Mesh-reinforced cruroplasty for type III-IV hiatus hernia repair: a single-center experience using the Patient-Tailored Algorithm
Abstract
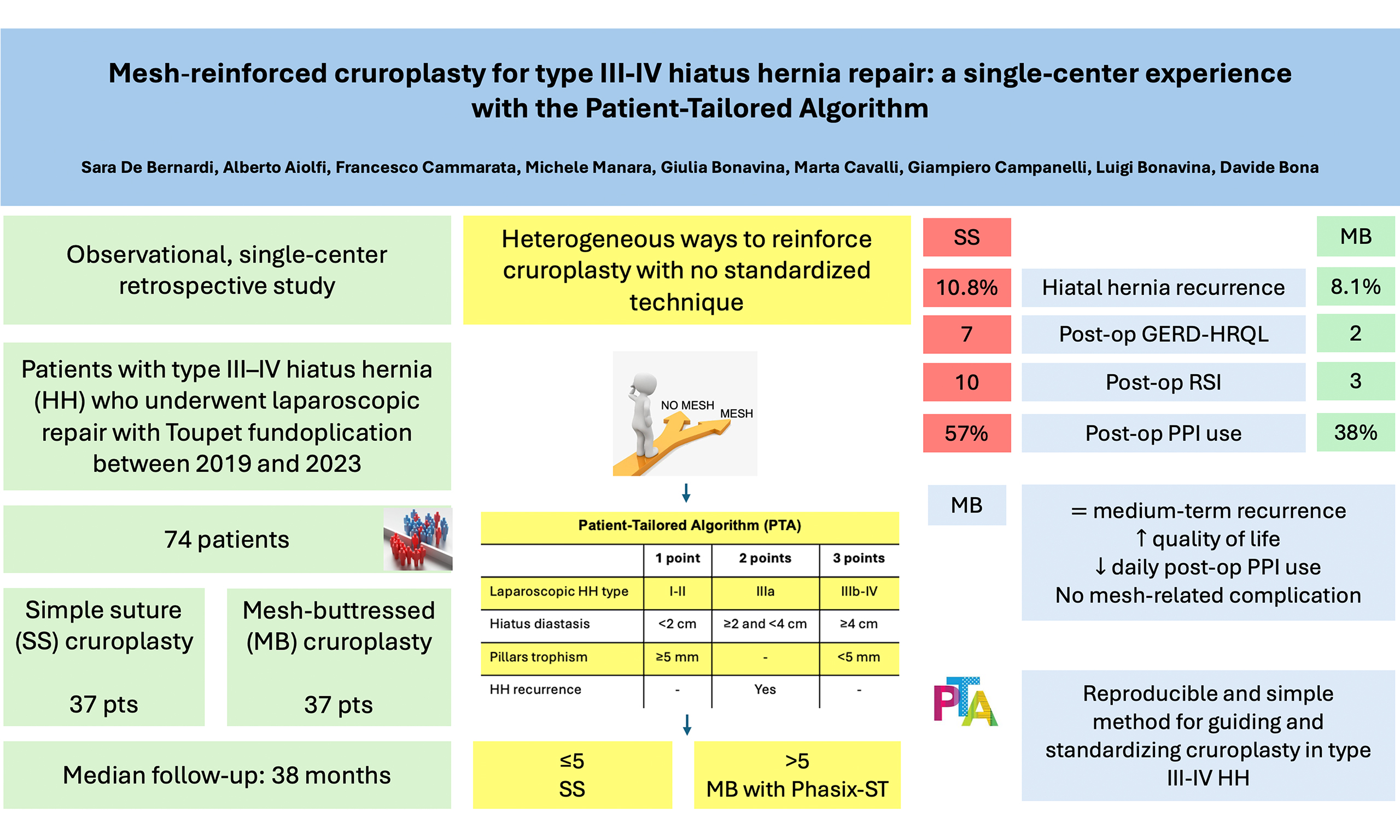
Aim: Laparoscopic hiatus hernia (HH) repair with cruroplasty is an effective treatment for symptomatic patients with type III-IV HH. Various techniques have been described for posterior cruroplasty, ranging from simple suture (SS) repair to suture reinforced with mesh. Mesh-buttressed (MB) cruroplasty aims to reduce HH recurrence, but there is no consensus on indications for mesh placement, mesh type, fixation method, or materials. This study aimed to assess the medium-term effectiveness of MB cruroplasty guided by the Patient-Tailored Algorithm (PTA) in laparoscopic repair of type III-IV HH.
Methods: We conducted a single-center, retrospective observational study from November 2019 to April 2023, including patients with type III-IV HH. The institutional PTA, based on intraoperative measurable parameters (HH type, hiatus diastasis, pillars trophism, and redo surgery), was utilized to guide the decision-making process. If the PTA score exceeded 5, MB cruroplasty using a 10 × 7 cm keyhole-shaped Phasix-ST mesh was performed. The primary outcome was HH recurrence, defined as a combination of symptoms and anatomical gastric migration
Results: Seventy-four patients with a minimum follow-up of 12 months were included. The median age was 66 years and 69% were female. The median follow-up was 38 months (range 12-98). MB cruroplasty was performed in 37 patients (50%). HH recurrence occurred in 7 patients (9.5%), with a clinical trend toward higher recurrence after SS compared with MB (10.8% vs. 8.1%). Postoperative quality of life, measured with the disease-specific GERD-HRQL, improved significantly compared to baseline (20 vs. 3, P = 0.004). In the MB group, both GERD-HRQL and Reflux Symptom Index (RSI) scores improved significantly compared to SS (GERD-HRQL: 2 vs. 7, P = 0.048; RSI: 3 vs. 10, P = 0.003). Trends toward improved SF-36 scores and reduced postoperative proton pump inhibitor (PPI) use were also observed.
