Concentrations and human exposure to hexabromocyclododecane and tetrabromobisphenol A from the indoor environment in Bangkok metropolitan area, Thailand
Abstract
Aim: This study investigated hexabromocyclododecane (HBCDD) and tetrabromobisphenol A (TBBPA) concentrations in indoor dust from houses, offices, and cars and estimated toddler and adult exposure to HBCDD and TBBPA through dust ingestion.
Methods: The concentrations of HBCDD and TBBPA were measured in 47 indoor dust samples collected from the Bangkok metropolitan area, Thailand. All samples were analyzed for HBCDD and TBBPA using LC-MS/MS. The estimated daily intake (EDI) through dust ingestion was calculated from the median and 95th percentile concentrations of HBCDD and TBBPA.
Results: HBCDD was detected in 47% of samples, and TBBPA was detected in all samples. The median concentrations of HBCDD were 6.7 ng g-1, <0.7 ng g-1, and <0.7 ng g-1 in cars, houses, and offices, respectively. The isomer composition of ∑HBCDD in dust was: α-HBCDD (40%-54%), γ-HBCDD (19%-40%), and β-HBCDD (17%-28%). In contrast, TBBPA was observed at higher concentrations, with median values of 674, 67, and 22 ng g-1 in offices, houses, and cars, respectively. Under a median exposure scenario, toddlers were exposed to 0.05 ng kg-1 bw day-1 for HBCDD and 0.25 ng kg-1 bw day-1 for TBBPA, with adults exposed to 0.01 and 0.06 ng kg-1 bw day-1 for HBCDD and TBBPA respectively.
Conclusion: Concentrations of HBCDD in dust from Thai cars, homes, and offices are lower than those of TBBPA following the listing of HBCDD in the Stockholm Convention on Persistent Organic Pollutants and limited use of HBCDD in Thailand in applications such as building insulation foam. Concentrations of TBBPA in office dust significantly exceeded (P < 0.05) those in house and car dust owing to the greater number of electronic appliances and poor natural ventilation in offices. EDIs for Thai toddlers exceeded those of adults under both median and high-end exposure scenarios. However, EDIs of HBCDD and TBBPA for the general Thai population were below the corresponding oral reference dose guidelines.
Keywords
INTRODUCTION
Indoor environments are prominent sources of chemicals that contribute significantly to total human exposure because people spend most of their day indoors. Indoor dust is of great interest because it is omnipresent and acts as a repository for chemicals[1], such as hexabromocyclododecane (HBCDD) and tetrabromobisphenol A (TBBPA). Thus, humans can be exposed to indoor pollutants[2,3], which may cause significant adverse impacts on human health[4]. HBCDD is a brominated flame retardant (BFR) that is primarily used in building insulation material made from expanded and extruded polystyrene foam, as well as a back-coating for synthetic fabrics used as covers for sofas, chairs, etc. Minor uses of HBCDD are in high-impact polystyrene (HIPS) used in electrical and electronic appliances[5-7]. In addition to displaying environmental persistence and bioaccumulation potential, HBCDD can disrupt the thyroid and reproductive systems and affect the nervous and reproductive systems[3,8-9] and has thus been listed under Annex A of the Stockholm Convention on persistent organic pollutants since 2013[10].
TBBPA is predominantly used as a reactive flame retardant in resins for application on printed circuit boards. However, it is used - albeit to a relatively minor extent - as an additive flame retardant in acrylonitrile butadiene styrene (ABS) and high-impact polystyrene. As additive FRs are not covalently bound to polymer components, they can be emitted more readily than reactive FRs from products and contaminate various environmental media[11-14]. This is of potential concern, as adverse effects of exposure to TBBPA include the disruption of the endocrine and thyroid hormone systems and neurotoxicity[6,14]. HBCDD and TBBPA are released into the environment during initial manufacturing and migration from consumer products and building materials, as well as during their recycling and disposal[11,15,16]. HBCDD and TBBPA are of increasing concern because of their chemical properties, such as low water solubility and low vapor pressure. Thus, they are persistent, transported long distances in the environment, highly toxic, and bioaccumulative[11,17].
Indoor dust is a significant source of human exposure to HBCDD and TBBPA, especially for toddlers and children, since dust associated with indoor pollutants can enter the body through ingestion from hand-to-mouth behavior[6-8,18-20]. Moreover, indoor pollution is important for human health since most people spend most of their time indoors[2]. Thus, toddlers or young children are more likely to be exposed to these substances through dust ingestion than adults[3,6,8]. Previous studies have revealed the presence of both HBCDD and TBBPA in indoor dust from several countries. House dust in the UK was contaminated at a concentration of 570,000 ng g-1 of HBCDD[21] and offices in France were contaminated at a concentration of 10,188 ng g-1[5]. TBBPA was present at 7260 ng g-1 in houses in the USA[22] and 7951 ng g-1 in Korean offices[6]. Notwithstanding these findings, there are, to our knowledge, few data on this issue in Southeast Asia and no data on concentrations of HBCDD and TBBPA in house dust from Thailand. Therefore, this article reports concentrations of HBCDD and TBBPA in floor dust collected from houses, offices, and cars in Bangkok. These concentrations are compared with those reported previously for other countries and used to evaluate human exposure to HBCDD and TBBPA through dust ingestion for Thai toddlers and adults.
