Bench-to-bedside translation of podophyllotoxin-based nanomedicines for cancer treatment: utopias and reality?
Abstract
Cancers represent a complex and multifaceted health challenge, marked by elevated disease burden and fatality rates stemming from both genetic alterations and epigenetic modifications. Nanomedicine, as an emerging field in oncological investigation, optimizes the site-specific delivery of chemotherapeutic compounds through innovative engineering approaches. This technological advancement enables the development of revolutionary strategies for designing precision-targeted treatments that maximize safety and effectiveness. Podophyllotoxin (PPT), a potent aryltetralin-class cytotoxic agent isolated from Podophyllum plants, has become a focal point in anticancer pharmaceutical development. Early research efforts focused on direct PPT administration for tumor management, yet clinical implementation through conventional delivery methods has been constrained by multiple factors including pronounced toxicity profiles, limited aqueous solubility, and narrow therapeutic windows. Advances in nanotechnology and biomaterials effectively enhance PPT therapy's potential for cancer treatment in clinical settings. Various PPT-based nanomedicines have been explored in recent years (2022-2025), including carrier-free nanodrugs, liposomes, polymeric micelles, polymer-drug conjugates, host-guest drug delivery systems, and peptide-based nanoparticles, which utilize passive targeting, active targeting, and stimulus-responsive targeting mechanisms to improve tumor-directed drug delivery. Nevertheless, due to the complexity of the tumor microenvironment, single PPT nanomedicine has suboptimal therapeutic efficacy. PPT-based "combo" drug delivery system facilitates innovative multi-dimensional therapies - such as chemotherapy combined with photodynamic therapy, photothermal therapy, immunotherapy, and chemodynamic therapy - to deliver superior therapeutic benefits and jump the “valley of death”. This review explores delivery strategies of PPT-based nanomedicines and may spark new ideas for multimodal treatment protocols, providing an entry point for professionals to thrive in this exciting field.
Keywords
INTRODUCTION
Malignancy poses a significant threat to human health[1,2]. Surgical intervention, chemotherapy, radiation therapy, and immunotherapy are frequently utilized modalities for cancer treatment, either in combination or as standalone approaches[3-6]. Surgery serves as the primary approach for early-stage solid tumors; however, its efficacy in advanced stages, characterized by the presence of metastatic tumors, is considerably limited[7,8]. In the 1940s, Louis Goodman and Alfred Gilman pioneered the use of nitrogen mustards in the treatment of non-Hodgkin’s lymphoma, marking a significant milestone in the history of chemotherapy[9]. Currently, chemotherapy (such as paclitaxel, vincristine, and irinotecan), an irreplaceable treatment, remains the most widely utilized therapeutic modality for various types of tumors due to its effectiveness in targeting both primary lesions and distant metastases[10-13]. Despite notable advancements in treatment modalities, clinical outcomes associated with chemotherapy continue to be suboptimal[14,15]. This is attributable to several factors, including narrow therapeutic windows, prominent tissue damage, inadequate curative effects, short circulation half-lives, suboptimal drug pharmacokinetics, and difficulties in achieving effective drug delivery within solid tumors[16]. Consequently, there is a clear need for ground-breaking tactics and more effective therapies that focus on new mechanisms and enhanced drug delivery technologies.
