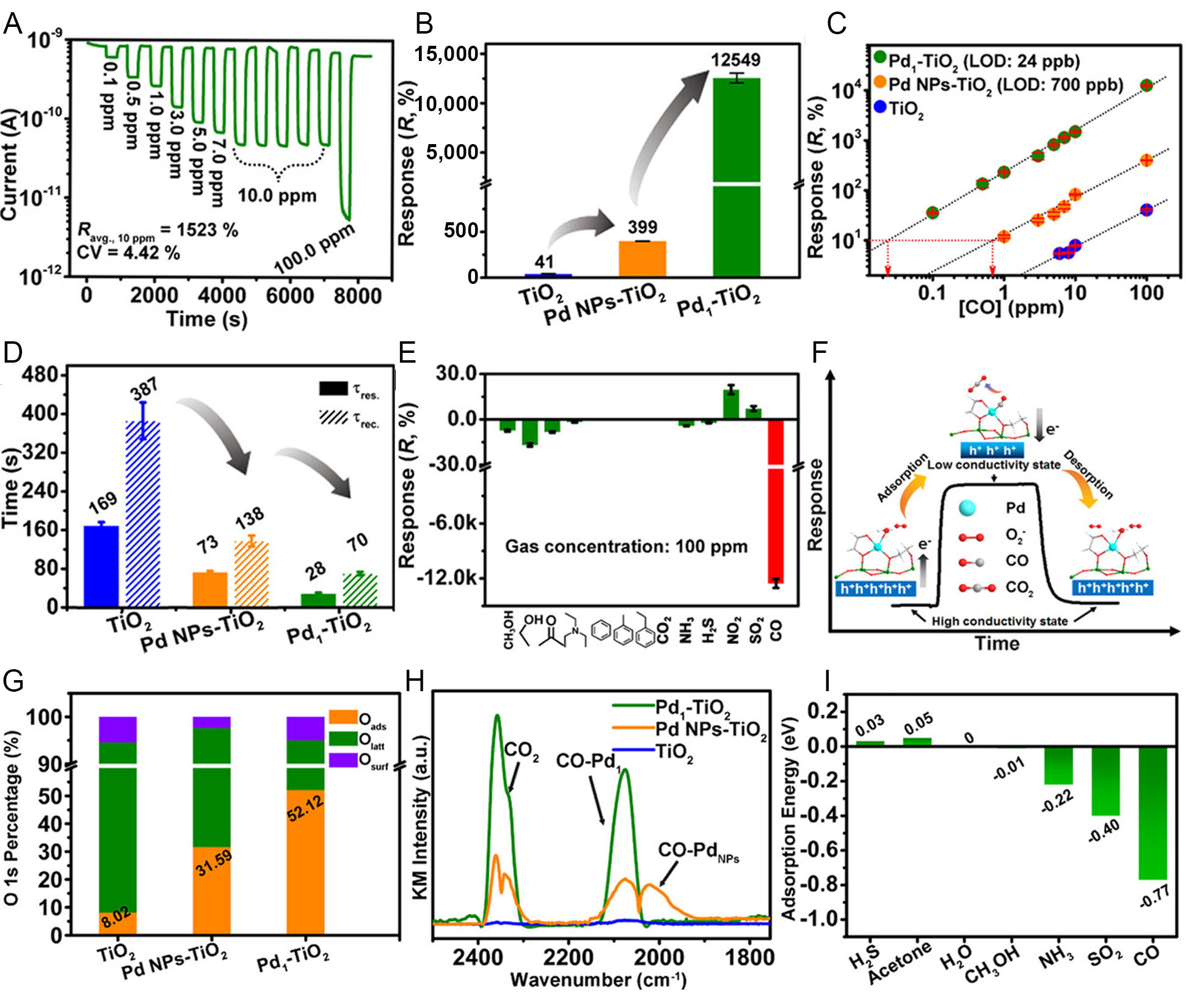
fig5
Figure 5. Enhanced CO sensing performance of atomically dispersed Pd on TiO2 and underlying mechanistic insights. (A) Response-recovery curves of Pd1-TiO2 toward CO at concentrations from 0.1 to 100 ppm; (B) Comparison of CO (100 ppm) responses for TiO2, Pd nanoparticles-loaded TiO2 (Pd NPs-TiO2), and Pd1-TiO2 sensors; (C) Linear correlation between response and CO concentration for TiO2, Pd NPs-TiO2, and Pd1-TiO2; (D) Response and recovery time of different TiO2-based sensors toward 100 ppm CO; (E) Selectivity of Pd1-TiO2 toward CO against various interference gases; (F) Proposed CO sensing mechanism highlighting the Pd-O-Ti interfacial active sites; (G) Relative ratios of surface oxygen species derived from O 1s X-ray photoelectron spectroscopy spectra; (H) In situ diffuse reflectance infrared Fourier transform spectroscopy of different sensors; (I) Density functional theory-calculated adsorption energies of various gases on Pd1-TiO2. Reproduced with permission from Ref.[9]. Copyright 2021, American Chemical Society. LOD: Limit of detection; CV: coefficientof variation; NP: nanoparticle; KM: Kubelka-Munk.