The importance of patient fitness in expert and multidisciplinary multiparametric management of HCC: a narrative review
Abstract
Hepatocellular carcinoma (HCC) management is a challenging task. Despite continuous efforts to develop more effective treatments, patient prognosis often remains poor. Recently, a new management concept - the “multiparametric therapeutic Hierarchy” - has been proposed, with the hypothetical advantage of avoiding the risk of undertreatment inherent in “classical” HCC management modalities while also minimizing the risk of overtreatment that may occur when inexperienced clinicians adopt overly aggressive treatment hierarchies. The “multiparametric” concept emphasizes a more comprehensive evaluation of each patient, focusing on their individual clinical presentation. Among the various factors considered, patient fitness plays a critical role. Here, fitness is understood as the set of patient-specific characteristics that can influence the outcomes of therapies within the hierarchical framework (or even preclude certain therapies altogether). This multifaceted concept extends beyond traditional “Performance Status” measurements, incorporating considerations of frailty and comorbidity. In this review, we explore the available evidence regarding the role of patient fitness within the multiparametric therapeutic hierarchy for HCC. Finally, we discuss fitness considerations across different levels of the therapeutic hierarchy, highlighting current evidence, existing challenges, and limitations, with the aim of providing new insights for physicians involved in HCC management.
Keywords
INTRODUCTION
Hepatocellular carcinoma (HCC) poses a significant challenge for clinicians managing this disease. This is largely due to its highly variable clinical presentations and frequent occurrence in patients with advanced liver disease. In these individuals, outcomes are further influenced by multiple factors, such as tumor burden, comorbidities, clinical frailty[1], and the underlying liver disease[2]. Despite continuous advancements and the growing availability of more refined treatment options, HCC often still carries a poor prognosis[3]. This remains true even though staging systems and treatment guidelines, such as the Barcelona Clinic Liver Cancer (BCLC), have substantially improved since their introduction thirty years ago[4]. The latest updates to these systems have incorporated new therapies and approaches shown to dynamically impact prognosis at all stages of the disease[3]. Nevertheless, they continue to rely on a rigid stage-based hierarchy, where tumor staging represents the conceptual starting point for patient management[5]. Although this approach provides clinicians with an evidence-based framework for selecting the most appropriate treatment for each tumor stage, in practice, it carries a non-negligible risk of undertreatment. Because this strategy is closely tied to the initial assessment of the patient, it is often ill-suited to account for dynamic changes in prognosis introduced by novel therapeutic options. Consequently, treatment decisions in clinical practice frequently diverge from guideline recommendations[6,7]. In recent years, several alternative management frameworks have been proposed to address these discrepancies. One such recent proposal is the multiparametric therapeutic hierarchy introduced by the HCC Special Interest Group of the Italian Association for the Study of the Liver (AISF)[5]. This approach places the patient’s characteristics at the center of the decision-making process, evaluating all possible variables to allocate the patient to the most appropriate therapy - whether curative, aimed at downstaging, or palliative - while continuously reassessing treatment allocation based on response[5]. However, this approach, while theoretically minimizing the risk of undertreatment, requires an extensive, multiparametric, and multidisciplinary evaluation of every clinical aspect of the patients, such as liver disease severity, tumor characteristics, and the feasibility of each treatment option, all hierarchically ranked according to their curative potential[3]. In fact, the most recent guidelines from the European Association for the Study of the Liver have begun to move away from a purely stage-based algorithm, increasingly favoring multiparametric expert evaluations for all HCC patients[8]. Nevertheless, these guidelines still lack a fully comprehensive framework, particularly concerning the concept of patient fitness. Patient fitness, defined as the combination of individual characteristics that not only influence outcomes but may also preclude certain therapies, plays a crucial role in expert, multidisciplinary, multiparametric HCC management. In oncology, fitness extends beyond traditional performance status (PS) measures and includes frailty (a state of decreased physiological reserve) and comorbidities. In HCC, where treatment options range from curative interventions to purely palliative care, patient fitness often becomes the decisive factor in treatment selection and success.
The aim of this review is to investigate the available evidence on patient fitness evaluation and its role within the above-mentioned multiparametric therapeutic hierarchy.
FITNESS
Fitness
As in the management of other solid cancers, the concept of patient fitness has been included as a key factor in the proposed multiparametric therapeutic hierarchy for treatment allocation[5]. Fitness can be defined as a patient’s ability to undergo a given treatment based on biological, physical, and medical conditions. Age, frailty, and comorbidities are among the variables that determine a patient’s fitness for treatment. This parameter was introduced to avoid the potentially misleading interpretation of PS when used to assess a patient’s overall condition[5]. In fact, patients should be deemed fit or unfit for treatment based on their general and medical conditions, independent of the malignant disease. By contrast, PS evaluates a patient’s physical abilities and overall health specifically in relation to neoplastic disease. Notably, in the original multiparametric therapeutic hierarchy algorithm, PS is incorporated as a “critical tumor feature” rather than as a general fitness parameter [Figure 1]. The most commonly used measure of PS is the Eastern Cooperative Oncology Group (ECOG) criteria, developed in 1974 as a standardized tool to assess treatment toxicity and response (with scores ranging from 0 to 5)[9]. Additionally, the Karnofsky Performance Scale mirrors this approach[10], classifying patients as “fully active” at a score of 0, “completely disabled” at 4, and “dead” at 5.
Figure 1. Fitness evaluation within the multiparametric therapeutic hierarchy. Proposal for modifying the original algorithm to provide a more detailed assessment of patient fitness across various treatment stages. At each stage, the impact of fitness on treatment choices varies depending on the radicality/invasiveness of the intervention - higher in liver transplantation, moderate in liver resection, and low/marginal in locoregional/systemic therapies. (Modified from Vitale et al.[5]). CCI: Charlson comorbidity index.
Older age in HCC fitness evaluation
Age is an unavoidable component in assessing fitness among patients with HCC[5]. In particular, in more radical approaches, advanced age (i.e., > 75 years) could represent a relative or even absolute contraindication. However, it is intuitive that biological age, rather than biographical age, is the most important consideration[5]. Indeed, the presence and number of comorbidities influence treatment choice and even the feasibility of treatment more than age alone. This is especially critical when evaluating liver transplantation (LT). With the aging global population, LT in elderly patients is increasingly discussed. Nowadays, there is a growing consensus that, after carefully excluding significant comorbidities, LT is feasible in elderly patients without a specific age cut-off, providing similar short- and long-term benefits compared to younger recipients. Nevertheless, questions remain regarding cost-effectiveness, quality-of-life outcomes, and optimal patient selection[11]. Less radical approaches are generally feasible in older patients. For example, in early-stage HCC, radiofrequency (RF) ablation or surgery (in carefully selected cases) can be considered, while in intermediate/advanced stages, more conservative approaches such as transarterial chemoembolization (TACE) or systemic therapy may be suitable[12,13]. In general, evidence supporting HCC therapies in elderly patients mostly comes from retrospective or real-world studies, as this population has often been excluded from clinical trials. Further studies are needed to address these gaps[12]. Nonetheless, there is broad consensus that multidisciplinary expert management is crucial for these subjects. The role of age will be further discussed in subsequent sections.
Comorbidities in HCC
Comorbidities play an important role in determining fitness and treatment eligibility. One of the earliest definitions of comorbidity is “any distinct additional clinical entity that has existed or that may occur during the clinical course of a disease that is under study”[14].
The presence of comorbidities can hinder treatment options for HCC patients at any stage. For example, severe cardiac, pulmonary, or renal disease, as well as significant frailty, can limit treatment choices regardless of liver function.
LT or resection may be contraindicated in patients with high cardiovascular risk or severe obesity[15,16]. Certain cardiovascular conditions may also restrict targeted therapies, while solid organ transplantation or autoimmune diseases can contraindicate the use of combined immune checkpoint inhibitors and anti-angiogenic drugs in advanced settings[17,18].
Whether individual comorbidities that contraindicate specific treatments should be evaluated separately, or whether a composite score capturing the overall effect of comorbidities is preferable, remains an open question. Among available scoring systems, the Charlson comorbidity index (CCI) is the most widely used and is often considered the gold standard for assessing comorbidities in both research and clinical practice[19]. In HCC, CCI has been correlated with post-treatment outcomes and prognosis[20-23], but it remains unclear whether it should be used in its original form or adapted for this patient population[20,24].
Liver Function. In the context of HCC, impaired liver function is not considered a comorbidity, as chronic liver disease is inherently present in most patients. Nevertheless, liver function strongly affects prognosis and treatment strategies, regardless of tumor stage[25]. The liver’s functional status is critical for determining whether treatments that could further impair liver function are feasible. This principle applies both to early-stage tumors, where surgical resection may be contraindicated, and to advanced stages, where locoregional or systemic therapies may be limited by the risk of hepatic decompensation[26,27].
Hepatic decompensation, more so than tumor progression, has been shown to negatively impact overall survival in both early[28,29] and intermediate/advanced HCC stages[2,30-32], although this impact has not been adequately quantified in randomized controlled trials.
Optimal patient care requires a coordinated, multidisciplinary approach to optimize treatment and improve overall outcomes[33-35].
Because of its importance, liver function assessment is considered a separate step in the therapeutic hierarchy and will be addressed in a dedicated review[5].
Definition of frailty
When evaluating the feasibility of HCC treatment, frailty is another critical factor to consider. Originally defined in geriatrics, frailty refers to a biological syndrome characterized by diminished physiological reserves and overall homeostasis, resulting in increased vulnerability to health stressors[36]. “Global” frailty arises from multidimensional impairments across one or more physiological systems, including the musculoskeletal, cardiovascular, neurologic, endocrine, and immune systems. Frailty is a dynamic process that can be caused or accelerated by extrinsic factors such as significant comorbidities, smoking, alcohol consumption, and socioeconomic status. In geriatric patients, the Comprehensive Geriatric Assessment (CGA) is regarded as the gold standard for evaluating frailty[37]. This multidomain assessment covers various areas, including comorbidities, mobility (muscle strength, balance, and motor skills), cognition and mood, nutritional status, socioeconomic factors, and medication use. Several screening tools for frailty have also been developed, such as the G8 screening tool, the Rockwood Clinical Frailty Scale, and the Vulnerable Elders Survey (VES-13)[37]. The G8 tool, an eight-item questionnaire encompassing the key domains of the CGA, is frequently used in oncology. Other tools, such as the Fried frailty phenotype[36], incorporate objective measures like grip strength, balance tests, and gait speed. These scores are valuable not only for evaluating frailty and determining suitability for anti-cancer therapies but also for identifying modifiable factors (e.g., poor nutrition) that can improve patient outcomes. However, there is no universally recommended approach for assessing frailty. Moreover, the complex interplay between polypharmacy, comorbidities, and frailty poses significant challenges. This is especially important in HCC, which predominantly affects patients over 65 years of age, a population inherently more susceptible to these intersecting clinical factors.
Frailty in cirrhosis
In most cases, HCC occurs in the context of liver cirrhosis, which itself can cause frailty. Frailty has become a significant contributor to morbidity and mortality in patients with cirrhosis[38-44]. In patients with liver disease, particularly if advanced, hepatic-specific factors (i.e., impaired protein synthesis, ammonia-associated muscle toxicity, and encephalopathy-related physical inactivity) are often the main drivers of frailty, leading to loss of muscle mass and function (physical frailty). In cirrhotic patients, sarcopenia represents the morphological correlate of frailty and serves as a negative prognostic factor, predicting poorer outcomes after LT[45-49]. Considering the close relationship between sarcopenia and frailty, most available tools emphasize assessing muscle function loss. Among global frailty assessments, the frailty index, Clinical Frailty Scale, and Hospital Frailty Risk Score have been evaluated[41,50,51]. Physical frailty has been assessed using the liver frailty index (LFI), Fried frailty phenotype, short physical performance battery (SPPB), activities of daily living (ADL), 6-minute walk test (6MWT), gait speed (used alone), and grip strength (used alone)[40,42,44,52-55]. All these tests, except for ADL, are performance-based[40,54,55] and require active patient participation.
