Volume 4 (2017) – 41 articles
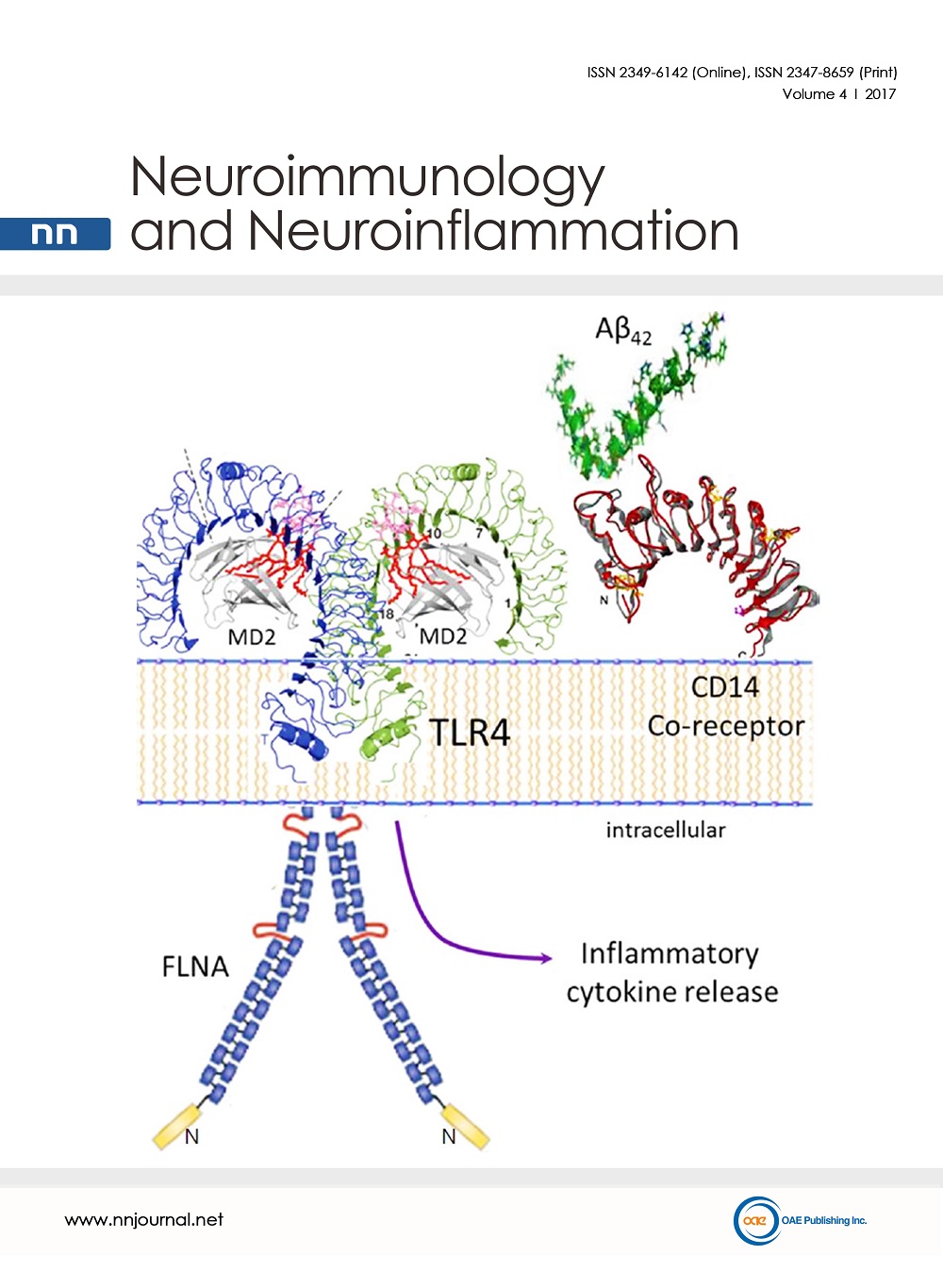
Cover Picture: Alzheimer’s disease (AD) is a neurodegenerative disease with proteopathy characterized by abnormalities in amyloid beta (Aβ) and tau proteins. Defective amyloid and tau propagate and aggregate, leading to eventual amyloid plaques and neurofibrillary tangles. New data show that a third proteopathy, an altered conformation of the scaffolding protein filamin A (FLNA), is critically linked to the amyloid and tau pathologies in AD. Altered FLNA is pervasive in AD brain and without apparent aggregation. In a striking interdependence, altered FLNA is both induced by Aβ and required for two prominent pathogenic signaling pathways of Aβ. Aβ monomers or small oligomers signal via the α7 nicotinic acetylcholine receptor (α7nAChR) to activate kinases that hyperphosphorylate tau to cause neurofibrillary lesions and formation of neurofibrillary tangles. Altered FLNA also enables a persistent activation of toll-like-receptor 4 (TLR4) by Aβ, leading to excessive inflammatory cytokine release and neuroinflammation. The novel AD therapeutic candidate PTI-125 binds and reverses the altered FLNA conformation to prevent Aβ’s signaling via α7nAChR and aberrant activation of TLR4, thus reducing multiple AD-related neuropathologies. As a regulator of Aβ’s signaling via α7nAChR and TLR4, altered FLNA represents a novel AD therapeutic target.
view this paper
Read Online Viewed:
Download This Volume Viewed:





