Risk factors for post-endoscopic retrograde cholangiopancreatography pancreatitis in the Chinese population: a systematic review and meta-analysis
Abstract
Aim: To systematically identify and evaluate key risk factors for post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis in the Chinese population, providing evidence-based guidance to inform clinical practice, particularly strategies for prevention.
Methods: A comprehensive literature search was conducted across PubMed, China National Knowledge Infrastructure, and other databases from October 2003 to October 2023. Data from 38 rigorously appraised investigations - comprising randomized controlled trials, case-control analyses, and cohort studies - were synthesized in RevMan 5.3 using both fixed- and random-effects models as appropriate. Risk factors were evaluated using odds ratios (ORs) with 95% confidence intervals (CIs), adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
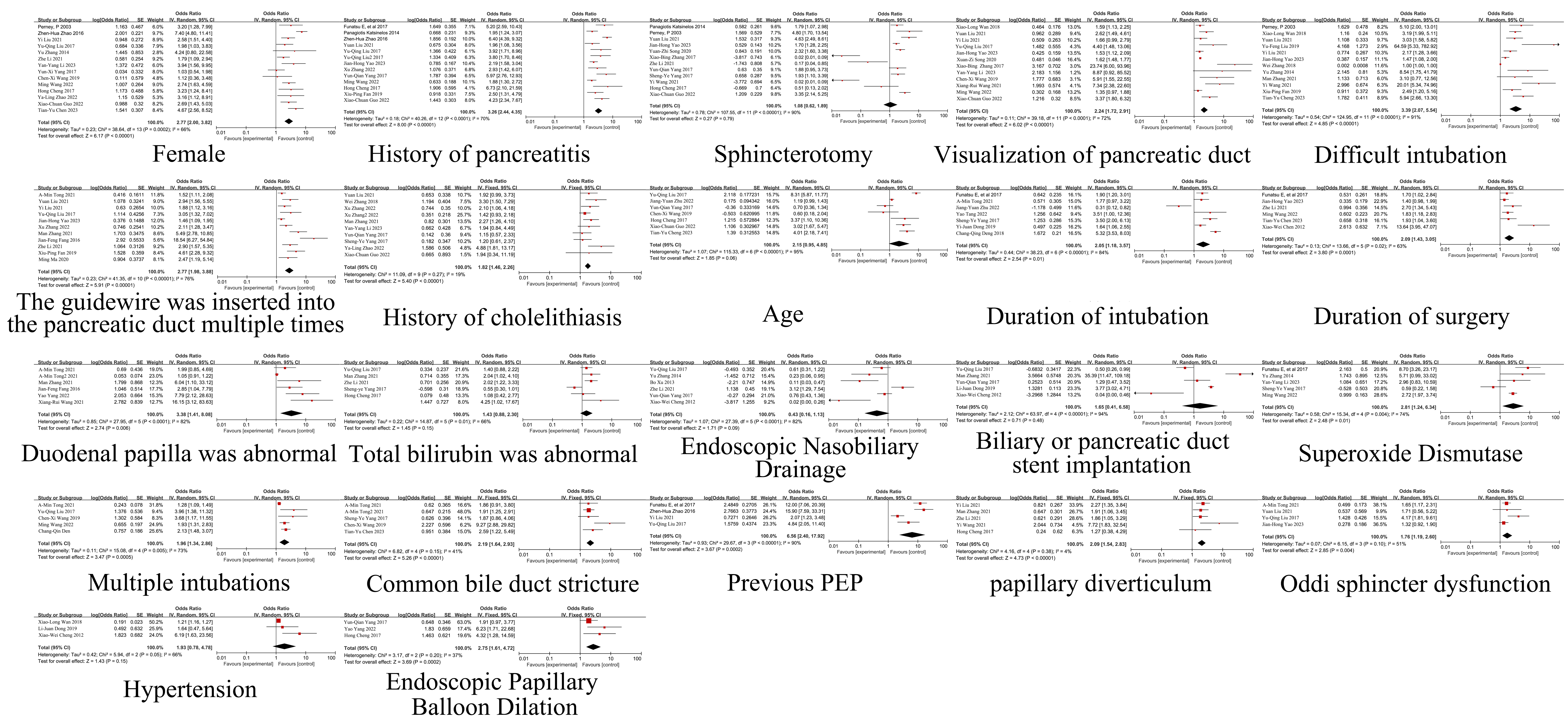
Results: The meta-analysis identified 22 significant risk factors. The strongest predictors included prior post-ERCP pancreatitis (OR = 6.56, 95%CI: 2.40-17.92), duodenal papillary abnormalities (OR = 3.38, 95%CI: 1.41-8.08), and difficult cannulation (OR = 3.39, 95%CI: 2.07-5.54). Other notable factors were endoscopic papillary balloon dilation (OR = 2.75, 95%CI: 1.61-4.72), elevated superoxide dismutase (SOD) levels (OR = 2.81, 95%CI: 1.24-6.34), female gender (OR = 2.77, 95%CI: 2.00-3.82), and prolonged cannulation time (OR = 2.05, 95%CI: 1.18-3.57). In contrast, hypertension (OR = 1.93, 95%CI: 0.78-4.78) and biliary stenting (OR = 1.65, 95%CI: 0.41-6.58) were not significantly associated with ERCP.
Conclusion: Procedural, anatomical, and biochemical factors were significantly associated with increased post-ERCP pancreatitis risk in Chinese patients. Early identification of high-risk individuals, especially those with prior pancreatitis or papillary abnormalities, and tailored interventions are critical for reducing incidence. These findings provide an evidence-based framework to enhance clinical outcomes in ERCP procedures.
Keywords
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is both a diagnostic and therapeutic procedure that uses a duodenoscope to locate the duodenal papilla, followed by the injection of contrast agents to visualize the bile and pancreatic ducts[1]. It is commonly used for diagnosing and treating biliary and pancreatic disorders, particularly in managing common bile duct stones and biliary strictures[2]. Due to its cost-effectiveness, short procedure time, and reduced post-procedure hospital stay, ERCP has become a first-line treatment for these conditions[2]. Between 2006 and 2012, the number of ERCP procedures performed in China increased from 63,787 to 195,643, with over 95% of these procedures being therapeutic[3]. ERCP is now routinely performed in tertiary hospitals across China, and some secondary hospitals also offer the procedure[3].
Despite its advantages, such as minimal invasiveness and rapid recovery, Post-ERCP pancreatitis (PEP) remains the most common and potentially severe complication. According to a systematic review and meta-analysis of 145 randomized controlled trials (RCTs), the incidence of PEP was 10.2%, which climbed to 14.1% in high-risk patient groups[4]. Similarly, the incidence of severe pancreatitis after ERCP is also not negligible, with a cumulative incidence of 0.5% in 91 RCTs involving 14,441 patients[5]. The pathogenesis of PEP is multifactorial, involving chemical, mechanical, enzymatic, hydrostatic, and thermal effects on the pancreatic parenchyma[6]. PEP susceptibility is shaped by both patient-specific and procedure-related determinants. Patient-level predictors comprise suspected sphincter of Oddi dysfunction, a history of pancreatitis or prior PEP, recurrent attacks, normobilirubinemia, younger age, and female sex. Procedural risk factors include difficult cannulation, guidewire passage through the pancreatic duct, sphincterotomy, papillotomy, repeated or aggressive pancreatic duct contrast injection, and short-duration balloon dilation of the biliary sphincter[7]. Damage to the ampulla and surrounding areas during ERCP, as well as increased pressure within the main pancreatic duct, are key pathophysiological mechanisms that trigger PEP[8]. However, these predictive factors are primarily based on Western cohorts, raising concerns about their applicability to Asian populations.
The incidence of PEP in the Chinese population differs from that observed in other countries or regions. A 2015 meta-analysis reported the incidence of PEP in North American RCTs to be 13%, in European RCTs to be 8.4%, and in Asian RCTs to be 9.9%[9]. A subsequent 2023 meta-analysis found the incidence of PEP in Asian populations to be approximately 3.86% (95%CI: 2.18%-5.93%), which is higher than that reported in North America (2.93%, 95%CI: 1.35%-5.00%) and South America (3.24%, 95%CI: 0.69%-7.11%)[10]. These findings suggest that genetic diversity and distinct clinical practices may influence the risk of PEP. Furthermore, studies indicate that medical centers with higher annual case volumes achieve better cannulation success rates, more effective complication management, and shorter procedure times, suggesting that experienced operators can reduce the risk of PEP[11].
The use of non-steroidal anti-inflammatory drugs (NSAIDs) has significantly increased in recent years. Between 2013 and 2020, NSAID use in ERCP procedures rose from 62% to 93%, markedly improving the effectiveness of PEP prevention[12]. Additionally, traditional Chinese medicine (TCM) has shown promise in preventing PEP, significantly reducing the incidence of post-procedure hyperamylasemia and serum amylase levels, without apparent adverse effects. The findings indicate a promising pharmacotherapeutic option for routine clinical use; nevertheless, confirmation through further large-scale, multicentre RCTs remains essential[13].
Although numerous studies have been conducted in China on ERCP-induced pancreatitis, the findings regarding the risk factors for PEP have been inconsistent. Clinically, targeted preventive interventions for PEP have yet to reach a consensus. To address this, we employed evidence-based medicine and meta-analysis techniques to evaluate the current state of PEP in China from 2003 to 2023, providing a valuable reference for the prevention and treatment of PEP in the Chinese population.
METHODS
Study design and registration
The systematic review and meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) reporting standards. A predefined protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42024514093) prior to the study’s initiation to ensure transparency and minimize bias (accessible at https://www.crd.york.ac.uk/PROSPERO/). The protocol outlined the objectives, search strategies, inclusion/exclusion criteria, and analytical methods, with any deviations from the original plan documented and justified.
Eligibility criteria
Study eligibility was determined according to the PICOS framework - Population, Intervention, Comparator, Outcomes, and Study design. The target population comprised Chinese patients undergoing diagnostic or therapeutic ERCP. Interventions or exposures included procedural techniques [e.g., endoscopic sphincterotomy (EST), pancreatic duct cannulation], biochemical markers (e.g., SOD levels), or clinical parameters (e.g., history of pancreatitis). Patients who underwent ERCP yet remained free of PEP served as the comparison group. The principal endpoint was PEP occurrence, operationalized according to ASGE criteria - new-onset abdominal pain after the procedure together with amylase or lipase values ≥ three times the upper reference limit and compatible imaging findings. Eligible evidence comprised RCTs, prospective and retrospective cohort studies, and case-control investigations. Non-research articles, animal studies, conference abstracts, and studies lacking extractable risk estimates [e.g., incomplete odds ratios (ORs) or confidence intervals (CIs)] were excluded. Studies finally included were those published in either Chinese or English and evaluated as moderate to high quality based on NOS and Cochrane risk of bias tools (section 2.8).
