance in NSCLC
We then analyzed the profiles of oncogenic kinases in EGFR-TKI resistant and sensitive cells. The differentially expressed genes (DEGs) between H1975 and H1975OR cells are depicted in Figure 1C. In particular, we found a total of 16 significantly upregulated genes and 1 significantly downregulated gene in H1975OR cells, with FAK being the most significantly upregulated transcript [Figure 1C]. Figure 1D highlights the various pathways enriched among DEGs through KEGG pathway analysis. Among these pathways, the mitogen-activated protein kinase (MAPK) signaling, PI3K-AKT, and rat sarcoma virus oncogene (RAS) pathways have been identified as significantly associated with EGFR-TKI resistance, consistent with previous studies[29]. Additionally, protein-protein interaction (PPI) Network analysis identified FAK as a central or “hub” gene among the DEGs [Figure 1E].
Since the phosphorylated form represents the active state of protein kinases, we used the PamChip array to analyze kinase phosphorylation profiles in EGFR-TKI resistant cells. In particular, the PamChip technology utilizes peptide sequences derived from known kinase substrates, representing various canonical kinase-dependent pathways, as described previously[30]. This approach enables the assessment of kinase activity across multiple pathways simultaneously. Our analysis revealed that p-FAK was among the most significantly upregulated kinases in both EGFR-TKI resistant variants [Figure 1F], supporting its potential role in drug resistance mechanisms.
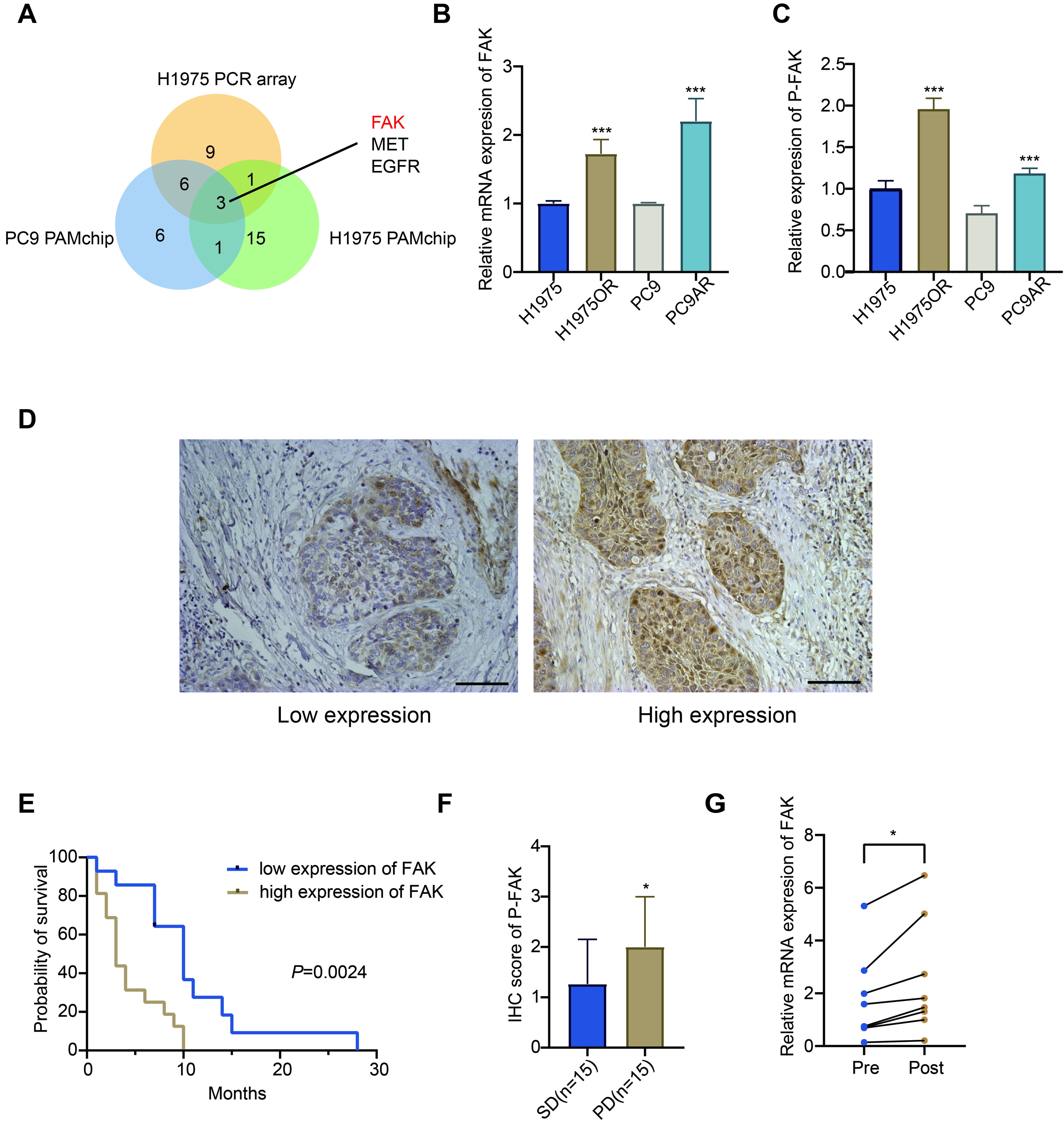
To further understand the functional enrichment of resistance mechanisms, Gene Set Enrichment Analysis (GSEA) was applied. GSEA is a computational method that determines whether an a priori defined set of genes displays statistically significant differences between two biological states - in this case, between EGFR-TKI sensitive and drug resistant cells. This GSEA analysis indicated a significant activation of EGFR-TKI resistance-related pathways in the H1975OR cells [Supplementary Figure 2A]. Specifically, GSEA enrichment identified several genes within the EGFR-TKI resistance axis, as shown in Supplementary Figure 2B. This enrichment of genes underscores their contribution to resistance phenotypes, providing potential therapeutic targets. Furthermore, an intersection analysis of the PamChip phosphorylation data with the gene expression results identified EGFR, MET, and FAK as the sole genes exhibiting upregulated expression at both the transcript and protein phosphorylation levels across the two TKI-resistant tumor cell line models [Figure 2A]. Consistently, the MET gene has been reported to be implicated in EGFR-TKI resistance in multiple studies[31,32].
Figure 2. FAK emerges as a biomarker of poor prognosis in NSCLC patients treated with EGFR-TKI. (A) Venn diagram showing genes upregulated in both the PCR array and PAMchip analyses; (B) mRNA expression of FAK was compared between H1975 and H1975OR cells using RT-qPCR. Data were analyzed by DDCt method, as described previously[51]; (C) p-FAK levels were measured by ELISA assay in TKI-resistant vs. TKI-sensitive cells; (D) Representative IHC images illustrating p-FAK expression in NSCLC patient tissue samples: left panel showing low expression, right panel showing high expression. Scale bars: 100 µm; (E) Survival analysis of NSCLC patients treated with EGFR-TKI, comparing low vs. high p-FAK levels; (F) IHC scoring of p-FAK in 30 NSCLC tissue samples. Patients with PD or SD upon EGFR-TKI treatment were compared; (G) Expression of FAK mRNA in 14 NSCLC biopsy samples, before (pre) and after (post) treatment. Data were analyzed by a standard curve method. Statistical significance: P-values were set as follows: *P < 0.05, ***P < 0.001. The graphs were created with GraphPad Prism. FAK: Focal adhesion kinase; NSCLC: non-small cell lung cancer; EGFR: epidermal growth factor receptor; TKI: tyrosine kinase inhibitor; PCR: polymerase chain reaction; mRNA: messenger RNA; H1975OR: H1975 Osimertinib resistant; RT-qPCR: reverse transcription quantitative polymerase chain reaction; DDCt: Delta Delta Ct; p-FAK: phosphorylated focal adhesion kinase; ELISA: enzyme-linked immunosorbent assay; IHC: immunohistochemistry; PD: progressive disease; SD: stable disease; MET: MET proto-oncogene, receptor tyrosine kinase.