Among these tools, the LFI has been thoroughly investigated. It was developed to predict mortality in patients with cirrhosis awaiting LT and is calculated using three components: grip strength (average of three trials measured with a hand dynamometer in the dominant hand), timed chair stands (time required to perform five chair stands with arms folded across the chest), and balance tests (total time the subject can maintain balance in three positions [side-by-side, semi-tandemm and tandem] for up to 10 s each)[39]. In outpatients with cirrhosis, an LFI score ≥ 4.5 identifies frail patients with a 1.9-fold increased adjusted risk of waitlist mortality (95%CI: 1.4-2.6) compared to non-frail patients[52]. By providing an objective measure, the LFI improves clinicians’ subjective predictions of waitlist mortality[56]. Additionally, LFI o predicts greater post-LT healthcare utilization (including longer hospital stays, more intensive care unit days, and more inpatient days within 90 days post-LT)[57]. However, identifying a specific LFI threshold above which mortality risk significantly increases remains challenging[58]. Evaluating longitudinal changes in LFI may be more informative for outcome prediction in patients with decompensated cirrhosis. For example, a 0.1-unit increase in LFI over 3 months is associated with a 2-fold higher risk of death/delisting (95%CI: 1.4-3.1)[59], whereas a 0.1-unit decrease reduces the overall mortality risk by 6% (cause-specific hazard ratio: 0.94; 95%CI: 0.92-0.97; P < 0.001)[60]. Attempts to identify an LFI cut-off beyond which transplantation becomes futile due to high post-LT mortality have been unsuccessful. A recent study showed that no LFI threshold was identified at which post-LT mortality exceeded pre-LT mortality, suggesting that transplantation still offers survival benefits even in patients with advanced frailty[61].
Other assessments using the Fried frailty phenotype, SPPB, 6MWT, and gait speed have demonstrated similar associations with mortality[38,42,44,53]. Ambulatory assessments using the Clinical Frailty Scale, frailty index, Fried frailty phenotype, and gait speed have also been shown to predict future hospitalizations and length of stay[38,41,44,51].
Frailty in HCC
Unlike patients with cirrhosis, there is limited evidence on the role of frailty in predicting prognosis and guiding treatment allocation in patients with HCC. Frailty, as assessed by the Clinical Frailty Scale, has been independently associated with cancer-specific survival in patients undergoing liver resection (LR)[62]. Similarly, a poor prognosis after hepatectomy has been reported among frail patients defined using the Kihon Checklist and Barthel Index[63,64]. Moreover, the Hospital Frailty Risk Score has been linked to longer hospital stays and higher mortality rates in patients with HCC[65]. However, data on the usefulness of LFI in treatment allocation or survival prediction in HCC patients are lacking. In this context, frailty has often been indirectly evaluated through the assessment of sarcopenia, its morphological correlate[66]. A systematic review and meta-analysis demonstrated that sarcopenia in HCC patients is associated with decreased overall survival, a higher risk of recurrence following LR and LT, lower objective response rates, and increased rates of drug-related adverse events[67]. Interestingly, a recent study evaluating the impact of skeletal muscle index (SMI) on the survival of patients with HCC who underwent transplantation beyond the Milan criteria concluded that greater muscle mass is associated with a better long-term prognosis[68]. Nevertheless, although closely related, sarcopenia and frailty are not synonymous. Sarcopenia reflects mainly the physical dimension of frailty, whereas frailty is a broader, multidimensional concept that encompasses physical, psychological, and social components. While hepatology studies commonly focus on physical frailty alone, a comprehensive assessment of global frailty may provide more accurate prognostic information. However, there is still a lack of data regarding which frailty score is most appropriate for HCC patients and the optimal cut-off values to guide treatment selection.
Frailty and sarcopenia
As stated above, sarcopenia and frailty are closely related, making it valuable to discuss sarcopenia within the framework of frailty[69]. Along with aging and comorbidities, sarcopenia is a key determinant of frailty. According to a widely accepted definition, sarcopenia is a progressive and generalized skeletal muscle disorder characterized by the pathological loss of muscle mass and function, associated with an increased risk of adverse outcomes including falls, fractures, disability, and mortality[70]. It can develop primarily as a consequence of aging or secondarily due to underlying conditions[70]. The causes of secondary sarcopenia include activity-related factors (e.g., bed rest, ataxia), nutrition-related factors (e.g., malnutrition), and disease-related factors (e.g., malignancies, severe organ failure, chronic inflammatory diseases, endocrine diseases)[70]. Despite its comprehensive theoretical definition, in clinical practice, sarcopenia is often assessed only by measuring skeletal muscle mass, rather than incorporating muscle strength and physical performance (LAI). Thus, an operational definition of sarcopenia based primarily on reduced muscle mass has been widely adopted, including by the American Association for the Study of Liver Diseases[71].
Skeletal muscle mass can be quantitatively assessed using various methods, such as dual-energy X-ray absorptiometry, bioelectrical impedance analysis, and cross-sectional imaging (CT or MRI scans)[71]. CT imaging is currently considered the gold standard for muscle mass assessment in cirrhosis and is typically reported as the SMI, calculated as the total skeletal muscle area at the L3 vertebral level normalized to height[71]. Diagnostic SMI cut-off values for sarcopenia are gender-specific and vary across regions and populations. For example, the American Association for the Study of Liver Disease (AASLD) defines sarcopenia as SMI < 39 cm2/m2 in women and < 50 cm2/m2 in men, whereas the Japanese Society of Hepatology uses L3-SMI < 38 cm2/m2 in women and < 42 cm2/m2 in men[72].
Overall, sarcopenia is highly prevalent among cirrhotic patients with HCC, and its pathogenesis is influenced by both cirrhosis- and tumor-related mechanisms. Cirrhosis-specific factors include malnutrition (e.g., decreased calories intake, deficiencies in branched-chain amino acids (BCAAs), zinc, magnesium, and vitamin D), a hypercatabolic state (e.g., chronic inflammation, impaired glycogen synthesis, insulin resistance, increased proteolysis, lipid peroxidation, hyperammonemia, upregulated myostatin), and hormonal dysfunction (e.g., low insulin-like growth factor and testosterone levels)[73]. Furthermore, HCC treatments themselves may contribute to or exacerbate muscle loss. A recent meta-analysis of 57 studies involving 9,790 HCC patients reported a pooled prevalence of sarcopenia of 41.7% (95%CI: 36.2-47.2). Sarcopenia was significantly associated with poorer overall survival (HR: 1.93; 95%CI: 1.73-2.17; P < 0.001), higher risk of tumor recurrence (HR: 1.75; 95%CI: 1.56-1.96; P < 0.001), lower objective response rates (OR: 0.37; 95%CI: 0.17-0.81; P = 0.012), and more drug‐related adverse events (OR: 2.23; 95%CI: 1.17-4.28; P = 0.015)[67], findings consistent with previous investigations[74,75].
Despite their close relationship, sarcopenia and frailty are distinct in definition and therapeutic focus. While sarcopenia emphasizes muscle mass and function, frailty encompasses a broader range of functional declines, including muscle weakness and slowness[76]. Therefore, evaluating frailty may be more appropriate for assessing overall fitness in HCC patients.
FRAILTY AND FITNESS AS DYNAMIC CONCEPTS
Cancer prehabilitation
Most pathogenic mechanisms and determinants of frailty can be targeted through specific clinical interventions, making frailty a dynamic feature in the global assessment and management of patients with HCC[71,77]. Cancer prehabilitation refers to a multimodal pre-treatment approach designed to optimize a patient’s health and functional reserve prior to cancer treatment. This strategy aims to maximize treatment opportunities while minimizing anticipated treatment-related impairments, morbidity, and mortality[77]. In 2017, the World Health Organization (WHO) launched the “Rehabilitation 2030” initiative - a call to action to advance global access to high-quality rehabilitation services as essential components of health care for individuals with noncommunicable diseases[78]. Oncology was designated a priority area, not only due to the severe impact of cancer and related therapies on patients’ physical and functional capacities, but also because of the demonstrated effectiveness of rehabilitation in mitigating these effects[78].
Despite the well-established negative prognostic impact of frailty in HCC patients, data on the feasibility and efficacy of frailty-focused therapeutic interventions in this population remain scarce and heterogeneous[77,79,80]. The lack of standardized operational definitions, consistent outcome assessments, and consensus on the optimal components of prehabilitation programs continues to hinder efforts to establish a clear causal relationship between prehabilitation and improved outcomes. Furthermore, various clinical, methodological, socioeconomic, and psychological factors complicate the implementation of such interventions, particularly in HCC patients[71,81,82]. Nevertheless, evidence supporting the effectiveness of prehabilitation in enhancing clinical outcomes can be pragmatically derived from studies involving patients with other solid organ cancers or liver cirrhosis[71,81-84].
Frailty treatment
In real-world clinical practice, frailty is a major challenge, as it represents a multidimensional construct requiring an interdisciplinary, time-consuming, and resource-intensive approach throughout all stages of care, from diagnosis to treatment and outcome assessment[71,77,78,80,85]. To ensure optimal resource allocation, implementing an effective and comprehensive screening program is a crucial prerequisite[71]. A three-level framework for disease prevention and health promotion seems to be the most cost-effective approach. Each level targets different stages of disease and thus requires varying intensities of assessment and intervention. According to the 2021 Practice Guidance on Malnutrition, Frailty, and Sarcopenia in Patients with Cirrhosis by the AASLD[71]: “Primary prevention” involves routine screening to identify patients with frailty or at high risk; “Secondary prevention” entails initiating therapy in patients diagnosed with frailty; “Tertiary prevention” focuses on intensifying therapy in patients with frailty who do not respond to first-line therapy.
Currently, the most significant determinants of frailty targeted by rehabilitative interventions in cancer and cirrhotic patients include major comorbidities, liver function, malnutrition, muscle dysfunction, and psychosocial disorders[71,79,80,85].
Obviously, a “one-size-fits-all” prehabilitation program is unrealistic, given the complexity and heterogeneity of both HCC patients and HCC therapies[71,79,80,85]. Instead, an individualized, dynamic, patient- and therapy-tailored approach should be pursued. Moreover, these interventions should primarily target high-risk patients identified not only during initial screening but also throughout the treatment pathway via repeated reassessment, as these individuals stand to benefit the most from prehabilitation[71,77,79,80,85].
Muscle dysfunction
Exercise training is a rehabilitative intervention with well-established benefits in patients with various chronic conditions and solid tumors. However, its implementation in the management of patients with chronic liver disease has long been limited by concerns about its feasibility and safety[86-88]. Recent systematic reviews evaluating exercise training as an intervention for frailty in cirrhotic patients awaiting LT have consistently reported that physical rehabilitation is feasible, with good patient adherence, minimal musculoskeletal adverse events, and no evidence of exercise-related cirrhosis decompensation or portal hypertension complications[86-88]. The reviewed studies uniformly excluded patients with severe cardiopulmonary disease or those without adequate primary or secondary prophylaxis for esophageal varices. Additionally, treatments for ascites and hepatic encephalopathy had to be optimized before enrollment[88]. Other factors considered in pre-exercise risk assessments included recent alcohol consumption, recurrent falls, significant anemia, and physical or mental disabilities[86,87]. The vast majority of studies reported significant improvements in functional, physical, or aerobic capacity[86-88]. Furthermore, improvements in physical activity levels and frailty were associated with favorable clinical outcomes[88]. In an ambispective cohort study of 517 patients, Lin et al. found that a median improvement of 0.3 points in the LFI among frail patients participating in a home-based prehabilitation program was significantly associated with improved survival. Moreover, adherence to the physical therapy program was independently associated with increased survival[89].