Information sources
Systematic searches were conducted in the following databases: PubMed
Search
The search strategies for PubMed, SinoMed, Cochrane Library, and Web of Science utilized both MeSH terms and keywords. Grey literature and unpublished trials were identified through the WHO International Clinical Trials Registry Platform: [“Endoscopic Retrograde Cholangiopancreatography”(Mesh) OR “ERCP”] AND [“Pancreatitis”(Mesh) OR “Post-ERCP Pancreatitis”] AND [“Risk Factors”(Mesh) OR “Epidemiologic Factors”]. The Chinese search terms were (“zhong zheng yi xian yan” OR “yi xian yan” OR “ji xing yi xian yan” OR “zhong du ji xing yi xian yan” OR “ji xing chu xue huai si xing yi xian yan” OR “ji xing chu xue xing huai si xing yi xian yan” OR “zhong xing ji xing yi xian yan” OR “ji xing zhong zheng yi xian yan”) AND (“yi dan guan zao ying shu, nei kui jing ni xing” OR “ERCP” OR “jing nei jing ni xing yi dan guan zao ying” OR “nei kui jing ni xing yi dan guan zao ying” OR “nei jing xia ni xing yi dan guan zao ying shu” OR “ni xing yi dan guan zao ying shu”) AND (“wei xian yin su” OR “ying xiang yin su” OR “bing yin” OR “xiang guan yin su”) AND (“bing li dui zhao yan jiu” OR “bing li dui zhao” OR “dui lie yan jiu” OR “dui lie”) Filters were applied to restrict results to human studies and publications from October 1, 2003, to October 31, 2023. Equivalent Chinese-language search terms were used in domestic databases.
Study selection
Two independent reviewers conducted title and abstract screening using Rayyan QCRI software. Full-text articles were then assessed against the eligibility criteria. Discrepancies were resolved through consensus discussions with a third investigator. Studies that met the inclusion criteria proceeded to data extraction and quality assessment.
Data collection process
Data extraction was performed using standardized forms that were pilot-tested on 5% of the included studies. Dual independent extraction was conducted for study characteristics (author, year, sample size), risk factor definitions, and outcome measures. If studies reported risk ratios, these were converted to ORs. ORs were synthesized under a random-effects model, and any data ambiguities were resolved by contacting the respective study authors for clarification.
Data items
The extracted variables included demographic parameters (age, gender), procedural factors (cannulation attempts, duct visualization), biochemical markers (SOD levels), and clinical history (prior pancreatitis, biliary pathology). Operational definitions were consistent with the American Society for Gastrointestinal Endoscopy criteria for diagnosing PEP.
Risk of bias in individual studies
The methodological quality was assessed using the Newcastle-Ottawa Scale (NOS) for observational studies, which evaluates studies based on three domains: selection (0-4 stars), comparability (0-2 stars), and outcome/exposure assessment (0-3 stars). RCTs were appraised with the Cochrane Risk of Bias 2.0 instrument, which evaluates bias related to randomization, protocol deviations, attrition, outcome assessment, and selective reporting. Scores of 5-7 denoted moderate methodological quality, whereas 8-9 signified high quality. For the sensitivity analyses, we excluded studies that attained ≤ 5 points on the Newcastle-Ottawa Scale or were judged high-risk by the RoB 2.0 tool to safeguard the robustness of our pooled estimates.
Summary measures
Effect sizes were ORs. Both ORs and their 95%CIs were extracted from individual studies and transformed to natural logarithms. Continuous variables were expressed as mean differences, where applicable.
Synthesis of results
All analyses were performed using RevMan 5.3 (Cochrane Collaboration). A two-sided P value ≤ 0.05 was considered statistically significant unless otherwise specified.
Heterogeneity was quantified using the I² statistic - classified as low (≤ 50 %), moderate (51%-75%), or high (> 75 %). Where I2 exceeded 50%, pooled ORs with 95%CI were estimated under a random-effects framework to accommodate clinical variability; otherwise, a fixed-effects model was applied. Sensitivity analyses were conducted by excluding one study at a time and replacing a random-effects model with a fixed-effects one or vice versa to examine the robustness of the findings.
Risk of bias across studies
Potential publication bias was interrogated for predictors represented by ten or more studies - including female sex, prior pancreatitis, EST, pancreatic-duct opacification, difficult cannulation, repeated guidewire passage into the pancreatic duct, and antecedent biliary calculi - by examining funnel plot symmetry and applying Egger’s regression analysis. When funnel plot asymmetry was detected, a trim-and-fill analysis was performed to estimate adjusted effect sizes and assess the potential impact of publication bias on the overall results.
Additional analyses
Pre-specified subgroup analyses were conducted to examine potential differences across characteristics [age (≥ 60 years vs. < 60 years, sex, ***] and procedural variations (with or without pancreatic stenting). Meta-regression was conducted to explore sources of heterogeneity, including sample size and study design.
RESULTS
Study selection
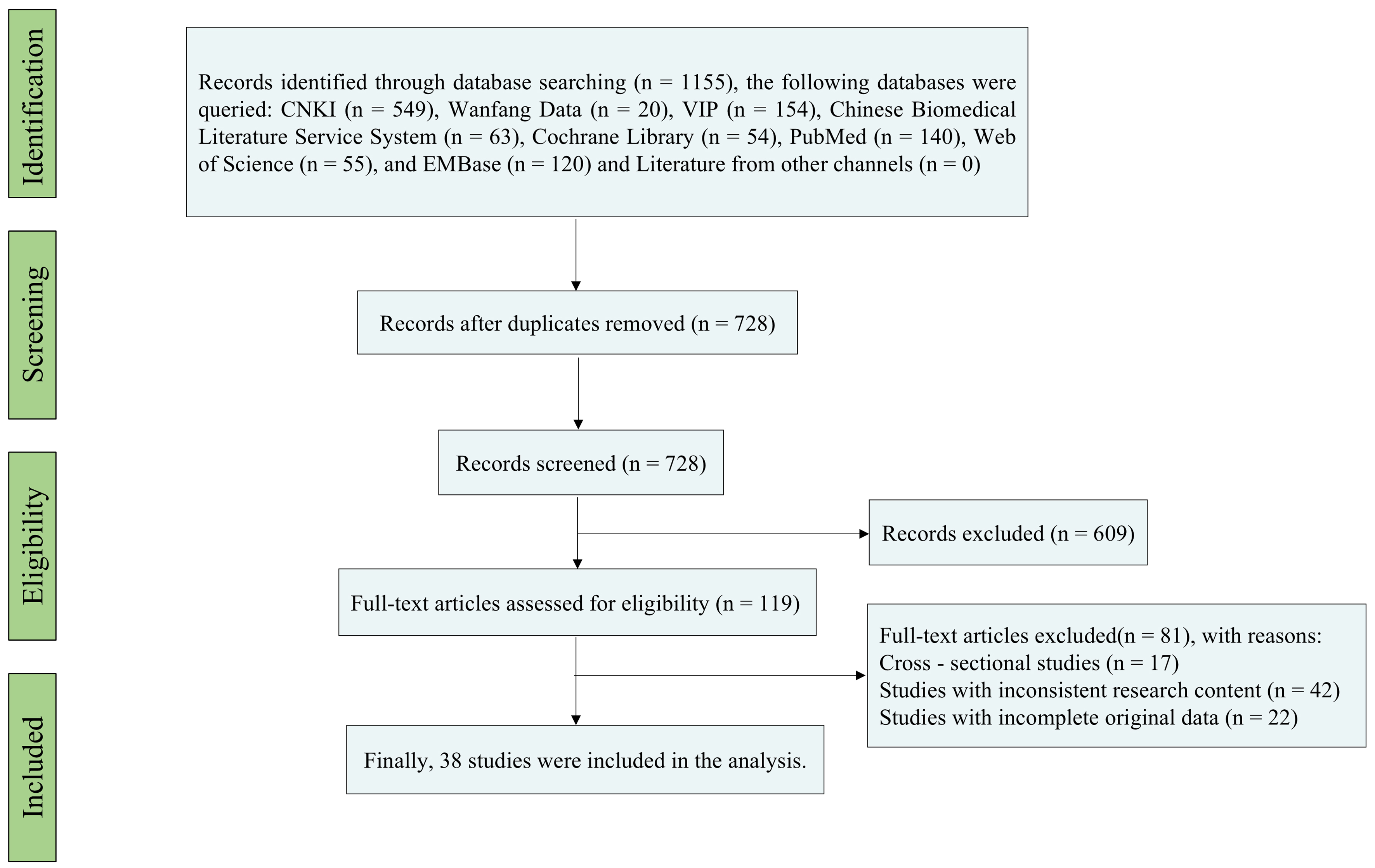
A comprehensive search of seven electronic databases retrieved 1,155 records. After de-duplication (n = 427), 728 titles and abstracts were screened, of which 421 were excluded as irrelevant or ineligible. Full-text evaluation of 119 articles led to the additional exclusion of 81 studies. Thirty-eight studies met the eligibility criteria and were included in the meta-analysis. The selection process is detailed in Figure 1.
Figure 1. Flowchart of Literature Screening. This PRISMA flowchart outlines the systematic selection process of relevant literature. A total of 1,155 studies were identified through initial database searches, with 427 duplicates removed. After screening titles and abstracts, 728 records were reviewed, and 119 full-text articles were assessed for eligibility. Thirty-eight studies met the inclusion criteria for the meta-analysis, focusing on PEP risk factors in the Chinese population. Excluded studies were those with non-relevant outcomes, non-Chinese cohorts, or insufficient data.
Study characteristics
Thirty-eight observational studies (2003-2023) were analyzed [Table 1]. These studies investigated 22 predefined risk factors based on the PICOS criteria, including patient demographics (e.g., female gender, age), procedural factors (e.g., cannulation attempts, procedure duration), and biochemical markers (e.g., SOD levels). Quality assessment scores ranged from 5 to 9 on the NOS, indicating moderate to high methodological rigor.