The close association between genes enriched in the EGFR-TKI resistance pathway and FAK suggests that FAK might orchestrate critical resistance-associated pathways. This supports the hypothesis that FAK plays a pivotal role in mediating resistance mechanisms in EGFR-TKI-treated cells, potentially offering new avenues for therapeutic intervention to overcome resistance.
Next, RT-qPCR analysis confirmed that the FAK gene was highly expressed in EGFR-TKI resistant cell lines [Figure 2B]. To validate these findings at the active protein level, an ELISA assay was conducted, confirming the increased levels of p-FAK in the EGFR-TKI resistant cell lines [Figure 2C].
FAK expression is upregulated in NSCLC patients and is correlated with poor prognosis of NSCLC patients treated with EGFR-TKI
The expression of FAK in NSCLC tissues was initially analyzed in the TCGA database, where we found that FAK messenger RNA (mRNA) levels were significantly higher in LUAD tissue [Supplementary Figure 3A] and LUSC tissue [Supplementary Figure 3B] compared to normal tissue. However, using TCGA-lung adenocarcinoma (LUAD) [Supplementary Figure 3C] and TCGA-lung squamous cell carcinoma (LUSC)
To further investigate the specific clinical impact of p-FAK in patients treated with EGFR-TKIs, we conducted IHC analysis in a cohort of NSCLC patients who received EGFR-TKI therapy. Table 1 depicts the characteristics of this cohort, and Figure 2D shows representative images of low and high p-FAK expression in two representative tissues. In this cohort, factors such as gender, age, smoking history, and pathological type were not found to be associated with OS [Supplementary Table 1]. However, survival analysis revealed a negative correlation between p-FAK levels and OS in patients treated with EGFR-TKI (P = 0.0024; Figure 2E). In addition to p-FAK, the performance status (PS) and the stage of NSCLC were identified as significant risk factors for OS [Supplementary Table 1]. However, we did not observe a correlation between p-FAK levels and these, or other clinical-pathological features of lung cancer, except for the observation that older patients exhibited lower p-FAK expression, as reported in Table 1. This finding was unexpected and has not been previously reported; however, age was not correlated with FAK expression nor response to EGFR-TKIs in previous studies[33,34]. Remarkably, p-FAK was identified not only as a marker for OS, but also as a predictor of treatment response. Among the 30 patients treated with EGFR-TKIs, p-FAK levels were significantly higher in the progressive disease (PD) group compared to the stable disease (SD) group [Figure 2F].
Association between p-FAK expression and clinicopathological features of lung cancer
| Characteristic | Patients | p-FAK expression | P-value | |
| n (%) | Low expression | High expression | ||
| Gender | 0.355 | |||
| Male | 21 (70) | 11 | 10 | |
| Female | 9 (30) | 3 | 6 | |
| Age (years) | 0.031* | |||
| ≤ 65 | 22 (73) | 9 | 13 | |
| > 65 | 8 (27) | 5 | 3 | |
| Stage | 0.064 | |||
| I/II | 8 (27) | 6 | 2 | |
| III/IV | 22 (73) | 8 | 14 | |
| PS | 0.094 | |||
| 0/1 | 27 (90) | 14 | 13 | |
| 2 | 3 (10) | 0 | 3 | |
| Smoking | 0.529 | |||
| Yes | 25 (83) | 11 | 14 | |
| No | 5 (17) | 3 | 2 | |
| Pathological type | 0.529 | |||
| SPINO | 7 (23) | 4 | 3 | |
| ADC | 21 (70) | 9 | 12 | |
| NAS | 2 (7) | 1 | 1 | |
Lastly, we analyzed the modulation of FAK mRNA levels in 7 patients before and after EGFR-TKI treatment. Despite the limited sample size, pre-treatment FAK mRNA levels in these patients correlated with p-FAK levels [Supplementary Figure 4]. Notably, in all these patients who experienced disease progression post-treatment, FAK transcript levels were significantly elevated [Figure 2G]. These findings suggest that increased FAK levels may serve as a potential monitoring biomarker for acquired resistance to EGFR-TKIs in serial biopsies from NSCLC patients.
Notably, p-FAK expression levels were found to be consistently elevated following treatment with EGFR-TKI also in our NSCLC models, as shown in Figure 3A-D. Drug resistance in cancer cells is often linked to dynamic changes in gene expression that occur as an adaptive response to therapeutic agents. In drug-sensitive cells, initial exposure to a drug may induce a transient upregulation of certain genes as part of a stress response. However, when gene upregulation persists and becomes stabilized in drug-resistant variants, it suggests that the gene may play a protective role, facilitating cellular adaptation and survival in the presence of the drug[35,36]. Therefore, we hypothesize that the persistent upregulation and activation of FAK are the result of signaling cascades promoting cellular survival in drug-resistant cells, as described in previous studies in platinum and taxane-resistant tumors[37,38]. This prompted the following studies with new FAK inhibitors.