EXPERIMENTAL
Chemicals and reagents
Solvents used for sample extraction and clean-up processes and analysis (e.g., hexane, dichloromethane, and methanol) were all HPLC grade chemicals purchased from Merck (Darmstadt, Germany). Concentrated sulfuric acid (98% purity) was obtained from Merck (Darmstadt, Germany). Standard solutions of α-, β-, and γ-HBCDD and TBBPA and isotopically labeled HBCDD, including 13C12-α-HBCDD, 13C12-β-HBCDD,
Sample collection
Dust samples (n = 47) were collected between November 2020 and January 2021 from the Bangkok metropolitan area in Thailand. Samples were collected from three categories of indoor microenvironments: houses (n = 20), offices (n = 5), and cars (n = 22). The house and office dust sampling procedure was conducted according to previously reported protocols[5,17,21,23]. Briefly, in living rooms and offices, for floor dust, 1 m2 of carpeted flooring was vacuumed for 2 min, while, for bare floors, 4 m2 was sampled for 4 min. The sampling procedure in cars was based on a previous study[5]. Car dust samples were collected from private cars with an engine of 1550 cc -3000 cc. Most of the cars were Asian brands, and the age of the car ranged from 1 month to 20 years (average age of the car, 8 years ± 6 years). Dust samples were vacuumed from the surface of the seat and dashboard for 2 min. All samples were collected using a nylon sock with a 25 μm pore size inserted into the nozzle of a portable vacuum cleaner tube. After each sampling, the sock was closed, wrapped with aluminum foil, and sealed in a plastic Ziplock bag. Samples were then placed in a clean glass container box and transported in a cooler with ice to the laboratory. The vacuum cleaner tube was cleaned thoroughly with water and an isopropanol-impregnated disposable wipe to prevent contamination. In the laboratory, each collected dust sample was passed through a pre-cleaned 250 µm mesh to remove coarse particles, packed in clean aluminum foil, sealed in plastic Ziplock bags, and stored at -20 °C until analysis.
Sample extraction and clean-up
Dust samples were extracted using a previously described method[17,21,23]. Before extraction, approximately 0.1 g of the sieved dust sample was spiked with 25 ng of each internal (or surrogate) standard (13C12-α-HBCDD, 13C12-β-HBCDD, 13C12-γ-HBCDD, and 13C12-TBBPA). Then, 7 mL of hexane:dichloromethane mixture (1:1, v/v) was added to each sample. The sample was vortexed for 5 min and sonicated in an ultrasonic bath at 20 °C for 30 min. The resultant extracts were centrifuged for 5 min at 3500 rpm to separate the supernatant and residual sample. The extraction process was repeated thrice. The combined supernatants were reduced to approximately 0.5 mL under a gentle nitrogen gas flow and reconstituted with 1 mL of hexane. The sample extract was then treated with concentrated sulfuric acid. The extracts were purified for the clean-up process using an SPE cartridge packed with 4 g of pre-cleaned acidified silica (44% concentrated sulfuric acid, w/w). Then, 3 mL of hexane:dichloromethane mixture (1:1, v/v) was used to pre-condition the cartridge and then discarded. After that, the extract (1 mL) was loaded onto cartridges with 2 mL × 1 mL hexane rinses. Cartridges were eluted with 25 mL of hexane:dichloromethane mixture (1:1, v/v). After the clean-up process, eluates were evaporated using a stream of nitrogen and reconstituted in 200 µL of methanol containing 25 pg/µL d18-γ-HBCDD as a recovery determination (or syringe) standard for LC-MS/MS analysis.
Instrumental analysis
Instrumental analysis was performed using an Agilent 1200SL HPLC system coupled with an Agilent 6400 tandem mass spectrometer. HBCDD isomers (α-, β-, and γ-HBCDD) and TBBPA were separated on an Agilent Pursuit XRS3 C18 reversed-phase analytical column (150 mm × 2.0 mm i.d., 3 μm particle size) maintained at 40 °C. The mobile phase of (A) 1:1 methanol/water and (B) methanol at a flow rate of 0.15 mL/min were applied to elute the target compound and 10 µL of each sample was injected. Mass spectrometry was performed in electrospray ionization negative mode. Nebulizer pressure was set at 50 psi and capillary voltage at 3500 V. The drying gas (nitrogen) was used at a flow rate of 10 L/min and set to 300 °C. MRM mode was used based on m/z 640.4-78.8 and m/z 652.4-79 for native and 13C-HBCDD labeled diastereomers, respectively. HBCDD isomers were baseline separated with retention times of 14.0, 14.6, and 15.0 min for α-, β-, and γ-HBCDD, respectively. For TBBPA, the quantitative determination of MRM was m/z 540.8-78.8 for native and m/z 552.8-78.8 for 13C-TBBPA. The retention time of TBBPA was 11.1 min.