Basic characteristics and quality scores of the included literature
| Number | Included study | Year | Risk factors | Quality score |
| 1 | Yang[77] | 2017 | Age, gender, history of common bile duct stones, EST, ENBD, EPBD common bile duct stent pancreatitis | 8 |
| 2 | Liu[78] | 2017 | Gender, age, sphincter of Oddi dysfunction, history of PEP, history of pancreatitis, jaundice, pancreatic duct stent, and ENBD | 6 |
| 3 | Wan[79] | 2018 | Age, hypertension, difficult intubation, and pancreatography | 5 |
| 4 | Zhang[80] | 2014 | Gender, SOD, difficult intubation, and ENBD | 9 |
| 5 | Tong[81] | 2021 | Intubation time, number of intubations, guidewire entry into the pancreatic duct, sphincter of Oddi dysfunction, papilla classification, and ductal stenosis | 6 |
| 6 | Deng[82] | 2018 | Number of cannulation, duration of cannulation, number of entries into the pancreatic duct and visualization of the pancreatic duct | 8 |
| 7 | Zhang[83] | 2018 | Bile duct stenosis, difficult intubation, and intubation time | 5 |
| 8 | Fang[84] | 2016 | Type of nipple and number of pancreatic duct entry | 7 |
| 9 | Fan[85] | 2019 | History of pancreatitis, difficult intubation, and number of pancreatic duct entries | 6 |
| 10 | Liu[56] | 2019 | Difficulty in intubation | 5 |
| 11 | Cheng[86] | 2017 | Gender, age, history of pancreatitis, EST, balloon dilation, bilirubin, and diverticulum | 5 |
| 12 | Perney et al.[87] | 2003 | Gender, difficult intubation, sphincterotomy, stone extraction | 6 |
| 13 | Chen[88] | 2023 | Gender, age, stenosis at the lower end of the common bile duct, difficulty in intubation, and operation time | 5 |
| 14 | Funatsu et al.[89] | 2017 | Previous PEP, history of pancreatitis, SOD, intubation time, and operation time | 5 |
| 15 | Zhang[90] | 2021 | Papilla type, guidewire entry into the pancreatic duct, biliary stent, gallstones, cholangitis, ALP, bilirubin, and difficult intubation | 6 |
| 16 | Zhao et al.[91] | 2016 | Gender, history of pancreatitis, and previous PEP | 8 |
| 17 | Katsinelos et al.[92] | 2014 | History of pancreatitis, sphincterotomy | 6 |
| 18 | Chen et al.[93] | 2012 | Hypertension, ENBD, biliary stent, operation time | 7 |
| 19 | Dong et al.[62] | 2019 | Hypertension, pancreatic duct stent, and intubation time | 5 |
| 20 | Guo[94] | 2022 | Age, sex, history of pancreatitis, choledocholithiasis, pancreatic duct visualization, and sphincterotomy | 7 |
| 21 | Liu et al.[95] | 2021 | Visualization of the pancreatic duct, sphincterotomy of Oddi, difficult cannulation, and multiple guidewire insertions into the pancreatic duct | 5 |
| 22 | Wang[96] | 2019 | Age, gender, common bile duct stenosis, number of intubations, and pancreatic duct visualization | 5 |
| 23 | Yao et al.[97] | 2023 | History of pancreatitis, sphincter of Oddi dysfunction, duration of surgery, EST | 6 |
| 24 | Zhao[98] | 2022 | Gender and bile duct stones | 5 |
| 25 | Wang[99] | 2021 | Peripapillary diverticulum, sphincterotomy, difficult intubation, visualization of the pancreatic duct | 6 |
| 26 | Li[100] | 2021 | Gender, bilirubin, operation time, guidewire entry into the pancreatic duct, and ENBD | 6 |
| 27 | Song[101] | 2020 | History of pancreatitis, visualization of the pancreatic duct, and sphincterotomy | 7 |
| 28 | Xu[102] | 2013 | ENBD | 5 |
| 29 | Yang[103] | 2017 | Common bile duct stones, common bile duct stenosis, SOD, ALP, intubation time | 7 |
| 30 | Li et al.[104] | 2023 | Gender, pancreatic duct visualization, SOD, and bile duct dilatation | 8 |
| 31 | Zhang et al.[105] | 2017 | Difficult intubation, visualization of the pancreatic duct, EST | 5 |
| 32 | Zhang et al.[106] | 2022 | History of pancreatitis, gallstones, and guidewire into the pancreatic duct | 6 |
| 33 | Zhu[107] | 2022 | Age and duration of intubation | 6 |
| 34 | Yang[108] | 2022 | Intubation time, EPBD | 5 |
| 35 | Ma and Zhou[109] | 2020 | The guidewire entered the pancreatic duct, ALP, and bilirubin | 7 |
| 36 | Wang et al.[110] | 2022 | Sex, SOD, number of intubations, history of pancreatitis, and duration of operation | 7 |
| 37 | Liu[53] | 2021 | Gender, history of PEP, guidewire entry into the pancreatic duct, visualization of the pancreatic duct, and difficulty in intubation | 6 |
| 38 | Wang[111] | 2021 | Visualization of pancreatic duct | 6 |
Risk of bias within studies
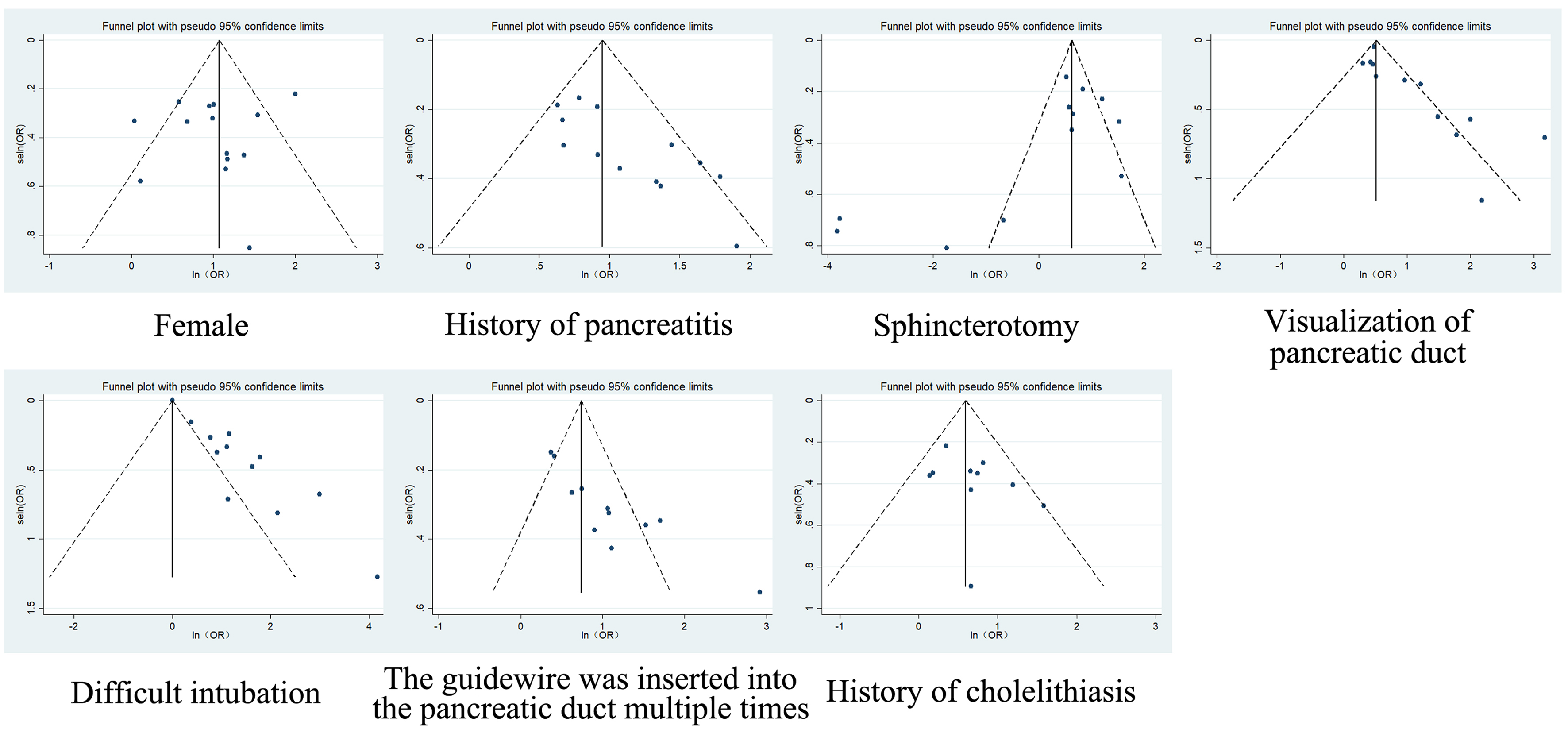
Funnel plots revealed publication bias in pancreatic duct visualization, difficult cannulation, and multiple guidewire passages into the pancreatic duct. For 18 factors with high heterogeneity (I2 > 50%), random-effects models were applied, while fixed-effects models were used for four factors with low heterogeneity (e.g., bile duct stenosis). Sensitivity analysis confirmed the robustness of the results, although heterogeneity was likely due to methodological or population differences. Retrospective study designs predominated, introducing the risk of selection and recall bias, highlighting the need for standardized prospective protocols.
Results of individual studies
Forest plot analysis revealed significant heterogeneity (I2 = 51%-95%) for 18 risk factors, necessitating the use of random-effects models. Fixed-effects models were applied to four factors with low heterogeneity (I2 ≤ 50%). Key findings included a significantly elevated ORs for PEP associated with prior pancreatitis (OR = 3.26, 95%CI: 2.44-4.35) and pancreatic duct visualization (OR = 2.24, 95%CI: 1.72-2.91). Non-significant associations were observed for hypertension (OR = 1.93, 95%CI: 0.78-4.78) and biliary stenting (OR = 1.65, 95%CI: 0.41-6.58).
Synthesis of results
The meta-analysis identified 22 statistically significant risk factors [Table 2 and Figure 2]. The strongest predictors included prior PEP (OR = 6.56, 95%CI: 2.40-17.92), papillary abnormalities (OR = 3.38, 95%CI: 1.41-8.08), and difficult cannulation (OR = 3.39, 95%CI: 2.07-5.54). Moderate heterogeneity (I2 = 51%-76%) characterized factors such as elevated SOD levels (OR = 2.81, 95%CI: 1.24-6.34) and Endoscopic papillary balloon dilation (EPBD) (OR = 2.75, 95%CI: 1.61-4.72). Fixed-effects models confirmed bile duct stenosis (OR = 2.19, 95%CI: 1.64-2.93) and periampullary diverticulum (PAD) (OR = 2.09, 95%CI: 1.54-2.83) as stable predictors.
Figure 2. Meta-analysis of the associations between influence factors and risk of post-ERCP pancreatitis. This figure presents forest plots showing the pooled ORs with 95%CIs for 22 identified risk factors for post-ERCP pancreatitis. Significant predictors include prior PEP, papillary abnormalities, and difficult cannulation. Random-effects models were applied to account for substantial heterogeneity (I2 = 51%-95%), while fixed-effects models confirmed the stability of the associations. ERCP: Endoscopic retrograde cholangiopancreatography; ORs: odds ratios; PEP: post-ERCP pancreatitis.
Meta-analysis of risk factors
| Influence factor | Heterogeneity test | Results of meta-analysis | Model | Number of pooled studies | ||
| I 2 | P | Pooled effect size | 95%CI | |||
| Female | 66% | 0.0002 | 2.77 | (2.00, 3.82) | Random | 14 |
| History of pancreatitis | 70% | < 0.0001 | 3.26 | (2.44, 4.35) | Random | 12 |
| Sphincterotomy | 90% | < 0.00001 | 1.08 | (0.62, 1.89) | Random | 12 |
| Pancreatic duct imaging | 72% | < 0.0001 | 2.24 | (1.72, 2.91) | Random | 12 |
| Difficult cannulation | 91% | < 0.00001 | 3.39 | (2.07, 5.54) | Random | 12 |
| Multiple cannulation of pancreatic duct | 76% | < 0.00001 | 2.77 | (1.98, 3.88) | Random | 11 |
| History of biliary stones | 19% | 0.27 | 1.82 | (1.46, 2.26) | Fixed | 9 |
| Age | 95% | < 0.00001 | 2.15 | (0.95, 4.85) | Random | 7 |
| Cannulation time | 84% | < 0.00001 | 2.05 | (1.18, 3.57) | Random | 7 |
| Operation time | 63% | 0.02 | 2.09 | (1.43, 3.05) | Random | 6 |
| Abnormal papilla | 82% | < 0.0001 | 3.38 | (1.41, 8.08) | Random | 5 |
| Abnormal total bilirubin | 66% | 0.01 | 1.43 | (0.88, 2.30) | Random | 6 |
| ENBD | 82% | < 0.0001 | 0.43 | (0.16, 1.13) | Random | 6 |
| Biliary/pancreatic duct stent insertion | 94% | < 0.00001 | 1.65 | (0.41, 6.58) | Random | 5 |
| Sphincter of Oddi dysfunction | 74% | 0.004 | 2.81 | (1.24, 6.34) | Random | 5 |
| Multiple cannulations | 73% | 0.005 | 1.96 | (1.34, 2.86) | Random | 5 |
| Common bile duct stricture | 41% | 0.15 | 2.19 | (1.64, 2.93) | Fixed | 4 |
| Previous PEP | 90% | < 0.00001 | 6.56 | (2.40, 17.92) | Random | 4 |
| Periampullary diverticulum | 4% | 0.38 | 2.09 | (1.54, 2.83) | Fixed | 5 |
| Oddi dysfunction | 51% | 0.10 | 1.76 | (1.19, 2.60) | Random | 4 |
| Hypertension | 66% | 0.05 | 1.93 | (0.78, 4.78) | Random | 3 |
| EPBD | 37% | 0.20 | 2.75 | (1.61, 4.72) | Fixed | 3 |
Risk of bias across studies
Funnel plot asymmetry [Figure 3] indicated potential publication bias for risk factors such as pancreatic duct visualization, difficult cannulation, and repeated guidewire insertion.