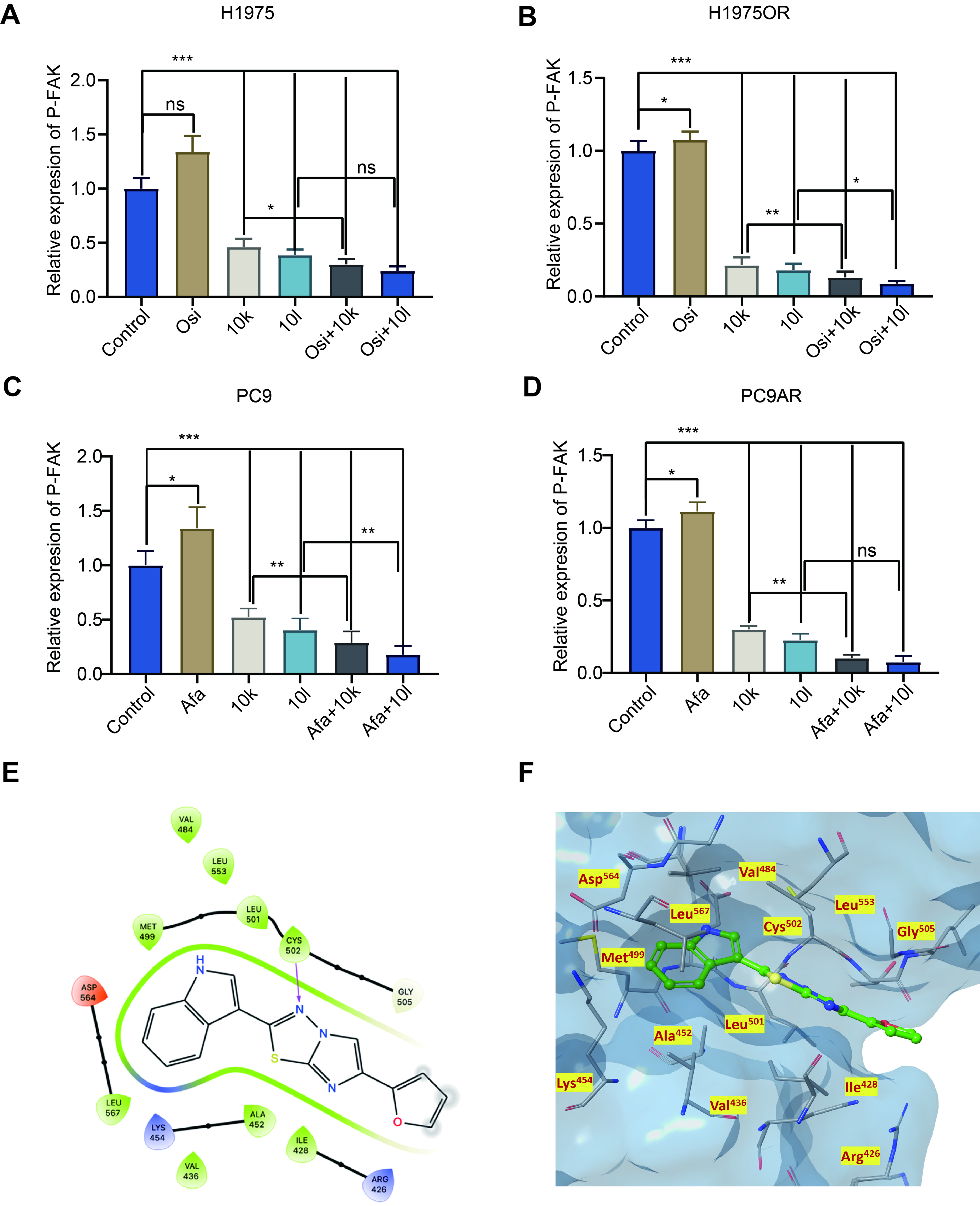
Figure 3. Structure and kinase inhibitory activities of the new FAK inhibitors. (A-D) NSCLC cells (PC9, PC9AR, H1975, and H1975OR) were treated with Afatinib, Osimertinib, 10k, 10l, or their combinations for 24 h, followed by protein extraction. The levels of p-FAK were measured using an ELISA assay. NSCLC Cells were treated with each compound at concentrations corresponding to their respective IC50 values for the indicated cell lines; (E) 2D ligand interaction diagram for compound 10k; (F) 3D complex of the FAK catalytic domain (PDB code: 6YOJ) with compound 10k; nitrogen, oxygen, and sulfur atoms are shown in blue, red, and yellow, respectively. Statistical significance: P-values were set as follows: *P < 0.05, **P < 0.01, ***P < 0.001, “ns” indicates a non-significant difference. The graphs were created with GraphPad Prism. FAK: Focal adhesion kinase; NSCLC: non-small cell lung cancer; PC9AR: PC9 Afatinib resistant; H1975OR: H1975 Osimertinib resistant; P-FAK: phosphorylated focal adhesion kinase; ELISA: enzyme-linked immunosorbent assay; IC50: half-maximal inhibitory concentration; 2D: two-dimensional; 3D: three-dimensional; PDB: Protein Data Bank; Afa: Afatinib; Osi: Osimertinib.
The structure and kinase inhibitory activities of the new FAK inhibitors 10k and 10l
In a previous study, we synthesized imidazo[2,1-b][1,3,4]thiadiazole derivatives as FAK inhibitors[24], and their chemical structures are shown in Supplementary Figure 5A. We initially tested these compounds in pancreatic cancer cell lines, where derivatives 10k and 10l exhibited significant cytotoxic activity. In the current study, we tested the antitumor activities of 10k and 10l in NSCLC cells. Additionally, we also performed additional molecular docking studies to investigate the binding modes of 10k and 10l to the FAK protein. This analysis revealed that both derivatives can bind to the catalytic domain of FAK, interacting with key amino acids at the binding site. In particular, derivative 10k, with the imidazo[2,1-b][1,3,4]thiadiazole core, interacts with the hinge region by forming hydrogen bonds with the C=O group of the Cys502 residue. Additionally, the gatekeeper residue (Met499) and a solvent region (Arg426, Ile428, Ala452, and Leu567) participate in electrostatic interactions with 10k [Figure 3E and F]. Interestingly, Cys502 was not favored for binding by either of the two inhibitors. However, as shown in Supplementary Figure 5B and C, compound 10l forms a hydrogen bond with Glu506 through the oxygen atom of its furan ring. We then evaluated the inhibitory effects of 10k and 10l on FAK using ELISA assays. Treatment of NSCLC cell lines with 10k and 10l led to a significant reduction in p-FAK levels [Figure 3A-D]. While Osimertinib and Afatinib increased p-FAK expression, the combination of 10k or 10l with these EGFR-TKIs resulted in a more pronounced reduction in p-FAK levels compared to monotherapy treatments [Figure 3A-D]. In conclusion, 10k and 10l markedly reduced p-FAK levels and these findings prompted further investigation to explore whether this inhibition might affect various signaling pathways downstream of FAK, particularly those involved in cell survival and migration.
The new FAK inhibitors 10k and 10l inhibit cell proliferation and migration in both EGFR-TKI sensitive and resistant tumor cells
The compounds 10k and 10l inhibited cell proliferation in a dose-dependent manner, with IC50 values ranging from 2 to 9 μM across PC9, PC9AR, H1975, and H1975OR cell lines [Supplementary Table 2]. The IC50 values of both compounds were comparable between EGFR-TKI-sensitive and drug-resistant cell lines
The FAK signaling pathway plays a crucial role in cell migration across various tumor types, including NSCLC[9]. To evaluate the effects of 10k and 10l on cell migration, we conducted a wound healing assay. As shown in Supplementary Figure 6E and F, both 10k and 10l significantly inhibited the migration of PC9, PC9AR, H1975 and H1975OR cells. Additionally, treatment with them led to downregulation of the mRNA levels of a key regulator of cell migration, matrix metalloproteinase-9 (MMP9, Supplementary Figure 7).