Conclusion: MB cruroplasty is associated with favorable medium-term outcomes, including reduced HH recurrence, improved quality of life, and decreased daily postoperative PPI use in patients with type III-IV HH. The PTA provides a simple, reproducible intraoperative strategy for guiding cruroplasty and standardizing surgical decision making in type III-IV HH repair.
Keywords
INTRODUCTION
The surgical management of hiatus hernia (HH) is complex and remains a widely discussed topic. Despite extensive literature on laparoscopic repair, there is still no consensus on many technical aspects, which limits the standardization and reproducibility of the procedure. This lack of consensus contributes to the wide range of reported HH recurrence rates after surgery, which vary from 1.2% to 66%[1-7]. Such variability largely reflects heterogeneous definitions of HH recurrence, representing a major limitation when comparing studies, interpreting results, and evaluating different surgical techniques.
Over the years, several methods have been proposed to reinforce cruroplasty, including mesh placement, pledgets, gastropexy, relaxing incisions, ligamentum teres augmentation, and anterior cruroplasty[8,9]. Among these, the use of mesh to reinforce cruroplasty and potentially reduce recurrence has generated considerable discussion, yet clear guidelines on indications for mesh reinforcement remain lacking. In this context, we previously proposed a Patient-Tailored Algorithm (PTA) based on four criteria (laparoscopic classification of HH, hiatus diastasis, pillars trophism, and recurrence) to guide the decision-making process for mesh-buttressed (MB) cruroplasty[10-12].
The aim of this study was to evaluate the medium-term efficacy of PTA-guided MB cruroplasty compared to simple suture (SS) cruroplasty in laparoscopic repair of type III-IV HH.
METHODS
This single-center, retrospective observational study was approved by the local Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
All HH cases treated laparoscopically at IRCCS Ospedale Galeazzi-Sant’Ambrogio were recorded in a prospectively maintained database. We screened patients from November 2019 to April 2023 to include adults (≥ 18 years) who underwent SS or MB cruroplasty with Toupet fundoplication. The inclusion criteria were: (a) adults (≥ 18 years); (b) diagnosis of type III or IV HH; (c) surgical indications according to the latest Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines[13,14]; and (d) a minimum follow-up of 12 months. Exclusion criteria were: (a) previous gastric surgery preventing fundoplication; (b) patients deemed ineligible for surgery; and (c) follow-up < 12 months. The primary outcome was HH recurrence, defined as the reappearance of GERD symptoms combined with evidence of
Data collection
Baseline demographics and patient characteristics were collected, including age, sex, body mass index (BMI, kg/m2), comorbidities, proton pump inhibitor (PPI) usage, HH size, operative parameters (duration, hiatus diastasis, pillars trophism, laparoscopic HH classification), and short-term outcomes (90-day morbidity and mortality). HH-related symptoms were evaluated using specific questionnaires: the Reflux Symptom Index (RSI)[16], GastroEsophageal Reflux Disease Health-Related Quality of Life (GERD-HRQL)[17,18], and 36-Item Short Form Health Survey (SF-36)[19]. Preoperative evaluation routinely included chest X-ray, barium swallow study, and UGI endoscopy. Selected patients additionally underwent high-resolution manometry (HRM), 24-hour pH-impedance testing, and/or chest and upper abdominal CT scans. Perioperative complications were classified according to the Clavien-Dindo classification[20].
Surgical technique
All surgeries were performed using a standardized laparoscopic procedure. Patients were positioned in reverse Trendelenburg, with the lead surgeon standing between the patient’s legs. Five trocars were inserted, after which HH classification was assigned. The gastrohepatic ligament was divided to expose the anterior esophagogastric junction and the angle of His. Circumferential esophageal dissection was performed to create a retroesophageal window for fundoplication. Mediastinal dissection and caudal mobilization of the esophagus ensured at least 3 cm of tension-free intra-abdominal esophagus. The hernia sac and any associated lipoma were excised. Using a laparoscopic ruler, the transverse diameter of the hiatal opening, pillars trophism, and right pillar length were measured to calculate the PTA [Supplementary Table 1] and hiatal surface area (HSA)[21].
For PTA ≤ 5, posterior cruroplasty was performed with interrupted non-absorbable sutures tied extracorporeally (Prolene® 2-0, Ethicon). Closure began posteriorly at the junction of the crura and proceeded anteriorly. For PTA ≥ 6, a 10 × 7 cm keyhole-shaped biosynthetic absorbable mesh (Phasix ST®, Bard), made of poly-4-hydroxybutyrate (P4HB) with a hydrogel barrier, was positioned over the hiatoplasty and fixed with two resorbable sutures (Vicryl® 2-0, Ethicon).
After dividing the upper short gastric vessels using a harmonic scalpel, a 270° Toupet fundoplication was performed following the “critical view” concept[11]. Once the gastric fundus was mobilized, four “cardinal” non-absorbable stitches (Prolene® 2-0, Ethicon) were placed to stabilize the wrap while minimizing manipulation of the fundus. The two cranial sutures included the esophagus, gastric fundus, and respective crus; the two distal sutures, placed 3 cm below, included the esophagus and gastric wall. A running barbed absorbable suture (3-0 polybutester/V-LocTM) secured the gastric wall to each side of the esophagus while preserving the anterior vagus nerve. The nasogastric tube was routinely removed at the end of the procedure, and a mediastinal drain was optionally left in place for 24 h. To prevent postoperative nausea and vomiting, patients received intravenous Ondansetron 4 mg and Dexamethasone 8 mg. On postoperative day 1, a chest X-ray and gastrographin swallow study confirmed the correct position of the gastroesophageal junction and excluded leakage. If unremarkable, patients were started on a semiliquid diet and typically discharged on postoperative day 2[10,12,22,23].