Additional analyses
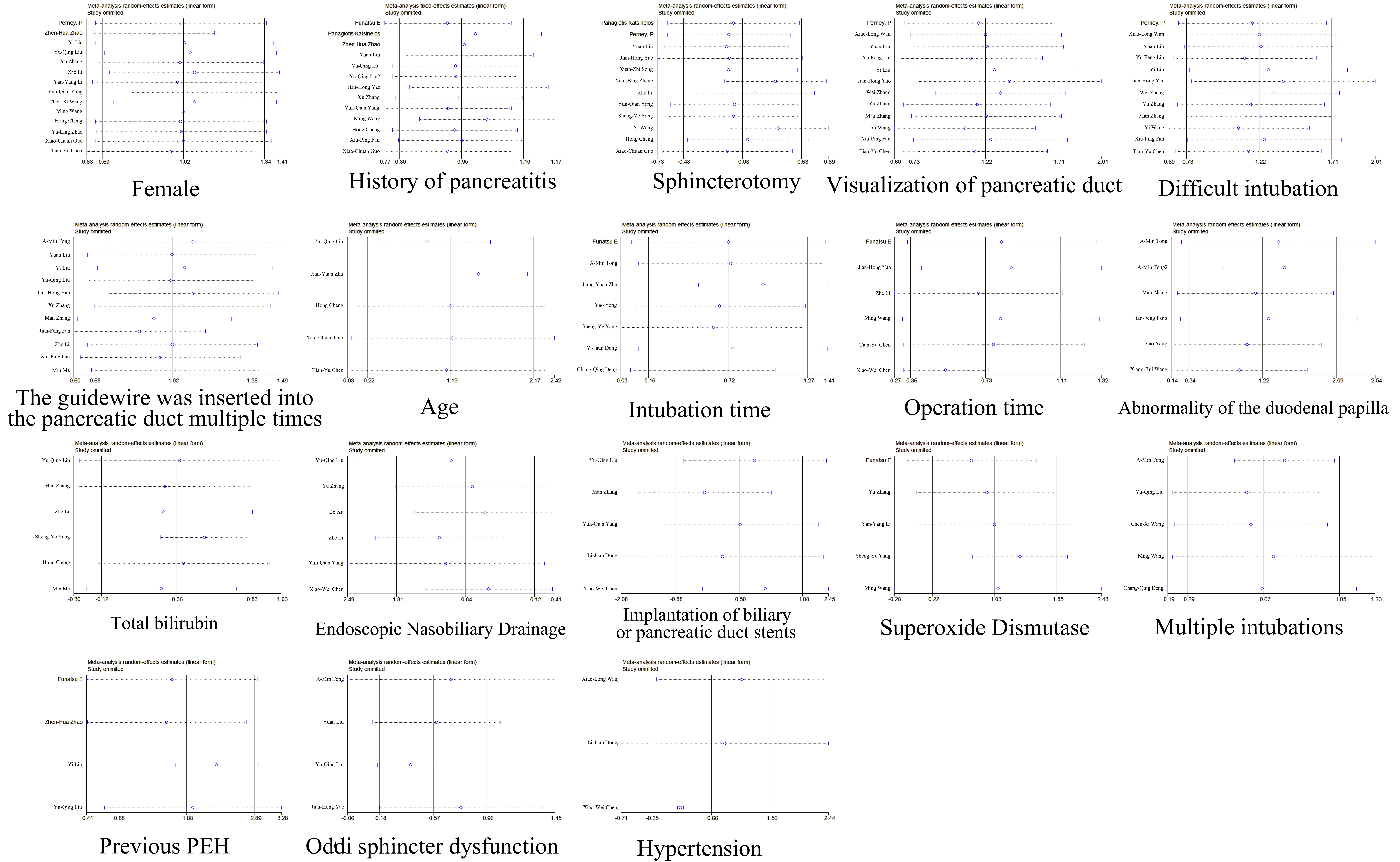
Sensitivity analyses using the leave-one-out approach confirmed the robustness of all significant predictors. The results remained within the 95%CI boundaries throughout, and the exclusion of any single study did not alter the overall conclusions [Figure 4].
Figure 4. Sensitivity analysis of different factors associated with risk of post-ERCP pancreatitis. This figure demonstrates the robustness of significant predictors. Sequential exclusion of individual studies showed that the OR fluctuations remained within the 95%CI bounds, confirming the stability of the findings. ERCP: Endoscopic retrograde cholangiopancreatography; OR: odds ratio.
DISCUSSION
This review synthesizes current evidence to construct an integrated risk-stratification schema for PEP in Chinese patients, pinpointing 22 statistically significant determinants across procedural, anatomical, and biochemical categories. The findings underscore the multifactorial etiology of PEP, with prior PEP history (OR = 6.56), duodenal papillary abnormalities (OR = 3.38), and difficult cannulation (OR = 3.39) emerging as dominant risk factors. Notably, the study reveals ethnic-specific patterns, including an attenuated association between EST and PEP compared to global data, suggesting potential population-specific protective mechanisms. These insights carry critical implications for risk mitigation strategies, emphasizing preoperative identification of high-risk cohorts - particularly patients with prior pancreatitis or papillary anomalies - and precision interventions such as optimized cannulation protocols and targeted biochemical monitoring.
The adoption of next-generation innovations - chiefly artificial intelligence (AI)-assisted endoscopic platforms and novel biomaterials - has the potential to sharpen PEP risk prediction and further enhance procedural safety. Innovations highlighted in contemporary research, including AI-driven image analysis and multifunctional capsule endoscopic platforms, exemplify translational opportunities to augment real-time decision making during ERCP. Such interdisciplinary advancements could synergize with the risk stratification model proposed here, enabling dynamic assessment of cannulation difficulty or papillary morphology while minimizing mechanical trauma. Future investigations should prioritize the development of ethnicity-specific predictive algorithms and mechanistic studies to decode the observed SOD-PEP paradox, ultimately bridging evidence-based risk mitigation with next-generation minimally invasive technologies.
Gender (Female)
In this study, the incidence of PEP in women was found to be 2.77 times higher than in men. This suggests that women are at a higher risk of developing PEP compared to men, a finding consistent with previous studies. Among female patients, SOD levels were found to be higher, which may explain the increased incidence of PEP in women. The more frequent occurrence of SOD in elderly women, compared to men, is thought to elevate the risk of post-procedure pancreatitis[14].
History of pancreatitis
A history of previous pancreatitis is a known risk factor for the development of PEP[15]. Patients with a history of pancreatitis have often sustained significant damage to the pancreatic parenchyma, resulting in impaired pancreatic secretory function[16]. The data from this meta-analysis indicate that patients with a history of pancreatitis are 3.26 times more likely to develop PEP compared to those without such a history, which aligns with findings from other studies. Consequently, the risk of developing pancreatitis following the procedure is significantly elevated[15].
EST
Upon pooling the available data, EST exhibited no statistically significant association with the incidence of PEP. EST, a routine procedure in ERCP, remains controversial regarding its role as a risk factor for PEP. Some studies suggest that the thermal effects of EST may cause edema in the tissues surrounding the pancreatic duct, obstructing pancreatic fluid drainage and potentially triggering PEP[17]. However, EST may also reduce pancreatic duct pressure, alleviate the severity of pancreatitis, and provide a protective effect by lowering the tension at the duct opening[18]. Furthermore, although some studies indicate that EST may reduce the incidence of PEP, its potential complications, such as bleeding and perforation, could counterbalance these benefits[19]. Therefore, the relationship between EST and PEP occurrence warrants further investigation through larger sample studies.
Pancreatic duct injection
In this study, pancreatic duct injection was identified as a risk factor for PEP. The injection of contrast agents during ERCP can directly damage pancreatic tissue, and if performed too rapidly or under excessive pressure, it can cause further mechanical injury to the pancreatic duct and acini, thus triggering PEP[20]. During ERCP procedures, repeated placement of the guidewire within the pancreatic duct increases the risk of duct injury and contrast agent entry, leading to more frequent pancreatic duct visualization, which significantly elevates the risk of PEP[21]. The placement of a metal biliary stent can disrupt biliary and pancreatic fluid drainage, causing pressure imbalance and pancreatic juice reflux, which further increases the likelihood of abnormal pancreatic duct imaging and the risk of PEP[21].
At the molecular scale, experimental evidence suggests that administration of contrast media or isotonic saline activates NF-κB p65 and STAT3 signalling, alters the expression of apoptosis-related proteins, and downregulates tight-junction components, collectively fostering the initiation and progression of PEP[20].
Moreover, increased pancreatic duct pressure has been confirmed as a key trigger for PEP. Animal studies have demonstrated that elevated intraductal pressure can activate the Ca2+/calmodulin-dependent phosphatase signaling pathway, leading to calcium homeostasis disruption in pancreatic acinar cells, mitochondrial dysfunction, and tight junction damage[22]. This cascade of events ultimately results in pancreatic edema, inflammation, and necrosis, closely mirroring the pathological features of clinical PEP.
Cannulation factors (Difficult cannulation, multiple cannulations)
In this study, difficult cannulation and multiple cannulations were identified as risk factors for PEP. Difficult cannulation is widely recognized as an independent risk factor for PEP[23].
Multiple guidewire insertions into the pancreatic duct
In this study, multiple guidewire insertions into the pancreatic duct were identified as a risk factor for PEP. Research has shown that repeated unintended guidewire insertions into the pancreatic duct, often due to difficult cannulation, are relatively common. The risk of PEP increases with the frequency and extent of these insertions, which contribute to a higher incidence of PEP and induce acute postoperative pancreatic inflammation[27].
History of biliary stone disease
A history of biliary stone disease is a recognized risk factor for PEP. When the number of stones is large, multiple interventions may be required, which increases the risk of mucosal injury and infection[28]. Asymptomatic stones, due to the lack of inflammatory adhesions, have fragile bile duct walls, making them more prone to perforation or bleeding during surgery[29]. Additionally, factors such as stone fragments or inflammatory mediators being refluxed into the pancreatic duct during the procedure may trigger pancreatitis[30]. Moreover, biliary obstruction caused by stones can lead to bacterial overgrowth, further increasing the risk of infection and PEP[31].
Age
Accumulating evidence indicates that patients younger than 60 years are at heightened risk of developing PEP[32]. Younger age appears to predispose patients to more complicated manifestations of PEP[32,33]. Pancreatic exocrine output increases until roughly 43 years of age and then gradually wanes, diminishing the gland’s responsiveness to mechanical stimuli such as ERCP[34]. Experts suggest that with advancing age, pancreatic exocrine function diminishes, potentially due to pancreatic atrophy and decreased enzyme activity. This may explain the reduced incidence of PEP in older individuals[34]. However, in this study, no significant association was found between age and the occurrence of PEP, indicating that larger sample size studies are needed for further validation.
Cannulation time
Consistent with this study, cannulation time is a recognized risk factor for PEP[25]. Prolonged cannulation time increases the risk of adverse events, including pancreatitis, following ERCP[35]. Some studies suggest that extended cannulation time may lead to ductal contrast injection, SOD spasm, and papillary edema, which in turn cause pancreatic duct obstruction and trigger pancreatitis[36]. Evidence from a retrospective analysis suggests that PEP stems from the interplay of multiple contributory factors, culminating in acinar cell injury and the downstream inflammatory cascade[37]. Other literature indicates that cannulation time decreases with increasing physician experience, suggesting that improper cannulation or lack of skill may contribute to prolonged cannulation time[38]. As such, cannulation time can, to some extent, serve as a representative factor for related procedural issues.
