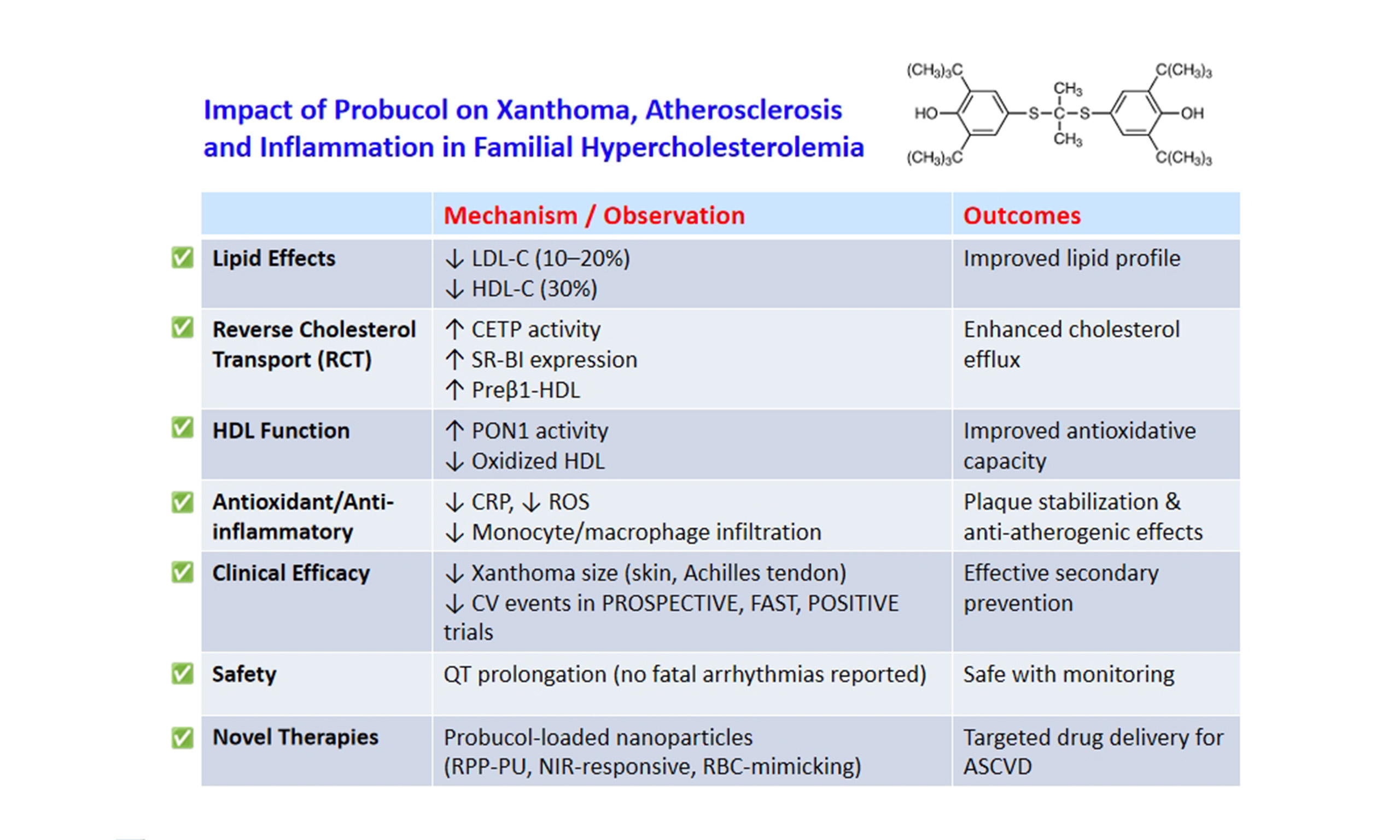
Impact of probucol on xanthoma reduction and its role in cardiovascular event prevention in familial hypercholesterolemia patients
Abstract
Although statins, ezetimibe, and PCSK9 inhibitors effectively reduce LDL-C levels and mitigate CHD risk, some residual risks persist. Probucol, a lipid-lowering agent with potent antioxidant properties, is prescribed in Asia for dyslipidemia treatment, ASCVD prevention, and xanthoma improvement. Its use was discontinued in Western countries due to concerns over HDL-C reduction and QT prolongation; however, recent research supports its therapeutic benefits. Probucol decreases HDL-C by promoting RCT through CETP and SR-BI activation while also enhancing HDL’s antioxidative function by elevating PON1 activity and lowering oxidized HDL levels. Studies on FH patients and those undergoing coronary revascularization indicate its role in secondary CV prevention. An integrated analysis of the PROSPECTIVE and IMPACT on IMT studies has suggested that, despite reducing HDL-C, probucol may offer protective effects against CHD. Potential applications of probucol for other diseases and recent advances of nanoparticle-based probucol delivery systems for the treatment of atherosclerosis and inflammation are also discussed.
Keywords
INTRODUCTION
Studies have confirmed that statins and other lipid-modifying agents that regulate the LDL receptor play a role in decreasing the likelihood of ASCVD development[1]. Additionally, ezetimibe, an inhibitor of intestinal cholesterol absorption, has been linked to lower CV event rates, as reported in the IMPROVE-IT study[2]. When combined with simvastatin, ezetimibe further reduced LDL-C levels, leading to a 7% decline in CV incidents. Achieving an LDL-C level of 53.7 mg/dL, which is lower than previous recommendations, was found to provide additional protective effects.
Moreover, findings from the recent FOURIER study[3] indicated that the PCSK9 inhibitor evolocumab, which controls LDL receptor expression at the plasma membrane, significantly decreased LDL-C levels by 59% compared to placebo, bringing the median baseline LDL-C from 92 mg/dL down to 30 mg/dL. This drop correlated with a 15% decline in primary endpoint risk. Another PCSK9 inhibitor, alirocumab, exhibited significant advantages in secondary ASCVD event prevention, as evidenced in the ODYSSEY-OUTCOMES study[4].
Inclisiran, an siRNA therapy that suppresses hepatic PCSK9 production, has demonstrated a sustained reduction in LDL-C levels with only a single administration every six months[5]. Research is currently ongoing to determine its impact on ASCVD outcomes.
Furthermore, ACL functions as an enzyme that operates upstream of HMG-CoA reductase. Bempedoic acid, an ACL inhibitor, has demonstrated effectiveness in lowering LDL-C levels, even among individuals on statin therapy, with minimal occurrence of muscle-related side effects. In patients who cannot tolerate statins, bempedoic acid has been associated with a reduced risk of major CV events[6].
Other studies show that individuals with ASCVD benefit from further LDL-C reduction beyond current guideline targets. While PCSK9 inhibitors have successfully decreased LDL-C concentrations to around
Because of its potent antioxidant properties, probucol was initially developed by Dow Chemical for industrial application to prevent degradation during the production of rubber tires. Safety studies in rats, a rodent species in which CETP is absent and most cholesterol is transported in HDL, showed that it lowered total plasma cholesterol. On this basis, probucol was then administered to humans, found to lower both LDL-C and HDL-C[7], and introduced into clinical practice prior to the availability of statins. Of concern, however, were the observed lowering of HDL-C and prolongation of the QT interval. Moreover, when the results of the PQRST angiographic study[8] that examined the impact of probucol on femoral artery lumen diameter were shown to be inconclusive, it was withdrawn from the market in the United States and Western countries in 1995. However, undeterred by the results of PQRST and confident based on clinical experience and the lack of evidence that the prolonged QT interval had adverse effects, physicians in Asia and South Africa continued to prescribe probucol in combination with newly available statins for FH patients[9].
A key feature that sets probucol apart is its capability to enhance RCT by increasing plasma CETP activity and boosting hepatic SR-BI expression[10,11]. In our past reviews on probucol[12,13], we compiled insights from prior fundamental studies and clinical investigations. This article highlights the latest research on probucol, with recent clinical evidence strongly suggesting that its role in lowering ASCVD risk and promoting xanthoma regression should be re-evaluated.
LESSONS FROM CETP DEFICIENCY AND THE FAILURE OF CETP INHIBITORS
A previous report documented a case of HALP (elevated HDL-C) associated with early-onset corneal opacity[14], followed by another case linked to angina pectoris. These findings indicate that HALP may not always be protective against atherosclerosis and, in certain contexts, may contribute to atherogenesis. It is now known that HALP is caused by a variety of genetic and environmental factors such as drugs, pregnancy, alcohol intake, and liver diseases. Primary forms of HALP are caused by mutations in genes coding CETP, HL, apolipoprotein C-III, scavenger receptor SRB-1, and EL[15]. Among these, plasma CETP deficiency is the most common in Asian populations, especially in Japan, where many cases have been observed in whom the prevalence of CHD is increased[16,17]. Indeed, recent Mendelian randomization data have shown a lack of atheroprotective effects of HDL-C[18].