Quality assurance/quality control
All glassware was cleaned, rinsed with distilled water, rinsed with solvent, and then oven-dried for 5 h prior to use. For every batch of ten samples, one laboratory procedural blank was used to check for contamination during extraction and purification. In addition, field blanks (n = 5) were also analyzed. These were obtained by spreading anhydrous sodium sulfate on pre-cleaned clean floors, vacuuming, and passing through all analytical processes as per real samples. The target substances were not detected in any procedural or field blanks. For further quality assurance, the certified reference material SRM 2585 (organics in indoor dust; n = 5) was analyzed to evaluate the accuracy and precision of our analytical procedure. The measured value ranged 80%-123% of the certified values with relative standard deviation values below 15%. The limits of quantification (LOQs) of each compound were determined using a signal-to-noise ratio (S/N) of 10:1. LOQ values were 0.7 ng g-1 and 0.1 ng g-1 for HBCDD and TBBPA, respectively. The recovery of internal standards added to dust samples ranged 76%-120%, with a mean value of 98% ± 13% for HBCDD. The TBBPA recovery value ranged 70%-122%, with a mean value of 89% ± 16%.
Estimation of daily exposure
Estimated daily intake (EDI, ng kg-1 bw day-1) values for HBCDD and TBBPA through dust ingestion for toddlers and adults were evaluated under the median and high-end exposure scenarios. EDI values were calculated using Equations (1) and (2)[11,15,19]:
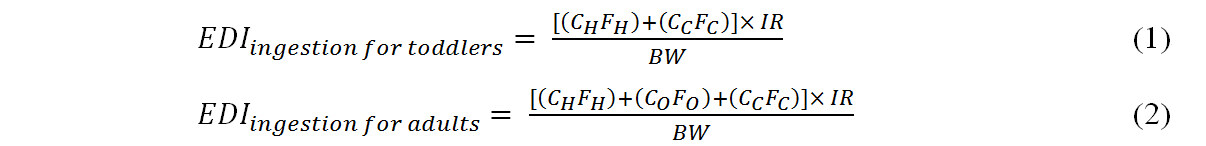
where CH, CO, and CC are the concentrations of HBCDD and TBBPA (ng g-1) in houses, offices, and cars, respectively. FH, FO, and FC are the exposure fractions (fraction of time spent in houses, offices, and cars), and IR is the dust ingestion rate (g day-1). BW is the body weight (kg) assumed for toddlers (12 kg)[24-26] and adults (63 kg)[8,11,18]. We assumed 100% absorption of HBCDD and TBBPA. The median and high dust ingestion rates assumed were 0.05 g day-1 and 0.2 g day-1 for toddlers and 0.02 g day-1 and 0.05 g day-1 for adults, respectively[5,16,19,27]. The exposure fraction assumed for toddlers was 86.1% of their time spent at home and 4.1% in cars. The figures for adults were 63.8% at home, 22.3% in offices, and 4.1% in cars[5,19,20].
Statistical analyses
Descriptive statistics (minimum, maximum, mean, median, and standard deviation) were calculated using Microsoft Office Excel 2016. Statistical comparisons were performed using IBM SPSS version 21. The distribution of concentrations of both TBBPA and HBCDD in the dust samples was evaluated for normal distribution using the Shapiro-Wilk test. This showed that sample concentrations did not display normal distribution. Therefore, concentrations were log-transformed. After log-transformation, TBBPA data were normally distributed, and the differences in TBBPA concentrations among our three indoor environment categories were evaluated using one-way ANOVA of log-transformed data. However, even after log-transformation, concentrations of HBCDD in house, office, and car dust remained not normally distributed. Therefore, the non-parametric Kruskal-Wallis test was used to evaluate any significant differences in concentrations of HBCDD among car, house, and office dust. Statistical significance was set at P < 0.05. When conducting statistical analysis, we assumed a zero concentration where a given contaminant in a sample was below the LOQ.
RESULTS
Concentrations of HBCDD and TBBPA
HBCDD and TBBPA were measured in floor dust samples collected from three categories of indoor environment: houses, offices, and cars. HBCDD was detected in 22 of 47 samples, with detection frequencies of 17%, 40%, and 77% in houses, offices, and cars, respectively. Concentrations of ∑HBCDD (the sum of α-, β-, and γ-HBCDD isomers) in dust samples ranged from < 0.7 ng g-1 to 215 ng g-1 (median 6.7 ng g-1) in cars, < 0.7 ng g-1 to 38 ng g-1 (median < 0.7 ng g-1) in houses, and < 0.7 ng g-1 to 21 ng g-1 (median < 0.7 ng g-1) in offices, as shown in Figure 1A. Concentrations were not significantly different among the three microenvironment categories (P = 0.658). For the diastereomers of HBCDD, our study showed that the average isomer composition of ∑HBCDD in house dust samples was α-HBCDD (42%), β-HBCDD (18%), and γ-HBCDD (40%). For office dust, the distribution was 52%, 20%, and 28% for α-, β-, and γ-HBCDD, respectively. In car dust, the distribution was α-HBCDD (54%), β-HBCDD (27%), and γ-HBCDD (19%) [Figure 2].
Figure 1. Box plot of (A) HBCDD and (B) TBBPA concentrations in different indoor environments. The lower and upper boundaries of the boxes are the 25th and 75th percentiles. Error bars represent the minimum and maximum values. HBCDD: Hexabromocyclododecane; TBBPA: tetrabromobisphenol A.
Figure 2. Relative percent contribution of individual HBCDD isomers to the average concentration of ∑HBCDD in indoor dust from houses, offices, and cars in the Bangkok metropolitan area, Thailand. HBCDD: Hexabromocyclododecane.
