Robotic Heller myotomy and fundoplication - techniques and outcomes
Abstract
Achalasia, a primary esophageal motility disorder characterized by impaired peristalsis and the inability of the lower esophageal sphincter to relax, affects approximately 0.5-1.2 individuals per 100,000 annually. Traditional treatments have included endoscopic interventions and Heller myotomy with partial fundoplication, long regarded as the gold-standard surgical option. The advent of minimally invasive techniques, particularly robotic Heller myotomy performed since 2001, has introduced significant advancements in the treatment of achalasia. This review examines the evolution of Heller myotomy, focusing on the robotic approach, which offers distinct advantages such as enhanced surgical precision, a lower risk of mucosal perforation, and shorter hospital stays. By comparing robotic-assisted Heller myotomy (RAHM) with laparoscopic and open approaches, this review underscores the effectiveness of the robotic method in improving operative outcomes and offering a safer, more efficient treatment for patients with achalasia. Through an overview of diagnostic strategies, surgical techniques, and postoperative management, this review underscores the growing role of RAHM as a pivotal shift toward optimizing care for patients with this complex esophageal disorder.
Keywords
INTRODUCTION
Achalasia is a disorder of the esophageal smooth muscle characterized by impaired peristalsis and the lower esophageal sphincter (LES)’s inability to relax[1]. It is the most prevalent primary esophageal motility disorder, with an annual incidence of approximately 0.5-1.6 cases per 100,000 individuals[2,3]. The predominant symptoms include progressive dysphagia, regurgitation, and chest pain or pressure[4,5]. Additional manifestations may include heartburn, weight loss, and respiratory complications such as cough and aspiration pneumonia[4,5]. While its precise etiology remains unclear, achalasia is believed to arise from viral infection or environmental triggers that incite inflammation in the esophageal myenteric plexus. This inflammatory process may initiate an autoimmune reaction, resulting in chronic inflammation and the progressive degeneration of ganglion cells within the plexus[6,7]. Recent evidence suggests a potential link between SARS-CoV-2 infection and the onset of achalasia, with case series reporting new diagnoses following COVID-19 infection. These observations support the hypothesis that viral triggers may contribute to disease pathogenesis through immune-mediated injury to the myenteric plexus[8,9].
The primary goal of achalasia therapy is to decrease LES resting pressure. Endoscopic interventions such as pneumatic dilation and botulinum toxin injection are commonly employed[10,11], but their long-term efficacy is limited, often requiring repeated procedures and sometimes leading to complications such as submucosal fibrosis[12]. For decades, the gold-standard surgical intervention has been Heller myotomy with partial fundoplication, which achieves 70%-90% symptom improvement over 6-10 years[5,13]. Originally described in 1913 by Dr. Ernst Heller, the procedure has evolved significantly, particularly with the introduction of the minimally invasive laparoscopic method in the 1990s[14]. Since the first robotic Heller myotomy was performed in 2001, the robotic approach has gained increasing adoption[15]. Robotic surgery offers several advantages for foregut procedures, including three-dimensional visualization, articulating instruments, high-definition magnification, and enhanced stability through EndoWrist technology[16-18]. These features are particularly valuable in Heller myotomy, as they improve the precision of muscle fiber dissection and submucosal visualization, thereby reducing the risk of perforation[19,20].
With broader training opportunities and greater availability of surgical robots, the use of robotic approaches, including robotic Heller myotomy, is steadily increasing[16]. This review discusses the techniques of robotic Heller myotomy with partial fundoplication and summarizes reported outcomes, comparing them with laparoscopic and open approaches.
DIAGNOSIS AND WORK-UP
The diagnosis of achalasia begins with a thorough history and physical examination. The hallmark symptom is dysphagia, which typically starts with difficulty swallowing solid foods, progresses to liquids, and eventually leads to regurgitation of ingested material[11,21,22]. Symptoms progress slowly, with onset often occurring several years before clinical presentation[11,23]. Patients may report needing to eat more slowly than family or friends, walking or stretching side-to-side after meals to facilitate the passage of food into the stomach, and thereby relieve dysphagia[21,22]. Additional symptoms may include weight loss, aspiration, cough, and pneumonia. In advanced disease, stasis of food and gastric acid may cause heartburn, sometimes leading to a delayed diagnosis during proton pump inhibitor trials.
The differential diagnosis for such symptoms includes achalasia, pseudoachalasia, gastroesophageal reflux disease (GERD), other esophageal motility disorders, and esophageal malignancy. Excluding other causes and confirming achalasia typically requires a combination of endoscopy, barium esophagram, and manometry[5]. Upper endoscopy is often considered the highest-yield initial test, as it can easily detect malignancy, ulcers, strictures, sequelae of pathologic reflux, and candidiasis. Endoscopic features of achalasia may include a dilated, tortuous esophagus containing retained food, narrowing at the LES that is difficult to traverse, and absence of peristalsis in many cases[5,24]. However, these findings are not remarkably sensitive or specific, as they typically appear only in advanced disease[5,22]. Barium esophagram is also useful in the work-up of dysphagia. In achalasia, it characteristically demonstrates an aperistaltic esophageal body with distal narrowing that produces a “bird’s beak” appearance[22]. In advanced stages, the esophagus may assume a sigmoid configuration[25]. A timed barium esophagram, in which the height of the barium column above the gastroesophageal junction (GEJ) is measured at one-, two-, and five-minute intervals post-ingestion of iodinated contrast, can quantify impaired esophageal emptying[5]. However, a normal esophagram does not exclude achalasia, since features may be absent in early disease when some myenteric ganglia remain functional, allowing for residual peristalsis[21,22].
Pseudoachalasia refers to distal esophageal narrowing caused by conditions other than primary denervation. The most common etiology is gastroesophageal carcinoma, though benign conditions such as amyloidosis, sarcoidosis, and pancreatic pseudocysts have also been described[26]. Pseudoachalasia should be suspected in older patients with rapidly progressive symptoms[22,27]. In addition to endoscopy for intraluminal disease, computed tomography of the chest and abdomen may help identify extraluminal compression[11,27].