Follow-up
Follow-up included:
outpatient visits at 1, 3, 6, and 12 months postoperatively, and annually thereafter;
• barium swallow study, routinely at 6 months;
• UGI endoscopy, performed 1 year after surgery.
Subsequent imaging (UGI endoscopy and/or barium swallow) was performed annually or whenever patients reported symptoms. Quality of life was assessed at baseline and follow-up using the RSI[24], GERD-HRQL[18], and SF-36[25] questionnaires.
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median (interquartile range, IQR). Categorical variables were reported as counts and percentages. Unpaired t-tests were used for continuous variables, and Fisher’s exact test was applied to categorical variables. Two-sided p-values were computed. Although no variable had a P-value < 0.2 in univariate analysis, several clinically relevant parameters were included in a binary logistic regression to identify independent predictors of HH recurrence. These included age, laparoscopic HH classification, hiatus diastasis, pillars trophism, and mesh placement. Multicollinearity testing was performed to identify correlations among variables. For multivariate analysis, age and hiatus diastasis were categorized: age (< 65 years vs. ≥ 65 years) and hiatus diastasis (< 2 cm, ≥ 2 cm but < 4 cm,
RESULTS
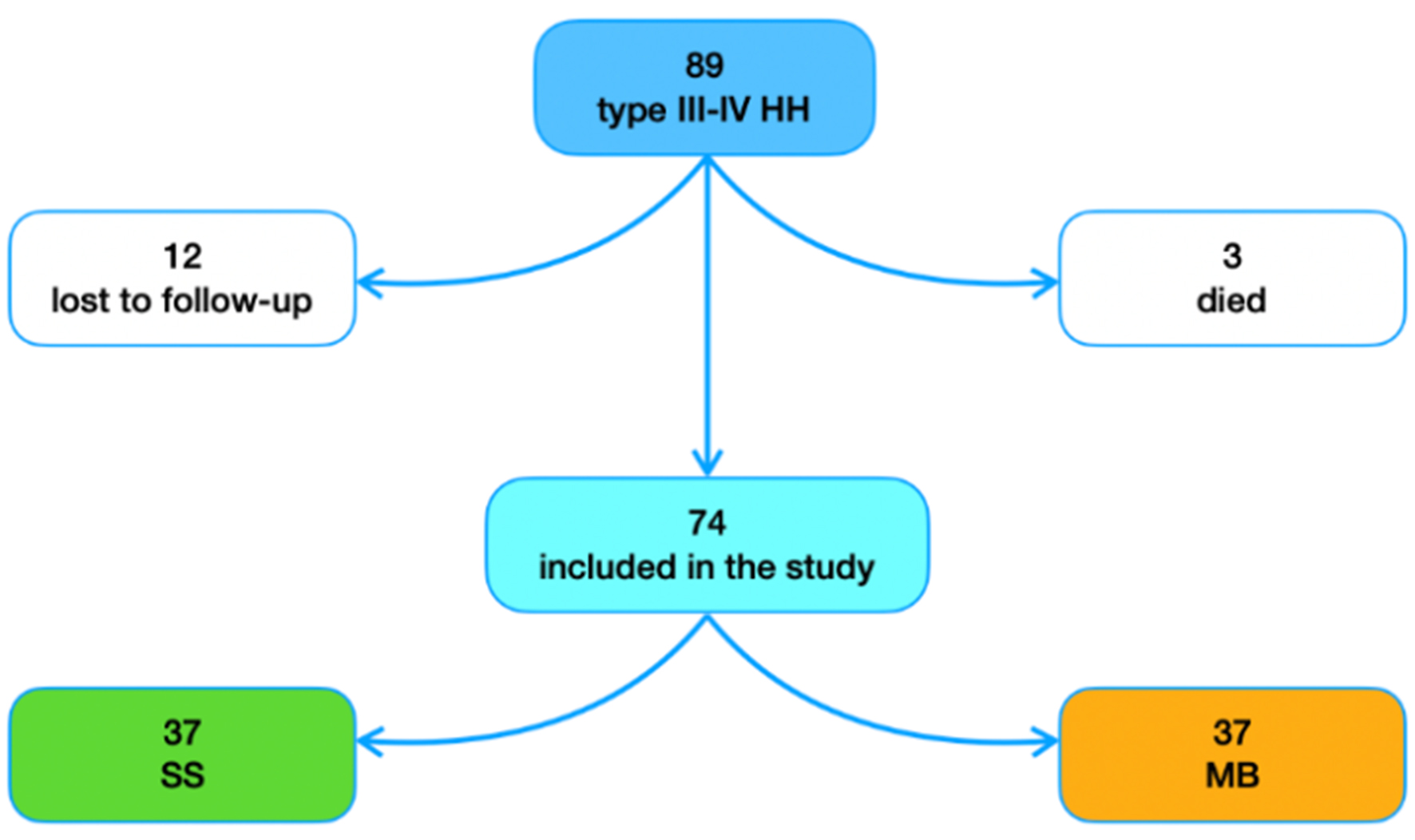
During the study period, 89 patients meeting the inclusion criteria underwent laparoscopic HH repair with Toupet fundoplication at our institution. During follow-up, three patients died from unrelated reasons and 12 patients were lost to follow-up. Thus, 74 patients were included in the final analysis [Figure 1]. All procedures were performed in a standardized manner by two surgeons (D.B. and A.A.).