Natural products have attracted considerable interest from chemists and pharmacologists due to their diverse chemical and biological activities[17,18]. For years, researchers have explored natural products as solutions to diseases; healthcare professionals are increasingly recognizing the potential of plant secondary metabolites in diseases (including cancer) prevention and treatment[19-25]. Podophyllum plants have long been used in traditional folk medicine for their notable medicinal properties[26]. Podophyllotoxin (PPT, PODO, POD; C22H22O8; Figure 1A) is an aryltetralin-type lignan derived from Podophyllum species, first described by Charles Linnaeus in 1753[27]. PPT, one of the most promising chemotherapeutic agents, possesses a chemical structure characterized by cohesive ring systems and a distinctive spatial configuration. Its multiple chiral centers play a significant role in contributing to its optical activity and biological efficacy. This complex structure underlies its pharmacological effects and influences physical properties such as hydrophobicity, which affects its behavior and applications in vivo[28,29]. PPT was added to the United States Pharmacopoeia in 1820 and is effective in treating venereal warts[30]. Currently, pure PPT serves as an active ingredient in the product Podofilox, which contains 0.5% PPT and is utilized for the treatment of genital warts caused by human papillomavirus[31]. Due to its significant antitumor efficacy, PPT, a tubulin inhibitor, has garnered substantial interest in research focused on targeting various cancers, including lung cancer, breast cancer, hepatocellular carcinoma, leukemia, and colorectal cancer[32-36]. PPT inhibits cell growth by blocking tubulin polymerization, disrupting mitotic spindle formation[37]. Initial studies aimed to use PPT directly for tumor treatment; however, various limitations (including poor water solubility, toxic side effects, and inadequate stability) hindered its effectiveness[38-40]. To enhance the anticancer efficacy of PPT, structural modifications have been made to develop chemotherapy derivatives that exhibit reduced side effects and improved effectiveness[41,42]. The main goal of altering the ring structure is to boost cytotoxic activity and inhibit topoisomerase II, primarily targeting the A, C, D, and E rings. Consequently, this effort has led to the development of etoposide, teniposide, and etopophos, all of which have been used in clinical settings[43-45] [Figure 1B and C]. Nevertheless, these drugs continue to be associated with significant adverse effects, including nausea, cardiomyopathy, and myelosuppression[46-48].
Figure 1. Chemical structure of PPT (A); FDA-approved derivatives etoposide, teniposide, and etopophos (B); Structure-activity relationships pertaining to PPT (C); Timeline of FDA-approved landmark nanomedicines for cancer treatment (D).
To address the limitations, there is a pressing need for drug targeting strategies that ensure tumor-specific delivery and improve the efficacy-toxicity balance of chemotherapeutic agents. As an emerging field within cancer research, nanomedicine paves the way for groundbreaking strategies in the design of effective and safe targeted therapies, and the demand for such advancements has never been greater[49-55]. Nanotechnology-based drug delivery systems (DDSs) (mainly 10-200 nm) have been extensively explored for their potential in targeted chemotherapy, aimed at treating, monitoring, and diagnosing malignancies[56-60]. To date, over 10 nanomedicines are approved by the U.S. Food and Drug Administration (FDA) for cancer treatment[61,62] [Figure 1D]. Insights from these approved nanomedicines have prompted and inspired the exploration of PPT-based nanoplatforms in cancer and beyond. Over the past few years, significant progress has been made in our understanding of the mechanisms, pharmacology, and therapeutic activities of PPT-based nanomedicines. An increasing number of PPT-based nanoplatforms have been reported, overcoming the unfavorable pharmacokinetic characteristics, as well as toxicity and biosafety concerns of the parent PPT. We sincerely hope that PPT-based nanoplatforms will be proven to be safe and effective anticancer drugs in the next decade. To move the field forward and ensure its transformative impact, it is crucial to consider various approaches. These PPT-based nanoplatforms employ diverse methods - such as passive targeting, active targeting, and stimuli-responsive targeting - to deliver chemotherapy drugs directly to tumors while reducing exposure to non-target tissues[63-65]. Conducting further fundamental research in this area may further clarify the potential applications of PPT-based nanomedicines in cancer treatment.