TBBPA was detected at concentrations above the LOQ in all samples. Median concentrations were 22 ng g-1 (4-242 ng g-1), 67 ng g-1 (11-230 ng g-1), and 674 ng g-1 (50-5319 ng g-1) in car, house, and office dust, respectively [Figure 1B], with the concentration in office dust significantly exceeding those detected in house and car dust (P = 0.000).
Estimated daily intake of HBCDD and TBBPA
EDI values of HBCDD and TBBPA through dust ingestion for toddlers and adults living in the Bangkok metropolitan area, Thailand, were calculated based on the concentrations of these substances in house, office, and car dust. EDI values were calculated using both median and high-end exposure scenarios, as explained in the experimental section, based on ingestion of house and car dust for toddlers and house, car, and office dust for adults. Consistent with other studies elsewhere, these calculations revealed that toddlers had much higher EDIs of HBCDD and TBBPA than adults. The estimated median exposure to HBCDD was 0.05 ng kg-1∙bw∙day-1 for toddlers and 0.01 ng∙kg-1∙bw∙day-1 for adults [Figure 3A], while, under the high-end exposure scenario, toddlers were exposed to 16 times more HBCDD. For TBBPA, the median EDI for toddlers was four times higher than for adults. High-end EDIs were 1.14 ng kg-1 bw day-1 and 0.89 ng kg-1 bw day-1 for toddlers and adults, respectively [Figure 3B].
DISCUSSION
Concentrations of HBCDD and TBBPA in indoor dust
The concentrations of HBCDD and TBBPA detected in dust collected from houses, offices, and cars in studies reported previously for several countries are shown in Table 1. Generally, the median concentration of HBCDD in dust was highest in cars, followed by offices and houses (e.g., in France)[5]. However, in some countries, HBCDD concentrations were highest in office dust[1,6]. Of note is that the median HBCDD concentration of car dust in our study was lower than that reported for other countries. Median concentrations of HBCDD in car dust from the UK[17], France[5], Kazakhstan[5], and Korea[6] were 13,000, 4539, 2065, and 297 ng∙g-1, respectively, compared to 6.7 ng g-1 in our study. Overall, concentrations of HBCDD in our study of car, house, and office dust in Thailand were among the lowest globally[5,6,16,21-22,28-29]. This is likely because HBCDD usage in Thailand is reported to be low[30]. In general, the main diastereomer in HBCDD commercial mixtures is γ-HBCDD with smaller amounts of α-HBCDD and β-HBCDD[31]. However, our study showed that the composition of ∑HBCDD in Thai dust was α-HBCDD (40%-54%), followed by γ-HBCDD (19%-40%) and β-HBCDD (17%-28%). This HBCDD isomer profile is similar to that previously reported in indoor dust from other countries. Previous studies have highlighted the photolytically induced conversion of γ-HBCDD to α-HBCDD in dust samples[5-7,18,19,31].
Median concentrations (ng g-1) of HBCDD and TBBPA from houses, offices, and cars in different countries
| Sample type | Location | Sampling year | Sample size (n) | α-HBCDD | β-HBCDD | γ-HBCDD | ΣHBCDD (Range) | TBBPA (Range) | Ref. |
| House dust | Thailand | 2019 and 2020 | 20 | < LOQ | < LOQ | < LOQ | < LOQ (< LOQ-38) | 67 (11-230) | This study |
| Spain | 2016 and 2017 | 10 | 74.6 | 19.6 | 34.9 | 129 (12-1321) | - | Corsolini et al.[16], 2021 | |
| UK | 2019 | 14 | 130 | 66 | 72 | 280 (76-570,000) | 35 (< 0.5-71) | Drage et al.[21], 2020 | |
| Korea | 2011 | 42 | 144 | 12 | 60 | 278 (< LOD-3,132) | 69 (< LOD-2,092) | Kweon et al.[29], 2018 | |
| China | 2014 | 20 | - | - | - | 34 (11-165) | 5 (3-42) | Sun et al.[38], 2018 | |
| China | 2014 | 30 | 64 | 21 | 64 | 156 (74-995) | 20 (7-113) | Wang et al.[7], 2018 | |
| USA | 2013 | 10 | 52 | 39 | 74 | 326 (104-636) | 187 (0-7,260) | Allgood et al.[22], 2017 | |
| China | Not recorded (NR) | 15 | - | - | - | 0.20 (0.08-1.4) | - | Peng et al.[19], 2017 | |
| Korea | 2009 and 2016 | 46 | 43 | 6 | 46 | 106 (19-2645) | 79 (14-1212) | Barghi | |
| Turkey | 2012 | 10 | - | - | - | 251 (50-8800) | - | Kurt-Karakus | |