Recently, Marcantei et al., in a systematic review involving 809 patients with HCC (514 in the exercise training group vs. 295 controls), showed that physical prehabilitation either prevented deterioration or significantly improved physical function, prevented muscle mass loss, and improved body composition. Overall, exercise training was found to be feasible, safe, and effective not only in outpatient settings but also, importantly, during hospitalization. Therefore, it should be started at diagnosis and continued during and after treatment, including in cases involving surgery[80].
In 2024, Hallsworth et al. reported on a home-based telehealth exercise intervention in HCC patients. Despite the small sample size (n = 15), the study successfully engaged older and medically complex patients (mean age 74 years; 68% with cardiovascular disease; 48% with diabetes, 89% with musculoskeletal disorders, 37% frail and 63% pre-frail). The intervention was feasible, with a 74% adherence rate and no serious adverse events, and it significantly improved physical function, grip strength, and balance[90].
Regarding exercise prescription, the AASLD[71] recommends a combination of aerobic and resistance training for cirrhotic patients. The rationale is that aerobic training improves muscular endurance and cardiopulmonary fitness, while resistance training specifically enhances skeletal muscle strength and mass. Guided by the principles of frequency, intensity, time, and type (FITT), the recommendations are as follows:
· Frequency - Aerobic (4-7 day/week); Resistance (2-3 day/week)
· Intensity - Use the talk test to ensure adequate intensity (patients should be short of breath but able to speak a full sentence); perform 3 sets of 10-15 repetitions per session
· Time - Start gradually and increase progressively
- Aerobic: 150 min per week
- Resistance: at least once per week
· Type - aerobic (e.g., walking), resistance (e.g., functional activities such as stair climbing or progressive weight training), flexibility and balance exercises (e.g., stretching, balance training)
Similarly, the European Society for Clinical Nutrition and Metabolism (ESPEN) practical guidelines for cancer patients[83] recommend (F) three sessions per week of (I) moderate-intensity training (50%-75% of maximum heart rate or aerobic capacity), (T) lasting 10-60 min per session, (T) combining aerobic and resistance exercises. The exercise prescriptions derived from systematic reviews of LT candidates and HCC patients were generally in line with these recommendations[86-88]. Exercise training can be delivered not only in-person but also through remote, home-based programs supervised by caregivers and/or monitored via telehealth, offering comparable outcomes and safety profiles[80,86-88,90].
Treatment of malnutrition
Similar to exercise training, there are few interventional studies investigating the effects of nutritional therapy on long-term outcomes in patients with HCC[77,91,92]. Nutritional interventions in this population should be evaluated within an operational framework that takes into account the morbidity of anti-cancer treatments, the underlying causes of chronic liver disease, and liver metabolic dysfunction.
Chemotherapy and surgery are known risk factors for the development or worsening of malnutrition. Accordingly, the guidelines for the prevention and treatment of malnutrition in cancer patients should also be applied to HCC patients[83]. These guidelines recommend interventions to increase oral intake, ensure adequate protein consumption, and correct vitamin or mineral deficiencies[83].
Patients with alcohol-related cirrhosis tend to present with more severe undernutrition, due to unhealthy lifestyles and frequent socioeconomic challenges[71,93]. Their caloric intake is often markedly reduced because of prevalent alcohol consumption, resulting in deficiencies in protein, fat, and various micronutrients (including folate, thiamine, zinc, selenium, vitamin D, vitamin B, and vitamin E)[71,92,93]. Additionally, alcohol-associated skeletal myopathy is an important contributor to sarcopenia in these patients[71]. In contrast, patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) exhibit prognostically unfavorable nutritional disorders, but with relatively opposite features[71,93]. Obesity is an independent risk factor for worse clinical outcomes both in chronic liver disease[93]and in liver surgery[94]. Intensive lifestyle interventions aiming for weight loss of ≥ 10% have been shown to significantly improve liver function and histology, as well as to reduce portal hypertension, without severe adverse events[71,84,93]. When caloric restriction is prescribed for MASLD, it is essential to ensure adequate protein intake (1.2-
The metabolic dysfunction associated with chronic liver disease is characterized by reduced macro/micronutrient availability, a persistent catabolic state, increased oxidative stress, and impaired biosynthetic and detoxification capacities[71,84,92,93]. Furthermore, portal hypertension and related complications can lead to reduced oral intake and malabsorption. The severity of malnutrition strongly correlates with the degree of liver disease, and a malnourished or sarcopenic state represents an independent risk factor for liver decompensation, complications of portal hypertension, hospitalization, and mortality[71,84,92,93]. Compared with other cancer patients, those with HCC often require higher caloric and protein intake (adjusted for BMI and Child-Pugh class; at least 35 kcal/kg/day in non-obese patients; and 1.2-1.5 g/kg/day of protein). In addition, specific dietary routines, such as frequent meals and late-night snacks, are recommended to counteract the accelerated starvation state[71,84,93].
Moreover, in the presence of malnutrition or sarcopenia, empirical oral multivitamin supplementation is pragmatically advised[71]. Nutritional supplements can help support or complement the prescribed diet. Enteral nutrition should be considered when oral intake is insufficient to meet nutritional targets, or when oral intake is not possible due to impaired cognitive status, severe gastrointestinal symptoms, or other clinical restrictions[71,82]. Delaying surgery to optimize preoperative malnutrition is necessary to mitigate the risk of postoperative morbidity and mortality, and has been proven safe in terms of tumor progression[82]. Patients with cirrhosis exhibit early onset of gluconeogenesis after short-term fasting, contributing to their increased protein requirements and muscle depletion. In cirrhotic patients, a randomized controlled trial reported that a late-evening nutritional supplement over 12 months improved body protein stores[95].
Specific nutritional interventions
Clinical research has explored the role of specific nutritional interventions in HCC patient management, focusing in particular on BCAAs and immunonutrition.
BCAAs
Cirrhotic patients often experience impaired protein metabolism, characterized by increased oxidation and reduced endogenous utilization of BCAAs[91,96]. This deficit represents a major pathogenic factor contributing to the development of hepatic encephalopathy and sarcopenia[91,96]. According to a recent systematic meta-analysis[97], the most consistent evidence supporting long-term BCAA supplementation is that it can improve event-free survival (P = 0.008; RR = 0.61; 95%CI: 0.42-0.88) and overall survival (P = 0.05; RR = 0.58; 95%CI: 0.34-1.00) in cirrhotic patients. However, when examining the effects of BCAAs in patients undergoing specific HCC treatments (e.g., systemic therapy, radiotherapy, transarterial/ablative treatments, LR, LT), results were heterogeneous[91,96,97]. A consistent improvement in overall survival and nutritional status was observed only in patients receiving systemic therapy[96,97]. Conversely, in patients treated with resection, locoregional therapies, or LT, no significant benefit in terms of overall survival or recurrence-free survival was demonstrated[97-99]. Nonetheless, BCAAs appear to contribute to patient optimization, improving nutritional status, preserving or enhancing liver function, and potentially preventing therapy-related liver injury[96,98,99]. A recent meta-analysis involving 1,389 liver cancer patients undergoing hepatic resection or LT reported that perioperative BCAA administration was associated with reduced postoperative infections (RR = 0.58; 95%CI: 0.39 - 0.84; P = 0.005) and ascites (RR = 0.57; 95%CI: 0.38-0.85; P = 0.005), as well as shorter hospital stays and increased body weight, without impacting cancer recurrence or overall survival[100].
Immunonutritional interventions
Immunonutrition involves supplementation with multiple nutrients that support metabolic homeostasis and immune function, promote protein synthesis and antioxidant activity, and reduce the metabolic response to stress, thus modulating the postoperative inflammatory response[101,102]. These formulas typically include ω-3-fatty acids (ω-3 FAs), glutamine (Gln), arginine (Arg), and nucleotides, and have mainly been tested in surgical patients[101,102]. Two recent meta-analyses consistently demonstrated that perioperative immunonutrition in patients undergoing LR was associated with a significantly decreased risk of overall postoperative complications, surgical-site infections, and shorter hospitalization. In contrast, no significant effects on liver failure, overall survival, or disease-free survival were observed[101,102]. Additionally, another meta-analysis investigating the effect of ω-3 FAs on liver function and inflammatory reaction in patients undergoing LR showed that their administration had a positive impact on postoperative liver function and inflammation[103]. Meanwhile, evidence supporting immunonutrition in LT patients is limited and conflicting. A 2015 meta-analysis[104] suggested a heterogeneous protective effect against infectious complications but found no significant impact on mortality, graft function recovery, or graft rejection risk. Similarly, two randomized trials did not demonstrate any significant clinical benefits of peritransplant immunonutrition[105,106].
Psychosocial disorders and comorbidities
Patients with HCC often experience a severe psychosocial burden and negative lifestyle habits, all of which can contribute to the development of frailty. A comprehensive, multimodal prehabilitation program typically includes both medical risk factor optimization and psychosocial interventions[77,85]. These interventions not only directly address features of frailty but also synergistically enhance the beneficial effects of exercise and nutritional therapy[71,82,85]. For HCC patients, cessation of smoking and alcohol consumption is a fundamental component. Moreover, these patients frequently face socioeconomic deprivation, depressive disorders, and anxiety, resulting in lower quality of life and significantly higher levels of distress compared to patients with other cancers[107]. In addition, the complexity and multimodality of HCC therapies themselves contribute to increased stress, uncertainty, and anxiety[108]. In 2021, the first systematic review on Psycho-Oncological Interventions for Patients with Hepatobiliary Cancers was performed[107]. Only one randomized controlled trial involving 136 HCC patients evaluated the effect of psychological-educational care over 1 year after hepatectomy. The intervention included health education, personal interviews and support, guided patient meetings, and telephone follow-ups. After 12 months, the intervention group showed significantly reduced rates of depression and anxiety, improved quality of life, and increased overall survival compared to the control group receiving standard supportive care[109]. Another trial involving 97 patients undergoing postoperative chemotherapy after LR assessed the effect of a 5A nursing intervention on quality of life and self-care efficacy. The intervention significantly improved self-care abilities and quality of life, alleviated cancer-related fatigue during treatment, and increased patient satisfaction with nursing care[110]. Overall, although data on psychosocial interventions in HCC patients are extremely limited, the high physical, psychological, and social needs observed throughout their cancer trajectory underscore the importance of further investigations in this area. These findings promote the incorporation of psychosocial prehabilitation strategies into clinical practice, as has been done in other oncological populations[111].
Enhanced recovery after surgery protocols
Enhanced Recovery After Surgery (ERAS) protocols currently represent the most consistent, effective, and evidence-based approach for implementing prehabilitation programs in clinical practice. These multimodal pathways comprise interventions at every stage of surgical patient management, aiming to facilitate a rapid recovery of physical function by attenuating the perioperative stress response to major surgery. The ERAS Society has recently released updated guidelines for perioperative care in liver surgery[82] and LT[81]. The recommendations regarding prehabilitation encompass preoperative counseling, lifestyle changes, physical exercise, and nutritional interventions. In 2024, Delabays et al. conducted a systematic review and meta-analysis to assess the impact of ERAS pathways on outcomes in cirrhotic patients undergoing liver surgery[112]. Among 646 patients (327 in the ERAS group and 319 in the non‐ERAS group), those managed with ERAS protocols showed a significant decrease in the risk of overall complications (OR 0.43; 95%CI: 0.31-0.61; P < 0.001) and experienced shorter hospitalization compared to the non‐ERAS group, although no significant differences were observed in 90‐day postoperative mortality, reoperation rates, readmissions, or liver failure. These findings align with the broader evidence supporting the benefits of ERAS protocols in other types of surgery, as well as in liver surgery overall[113]. Another meta-analysis[114] examining LT outcomes in 284 patients managed with fast-track protocols derived from ERAS guidelines reported a significant reduction in overall complication rates after LT (OR 0.41; 95%CI: 0.23-0.74; P = 0.003).