According to data from the Framingham Heart Study[19] and the LURIC Study[20], an inverse relationship exists between plasma CETP levels and CHD occurrence. Assessing the role of CETP in ASCVD risk continues to be problematic because RCT is a complex process involving multiple steps and players, not limited to HDL-cell receptor interactions[21]. While plasma CETP concentrations in LURIC and the Framingham Heart Studies have been shown to correlate with CHD occurrence, the level of CETP activity in intact plasma (CET) is dependent on the abundance and availability of its potentially atherogenic apoB-containing substrate, i.e., CE acceptor lipoproteins such as immunochemically distinct LpB:C particles[22]. The complexity of RCT is illustrated by the fact that in the presence of near-total CETP inhibition with obicetrapib, LDL-C levels declined[23].
Further analysis from the Copenhagen City Heart and Copenhagen General Population Studies revealed a U-shaped correlation between HDL-C concentrations and both all-cause and CV mortality across genders[24]. Compared with individuals at minimal risk, the HRs for all-cause mortality were 1.36 for men with HDL-C levels between 97 and 115 mg/dL, rising to 2.06 for those exceeding 116 mg/dL. Among women, HRs were 1.10 for HDL-C levels in the range of 116-134 mg/dL and 1.68 for levels above
Numerous CETP inhibitors have been designed to elevate serum HDL-C levels while lowering LDL-C concentrations. Those progressing to late-stage clinical trials can be classified into CETP inhibitors, including torcetrapib, anacetrapib, and evacetrapib, and CETP modulators such as dalcetrapib. Studies assessing CETP inhibitors and modulators in combination with statin therapy have reported either neutral or adverse outcomes regarding CV event prevention and carotid atherosclerosis, even though they significantly increased HDL-C levels[27-30].
The lack of success of CETP inhibitors was primarily linked to either an unchanged or heightened ASCVD risk. The ILLUMINATE study[27] demonstrated that torcetrapib led to elevated systolic and diastolic blood pressure due to excessive aldosterone secretion. However, other CETP inhibitors neither triggered this effect nor altered plasma aldosterone levels[31]. The outcome failures of torcetrapib, dalcetrapib, and evacetrapib may have been influenced by off-target effects such as blood pressure elevation, particularly in the case of torcetrapib[32]. In contrast, anacetrapib, which did not affect blood pressure, demonstrated a modest decline in ASCVD events[33], although its development was halted by Merck due to long-term retention in adipose tissues[34]. These findings still argue that CETP inhibition itself may not be entirely ineffective in preventing cardiovascular outcomes.
These observations imply that rather than suppressing CETP, a probucol-driven enhancement of RCT could offer a promising approach for both ASCVD prevention and regression[35].
IMPACT OF PROBUCOL ON LIPOPROTEIN METABOLISM
Probucol is a lipophilic compound that distributes across various lipoprotein classes. It reduces serum LDL-C levels by approximately 10%-20% while decreasing HDL-C by around 30%[36]. Additionally, probucol promotes the excretion of cholesterol into bile[37]. Studies have demonstrated that it lowers LDL-C levels even in rabbits lacking LDL receptors[38,39] and in individuals with FH, both in homozygous and heterozygous forms[40-42]. These observations indicate that probucol’s LDL-C-lowering action is mediated through an LDL receptor-independent mechanism.
The ABCA1 is essential for generating disc-shaped HDL by transferring lipids to lipid-free apoA-I. 2-Bromopalmitate has been shown to hinder the formation of larger HDL particles while favoring the production of smaller-sized HDL. Probucol exerts a comparable effect to 2-bromopalmitate, leading to a shift in HDL formation toward smaller, discoidal particles that more avidly take up free cholesterol from peripheral tissues[43]. A consistent problem inherent in characterizing the role of CETP in lipoprotein transport is the fact that different laboratory methods to assess CE transfer (CET) activity provide contrasting results depending on whether endogenous or exogenous substrates are employed[44]. When CET is estimated in intact plasma of FH patients as the loss of CE from HDL to acceptor lipoproteins as opposed to measuring the concentration of CETP by immunoassay, CET is increased[45] and returned to normal after treatment with probucol[46].
A deficiency of SR-BI in mice has been observed to convert normal HDL into significantly larger, CE-rich, and dysfunctional HDL particles, heightening the risk of atherosclerosis. While multiple studies in rodents and tissue culture models have shown that the action of probucol enhances SR-BI-mediated delivery of CE to HDL, little is known about the extent to which the behavior and functionality of lipoproteins are altered when lipophilic probucol is incorporated into their structure. Research has documented the impact of probucol on impaired HDL function in SR-BI knockout mice[47]. HDL particles from SR-BI-deficient mice were examined through shotgun proteomics and functional assessments. Probucol-treated mice displayed notable modifications in HDL protein composition. The levels of apoA-I and PON1 in HDL were considerably lower in SR-BI-deficient mice compared to wild-type counterparts. Conversely, SR-BI null mice exhibited increased levels of acute-phase protein SAA, apoA-IV, and the proteinase inhibitor A1AT in HDL, which correlated with dysfunctional HDL properties. When compared with HDL from SR-BI+/+ mice, HDL obtained from SR-BI-/- mice retained significantly higher cholesterol content. Furthermore, HDL from SR-BI-/- mice exhibited diminished antioxidant and anti-inflammatory properties. However, administration of probucol to SR-BI-/- mice restored the relative levels of apoA-I, PON1, SAA, apoA-IV, and A1AT in HDL, leading to enhanced HDL functionality. These findings suggest that probucol has the capability to convert dysfunctional HDL in SR-BI-/- mice into a functional, atheroprotective form.
PROBUCOL AS A POWERFUL STIMULATOR OF RCT AND XANTHOMA REGRESSION
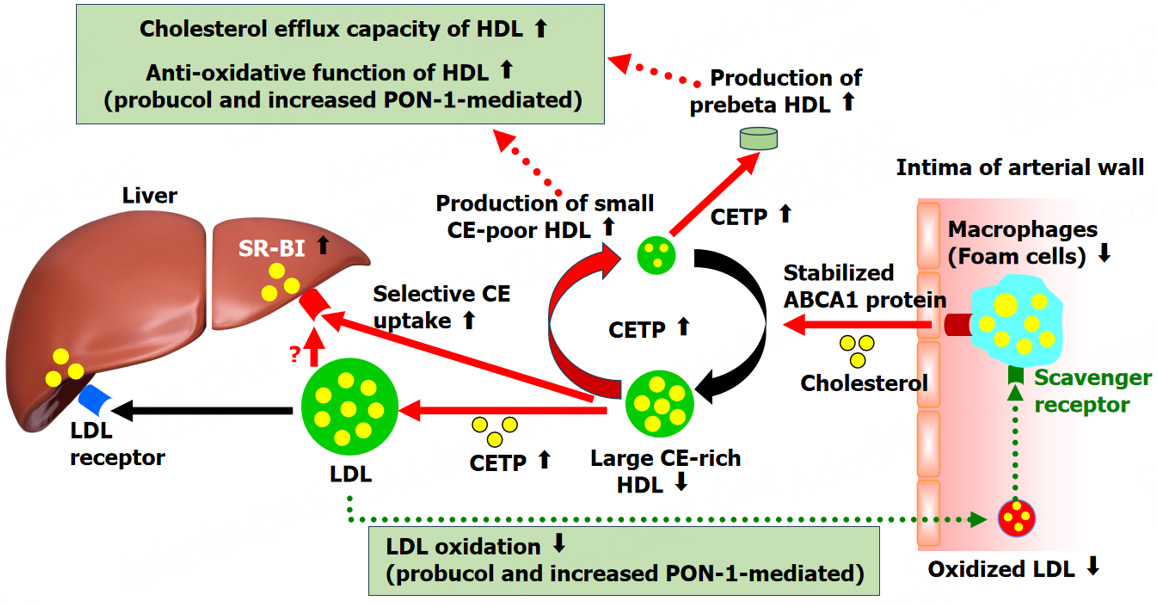
The decline in serum HDL-C caused by probucol is largely due to its role in enhancing RCT. Cholesterol within foam cells of lipid-rich plaques is absorbed by HDL and its primary protein, apoA-I, through the actions of ABCG1 and ABCA1. This cholesterol is subsequently esterified by LCAT, forming CE. In the bloodstream, CETP promotes the exchange of CE from HDL to VLDL, IDL, and LDL in return for TG. The liver then removes VLDL that has undergone remodeling by lipoprotein lipase, IDL, and LDL through apoB-LDL receptors, while hepatic SR-BI selectively absorbs CE from HDL. Excess cholesterol derived from foam cells in atherosclerotic plaques is transported by HDL to the liver for elimination via bile, a process known as RCT.