Duodenal papillary abnormalities
Duodenal papillary abnormalities are a recognized risk factor for pancreatitis following ERCP. These abnormalities include papillary microstone impaction, papillary inflammatory stricture, and anomalous pancreaticobiliary duct junctions. When such abnormalities occur, they can lead to biliary and pancreatic duct obstruction, increasing biliary pressure and elevating the risk of pancreatitis after ERCP[39]. Additionally, the size and anatomical variations of the papilla can affect the success rate of cannulation[40]. Small papillae are associated with a higher risk of PEP[41]. Abnormal papillary shapes can lead to increased incidences of pancreatitis, more attempts at cannulation, longer cannulation times, and a higher rate of inadvertent pancreatic duct cannulation, further raising the risk of PEP[42]. These known risk factors make the occurrence of PEP more likely after the procedure, thereby increasing the risk of pancreatitis following ERCP.
Total bilirubin abnormalities
In this study, total bilirubin abnormalities were not found to be statistically significant when considered alongside other variables, suggesting that they are not an independent risk factor. However, elevated total bilirubin serves as a highly specific marker of residual common bile duct stones in cholelithiasis-related pancreatitis and can therefore guide the selective use of ERCP, averting unnecessary procedures[43].
Endoscopic nasobiliary drainage
In this study, the analysis of data related to endoscopic nasobiliary drainage (ENBD) did not yield statistically significant results. This suggests that, based on the data from this study, there is no significant correlation between ENBD and the occurrence of PEP. However, some existing studies present differing viewpoints. One study indicated that the insertion of an ENBD catheter had a preventive effect on PEP, with the mechanism likely being that ENBD can effectively prevent pancreatic duct obstruction, thereby reducing the risk of PEP[42,44]. Nonetheless, it should be noted that in clinical practice, there are certain drawbacks to placing an ENBD catheter. These include potential discomfort, the risk of pressure ulcers, and the possibility of the catheter being removed by non-compliant patients[45]. Given these considerations, further clinical research is needed to determine whether ENBD serves as a protective or risk factor in the occurrence of PEP. More in-depth analysis from various perspectives is necessary to clarify its exact role and provide more reliable guidance for clinical practice.
Biliary or pancreatic stent placement
The combined results for this factor were not statistically significant, suggesting that biliary or pancreatic stent placement is not significantly associated with the occurrence of PEP. Part of the reason for this may be the current understanding of the challenges in pancreatic stent placement, along with skepticism regarding its effectiveness in preventing PEP. Some studies suggest that stent placement may fail if the duct is small or curved, potentially increasing the risk[46]. However, a related study by Martin Freeman indicates that pancreatic stent placement remains a critical intervention for high-risk patients, young patients with normal pancreatic function, or those who encounter cannulation difficulties during surgery[47]. Although this study initially concluded that biliary or pancreatic stent placement is not significantly associated with the occurrence of PEP, the complexity of research in this area and the limitations of the current sample size suggest that this conclusion requires further validation through large-scale studies.
SOD
SOD abnormalities are a well-established risk factor for PEP[48]. During ERCP, abnormal increases in biliary and pancreatic duct pressure can lead to ductal obstruction. This obstructive state can cause local circulatory disturbances, resulting in insufficient oxygen supply to the pancreatic tissue. In this hypoxic microenvironment, the redox balance within cells is disrupted, leading to the excessive production of oxygen free radicals[49]. Normally, SOD, a key antioxidant enzyme, clears oxygen free radicals in a timely manner, maintaining the body’s oxidative-antioxidant balance. However, when SOD levels become excessively high, its overactivity in clearing free radicals can disrupt cellular redox signaling pathways, overstimulating the oxidative stress response[50]. This enhanced oxidative stress further promotes the release of inflammatory mediators, exacerbating the inflammatory cascade and causing direct cytotoxic effects on pancreatic tissue[51]. As a result, pancreatic acinar cells are damaged, and pancreatic parenchymal inflammation occurs, significantly increasing the risk of pancreatitis.
Common bile duct stricture
Common bile duct stricture is a recognized risk factor for PEP. Bile duct stricture can lead to biliary obstruction, increasing the risk of elevated biliary pressure and bile stasis during the ERCP procedure. This can result in bile reflux into the pancreatic duct, triggering pancreatitis[52].
Previous PEP
In this study, a history of previous PEP was identified as a risk factor for PEP. This finding is consistent with the 2020 European Society of Gastrointestinal Endoscopy guidelines, which identified a history of previous PEP as an independent risk factor for PEP. The underlying reason may be that patients with a history of previous PEP often have a higher number of comorbidities and susceptibility factors, making them more sensitive to the procedure and increasing the likelihood of developing PEP[53].
PAD
PAD is considered a risk factor for PEP. PAD is a cystic structure closely associated with the biliary tract, where bile may accumulate. It has long been recognized as a risk factor for PEP[54]. Historically, PAD was considered one of the major causes of ERCP failure, likely due to the location of the diverticulum near the papillary orifice[55]. This proximity could lead to prolonged procedure times and a higher failure rate of cannulation. If the diverticulum is too close to the orifice, it may exert pressure on the distal bile duct, disrupting pancreatic juice drainage and thereby leading to pancreatitis[56]. However, some studies suggest that the presence of PAD does not affect the technical success of ERCP[56]. Research has shown that there is no significant difference between PAD and non-PAD groups among patients undergoing ERCP for various indications[57]. Therefore, the impact of PAD on the success of ERCP and the occurrence of complications remains controversial.
Sphincter of oddi dysfunction
Sphincter of Oddi dysfunction is a recognized risk factor for PEP. Oddi dysfunction may contribute to the pathogenesis of acute pancreatitis. When the function of the Sphincter of Oddi is impaired, it can lead to impaired drainage of pancreatic juice and bile, potentially triggering pancreatitis[58,59]. A study by Chen et al. demonstrated in animal experiments that Sphincter of Oddi dysfunction is an independent risk factor for inducing pancreatitis[60].
Hypertension
Based on the hypertension data from this study, no statistically significant association was observed between hypertension and the development of PEP. This finding aligns with the study by Liu[61], while the research by Dong et al. suggests that hypertension may be a risk factor for PEP[62]. Consequently, the role of hypertension as a risk factor for PEP remains controversial and warrants further investigation. However, renin-angiotensin system inhibitors, including captopril, ramipril, enalapril, lisinopril, quinapril, benazepril, losartan, and valsartan, have been implicated in cases of drug-induced acute pancreatitis[63]. Moreover, certain combinations of antihypertensive drugs, such as ramipril-hydrochlorothiazide, along with medications like formoterol and budesonide, have been associated with an increased risk of PEP following ERCP[64]. The evidence implies that certain classes of antihypertensive agents may heighten the risk of PEP. Given the limitations of this preliminary study, including potential sample selection bias and small sample size, further studies with larger cohorts are needed to determine whether hypertension is a risk factor for PEP.
Endoscopic papillary balloon dilation
In this study, EPBD was identified as a risk factor for PEP. The sensitivity of EPBD-related PEP may vary across different populations. Some studies suggest that balloon dilation itself does not directly cause PEP, but insufficient dilation may damage the pancreatic duct, and post-dilation papillary edema or spasm may contribute to the development of PEP[65].
Therapeutic potential and research imperatives of TCM for PEP prevention
Beyond conventional pharmacotherapy, TCM has emerged as a potential strategy for PEP prophylaxis. Meta-analyses indicate that TCM formulations (e.g., Gegen Qinlian decoction, Shakuyakukanzoto) may reduce the incidence of PEP and hyperamylasemia by modulating inflammatory pathways such as NLRP3 inflammasome-mediated pyroptosis and suppressing oxidative stress[66]. Experimental studies demonstrate that topical application of TCM extracts (e.g., Shakuyakukanzoto spray) to the duodenal papilla inhibits sphincter of Oddi spasm, thereby reducing pancreatic duct pressure and serum amylase elevation[67]. Mechanistically, TCM compounds exhibit antioxidative properties that counteract free radical-mediated acinar cell injury, a key pathogenic mechanism in PEP[66].
However, clinical adoption remains constrained by heterogeneity in TCM formulations, limited multicenter randomized trials, and insufficient standardization of dosing protocols. While preliminary evidence suggests efficacy comparable to rectal NSAIDs in specific cohorts, large-scale validation is needed to establish safety profiles, optimize delivery methods (e.g., enteral vs. topical), and identify patient subgroups most likely to benefit[68]. Future research should prioritize high-quality RCTs evaluating TCM as adjunctive therapy alongside established PEP prophylaxis protocols.
Comparison of PEP risk factors in chinese vs. global populations
Our findings reveal distinctive patterns in PEP risk factors within the Chinese population compared to global cohorts. While female gender and difficult cannulation universally elevate PEP risk globally (OR 1.47-1.70 and 2.60-3.39, respectively), their prevalence in Chinese patients (OR 2.77 and 3.39) exceeds pooled estimates from multinational meta-analyses[69]. Notably, the attenuated association between EST and PEP in our cohort contrasts with Western reports where EST remains a significant procedural risk (OR 1.08 vs. global OR 1.98-2.64)[69,70]. This divergence may arise from ethnic variations in pancreatic ductal anatomy or differential operator techniques in sphincterotomy execution. Furthermore, the prominence of elevated SOD as a biochemical predictor (OR 2.81) is underrepresented in non-Asian studies, suggesting population-specific pathophysiological pathways[71]. Conversely, prior pancreatitis consistently confers high PEP risk across ethnicities, though its effect size in Chinese patients (OR 6.56) surpasses global estimates (OR 3.26-4.40)[69,72]. These distinctions underscore the necessity of ethnicity-tailored risk stratification, particularly as emerging machine learning algorithms (e.g., RF AUC 0.947 in Chinese cohorts vs. 0.70-0.74 in multinational models) exhibit population-dependent performance variances[73,74]. Future guidelines should integrate region-specific procedural factors - such as the reduced PEP incidence in acute cholangitis patients observed in East Asia (Saito et al., 2022) - to optimize prophylaxis strategies[75].
Methodological limitations of the meta-analysis
This study has several methodological limitations requiring careful consideration. Substantial heterogeneity (I2 > 50%) was observed for 18 of the 22 risk factors analyzed, with I² values ranging from 51% to 95%, attributable to clinical and methodological variations across studies. These include differences in ERCP procedural techniques (e.g., cannulation protocols, stent placement criteria), definitions of risk exposures (e.g., “difficult cannulation”, “papillary abnormalities”), and PEP diagnostic thresholds across institutions. Although random-effects models were employed to incorporate this heterogeneity, residual variability may influence the precision of pooled estimates. Furthermore, variations in study quality were evident, with NOS scores spanning 5-9 points, reflecting disparities in design robustness, particularly among retrospective studies that may be susceptible to selection and recall biases. The predominance of single-center observational studies also limits generalizability. Publication bias, indicated by funnel plot asymmetry for factors including pancreatic duct visualization, difficult cannulation, and multiple guidewire passages [Figure 3], suggests possible underrepresentation of smaller studies reporting null associations. While trim-and-fill analysis was performed to adjust effect sizes, the exclusion of non-English literature and reliance on Chinese databases (CNKI, Wanfang) may have omitted relevant data, potentially skewing risk estimates. These limitations underscore the need for cautious interpretation of results and highlight the imperative for future prospective, multicenter studies with standardized PEP diagnostic criteria and risk exposure definitions.