| South Africa | 2012 | 7 | - | - | - | - | 120 (< 0.35-3767) | Abafe and Martincigh[11], 2016 | |
| France | 2014 | 9 | 559 | 144 | 422 | 1125 (363-1865) | 44 (7-165) | Abdallah et al.[5], 2016 | |
| Kazakhstan | 2014 | 10 | 78 | 20 | 189 | 287 (112-450) | 13 (< 0.06-83) | Abdallah et al.[5], 2016 | |
| Nigeria | 2014 | 10 | 199 | 81 | 125 | 405 (41-1863) | 50 (19-127) | Abdallah et al.[5], 2016 | |
| Egypt | 2013 | 17 | - | - | - | - | 6.2 (1.4-153) | Hassan and Shoeib[15], 2015 | |
| USA | 2012 | 30 | 7.9 | 27.8 | 70 | 338 (78-2528) | 7.9 (< 0.2-245) | Stapleton et al.[28], 2014 | |
| Germany | 2013 | 20 | 180 | 35 | 114 | 345 (53-4041) | 28 (2.9-233) | Fromme et al.[8], 2014 | |
| USA | 2011 | 16 | 62 | 16 | 73 | 160 (39-1800) | 200 (22-2000) | Dodson et al.[32], 2012 | |
| New Zealand | NR | 34 | 99 | 12 | 96 | 190 (20-4100) | - | Ali et al.[35], 2012 | |
| UK | 2006 and 2007 | 45 | 380 | 93 | 670 | 1300 (140-140,000) | 62 (< MQL-382) | Abdallah et al.[17], 2008 | |
| Office | Thailand | 2019 and 2020 | 5 | < LOQ | < LOQ | < LOQ | < LOQ (< LOQ-21) | 674 (50-5319) | This study |
| China | 2014 | 20 | - | - | - | 59 (18-224) | 9 (2-14) | Sun et al.[38], 2018 | |
| China | 2014 | 27 | 103 | 35 | 258 | 40 (8-171) | 40 (8-171) | Wang et al.[7], 2018 | |
| Korea | 2009 and 2016 | 18 | 137 | 34 | 294 | 496 (117-2519) | 464 (138-7951) | Barghi et al.[6], 2017 | |
| Turkey | 2012 | 9 | - | - | - | 424 (0.25-94,000) | - | Kurt-Karakus | |
| South Africa | 2012 | 7 | - | - | - | - | 492 (< 1.15-2063) | Abafe and Martincigh[11], 2016 | |
| France | 2014 | 11 | 2722 | 442 | 1329 | 4493 (1069-10188) | 79 (32-1255) | Abdallah et al.[5], 2016 | |
| Kazakhstan | 2014 | 10 | 106 | 32 | 123 | 261 (195-440) | 4 (< 0.06-30) | Abdallah et al.[5], 2016 | |
| Nigeria | 2014 | 10 | 76 | 28 | 55 | 159 (62-943) | 30 (< 0.06-149) | Abdallah et al.[5], 2016 | |
| UK | 2006 and 2007 | 28 | 220 | 84 | 470 | 760 (90-6600) | 36 (< 0.05-140) | Abdallah et al.[17], 2008 | |
| Cars | Thailand | 2019 and 2020 | 22 | 2.68 | 0.88 | 2.09 | 6.7 (< LOQ-215) | 22 (4-242) | This study |
| Korea | 2009 and 2016 | 19 | 185 | 21.41 | 98.98 | 297 (58-4172) | 81 (46-651) | Barghi et al.[6], 2017 | |
| Greece | NR | 30 | 90.3 | 15.8 | 46.4 | 155 (< LOQ-1745) | < LOQ (< LOQ-1064) | Besis et al.[18], 2017 | |
| China | NR | 15 | - | - | - | 0.1 (0.05-0.3) | - | Peng et al.[19], 2017 | |
| France | 2014 | 7 | 2221 | 629 | 1689 | 4539 (1458-7900) | 47 (9-66) | Abdallah et al., 2016[5] | |
| Kazakhstan | 2014 | 11 | 609 | 231 | 1225 | 2065 (559-3962) | < 0.06 (< 0.06-5) | Abdallah et al.[5], 2016 | |
| Nigeria | 2014 | 10 | 123 | 65 | 107 | 295 | < 0.06 (< 0.06-6) | Abdallah et al.[5], 2016 | |
| South Africa | 2012 | 14 | - | - | - | - | 1156 (< LOQ-4578) | Abafe and Martincigh | |
| Egypt | 2013 | 9 | - | - | - | 38 (17.39-105) | - | Hassan and Shoeib | |
| Czech Republic | 2008 | 25 | < 0.3-275 | < 0.3-57.3 | < 0.3-739.5 | 92.6 (< 0.3-949.5) | - | Kalachova | |
| UK | 2006 and 2007 | 20 | 2000 | 740 | 9600 | 13,000 (9190-69,000) | 8 (< LOQ-25) | Abdallah |
For TBBPA, previous studies from Korea[6] and South Africa[11] reported a high median concentration in office dust, similar to our study [Table 1]. Considering the use of TBBPA as an additive in HIPS and ABS resin in the plastic casings of electrical and electronic items[11], the significantly higher concentrations detected in office compared to house and car dust in our study is likely because offices contain a greater number of electrical and electronic devices (computers, printers, photocopiers, etc.)[6,7,11]. For house dust, three previous studies recorded median concentrations of TBBPA in Korean (79 ng∙g-1 and 69 ng∙g-1)[6,29] and UK (62 ng∙g-1)[17] houses that are similar to the median concentration of 67 ng g-1 in our study. The concentration of TBBPA in Thai houses exceeded that in dust samples collected in Germany[8], China[7], and in a recent study in the UK[21]. In contrast, the median concentration of TBBPA in Thai houses was two and three times lower than that in South African[11] and American[22,32] houses, respectively.