Ultimately, objective assessment of esophageal motor function is necessary to establish a diagnosis of achalasia[21]. Manometry remains the gold standard for diagnosing achalasia and other esophageal dysmotility disorders[21,22,27]. Recently, the Chicago Achalasia Risk Score (CARS) has been introduced as a predictive model to improve diagnostic confidence in suspected cases[28]. This scoring system integrates clinical and radiologic data to estimate the likelihood of achalasia, which can be particularly useful in settings where high-resolution manometry is not readily available. Classic manometric findings include failure of LES relaxation during swallowing and absence of esophageal peristalsis. High-resolution manometry further classifies achalasia into three subtypes, which strongly predict treatment response. According to the Chicago Classification, Type I achalasia is characterized by complete aperistalsis with impaired LES relaxation (“classic achalasia”); Type II is marked by panesophageal pressurization (“achalasia with esophageal compression”); and Type III is defined by premature distal esophageal spasms (“spastic achalasia”)[5,21].
INDICATIONS FOR SURGICAL TREATMENT
The natural course of achalasia is progressive and can ultimately lead to the development of megaesophagus, which may necessitate esophagectomy. Therefore, early intervention is indicated for all diagnosed patients. Currently, no available treatment can restore the function of the esophageal myenteric plexus, and thus, none are considered curative. The primary objective of current therapies is to facilitate the passage of food boluses across the LES[22,29]. Various treatment options exist, and selection should be based on both the patient’s operative risk and their personal preferences.
Pharmacologic therapies provide a non-operative option but have limited effectiveness. These include smooth muscle relaxants and botulinum toxin injections[30]. Smooth muscle relaxants, such as nitrates, calcium channel blockers, anticholinergics, and phosphodiesterase inhibitors, result in a short-term
Compared with pharmacologic treatments, pneumatic dilation yields superior outcomes, achieving clinical success in 74%-90% of patients at 1-2 years and providing long-term symptom relief in 50%-85% of cases[5]. Twelve-month remission rates are approximately 73% for pneumatic dilation compared with around 38% for botulinum toxin injections[5]. However, complications are more significant with pneumatic dilation than with pharmacologic therapies. Major bleeding occurs in about 2% of cases, but the most serious complication is esophageal perforation, with an incidence of 1.9%-2.8% even in experienced hands[5].
First introduced in 2008, peroral endoscopic myotomy (POEM) has emerged as an endoscopic alternative to surgical myotomy[31]. POEM involves sharp endoscopic dissection of the submucosal plane proximal to the GEJ. Although initially described as a selective myotomy of the inner circular muscle fibers[32], POEM is now most commonly performed as a full-thickness myotomy, especially at the GEJ, to improve LES disruption and symptom relief[33]. The proximal mucosal incision is then closed with clips or sutures[34]. Patients with type III achalasia may benefit the most from POEM, as the antegrade nature of the procedure allows the myotomy to begin more proximally in the esophagus when necessary[11]. POEM achieves clinical success rates of 83%-92% at 1-2 years post-treatment, superior to pneumatic dilation and comparable to laparoscopic Heller myotomy (LHM)[5,35,36]. Nevertheless, complications do occur. Minor adverse events, such as transient chest pain and mucosal tears, are the most common[35,36]. The most clinically significant complication of POEM is gastroesophageal reflux, which includes both symptomatic GERD and endoscopic evidence of reflux esophagitis. Studies report that GERD symptoms and mild erosive esophagitis (LA Grade A/B) are more frequent following POEM, affecting 44%-58% of patients at 1-2 years post-treatment. In contrast, the incidence of severe esophagitis (LA Grade C/D) appears comparable between POEM and LHM with fundoplication[35,36]. Serious adverse events such as perforation, bleeding, and pneumothorax are rare (0.5%-3%) and occur less frequently with POEM than with LMH[35,36].
Historically, surgical myotomy of the LES has been the most common definitive treatment[11]. Over 90% of patients report improvement in dysphagia, chest pain, and vomiting[11,37,38]. Compared with dilation or endoscopic myotomy, surgical myotomy enables the simultaneous creation of a partial fundoplication, which, in one prospective study, reduced the rate of post-intervention gastroesophageal reflux from 48% to 9%[39]. Werner et al. reported a higher incidence of reflux esophagitis in patients who underwent POEM compared to those treated with LHM, likely attributed to the absence of an anti-reflux procedure in POEM. Interestingly, this difference was more pronounced at the 3-month follow-up but diminished by the 2-year mark, despite higher use of proton pump inhibitors among POEM patients at 2 years[36]. While both POEM and LHM are first-line treatment options, certain patient subsets may benefit more from LHM with fundoplication. For example, patients with large hiatal hernias, obesity, or established reflux disease may not be ideal candidates for POEM due to the increased risk of post-myotomy reflux. The addition of an anti-reflux procedure during LHM helps mitigate this concern.
Since the implementation of minimally invasive approaches for Heller myotomy, a growing body of research has demonstrated their safety and efficacy, leading them to largely replace the open surgical approach[38,40]. Studies have highlighted multiple benefits of the laparoscopic approach compared to the thoracoscopic approach, including shorter operative time, reduced length of hospital stay, greater improvement in dysphagia, lower incidence of postoperative reflux, and reduced rates of infection, pulmonary complications, and postoperative interventions[41]. Most recently, the introduction of robotic-assisted laparoscopic surgery has marked the latest advancement in Heller myotomy and fundoplication, offering improved visualization and dexterity, and potentially reducing the risk of iatrogenic perforation[42]. A comparative summary of achalasia treatment modalities is presented in Table 1.