In conclusion, this synthesis outlines a distinct set of procedural, anatomical, and biochemical risk factors for PEP in Chinese patients, highlighting cannulation difficulty, papillary abnormalities, and elevated SOD levels as particularly notable. Interestingly, the lack of a clear association between EST and PEP risk in this population stands in contrast to global data, suggesting the presence of ethnic-specific protective mechanisms that warrant further investigation. Future research should focus on prospectively validating these risk factors, developing ethnicity-tailored risk prediction models, and exploring the mechanisms behind the SOD-PEP paradox. Additionally, the integration of AI for real-time assessment of cannulation difficulty and genetic studies on PEP susceptibility loci offer promising avenues to enhance ERCP safety, particularly for high-risk populations[76].
DECLARATIONS
Acknowledgements
The authors thank all contributors, including teams at China Three Gorges University and Qiqihar Medical College. Special thanks are extended to FigDraw (www.figdraw.com) for technical assistance in creating scientific illustrations, which enhanced graphical clarity. The authors also acknowledge the authors of the included studies and colleagues at Yichang Central People’s Hospital.
Authors’ contributions
Responsible for the study’s conception, design, and data analysis: Yang Y, Hou Y
Conducted the literature search and data extraction: Li B, Niu M, Yi L, Fu Y, Gou Z, Wang Y, Xue Y, Xi J
Provided study supervision and critically revised the manuscript for important intellectual content: Hu M, Xing R
All authors approved the final version of the manuscript for publication.
Availability of data and materials
The datasets analyzed during this meta-analysis were derived from the published studies referenced in this article. All extracted data and analytical methods are described within the manuscript. Further details regarding the original studies can be obtained from the corresponding author upon reasonable request.
Financial support and sponsorship
The study was supported by the Hubei Provincial Natural Science Foundation (Grant No.2025AFB806), Yichang Science and Technology Innovation Fund (Grant No. A25-2-010), and the Doctoral Start-up Fund of Yichang Central People’s Hospital.
Conflicts of interest
Hu M is a Junior Editorial Board member of the journal Mini-invasive Surgery. Hu M was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study was reviewed and approved by the Medical Ethics Committee of Yichang Central People’s Hospital (2024-382-01). All procedures complied with the Declaration of Helsinki and relevant national regulations.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Drabek J, Keil R, Stovicek J, et al. The role of endoscopic retrograde cholangiopancreatography in choledochal cysts and/or abnormal pancreatobiliary junction in children. Prz Gastroenterol. 2017;12:303-9.
2. Aydelotte JD, Ali J, Huynh PT, Coopwood TB, Uecker JM, Brown CV. Use of magnetic resonance cholangiopancreatography in clinical practice: not as good as we once thought. J Am Coll Surg. 2015;221:215-9.
3. Hu LH, Xin L, Liao Z, et al; Endoscopy Audit of the Chinese Society of Digestive Endoscopy. ERCP development in the largest developing country: a national survey from China in 2013. Gastrointest Endosc. 2016;84:659-66.
4. Akshintala VS, Kanthasamy K, Bhullar FA, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: an updated systematic review and meta-analysis of 145 randomized controlled trials. Gastrointest Endosc. 2023;98:1-6.e12.
5. Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-18.
6. Tryliskyy Y, Bryce GJ. Post-ERCP pancreatitis: pathophysiology, early identification and risk stratification. Adv Clin Exp Med. 2018;27:149-54.
7. Elmunzer BJ. Reducing the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis. Dig Endosc. 2017;29:749-57.
8. Morales SJ, Sampath K, Gardner TB. A review of prevention of post-ERCP pancreatitis. Gastroenterol Hepatol. 2018;14:286-92.
9. Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143-9.e9.
10. Alsakarneh S, Jaber F, Ahmed K, et al. Incidence and cross-continents differences in endoscopic retrograde cholangiopancreatography outcomes among patients with cirrhosis: a systematic review and meta-analysis. Gastroenterology Res. 2023;16:105-17.
11. Syrén EL, Sandblom G, Enochsson L, et al. Outcome of ERCP related to case-volume. Surg Endosc. 2022;36:5339-47.
12. Sperna Weiland CJ, Engels MML, Poen AC, et al; Dutch Pancreatitis Study Group. Increased use of prophylactic measures in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis. Dig Dis Sci. 2021;66:4457-66.
13. Xu Y, Li Y, Wang D. Meta-analysis on the efficacy and safety of traditional Chinese medicine in preventing post-ERCP pancreatitis. J Hexi Univ. 2021;37:20-28.
14. Ergin E, Oruç N, Ersöz G, Tekeşin O, Özütemiz Ö. Prognosis and risk factors of ERCP pancreatitis in elderly. Sci Rep. 2021;11:15930.
15. Pekgöz M. Post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review for prevention and treatment. World J Gastroenterol. 2019;25:4019-42.
16. Wen K, Cen C, Wu L, et al. Relationship between pancreatic parenchyma loss and early postoperative hyperglycemia in patients with benign pancreatic diseases. Abdom Radiol. 2021;46:4210-7.
17. García-Cano J, Viñuelas Chicano M. Should we administer rectal anti-inflammatory drugs in all ERCPs in order to prevent pancreatitis? Rev Esp Enferm Dig. 2020;112:167-9.
18. Akashi R, Kiyozumi T, Tanaka T, Sakurai K, Oda Y, Sagara K. Mechanism of pancreatitis caused by ERCP. Gastrointest Endosc. 2002;55:50-4.
19. Cui PJ, Yao J, Zhao YJ, Han HZ, Yang J. Biliary stenting with or without sphincterotomy for malignant biliary obstruction: a meta-analysis. World J Gastroenterol. 2014;20:14033-9.
20. Zhang D, Man X, Li L, Tang J, Liu F. Radiocontrast agent and intraductal pressure promote the progression of post-ERCP pancreatitis by regulating inflammatory response, cellular apoptosis, and tight junction integrity. Pancreatology. 2022;22:74-82.
21. Shin SH, So H, Cho S, et al. The number of wire placement in the pancreatic duct and metal biliary stent as risk factors for post-endoscopic retrograde cholangiopancreatography pancreatitis. J Gastroenterol Hepatol. 2020;35:1201-7.
22. Wen L, Javed TA, Yimlamai D, Mukherjee A, Xiao X, Husain SZ. Transient high pressure in pancreatic ducts promotes inflammation and alters tight junctions via calcineurin signaling in mice. Gastroenterology. 2018;155:1250-63.e5.
23. Chi JY, Ma LY, Zou JC, Ma YF. Risk factors of pancreatitis after endoscopic retrograde cholangiopancreatography in patients with biliary tract diseases. BMC Surg. 2023;23:62.
24. Concepción-Martín M, Gómez-Oliva C, Juanes A, et al. Somatostatin for prevention of post-ERCP pancreatitis: a randomized, double-blind trial. Endoscopy. 2014;46:851-6.
25. Zhu P. Analysis of risk factors and construction of predictive model for post-endoscopic retrograde cholangiopancreatography pancreatitis. Pak J Med Sci. 2023;39:1642-6.
26. Mohammad Alizadeh AH, Afzali ES, Zafar Doagoo S, et al. Preventive role of wire-guided cannulation to reduce hyperamylasemia and pancreatitis following endoscopic retrograde cholangiopancreatography. Diagn Ther Endosc. 2012;2012:821376.
27. Jamry A. Risk factors of pancreatitis after endoscopic sphincterotomy. Review of literature and practical remarks based on approximately 10,000 ERCPs. Pol Przegl Chir. 2017;89:29-33.
28. Sánchez-Ocaña R, Foruny Olcina JR, Vila Costas J, et al. SEED consensus document on SpyGlass-DS. Gastroenterol Hepatol. 2023;46:69-79.
29. Kayashima A, Horibe M, Iwasaki E, et al. Non-interventional management of asymptomatic diminutive choledocholithiasis versus endoscopic extraction in consecutive patients. Dig Dis Sci. 2023;68:4456-65.
30. Facundo HG, Montoliu RR, Llanos DRC, et al. Cholecystectomy 7 days vs 4 weeks after mild biliary pancreatitis; looking a decrease the incidence of persistent choledocholithiasis and ERCP: a multicentric randomized clinical trial. Int J Surg. 2022;98:106207.
31. Huang RJ, Barakat MT, Girotra M, Banerjee S. Practice patterns for cholecystectomy after endoscopic retrograde cholangiopancreatography for patients with choledocholithiasis. Gastroenterology. 2017;153:762-71.e2.
32. Lin Y, Liu X, Cao DQ, et al. Analysis of risk factors and prevention strategies of post-ERCP pancreatitis. Eur Rev Med Pharmacol Sci. 2017;21:5185-90.
33. Chandrasekhara V, Khashab MA, Muthusamy VR, et al; ASGE Standards of Practice Committee. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47.
34. Laugier R, Bernard JP, Berthezene P, Dupuy P. Changes in pancreatic exocrine secretion with age: pancreatic exocrine secretion does decrease in the elderly. Digestion. 1991;50:202-11.
35. Cankurtaran RE, Ersoy O. Adverse events in endoscopic retrograde cholangiopancreticography for choledocholithiasis: a holistic perspective. Cureus. 2024;16:e53375.
36. Tintara S, Buxbaum J. Updates in post-endoscopic retrograde cholangiopancreatography pancreatitis. Gastroenterol Clin North Am. 2025;54:97-112.
37. Hayashi S, Nishida T, Shimakoshi H, et al. Combination of two-hour post-endoscopic retrograde cholangiopancreatography amylase levels and cannulation times is useful for predicting post-endoscopic retrograde cholangiopancreatography pancreatitis. World J Gastrointest Endosc. 2016;8:777-84.
38. Liu WH, Huang XY, Hu X, et al. Initial experience of visualized biliary cannulation during ERCP. Endoscopy. 2023;55:1037-42.
39. Fei L. Endoscopic management of bile leakage after laparoscopic operation of gallbladder and biliary tract. Lingnan Modern Clinical Surgery. 2015;15:276-9. (In Chinese).
40. Berry R, Han JY, Tabibian JH. Difficult biliary cannulation: historical perspective, practical updates, and guide for the endoscopist. World J Gastrointest Endosc. 2019;11:5-21.
41. Chen PH, Tung CF, Peng YC, Yeh HZ, Chang CS, Chen CC. Duodenal major papilla morphology can affect biliary cannulation and complications during ERCP, an observational study. BMC Gastroenterol. 2020;20:310.
42. Xu XD, Dai JJ, Qian JQ, Wang WJ. Prevention of pancreatitis after papillary balloon dilatation by nasobiliary drainage: a randomized controlled trial. Dig Dis Sci. 2015;60:1087-91.
43. Chan T, Yaghoubian A, Rosing D, et al. Total bilirubin is a useful predictor of persisting common bile duct stone in gallstone pancreatitis. Am Surg. 2008;74:977-80.
44. Sato D, Shibahara T, Miyazaki K, et al. Efficacy of endoscopic nasobiliary drainage for the prevention of pancreatitis after papillary balloon dilatation: a pilot study. Pancreas. 2005;31:93-7.
45. Xu XD, Dai JJ, Qian JQ, Wang WJ. Nasobiliary drainage after endoscopic papillary balloon dilatation may prevent postoperative pancreatitis. World J Gastroenterol. 2015;21:2443-9.
46. Freeman ML, Overby C, Qi D. Pancreatic stent insertion: consequences of failure and results of a modified technique to maximize success. Gastrointest Endosc. 2004;59:8-14.
47. Freeman ML. Pancreatic stents for prevention of post-ERCP pancreatitis: the evidence is irrefutable. J Gastroenterol. 2014;49:369-70.
48. Kodydkova J, Vavrova L, Stankova B, Macasek J, Krechler T, Zak A. Antioxidant status and oxidative stress markers in pancreatic cancer and chronic pancreatitis. Pancreas. 2013;42:614-21.