The probucol-induced decrease in HDL-C is driven by activation of RCT and SR-BI pathways[48-51]. By stimulating CETP, probucol facilitates the movement of CE from HDL, leading to the generation of small, CE-depleted HDL particles with an enhanced ability to promote cholesterol efflux. As a result, probucol significantly boosts RCT, expediting the excretion of cholesterol into bile[37]. Additionally, plasma LCAT activity showed a notable increase in FH patients receiving probucol therapy[51]. Probucol was found to enhance LCAT activity in Lp A-I by 4.5-fold. It also promoted cholesterol efflux from cholesterol-loaded adipose cells via Lp A-I and Lp A-IV, but not through Lp AI:AII. Therefore, the reduction in HDL-C induced by probucol does not signify a pro-atherogenic change but rather supports an anti-atherogenic effect by stimulating RCT from peripheral tissues.
Although probucol lowers serum HDL-C levels[40,52,53], it has been demonstrated to aid in the regression of xanthelasma and Achilles tendon xanthomas in individuals with FH. Consequently, dermatologists in Japan commonly prescribe probucol for xanthelasma patients. A reduction in Achilles tendon thickness in FH individuals has been observed to correlate positively with declining HDL-C levels[40], implying that HDL-C reduction may indicate enhanced RCT. Probucol also promotes the generation of lipid-poor preβ1-HDL, a crucial factor in cholesterol efflux[54] from peripheral tissues.
However, probucol has been noted to suppress ABCA1-mediated cholesterol efflux via apoA-I[55]. Conversely, a later investigation revealed that probucol oxidation derivatives, such as spiroquinone and diphenoquinone, elevated functional ABCA1 protein levels in macrophages and liver cells, suggesting a potential role in accelerating RCT in mice that lack CETP[56].
Studies have shown that probucol more strongly suppresses cholesterol efflux to apoA-I in THP-1 non-foam cells than in foam cells[57]. Foam cells treated with probucol generated larger nascent HDL particles through an ABCA1-independent mechanism compared to non-foam cells exposed to probucol. Additionally, probucol enhanced the release of functional nascent HDL particles from foam cells. The variation in inhibitory effects on apoA-I-mediated cholesterol efflux was linked to modifications in the plasma membrane cholesterol pool and intracellular cholesterol accumulation, independent of ABCA1[58], providing further evidence that probucol alters lipoprotein composition. In ABCA1/ABCG1 double knockout mice, a reduction in serum cholesterol levels was observed along with an elevation in CE content in the spleen and liver, accompanied by increased macrophage infiltration. Treatment with probucol significantly lowered CE content in these tissues and reduced macrophage infiltration[59].
Preβ1-HDL concentrations are elevated in individuals with hypercholesterolemia and high CETP activity. In patients receiving probucol, preβ1-HDL levels remain elevated due to increased CETP activity and enhanced CETP-driven HDL conversion[54]. ANGPTL3 is recognized for its role in inhibiting EL, an enzyme that breaks down HDL phospholipids and contributes to preβ1-HDL formation. Probucol decreases ANGPTL3 levels and the content of the phospholipids sphingomyelin, lecithin (L), and the FC/L ratio in HDL2[46] while promoting an increase in preβ1-HDL levels in hypercholesterolemic individuals[60].
The reduction in HDL-C levels induced by probucol is also linked to an upregulation of hepatic SR-BI expression[10,11]. The impact of probucol on hepatic SR-BI levels has been documented in rabbits maintained on a high-cholesterol diet[61]. Probucol notably restored hepatic SR-BI expression following dietary cholesterol elevation, resulting in a twofold rise in hepatic HDL-CE uptake and a reduction in atherosclerosis progression.
Taken together, although reduced HDL-C is generally considered detrimental in terms of ASCVD protection, the paradoxical HDL-C-lowering effect of probucol may actually reflect an enhancement of RCT via increased cholesterol efflux from foam cells and CETP and SR-BI-mediated enhanced cholesterol transfer to the liver, leading to xanthoma regression and the prevention or regression of atherosclerosis, despite ongoing controversies.
PROBUCOL: A HIGHLY EFFECTIVE ANTIOXIDANT DRUG
Probucol mitigates oxidative modification of LDL at low doses without altering the lipoprotein profile[62], primarily by suppressing free radical-induced lipid peroxidation in lipoproteins[63-65]. The influence of probucol on the serum oxidation index and lipid parameters was assessed in patients with acute coronary syndrome who were randomly allocated to either a control group (atorvastatin 20 mg/day) or a treatment group (atorvastatin 20 mg/day + probucol 750 mg/day)[66]. After 12 weeks, the atorvastatin + probucol group exhibited significantly lower oxidized LDL levels and higher PON1 levels compared to those receiving atorvastatin alone. These results indicate that adding probucol to atorvastatin therapy enhances the reduction of oxidized LDL and increases PON1 levels more efficiently than atorvastatin alone.
HDL plays a crucial role in atherosclerosis prevention through its antioxidative, anti-inflammatory, and antithrombotic properties, in addition to being a key regulator of RCT[67]. Probucol enhances the activity of HDL-associated PON1, thereby protecting LDL from lipid peroxidation[68,69]. We investigated the protective effects of probucol-treated HDL against LDL oxidation induced by AAPH[70]. In FH patients, probucol treatment led to a 47% decline in serum HDL-C, with their HDL particles appearing smaller than those in untreated individuals. Notably, HDL from probucol-treated patients exhibited a 112% prolongation in the time required for LDL oxidation to initiate. This effect may be attributed to the incorporation of probucol into HDL core lipids and the enhanced function of HDL-associated PON1.
Oxidized HDL has been identified as a proatherogenic factor, contributing to lipid accumulation within macrophages and initiating the ER stress-CHOP-mediated apoptotic cascade through increased oxidative stress[71]. This process is set in motion by oxidized HDL binding to TLR4. As a reactive oxygen species scavenger, probucol mitigates oxidized HDL-driven macrophage apoptosis and oxidative stress.
Additionally, a novel sandwich ELISA was developed to detect oxidized HDL using antibodies that recognize oxidized phosphatidylcholine, a key phospholipid component of HDL[72]. In FH patients, probucol treatment resulted in significantly lower oxidized HDL levels and a reduced oxidized HDL-to-HDL-phospholipid ratio compared to those who did not receive probucol.
Collectively, these findings suggest potential molecular pathways that underlie the anti-atherosclerotic effects of probucol, as depicted in Figure 1.
The observed reduction in serum HDL-C levels following probucol treatment may indicate an enhancement of RCT and improved HDL functionality in atherosclerosis prevention. Probucol alters CET activity and promotes the generation of preβ1-HDL and small, cholesterol-depleted HDL particles, both of which are highly efficient in cholesterol efflux. It facilitates RCT by modifying CE transfer from HDL to apoB-containing lipoproteins and upregulating hepatic SR-BI receptor expression. Consequently, enhanced hepatic uptake of cholesteryl ester via SR-BI from HDL and through both LDL receptor-mediated and non-receptor-mediated pathways accelerates cholesterol clearance into bile, despite a reduction in circulating HDL-C levels.
While probucol has been noted to inhibit ABCA1-mediated cholesterol efflux, its metabolites have been found to increase ABCA1 protein stability. Additionally, probucol alters both the surface and core lipid composition of HDL, enhancing its antioxidative potential by boosting PON-1 activity and incorporating probucol itself into the HDL core. These mechanisms may contribute to the probucol-induced xanthoma regression and its protective effects against atherosclerosis.
RECENT CLINICAL FINDINGS ON PROBUCOL FOR ATHEROSCLEROSIS PREVENTION AND REGRESSION
Research has demonstrated that probucol effectively slows atherosclerosis progression in WHHL rabbits, which are deficient in LDL receptors[73]. In cholesterol-fed rabbits, probucol significantly decreased aortic lesion size, monocyte adhesion to endothelial cells, and macrophage infiltration in the intima, showing greater efficacy than atorvastatin[74]. LDL extracted from probucol-treated rabbits exhibited enhanced antioxidative properties compared to LDL from atorvastatin-treated or control rabbits. Additionally, probucol induced more substantial reductions in atherosclerosis and aortic macrophage accumulation than atorvastatin in WHHL rabbits[75].