Comparative summary of achalasia treatment modalities
| Treatment | Mechanism | Clinical success | Reflux risk | Major complications |
| Smooth muscle relaxants (e.g., nitrates, CCBs) | Pharmacologic LES relaxation | 0%-8% | Low | Headache, hypotension, edema |
| Botulinum toxin injection | Inhibits ACh release at LES | ~78.7% at 30 days; ~40.6% at 12 months | Moderate | Chest pain, rare allergic or mediastinal reactions |
| Pneumatic dilation | Mechanical disruption of LES via balloon | 74%-90% at 1-2 years | Moderate | Esophageal perforation (1.9%-2.8%), bleeding (~2%) |
| POEM | Endoscopic submucosal myotomy | 83%-92% | High | Reflux esophagitis; rare perforation, pneumothorax (0.5%-3%) |
| LHM + fundoplication | Surgical LES myotomy + anti-reflux wrap | > 90% | Low | Mucosal perforation (up to 15.8%) |
| RAHM + fundoplication | Same as LHM performed with a robotic platform | > 90% | Low | Lower perforation rate vs. LHM (0%-2.5%) |
SURGICAL TECHNIQUE
Patients are typically advised to follow a clear liquid diet for 24-48 h prior to surgery to minimize esophageal residue. In cases of advanced achalasia with sigmoid esophagus or significant food stasis, this duration may be selectively extended. A full liquid diet may also be recommended for 2-3 days prior to surgery to further aid clearance. Even in severe cases, preoperative admission for endoscopic disimpaction is rarely required; when necessary, it is performed to reduce aspiration risk during anesthesia induction and to facilitate bowel decompression. Antifungal treatment may also be indicated in patients with candidiasis secondary to food stasis.
During rapid sequence induction, placing the patient supine in the reverse Trendelenburg position reduces the risk of aspiration. After intubation, a gentle endoscopic assessment with minimal insufflation is crucial to exclude occult malignancies, decompress the stomach, and clear any esophageal debris. To stabilize the esophagogastromyotomy, the endoscope may be left in place, or alternatively, a 36 Fr orogastric tube can be inserted. For patient stability during hiatal dissection, particularly in reverse Trendelenburg, a footboard is recommended.
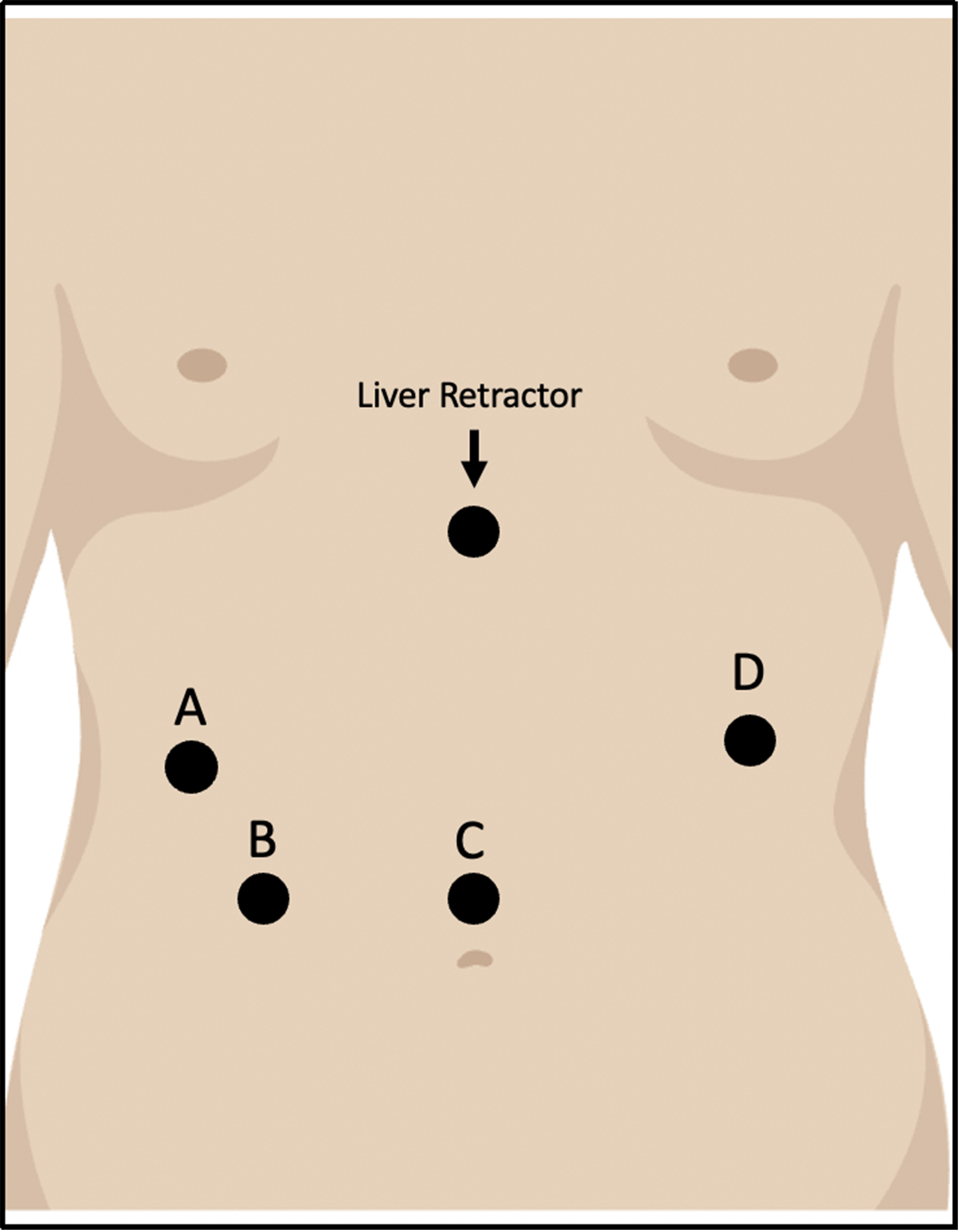
The procedure then proceeds with the placement of four or five robotic trocars. First, an 8 mm camera trocar is inserted laparoscopically in the midline, about 15 cm below the xiphoid process. The other 8 mm robotic trocars are positioned about 7 cm apart in line with the camera port. A 12 mm assistant port may be added in the right lower abdominal quadrant. Lastly, a 5 mm incision is made below the xiphoid process for the liver retractor [Figure 1].
Figure 1. Trocar positions for RAHM. A and D: Working ports; B: Auxiliary port; C: Camera port. RAHM: Robotic-assisted Heller myotomy.
Hiatal dissection begins with division of the pars flaccida of the gastrohepatic ligament, followed by mobilization of the right crus while preserving the overlying peritoneum to minimize the risk of crural closure dehiscence. Dissection may be carried out circumferentially or limited to the anterior esophageal hiatus, preserving the phrenoesophageal membrane along the posterolateral crura. Identifying and protecting the anterior and posterior vagal nerves throughout the procedure is critical. The anterior vagus can be safely mobilized from the esophageal wall to allow extension of the myotomy beneath it. The anterior esophageal wall is then dissected into the mediastinum until at least 8 cm of esophageal length is exposed.