Overall, the implementation of ERAS protocols in the therapeutic management of patients with HCC should be encouraged and prioritized, given their feasibility, safety, efficacy, and cost-effectiveness.
FITNESS AND FRAILTY EVALUATION IN THE THERAPEUTIC HIERARCHY
In the multiparametric, multidisciplinary expert decision-making framework that guides each step of the exclusionary ordinal therapeutic hierarchy for HCC treatment[5], the assessment of patient fitness is the first and foremost consideration, even before evaluating tumor characteristics. This prioritization is necessary because severe frailty or certain comorbidities may preclude the feasibility of a given therapeutic approach, regardless of tumor features or technical feasibility. Naturally, the impact of comorbidities and frailty varies depending on the type of therapeutic approach. Below, we outline the most important factors to consider when evaluating patient fitness at each stage of the ordinal therapeutic hierarchy. Table 1 summarizes the critical roles of fitness and frailty in influencing therapeutic choices for HCC. Figure 1 presents a proposed algorithm for a more granular evaluation of patient fitness within the multiparametric therapeutic hierarchy, which will be discussed in the following paragraphs[5].
Potential treatment implications of frailty and comorbidities
| 1. Surgical interventions · Frail patients could show significantly higher early mortality after hepatectomy · Increased risk of postoperative complications · Extended recovery periods · Higher likelihood of failure to rescue |
| 2. Locoregional therapies · Reduced tolerability to transarterial therapies · Increased risk of adverse events · Lower complete response rates |
| 3. Systemic therapy · Higher rates of dose modifications · Increased treatment discontinuation · Reduced survival benefit |
| 4. Liver transplantation · Increased risk of waitlist mortality · Greater risk of hospitalizations and mortality after hospitalization · Higher risk of mortality and poor functional recovery after transplant |
LT
LT represents the most curative therapy for HCC, offering both radical treatment for tumors confined to the liver and resolution of underlying liver disease[115,116]. However, it is also a highly invasive and aggressive procedure, carrying a substantial risk of perioperative morbidity and mortality - risks that are significantly heightened by the presence of comorbidities and/or frailty, which can in turn diminish the benefit of transplantation[116,117]. Among treatment settings (along with LR), LT is supported by the most robust and granular data, with frailty/comorbidity indices demonstrating reliable predictive value for outcomes[117]. While there is no formal age limit for LT, it is generally recommended that candidates over 65 years undergo more stringent evaluation[117]. This recommendation stems primarily from the higher prevalence of specific comorbidities in elderly patients (e.g., cardiovascular diseases, diabetes, chronic respiratory diseases, chronic kidney disease, and obesity), which tend to reduce the benefits of LT more than age itself[15,118-126]. There is increasing interest in validating deep learning and AI-based algorithms for predicting major cardiovascular events in LT recipients[127,128]. Nevertheless, more traditional assessment tools, such as the CCI, have also demonstrated good reliability in the LT setting[20].
An unresolved challenge remains in understanding the impact of HCC recurrence after LT on efficacy, toxicity profiles, and overall survival. Further investigation is needed to establish optimal management strategies for these patients[129-131].
LR
The considerations regarding fitness for LT can largely be applied to LR as well. Thanks to advancements in minimally invasive surgical approaches (i.e., mini-invasive LR) and improved anesthesiological management, there is virtually no age limit for LR, provided that candidates are appropriately selected[132-136]. However, while a single comorbidity does not necessarily contraindicate LR, the cumulative burden of multiple comorbidities can significantly increase morbidity and mortality. This is especially true for patients with metabolic syndrome (obesity, dyslipidemia, type 2 diabetes, arterial hypertension), and more specifically in those with MASLD-related HCC[137-140]. Frailty and sarcopenia also clearly affect treatment outcomes[141-144]. Beyond the traditional ASA (American Society of Anesthesiologists) score, several additional parameters have been investigated to better assess surgical risk[62,145-150]. Unlike in the LT setting, where comorbidities may contraindicate transplantation, in LR, these conditions do not necessarily preclude surgery, and there are no clearly defined cut-off values in the proposed scoring systems to serve as absolute contraindications. Therefore, the selection of candidates for LR should rely on a comprehensive, multidisciplinary evaluation that takes into account all relevant aspects - clinical status, surgical factors, and the availability of efficacious alternatives.
Anesthesiological assessment in surgical fitness evaluation
Anesthesiological evaluation is essential in assessing surgical fitness for both LR and transplantation. While both procedures require thorough preoperative assessment, their risk profiles and perioperative implications differ substantially. For LR, anesthesiologists focus on evaluating cardiopulmonary reserve, frailty, and liver function reserve - especially in patients with chronic liver disease. Tools such as Cardiopulmonary Exercise Testing (CPET), spirometry, and the ASA classification are commonly employed to quantify surgical risk and identify candidates who may benefit from prehabilitation[151,152].
In contrast, anesthesiological assessment for LT also involves evaluating the systemic effects of chronic liver dysfunction (e.g., portopulmonary hypertension, hepatopulmonary syndrome, coagulopathy) and the patient’s ability to tolerate prolonged anesthesia and significant hemodynamic shifts during surgery[151]. The complexity of transplantation, with its multi-organ implications, necessitates a more extensive perioperative evaluation, including echocardiography, pulmonary function tests, and detailed nutritional and sarcopenia assessments[39].
According to the 2024 EASL guidelines, pretransplant evaluation should be conducted within a multidisciplinary framework and tailored to optimize patient survival and graft function. Anesthesiologists contribute decisively to this process, helping to inform transplant candidacy decisions and identify reversible contraindications. Their role is particularly pronounced in borderline or high-risk patients, where perioperative outcomes hinge on meticulous physiological optimization[8].
Locoregional therapies
As we move “down” the therapeutic hierarchy, we gain greater applicability at the cost of treatment radicality. Percutaneous ablation (PA), whether using mono- or bipolar RF or microwave (MW) techniques, is generally well tolerated[153,154], even in frail patients or those with significant comorbidities. In recent years, laparoscopic ablation (LA) has also emerged as a more effective option than other lower-tier therapies, such as TACE, and is now included in the therapeutic hierarchy when technical limitations preclude PA[155,156]. Regarding patient fitness, PA is typically feasible even in patients with severe comorbidities and of any age, largely because it does not require general anesthesia and can usually be performed under mild systemic analgesia with sedation. In contrast, LA requires general anesthesia, making careful anesthetic management crucial in high-risk patients (frail/elderly), as is the case with any laparoscopic procedure[157]. Special attention is needed in patients with previous biliary interventions (e.g., biliary anastomosis or sphincterotomy), as they are at higher risk for biliary abscesses. However, this risk can be mitigated through extended antibiotic prophylaxis[157,158]. Finally, it should be noted that monopolar RF can interfere with pacemakers, whereas bipolar RF and MW do not[159].
Intra-arterial therapies
Intra-arterial therapies encompass a range of locoregional techniques that target HCC via its vascular supply. These include transarterial embolization (TAE), which reduces tumor blood flow by blocking the feeding artery with a gelatin sponge; TACE, which combines arterial embolization with the administration of high concentrations of chemotherapeutic agents; and transarterial radioembolization (TARE), which delivers high-dose beta radiation-emitting particles into the tumor’s capillary network. In addition, nonselective hepatic artery infusion chemotherapy (HAIC) is also considered a therapeutic option[159-162].
Regarding frailty and comorbidities, there are no major contraindications for intravascular treatments, given their minimally invasive nature and the absence of a need for major anesthesia. Furthermore, advanced age is not a limiting factor, as evidence indicates it is not correlated with higher rates of adverse events or lower overall survival compared to younger patients[163-167]. However, it should be noted that chronic kidney disease with a reduced glomerular filtration rate could be a relative contraindication due to the interventional radiology component of these procedures[168-170].
Systemic therapies
The therapeutic landscape of HCC has drastically changed in recent years, largely due to the continuous development and availability of new systemic treatments. These advances have expanded treatment options for patients deemed unsuitable for surgical or locoregional treatments and have added complexity to patient management and decisions regarding treatment futility[2,171]. When selecting systemic therapy, multiple factors must be carefully evaluated to determine the most appropriate and effective option. Beyond considerations of first-, second-, or third-line therapies - based on efficacy, mechanism of action, and patient and tumor characteristics, which are beyond the scope of this review - the feasibility of systemic treatments crucially depends on the patient’s overall fitness. As previously discussed, systemic therapies are typically reserved for patients with advanced disease who are not candidates for more radical therapies due to technical or biological factors (e.g., liver function, general fitness, or tumor burden)[2,171-175]. Consequently, patients considered for systemic therapy are often among the most frail and comorbid[5]. Importantly, patient fitness varies depending on the specific systemic treatment under consideration. Table 2 summarizes potential mechanisms by which frailty may negatively impact systemic therapies.
Potential mechanisms through which frailty may negatively impact systemic therapies
| Treatment tolerance: · Increased severity of adverse events · Higher rates of dose reductions and treatment discontinuation · Greater risk of treatment-related hospitalizations · Reduced completion of planned treatment cycles |
| Functional reserve: · Diminished ability to recover from treatment-related toxicities · Increased risk of functional decline during therapy · Compromised resistance to metabolic stress · Reduced capacity to maintain daily activities during treatment |
| Treatment delivery: · More frequent dose modifications required · Increased likelihood of treatment delays · Higher risk of permanent discontinuation · Challenges in maintaining dose intensity |
| Clinical outcomes: · Higher rates of early treatment discontinuation · Increased mortality risk · Reduced overall survival · Compromised quality of life during treatment |
The primary limitation arises from the phase III registration trials, which have predominantly included patients with an Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0-2 (and more recently, 0-1). Therefore, a poor PS should be regarded as at least a relative contraindication, given the limited evidence from real-world studies in patients with more advanced functional impairments - and such data are not available for every systemic treatment[176]. With respect to age, older patients have been included in both real-world studies and post hoc analyses of clinical trials, which have demonstrated clinical benefits[177-182]. Thus, age alone should not be considered a barrier to systemic therapy. Instead, attention should be focused on comorbidities, which tend to increase with age and may contraindicate specific systemic treatments based on drug mechanisms and related side effects. For example, cardiovascular diseases may limit the use of tyrosine kinase inhibitors (TKIs) and anti-VEGF agents, given the high rates of uncontrolled arterial hypertension and cardiovascular events associated with these regimens[183,184]. Autoimmune diseases represent another important group of comorbidities. Overall, data regarding the use of systemic therapies in patients with autoimmune diseases are limited. While there are no specific restrictions on the use of TKIs or anti-VEGF agents in this population[17], the safety of immunotherapy is less well established, as patients with autoimmune conditions are typically excluded from clinical trials[185]. Some authors, based on experience in other oncological settings, have suggested cautious use of these agents due to the potential risk of exacerbating autoimmune diseases[186,187]. However, there is currently insufficient evidence to recommend immunotherapy in patients with autoimmune liver diseases, and further trials are needed[17]. The same caution applies to extrahepatic autoimmune diseases, for which data in HCC are entirely lacking; thus, the use of immunotherapies should be carefully evaluated on a case-by-case basis[17].
ACTUAL LIMITATIONS, CONTROVERSIES AND UNRESOLVED ISSUES
Several critical gaps persist in our understanding of the relationship between patient fitness and treatment outcomes in HCC. First, many pivotal HCC trials have historically excluded or underrepresented frail patients, typically enrolling only those with an ECOG PS of 0-1. Second, the lack of standardized frailty assessment tools specifically validated for HCC patients represents a significant limitation. While various instruments exist, including the LFI and general geriatric assessment tools, their predictive value for treatment outcomes and the optimal timing for their implementation remain inadequately studied in the context of systemic therapy for HCC. Moreover, optimal cut-off values remain to be defined[188]. For example, the proposed algorithm [Figure 1] for evaluating patient fitness incorporates items such as the “Relevance” of frailty assessment for any therapy and the CCI, which are quantitative. However, no specific thresholds can currently be recommended due to the lack of supporting data. Therefore, prospective or interventional studies directly comparing different frailty indices in HCC patients are warranted. Such studies could also provide an opportunity to design and validate HCC-specific fitness tools or scores (e.g., integrating age, CCI, LFI, and muscle mass index), which could improve clinical assessment. Third, prospective studies investigating the relationship between baseline fitness measures and treatment-related adverse events are scarce. This gap is particularly relevant for newer therapeutic options, such as immunotherapy combinations and targeted agents, where the impact of pre-existing frailty on toxicity profiles remains poorly characterized. Fourth, there is limited evidence regarding the dynamic nature of fitness during treatment. Most available data are derived from baseline assessments, while changes in functional status during therapy and their implications for treatment modification remain largely unexplored.