A recent study in mice examined the role of probucol in AAA[76]. Probucol reduced AAA incidence by preventing elastin degradation and inhibiting inflammatory cell infiltration via heme oxygenase-1 activation. Moreover, elevated homocysteine levels are recognized as a risk factor for atherosclerosis, contributing to oxidative stress and chronic inflammation. In rat aortic smooth muscle cells, homocysteine promotes CRP production[77]. Probucol was found to suppress CRP mRNA expression induced by homocysteine in cultured rat aortic smooth muscle cells and hyperhomocysteinemic rats, leading to a reduction in serum CRP levels in these animals.
In a previous review, we summarized clinical investigations assessing the impact of probucol on the prevention of atherosclerosis in the femoral, carotid, and coronary arteries, as well as its role in reducing restenosis after PCI[12]. This article highlights the results of key clinical studies on the subject and the comparison of probucol clinical trials is summarized in Table 1.
Comparison of probucol clinical trials
| Trial | Population | Design | Sample size | Duration | Primary endpoint | Key findings | Safety |
| SECURE | Post-PCI patients with moderate stenosis | RCT (double-blind) | 119 | 9 months | Change in plaque volume (IVUS) | Plaque regression in both groups; no added benefit from probucol | No safety concerns; well tolerated |
| PQRST | Primary hypercholesterolemia | RCT (placebo-controlled) | 303 | 3 years | Femoral artery lumen volume | No difference in lumen volume; HDL-C reduction by 24%-34% with probucol | Well tolerated; HDL reduction noted |
| FAST | Asymptomatic hypercholesterolemia | RCT | 246 | 2 years | Carotid IMT, CV events | IMT declined in both pravastatin and probucol groups; CV events lowest with probucol (2.4%) | No major AEs; probucol effective independent of lipid changes |
| POSITIVE | FH patients in Japan | Retrospective cohort | 410 | ~8-10 years | Time to first CV event | Significant reduction in events in secondary prevention (HR 0.13) | No increase in AEs; long-term use tolerated |
| Long-term revascularization study | Post-PCI/CABG patients | Retrospective (propensity-matched) | 1,694 | > 10 years | All-cause mortality | Probucol reduced mortality risk (HR 0.45) | No increase in QT-related AEs; favorable long-term safety |
| PROSPECTIVE | CHD patients in Japan | RCT (open-label) | 876 | 3 years | Composite CV/Cerebrovascular events | Lower event rate in probucol group (7.8% vs. 10.2%), not statistically significant | No major AEs; QT prolongation not observed |
| IMPACT on IMT | Stable CHD patients (Asia) | RCT (open-label, blinded endpoint) | 281 | 3 years | Carotid IMT | IMT regressed in all groups; no additional benefit from probucol or cilostazol | Well tolerated; no QT-related issues reported |
| Integrated analysis of PROSPECTIVE and IMPACT on IMT | CHD patients in Asia | RCT (open-label) | 1,025 | 3 years | Composite CV/Cerebrovascular events and carotid IMT | Tendency to show the effect of probucol on CV events (HR 0.67); significant decrease in CV events in patients with reduced levels of HDL-C | Well tolerated; no significant increase in AEs, including severe ventricular arrhythmias |
| PICASSO | High-hemorrhage risk stroke patients (Asia) | RCT (2 × 2 factorial) | 1,512 | 1.9 years (median) | Stroke, MI, vascular death; hemorrhagic stroke | Probucol reduced vascular events (HR 0.69); no increase in hemorrhagic stroke | Safe profile; dizziness and GI symptoms noted |
SECURE study
The SECURE study[78] evaluated 119 PCI patients who received either a probucol/cilostazol combination therapy or cilostazol alone, measuring coronary plaque characteristics through intravascular ultrasound at baseline and after nine months. All patients were prescribed simvastatin (20 mg/day). No significant differences were observed in plaque volume, composition, or primary endpoint between the groups. While plaque regression occurred in over 70% of participants, probucol did not show additional anti-atherogenic effects. However, its inclusion as an adjunct therapy was linked to plaque regression.
PQRST
The PQRST trial was the first randomized controlled study designed to evaluate whether a three-year course of probucol therapy influenced femoral atherosclerosis in patients with hypercholesterolemia[8]. Although LDL-C levels decreased by 12% and HDL-C by 24%, lumen volume remained unchanged. Since vascular remodeling may occur without affecting lumen size, the study’s neutral results could have limited diagnostic relevance.
FAST
The FAST trial investigated the impact of probucol on carotid IMT and CV events in 246 patients with hypercholesterolemia over a two-year period[79]. Participants were assigned to receive probucol
POSITIVE
This retrospective analysis examined the long-term effects of probucol treatment in 410 patients with FH[80]. The primary endpoint assessed was the duration until the first CV event requiring hospitalization. In secondary prevention, probucol significantly lowered the risk (HR 0.13, 95%CI: 0.05-0.34, P < 0.001). However, no substantial benefit was observed in primary prevention, possibly due to the higher LDL-C levels (~30 mg/dL) observed in the probucol group.
Probucol study on long-term survival following complete revascularization
An investigation into the long-term survival effects of probucol therapy included 1,694 patients who had undergone complete revascularization via PCI and/or bypass surgery. Findings revealed a significant decrease in all-cause mortality among probucol-treated individuals (HR 0.45, P = 0.002), along with a slight reduction in CV mortality[81]. These results suggest that prolonged probucol therapy may be beneficial in preventing recurrent CV events in high-risk populations, such as FH patients and those who have undergone revascularization.
PROSPECTIVE
The PROSPECTIVE[82,83] assessed whether the addition of probucol to statins or other LLT could reduce ASCVD events and carotid atherosclerosis in 876 Japanese patients with a history of CHD and dyslipidemia. Participants were randomly assigned to receive either LLT alone or LLT combined with probucol
IMPACT on IMT study
The IMPACT on IMT Study, conducted alongside PROSPECTIVE, explored the influence of probucol on carotid IMT in 281 hypercholesterolemic patients with CHD who were on statin therapy[84]. Participants were divided into three groups: statin alone, statin combined with probucol, and statin plus both probucol and cilostazol, the inhibitor of platelet aggregation. The primary focus was on changes in mean carotid IMT over three years, while secondary measures included biomarker analysis, MACCEs, and safety evaluations. IMT showed regression in all groups, with no additional improvement observed with probucol or cilostazol. The control group experienced more MACCEs, but this difference was not statistically significant.
Integrated analysis of PROSPECTIVE and IMPACT on IMT
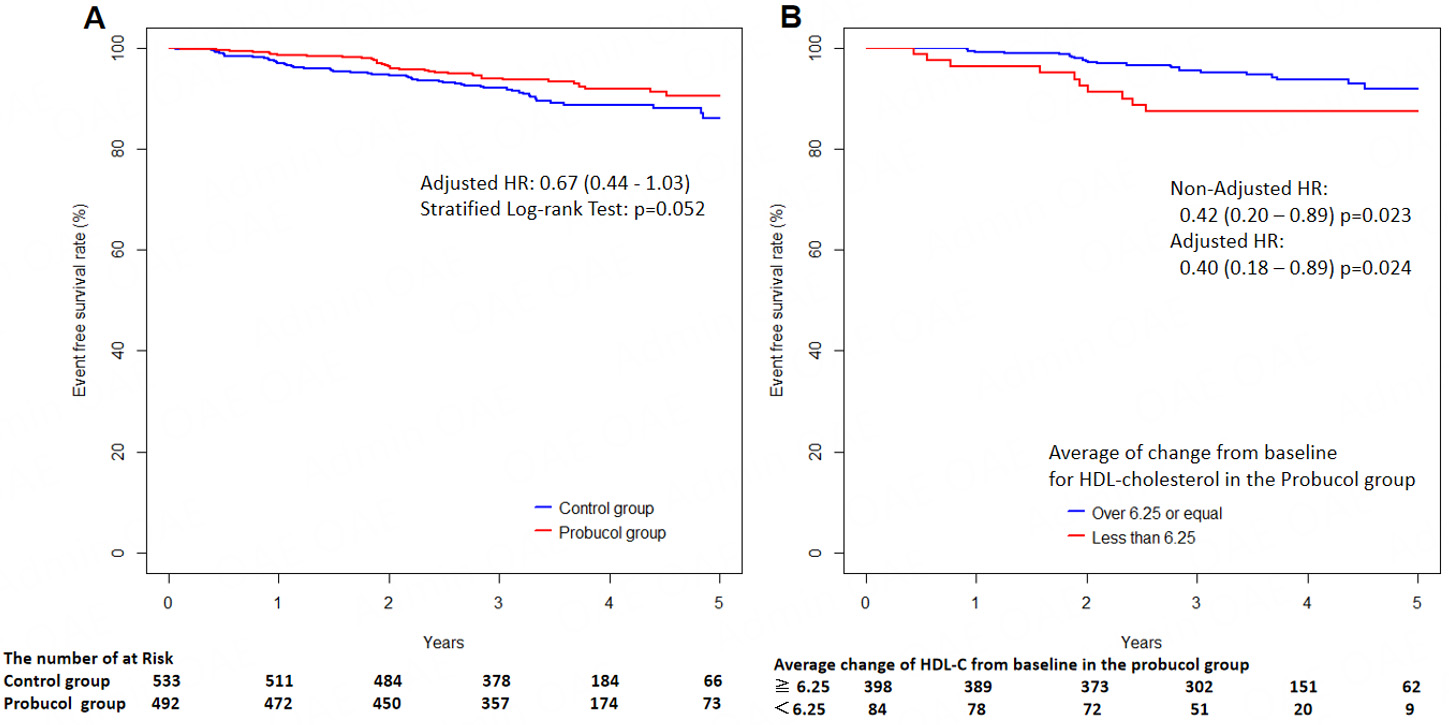
A combined analysis of data from the PROSPECTIVE and IMPACT on IMT trials assessed probucol’s effects on CV outcomes and carotid IMT in 1,025 Asian individuals with CAD[85]. The adjusted HR for CV events was 0.67 (95%CI: 0.44-1.03), indicating a likely benefit (Figure 2A, reproduced from reference[85]). While carotid IMT changes remained comparable across groups, a greater HDL-C reduction (≥ 6.25 mg/dL) was linked to a significant decline in CV events (Figure 2B, reproduced from reference[85]). There was no increase in severe adverse effects, including serious ventricular arrhythmias, among those receiving probucol. These findings suggest a possible reduction in CV risk with probucol, but further large-scale studies are needed for validation.