Myotomy
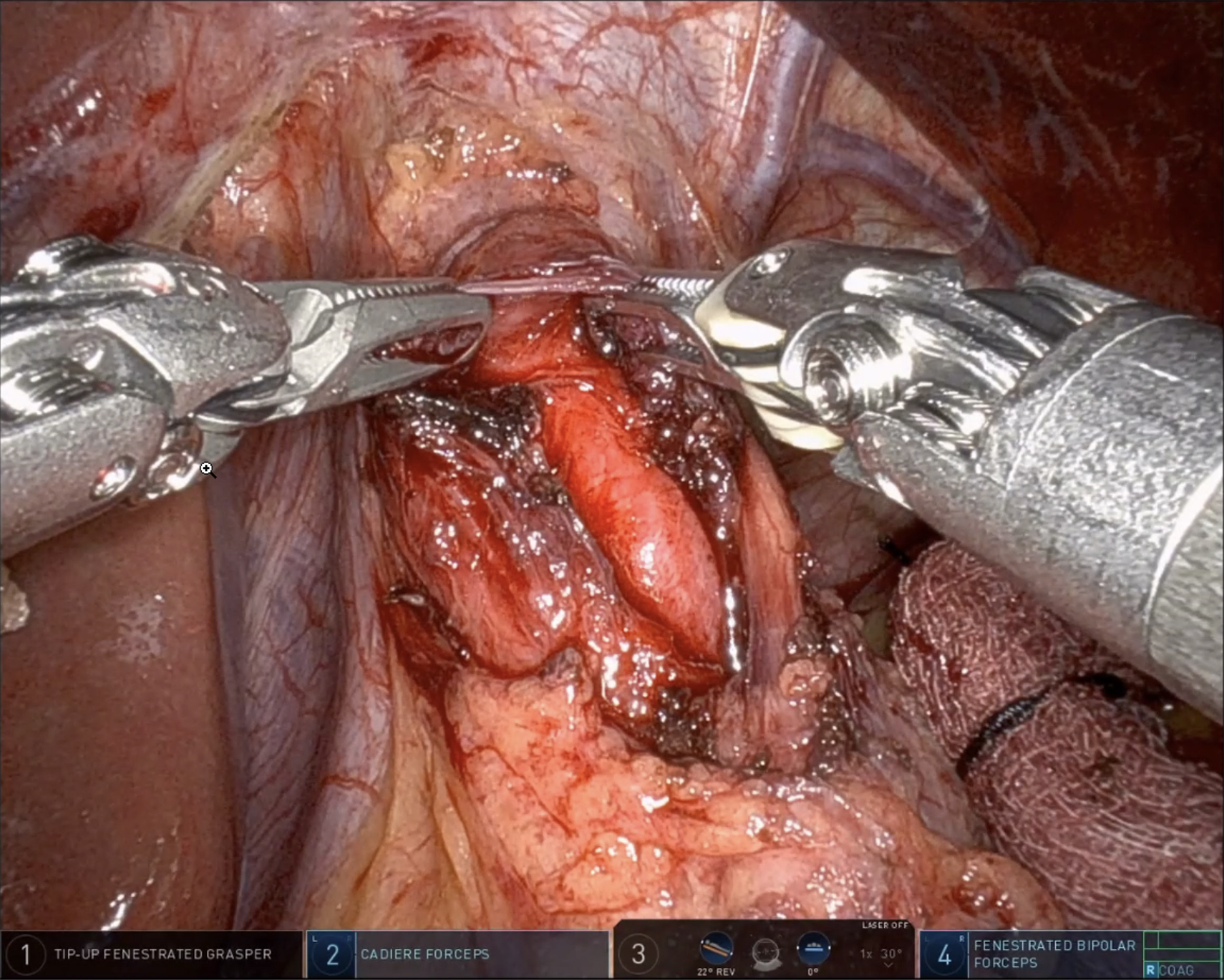
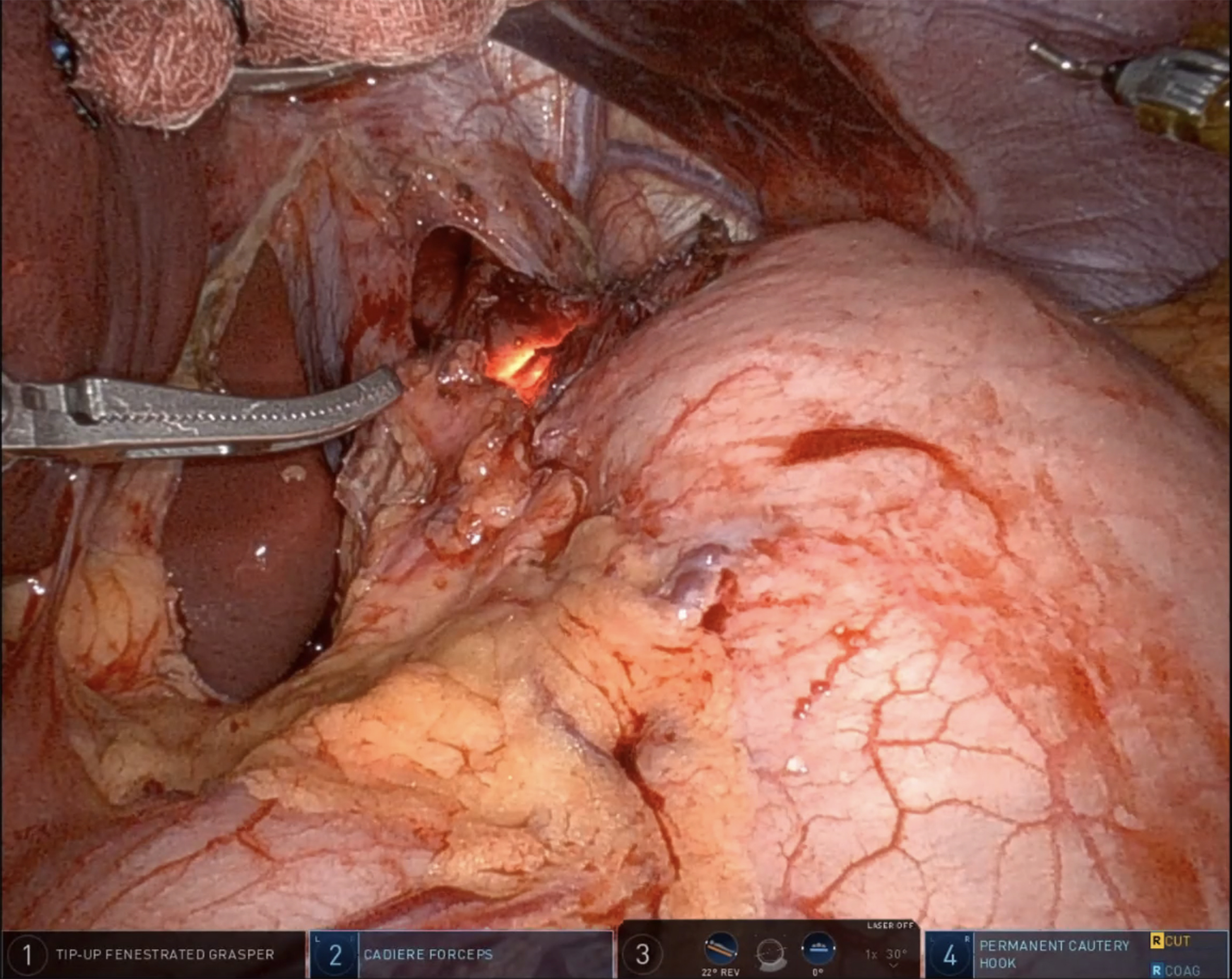
After mobilization of the fundus, removal of the gastric fat pad enhances visualization of the GEJ. Using both blunt and energy dissection, the myotomy begins between the 11 and 1 o’clock positions on the esophagus. Traditionally, the myotomy extends approximately 6 cm above and 2.5-3 cm below the GEJ. However, recent evidence from the POEM literature suggests that shorter myotomies (4-5 cm in total) may be adequate in non-spastic achalasia and are associated with a lower risk of postoperative complications, such as blown-out myotomy or increased reflux[33]. The decision to perform an extended esophageal myotomy should be individualized based on the achalasia subtype and intraoperative findings. During the myotomy, both longitudinal and circular muscle fibers are divided until the submucosa of the esophagus is exposed [Figure 2]. On the gastric side, the muscle may be thinner and more adherent to the mucosa, especially in patients with a history of botulinum toxin injections. Meticulous attention is therefore essential to avoid mucosal injury. At the conclusion of the myotomy, a leak test is performed either by endoscopic insufflation or by instilling methylene blue [Figure 3]. Any mucosal perforation should be addressed immediately, and a subsequent anterior fundoplication can be applied as a Thal patch to reinforce the repair. To evaluate the adequacy