Moreover, the complex interaction between liver dysfunction, tumor burden, and fitness measures introduces significant confounding that is not adequately addressed in the current literature. This complexity particularly hampers the interpretation of treatment outcomes and the development of predictive models. These limitations underscore the urgent need for dedicated research addressing the role of fitness assessment in treatment decision making and outcome prediction for HCC patients.
Finally, while the multiparametric therapeutic hierarchy approach for HCC treatment is a promising framework, its feasibility in low-resource settings poses significant challenges. Although this approach emphasizes personalized treatment based on survival benefit, practical implementation in these settings requires addressing resource constraints, potential biases, and the need for multidisciplinary teams, all of which are difficult to achieve with limited resources. Table 3 summarizes possible interventions to overcome or manage frailty and comorbidities in HCC patients.
Possible interventions to overcome or manage frailty and comorbidities in HCC
| Management implications |
| 1. Treatment selection · Frailty: Consider less aggressive interventions · Comorbidities: May (relatively or absolutely) contraindicate specific treatments |
| 2. Risk stratification · Frailty: Focus on functional reserve assessment · Comorbidities: Emphasize organ system evaluation |
| 3. Supportive care · Frailty: Rehabilitation and nutritional support are crucial · Comorbidities: Specific organ system support required |
| Recommendations for clinical practice |
| 1. Comprehensive assessment · Standardized frailty evaluation · Detailed comorbidity documentation · Regular reassessment during treatment |
| 2. Treatment adaptation · Individualized approach based on fitness profile · Consider modified treatment protocols · Enhanced monitoring for vulnerable patients |
| 3. Supportive interventions · Prehabilitation for frail patients · Optimization of comorbid conditions · Involvement of a multidisciplinary team |
CONCLUSIONS
Patient fitness, encompassing both frailty and comorbidity burden, fundamentally influences HCC treatment outcomes. Understanding the distinct impacts of these components enables more precise risk stratification and treatment selection. Future research should focus on developing tailored interventions for patients with different fitness profiles to optimize outcomes while minimizing treatment-related complications.
DECLARATIONS
Acknowledgments
Members of the Associazione Italiana per lo Studio del Fegato (AISF) HCC Special Interest Group: ASST Papa Giovanni XXIII Bergamo, Italy: Mauro Viganò; Campus Bio-Medico University Rome, Italy: Giovanni Galati; Cardarelli Hospital Naples, Italy: Marco Guarracino, Raffaella Tortora; Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Italy: Nicoletta De Matthaeis, Simone Famularo, Felice Giuliante, Luca Miele, Francesca R. Ponziani; Foggia University Hospital, Italy: Rodolfo Sacco; Hospital of Faenza, Italy: Francesco G. Foschi; Humanitas University Milan, Italy: Luca Viganò; Imperial College London, UK: David J. Pinato; IS.ME.T.T. Palermo, Italy: Salvatore Gruttadauria, Duilio Pagano; Istituto Tumori Milano, Italy: Sherrie Bhoori, Carlo Sposito; National Institute of Gastroenterology Saverio de Bellis Bari, Italy: Raffaele Cozzolongo; Niguarda Ca’ Granda Hospital Milan, Italy: Leonardo Centonze, Chiara Mazzarelli; Polytechnic University of Marche: Daniele Nicolini; ce University of Rome, Italy: Quirino Lai, Fabio Melandro; University Hospital of Verona, Italy: Paola Violi; University of Bari, Italy: Maria Rendina, Francesco D’Amico; University of Bologna, Italy: Matteo Cescon, Fabio Piscaglia, Matteo Renzulli, Nicolò Brandi, Francesco Tovoli, Franco Trevisani; University of Genoa, Italy: Edoardo G. Giannini, Giulia Pieri, Maria Corina Platz Torres; University of Milan, Italy: Massimo Iavarone, Angelo Sangiovanni; University of Milano Bicocca, Italy: Fabrizio Romano; University of Modena and Reggio Emilia, Italy: Stefano Di Sandro; University of Naples Federico II, Italy: Maria Guarino, Filomena Morisco; University of Padua, Italy: Patrizia Burra, Umberto Cillo, Fabio Farinati, Michele Finotti, Martina Gambato, Filippo Pelizzaro, Francesco P. Russo, Alessandro Vitale; University of Palermo, Italy: Giuseppe Cabibbo, Ciro Celsa; University of Pisa, Italy: Paola Carrai, Laura Crocetti, Davide Ghinolfi; University of Salerno, Italy: Mario Masarone, Marcello Persico; University of Tor Vergata Rome, Italy: Ilaria Lenci, Tommaso M. Manzia, Bruno Sensi; University of Udine, Italy: Umberto Baccarani, Riccardo Pravisani; Vita-Salute San Raffaele University Milan, Italy: Andrea Casadei Gardini; Università degli Studi Link Campus University Roma: Giuseppina Brancaccio; Dipartimento di Chirurgia Frosinone: Giovanni Battista Levi Sandri.
The authors thank Dr. Berardo Guzzi (English Certificate of Proficiency released by the University of Cambridge) for his assistance with English.
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Masarone M, Cabibbo G, Pravisani R, Pellizzaro F, Viganò M, Vitale A, Persico M
Performed data acquisition, as well as providing administrative, technical, and material support: Torre P, Loglio A
Availability of data and materials
Not applicable.
Financial support and sponsorship
Masarone M is funded by Italian Ministry of the University and Research: Progetti di Rilevante Interesse Nazionale (PRIN) 2022 - Grant number: (202222FCC).
Conflicts of interest
Vitale A is an Editorial Board member of Hepatoma Research and a Guest Editor of the Special Issue to which this article belongs. Pelizzaro F is a Junior Editorial Board member of Hepatoma Research. Vitale A and Pelizzaro F were not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
2. Cabibbo G, Aghemo A, Lai Q, Masarone M, Montagnese S, Ponziani FR; Italian Association for the Study of the Liver (AISF). Optimizing systemic therapy for advanced hepatocellular carcinoma: the key role of liver function. Dig Liver Dis. 2022;54:452-60.
3. Vitale A, Trevisani F, Farinati F, Cillo U. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. 2020;72:2206-18.
4. Teufel A, Kudo M, Qian Y, et al. Current trends and advancements in the management of hepatocellular carcinoma. Dig Dis. 2024;42:349-60.
5. Vitale A, Cabibbo G, Iavarone M, et al; HCC Special Interest Group of the Italian Association for the Study of the Liver. Personalised management of patients with hepatocellular carcinoma: a multiparametric therapeutic hierarchy concept. Lancet Oncol. 2023;24:e312-22.
6. Kim KM, Sinn DH, Jung SH, et al. The recommended treatment algorithms of the BCLC and HKLC staging systems: does following these always improve survival rates for HCC patients? Liver Int. 2016;36:1490-7.
7. Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-51.
8. Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J Hepatol. 2025;82:315-74.
9. Oken MM, Creech RH, Tormey DC et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-55.
10. Karnofsky D A. The clinical evaluation of chemotherapeutic agents in cancer. Eval Chemother Agents 1949:191-205. Available from: https://scispace.com/papers/the-clinical-evaluation-of-chemotherapeutic-agents-in-cancer-12buycduj2. [Last accessed on 11 Jul 2025].
11. Dolnikov S, Adam R, Cherqui D, Allard MA. Liver transplantation in elderly patients: what do we know at the beginning of 2020? Surg Today. 2020;50:533-9.
12. Cho E, Cho HA, Jun CH, Kim HJ, Cho SB, Choi SK. A review of hepatocellular carcinoma in elderly patients focused on management and outcomes. In Vivo. 2019;33:1411-20.
13. Arora SP, Liposits G, Caird S, et al. Hepatocellular carcinoma in older adults: a comprehensive review by Young International Society of Geriatric Oncology. J Geriatr Oncol. 2020;11:557-65.
14. Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23:455-68.
15. VanWagner LB, Ning H, Whitsett M, et al. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: The CAR-OLT score. Hepatology. 2017;66:1968-79.
16. Dick AA, Spitzer AL, Seifert CF, et al. Liver transplantation at the extremes of the body mass index. Liver Transpl. 2009;15:968-77.
17. Rimassa L, Personeni N, Czauderna C, Foerster F, Galle P. Systemic treatment of HCC in special populations. J Hepatol. 2021;74:931-43.
18. Lai Q, Sapisochin G, Gorgen A, et al. Evaluation of the intention-to-treat benefit of living donation in patients with hepatocellular carcinoma awaiting a liver transplant. JAMA Surg. 2021;156:e213112.
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-83.
20. Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transpl. 2007;13:1515-20.
21. Alaimo L, Endo Y, Lima HA, et al. A comprehensive preoperative predictive score for post-hepatectomy liver failure after hepatocellular carcinoma resection based on patient comorbidities, tumor burden, and liver function: the CTF score. J Gastrointest Surg. 2022;26:2486-95.
22. Chang YS, Huang JS, Yen CL et al. The Charlson comorbidity index is an independent prognostic factor for treatment-naïve hepatocellular carcinoma patients with extrahepatic metastases. Hepatogastroenterology. 2015;62:1011-5.
23. Shinkawa H, Tanaka S, Takemura S, et al. Predictive value of the age-adjusted Charlson comorbidity index for outcomes after hepatic resection of hepatocellular carcinoma. World J Surg. 2020;44:3901-14.
24. Choi J, Choi EW, Choi Y, et al. Modified Charlson comorbidity index as a survival prediction tool for older patients after liver transplantation. Ann Surg Treat Res. 2023;104:358-63.
25. Cabibbo G, Maida M, Genco C, Antonucci M, Cammà C. Causes of and prevention strategies for hepatocellular carcinoma. Semin Oncol. 2012;39:374-83.
26. Aliberti C, Carandina R, Lonardi S, et al. Transarterial chemoembolization with small drug-eluting beads in patients with hepatocellular carcinoma: experience from a cohort of 421 patients at an italian center. J Vasc Interv Radiol. 2017;28:1495-502.
27. Farinati F, Vanin V, Giacomin A, et al; Italian Liver Cancer (ITA. LI.CA) group. BCLC stage B hepatocellular carcinoma and transcatheter arterial chemoembolization: a 20-year survey by the Italian Liver Cancer group. Liver Int. 2015;35:223-31.
28. Cabibbo G, Petta S, Barbara M, et al; Italian Liver Cancer (ITA.LI.CA) group. Hepatic decompensation is the major driver of death in HCV-infected cirrhotic patients with successfully treated early hepatocellular carcinoma. J Hepatol. 2017;67:65-71.
29. Cabibbo G, Celsa C, Calvaruso V, et al; Rete Sicilia Selezione Terapia - HCV (RESIST-HCV) and Italian Liver Cancer (ITA.LI.CA.) Group. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265-73.
30. Celsa C, Cabibbo G, Fulgenzi CAM, et al. Hepatic decompensation is the major driver of mortality in patients with HCC treated with atezolizumab plus bevacizumab: the impact of successful antiviral treatment. Hepatology. 2025;81:837-52.
31. Reig M, Cabibbo G. Antiviral therapy in the palliative setting of HCC (BCLC-B and -C). J Hepatol. 2021;74:1225-33.
32. Cabibbo G, Celsa C, Battaglia S, et al. Early hepatic decompensation identifies patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab or sorafenib at highest risk of death. Clin Cancer Res. 2025;31:543-50.