The new FAK inhibitors 10k or 10l increase apoptosis and necrosis of EGFR-TKI sensitive and resistant cells
After treatment with 10k or 10l, we observed significant changes in cell morphology, including cell shrinkage, increased cytoplasmic density, and fragmentation, which are characteristic features of cell death induction [Supplementary Figure 8A]. To determine whether the inhibition of cell proliferation by 10k or 10l was due to apoptosis or necrosis, we evaluated the levels of Annexin V, PI, and BCL2 in NSCLC cell lines. Annexin V is a marker of apoptosis, PI indicates necrosis, whereas BCL2 is a well-established anti-apoptotic protein. Following treatment with 10k or 10l, NSCLC cells were double-stained with Annexin V/PI. Representative images of Annexin V staining levels after drug treatment are shown in Supplementary Figure 8B. We found that both 10k and 10l increased Annexin V and PI levels in all tested cell lines
The new FAK inhibitors 10k or 10l restore sensitivity of EGFR-TKI resistant cells to Afatinib and Osimertinib
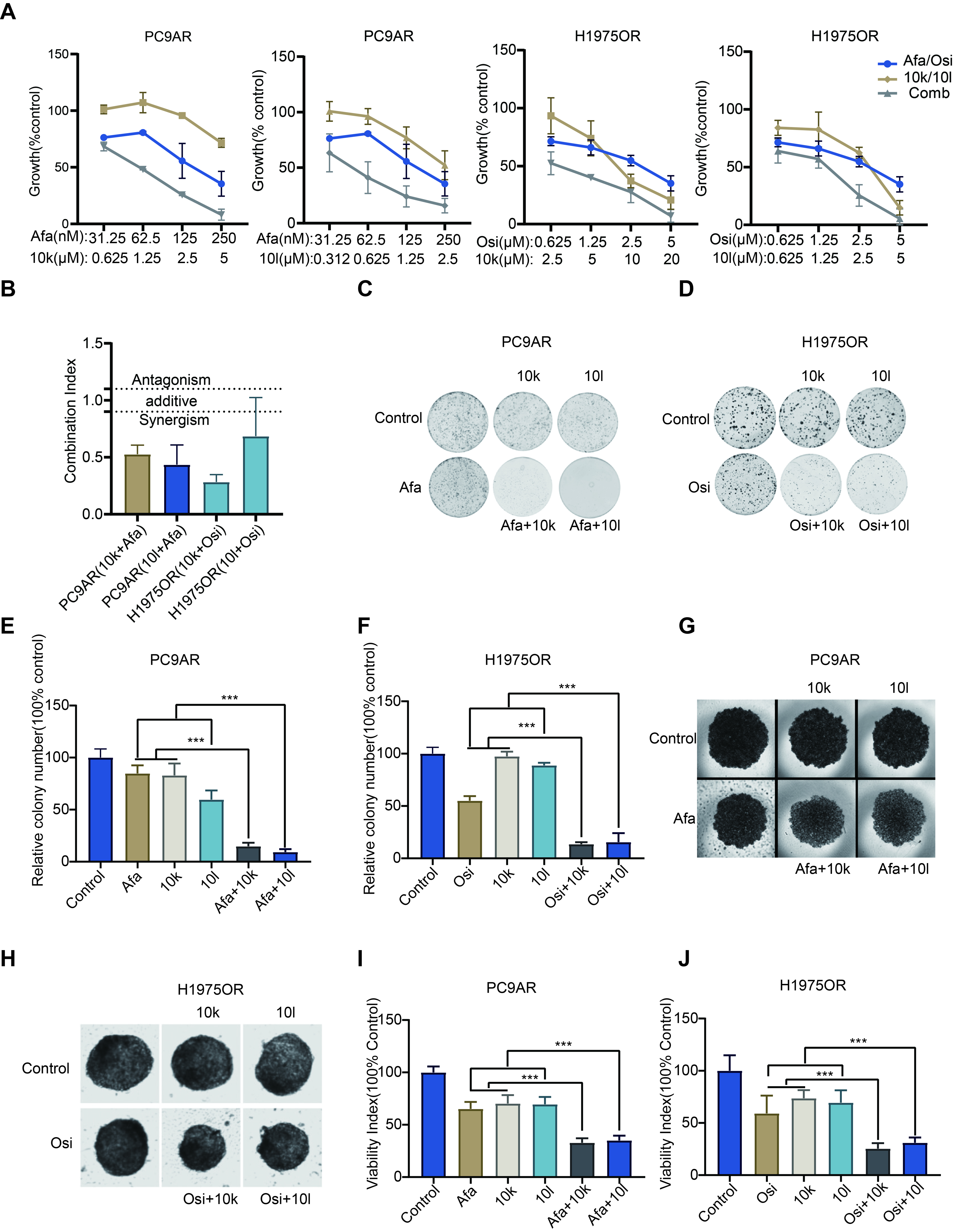
Our findings indicate that highly activated FAK may contribute to EGFR-TKI resistance. Hence, we examined whether blocking FAK with 10k or 10l can restore the sensitivity of EGFR-TKI resistant cells to Afatinib and Osimertinib. As shown in Figure 4A, the combination of Afatinib and Osimertinib with 10k or 10l was more efficacious in blocking the growth of PC9AR or H1975OR cells at different concentrations, compared with single drug treatments. All combination indices (CIs) of different combinations were very low and significantly below 1, hence indicating a synergistic interaction [Figure 4B].
Figure 4. The new FAK inhibitors 10k and 10l enhance EGFR-TKI sensitivity in drug resistant cells. (A) PC9AR and H1975OR cells were treated with the indicated concentrations of the tested drugs, either alone or in combination, and growth rates were measured using the SRB assay after 72 h. The compounds 10k and 10l treated the same cell line in one experiment; (B) CIs at 50 %, 75%, and 90% values of fraction affected revealed a synergistic interaction between the drugs; (C and D) PC9AR and H1975OR cells were plated in 6-well plates (1 × 103 cells/well) and treated for 10 days with indicated compounds. Representative images of colony formation are shown; (E and F) Quantitative analysis of colony formation results. PC9AR cells: treated with Afatinib, 10k, 10l at 0.125 × IC50 (PC9AR cells) or combination, H1975OR cells: treated with Osimertinib, 10k, 10l at 0.125 × IC50 (H1975OR cells) or combination; (G and H) Spheroids generated from PC9AR and H1975OR cells were treated for 4 days with indicated compounds. PC9AR cells: treated with Afatinib at 0.125 × IC50 (PC9AR cells) and 10k or 10l at 1.5 × IC50 (PC9AR cells) or combination, H1975OR cells: treated with Osimertinib at 0.125 × IC50 (H1975OR cells) and 10k, 10l at 1 × IC50 (H1975OR cells) or combination. Representative images of spheroids are shown; (I and J) Viability of spheroids was assessed using the resazurin assay. Statistical significance was set as follows: ***P < 0.001. The graphs were created with GraphPad Prism. FAK: Focal adhesion kinase; EGFR: epidermal growth factor receptor; TKI: tyrosine kinase inhibitor; PC9AR: PC9 Afatinib resistant; H1975OR: H1975 Osimertinib resistant; SRB: sulforhodamine B; CIs: combination indices; IC50: half-maximal inhibitory concentration; Afa: Afatinib; Osi: Osimertinib.
Moreover, we evaluated the anticancer activity of the drug combinations using colony formation [Figure 4C-F] and the cell spheroid assay [Figure 4G-J]. All drug combinations more effectively blocked colony formation and the growth of cell spheroids compared to single drugs. In particular, the combination of Afatinib and Osimertinib with 10k or 10l was synergistic also in three-dimensional tumor spheroid models. Furthermore, we detected significantly higher levels of Annexin V and PI in EGFR-TKI resistant cells treated with different drug combinations compared to single drug treatments [Supplementary Figure 10A-D]. In summary, these findings demonstrate that the combination of 10k or 10l with EGFR-TKIs markedly enhances the inhibition of cell proliferation and increases the apoptosis and necrosis in EGFR-TKI resistant NSCLC cell models.