of the myotomy, some centers employ Endoluminal Functional Lumen Imaging Probe (EndoFLIP) intraoperatively[43]. This device measures GEJ distensibility before and after the procedure, confirming sufficient lower LES disruption. Experienced surgeons may also rely on direct visualization and tactile feedback to assess the completeness of the myotomy.
Figure 2. Blunt dissection of the longitudinal and circular esophageal muscle fibers to expose the submucosa. Image captured during a robotic Heller myotomy performed by the corresponding author (Shah RD).
Fundoplication
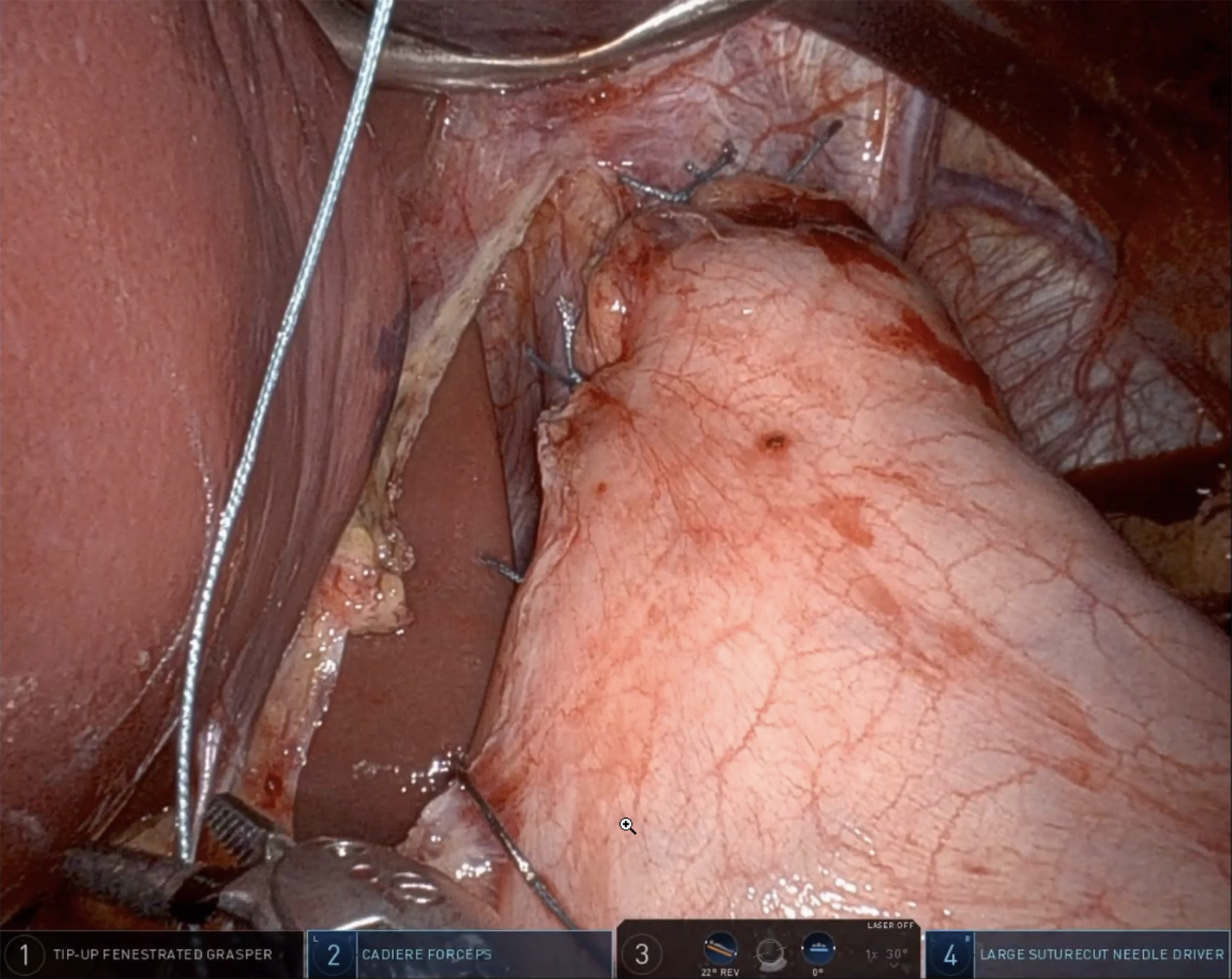
Following myotomy, a fundoplication is performed to reduce the risk of developing GERD. The choice of technique depends on several factors, including the surgeon’s preference, the patient’s clinical presentation, and findings from preoperative assessments. The short gastric vessels should be ligated with a vessel sealer to enable a tension-free fundoplication. The two most common techniques are Dor and Toupet fundoplication. Dor fundoplication involves a 180° anterior wrap of the gastric fundus over the myotomy site, which is then secured to both the crura and the edges of the myotomy [Figure 4]. In contrast, Toupet fundoplication consists of a 270° posterior wrap, in which the left and right segments of the fundus are sutured separately to the esophagus with non-absorbable sutures, leaving a 1-2 cm anterior gap between the gastric segments.