33. Reiberger T, Lens S, Cabibbo G, et al. EASL position paper on clinical follow-up after HCV cure. J Hepatol. 2024;81:326-44.
34. Piano S, Reiberger T, Bosch J. Mechanisms and implications of recompensation in cirrhosis. JHEP Rep. 2024;6:101233.
35. Wu J, Liu W, Qiu X, et al. A noninvasive approach to evaluate tumor immune microenvironment and predict outcomes in hepatocellular carcinoma. Phenomics. 2023;3:549-64.
36. Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-56.
37. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459-544.
38. Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870-9.
39. Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66:564-74.
40. Tandon P, Reddy KR, O’Leary JG, et al; North American Consortium for the Study of End-Stage Liver Disease. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017;65:217-24.
41. Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol. 2016;111:1759-67.
42. Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373-8.
43. Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017;23:899-905.
44. Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol. 2016;111:1768-75.
45. Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-73.
46. Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, et al. Inclusion of sarcopenia within MELD (MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015;6:e102.
47. Tantai X, Liu Y, Yeo YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;76:588-99.
48. Montano-Loza AJ. Skeletal muscle abnormalities and outcomes after liver transplantation. Liver Transpl. 2014;20:1293-5.
49. DiMartini A, Cruz RJ Jr, Dew MA, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19:1172-80.
50. Kremer WM, Nagel M, Reuter M, et al. Validation of the clinical frailty scale for the prediction of mortality in patients with liver cirrhosis. Clin Transl Gastroenterol. 2020;11:e00211.
51. Bhanji RA, Narayanan P, Moynagh MR, et al. Differing impact of sarcopenia and frailty in nonalcoholic steatohepatitis and alcoholic liver disease. Liver Transpl. 2019;25:14-24.
52. Lai JC, Rahimi RS, Verna EC, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology. 2019;156:1675-82.
53. Essam Behiry M, Mogawer S, Yamany A, et al. Ability of the short physical performance battery frailty index to predict mortality and hospital readmission in patients with liver cirrhosis. Int J Hepatol. 2019;2019:8092865.
54. Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62:584-90.
55. Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky performance status before and after liver transplantation predicts graft and patient survival. J Hepatol. 2018;69:818-25.
56. Lai JC, Covinsky KE, McCulloch CE, Feng S. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol. 2018;113:235-42.
57. Lai JC, Shui AM, Duarte-Rojo A, et al; from the Multi‐Center Functional Assessment in Liver Transplantation (FrAILT) Study. Frailty, mortality, and health care utilization after liver transplantation: from the multicenter functional assessment in liver transplantation (FrAILT) study. Hepatology. 2022;75:1471-9.
58. Kardashian A, Ge J, McCulloch CE, et al. Identifying an optimal liver frailty index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology. 2021;73:1132-9.
59. Lai JC, Dodge JL, Kappus MR, et al; Multi-Center Functional Assessment in Liver Transplantation (FrAILT) Study. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol. 2020;73:575-81.
60. Wang M, Shui AM, Ruck J, et al. Clinically relevant cut-points for changes in the liver frailty index are associated with waitlist mortality in patients with cirrhosis. Liver Transpl. 2024;30:991-1001.
61. Wang M, Chiou SH, Ganger D, et al. Liver transplantation provides survival benefit at all levels of frailty: from the multicenter functional assessment in liver transplantation study. Hepatology. 2025;81:1269-75.
62. Yamada S, Shimada M, Morine Y, et al. Significance of frailty in prognosis after hepatectomy for elderly patients with hepatocellular carcinoma. Ann Surg Oncol. 2021;28:439-46.
63. Okada T, Tanaka S, Shinkawa H, et al. Impact of frailty on long-term outcomes after liver resection for hepatocellular carcinoma in elderly patients: a prospective study. Asian J Surg. 2024;47:147-53.
64. Yang T, Liu DQ, Qiu W, et al. The barthel index predicts surgical textbook outcomes following hepatectomy for elderly patients with hepatocellular carcinoma: a multicenter cohort study. Am J Surg. 2024;237:115761.
65. Ramai D, Dang-Ho KP, Kewalramani A, et al. Hospital frailty risk score is independently associated with mortality and encephalopathy in hospitalized patients with hepatocellular carcinoma. Biomedicines. 2021;9:1693.
66. Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S147-62.
67. Guo Y, Ren Y, Zhu L, Yang L, Zheng C. Association between sarcopenia and clinical outcomes in patients with hepatocellular carcinoma: an updated meta-analysis. Sci Rep. 2023;13:934.
68. Beumer BR, van Vugt JLA, Sapisochin G, et al; Collaborators. Impact of muscle mass on survival of patients with hepatocellular carcinoma after liver transplantation beyond the Milan criteria. J Cachexia Sarcopenia Muscle. 2022;13:2373-82.
69. Cederholm T. Overlaps between frailty and sarcopenia definitions. Nestle Nutr Inst Workshop Ser. 2015;83:65-9.
70. Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31.
71. Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611-44.
72. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951-63.
73. Ii ER, Satapathy SK. Sarcopenia in the cirrhotic patient: current knowledge and future directions. J Clin Exp Hepatol. 2023;13:162-77.
74. Jiang C, Wang Y, Fu W, et al. Association between sarcopenia and prognosis of hepatocellular carcinoma: a systematic review and meta-analysis. Front Nutr. 2022;9:978110.
75. Liu J, Luo H, Huang L, Wang J. Prevalence of sarcopenia among patients with hepatocellular carcinoma: a systematic review and metaanalysis. Oncol Lett. 2023;26:283.
76. Nikolaou D. Sarcopenia and frailty: the detectable overlapping and the possible diagnostic approaches. JFSF. 2016;01:08-12.
77. Kichena S, Kamani A, Fricke B. Potential of prehabilitation in hepatocellular carcinoma: a narrative review of available evidence. Ann Palliat Med. 2024;13:101-11.
78. Stout NL, Santa Mina D, Lyons KD, Robb K, Silver JK. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J Clin. 2021;71:149-75.
79. Christopher CN, Kang DW, Wilson RL, et al. Exercise and nutrition interventions for prehabilitation in hepato-pancreato-biliary cancers: a narrative review. Nutrients. 2023;15:5044.
80. Marcantei C, Couret A, King J, Mazeaud S, Armand A, Ennequin G. Effects of exercise training on muscle mass and physical function in patients with hepatocellular carcinoma after diagnosis: a systematic review. Dig Dis Sci. 2024;69:2667-80.
81. Brustia R, Monsel A, Skurzak S, et al. Guidelines for perioperative care for liver transplantation: enhanced recovery after surgery (ERAS) recommendations. Transplantation. 2022;106:552-61.
82. Joliat GR, Kobayashi K, Hasegawa K, et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations 2022. World J Surg. 2023;47:11-34.
83. Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: clinical nutrition in cancer. Clin Nutr. 2021;40:2898-913.
84. Bischoff SC, Bernal W, Dasarathy S, et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. 2020;39:3533-62.
85. Toh EQ, Wong HPN, Wang JDJ, Liau MYQ, Tan YF, Shelat VG. Prehabilitation programs in liver resection: a narrative review. Chin Clin Oncol. 2024;13:9.
86. Jetten WD, Hogenbirk RNM, Van Meeteren NLU, Cuperus FJC, Klaase JM, De Jong R. Physical effects, safety and feasibility of prehabilitation in patients awaiting orthotopic liver transplantation, a systematic review. Transpl Int. 2022;35:10330.
87. Benmassaoud A, Martel M, Carli F, et al. Prehabilitation in patients awaiting liver transplantation. Transplant Rev. 2024;38:100835.
88. Loschi TM, Baccan MDTA, Della Guardia B, Martins PN, Boteon APCS, Boteon YL. Exercise training as an intervention for frailty in cirrhotic patients on the liver transplant waiting list: a systematic review. World J Hepatol. 2023;15:1153-63.
89. Lin FP, Visina JM, Bloomer PM, et al. Prehabilitation-driven changes in frailty metrics predict mortality in patients with advanced liver disease. Am J Gastroenterol. 2021;116:2105-17.
90. Hallsworth K, McCain MV, Fallen-Bailey R, Brown MC, Orange ST, Reeves HL. Is home-based, virtually delivered, group exercise feasible and acceptable for older patients with hepatocellular carcinoma? A non-randomised feasibility study (TELEX-Liver Cancer). BMJ Open. 2024;14:e082155.
91. Colosimo S, Bertoli S, Saffioti F. Use of branched-chain amino acids as a potential treatment for improving nutrition-related outcomes in advanced chronic liver disease. Nutrients. 2023;15:4190.
92. Ruiz-Margáin A, Román-Calleja BM, Moreno-Guillén P, et al. Nutritional therapy for hepatocellular carcinoma. World J Gastrointest Oncol. 2021;13:1440-52.
93. Espina S, Casas-Deza D, Bernal-Monterde V, Domper-Arnal MJ, García-Mateo S, Lué A. Evaluation and management of nutritional consequences of chronic liver diseases. Nutrients. 2023;15:3487.
94. Berardi G, Ratti F, Sposito C, et al. Model to predict major complications following liver resection for HCC in patients with metabolic syndrome. Hepatology. 2023;77:1527-39.
95. Plank LD, Gane EJ, Peng S, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557-66.
96. Zhang Y, Zhan L, Zhang L, Shi Q, Li L. Branched-chain amino acids in liver diseases: complexity and controversy. Nutrients. 2024;16:1875.
97. van Dijk AM, Bruins Slot AS, Portincasa P, et al. Systematic review with meta-analysis: branched-chain amino acid supplementation in liver disease. Eur J Clin Invest. 2023;53:e13909.
98. Sideris GA, Tsaramanidis S, Vyllioti AT, Njuguna N. The role of branched-chain amino acid supplementation in combination with locoregional treatments for hepatocellular carcinoma: systematic review and meta-analysis. Cancers. 2023;15:926.
99. Chen L, Chen Y, Wang X, et al. Efficacy and safety of oral branched-chain amino acid supplementation in patients undergoing interventions for hepatocellular carcinoma: a meta-analysis. Nutr J. 2015;14:67.
100. Yap KY, Chi H, Ng S, Ng DH, Shelat VG. Effect of perioperative branched chain amino acids supplementation in liver cancer patients undergoing surgical intervention: a systematic review. World J Gastrointest Surg. 2023;15:2596-618.
101. Wong CS, Praseedom R, Liau SS. Perioperative immunonutrition in hepatectomy: a systematic review and meta-analysis. Ann Hepatobiliary Pancreat Surg. 2020;24:396-414.
102. Gao B, Luo J, Liu Y, et al. Clinical efficacy of perioperative immunonutrition containing omega-3-fatty acids in patients undergoing hepatectomy: a systematic review and meta-analysis of randomized controlled trials. Ann Nutr Metab. 2020;76:375-86.
103. Zhang C, Lin J, Li F, et al. Effect of ω-3 polyunsaturated fatty acids on liver function and inflammatory reaction in patients undergoing hepatectomy: a systematic review and meta-analysis of randomized control trials. Expert Rev Gastroenterol Hepatol. 2019;13:375-84.
104. Lei Q, Wang X, Zheng H, Bi J, Tan S, Li N. Peri-operative immunonutrition in patients undergoing liver transplantation: a meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2015;24:583-90.
105. Plank LD, Mathur S, Gane EJ, et al. Perioperative immunonutrition in patients undergoing liver transplantation: a randomized double-blind trial. Hepatology. 2015;61:639-47.
106. Ss S, Pottakkat B. Effect of immunonutrition on the liver function status of end-stage liver disease patients waiting/referred for liver transplant: a randomized controlled trial. Cureus. 2023;15:e36923.