Among the 74 patients, 69% were female. The mean age was 65.9 years, and the mean preoperative BMI was 27.6 kg/m2. The most common comorbidities were hypertension (51%), dyslipidemia (15%), and thyroid disease (10%). The main symptoms were heartburn (58%), regurgitation (39%), and epigastric pain (38%), while atypical symptoms were reported in a minority of patients [Table 1]. The mean HH size measured during UGI endoscopy was 6.5 cm, and esophagitis of any grade (according to the Los Angeles classification) was observed in about 14% of cases. HRM and pH-impedance testing were performed selectively as part of the diagnostic work-up.
Demographic characteristics, preoperative, intraoperative, and postoperative data
| n = 74 | |||
| Age, years (mean ± SD) | 65.9 ± 10.3 | ||
| Sex, female, n (%) | 51 (69.0) | ||
| Follow-up, months, median (IQR) | 38 (25-62) | ||
| BMI, kg/m2 (mean ± SD) | Pre | 27.6 ± 3.8 | |
| Post | 26.2 ± 4.5 | ||
| P-value | 0.10 | ||
| Symptoms, n (%) | Heartburn | 43 (58) | |
| Epigastric pain | 28 (38) | ||
| Regurgitation | 29 (39) | ||
| Cough | 20 (27) | ||
| Dysphagia | 25 (34) | ||
| Dyspepsia | 19 (26) | ||
| EGDS | HH size, cm (mean ± SD) | 6.5 ± 2.1 | |
| Esophagitis, n (%) | L.A. A | 4 (5.4) | |
| L.A. B | 5 (6.8) | ||
| L.A. C | 0 | ||
| L.A. D | 1 (1.4) | ||
| Barrett’s esophagus, n (%) | 0 | ||
| Surgical procedure | HH laparoscopic classification, n (%) | IIIA | 47 (63) |
| IIIB | 16 (22) | ||
| IV | 11 (15) | ||
| Hiatus diastasis, n (%) | < 2 cm | 14 (18.9) | |
| ≥ 2 and < 4 cm | 53 (71.6) | ||
| ≥ 4 cm | 7 (9.5) | ||
| Pillars trophism, n (%) | ≥ 5 mm | 64 (86.5) | |
| < 5 mm | 10 (13.5) | ||
| Previous antireflux procedure, n (%) | 10 (13.5) | ||
| Mesh placement, n (%) | 37 (50) | ||
| OT, min (mean ± SD) | 126 ± 39 | ||
| HSA, cm2 (mean ± SD) | 6.2 ± 2.7 | ||
| Concordance rate between PTA and HSA, n (%) (n = 49) | 42 (86) | ||
| PPI use, n (%) | Pre | 55 (74.3) | |
| Post | 35 (47.3) | ||
| P-value | 0.001 | ||
| Quality of life | GERD-HRQL, median (IQR) | Pre | 20 (6-35) |
| Post | 3 (0-19) | ||
| P-value | 0.004 | ||
| RSI, median (IQR) | Pre | 14 (5-22) | |
| Post | 6 (2-11) | ||
| P-value | 0.008 | ||
| SF-36, median (IQR) | Pre | 63.5 (36.5-82.2) | |
| Post | 72.3 (47-85.6) | ||
| p-value | 0.325 | ||
| Recurrence, n (%) | 7 (9.5) | ||
The mean operative time was 126 min. According to the preoperative PTA score, 50% of patients with a score ≤ 5 underwent SS cruroplasty, while the remaining 50% received MB cruroplasty. The mean HSA was 6.2 cm2. Concordance between HSA and PTA in indicating MB cruroplasty was 86%.
No intraoperative complications occurred, and no cases required conversion to open surgery. The 90-day postoperative complication rate was 5.4% (n = 4). Complications according to the Clavien-Dindo classification included: grade IIIa (pneumothorax, pleural effusion; n = 3) and grade IIIb (hydropneumothorax with pleural empyema; n = 1). No mortality (grade V) was observed.
The median follow-up was 38 months (range, 12-98). Compared to baseline, there was a significant reduction in postoperative PPI use (74% vs. 47%; P = 0.001) and significant improvements in quality of life, as measured by both GERD-HRQL (20 vs. 3; P = 0.004) and RSI (14 vs. 6; P = 0.008). Overall, seven patients (9.5%) were diagnosed with HH recurrence; none required redo surgery. No mesh-related complications were reported during follow-up.