Postoperative management
After the procedure, patients are awakened, extubated, and transferred either to the ward or the ICU. Use of a nasogastric tube is strongly discouraged in the postoperative period to avoid inadvertent disruption of the esophageal mucosa. Postoperative nausea and vomiting can be minimized with an enhanced recovery after surgery (ERAS) protocol that incorporates preoperative, intraoperative, and postoperative antiemetics, thereby reducing the need for round-the-clock intravenous antiemetic administration to control nausea and retching[44]. Early ambulation is encouraged. A swallow esophagram is conducted on postoperative day one to evaluate for leaks. If a leak is detected, further intervention may be necessary, such as esophageal stenting, laparoscopic primary suturing, or complete laparoscopic repair. Once patients tolerate a liquid diet and achieve adequate pain relief, they are discharged with simethicone for bloating and a proton pump inhibitor for reflux management. Another important outcome measure in achalasia care is the need for reintervention due to incomplete myotomy. Although Heller myotomy often alleviates symptoms, some patients may require additional postoperative treatments, including simple dilatation, pneumatic dilatation, or surgical revision[45].
OUTCOMES
Table 2 summarizes studies comparing robotic-assisted Heller myotomy (RAHM) with other interventional techniques for achalasia, including LHM, POEM, and the open approach. Among studies evaluating operative time, six reported significant variations. Of these, four found RAHM to be significantly faster than LHM, while two reported longer operative times for RAHM compared to POEM, which does not require a partial fundoplication following myotomy. Beyond operative duration, Jiang et al. examined the learning curve for both robotic and LHM. They reported that proficiency in RAHM is typically reached after approximately 16-18 cases, compared with 19-20 cases for LHM[17]. Although this difference may not be clinically significant, it likely reflects that surgeons training in RAHM are already proficient in LHM, allowing them to focus on refining robotic techniques.
Literature review of RAHM compared with other surgical techniques
| Author | Year | Groups | Operative time (min) | Perforation rate (%) | Mortality (%) | LOS (days) |
| Ali et al.[46] | 2020 | RAHM vs. LHM vs. POEM | 183.5 vs. 157 vs. 169* | 0 vs. 17.5 vs. 1.1* | 0 vs. 0 vs. 0 | 1 vs. 1 vs. 1 |
| Arcerito et al.[18] | 2022 | RAHM vs. LHM | 105 vs. 158* | 0 vs. 4.2 | 0 vs. 0 | Nr |
| Chacko et al.[47] | 2022 | RAHM vs. LHM | Nr | 1.4 vs. 1.7 | 0 vs. 0.2 | 2.6 vs. 3 |
| Delgado-Miguel et al.[48] | 2023 | RAHM vs. LHM | 178 vs. 212* | 0 vs. 16.7 | Nr | 2 vs. 4* |
| Horgan et al.[49] | 2005 | RAHM vs. LHM | 141 vs. 222* | 0 vs. 16* | 0 vs. 0 | 1.5 vs. 2.2 |
| Huffmanm et al.[50] | 2007 | RAHM vs. LHM | 355 vs. 287 | 0 vs. 8.1 | Nr | 2.8 vs. 2.6 |
| Jiang et al.[17] | 2023 | RAHM vs. LHM | 130 vs. 163* | 2.5 vs. 19.2* | Nr | Nr |
| Khashab et al.[13] | 2017 | RAHM vs. POEM | 263 vs.106* | 0 vs. 7.7 | 0 vs. 0 | 2.3 vs. 1.9 |
| Kim et al.[19] | 2019 | RAHM vs. LHM | 158 vs. 157 | 2.7 vs. 11.4 | Nr | 2 vs. 2.2 |
| Perry et al.[20] | 2014 | RAHM vs. LHM | 133 vs. 121 | 0 vs. 15.8* | 0 vs. 0 | 1 vs. 2* |
| Rabe et al.[51] | 2023 | RAHM vs. LHM | 112 vs. 117 | 0 vs. 6.5 | Nr | 2 vs. 3 |
| Raja et al.[52] | 2022 | RAHM vs. LHM | 121 vs. 142 | 0 vs. 1.5 | Nr | 1 vs. 1 |
| Sanchez et al.[53] | 2012 | RAHM vs. LHM | 79 vs. 76 | 0 vs. 5.5 | 0 vs. 0 | 2 vs. 2.2 |
| Shaligram et al.[54] | 2012 | RAHM vs. LHM vs. Open | Nr | Nr | 0 vs. 0.14 vs. 0.24 | 2.42 vs. 2.7 vs. 4.42* |
| Villamere et al.[55] | 2015 | RAHM vs. LHM | Nr | Nr | Nr | 2.3 vs. 2.8* |
Regarding mucosal perforation, studies consistently favored RAHM, with nearly all reporting statistically significant or near-significant reductions. This superiority is likely attributed to the high magnification and resolution of the robotic system, which enable precise visualization of individual muscle fibers during myotomy. Only three studies documented mucosal perforations with RAHM. Previous studies demonstrate that perforations commonly occur at the EGJ, where muscle fibers transition from a circular arrangement in the esophagus to an oblique orientation in the stomach. Creating a submucosal plane at the EGJ is technically challenging during laparoscopy, but this difficulty is mitigated with robotic systems, which offer enhanced visualization and precise motor control[17].