107. Graf J, Stengel A. Psychological burden and psycho-oncological interventions for patients with hepatobiliary cancers-a systematic review. Front Psychol. 2021;12:662777.
108. Li L, Zhang HZ, Ge Y, et al. The perioperative experience and needs of hepatocellular carcinoma patients in interventional therapy: a phenomenological qualitative study. Eur J Gastroenterol Hepatol. 2024;36:423-9.
109. Wang J, Yan C, Fu A. A randomized clinical trial of comprehensive education and care program compared to basic care for reducing anxiety and depression and improving quality of life and survival in patients with hepatocellular carcinoma who underwent surgery. Medicine. 2019;98:e17552.
110. Zhang X, Lai M, Wu D, Luo P, Fu S. The effect of 5A nursing intervention on living quality and self-care efficacy of patients undergoing chemotherapy after hepatocellular carcinoma surgery. Am J Transl Res. 2021;13:6638-45.
111. Grimmett C, Heneka N, Chambers S. Psychological interventions prior to cancer surgery: a review of reviews. Curr Anesthesiol Rep. 2022;12:78-87.
112. Delabays C, Demartines N, Joliat GR, Melloul E. Enhanced recovery after liver surgery in cirrhotic patients: a systematic review and meta-analysis. Perioper Med. 2024;13:24.
113. Noba L, Rodgers S, Chandler C, Balfour A, Hariharan D, Yip VS. Enhanced recovery after surgery (ERAS) reduces hospital costs and improve clinical outcomes in liver surgery: a systematic review and meta-analysis. J Gastrointest Surg. 2020;24:918-32.
114. Tinguely P, Morare N, Ramirez-Del Val A, et al. Enhanced recovery after surgery programs improve short-term outcomes after liver transplantation-a systematic review and meta-analysis. Clin Transplant. 2021;35:e14453.
115. Cillo U, Vitale A, Polacco M, Fasolo E. Liver transplantation for hepatocellular carcinoma through the lens of transplant benefit. Hepatology. 2017;65:1741-8.
116. Cillo U, Vitale A, Volk ML, et al. The survival benefit of liver transplantation in hepatocellular carcinoma patients. Dig Liver Dis. 2010;42:642-9.
117. European Association for the Study of the Liver. Electronic address: [email protected]. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-85.
118. Aduen JF, Sujay B, Dickson RC, et al. Outcomes after liver transplant in patients aged 70 years or older compared with those younger than 60 years. Mayo Clin Proc. 2009;84:973-8.
119. Su F, Yu L, Berry K, et al. Aging of liver transplant registrants and recipients: trends and impact on waitlist outcomes, post-transplantation outcomes, and transplant-related survival benefit. Gastroenterology. 2016;150:441-53.e6.
120. Gulla A, Jakiunaite I, Juchneviciute I, Dzemyda G. A narrative review: predicting liver transplant graft survival using artificial intelligence modeling. Front Transplant. 2024;3:1378378.
121. Romero-Cristóbal M, Díaz-Fontenla F, Fernández-Yunquera A, et al. Demographic trends in liver transplant survivors after 3 decades of program implementation: the impact of cohort and period effects on life expectancy. Transplant Direct. 2024;10:e1684.
122. Yong CM, Sharma M, Ochoa V, et al. Multivessel coronary artery disease predicts mortality, length of stay, and pressor requirements after liver transplantation. Liver Transpl. 2010;16:1242-8.
123. Martinez-Perez S, McCluskey SA, Davierwala PM, et al. Perioperative cardiovascular risk assessment and management in liver transplant recipients: a review of the literature merging guidelines and interventions. J Cardiothorac Vasc Anesth. 2024;38:1015-30.
124. Pagano G, Sastre L, Blasi A, et al. CACS, CCTA and mCAD-LT score in the pre-transplant assessment of coronary artery disease and the prediction of post-transplant cardiovascular events. Liver Int. 2024;44:1912-23.
125. Alvarez J, Mei X, Daily M, et al. Tipping the scales: liver transplant outcomes of the super obese. J Gastrointest Surg. 2016;20:1628-35.
126. Cullaro G, Verna EC, Lee BP, Lai JC. Chronic kidney disease in liver transplant candidates: a rising burden impacting post-liver transplant outcomes. Liver Transpl. 2020;26:498-506.
127. Soldera J, Corso LL, Rech MM, et al. Predicting major adverse cardiovascular events after orthotopic liver transplantation using a supervised machine learning model: a cohort study. World J Hepatol. 2024;16:193-210.
128. Abdelhameed A, Bhangu H, Feng J, et al. Deep learning-based prediction modeling of major adverse cardiovascular events after liver transplantation. Mayo Clin Proc Digit Health. 2024;2:221-30.
129. Mancuso A, Mazzola A, Cabibbo G, et al. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:324-30.
130. Bang K, Casadei-Gardini A, Yoo C, et al. Efficacy and safety of lenvatinib in patients with recurrent hepatocellular carcinoma after liver transplantation. Cancer Med. 2023;12:2572-9.
131. Cillo U, Carraro A, Avolio AW, et al; Italian Board of Experts in Liver Transplantation (I-BELT). Immunosuppression in liver transplant oncology: position paper of the Italian Board of Experts in Liver Transplantation (I-BELT). Updates Surg. 2024;76:725-41.
132. Kaibori M, Yoshii K, Yokota I, et al; Liver Cancer Study Group of Japan. Impact of advanced age on survival in patients undergoing resection of hepatocellular carcinoma: report of a Japanese nationwide survey. Ann Surg. 2019;269:692-9.
133. Tan LLY, Chew VTW, Syn N, et al. Effect of age on the short- and long-term outcomes of patients undergoing curative liver resection for HCC. Eur J Surg Oncol. 2022;48:1339-47.
134. Okinaga H, Yasunaga H, Hasegawa K, Fushimi K, Kokudo N. Short-term outcomes following hepatectomy in elderly patients with hepatocellular carcinoma: an analysis of 10,805 septuagenarians and 2,381 Octo- and Nonagenarians in Japan. Liver Cancer. 2018;7:55-64.
135. Lee JS, Park DA, Ryoo S, Park J, Choi GH, Yoo JJ. Efficacy and safety of surgical resection in elderly patients with hepatocellular carcinoma: a systematic review and meta-analysis. Gut Liver. 2024;18:695-708.
136. Lu Y, Liang L, Lu WF, et al. The prognosis of elderly patients with hepatocellular carcinoma after curative hepatectomy a multicenter competing risk analysis. Clin Res Hepatol Gastroenterol. 2023;47:102147.
137. Cauchy F, Zalinski S, Dokmak S, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg. 2013;100:113-21.
138. Viganò L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: a multicenter matched analysis with HCV-related HCC. J Hepatol. 2015;63:93-101.
139. Berardi G, Cucchetti A, Sposito C, et al. Recurrence and tumor-related death after resection of hepatocellular carcinoma in patients with metabolic syndrome. JHEP Rep. 2024;6:101075.
140. Molinari M, Kaltenmeier C, Samra PB, et al. Hepatic resection for hepatocellular carcinoma in nonalcoholic fatty liver disease: a systematic review and meta-analysis of 7226 patients. Ann Surg Open. 2021;2:e065.
141. Lanari J, Lupi A, Billato I, et al. Textbook outcome and nomogram-guided approaches for enhancing surgical success in elderly HCC patients: deciphering the influence of sarcopenia. Updates Surg. 2024;76:2645-54.
142. Polvieng T, Hongjinda S, Thienhiran A, Burasakarn P, Fuengfoo P. Effect of sarcopenia on the prognosis of clinical outcomes in patients with hepatocellular carcinoma after hepatic resection. Am Surg. 2024;90:1447-55.
143. Kobayashi A, Kaido T, Hamaguchi Y, et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg. 2019;269:924-31.
144. Giakoustidis A, Papakonstantinou M, Chatzikomnitsa P, et al. The effects of sarcopenia on overall survival and postoperative complications of patients undergoing hepatic resection for primary or metastatic liver cancer: a systematic review and meta-analysis. J Clin Med. 2024;13:3869.
145. Gani F, Cerullo M, Amini N, et al. Frailty as a risk predictor of morbidity and mortality following liver surgery. J Gastrointest Surg. 2017;21:822-30.
146. Tanaka S, Ueno M, Iida H, et al. Preoperative assessment of frailty predicts age-related events after hepatic resection: a prospective multicenter study. J Hepatobiliary Pancreat Sci. 2018;25:377-87.
147. Osei-Bordom D, Hall L, Hodson J, et al. Impact of frailty on short-term outcomes after laparoscopic and open hepatectomy. World J Surg. 2022;46:2444-53.
148. Voron T, Tselikas L, Pietrasz D, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2015;261:1173-83.
149. Berardi G, Antonelli G, Colasanti M, et al. Association of sarcopenia and body composition with short-term outcomes after liver resection for malignant tumors. JAMA Surg. 2020;155:e203336.
150. Marasco G, Dajti E, Serenari M, et al. Sarcopenia predicts major complications after resection for primary hepatocellular carcinoma in compensated cirrhosis. Cancers. 2022;14:1935.
151. Snowden CP, Prentis JM, Anderson HL, et al. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251:535-41.
152. Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin. 2015;33:17-33.
153. Yu Q, Liu C, Navuluri R, Ahmed O. Percutaneous microwave ablation versus radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Abdom Radiol. 2021;46:4467-75.
154. South E, Wade R, Anwer S, et al. The effectiveness of ablative and non-surgical therapies for early hepatocellular carcinoma: systematic review and network meta-analysis of randomised controlled trials. Cancer Med. 2023;12:20759-72.
155. Cillo U, Bertacco A, Fasolo E, et al. Videolaparoscopic microwave ablation in patients with HCC at a European high-volume center: results of 815 procedures. J Surg Oncol. 2019;120:956-65.
156. Cillo U, Noaro G, Vitale A, et al; HePaTIC Study Group. Laparoscopic microwave ablation in patients with hepatocellular carcinoma: a prospective cohort study. HPB. 2014;16:979-86.
157. Gerges FJ, Kanazi GE, Jabbour-Khoury SI. Anesthesia for laparoscopy: a review. J Clin Anesth. 2006;18:67-78.
158. Hoffmann R, Rempp H, Schmidt D, Pereira PL, Claussen CD, Clasen S. Prolonged antibiotic prophylaxis in patients with bilioenteric anastomosis undergoing percutaneous radiofrequency ablation. J Vasc Interv Radiol. 2012;23:545-51.
159. Skonieczki BD, Wells C, Wasser EJ, Dupuy DE. Radiofrequency and microwave tumor ablation in patients with implanted cardiac devices: is it safe? Eur J Radiol. 2011;79:343-6.
160. Ibrahim SM, Lewandowski RJ, Sato KT, et al. Radioembolization for the treatment of unresectable hepatocellular carcinoma: a clinical review. World J Gastroenterol. 2008;14:1664-9.
161. Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol. 2022;40:468-80.
162. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5:953-60.
163. Fründt TW, Casar C, von Felden J, et al. Equal efficacy and safety profile in elderly patients with hepatocellular carcinoma receiving palliative treatment. Cancers. 2022;14:768.
164. Tang Q, Huang W, Liang J, Xue J. Efficacy and safety of transarterial chemoembolization in elderly patients of advanced hepatocellular carcinoma with portal vein tumor thrombus: a retrospective study. Front Oncol. 2021;11:646410.
165. Sun Z, Jiao D, Fang Y, et al. Efficacy and safety of raltitrexed-eluting CalliSpheres® bead transarterial chemoembolization in patients with intermediate-stage hepatocellular carcinoma: a single-arm, prospective study. Ther Adv Med Oncol. 2024;16:17588359241229661.
166. Schindler P, Kaldewey D, Rennebaum F, et al. Safety, efficacy, and survival of different transarterial chemoembolization techniques in the management of unresectable hepatocellular carcinoma: a comparative single-center analysis. J Cancer Res Clin Oncol. 2024;150:235.