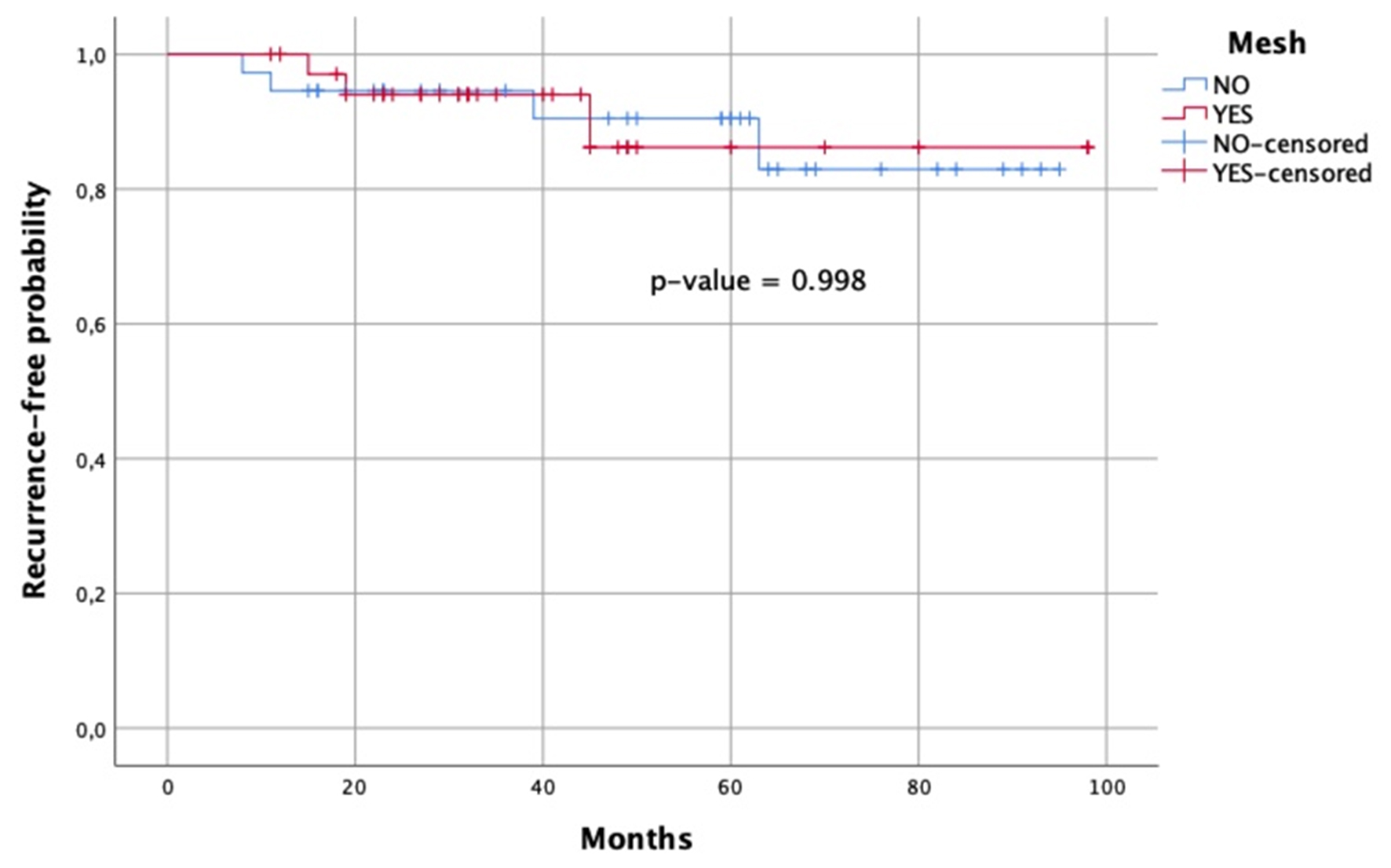
Patients were stratified into SS and MB groups. Demographic, preoperative, intraoperative, and postoperative characteristics are reported in Table 2. Compared with SS cruroplasty, MB cruroplasty showed a trend toward lower HH recurrence (10.8% vs. 8.8%). Notably, both GERD-HRQL (2 vs. 7; P = 0.048) and RSI (3 vs. 10; P = 0.008) were significantly improved in the MB group compared with SS. Trends toward improved SF-36 scores and reduced PPI use were also observed with MB cruroplasty. Multivariate logistic regression, adjusting for relevant covariates, did not identify significant factors associated with HH recurrence [Table 3]. The recurrence-free probability for mesh vs. no mesh repair was as follows: at 12 months, 1.00 vs. 0.95; at 24 months, 0.94 vs. 0.95; at 36 months, 0.94 vs. 0.94; at 48 months, 0.86 vs. 0.91; and at 72 months, 0.86 vs. 0.83 [Figure 2].
Figure 2. Kaplan-Meier survival curve of patients treated with mesh (red line) vs. no mesh (blue line). The x-axis represents time in months, and the y-axis represents recurrence-free probability.