49. Rosenbaum DM, Kalberg J, Kessler JA. Superoxide dismutase ameliorates neuronal death from hypoxia in culture. Stroke. 1994;25:857-62.
50. Dabrowski A, Gabryelewicz A. Oxidative stress. An early phenomenon characteristic of acute experimental pancreatitis. Int J Pancreatol. 1992;12:193-9.
51. Pérez S, Pereda J, Sabater L, Sastre J. Redox signaling in acute pancreatitis. Redox Biol. 2015;5:1-14.
52. Wisløoff F, Jakobsen J, Osnes M. Stenosis of the common bile duct in chronic pancreatitis. Br J Surg. 1982;69:52-4.
53. Liu Y, Zhang H, Zhang B. Analysis of risk factors for acute pancreatitis in elderly patients after ERCP surgery. Available from https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCUQwMjM0MzQxMhoIdWNsNjgyMjU%3D [accessed 4 Auguest 2025].
54. Jayaraj M, Mohan BP, Dhindsa BS, et al. Periampullary diverticula and ERCP outcomes: a systematic review and meta-analysis. Dig Dis Sci. 2019;64:1364-76.
55. Shi HX, Ye YQ, Zhao HW, et al. A new classification of periampullary diverticulum: cannulation of papilla on the inner margins of the diverticulum (Type IIa) is more challenging. BMC Gastroenterol. 2023;23:252.
56. Liu Y. Analysis of the causes and prognosis of severe acute pancreatitis complicated after ERCP procedure. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ueW-MCsl3UZNUUM2X_s-ZT3ITqPUaFMigyNnBhpuQ_eGusUZt84x3lgoUy3caIOkD6Q3o3eva7AD-UXhhr5DdPDOadMYlOvi1xJhz3qFSkr4rbkW0h1SnJ5LrxS0h5cUS-FnKXjhOFjJsH52N5acFVeb1uDfipUSLeMEUBS0IcCi-VRIx7x0cXv073Kswzo_eg=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
57. Tyagi P, Sharma P, Sharma BC, Puri AS. Periampullary diverticula and technical success of endoscopic retrograde cholangiopancreatography. Surg Endosc. 2009;23:1342-5.
58. Seetharam P, Rodrigues G. Sphincter of Oddi and its dysfunction. Saudi J Gastroenterol. 2008;14:1-6.
59. Ren LK, Cai ZY, Ran X, et al. Evaluating the efficacy of endoscopic sphincterotomy on biliary-type sphincter of Oddi dysfunction: a retrospective clinical trial. World J Clin Cases. 2021;9:9835-46.
60. Chen JW, Thomas A, Woods CM, Schloithe AC, Toouli J, Saccone GT. Sphincter of Oddi dysfunction produces acute pancreatitis in the possum. Gut. 2000;47:539-45.
61. Liu Z, He Y, Gong J. Research progress on the relationship between sphincter of Oddi dysfunction and acute pancreatitis. Chongqing Medicine 2010;39:613-5. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ue8bcNCwSDmi9SNVC_sdZBI1IllT-vPREKym8qMQ7nY3GvJgScLR534FxeFsGkhVeVLBOj-wIyg4zCWGvyUDXtiZPbjyojzuUeDmyPn4Ep2rMxy9sykEr4warIJR5RoR2MaJxy7OjV_kLXC8CQvONavFstuyd-tVRhEAuPMHa-60HAD9sfAfDNq&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
62. Dong L, Liu X, Zhao W, Dang X, He J. Analysis of risk factors for pancreatitis after placement of pancreatic duct stent by endoscopic retrograde cholangiopancreatography. Med J Chin PLA. 2019;62:545-7. (in Chinese). Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8uf53u8niufS6F5Uw9dWjPz4mXdiD7g3yPtov9pwcAMjvvIhU-FSetqQpoCY_Rvts57mYGMcegjNTiWAvHQYJUPI4pPyrEFDKCmh0CMKWAr6q_pDPxtqG4BgCMBbwoPwelPfdC7VIKUB2bYmehpvgxEU2a_8IxlzcAxWCtzrJsWtW8k1xU6JfePJjQ-vbQJYnhE=&uniplatform=NZKPT&language=CHS. [Last accessed on 8 Aug 2025].
64. García Gavilán MD, Moreno García AM, Rosales Zabal JM, Navarro Jarabo JM, Sánchez Cantos A. Case of drug-induced acute pancreatitis produced by horsetail infusions. Rev Esp Enferm Dig. 2017;109:301-4.
65. Fujisawa T, Kagawa K, Hisatomi K, Kubota K, Nakajima A, Matsuhashi N. Is endoscopic papillary balloon dilatation really a risk factor for post-ERCP pancreatitis? World J Gastroenterol. 2016;22:5909-16.
66. Park JM, Lee S, Chung MK, et al. Antioxidative phytoceuticals to ameliorate pancreatitis in animal models: an answer from nature. World J Gastroenterol. 2014;20:16570-81.
67. Fujinami H, Kajiura S, Ando T, Mihara H, Hosokawa A, Sugiyama T. Direct spraying of shakuyakukanzoto onto the duodenal papilla: a novel method for preventing pancreatitis following endoscopic retrograde cholangiopancreatography. Digestion. 2015;91:42-5.
68. Cui Y, Li J, Cai M, Zhang Y, Zhao B, Liu J. Gegen Qinlian decoction prevents post-ERCP pancreatitis by regulating NLRP3 inflammasome-mediated pyroptosis. Front Pharmacol. 2025;16:1588585.
69. Beran A, Aboursheid T, Ali AH, et al. Predictors of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis: A Comprehensive Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2024:Epub ahead of print.
70. Pereira Funari M, Ottoboni Brunaldi V, Mendonça Proença I, et al. Pure cut or endocut for biliary sphincterotomy? Am J Gastroenterol. 2023;118:1871-9.
71. Bytyçi I, Penson PE, Mikhailidis DP, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43:3213-23.
72. Bishay K, Meng ZW, Khan R, et al. Adverse events associated with endoscopic retrograde cholangiopancreatography: systematic review and meta-analysis. Gastroenterology. 2025;168:568-86.
73. Wang G, Sun Q, Zhu H, et al. Development and external validation of a model for post-endoscopic retrograde cholangiopancreatography pancreatitis. iScience. 2025;28:112570.
74. Brenner T, Kuo A, Sperna Weiland CJ, et al. Development and validation of a machine learning-based, point-of-care risk calculator for post-ERCP pancreatitis and prophylaxis selection. Gastrointest Endosc. 2025;101:129-38.e0.
75. Saito H, Sakaguchi M, Kadono Y, et al. Disease-based risk stratification of post-endoscopic retrograde cholangiopancreatography pancreatitis for common bile duct stones. Dig Dis Sci. 2022;67:305-14.
76. George AA, Tan JL, Kovoor JG, et al. Artificial intelligence in capsule endoscopy: development status and future expectations. Mini-invasive Surg. 2024;8:4.
77. Yang Y. Analysis of risk factors associated with post-ERCP pancreatitis and hyperamylasemia. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ueG-Jp5VvQ0oMZCZdGwUyqUC8ZLlDLg76sIV8QUT9BwjqyhWR2k3mmo4xlkiVO9GIvzFQAjBTBlD_ir0QoODsB9rGKjRy1-FzyzFyRYTnrsZn52P2ZST19mD2xqXsK4Va6KLcfLoKgf_LfutQM4YHQKu9fyEowe9JGE7bLIpFd_Vof5zW4s9UOTbg0Gz_dp07Q=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
78. Liu Y. Analysis and prevention of related factors of post-ERCP pancreatitis. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ueG-Jp5VvQ0oMZCZdGwUyqUC8ZLlDLg76sIV8QUT9BwjqyhWR2k3mmo4xlkiVO9GIvzFQAjBTBlD_ir0QoODsB9rGKjRy1-FzyzFyRYTnrsZn52P2ZST19mD2xqXsK4Va6KLcfLoKgf_LfutQM4YHQKu9fyEowe9JGE7bLIpFd_Vof5zW4s9UOTbg0Gz_dp07Q=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
79. Wan X. Analysis of risk factors for post-ERCP pancreatitis and evaluation of prediction effects. Chin J Integr Med 2018;26:677-80. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ucq6td3A4cPyVrgLGsXRN9bLcYxBQAkKW9_hOeDhWyrvsjLoZrnJGMiEIsbhIUzHPRZpYZxba4Ms0dY7kq0U-QYYZyluGDMYB28sXp97XYTAOhNTOIvTzXJvR0wF28pDKBOm22w0m0uncsW6GZzPYYHiYnN_ME9knVqEfPjQspdWc1dco-QewKyggwK0_Jdph0=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
80. Zhang Y. Analysis of risk factors for post-ERCP pancreatitis and its prevention. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ucbVo2J5599J4RXUI5HDIiRc_VE1q3pLt8A0qHnBJj2BMMLhj7o-KuNWjlEa7T0dK6w0FZvc3RECUQiq3rlz-cVco7UE_GsunVVdy8E-rk9bj9r-bOAtSpoKq5hOZDo6Zr8efAnUcqB0PiJE-iMTDM29_A9w3DjnOClxZvtOFGRi5Mx-RZLWeBYMXsKhKyF4Uo=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
82. Deng C. Analysis of surgical operation-related factors for post-ERCP pancreatitis. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ud4JEV6430Nhk9PtN8PW0iEqhb5JWppdgaGAKGi674RibYfqZMmyYlrRttCNqAxKiRPWNPgGW68o4R43OUbGryEZ0LYKcfrhptS2HVoK8afxeuNixJdQdvcJgD8OHPwzul36Dbp3em85MLAOo8V4c9xEqORsuED1IC9S363Pr7P5jkSahTzs67K8baqmJgQdyo=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
83. Zhang W. Analysis of risk factors for post-ERCP pancreatitis and hyperamylasemia and study on the effect of prophylactic administration of epinephrine. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ucnfwPKxeWM5bMFLq0LkRxdkvez1WjFIuSxHOCYz5KI_g8yBt5yy7QiefvG8ykkrzrIGdRTS4yWhpYGSxnO3ywIJkQRT0UTT8LzRt3BB8QUD3Dlr6f5K8xBKqaIwxSBYHTWu1cAzmQaNKWSCwy9KZLwXSdUOcGyCvJyt-VpgPHl4KPmlM0j-F_QqNR_FQDI2dc=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
84. Fang J. Analysis of risk factors for post-ERCP pancreatitis and establishment of prediction model. Master’s thesis, Zhejiang University, Hangzhou, Zhejiang, China. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ud2RFRmYF2eD1TlrqEjJcs99OaFmxgdg89bUKvGBOid1VJN4bwJ3u9zRo5l56TOCmgMYzYxGKLOTE-M_ieBhlA5Kq8RGKoIOs5iKXwOSqJghQuNj7b3T_6hpTDCQNG-y5lBaFnR4vpzEu1GoBvd2-gv7M8MI8LhKhLdnSp-AwofFjZQ-eiMNR5m-FDRxGiU2R4=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
85. Fan X, Huang X, Li X, Li T. Analysis of relevant factors for pancreatitis or hyperamylasemia occurring after ERCP procedure. J Hebei Med Univ 2019;25:1166-9. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ud8FpqeAUxh0uTU7YLwFzjek6M5ygUhOASMRY54e09dsRwEoOJ7I6vXCueG-DKLbmDosMQcQDKe010s_2zMwmUITnaW8U_K9b2mr8-P92-FHJ6wuSX3ytoglbDF0NTVfKwNOl_qgg87DO3xGBK7Y3It7_qgvU2YuJksB8rpgnqI0ss8aG3-PGylXBE42UIzpe4=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
86. Cheng H. Analysis of risk factors for pancreatitis complicated after ERCP procedure. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ufhyTyofDLq-t9cXlU5IUMR6XOnAYVblqeVcjaTnt6TiuR6WywLzeRMKPrp407qwVBtVSMbrWHRqRQgyOJuJu8WtoLk9PT_iY716xZ4Is3-KUryuyDblDSrhWC1mdwDO-LiemLpaqZkRKuba7PVY9XUuU1gkEBzhtufEG_OCPaqzNKB_5FDcg6NRXIjo1I1A8U=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
87. Perney P, Berthier E, Pageaux GP, et al. Are drugs a risk factor of post-ERCP pancreatitis? Gastrointest Endosc. 2003;58:696-700.