With respect to postoperative length of stay, four studies reported significantly shorter hospitalizations following RAHM, and none found an increased stay compared with other techniques. Given that both LHM and RAHM are minimally invasive, comparable lengths of stay would generally be expected after uncomplicated surgeries. However, perforations, which occur more frequently in the LHM group, may prolong hospitalization. To complement these findings, Table 3 provides a visual summary of clinical outcomes across treatment modalities, including success rates, GERD risk, perforation rates, and hospital stay.
Summary of achalasia treatments (LHM, RAHM, POEM) across key clinical outcome metrics
| Treatment | Clinical success (%) | GERD risk (%) | Length of stay (days) | Perforation risk (%) |
| LHM + fundoplication | ≥ 90 | 11-29 | 2 | Up to 15.8 |
| RAHM + fundoplication | ≥ 90 | 11-29 | 1-2 | 0-2.5 |
| POEM | 83-92 | 44-58 | 1.9 | 0.5-3 |
CONCLUSION
Achalasia is a rare esophageal motility disorder that poses diagnostic and therapeutic challenges. Initial therapies, such as pneumatic dilation or botulinum toxin injection, offer limited long-term benefit. Surgical options, particularly Heller myotomy with partial fundoplication, have evolved significantly, with robotic-assisted techniques improving precision and reducing complications such as mucosal perforation. This comprehensive review highlights the robotic approach’s advantages in operative efficiency and postoperative outcomes, as well as demonstrating the effectiveness of robotic surgery in the management of achalasia.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study: Scheese D, Tragesser C, Patki T, Puig C, Shah RD
Performed data acquisition, analysis, and interpretation: Scheese D, Tragesser C, Patki T, Puig C, Shah RD
Drafted the work and substantively revised it: Scheese D, Tragesser C, Patki T, Puig C, Shah RD
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. O’Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2013;19:5806-12.
2. Duffield JA, Hamer PW, Heddle R, Holloway RH, Myers JC, Thompson SK. Incidence of achalasia in South Australia based on esophageal manometry findings. Clin Gastroenterol Hepatol. 2017;15:360-5.
3. Samo S, Carlson DA, Gregory DL, Gawel SH, Pandolfino JE, Kahrilas PJ. Incidence and prevalence of achalasia in Central Chicago, 2004-2014, since the widespread use of high-resolution manometry. Clin Gastroenterol Hepatol. 2017;15:366-73.
4. Pallabazzer G, Peluso C, de Bortoli N, et al. Clinical and pathophysiological outcomes of the robotic-assisted Heller-Dor myotomy for achalasia: a single-center experience. J Robot Surg. 2020;14:331-5.
5. Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. ACG Clinical Guidelines: diagnosis and management of achalasia. Am J Gastroenterol. 2020;115:1393-411.
6. Furuzawa-Carballeda J, Aguilar-León D, Gamboa-Domínguez A, et al. Achalasia - an autoimmune inflammatory disease: a cross-sectional study. J Immunol Res. 2015;2015:729217.
7. Wu XY, Liu ZQ, Wang Y, et al. The etiology of achalasia: an immune-dominant disease. J Dig Dis. 2021;22:126-35.
8. Furuzawa-Carballeda J, Icaza-Chávez ME, Aguilar-León D, et al. Is the Sars-CoV-2 virus a possible trigger agent for the development of achalasia? Neurogastroenterol Motil. 2023;35:e14502.
9. Samo S, Hamo F, Hamza A, Yadlapati R, Kahrilas PJ, Wozniak A. Rapid development of achalasia after SARS-CoV-2 infection: polymerase chain reaction analysis of esophageal muscle tissue. Am J Gastroenterol. 2024;119:987-90.
10. Pandolfino JE, Kahrilas PJ. Presentation, diagnosis, and management of achalasia. Clin Gastroenterol Hepatol. 2013;11:887-97.
12. Neubrand M, Scheurlen C, Schepke M, Sauerbruch T. Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy. 2002;34:519-23.
13. Khashab MA, Vela MF, Thosani N, et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2020;91:213-27.e6.
14. Xie J, Vatsan MS, Gangemi A. Laparoscopic versus robotic-assisted Heller myotomy for the treatment of achalasia: a systematic review with meta-analysis. Int J Med Robot. 2021;17:e2253.
15. Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery. Initial experience in foregut surgery. Surg Endosc. 2002;16:1790-2.
16. Engwall-Gill AJ, Soleimani T, Engwall SS. Heller myotomy perforation: robotic visualization decreases perforation rate and revisional surgery is a perforation risk. J Robot Surg. 2022;16:867-73.
17. Jiang X, Ye C, Jiang L, et al. Single-center experience of transitioning from video-assisted laparoscopic to robotic Heller myotomy with Dor fundoplication for esophageal motility disorders. BMC Surg. 2023;23:341.
18. Arcerito M, Jamal MM, Perez MG, Kaur H, Sundahl A, Moon JT. Esophageal achalasia: from laparoscopic to robotic Heller myotomy and Dor fundoplication. JSLS. 2022;26:e2022.00027.
19. Kim SS, Guillen-Rodriguez J, Little AG. Optimal surgical intervention for achalasia: laparoscopic or robotic approach. J Robot Surg. 2019;13:397-400.
20. Perry KA, Kanji A, Drosdeck JM, et al. Efficacy and durability of robotic Heller myotomy for achalasia: patient symptoms and satisfaction at long-term follow-up. Surg Endosc. 2014;28:3162-7.