Demographic characteristics, preoperative, intraoperative, and postoperative data of patients divided into SS and MB groups
| SS group | MB group | P-value | |||
| n | 37 | 37 | |||
| Age, years (mean ± SD) | 65 ± 11 | 67 ± 10 | 0.301 | ||
| Sex, female, n (%) | 26 (70) | 25 (68) | 1.000 | ||
| Follow-up, months, median (IQR) | 59 (23-68) | 33 (27-49) | 0.081 | ||
| BMI, kg/m2 (mean ± SD) | Pre | 28 ± 4 | 26 ± 4 | 0.109 | |
| Post | 27 ± 5 | 25 ± 4 | 0.260 | ||
| Symptoms, n (%) | Heartburn | 25 (68) | 18 (49) | 0.157 | |
| Epigastric pain | 14 (38) | 14 (38) | 1.000 | ||
| Regurgitation | 18 (49) | 11 (30) | 0.153 | ||
| Cough | 13 (35) | 7 (19) | 0.190 | ||
| Dysphagia | 12 (32) | 7 (19) | 0.287 | ||
| Dyspepsia | 12 (32) | 13 (35) | 1.000 | ||
| EGDS | HH size, cm (mean ± SD) | 6 ± 2 | 7 ± 2 | 0.072 | |
| Surgical procedure | HH laparoscopic classification, n (%) | IIIA | 32 (87) | 15 (41) | 0.000 |
| IIIB | 3 (8) | 13 (35) | 0.010 | ||
| IV | 2 (5) | 9 (24) | 0.046 | ||
| Hiatus diastasis, n (%) | < 2 cm | 13 (35) | 1 (3) | 0.001 | |
| ≥ 2 and < 4 cm | 24 (65) | 29 (78) | 0.302 | ||
| ≥ 4 cm | 0 | 7 (19) | 0.011 | ||
| Pillars trophism, n (%) | ≥ 5 mm | 37 (100) | 27 (73) | 0.001 | |
| < 5 mm | 0 | 10 (27) | |||
| Previous antireflux procedure, n (%) | 1 (3) | 9 (24) | 0.014 | ||
| Score, n (%) | ≤ 5 | 35 (95) | 0 | 0.000 | |
| > 5 | 2 (5) | 37 (100) | |||
| OT, min (mean ± SD) | 107 ± 31 | 145 ± 36 | < 0.0001 | ||
| HSA, cm2 (mean ± SD) | 4.3 ± 1.4 | 7.4 ± 2.7 | < 0.0001 | ||
| HSA concordance (%) | 42/49 (86) | ||||
| Recurrence, n (%) | 4 (10.8) | 3 (8.1) | 1.000 | ||
| Time from surgery to recurrence, months, median (IQR) | 25 (10-45) | 19 (17-32) | 0.829 | ||
| PPI use, n (%) | Pre | 32 (86.5) | 23 (62.1) | 0.032 | |
| Post | 21 (56.8) | 14 (37.8) | 0.162 | ||
| Quality of life | GERD-HRQL, median (IQR) | Pre | 20 (5-35) | 23 (10-34) | 0.774 |
| Post | 7 (0-25) | 2 (0-13) | 0.048 | ||
| RSI, median (IQR) | Pre | 15 (5-22) | 10 (5-17) | 0.731 | |
| Post | 10 (4-18) | 3 (1-8) | 0.003 | ||
| SF-36, median (IQR) | Pre | 31 (19-59) | 73 (64-79) | 0.321 | |
| Post | 65 (40-89) | 77 (61-84) | 0.140 | ||
Univariate vs. multivariate analysis
| Non-recurrence group | Recurrence group | Univariate analysis | Multivariate analysis | ||
| (n = 67) | (n = 7) | P-value | P-value | OR (95%CI) | |
| Age | |||||
| < 65 | 26 | 3 | 1.00 | 0.94 | 1.07 (0.21; 5.53) |
| ≥ 65 | 41 | 4 | |||
| HH laparoscopic classification | |||||
| IIIA | 42 | 5 | 1.00 | 0.99 | 0.99 (0.12; 8.11) |
| IIIB-IV | 25 | 2 | |||
| Diastasis | |||||
| < 2 cm | 13 | 1 | 1.00 | 0.61 | 1.90 (0.17; 21.58) |
| ≥ 2 and < 4 cm | 47 | 6 | |||
| ≥ 4 cm | 7 | 0 | |||
| Pillars trophism | |||||
| ≥ 5 mm | 58 | 6 | 1.00 | 0.98 | 0.97 (0.07; 13.52) |
| < 5 mm | 9 | 1 | |||
| Mesh placement | |||||
| No | 33 | 4 | 1.00 | 0.83 | 0.78 (0.08; 7.34) |
| Yes | 34 | 3 | |||
DISCUSSION
This study shows a promising effect of MB PTA-guided cruroplasty in patients with symptomatic type III-IV HH. Compared to SS cruroplasty, MB cruroplasty was associated with a trend toward lower medium-term HH recurrence and reduced PPI use, along with significant improvements in patient quality of life.
Laparoscopic posterior cruroplasty is a crucial step in HH repair. The choice of technique largely depends on the surgeon’s subjective assessment of crural weakness, as there is currently no standardized, objective method for crura repair. The use of mesh to reinforce diaphragmatic closure and reduce recurrence risk remains controversial due to the lack of clear guidelines. Conflicting evidence in the literature contributes to this uncertainty. Studies comparing MB cruroplasty with SS cruroplasty report heterogeneous outcomes, with some suggesting benefits in recurrence reduction and others showing no significant effect. For instance, a 2022 meta-analysis by Clapp et al., including 21 studies, reported a significantly lower recurrence rate in the bioabsorbable mesh group compared to the non-mesh group (8% vs. 18%, pooled P-value < 0.0001) with a median follow-up of 27 months[26]. Similarly, Hanna et al. (2024) found that mesh placement during primary HH repair reduced early recurrences but did not affect late recurrences, complications, or postoperative symptoms[27]. Conversely, Petric et al., in a meta-analysis of randomized control trials (RCTs) comparing sutured vs. mesh-augmented HH repair, found no significant differences in short-term recurrence (6-12 months, 10.1% mesh vs. 15.5% sutured, P-value = 0.22), long-term recurrence (3-5 years, 30.7% mesh vs. 31.3% sutured, P-value = 0.69), functional outcomes, or patient satisfaction[28]. Latorre‐Rodríguez et al. similarly reported no difference in recurrence rates between absorbable mesh and suture repair (20.6% vs. 20.8%)[29]. Another 2022 RCT meta-analysis also found comparable early [relative risk (RR) = 0.74, P-value = 0.46) and late (RR = 0.75, P-value = 0.48) recurrence rates regardless of mesh use. However, heterogeneous definitions of HH recurrence and inclusion of patients lost to follow-up limited these conclusions. By reanalyzing the same RCTs with a more uniform definition of recurrence (both anatomic and symptomatic), excluding patients lost to follow-up, and performing sensitivity analysis, Aiolfi et al. found a significant reduction in HH recurrence (RR = 0.35; P-value = 0.02), supporting mesh use for crural augmentation[30]. Nevertheless, it remains essential to weigh the potential benefits against possible mesh-related complications. Absorbable meshes are preferred, as the inflammation associated with implantation promotes native tissue strengthening and scar formation without causing the serious complications seen with non-resorbable meshes, such as erosion, migration, adhesions, infection, strictures, dysphagia, or chest pain[31-33].