The new FAK inhibitor 10k inhibited the growth of NSCLC cell xenograft models, modulated FAK and downstream pathways favoring apoptosis induction, and enhanced the sensitivity to Osimertinib in vivo
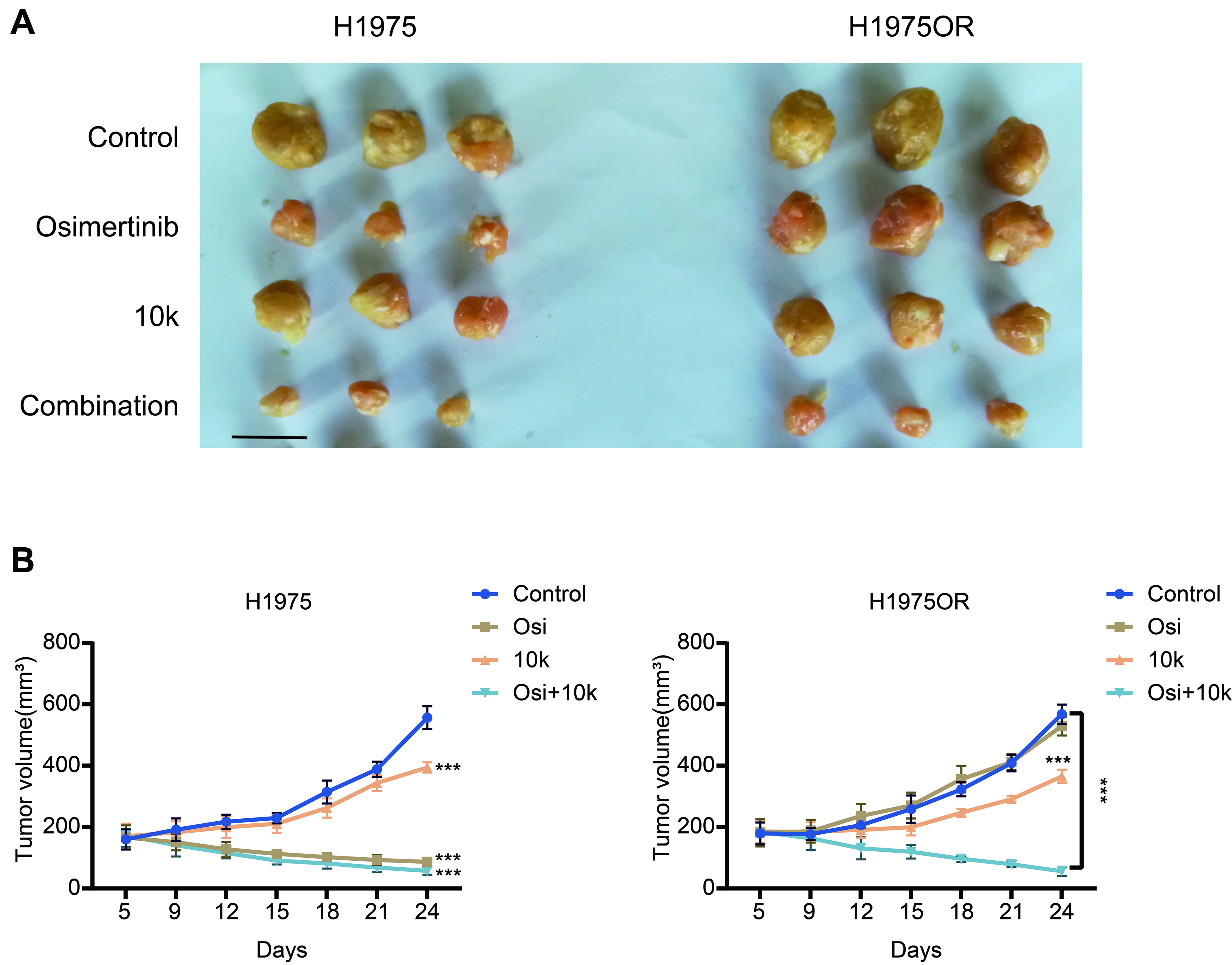
Since 10k displayed a superior CI with Osimertinib compared to 10l, and as Osimertinib is the most commonly administered (third-generation) EGFR-TKI, we selected 10k and Osimertinib for further in vivo studies. These studies demonstrated that 10k effectively inhibited tumor cell proliferation and growth in both H1975 and H1975OR xenograft models, as also assessed by reduction of Ki67 staining in H1975OR specimens [Supplementary Figure 11A], while Osimertinib was only able to inhibit the growth of H1975 tumors [Figure 5A and B]. In addition, the data presented in Supplementary Figure 11 show that 10k treatment suppressed the expression of both p-FAK and its downstream effector p-Akt in H1975OR tumors [Supplementary Figure 11B and C]. Consistently, caspase-3 activity assays indicated that 10k increased apoptosis in these tumor tissues [Supplementary Figure 11D]. Defactinib, a well-studied FAK inhibitor, was also tested in the same mouse xenograft model; we found that 10k exhibited similar inhibitory effects to Defactinib [Supplementary Figure 12A] with a Defactinib concentration used in our previous study[39]. As shown in Figure 5B, the combination of Osimertinib and 10k demonstrated a stronger inhibitory effect on H1975OR xenograft growth compared to either drug alone. Notably, data reported in Supplementary Figure 12B confirmed that 10k, Osimertinib, Defactinib, and the combination of 10k with Osimertinib did not exert any deleterious effect on the weight of mice at the indicated doses. These findings suggest that 10k effectively suppresses tumor growth in vivo and can markedly enhance the sensitivity of Osimertinib in TKI-resistant xenograft models, without causing untoward toxicity.
Figure 5. The novel FAK inhibitor 10k enhances the activity of Osimertinib in vivo. (A) Tumor-bearing mice with H1975 (left panel) or H1975OR (right panel) xenografts were treated with 10k (20 mg/kg), Osimertinib (3 mg/kg), or a combination of both drugs at the same doses for 2 weeks. After one additional week, tumors were excised from the mice and photographed. Scale bars: 1 cm; (B) Tumor volumes were measured at regular intervals during the treatment. Statistical significance: P-values was set as follows: ***P < 0.001. The graphs were created with GraphPad Prism. FAK: Focal adhesion kinase; H1975OR: H1975 Osimertinib resistant; Afa: Afatinib; Osi: Osimertinib.