21. Vaezi MF, Felix VN, Penagini R, et al. Achalasia: from diagnosis to management. Ann N Y Acad Sci. 2016;1381:34-44.
22. Pohl D, Tutuian R. Achalasia: an overview of diagnosis and treatment. J Gastrointestin Liver Dis. 2007;16:297-303.
23. Finley RJ, Rattenberry J, Clifton JC, Finley CJ, Yee J. Practical approaches to the surgical management of achalasia. Am Surg. 2008;74:97-102.
25. Chan EG, Sarkaria IS. Robotic assisted Heller myotomy: indications, techniques and outcomes. Shanghai Chest. 2021;5:11.
26. Schizas D, Theochari NA, Katsaros I, et al. Pseudoachalasia: a systematic review of the literature. Esophagus. 2020;17:216-22.
27. Haj Ali SN, Nguyen NQ, Abu Sneineh AT. Pseudoachalasia: a diagnostic challenge. When to consider and how to manage? Scand J Gastroenterol. 2021;56:747-52.
28. Ellison A, Peller M, Nguyen AD, et al. An endoscopic scoring system for achalasia: the CARS score. Gastrointest Endosc. 2024;100:417-28.e1.
29. Arora Z, Thota PN, Sanaka MR. Achalasia: current therapeutic options. Ther Adv Chronic Dis. 2017;8:101-8.
30. Rolland S, Paterson W, Bechara R. Achalasia: current therapeutic options. Neurogastroenterol Motil. 2023;35:e14459.
31. Bechara R, Onimaru M, Ikeda H, Inoue H. Per-oral endoscopic myotomy, 1000 cases later: pearls, pitfalls, and practical considerations. Gastrointest Endosc. 2016;84:330-8.
32. Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-71.
33. Elkholy S, Essam K, Wahba M, El-Sherbiny M. Endoscopic techniques to detect gastroesophageal junction in peroral endoscopic myotomy. VideoGIE. 2021;6:55-7.
34. Olson MT, Triantafyllou T, Singhal S. A decade of investigation: peroral endoscopic myotomy versus laparoscopic Heller myotomy for achalasia. J Laparoendosc Adv Surg Tech A. 2019;29:1093-104.
35. Ponds FA, Fockens P, Lei A, et al. Effect of peroral endoscopic myotomy vs pneumatic dilation on symptom severity and treatment outcomes among treatment-naive patients with achalasia: a randomized clinical trial. JAMA. 2019;322:134-44.
36. Werner YB, Hakanson B, Martinek J, et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med. 2019;381:2219-29.
37. Fukushima N, Masuda T, Yano F, et al. Over ten-year outcomes of laparoscopic Heller-myotomy with Dor-fundoplication with achalasia: single-center experience with annual endoscopic surveillance. Surg Endosc. 2021;35:6513-23.
39. Richards WO, Torquati A, Holzman MD, et al. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405-12; discussion 412.
40. Allaix ME, Patti MG. Heller myotomy for achalasia. From the open to the laparoscopic approach. World J Surg. 2015;39:1603-7.
41. Lin RP, Napolitano M, Sparks AD, Lee J. Laparoscopic Heller myotomy is associated with fewer postoperative complications compared to the thoracoscopic approach: a NSQIP study. J Am Coll Surg. 2021;233:S17-8.
42. Milone M, Manigrasso M, Vertaldi S, et al. Robotic versus laparoscopic approach to treat symptomatic achalasia: systematic review with meta-analysis. Dis Esophagus. 2019;32:1-8.
43. Law YY, Nguyen DT, Meisenbach LM, et al. Intraoperative diagnosis and treatment of Achalasia using EndoFLIP during Heller Myotomy and Dor fundoplication. Surg Endosc. 2022;36:2365-72.
44. Schwartz J, Gan TJ. Management of postoperative nausea and vomiting in the context of an Enhanced Recovery after Surgery program. Best Pract Res Clin Anaesthesiol. 2020;34:687-700.
45. Raja S, Schraufnagel DP, Blackstone EH, et al. Reintervention after Heller myotomy for achalasia: is it inevitable? Ann Thorac Surg. 2019;107:860-7.
46. Ali AB, Khan NA, Nguyen DT, et al. Robotic and per-oral endoscopic myotomy have fewer technical complications compared to laparoscopic Heller myotomy. Surg Endosc. 2020;34:3191-6.
47. Chacko J, Leeds SG, Aladegbami BG, Ogola GO, Ward MA. Overall complications following robotic Heller myotomy are lower compared with laparoscopy. Surg Laparosc Endosc Percutan Tech. 2022;32:319-23.
48. Delgado-Miguel C, Amarnath RP, Camps JI. Robotic-assisted vs. laparoscopic Heller’s myotomy for achalasia in children. J Pediatr Surg. 2024;59:1072-6.
49. Horgan S, Galvani C, Gorodner MV, et al. Robotic-assisted Heller myotomy versus laparoscopic Heller myotomy for the treatment of esophageal achalasia: multicenter study. J Gastrointest Surg. 2005;9:1020-9; discussion 1029.
50. Huffmanm LC, Pandalai PK, Boulton BJ, et al. Robotic Heller myotomy: a safe operation with higher postoperative quality-of-life indices. Surgery. 2007;142:613-8; discussion 618.
51. Rabe SM, Burmeister E, Niebisch S, Gockel I. Clinical and functional outcome following robotic Heller-myotomy with partial fundoplication in patients with achalasia. J Robot Surg. 2023;17:1689-96.
52. Raja S, Adhikari S, Blackstone EH, et al; Cleveland Clinic Esophageal Research Group. A comparative study of robotic and laparoscopic approaches to Heller myotomy. J Thorac Cardiovasc Surg. 2022;164:1639-49.e7.
53. Sánchez A, Rodríguez O, Nakhal E, et al. Robotic-assisted Heller myotomy versus laparoscopic Heller myotomy for the treatment of esophageal achalasia: a case-control study. J Robot Surg. 2012;6:213-6.
54. Shaligram A, Unnirevi J, Simorov A, Kothari VM, Oleynikov D. How does the robot affect outcomes? A retrospective review of open, laparoscopic, and robotic Heller myotomy for achalasia. Surg Endosc. 2012;26:1047-50.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].