As noted, there is no standardized method for posterior cruroplasty, and techniques vary according to surgeon experience, contributing to outcome heterogeneity. An early attempt at standardization was proposed by Granderath et al. (2007), who introduced the HSA calculation via a complex trigonometric formula[21]. They recommended mesh placement when HSA exceeded 4 cm2. To simplify decision-making, we developed the PTA, a tool based on easily assessable intraoperative features without complex computations. The PTA provides a simpler, reproducible, and direct method for guiding cruroplasty, demonstrating promising outcomes in improving quality of life and reducing HH recurrence[23]. Compared to HSA, the PTA is more accessible intraoperatively, with a high concordance rate exceeding 85%, and accounts for factors such as HH recurrence risk and crural frailty, which HSA does not.
In our study, the overall recurrence rate was 9.5%, lower than many reported rates. However, the lack of a uniform definition of recurrence contributes to such variability and makes robust comparisons difficult[9,34,35]. Surgeons at our center are part of a specialized referral unit for GERD surgery, which may also contribute to lower recurrence and complication rates. The use of Phasix-ST®, a biosynthetic absorbable mesh composed of P4HB with Sepra Technology (ST) coating, may further reduce complications. This mesh degrades over 12-18 months, acting as a scaffold to reinforce the hiatus[12], while the ST coating - composed of sodium hyaluronate and carboxymethylcellulose - minimizes visceral adhesions. This type of mesh demonstrates a favorable short- and medium-term safety and efficacy profile, preventing early recurrences while avoiding long-term complications. In our study, a trend toward lower HH recurrence was observed for MB vs. SS cruroplasty (8.8% vs. 10.8%), although the difference did not reach statistical significance, likely due to the small number of recurrences. Multivariate analysis suggested a possible clinical trend (point estimation 0.78), but the 95%CI included the null value. Increasing age was linearly associated with HH prevalence, though statistical significance was limited by the sample size[36,37]. Clinically, postoperative PPI use tended to be lower in the mesh group (37.8% vs. 56.8%, P-value = 0.16). MB cruroplasty was also associated with improved GERD-HRQL and RSI scores compared to SS, an important finding given that, according to the 2024 SAGES guidelines, HH repair primarily aims to improve quality of life[13].
Limitations of this study include its retrospective design, which may introduce attrition and selection bias. Variable follow-up durations could underestimate recurrence rates. The limited number of patients and recurrences restricted robust multivariate analysis. Additionally, as a tertiary referral center study, generalizability may be limited. Prospective, multicenter studies with larger samples are required to validate these preliminary findings.
In conclusion, MB cruroplasty using a biosynthetic absorbable mesh appears safe and promising for reducing HH recurrence, improving quality of life, and potentially decreasing PPI use. Careful patient selection is essential to optimize outcomes. The PTA offers a practical, reproducible approach to standardize type III-IV cruroplasty, reducing reliance on subjective surgical judgment.
DECLARATIONS
Authors’ contributions
Conceptualization, investigation, analysis, writing - original draft, review, and editing: Aiolfi A
Conceptualization, investigation, writing - original draft, review, and editing, supervision: Bona D
Data analysis, writing - review and editing: Cammarata F
Conceptualization, investigation, writing - review and editing, supervision: Campanelli G
Conceptualization, investigation, writing - original draft, review, and editing, supervision: Bonavina L
Writing - review and editing: Cavalli M
Data collection, writing - review and editing: Manara M, Bonavina G
Data collection, analysis, writing - original draft, review, and editing: De Bernardi S
All authors contributed to data interpretation, critical revisions and approved the final version of the manuscript.
Availability of data and materials
All data are available from the corresponding author upon reasonable request.
Financial support and sponsorship
None.
Conflicts of interest
Aiolfi A and Bona D are Editorial Board members of Mini-invasive Surgery. Bonavina L and Aiolfi A served as Guest Editors for the Special Issue Minimally Invasive Techniques for Repair of Hiatal Hernia. Aiolfi A, Bona D, and Bonavina L were not involved in any stage of the editorial process, including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study was approved by the Comitato Etico Territoriale Lombardia 1 (protocol number: OGSA#2025-00151) and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all patients prior to surgery during preoperative counseling.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Braghetto I, Lanzarini E, Musleh M, Korn O, Lasnibat JP. Thinking about hiatal hernia recurrence after laparoscopic repair: when should it be considered a true recurrence? A different point of view. Int Surg. 2018;103:105-15.
2. Dallemagne B, Kohnen L, Perretta S, Weerts J, Markiewicz S, Jehaes C. Laparoscopic repair of paraesophageal hernia. Long-term follow-up reveals good clinical outcome despite high radiological recurrence rate. Ann Surg. 2011;253:291-6.
3. Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg. 2011;213:461-8.
4. Luketich JD, Nason KS, Christie NA, et al. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg. 2010;139:395-404.e1.
5. Jones R, Simorov A, Lomelin D, Tadaki C, Oleynikov D. Long-term outcomes of radiologic recurrence after paraesophageal hernia repair with mesh. Surg Endosc. 2015;29:425-30.