In our study, the TBBPA concentration in dust from cars ranged from 4 ng g-1 to 242 ng g-1 (median
Estimated daily intake of HBCDD and TBBPA
Reassuringly, even under high-end exposure scenarios, the EDI of HBCDD for both adults and toddlers was substantially below the oral reference dose (RfD) guideline value predicted to be toxic to the liver (200,000 ng∙kg-1∙bw∙day-1) suggested by the US National Research Council[33-34]. The median exposure value in our result was similar to the EDIs of HBCDD from Egypt[15], which were 0.03 and < 0.01 ng kg-1 bw day-1 for toddlers and adults, respectively [Table 2]. Furthermore, our results show lower EDIs than those in several previous studies. Briefly, the concentrations of HBCDD in dust from other global studies were higher than that in Thailand. Therefore, toddlers living in France[5], the UK[17], Korea[6], Germany[8], and New Zealand[35] were exposed to concentrations of HBCDD that were 170, 145, 96, 35, and 16 times higher, respectively, than Thai toddlers.
Estimated daily intake (EDI) of HBCDD and TBBPA through dust ingestion of indoor environments in the Bangkok metropolitan area, Thailand
| Country | Type of dust | EDI of HBCDD (ng kg-1 bw day-1) | EDI of TBBPA (ng kg-1 bw day-1) | |||||||
| Toddler | Adults | Toddler | Adults | Ref. | ||||||
| Median exposure | High-end exposure | Median exposure | High-end exposure | Median exposure | High-end exposure | Median exposure | High-end exposure | |||
| Thailand | House, office, and cars | 0.05 | 0.80 | 0.01 | 0.03 | 0.25 | 1.14 | 0.06 | 0.89 | This study |
| Spain | House and laboratories | - | - | 0.11 | 0.25 | - | - | - | - | Corsolini |
| House, laboratories, and computer room | - | - | 0.24 | 0.61 | - | - | - | - | Corsolini | |
| China | Houses | 0.43 | - | 0.04 | - | 0.09 | - | 0.01 | - | Sun |
| office | - | - | 0.03 | - | - | - | 0.004 | - | Sun | |
| Korea | House, office, school, and cars | 4.79 | 7.91 | 0.90 | 1.01 | 0.61 | 1.32 | 0.10 | 0.13 | Barghi et al.[6], 2017 |
| Turkey | House and office | 0.95 | 3.46 | 0.02 | 43 | - | - | - | - | Kurt-Karakus |
| South Africa | House, office, and cars | - | - | - | - | 0.60 | 2.41 | 0.08 | 1.92 | Abafe and Martincigh |
| France | House, office, and cars | 8.5 | 61.6 | 0.65 | 2.93 | 0.47 | 5.61 | 0.03 | 0.27 | Abdallah et al.[5], 2016 |
| Kazakhstan | House, office, and cars | 0.83 | 4.88 | 0.06 | 0.23 | 0.07 | 0.83 | < 0.01 | 0.04 | Abdallah |
| Nigeria | House, office, and cars | 2.16 | 22.3 | 0.17 | 1.07 | 0.19 | 1.57 | 0.01 | 0.07 | Abdallah |
| Egypt | House, office, and cars | 0.03 | 1.2 | 0.003 | 0.05 | - | - | - | - | Hassan and Shoeib |
| Germany | House | 1.73 | 8.91 | 0.01 | 0.05 | 0.14 | 0.53 | 0.01 | 0.05 | Fromme |
| New Zealand | House | 0.78 | 29.3 | 0.05 | 1.26 | - | - | - | - | Ali |
| UK | House, office, and cars | 7.24 | 20.9 | 0.52 | 1.29 | 0.28 | 1.13 | 0.02 | 0.05 | Abdallah |
In our study, estimated TBBPA exposure of toddlers exceeded that of adults. However, the value of the high-end exposure scenario to TBBPA evaluated for toddlers and adults was lower than an RfD value (600,000 ng∙kg-1∙bw∙day-1), proposed in 2015 by US authors based on uterine hyperplasia in rats[36]. However, some recent studies found that a lower dose of TBBPA in the environment (30,000 ng kg-1 body weight) can affect health[37]. In comparison, Thai toddlers were exposed to TBBPA at concentrations three and two times higher than Chinese[38] and German[8] toddlers, respectively. This may be because the EDIs in the Chinese and German studies were based on house dust only. In contrast, our EDI was calculated based on dust from three indoor environment categories: houses, offices, and cars. In contrast, several previous studies, such as those in France[5], the UK[17], South Africa[11], and Korea[6], reported EDI values that exceed those of Thai toddlers. TBBPA concentrations in South Africa and Korea exceeded those in Thailand, although the EDIs in Korean studies included ingestion of dust from several indoor microenvironment categories, such as schools, which were not considered in our study. In addition, the body weight values were slightly different from those used in our study, resulting in the EDI values of these two countries exceeding those of Thailand.