Figure 2. Kaplan-Meier curves for 5-year incidence of endpoint events. (A) Comparison of event-free survival between the probucol and control groups following randomization; (B) Subgroup analysis of the probucol group based on HDL-C reduction, stratified by an average decrease of ≥ 6.25 mg/dL versus < 6.25 mg/dL from baseline. Adapted from[85] (Licensed under CC BY-NC-SA 4.0).
Picasso trial
The PICASSO Trial[86] compared the efficacy and safety of cilostazol and aspirin, with or without probucol, in ischemic stroke patients considered to be at high risk for cerebral hemorrhage[87]. Participants were randomly assigned to receive cilostazol, aspirin, cilostazol plus probucol, or aspirin plus probucol. The study's main outcomes included the occurrence of stroke, myocardial infarction, vascular mortality (efficacy), and hemorrhagic stroke (safety). Over 1.9 years, both composite vascular event rates and cerebral hemorrhage rates were similar between the cilostazol and aspirin groups. However, vascular events were significantly lower among those taking probucol (HR 0.69, 95%CI: 0.50-0.97), while cerebral hemorrhage showed a downward trend (HR 0.65, P = 0.55). These findings suggest probucol may provide cardiovascular protection for high-risk ischemic stroke patients.
Additionally, the ongoing PICASSO-IMT sub-study[88] is examining changes in carotid mean IMT over a 13-month period in response to these therapies.
CLINICAL EVIDENCE OF PROBUCOL IN CORONARY RESTENOSIS
A small-scale trial in Japan demonstrated that probucol may have an anti-restenotic effect following PCI[89]. The MVP Trial[90,91] evaluated whether probucol or antioxidants could influence restenosis rates by randomly assigning 317 patients to one of four groups: placebo, probucol (500 mg), a multivitamin mix (including beta carotene, vitamin C, and vitamin E), or a combination of probucol and multivitamins. After six months, patients receiving probucol showed a significantly smaller luminal diameter reduction. The restenosis rates observed were 20.7% for probucol, 28.9% for the combined therapy, 40.3% for the multivitamin group, and 38.9% for the placebo group, indicating that probucol may help mitigate restenosis. The PART further demonstrated that administering probucol four weeks prior to angioplasty and continuing for six months post-procedure led to a notable reduction in restenosis rates[92]. A meta-analysis involving 859 patients validated that probucol effectively reduced restenosis (> 50% stenosis), increased the minimal luminal diameter, and decreased the incidence of major adverse cardiac events (HR = 0.69,
To further explore restenosis prevention post-PCI, drug-eluting stents coated with probucol have been developed[94,95] and studied further to assess their safety and long-term efficacy in managing chronic total occlusions[96].
ADVERSE REACTIONS OF PROBUCOL
As mentioned above, probucol is known to have several notable adverse effects:
QT interval prolongation: There is a possibility that probucol can increase the risk of ventricular arrhythmias, including torsades de pointes. However, according to the results of the previous studies listed above, there have been no reports of fatal ventricular arrhythmias. In a long-term retrospective analysis of 1,694 patients who had undergone complete revascularization via PCI and/or bypass surgery[81], the use of probucol was associated with a significant decrease in all-cause mortality and a slight reduction in CV mortality without an increase in fatal ventricular arrhythmias. Furthermore, the POSITIVE Trial[80] also showed no significant difference in serious adverse events such as ventricular arrhythmias between the probucol-treated and untreated groups. Later clinical trials also confirmed the safety of probucol use. Therefore, probucol has a possibility of QT prolongation, but the long-term use of probucol has been proven to be safe. However, there is a consensus to monitor the QT interval while administering probucol for a longer period in Asian countries.
Decreased HDL-C levels: Unlike other lipid-lowering agents, probucol paradoxically lowers HDL cholesterol, which may reduce its cardioprotective effect.
Gastrointestinal disturbances: Such as nausea, diarrhea, and abdominal discomfort.
Liver dysfunction: Mild elevations in liver enzymes can occur.
Hypersensitivity reactions: Rare, but include rash or allergic responses.
Due to these adverse effects, monitoring - particularly for QT prolongation and HDL-C lowering - should be performed during the use of probucol.
POTENTIAL APPLICATIONS OF PROBUCOL FOR OTHER DISEASES
Probucol exhibits antioxidative, anti-inflammatory, and RCT-promoting effects, suggesting potential therapeutic applications in various conditions. For these reasons, it may provide benefits for individuals with NAFLD[97], contrast-induced acute kidney injury in CHD patients[98-100], and neurodegenerative disorders such as Alzheimer's and Parkinson's disease[101].
Lipoprotein glomerulopathy is a renal disorder marked by proteinuria, kidney dysfunction, and type III dyslipidemia, often associated with mutations in the apoE gene. Liao et al. observed similar renal abnormalities in LDL receptor and SR-BI double-knockout mice subjected to a high-fat diet[102]. In this experimental setting, probucol significantly mitigated kidney damage, suggesting its potential role in treating lipoprotein glomerulopathy.
NANOPARTICLE-BASED PROBUCOL DELIVERY SYSTEMS IN ATHEROSCLEROSIS AND RELATED CONDITIONS
Emerging research on nanoparticle-based probucol delivery systems has demonstrated encouraging results in atherosclerosis, inflammation, and hyperlipidemia-induced pancreatitis, often outperforming non-probucol nanoparticles[103-111].
Liang et al.[103] developed RPP-PU, a probucol-loaded, H2O2-sensitive nanoparticle mimicking red blood cells. In ApoE-/- mice, it prolonged drug release, reduced oxidative stress, lowered lipid and enzyme levels, and decreased plaque burden. It also suppressed inflammatory markers (e.g., MCP-1, ICAM-1) and foam cell formation, suggesting attenuated inflammation and slower plaque growth. Translation, however, faces manufacturing and safety challenges.
Chen et al.[104] created theranostic nanoparticles combining probucol with near-infrared dyes (ICG or IR783) for simultaneous therapy and imaging. They reduced foam cell formation, oxidative stress, and inflammation in vitro, and selectively targeted plaques in mice. The IR783 variant enabled NIR visualization of lesions. Barriers include regulatory approval, dye safety validation, and scalable production.
Zeng et al.[105] produced carrier-free nanoparticles from probucol and ginsenoside Rb1, cloaked with inflammatory cell membranes for lesion targeting. These bound activated endothelium, inhibited monocyte migration, and reduced oxidative and inflammatory markers in vitro. In ApoE-/- mice, they localized to plaques, lowered lipid and necrotic core content, and reduced macrophages, smooth muscle cells, and TNF-α without observed toxicity. Structural complexity may hinder scale-up and quality control.
Fu et al.[106] developed ceria-zoledronic acid nanozymes loaded with probucol and coated with platelet membranes. These showed ROS-scavenging and anti-inflammatory effects, accumulated in plaques, and significantly reduced foam cell formation and plaque progression in ApoE-/- mice, suggesting a versatile redox- and inflammation-targeted platform.
Chen et al.[107] designed platelet membrane-coated mesoporous polydopamine nanoparticles carrying probucol with NIR-triggered release. They suppressed foam cell formation and oxidative stress in vitro, and in a carotid ligation mouse model, accumulated in plaques and reduced intimal thickening, indicating potential for precise, responsive therapy.