Advancements in nanotechnology and biomaterials significantly enhance the potential of PPT for cancer treatment within clinical settings[66-70]. In recent years, a variety of PPT-based nanoplatforms have been investigated, including carrier-free nanodrugs, liposomes, polymeric micelles, polymer-drug conjugates, host-guest DDSs, protein-based and peptide-based nanoparticles (NPs, Figure 2). Nanotechnology-based DDSs provide key advantages over traditional methods, such as enhanced bioavailability, increased stability, enhanced cellular uptake, extended vascular circulation, controlled release, improved therapeutic indices, and combination treatment[71-76]. These benefits are particularly crucial for water-insoluble drugs (including PPT), offering a novel approach to their delivery[77,78]. Meanwhile, due to the inherent complexity of the tumor microenvironment, single PPT nanomedicine exhibits suboptimal therapeutic efficacy. Combination therapies (and heterodimers) utilizing multiple treatment modalities can enhance anticancer efficacy synergistically while reducing the dosage of each drug, thus minimizing side effects[79-85]. The PPT-based "combo" DDS enables innovative multi-dimensional therapies - such as chemotherapy in conjunction with photodynamic therapy (PDT), photothermal therapy (PTT), immunotherapy, and chemodynamic therapy (CDT) - thereby providing enhanced therapeutic benefits [Figure 2]. This comprehensive review explores delivery strategies for PPT-based nanoplatforms (between 2022 and 2025[86-105]; Table 1) and aims to inspire innovative ideas for multimodal treatment protocols. Major digital databases, including Web of Science,
Representative paradigms of PPT-based nanoplatforms for cancer treatment
| Type of nanoplatforms | Therapeutic agent | Size [nm] | PDI | Zeta [mV] | Therapy method | Results | Refs. |
| Carrier-free nanomedicines | PPT/Ce6 NPs | 147 | 0.15 | - | Chemotherapy, PDT | Enhancing chemo-photodynamic therapy for effective cancer treatment | [86] |
| PPT/PPa NPs | 130 | 0.17 | -14.8 | Chemotherapy, PDT | Superior tumor targeting, increased cytotoxicity, and improved PDT | [87] | |
| ECT Nano | 84.1 | < 0.2 | - | Chemotherapy | Nanomedicine for dual inhibition of topoisomerase I and tubulin | [88] | |
| FAP NPs | 96.4 | 0.08 | -31.0 | Chemotherapy | Improved blood circulation, greater tumor targeting, and enhanced in vivo antitumor efficacy | [89] | |
| PSSF/BL-193 NAs | 83.5 | 0.14 | -25.0 | Chemotherapy, Chemosensitization | Induces strong antitumor effects with reduced off-target toxicity | [90] | |
| PPT-SS1-PPT NPs | 105.7 | 0.15 | -35.8 | Chemotherapy | Superior antitumor efficacy and reduced off-target toxicity | [91] | |
| Liposomes | PAG/Ce6 LPs | 106 | 0.044 | -18.8 | Combined chemo-photodynamic therapy | Hypoxia-responsive, increased tumor accumulation, and enhanced efficacy | [92] |
| MM-LPs-POD | 101 | 0.129 | -27.0 | Chemotherapy, immune modulation | Enhanced tumor growth and lung metastasis suppression efficacy | [93] | |
| PPCNs | 75 | < 0.1 | 100 | Chemotherapy, immunotherapy | Immunological and antitumor effects, along with improved targeting and reduced side effects | [94] | |
| miR-424@PPCNs | 110 | < 0.1 | Positive | Chemotherapy, immunotherapy, and gene therapy | Reduced tumor growth and extended survival; decreasing immunosuppression | [95] | |
| Podo-NP | 93 | 0.2 | -1.65 | Chemotherapy, immunotherapy | Regress tumors and reverse immunosuppressive microenvironments | [96] | |
| Lc-PTOX-Lps | 169 | 0.19 | -24.37 | Chemotherapy | Improved effects on MCF-7 cells with lower toxicity to normal cells | [97] | |
| Polymeric micelles | HA-S-S-PPT micelles | 98.3 | 0.286 | -44.67 | Chemotherapy | pH/reduction dual-responsive, excellent antitumor efficacy, minimal systemic toxicity | [98] |
| HP micelles | 131 | 0.288 | -50.37 | Chemotherapy | pH-sensitive hyaluronic acid, improved chemotherapy | [99] | |
| FA-HA-SS-PPT NPs | 69.7 | 0.252 | -18.7 | Chemotherapy | GSH-responsive, dual targeting of FR+CD44 overexpressing tumors | [100] | |
| MTX-PPT-micelles | 107 | 0.235 | -11.5 | Chemotherapy, immunomodulatory | Inhibit proliferation, improve tumor immunosuppression | [101] | |