CONCLUSIONS
In this study, concentrations of HBCDD and TBBPA were measured in dust samples from houses, offices, and cars in the Bangkok metropolitan area of Thailand and used to estimate human exposure. The highest median concentration of HBCDD was observed in car dust (6.7 ng g-1), followed by houses and offices. Owing to the low application of HBCDD in Thailand in applications such as building insulation foam, concentrations of HBCDD in this study were among the lowest reported globally. In the case of TBBPA, the median concentration was highest in office dust (674 ng∙g-1), followed by house dust (67 ng∙g-1) and car (22 ng∙g-1) dust. This may reflect a high density of electronic and electrical items used in offices. However, owing to the relatively small number of samples collected in this study, additional samples should be studied to determine exposure by the Thai population to these substances. Estimated daily intakes through dust ingestion under both median and high-end exposure scenarios showed that toddlers were more highly exposed than adults. Reassuringly, the daily intakes of HBCDD and TBBPA for the general Thai population were well below the oral RfDs suggested by the US National Research Council and Wikoff et al.[36] (2015), respectively. However, it is important to note that the RfD for HBCDD was calculated by the US National Academy of Sciences (NAS) using data from Zeller and Kirsch (1970), which is an unpublished subchronic study performed on rats in 1970. The NAS concluded that confidence in this RfD for HBCDD is low because of a lack of other subchronic and chronic studies. Moreover, recent research has revealed that dysfunction of the liver is a health concern following exposure to TBBPA at 30,000 ng kg-1 body weight[37]. Therefore, chronic effects induced by continuous exposure to TBBPA and its derivatives should be further investigated. Continued monitoring of human exposure to both HBCDD and TBBPA via various pathways is thus prudent, especially for developmentally sensitive age groups such as toddler
DECLARATIONS
Acknowledgements
The authors are grateful to the Thailand Research Fund (RSA5880046), Royal Golden Jubilee (RGJ) PhD Program scholarship from the Thailand Research Fund (PHD/0129/2559), and Fundamental Fund (BRF2-NDFR29/2564) from Mahidol University for financial support.
Authors’ contributions
Made substantial contributions to the conceptualisation and design of the study, sample collection and analysis, performed data analysis and interpretation, provided content, and wrote and edited the manuscript for submission: Waiyarat S
Conceptualized and designed the study; data analysis; edited the manuscript; and provided administrative, technical, material support, and funding acquisition: Boontanon SK
Made a significant contribution to the methodology of sample analysis and validation of these procedures: Boontanon N
Made substantial contributions to the conceptualization, methodology of sample collection and analysis, and edited the manuscript: Harrad S, Abdallah MAE, Drage DS
Availability of Data and Materials
Not applicable.
Financial support and sponsorship
This study was supported by the Thailand Research Fund (RSA5880046), Royal Golden Jubilee (RGJ) PhD Program scholarship of the Thailand Research Fund (PHD/0129/2559) and Fundamental Fund (BRF2-NDFR29/2564) from Mahidol University.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
REFERENCES
1. Kurt-karakus PB, Alegria H, Jantunen L, et al. Polybrominated diphenyl ethers (PBDEs) and alternative flame retardants (NFRs) in indoor and outdoor air and indoor dust from Istanbul-Turkey: levels and an assessment of human exposure. Atmospheric Pollution Research 2017;8:801-15.
2. Melymuk L, Demirtepe H, Jílková SR. Indoor dust and associated chemical exposures. Current Opinion in Environmental Science & Health 2020;15:1-6.
3. Liu D, Wu P, Zhao N, et al. Differences of bisphenol analogue concentrations in indoor dust between rural and urban areas. Chemosphere 2021;276:130016.
4. Al-Harbi M, Al-Enzi E, Al-Mutairi H, Whalen JK. Human health risks from brominated flame retardants and polycyclic aromatic hydrocarbons in indoor dust. Chemosphere 2021;282:131005.
5. Abdallah MA, Bressi M, Oluseyi T, Harrad S. Hexabromocyclododecane and tetrabromobisphenol-A in indoor dust from France, Kazakhstan and Nigeria: implications for human exposure. Emerging Contaminants 2016;2:73-9.
6. Barghi M, Shin ES, Kim JC, Choi SD, Chang YS. Human exposure to HBCD and TBBPA via indoor dust in Korea: estimation of external exposure and body burden. Sci Total Environ 2017;593-594:779-86.
7. Wang J, Wang Y, Shi Z, Zhou X, Sun Z. Legacy and novel brominated flame retardants in indoor dust from Beijing, China: occurrence, human exposure assessment and evidence for PBDEs replacement. Sci Total Environ 2018;618:48-59.
8. Fromme H, Hilger B, Kopp E, Miserok M, Völkel W. Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany. Environ Int 2014;64:61-8.
9. Li F, Jin J, Tan D, et al. Hexabromocyclododecane and tetrabromobisphenol A in sediments and paddy soils from Liaohe River Basin, China: Levels, distribution and mass inventory. J Environ Sci (China) 2016;48:209-17.
10. U.S. EPA. Flame retardant alternatives for hexabromocyclododecane (HBCD). Available from: https://www.epa.gov/sites/default/files/2014-06/documents/hbcd_draft.pdf [Last accessed on 18 Apr 2022].
11. Abafe OA, Martincigh BS. Determination and human exposure assessment of polybrominated diphenyl ethers and tetrabromobisphenol A in indoor dust in South Africa. Environ Sci Pollut Res Int 2016;23:7038-49.
12. Abdallah M. Environmental occurrence, analysis and human exposure to the flame retardant tetrabromobisphenol-A (TBBP-A)-A review. Environ Int 2016;94:235-50.
13. Malkoske T, Tang Y, Xu W, Yu S, Wang H. A review of the environmental distribution, fate, and control of tetrabromobisphenol A released from sources. Sci Total Environ 2016;569-570:1608-17.