DISCUSSION
NSCLC patients can develop resistance to EGFR-TKIs through EGFR-independent mechanisms, such as activation of alternative bypass pathways, aberrant downstream signaling, or histologic transformation[5,31]. In the current study, FAK emerged as the most prominently activated protein kinase in two distinct models of EGFR-TKI-resistant NSCLC cells. Furthermore, we observed that both Afatinib and Osimertinib increased p-FAK levels in both EGFR-TKI-resistant and sensitive cells - a novel phenomenon which was not previously reported[40,41]. Notably, while some studies have documented increased p-FAK levels in OR H1975 or HCC827 cells[15], others have reported decreased p-FAK expression in gefitinib-resistant HCC827 cells[16]. However, these discrepancies align with findings indicating that drug resistance is a multifactorial and complex phenomenon, shaped by the acquisition of distinct molecular mechanisms in different tumor cell lines[42,43]. The mechanism by which FAK activation leads to EGFR-TKI resistance is rather complex. For example, evidence exists that abnormally activated osteopontin (OPN)/integrin αVβ3/FAK signaling is responsible for EGFR-TKI resistance[16]. Remarkably, Ibrutinib can reverse Osimertinib resistance through inhibition of Laminin α5/FAK signaling[40].
Our analysis of gene and protein kinase profiles using PCR arrays and PamChip revealed that FAK was a central hub gene among the DEGs in drug-resistant cells, and it displayed a strong correlation with genes enriched in the EGFR-TKI resistance pathway. Additionally, the MAPK, PI3K-AKT, and RAS signaling pathways were found to be activated in EGFR-TKI-resistant cells[29]. Previous studies have demonstrated that these pathways are closely associated with FAK, further supporting its key role in drug resistance mechanisms[9,21,29].
Several studies have shown that FAK was highly expressed in various cancer types. In addition, FAK upregulation and activation were associated with poor prognosis and aggressive disease progression[9,10]. Herein, we confirmed that FAK is significantly expressed in different types of NSCLC tissues (LUAD and LUSC) based on the TCGA dataset. Interestingly, while FAK expression was not correlated with poor prognosis for lung cancer patients in the TCGA dataset, our patient cohort revealed that FAK serves as a marker for prognosis and response to EGFR-TKIs. This can be explained by the fact that the lung cancer cases in the TCGA dataset include a heterogeneous mixture of mutations, with EGFR mutations likely comprising only a small subset and only a small subset of TCGA cases were treated with EGFR-TKIs. Conversely, our current study in NSCLC patients with EGFR mutations undergoing EGFR-TKI therapy, showed significantly higher FAK expression in patients with shorter OS and PD, compared to those with longer OS and SD. Thus, FAK emerges as a prognostic marker for poor outcomes in patients treated with EGFR-TKIs. Furthermore, our findings from repeated biopsies indicate that EGFR-TKI treatment leads to increased FAK expression levels. This elevated FAK expression can contribute to resistance to EGFR-TKIs, ultimately driving disease progression.
These consistent findings of FAK upregulation in EGFR-TKI-resistant cells and tumor tissues not only underscore its role in mediating chemoresistance but also position it as a promising therapeutic target. Targeting FAK or its downstream signaling pathways could potentially reverse resistance and enhance the efficacy of EGFR-TKI treatment. In previous studies, we screened a series of new FAK inhibitors with imidazo[1,2-b]pyridine pharmacophore structures, thereby demonstrating promising anticancer activity in several preclinical models of pancreatic cancer, including primary cultures[24]. In the current study, we assessed the antitumor activities of the most potent compounds 10k and 10l in our NSCLC models. These compounds demonstrated excellent antitumor activity, significantly reducing p-FAK levels, consistent with in silico studies suggesting these compounds as promising candidates for developing new inhibitors of FAK and its downstream oncogenic pathways. Specifically, they strongly inhibited cell proliferation, survival, and migration, while increasing apoptosis in various NSCLC cell lines with different EGFR mutations and EGFR-TKI resistance profiles. In particular, compounds 10k or 10l induced apoptosis and decreased the levels of the anti-apoptotic gene BCL2 in vitro and 10k decreased p-Akt, inducing activation of caspase-3, a key effector of apoptosis in vivo. Interestingly, it was previously shown that overexpression of BCL2 family (such as BCL2 and BCL-XL) in the apoptotic signaling pathway, blocked apoptosis and reduced the sensitivity of cells to EGFR-TKI-induced apoptosis[44-46]. Enhancing the sensitivity of tumor cells to apoptosis by treatment with BCL2 inhibitors, such as ABT737, or other apoptosis inducers can effectively overcome EGFR-TKI resistance[45,47]. Indeed, the combination of lapatinib (a dual EGFR and HER2 inhibitor) with BCL2/BCL-XL inhibitors has been shown to reverse matrix-induced lapatinib resistance[48], warranting future studies to evaluate the potential synergy between 10k or 10l and lapatinib.
We further investigated the roles of 10k and 10l in surmounting EGFR-TKI resistance, in combination with EGFR-TKIs. Through SRB assays, colony survival assays, and spheroid formation, we found that when combining them with EGFR-TKI, they displayed an enhanced inhibition of tumor cell proliferation when compared with single drugs in the resistant cells. Consistently, 10k and 10l exhibited excellent CIs when combined with Afatinib or Osimertinib. In addition, BCL2 was highly expressed in drug-resistant cells, and when used in combination with EGFR-TKIs, 10k or 10l synergistically induced apoptosis in these drug resistant cells. Remarkably, 10k was also able to significantly suppress tumor growth in vivo and enhance the Osimertinib sensitivity of TKI-resistant xenograft models. Thus, we demonstrated that 10k and 10l are promising FAK inhibitors in NSCLC that resensitize EGFR-TK-resistant tumor to Afatinib or Osimertinib treatment. However, our current and previous studies[24] demonstrated that they inhibited the activity of other kinases, including the EPH and STAT families, which have also been associated with acquired resistance to EGFR-TKIs[49,50]. Therefore, the superior synergistic activity of 10k and 10l in combination with EGFR-TKIs might not have depended solely on inhibition of FAK activity.
Our pharmacological study has several limitations. First, we did not investigate the precise mechanisms by which FAK overexpression drives the acquisition of resistance to EGFR-TKIs. To gain preliminary insight into downstream mechanisms, we assessed p-Akt inhibition, induction of apoptosis (via caspase-3 activity), and suppression of cell proliferation (via Ki-67 staining) following 10k treatment in our in vivo models. A deeper understanding of FAK-mediated, EGFR-coupled signaling cascades will be essential for developing strategies to overcome acquired EGFR-TKI resistance in NSCLC.
Second, although we demonstrated that our compounds can overcome EGFR-TKI resistance in vitro, we did not perform comprehensive analyses of treatment-induced changes in gene expression or signaling pathways. Future studies incorporating RNA sequencing and (phospho)-proteomics to map mechanistic pathways will be important to elucidate FAK regulation and clarify how its inhibition reverses resistance, ultimately guiding the optimization of therapeutic strategies for NSCLC patients.
Despite these limitations, our study has several strengths. For instance, by integrating data from diverse sources - clinical patient samples, public databases, and well-established in vitro models - we offer a robust multi-faceted analysis of the role of FAK in EGFR-TKI resistance. Additionally, the promising in vivo antitumor activity observed with our 10k compound suggests its potential as a viable therapeutic agent, laying a strong foundation for future translational investigations.
In conclusion, our results provide strong evidence that elevated FAK levels are a key driver of acquired EGFR-TKI resistance and can serve as a biomarker for poor prognosis in NSCLC patients treated with EGFR-TKIs. This underscores the importance of developing novel FAK inhibitors. The novel FAK inhibitors 10k or 10l, significantly reduced p-FAK levels and exhibited potent antitumor activity in both EGFR-TKI-sensitive and -resistant NSCLC cells harboring activating EGFR mutations, in vitro and in vivo. Additionally, 10k and 10l significantly enhanced the sensitivity of EGFR-TKI-resistant cells to EGFR-TKIs. These findings reveal a promising therapeutic avenue for patients who develop resistance to EGFR-TKIs, suggesting that compounds such as 10k and 10l could be used either as standalone treatments or in combination with EGFR-TKI therapy to enhance clinical outcomes.
DECLARATIONS
Acknowledgments
The authors would like to thank Ida van der Meulen and Sandra Veldt-Verkuijlen for the technical support. This work is dedicated to the memory of Prof. G. Cirrincione, whose leadership and scientific excellence within the EORTC-PAMM group greatly advanced collaborative multidisciplinary research. The graphical abstract was created with BioRender.com [Created in BioRender. Xu, G. (2025)].
Authors’ contributions
Methodology, investigation, data curation, visualization, writing - first draft and editing: Xu G
Methodology, investigation, data curation: Pecoraro C, Vahabi M
Writing - review and editing, funding acquisition, supervision: Deng D
Methodology, investigation, data curation: Cavazzoni A, Fiuji H, Lagrasta CAM
Methodology, visualization, writing: Cascioferro SM
Supervision, funding acquisition, conceptualization: Tiseo M
Methodology, visualization, writing: Carbone D
Writing - review and editing, data curation, funding acquisition, supervision: Avan A
Data curation, supervision, funding acquisition, conceptualization: Zucali PA
Writing - first draft and editing, data curation, supervision, conceptualization: Assaraf YG
Writing - review and editing, supervision, conceptualization: Peters GJ
Writing - review and editing, funding acquisition, supervision: Diana P
Writing - review and editing, data curation, supervision, funding acquisition, conceptualization: Giovannetti E
Availability of data and materials
The raw data supporting the findings of this study are available within this article and its Supplementary Materials. Further data are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This study was supported by research grants from Associazione Italiana per la Ricerca sul Cancro (IG-grant 24444, Giovannetti E), Dutch Cancer Society (KWF-13598/15305) and Bennink Foundation.
Conflicts of interest
Tiseo M received speaker and consultant fees from Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Takeda, Amgen, Merck, Sanofi, Janssen, and Daiichi Sankyo. Tiseo M also received institutional research grants from Astra-Zeneca, Boehringer Ingelheim and Roche, and travel support from Amgen and Takeda. Peters GJ is the Editor-in-Chief of the journal Cancer Drug Resistance, Giovannetti E is a Deputy Editor of the journal and Assaraf YG is a Editorial Board Member of the journal. They were not involved in any steps of editorial processing, notably including reviewers’ selection, manuscript handling and decision making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
The studies on clinical samples were approved by the local Ethics Committee as follows: NSCLC samples collected between 2004 and 2014 were waived by the Amsterdam UMC/VU Medical Center (Amsterdam, the Netherlands) biobank (protocol number BUP2016‐032). Of note, the use of pre‐existing archived and de‐identified samples does not fall within the scope of the Medical Research Involving Human Subjects Act in the Netherlands. Collection, storage and use of tissue and patient data were performed in agreement with the “Code for Proper Secondary Use of Human Tissue in the Netherlands” developed by the Dutch Federation of Medical Scientific Societies (FMWV); for the samples from Humanitas Clinical Institute (Rozzano, Milan, Italy), the study protocol was in accordance with the ethical guidelines established in the 1975 Declaration of Helsinki and compliant to the procedures of the local ethical committee (protocol no. 63/20). All participants provided a written informed consent at the time of recruitment. For the samples from Parma University Hospital (Parma, Italy), the study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University Hospital of Parma (protocol code 278/2018/OSS*/AOUPR). Lastly, the experimental in vivo protocol received approval from the Local Ethics Committee of Mashhad University of Medical Sciences (reference number: IR.MUMS.MEDICAL.REC.1403.294). The original documents regarding the ethical approval and consent (in their original language) are available upon request, in accordance with privacy and data protection regulations.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63.
2. Miao D, Zhao J, Han Y, et al. Management of locally advanced non-small cell lung cancer: state of the art and future directions. Cancer Commun. 2024;44:23-46.
3. Corvaja C, Passaro A, Attili I, et al. Advancements in fourth-generation EGFR TKIs in EGFR-mutant NSCLC: bridging biological insights and therapeutic development. Cancer Treat Rev. 2024;130:102824.
4. Liang SK, Wei PF, Hsieh MS, Wu CL, Shih JY. Next-generation sequencing reveals genetic heterogeneity and resistance mechanisms in patients with EGFR-mutated non-small cell lung cancer treated with afatinib. ERJ Open Res. 2024;10:00676-2023.
5. Piper Vallillo AJ, Viray H, Feldman J, Rangachari D. Management of treatment resistance in patients with advanced epidermal growth factor receptor-mutated lung cancer: personalization, parsimony, and partnership. J Clin Oncol. 2024;42:1215-21.
6. Nicoś M, Sroka-Bartnicka A, Kalinka E, Krawczyk P. Possibilities of overcoming resistance to osimertinib in NSCLC patients with mutations in the EGFR gene. Cancers. 2025;17:563.
7. Li Y, Juergens RA, Finley C, Swaminath A. Current and future treatment options in the management of stage III NSCLC. J Thorac Oncol. 2023;18:1478-91.
8. Li X, Combs JD 3rd, Salaita K, Shu X. Polarized focal adhesion kinase activity within a focal adhesion during cell migration. Nat Chem Biol. 2023;19:1458-68.
9. Hu HH, Wang SQ, Shang HL, et al. Roles and inhibitors of FAK in cancer: current advances and future directions. Front Pharmacol. 2024;15:1274209.
10. Shi Y, Ju M, Zhang Y, Liang L, Sun X, Di X. Comprehensive pan-cancer analysis of 33 human cancers reveals immunotherapeutic value of focal adhesion tyrosine kinase. Medicine. 2024;103:e37362.
11. Yang M, Xiang H, Luo G. Targeting focal adhesion kinase (FAK) for cancer therapy: FAK inhibitors, FAK-based dual-target inhibitors and PROTAC degraders. Biochem Pharmacol. 2024;224:116246.
12. Chuang HH, Zhen YY, Tsai YC, et al. FAK in cancer: from mechanisms to therapeutic strategies. Int J Mol Sci. 2022;23:1726.
13. Tozuka T, Noro R, Yoshida K, et al. Phosphoproteomic analysis identified mutual phosphorylation of FAK and Src as a mechanism of osimertinib resistance in EGFR-mutant lung cancer. JTO Clin Res Rep. 2024;5:100668.
14. Haderk F, Chou YT, Cech L, et al. Focal adhesion kinase-YAP signaling axis drives drug-tolerant persister cells and residual disease in lung cancer. Nat Commun. 2024;15:3741.
15. Kosibaty Z, Brustugun OT, Zwicky Eide IJ, et al. Ras-related protein Rab-32 and thrombospondin 1 confer resistance to the EGFR tyrosine kinase inhibitor osimertinib by activating focal adhesion kinase in non-small cell lung cancer. Cancers. 2022;14:3430.