88. Chen T. Analysis of factors contributing to acute pancreatitis and development of predictive model in patients with early hyperamylasemia after ERCP. (In Chinese). Available from: https://link.cnki.net/doi/10.26925/d.cnki.gbbyc.2023.000358. [Last accessed on 8 Aug 2025].
89. Funatsu E, Masuda A, Takenaka M, et al. History of post-endoscopic retrograde cholangiopancreatography pancreatitis and acute pancreatitis as risk factors for post-ERCP pancreatitis. Kobe J Med Sci. 2017;63:E1-8.
90. Zhang M. Nomogram chart predicts the risk of post-ERCP pancreatitis occurrence after the first procedure. Available from: https://d.wanfangdata.com.cn/thesis/CiBUaGVzaXNOZXdTMjAyNTA2MTMyMDI1MDYxMzE2MTkxNhIJRDAyODAwNjQxGghkaG9wdWtyYg==. [Last accessed on 4 Aug 2025].
91. Zhao ZH, Hu LH, Ren HB, et al. Incidence and risk factors for post-ERCP pancreatitis in chronic pancreatitis. Gastrointest Endosc. 2017;86:519-24.e1.
92. Katsinelos P, Lazaraki G, Gkagkalis S, et al. Predictive factors for post-ERCP pancreatitis: a large-scale single expertized endoscopist study. Surg Laparosc Endosc Percutan Tech. 2014;24:512-6.
93. Chen X, Hong W, Wu X, Huang Q, Zhu Q. Logistic regression analysis of risk factors for post-ERCP pancreatitis after therapeutic ERCP. J Pract Med 2012;28:614-5. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ucCzcPY6V6Q7rZ26qBezT8TZ_9nD1XKZzs9Yw19oy16P6bRA3BQ3I_Gm0avdUlIPPSGTLHlpN5xARr0mNjimvqN-prLFtt24m0DTM8B3NJ8m6kwPANf7096ThnLPWTwr8-Zb2jl4EAYxOv7F8iOsogVS0x0dKQqrmxF3vQKnfWFjqReT3uEs1HE&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
94. Guo X. Analysis of the influencing factors of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Med Res Home Abroad. 2022;20:128-31.
95. Liu Y, Xu J, Chen D, He X. Analysis of relevant factors and countermeasures for pancreatitis occurring after endoscopic retrograde cholangiopancreatography. Chin J Dig Endosc 2021;27:71-6. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ufXeWqk0EiPqRGxShcbLirEFgUEI7MAqmhqZdT27rcBNPLBwAlxv1MkQW59Neo-_3XwNXlGni8rZHHcQlUZgL74L8FOTV1hhbu8JLlyV9kcgm9vSygS9OYt_JTc322GQmUeYzWKu1tIGhWXPPH-awIG_ZViUWLReilJ4tWw0ujgB7YqUfX6uP-Fg-iM6D5Vyss=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
96. Wang C. Analysis of risk factors for pancreatitis induced by endoscopic retrograde cholangiopancreatography. Clin Res 2019;27:5-7. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ucEfCAlRqkrTTd-eIgybWnjCNFU7oUzZTMyPUR4SpTiQxAoqXXqfvJa-ItE4tiNpVbGoW7syJY3QzWFBzKmqDja2DGdrrqNbu052RVNmykrFNsth9QWGpRKv4_b37pVswdnvcKcq9BstsSovE-SAfC0HkJVNedAxaazzux5m1c_uCgpUq5yB4__SdU9px8OdDA=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
97. Yao J, Chai B, Cao X, Xu M, Zhu Q. Risk factors and construction of predictive model for post-endoscopic retrograde cholangiopancreatography pancreatitis. Nurs Res 2023;37:814-8. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ufOPkq-ln8N2FyWRZKyGNQevPdqyIIdYpa72FxFZeKhqsE2u-rttpO2b2NQaojtC3-LLvAIz4yxZK17ZyM4ljvNPmZ1E0zP2RNAtinlYK_1w3EgAYbpSAN2eG2R0Q0Vlmut1Nkgg2DdeEn8b3zanVzx5xMPBg7FsfnF3IVyBo4DEtPFvySNRRNoiPlbm94eT2Q=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
98. Zhao Y. Analysis of risk factors and establishment of risk scoring system for complications after ERCP in patients with common bile duct stones. (In Chinese).
99. Wang Y. Analysis of related factors for pancreatitis occurring after retrograde cholangiopancreatography for common bile duct stones. Smart Health 2021;7:93-5. Available from: https://link.cnki.net/doi/10.19335/j.cnki.2096-1219.2021.31.030. [Last accessed on 8 Aug 2025].
100. Li Z. Analysis of the incidence and related risk factors of postoperative complications after ERCP in high-altitude areas.
101. Song X. Risk factors for acute pancreatitis after ERCP. Henan Med Res 2020;29:2771-3. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ufFKFhZpTLpeGLT6HTxXRQeghvM-Yo_iGw7lfr3duty258Z8cytpdHQae_h8zhXrwsjzMFj1mq79vLY-b0dR1nv9t9ahoH5QWiuOj1uBl3vbiNYGha-gtuw55njgdzlL4f6s2SMps4U_XRcyv1Wn7T34inqXVxQwmPUgDKqHfMQW7S-XIKHAvthF8Nx9l5oqkw=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
102. Xu B. Analysis of related factors of post-ERCP pancreatitis after therapeutic ERCP for obstructive jaundice. Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8ucEz3WgnELOSEY-LspVuz1TclqRAO2cBcKxRpNn3Bg2Sn7bLvZ9agC060AJvyXW6PH3CKUpetDtMF4xjYsgf70alBSp6f79A32SoOKnUHhiJwrwbPEO6YewLWU_riy-DK3fe8NwKH6VkNHFXejEl1jGAP5y4r89gcYldge0XLxCn06lAFIyj1Xexw83FAiPrNs=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
103. Yang S. The influence of intubation time on postoperative pancreatitis after endoscopic retrograde cholangiopancreatography. Available from: https://d.wanfangdata.com.cn/thesis/CiBUaGVzaXNOZXdTMjAyNTA2MTMyMDI1MDYxMzE2MTkxNhIJRDAxMjc0MDA0Ggh6dGV2OWNiZQ%3D%3D. [Last accessed on 4 Aug 2025].
104. Li Y, Li K, Liu A, et al. The relationship between uneven dilation of the bile duct and post-ERCP pancreatitis. J Kunming Med Univ 2023;44:131-7. (in Chinese) Available from: https://kns.cnki.net/kcms2/article/abstract?v=oWJgMrFo8uegmACI_plE1AdnGC7YDQXtQC0bOfeZOaVhmYNp7YDPWPLBiW9YH71o2Ub3_Vb66Dw49Y7tN1PJ3dv_5TvSylAJtNQeRKqoYLYWh9G_Rl4srtx0gf9QpZg7dK9yUYOEA1JmXyJ5Sc_FRMU_vzAmNcCj0AXPZcR-Ak8JT_jyz3hdnFvB8jfqV_h6swVlgqhhJNY=&uniplatform=NZKPT&language=CHS. [Last accessed on 4 Aug 2025].
105. Zhang X, Tan N, Zhou Y, Yu Y, Ji G. Analysis of related factors for pancreatitis occurring postoperatively after retrograde cholangiopancreatography for common bile duct and main trunk stones. Contemp Chin Med. 2017;24:30-2. (In Chinese). Available from: https://kns.cnki.net/kcms2/article/abstract?v=FqAfUZ3F7badEGLN5tjbmH73C5-S7vo2Cf3qg9GaEks8wiYUM8PKqVOdNWQPbhGKoLmozUXeTGSkE0Sl9P16Ip1iwrioLc9jKUzkclVD4m4vFkygkjs3rbGWRbKHT9KSGXi0_VdFtrC0rpKdz4v1J-hTiIyKRSOjNVVG_xoThkgZm8BeSvNIdg==&uniplatform=NZKPT&language=CHS. [Last accessed on 8 Aug 2025].
106. Zhang X, Zhang J, Pei Z, et al. Study on the impact of gallbladder condition on post-ERCP pancreatitis and the development of prediction model. J Lanzhou Univ. 2022;48:44-9. (In Chinese) Available from: https://link.cnki.net/doi/10.13885/j.issn.1000-2812.2022.01.010. [Last accessed on 8 Aug 2025].
107. Zhu J, Huang Z, Wang G. Analysis of factors influencing the efficacy of endoscopic retrograde cholangiopancreatography in difficult cases and postoperative Ac pancreatitis with different assisting catheterizations. J Hebei Med Univ. 2022;28:1135-41. (In Chinese). Available from: https://kns.cnki.net/kcms2/article/abstract?v=FqAfUZ3F7bahRKyS80qC-CmU1aXgJAJy_SRssIfvyQvb2az0jCUtCgS1sMdMT3772syAWH0bj4iQLasmlznwCwsnlwyF34JP08YQYYUXm6HD0VwMoZOBOfKQbaeVID52hKy8D2b0-Uyn2lqt7COWrWYcVhK5YpTr7y6h8xM5Y4FrYFfhoL_SbA==&uniplatform=NZKPT&language=CHS. [Last accessed on 8 Aug 2025].
108. Yang Y. The influence of intubation time on postoperative pancreatitis in patients undergoing difficult ERCP intubation procedures. (In Chinese).
109. Ma M, Zhou Z. Comparative observation on acute pancreatitis and hyperamylasemia after endoscopic retrograde cholangiopancreatography. J Clin Hepatobiliary Dis. 2020;36:395-8. (In Chinese). Available from: https://kns.cnki.net/kcms2/article/abstract?v=FqAfUZ3F7ba7I7gmPMYnnT_r0RSrJMkxO8ApoFViP6TnvGlimcQbsMiICM1i9lYyMZYmM6s10e9Oo84w4zwnB_HjeyvOmm4-bZXwe3syT9SfNbtTZTWfJu7Llv5Tn-aq_t6MlXmrLJuoStzQ2OmY3t9ybUT4ayHHwPJ_GB640qr4ZwFaGbqEGw==&uniplatform=NZKPT&language=CHS. [Last accessed on 8 Aug 2025].
110. Wang M, Zhu H, Wu C, Li M, Shi H. Study on changes of inflammatory factors and immune function in elderly patients with common bile duct stones before and after ERCP treatment and risk factors for postoperative pancreatitis complications. Adv Mod Biomed. 2022;22:2265-93.
111. Wang X. Analysis of risk factors related to post-ERCP pancreatitis. Available from: https://d.wanfangdata.com.cn/thesis/CiBUaGVzaXNOZXdTMjAyNTA2MTMyMDI1MDYxMzE2MTkxNhIJRDAyNTk2MzYxGghxbWQ5bWZmNw%3D%3D. [Last accessed on 4 Aug 2025].
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].