Liang et al.[108] introduced a red blood cell membrane-coated nanoparticle using a star polymer scaffold to encapsulate probucol, improving loading, biocompatibility, and plaque targeting. In ApoE-/- mice, it reduced serum lipids, plaque collagen, inflammatory mediators, and foam cell formation, offering a multifaceted therapeutic strategy.
Wagle et al.[109] encapsulated probucol with lithocholic acid in sodium alginate, enhancing insulin secretion, antioxidant activity, and cellular uptake in islet cells. In diabetic mice, oral delivery lowered glucose, reduced inflammation, and improved survival, protecting β cells from oxidative damage. Clinical use requires evaluating bile acid toxicity and potential cardiovascular risks.
Wu et al.[110] formulated a self-assembled probucol nanosuspension with improved stability and pharmacokinetics, which reduced pancreatic damage and lung injury in hyperlipidemia-induced acute pancreatitis models, suggesting a novel anti-inflammatory approach beyond supportive care.
Wagle et al.[111] developed probucol-lithocholic acid nanoparticles for sensorineural hearing loss. In auditory cell models, they improved mitochondrial function, activated antioxidant pathways (e.g., Nrf2/HO-1), and enhanced cell survival, outperforming free probucol. However, clinical translation remains challenging due to difficulties in crossing the blood-labyrinth barrier, achieving sustained inner-ear release, and assessing potential ototoxicity.
Collectively, nanoparticle-based probucol platforms enable targeted, multi-modal interventions for vascular and metabolic diseases. However, translation to clinical practice will require addressing safety, manufacturing, scalability, and disease-specific delivery barriers.
FUTURE PERSPECTIVES AND POTENTIAL STRATEGY TO DISSEMINATE THIS TREATMENT
Since probucol has been used for many years in Asian countries, particularly Japan, substantial evidence indicates that it can reduce LDL-C and regress skin and tendon xanthomas in FH patients, despite lowering HDL-C. This HDL-C reduction is unlikely to increase ASCVD risk and may instead reflect enhanced cholesterol efflux from foam macrophages and activation of RCT. The Japan Atherosclerosis Society (JAS) Guidelines for the Prevention of Atherosclerotic Cardiovascular Disease, 2022 edition[112], and the JAS Guidelines for the Management of Dyslipidemia, 2023 edition[113], list probucol as a treatment option for dyslipidemia, especially FH. To further promote the use of probucol for xanthoma treatment and possible ASCVD prevention, a larger randomized trial in high-risk patients with established ASCVD, in addition to current lipid-lowering therapy, may be required.
CONCLUSION
This review summarizes clinical experience and laboratory findings obtained over four decades that not only support the continued role of probucol in promoting xanthoma regression and mitigating ASCVD risk in FH patients, but also suggest that it should be utilized more widely in Western countries for this purpose. Although it lowers HDL-C and prolongs the QT interval, evidence indicates that probucol contributes to ASCVD prevention and xanthoma improvement without increasing the incidence of fatal ventricular arrhythmias. These benefits stem from both its antioxidative and anti-inflammatory properties as well as alterations in lipoprotein transport that facilitate the removal of cholesterol from peripheral tissues and deliver it to the liver. The observed reduction in HDL-C likely reflects activation of the RCT and the SR-BI pathway. Given its established safety profile and proven efficacy, probucol merits reconsideration as a therapeutic option for addressing residual ASCVD risk.
DECLARATIONS
Acknowledgments
The authors express their gratitude to Dr. Yuji Matsuzawa (Sumitomo Hospital) for his valuable support in the probucol trial. The authors acknowledge the use of ChatGPT for language editing of the manuscript to enhance grammatical accuracy and clarity.
Authors’ contributions
Involved in the conception, execution and drafting of the manuscript: Yamashita S
Management and data analysis of clinical studies on probucol: Masuda D
Availability of data and materials
The data supporting the findings of this study were collected from publications listed in the References. Due to the nature of this review, individual-level data cannot be shared.
Financial support and sponsorship
This research was partially funded by a Joint Research Grant from Otsuka Pharmaceutical Co., Ltd.
Conflicts of interest
Both authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Fulcher J, O'Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397-405.
2. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97.
3. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-22.
4. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-107.
5. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-40.
6. Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388:1353-64.
7. Barnhart JW, Sefranka JA, McIntosh DD. Hypocholesterolemic effect of 4,4'-(isopropylidenedithio)-bis(2,6-di-t-butylphenol) (probucol). Am J Clin Nutr. 1970;23:1229-33.
8. Walldius G, Erikson U, Olsson AG, et al. The effect of probucol on femoral atherosclerosis: the Probucol Quantitative Regression Swedish Trial (PQRST). Am J Cardiol. 1994;74:875-83.
9. Yamashita S, Masuda D, Harada-Shiba M, et al. Effectiveness and safety of lipid-lowering drug treatments in japanese patients with familial hypercholesterolemia: familial hypercholesterolemia expert forum (FAME) study. J Atheroscler Thromb. 2022;29:608-38.
10. Rinninger F, Wang N, Ramakrishnan R, Jiang XC, Tall AR. Probucol enhances selective uptake of HDL-associated cholesteryl esters in vitro by a scavenger receptor B-I-dependent mechanism. Arterioscler Thromb Vasc Biol. 1999;19:1325-32.
11. Hirano K, Ikegami C, Tsujii K, et al. Probucol enhances the expression of human hepatic scavenger receptor class B type I, possibly through a species-specific mechanism. Arterioscler Thromb Vasc Biol. 2005;25:2422-7.
12. Yamashita S, Masuda D, Matsuzawa Y. Did we abandon probucol too soon? Curr Opin Lipidol. 2015;26:304-16.
13. Yamashita S, Matsuzawa Y. Where are we with probucol: a new life for an old drug? Atherosclerosis. 2009;207:16-23.
14. Matsuzawa Y, Yamashita S, Kameda K, Kubo M, Tarui S, Hara I. Marked hyper-HDL2-cholesterolemia associated with premature corneal opacity. A case report. Atherosclerosis. 1984;53:207-12.
15. Giammanco A, Noto D, Barbagallo CM, et al. Hyperalphalipoproteinemia and beyond: the role of HDL in cardiovascular diseases. Life. 2021;11:581.
16. Hirano K, Yamashita S, Nakajima N, et al. Genetic cholesteryl ester transfer protein deficiency is extremely frequent in the Omagari area of Japan. Marked hyperalphalipoproteinemia caused by CETP gene mutation is not associated with longevity. Arterioscler Thromb Vasc Biol. 1997;17:1053-9.
17. Zhong S, Sharp DS, Grove JS, et al. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest. 1996;97:2917-23.
18. Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572-80.
19. Robins SJ, Lyass A, Brocia RW, Massaro JM, Vasan RS. Plasma lipid transfer proteins and cardiovascular disease. The Framingham Heart Study. Atherosclerosis. 2013;228:230-6.
20. Ritsch A, Scharnagl H, Eller P, et al. Cholesteryl ester transfer protein and mortality in patients undergoing coronary angiography: the Ludwigshafen Risk and Cardiovascular Health study. Circulation. 2010;121:366-74.
21. Miller NE. CETP inhibitors and cardiovascular disease: time to think again. F1000Res. 2014;3:124.
22. Lee DM, Alaupovic P, Knight-Gibson C, Bagdade JD. Apolipoprotein-B subclasses as acceptors of cholesteryl esters transferred by CETP. Eur J Clin Invest. 2008;38:734-42.
23. Nicholls SJ, Nelson AJ, Ditmarsch M, et al. Obicetrapib on top of maximally tolerated lipid-modifying therapies in participants with or at high risk for atherosclerotic cardiovascular disease: rationale and designs of BROADWAY and BROOKLYN. Am Heart J. 2024;274:32-45.
24. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38:2478-86.
25. Hirata A, Okamura T, Sugiyama D, et al. The relationship between very high levels of serum high-density lipoprotein cholesterol and cause-specific mortality in a 20-year follow-up study of japanese general population. J Atheroscler Thromb. 2016;23:800-9.
26. Hirata A, Sugiyama D, Watanabe M, et al. Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: the EPOCH-JAPAN study. J Clin Lipidol. 2018;12:674-84.e5.
27. Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109-22.
28. Kastelein JJ, van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620-30.
29. Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089-99.
30. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-42.
31. Ferri N, Corsini A, Sirtori CR, Ruscica M. Present therapeutic role of cholesteryl ester transfer protein inhibitors. Pharmacol Res. 2018;128:29-41.