167. Zhang L, Hong W, Wang Z, Zheng C, Liang B, Shi H. Safety and effectiveness of transarterial chemoembolization in hepatocellular carcinoma patients aged greater versus less than 80 years. Clin Interv Aging. 2023;18:1883-92.
168. Lin WC, Chang CW, Chang CW, Wang TE, Chen MJ, Wang HY. Challenges of transarterial therapy for hepatocellular carcinoma in patients with chronic kidney disease. Medicine. 2019;98:e17007.
169. Hsu CY, Huang YH, Su CW, et al. Transarterial chemoembolization in patients with hepatocellular carcinoma and renal insufficiency. J Clin Gastroenterol. 2010;44:e171-7.
170. Yeh H, Chiang CC, Yen TH. Hepatocellular carcinoma in patients with renal dysfunction: pathophysiology, prognosis, and treatment challenges. World J Gastroenterol. 2021;27:4104-42.
171. Giannini EG, Aglitti A, Borzio M, et al; Associazione Italiana per lo Studio del Fegato (AISF) HCC Special Interest Group. Overview of immune checkpoint inhibitors therapy for hepatocellular carcinoma, and the ITA.LI.CA cohort derived estimate of amenability rate to immune checkpoint inhibitors in clinical practice. Cancers. 2019;11:1689.
172. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38:4317-45.
173. Vogel A, Martinelli E; ESMO Guidelines Committee. Electronic address: [email protected]; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32:801-5.
174. Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75:960-74.
175. Cabibbo G, Daniele B, Borzio M, et al. Multidisciplinary treatment of hepatocellular carcinoma in 2023: Italian practice Treatment Guidelines of the Italian Association for the Study of the Liver (AISF), Italian Association of Medical Oncology (AIOM), Italian Association of Hepato-Bilio-Pancreatic Surgery (AICEP), Italian Association of Hospital Gastroenterologists (AIGO), Italian Association of Radiology and Clinical Oncology (AIRO), Italian Society of Pathological Anatomy and Diagnostic Cytology (SIAPeC-IAP), Italian Society of Surgery (SIC), Italian Society of Gastroenterology (SIGE), Italian Society of Medical and Interventional Radiology (SIRM), Italian Organ Transplant Society (SITO), and Association of Patients with Hepatitis and Liver Disease (EpaC) - Part II - Non-surgical treatments. Dig Liver Dis. 2024;56:394-405.
176. Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65:1140-7.
177. Vithayathil M, D’Alessio A, Fulgenzi CAM, et al. Impact of older age in patients receiving atezolizumab and bevacizumab for hepatocellular carcinoma. Liver Int. 2022;42:2538-47.
178. Hajiev S, Allara E, Motedayеn Aval L, et al. Impact of age on sorafenib outcomes in hepatocellular carcinoma: an international cohort study. Br J Cancer. 2021;124:407-13.
179. Di Costanzo GG, Tortora R, De Luca M, et al. Impact of age on toxicity and efficacy of sorafenib-targeted therapy in cirrhotic patients with hepatocellular carcinoma. Med Oncol. 2013;30:446.
180. Li D, Toh HC, Merle P, et al. Atezolizumab plus bevacizumab versus sorafenib for unresectable hepatocellular carcinoma: results from older adults enrolled in the imbrave150 randomized clinical trial. Liver Cancer. 2022;11:558-71.
181. Alkadimi MA, Aldawoodi TA, Lucero KT, et al. Clinical characteristics, treatment patterns, and outcomes of patients with locally advanced/metastatic hepatocellular carcinoma treated at the veterans health administration. Oncologist. 2024;29:369-76.
182. Song BG, Goh MJ, Kang W, et al. Analysis of factors predicting the real-world efficacy of atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma. Gut Liver. 2024;18:709-18.
183. Carballo-Folgoso L, Álvarez-Velasco R, Lorca R, et al. Evaluation of cardiovascular events in patients with hepatocellular carcinoma treated with sorafenib in the clinical practice. The CARDIO-SOR study. Liver Int. 2021;41:2200-11.
184. Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: Improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20-8.
185. Nishida N, Kudo M. Liver damage related to immune checkpoint inhibitors. Hepatol Int. 2019;13:248-52.
186. Leonardi GC, Gainor JF, Altan M, et al. Safety of programmed death-1 pathway inhibitors among patients with non-small-cell lung cancer and preexisting autoimmune disorders. J Clin Oncol. 2018;36:1905-12.
187. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904-11.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data






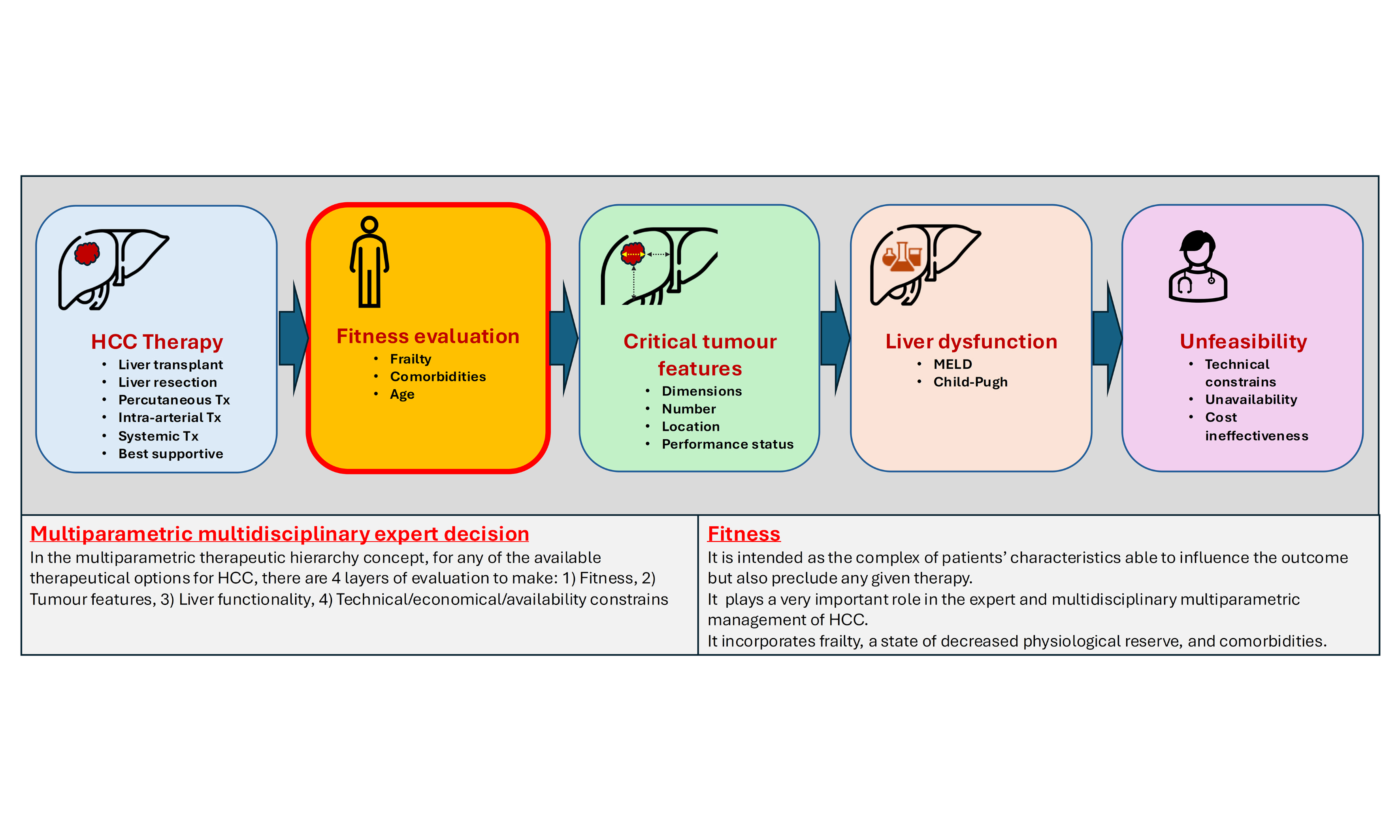
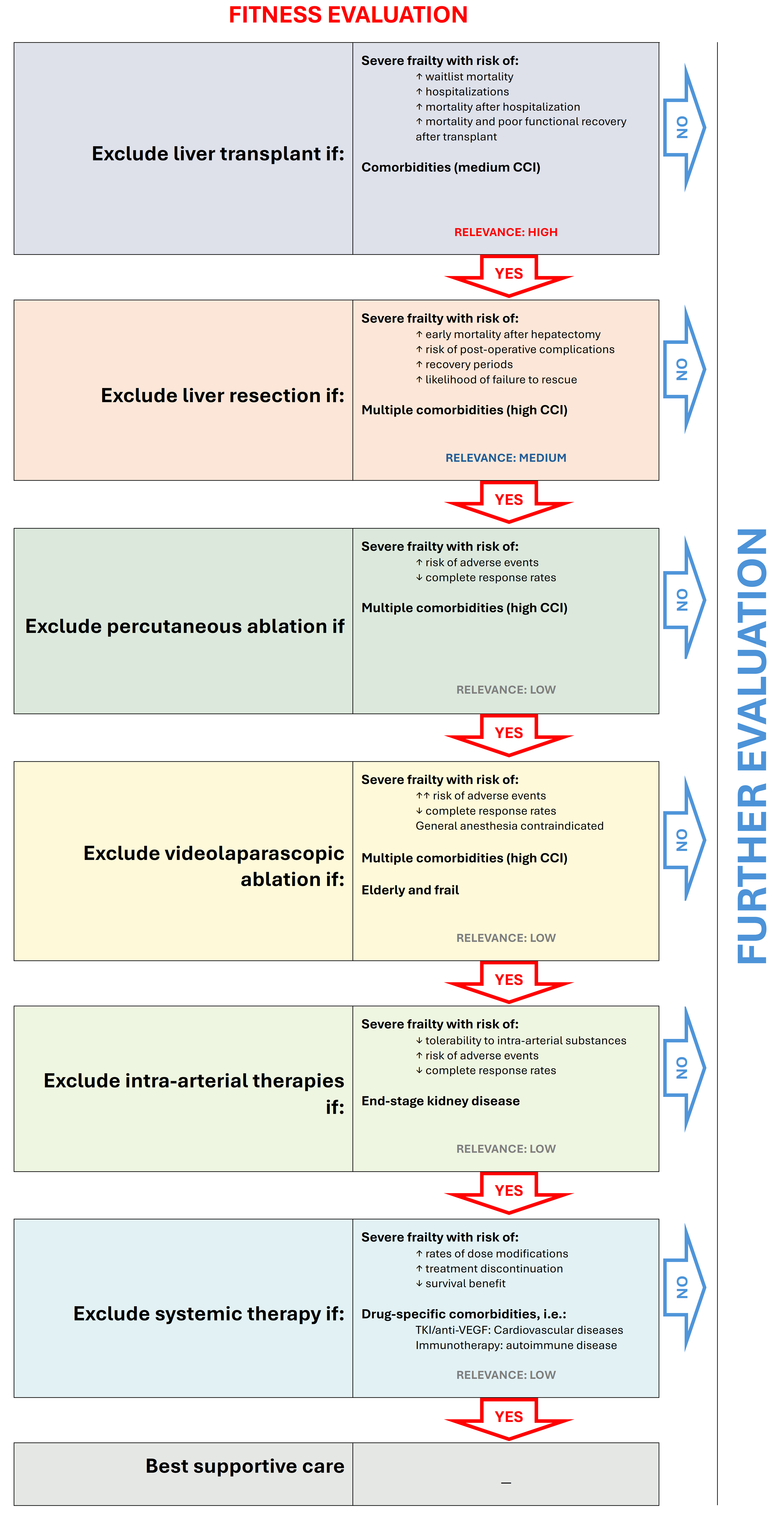










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].