14. Wu Y, Li Y, Kang D, et al. Tetrabromobisphenol A and heavy metal exposure via dust ingestion in an e-waste recycling region in Southeast China. Sci Total Environ 2016;541:356-64.
15. Hassan Y, Shoeib T. Levels of polybrominated diphenyl ethers and novel flame retardants in microenvironment dust from Egypt: an assessment of human exposure. Sci Total Environ 2015;505:47-55.
16. Corsolini S, Metzdorff A, Baroni D, et al. Legacy and novel flame retardants from indoor dust in Antarctica: sources and human exposure. Environ Res 2021;196:110344.
17. Abdallah MA, Harrad S, Covaci A. Hexabromocyclododecanes and tetrabromobisphenol-A in indoor air and dust in Birmingham, U.K: implications for human exposure. Environ Sci Technol 2008;42:6855-61.
18. Besis A, Christia C, Poma G, Covaci A, Samara C. Legacy and novel brominated flame retardants in interior car dust - implications for human exposure. Environ Pollut 2017;230:871-81.
19. Peng C, Tan H, Guo Y, Wu Y, Chen D. Emerging and legacy flame retardants in indoor dust from East China. Chemosphere 2017;186:635-43.
20. Yadav IC, Devi NL, Singh VK, Li J, Zhang G. Measurement of legacy and emerging flame retardants in indoor dust from a rural village (Kopawa) in Nepal: implication for source apportionment and health risk assessment. Ecotoxicol Environ Saf 2019;168:304-14.
21. Drage DS, Waiyarat S, Harrad S, Abou-elwafa Abdallah M, Boontanon SK. Temporal trends in concentrations of legacy and novel brominated flame retardants in house dust from Birmingham in the United Kingdom. Emerging Contaminants 2020;6:323-9.
22. Allgood JM, Jimah T, McClaskey CM, et al. Ogunseitan O.A. potential human exposure to halogenated flame-retardants in elevated surface dust and floor dust in an academic environment. Enviro Res 2017;153:55-62.
23. Harrad S, Goosey E, Desborough J, Abdallah MA, Roosens L, Covaci A. Dust from U.K. primary school classrooms and daycare centers: the significance of dust as a pathway of exposure of young U.K. children to brominated flame retardants and polychlorinated biphenyls. Environ Sci Technol 2010;44:4198-202.
24. Shen M, Ge J, Lam JCW, Zhu M, Li J, Zeng L. Occurrence of two novel triazine-based flame retardants in an E-waste recycling area in South China: implication for human exposure. Sci Total Environ 2019;683:249-57.
25. Li W, Li J, Deng M, Pan Y, Zeng L. Benzotriazoles and benzothiazoles prevail in indoor dust from an E-waste dismantling area in South China: elevated concentrations and implication for human exposure. Sci Total Environ 2020;723:137979.
26. Deng M, Han X, Ge J, et al. Prevalence of phthalate alternatives and monoesters alongside traditional phthalates in indoor dust from a typical e-waste recycling area: Source elucidation and co-exposure risk. J Hazard Mater 2021;413:125322.
27. Gwon HR, Oh HJ, Chang KH, et al. Occurrence, distribution, and potential exposure risk of organophosphate flame retardants in house dust in South Korea. Sci Total Environ 2021;770:144571.
28. Stapleton HM, Klosterhaus S, Keller A, et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol 2011;45:5323-31.
29. Kweon D, Kim M, Zoh K. Distribution of brominated flame retardants and phthalate esters in house dust in Korea. Environmental Engineering Research 2018;23:354-63.
30. Thailand National Metal and Materials Technology Center (MTEC), National Science and Technology Development Agency (NSTDA). Thailand’s POPs Inventory Assessment Report (Part 2: Thailand’s 2019 POPs industrial chemicals inventory). 1st ed (ISBN 978-616-12-0616-1). MTEC & NSTDA Pub; 2021. p. 63-73. Available from: https://www.mtec.or.th/annual-report2021/th/ [Last accessed on 18 Apr 2022].
31. Kalachova K, Hradkova P, Lankova D, Hajslova J, Pulkrabova J. Occurrence of brominated flame retardants in household and car dust from the Czech Republic. Sci Total Environ 2012;441:182-93.
32. Dodson RE, Perovich LJ, Covaci A, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol 2012;46:13056-66.
33. US-NRC (US-National Research Council). Toxicological risks of selected flame-retardant chemicals. Available from: https://www.ncbi.nlm.nih.gov/books/NBK225647/pdf/Bookshelf_NBK225647.pdf [Last accessed on 18 Apr 2022].
34. Zeller H, Kirsch P. Hexabromocyclododecane: 90-day feeding trials eith rats. 1970. Available from: https://www.ncbi.nlm.nih.gov/books/NBK225647/pdf/Bookshelf_NBK225647.pdf [Last accessed on 20 Apr 2022].
35. Ali N, Dirtu AC, Van den Eede N, et al. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere 2012;88:1276-82.
36. Wikoff D, Thompson C, Perry C, et al. Development of toxicity values and exposure estimates for tetrabromobisphenol A: application in a margin of exposure assessment. J Appl Toxicol 2015;35:1292-308.
37. Yao L, Wang Y, Shi J, et al. Toxicity of tetrabromobisphenol A and Its derivative in the mouse liver following oral exposure at environmentally relevant levels. Environ Sci Technol 2021;55:8191-202.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].