6. Lubezky N, Sagie B, Keidar A, Szold A. Prosthetic mesh repair of large and recurrent diaphragmatic hernias. Surg Endosc. 2007;21:737-41.
7. Zaninotto G, Portale G, Costantini M, et al. Objective follow-up after laparoscopic repair of large type III hiatal hernia. Assessment of safety and durability. World J Surg. 2007;31:2177-83.
8. Westcott LZ, Ward MA. Techniques for closing the hiatus: mesh, pledgets and suture techniques. Ann Laparosc Endosc Surg. 2020;5:16.
9. Gerdes S, Schoppmann SF, Bonavina L, Boyle N, Müller-Stich BP, Gutschow CA; Hiatus Hernia Delphi Collaborative Group. Management of paraesophageal hiatus hernia: recommendations following a European expert Delphi consensus. Surg Endosc. 2023;37:4555-65.
10. Aiolfi A, Cavalli M, Sozzi A, et al. Medium-term safety and efficacy profile of paraesophageal hernia repair with Phasix-ST® mesh: a single-institution experience. Hernia. 2022;26:279-86.
11. Bona D, Aiolfi A, Asti E, Bonavina L. Laparoscopic Toupet fundoplication for gastroesophageal reflux disease and hiatus hernia: proposal for standardization using the “critical view” concept. Updates Surg. 2020;72:555-8.
12. Aiolfi A, Cavalli M, Saino G, et al. Laparoscopic posterior cruroplasty: a patient tailored approach. Hernia. 2022;26:619-26.
13. Daly S, Kumar SS, Collings AT, et al. SAGES guidelines for the surgical treatment of hiatal hernias. Surg Endosc. 2024;38:4765-75.
14. Kohn GP, Price RR, DeMeester SR, et al; SAGES Guidelines Committee. Guidelines for the management of hiatal hernia. Surg Endosc. 2013;27:4409-28.
15. Lima DL, de Figueiredo SMP, Pereira X, et al. Hiatal hernia repair with biosynthetic mesh reinforcement: a qualitative systematic review. Surg Endosc. 2023;37:7425-36.
16. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274-7.
17. Balla A, Leone G, Ribichini E, et al. Gastroesophageal reflux disease - health-related quality of life questionnaire: prospective development and validation in Italian. Eur J Gastroenterol Hepatol. 2021;33:339-45.
18. Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20:130-4.
19. Velanovich V. Comparison of generic (SF-36) vs. disease-specific (GERD-HRQL) quality-of-life scales for gastroesophageal reflux disease. J Gastrointest Surg. 1998;2:141-5.
20. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-96.
21. Granderath FA, Schweiger UM, Pointner R. Laparoscopic antireflux surgery: tailoring the hiatal closure to the size of hiatal surface area. Surg Endosc. 2007;21:542-8.
22. Barazanchi AWH, Chui JN, Bhimani N, Leibman S, Smith G. Impact of pre-existing mesh at the hiatus at revisional hiatal hernia surgery. Dis Esophagus. 2024;37:doae050.
23. Paranyak M, Patel R. A prospective randomized trial on laparoscopic total vs partial fundoplication in patients with atypical symptoms of gastroesophageal reflux disease. Langenbecks Arch Surg. 2023;408:269.
24. Aiolfi A, Cavalli M, Saino G, et al. Laparoscopic toupet fundoplication for the treatment of laryngopharyngeal reflux: results at medium-term follow-up. World J Surg. 2020;44:3821-8.
25. Porta A, Aiolfi A, Musolino C, Antonini I, Zappa MA. Prospective comparison and quality of life for single-incision and conventional laparoscopic sleeve gastrectomy in a series of morbidly obese patients. Obes Surg. 2017;27:681-7.
26. Clapp B, Kara AM, Nguyen-Lee PJ, et al. Does bioabsorbable mesh reduce hiatal hernia recurrence rates? A meta-analysis. Surg Endosc. 2023;37:2295-303.
27. Hanna NM, Kumar SS, Collings AT, et al. Management of symptomatic, asymptomatic, and recurrent hiatal hernia: a systematic review and meta-analysis. Surg Endosc. 2024;38:2917-38.
28. Petric J, Bright T, Liu DS, Yun MW, Watson DI. Sutured versus mesh-augmented hiatus hernia repair: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2022;275:e45-51.
29. Latorre-Rodríguez AR, Rajan A, Mittal SK. Cruroplasty with or without mesh? A systematic literature review with a novel time-organized proportion meta-analysis. Surg Endosc. 2024;38:1685-708.
30. Aiolfi A, Bona D, Bonitta G, Manara M, Bonavina L. Comment on "Laparoscopic paraesophageal hernia repair: to mesh or not to mesh". Ann Surg Open. 2023;4:e304.
31. Granderath FA, Carlson MA, Champion JK, et al. Prosthetic closure of the esophageal hiatus in large hiatal hernia repair and laparoscopic antireflux surgery. Surg Endosc. 2006;20:367-79.
32. Aiolfi A, Bona D, Sozzi A, Bonavina L; PROMER Collaborative Group. PROsthetic MEsh Reinforcement in elective minimally invasive paraesophageal hernia repair (PROMER): an international survey. Updates Surg. 2024;76:2675-82.
33. Rajkomar K, Wong CS, Gall L, et al. Laparoscopic large hiatus hernia repair with mesh reinforcement versus suture cruroplasty alone: a systematic review and meta-analysis. Hernia. 2023;27:849-60.
35. Akmaz B, Hameleers A, Boerma EG, et al. Hiatal hernia recurrences after laparoscopic surgery: exploring the optimal technique. Surg Endosc. 2023;37:4431-42.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].