32. Forrest MJ, Bloomfield D, Briscoe RJ, et al. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br J Pharmacol. 2008;154:1465-73.
33. Bowman L, Hopewell JC, Chen F, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-27.
34. Krishna R, Gheyas F, Liu Y, et al. Chronic administration of anacetrapib is associated with accumulation in adipose and slow elimination. Clin Pharmacol Ther. 2017;102:832-40.
35. Yamashita S, Ruscica M, Macchi C, Corsini A, Matsuzawa Y, Sirtori CR. Cholesteryl ester transfer protein: an enigmatic pharmacology - Antagonists and agonists. Atherosclerosis. 2018;278:286-98.
36. Buckley MM, Goa KL, Price AH, Brogden RN. Probucol. A reappraisal of its pharmacological properties and therapeutic use in hypercholesterolaemia. Drugs. 1989;37:761-800.
37. Tawara K, Tomikawa M, Abiko Y. Mode of action of probucol in reducing serum cholesterol in mice. Jpn J Pharmacol. 1986;40:123-33.
38. Naruszewicz M, Carew TE, Pittman RC, et al. A novel mechanism by which probucol lowers low density lipoprotein levels demonstrated in the LDL receptor-deficient rabbit. J Lipid Res. 1984;25:1206-13.
39. Baker SG, Joffe BI, Mendelsohn D, et al. Treatment of homozygous familial hypercholesterolaemia with probucol. S Afr Med J. 1982;62:7-11.
40. Matsuzawa Y, Yamashita S, Funahashi T, Yamamoto A, Tarui S. Selective reduction of cholesterol in HDL2 fraction by probucol in familial hypercholesterolemia and hyperHDL2 cholesterolemia with abnormal cholesteryl ester transfer. Am J Cardiol. 1988;62:66B-72B.
41. Durrington PN, Miller JP. Double-blind, placebo-controlled, cross-over trial of probucol in heterozygous familial hypercholesterolaemia. Atherosclerosis. 1985;55:187-94.
42. Sanjurjo P, Martul P, Sasieta M, Lafuente P, Ariza F, Cabeza I. Treatment with probucol of children with familial hypercholesterolaemia. Acta Paediatr Scand. 1988;77:132-5.
43. Quach D, Vitali C, La FM, et al. Cell lipid metabolism modulators 2-bromopalmitate, D609, monensin, U18666A and probucol shift discoidal HDL formation to the smaller-sized particles: implications for the mechanism of HDL assembly. Biochim Biophys Acta. 2016;1861:1968-79.
44. Chapman MJ, Le Goff W, Guerin M, Kontush A. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur Heart J. 2010;31:149-64.
45. Bagdade JD, Ritter MC, Subbaiah PV. Accelerated cholesteryl ester transfer in plasma of patients with hypercholesterolemia. J Clin Invest. 1991;87:1259-65.
46. Bagdade JD, Kaufman D, Ritter MC, Subbaiah PV. Probucol treatment in hypercholesterolemic patients: effects on lipoprotein composition, HDL particle size, and cholesteryl ester transfer. Atherosclerosis. 1990;84:145-54.
47. Cao J, Xu Y, Li F, Shang L, Fan D, Yu H. Protein markers of dysfunctional HDL in scavenger receptor class B type I deficient mice. J Transl Med. 2018;16:155.
48. McPherson R, Hogue M, Milne RW, Tall AR, Marcel YL. Increase in plasma cholesteryl ester transfer protein during probucol treatment. Relation to changes in high density lipoprotein composition. Arterioscler Thromb. 1991;11:476-81.
49. Chiesa G, Michelagnoli S, Cassinotti M, et al. Mechanisms of high-density lipoprotein reduction after probucol treatment: changes in plasma cholesterol esterification/transfer and lipase activities. Metabolism. 1993;42:229-35.
50. Ishigami M, Yamashita S, Sakai N, et al. High-density lipoproteins from probucol-treated patients have increased capacity to promote cholesterol efflux from mouse peritoneal macrophages loaded with acetylated low-density lipoproteins. Eur J Clin Invest. 1997;27:285-92.
51. Adlouni A, El Messal M, Saïle R, Parra H, Fruchart J, Ghalim N. Probucol promotes reverse cholesterol transport in heterozygous familial hypercholesterolemia. Effects on apolipoprotein AI-containing lipoprotein particles. Atherosclerosis. 2000;152:433-40.
52. Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, Kishino B. Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol. 1986;57:29H-35H.
53. Fujita M, Shirai K. A comparative study of the therapeutic effect of probucol and pravastatin on xanthelasma. J Dermatol. 1996;23:598-602.
54. Miida T, Seino U, Miyazaki O, et al. Probucol markedly reduces HDL phospholipids and elevated prebeta1-HDL without delayed conversion into alpha-migrating HDL: putative role of angiopoietin-like protein 3 in probucol-induced HDL remodeling. Atherosclerosis. 2008;200:329-35.
55. Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler Thromb Vasc Biol. 2004;24:2345-50.
56. Yakushiji E, Ayaori M, Nishida T, et al. Probucol-oxidized products, spiroquinone and diphenoquinone, promote reverse cholesterol transport in mice. Arterioscler Thromb Vasc Biol. 2016;36:591-7.
57. Hafiane A, Pisaturo A, Ronca A, Incerti M, Kiss RS, Favari E. Probucol treatment is associated with an ABCA1-independent mechanism of cholesterol efflux to lipid poor apolipoproteins from foam cell macrophages. BBA Adv. 2021;1:100003.
58. Hafiane A, Ronca A, Kiss RS, Favari E. High density lipoprotein-based therapeutics: novel mechanism of probucol in foam cells. Front Cardiovasc Med. 2022;9:895031.
59. Hoekstra M, Van Eck M. Probucol-induced hypocholesterolemia is not associated with exacerbated foam cell formation in ABCG1 knockout mice. Atherosclerosis. 2020;296:91-2.
60. Miida T, Yamaguchi T, Tsuda T, Okada M. High prebeta1-HDL levels in hypercholesterolemia are maintained by probucol but reduced by a low-cholesterol diet. Atherosclerosis. 1998;138:129-34.
61. Hong SC, Zhao SP, Wu ZH. Effect of probucol on HDL metabolism and class B type I scavenger receptor (SR-BI) expression in the liver of hypercholesterolemic rabbits. Int J Cardiol. 2007;115:29-35.
62. Cristol LS, Jialal I, Grundy SM. Effect of low-dose probucol therapy on LDL oxidation and the plasma lipoprotein profile in male volunteers. Atherosclerosis. 1992;97:11-20.
63. Kaminnyi AI, Lankin VZ, Perepelitsa EI, et al. Relationship between free-radical lipid oxidation and efficiency of coronary angioplasty in coronary patients. Bull Exp Biol Med. 2007;144:664-6.
64. Carew TE, Schwenke DC, Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci USA. 1987;84:7725-9.
65. Kita T, Yokode M, Ishii K, et al. The role of oxidized lipoproteins in the pathogenesis of atherosclerosis. Clin Exp Pharmacol Physiol Suppl. 1992;20:37-42.
66. Guo YS, Wang CX, Cao J, et al. Antioxidant and lipid-regulating effects of probucol combined with atorvastatin in patients with acute coronary syndrome. J Thorac Dis. 2015;7:368-75.
67. Smith JD. Dysfunctional HDL as a diagnostic and therapeutic target. Arterioscler Thromb Vasc Biol. 2010;30:151-5.
68. Noto H, Kawamura M, Hashimoto Y, et al. Modulation of HDL metabolism by probucol in complete cholesteryl ester transfer protein deficiency. Atherosclerosis. 2003;171:131-6.
69. Hong SC, Zhao SP, Wu ZH. Probucol up-regulates paraoxonase 1 expression in hepatocytes of hypercholesterolemic rabbits. J Cardiovasc Pharmacol. 2006;47:77-81.
70. Inagaki M, Nakagawa-Toyama Y, Nishida M, et al. Effect of probucol on antioxidant properties of HDL in patients with heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2012;19:643-56.
71. Yao S, Tian H, Zhao L, et al. Oxidized high density lipoprotein induces macrophage apoptosis via toll-like receptor 4-dependent CHOP pathway. J Lipid Res. 2017;58:164-77.
72. Okada T, Sumida M, Ohama T, et al. Development and clinical application of an enzyme-linked immunosorbent assay for oxidized high-density lipoprotein. J Atheroscler Thromb. 2021;28:703-15.
73. Kita T, Nagano Y, Yokode M, et al. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci USA. 1987;84:5928-31.
74. Niimi M, Keyamura Y, Nozako M, et al. Probucol inhibits the initiation of atherosclerosis in cholesterol-fed rabbits. Lipids Health Dis. 2013;12:166.