16. Fu Y, Zhang Y, Lei Z, et al. Abnormally activated OPN/integrin αVβ3/FAK signalling is responsible for EGFR-TKI resistance in EGFR mutant non-small-cell lung cancer. J Hematol Oncol. 2020;13:169.
17. Aboubakar Nana F, Hoton D, Ambroise J, et al. Increased expression and activation of FAK in small-cell lung cancer compared to non-small-cell lung cancer. Cancers. 2019;11:1526.
18. Dy GK, Ylagan L, Pokharel S, et al. The prognostic significance of focal adhesion kinase expression in stage I non-small-cell lung cancer. J Thorac Oncol. 2014;9:1278-84.
19. Ocak S, Chen H, Callison C, Gonzalez AL, Massion PP. Expression of focal adhesion kinase in small-cell lung carcinoma. Cancer. 2012;118:1293-301.
20. Banerjee S, Krebs MG, Greystoke A, et al. Defactinib with avutometinib in patients with solid tumors: the phase 1 FRAME trial. Nat Med. 2025;31:3074-80.
21. Zhu SK, Wu Q, Liu GX, Geng AQ, Wang PA. Recent advances in focal adhesion kinase (FAK)-targeting antitumor agents. RSC Adv. 2025;15:20957-84.
22. Fennell DA, Baas P, Taylor P, et al. Maintenance defactinib versus placebo after first-line chemotherapy in patients with merlin-stratified pleural mesothelioma: COMMAND-A double-blind, randomized, phase II study. J Clin Oncol. 2019;37:790-8.
23. Gerber DE, Camidge DR, Morgensztern D, et al. Phase 2 study of the focal adhesion kinase inhibitor defactinib (VS-6063) in previously treated advanced KRAS mutant non-small cell lung cancer. Lung Cancer. 2020;139:60-7.
24. Pecoraro C, Carbone D, Scianò F, et al. Exploring the therapeutic potential of a novel series of imidazothiadiazoles targeting focal adhesion kinase (FAK) for pancreatic cancer treatment: synthesis, mechanistic insights and promising antitumor and safety profile. J Drug Target. 2024;32:1278-94.
25. Sciarrillo R, Wojtuszkiewicz A, Kooi IE, et al. Using RNA-sequencing to detect novel splice variants related to drug resistance in in vitro cancer models. J Vis Exp. 2016;9:54714.
26. Bijnsdorp IV, Giovannetti E, Peters GJ. Analysis of drug interactions. In: Cree IA, editor. Cancer cell culture. Totowa: Humana Press; 2011. pp. 421-34.
27. Churchman ML, Evans K, Richmond J, et al. Synergism of FAK and tyrosine kinase inhibition in Ph+ B-ALL. JCI Insight. 2016;1:86082.
28. Ichihara E, Westover D, Meador CB, et al. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer. Cancer Res. 2017;77:2990-3000.
29. Zhou X, Zeng L, Huang Z, et al. Strategies beyond 3rd EGFR-TKI acquired resistance: opportunities and challenges. Cancer Med. 2025;14:e70921.
31. Angelopoulos PA, Passaro A, Attili I, et al. Management of MET-driven resistance to osimertinib in EGFR-mutant non-small cell lung cancer. Genes. 2025;16:772.
32. Hu D, Hu Y, Lei S, Wu D, Wang Y. MET tyrosine kinase inhibitors in combination with EGFR tyrosine kinase inhibitors in NSCLC patients with EGFR mutations and acquired MET alterations: a systematic review and meta-analysis. BMC Cancer. 2025;25:732.
33. Wang QA, Tsai IL, Lin CY, et al. Multivariable model for predicting 5-year survival in patients with EGFR-mutated non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective study. Ther Adv Med Oncol. 2025;17:17588359251321901.
34. Viray H, Piper-Vallillo AJ, Widick P, et al. A real-world study of patient characteristics and clinical outcomes in EGFR mutated lung cancer treated with first-line osimertinib: expanding the FLAURA trial results into routine clinical practice. Cancers. 2024;16:1079.
35. Groenendijk FH, Bernards R. Drug resistance to targeted therapies: déjà vu all over again. Mol Oncol. 2014;8:1067-83.
36. Jin H, Wang L, Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat Rev Drug Discov. 2023;22:213-34.
37. Levy A, Alhazzani K, Dondapati P, et al. Focal adhesion kinase in ovarian cancer: a potential therapeutic target for platinum and taxane-resistant tumors. Curr Cancer Drug Targets. 2019;19:179-88.
38. Zhang Y, Ojalill M, Boyer A, et al. Nuclear focal adhesion kinase protects against cisplatin stress in ovarian carcinoma. Cancer Res Commun. 2024;4:3165-79.
39. Cavazzoni A, La Monica S, Alfieri R, et al. Enhanced efficacy of AKT and FAK kinase combined inhibition in squamous cell lung carcinomas with stable reduction in PTEN. Oncotarget. 2017;8:53068-83.
40. Li L, Li Z, Lu C, et al. Ibrutinib reverses IL-6-induced osimertinib resistance through inhibition of Laminin α5/FAK signaling. Commun Biol. 2022;5:155.
41. Vengoji R, Macha MA, Nimmakayala RK, et al. Afatinib and Temozolomide combination inhibits tumorigenesis by targeting EGFRvIII-cMet signaling in glioblastoma cells. J Exp Clin Cancer Res. 2019;38:266.
42. Assaraf YG, Brozovic A, Gonçalves AC, et al. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist Updat. 2019;46:100645.
43. Du Z, Kan H, Sun J, et al. Molecular mechanisms of acquired resistance to EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Drug Resist Updat. 2025;82:101266.
44. Kaloni D, Diepstraten ST, Strasser A, Kelly GL. BCL-2 protein family: attractive targets for cancer therapy. Apoptosis. 2023;28:20-38.
45. Alam M, Alam S, Shamsi A, et al. Bax/Bcl-2 cascade is regulated by the EGFR pathway: therapeutic targeting of non-small cell lung cancer. Front Oncol. 2022;12:869672.
46. Vogler M, Braun Y, Smith VM, et al. The BCL2 family: from apoptosis mechanisms to new advances in targeted therapy. Signal Transduct Target Ther. 2025;10:91.
47. Deng J, Shimamura T, Perera S, et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867-75.
48. Luo QY, Yang J, Di T, et al. The novel BCL-2/BCL-XL inhibitor APG-1252-mediated cleavage of GSDME enhances the antitumor efficacy of HER2-targeted therapy in HER2-positive gastric cancer. Acta Pharmacol Sin. 2025;46:1082-96.
49. Fan X, Wu S, Wu H, et al. STAT3 mediates CAF-induced osimertinib resistance via regulating protein secretion in non-small cell lung cancer. Front Pharmacol. 2025;16:1546491.
50. Amato KR, Wang S, Tan L, et al. EPHA2 blockade overcomes acquired resistance to EGFR kinase inhibitors in lung cancer. Cancer Res. 2016;76:305-18.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data




















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].