75. Li S, Liang J, Niimi M, et al. Probucol suppresses macrophage infiltration and MMP expression in atherosclerotic plaques of WHHL rabbits. J Atheroscler Thromb. 2014;21:648-58.
76. Chen C, Wang Y, Cao Y, et al. Mechanisms underlying the inhibitory effects of probucol on elastase-induced abdominal aortic aneurysm in mice. Br J Pharmacol. 2020;177:204-16.
77. Li Y, Zhao Q, Cao Y, et al. Probucol decreases homocysteine-stimulated CRP production in rat aortic smooth muscle cells via regulating HO-1/NADPH oxidase/ROS/p38 pathway. Acta Biochim Biophys Sin. 2021;53:212-9.
78. Ko YG, Choi SH, Chol Kang W, Kwon Lee B, Wook Kim S, Shim WH. Effects of combination therapy with cilostazol and probucol versus monotherapy with cilostazol on coronary plaque, lipid and biomarkers: SECURE study, a double-blind randomized controlled clinical trial. J Atheroscler Thromb. 2014;21:816-30.
79. Sawayama Y, Shimizu C, Maeda N, et al. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol. 2002;39:610-6.
80. Yamashita S, Bujo H, Arai H, et al. Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb. 2008;15:292-303.
81. Kasai T, Miyauchi K, Kubota N, Kajimoto K, Amano A, Daida H. Probucol therapy improves long-term (>10-year) survival after complete revascularization: a propensity analysis. Atherosclerosis. 2012;220:463-9.
82. Yamashita S, Masuda D, Ohama T, et al. Rationale and design of the PROSPECTIVE trial: probucol trial for secondary prevention of atherosclerotic events in patients with prior coronary heart disease. J Atheroscler Thromb. 2016;23:746-56.
83. Yamashita S, Arai H, Bujo H, et al. Probucol trial for secondary prevention of atherosclerotic events in patients with coronary heart disease (PROSPECTIVE). J Atheroscler Thromb. 2021;28:103-23.
84. Kang HJ, Kim MH, Sung J, et al. Effect of probucol and/or cilostazol on carotid intima media thickness in patients with coronary heart disease: a randomized, multicenter, multinational study. J Atheroscler Thromb. 2021;28:124-36.
85. Arai H, Bujo H, Masuda D, et al. Integrated analysis of two probucol trials for the secondary prevention of atherosclerotic cardiovascular events: PROSPECTIVE and IMPACT. J Atheroscler Thromb. 2022;29:850-65.
86. Hong KS, Kim BJ, Lee JY, Kwon SU; PICASSO Investigators. Rationale and design of the PreventIon of CArdiovascular events in iSchemic Stroke patients with high risk of cerebral hemOrrhage (PICASSO) study: a randomized controlled trial. Int J Stroke. 2015;10:1153-8.
87. Kim BJ, Lee EJ, Kwon SU, et al. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. Lancet Neurol. 2018;17:509-18.
88. Seo WK, Kim YJ, Lee J, Kwon SU; PICASSO Investigators. Design and rationale of the intima-medial thickness sub-study of the prevention of CArdiovascular events in iSchemic stroke patients with high risk of cerebral hemOrrhage (PICASSO-IMT) study. J Stroke Cerebrovasc Dis. 2017;26:1892-8.
89. Setsuda M, Inden M, Hiraoka N, et al. Probucol therapy in the prevention of restenosis after successful percutaneous transluminal coronary angioplasty. Clin Ther. 1993;15:374-382.
90. Tardif JC, Cöté G, Lespérance J, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 1997;337:365-72.
91. Côté G, Tardif JC, Lespérance J, et al. Effects of probucol on vascular remodeling after coronary angioplasty. Multivitamins and Protocol Study Group. Circulation. 1999;99:30-5.
92. Daida H, Kuwabara Y, Yokoi H, et al. Effect of probucol on repeat revascularization rate after percutaneous transluminal coronary angioplasty (from the Probucol Angioplasty Restenosis Trial [PART]). Am J Cardiol. 2000;86:550-2, A9.
93. Liu J, Li M, Lu H, et al. Effects of probucol on restenosis after percutaneous coronary intervention: a systematic review and meta-analysis. PLoS One. 2015;10:e0124021.
94. Harada Y, Colleran R, Kufner S, et al. Five-year clinical outcomes in patients with diabetes mellitus treated with polymer-free sirolimus- and probucol-eluting stents versus second-generation zotarolimus-eluting stents: a subgroup analysis of a randomized controlled trial. Cardiovasc Diabetol. 2016;15:124.
95. Kufner S, Sorges J, Mehilli J, et al. Randomized trial of polymer-free sirolimus- and probucol-eluting stents versus durable polymer zotarolimus-eluting stents: 5-year results of the ISAR-TEST-5 trial. JACC Cardiovasc Interv. 2016;9:784-92.
96. Zuhdi AS, Krackhardt F, Waliszewski MW, Ismail MD, Boxberger M, Wan Ahmad WA. Efficacy and safety of polymer-free ultrathin strut sirolimus-probucol coated drug-eluting stents for chronic total occlusions: insights from the coroflex ISAR 2000 worldwide registry. Cardiol Res Pract. 2018;2018:8053168.
97. Chen KQ, Ke BY, Cheng L, Guan MT, Wang ZB, Wang SZ. Research and progress of probucol in nonalcoholic fatty liver disease. Mini Rev Med Chem. 2023;23:1905-11.
98. Cui X, Xie B, Wang H, et al. Preventing contrast-induced acute kidney injury with probucol and hydration in patients with coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2023;102:e33273.
99. Navarese EP, Gurbel PA, Andreotti F, et al. Prevention of contrast-induced acute kidney injury in patients undergoing cardiovascular procedures-a systematic review and network meta-analysis. PLoS One. 2017;12:e0168726.
100. Pranata R, Yonas E, Vania R, Lukito AA. The role of probucol preventing contrast-induced nephropathy in patients undergoing invasive coronary procedures - Systematic review and meta-analysis of randomized controlled trials. Turk Kardiyol Dern Ars. 2021;49:51-9.
101. Sharif A, Mamo J, Lam V, et al. The therapeutic potential of probucol and probucol analogues in neurodegenerative diseases. Transl Neurodegener. 2024;13:6.
102. Liao J, Bai J, An X, et al. Lipoprotein glomerulopathy-like lesions in atherosclerotic mice defected with HDL receptor SR-B1. Front Cardiovasc Med. 2021;8:734824.
103. Liang X, Li H, Li X, et al. Highly sensitive H2O2-scavenging nano-bionic system for precise treatment of atherosclerosis. Acta Pharm Sin B. 2023;13:372-89.
104. Chen F, Chen J, Han C, et al. Theranostics of atherosclerosis by the indole molecule-templated self-assembly of probucol nanoparticles. J Mater Chem B. 2021;9:4134-42.
105. Zeng J, Zhang Y, Gao Y, et al. Biomimetic ginsenoside Rb1 and probucol Co-assembled nanoparticles for targeted atherosclerosis therapy via inhibition of oxidative stress, inflammation, and lipid deposition. ACS Nano. 2025;19:22968-87.
106. Fu X, Yu X, Jiang J, et al. Small molecule-assisted assembly of multifunctional ceria nanozymes for synergistic treatment of atherosclerosis. Nat Commun. 2022;13:6528.
107. Chen L, Yang J, Fu X, et al. A targeting mesoporous dopamine nanodrug platform with NIR responsiveness for atherosclerosis improvement. Biomater Adv. 2022;136:212775.
108. Liang X, Li H, Zhang A, et al. Red blood cell biomimetic nanoparticle with anti-inflammatory, anti-oxidative and hypolipidemia effect ameliorated atherosclerosis therapy. Nanomedicine. 2022;41:102519.
109. Wagle SR, Kovacevic B, Ionescu CM, et al. Pharmacological and biological study of microencapsulated probucol-secondary bile acid in a diseased mouse model. Pharmaceutics. 2021;13:1223.
110. Wu Z, Wang X, Jiang X. Study on the mechanism of probucol nanosuspension on hyperlipidemic pancreatitis and regulation of blood lipid function. J Nanosci Nanotechnol. 2021;21:1286-92.
111. Wagle SR, Kovacevic B, Ionescu CM, et al. Probucol-bile acid based nanoparticles protect auditory cells from oxidative stress: an in vitro study. Ther Deliv. 2024;15:237-52.
112. Okamura T, Tsukamoto K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2022. J Atheroscler Thromb. 2024;31:641-853.
113. Japan Atherosclerosis Society. Guide for Management of Dyslipidemia for Prevention of Atherosclerotic Cardiovascular Diseases. 2023 Edition (in Japanese).
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].