| Polymer-drug conjugates | GBEPPT | 52.8 | 0.116 | -6.84 | Chemotherapy | GGT-activated charge reversal and mitochondrial disturbance-induced mitocytosis | [102] |
| Peptide-based Nanoparticles | PEG-Pep-PPT NPs | 108.7 | 0.295 | -6.41 | Chemotherapy | Improve in vivo inhibition of tumor growth, intratumoral microvessel density, and decrease systemic toxicity | [103] |
| Protein-based Nanoparticles | Nano-PPT | 90.0 | 0.120 | 26.5 | Chemotherapy, immunotherapy | Enhance antitumor immunity via mechanotransduction | [104] |
| Host-guest drug delivery systems | ACB-L/Fe-SS-PPT NPs | 100 | < 0.1 | 14.0 | Chemo/chemodynamic/photothermal therapy | Targeting of cancer cells, NIR-enhanced Fe2+ triggered •OH generation, and GSH consumption for cancer treatment | [105] |
CARRIER-FREE NANOMEDICINES
The clinical translation of nanomedicine delivery systems faces barriers due to complex preparation processes and low drug loading capacities[106-110]. Since 2022, carrier-free nanomedicine has emerged as a cutting-edge research focus within the field of drug delivery, representing an innovative direction for drug development[111-115] [Figure 3].
Unlike traditional DDS, carrier-free nanomedicines do not depend on additional carrier materials to encapsulate or transport the prodrugs or pure drugs (e.g., single or multiple drugs)[116,117]. Instead, they primarily utilize the intrinsic physical and chemical properties of the drug molecules themselves to form nanoscale aggregates through self-assembly, with particle sizes typically ranging from 10 to 100 nanometers[118,119]. During the preparation process, drug molecules spontaneously aggregate to create stable nanostructures via intermolecular interactions such as hydrogen bonding, hydrophobic interactions, and
Carrier-free, noncovalent NPs for chemo-photodynamic combination therapy
The complexity, diversity, and heterogeneity of tumors can significantly hinder the effectiveness of monotherapy, presenting substantial challenges in identifying optimal treatment strategies[139,140]. Consequently, an increasing number of researchers have advocated for the combination of two or more therapeutic approaches to achieve enhanced antitumor effects[141]. This strategy, which integrates multiple treatments for tumor management, is referred to as tumor combination therapy[142]. Tumor combination therapy effectively addresses the limitations associated with single-agent therapies[143,144]. PDT is a non-invasive cancer treatment that utilizes photosensitizers, light, and oxygen [Figure 4A][79,145-150]. PDT for cancer, a dynamic multi-stage procedure, typically consists of four components [Figure 4B]. The initial phase involves the administration of an inactive form of PS to the patient through topical application or systemic injection. Subsequently, PS can be selectively taken up by tumor cells or localized within tumor tissues. The third stage involves activating the PS by exposing the tumor site to the appropriate wavelength of light, which depends on the specific PS used. Finally, reactive oxygen species (ROS) are generated, which have cytotoxic effects on tumor cells and can induce oxidative damage to blood vessels and elicit an inflammatory response leading to targeted cell death or destruction within tumor tissue. However, the development of efficacious photosensitizers for PDT has encountered numerous challenges, including issues pertaining to poor solubility and non-specific tissue accumulation[151,152]. The strategic combination of PDT with conventional chemotherapy holds potential to yield enduring and comprehensive treatment responses[153,154].
Figure 4. (A) Three key components of PDT. (B) The application of PDT in cancer treatment. Reproduced from Ref.[79] with permission from © 2024 The Authors. Published by Elsevier Ltd.
Intrigued by this motivation, Zhang et al. proposed and successfully developed self-sensitive supra-molecular nanoassemblies that enhance antitumor efficacy by co-assembling PPT and chlorine e6 (Ce6)
Figure 5. Illustration of the preparation process and the noncovalent NPs for combined tumor chemotherapy and PDT. (A) The preparation process of dual-drug nanoassemblies and its action mechanism; (B) In vivo therapeutic performances. **P < 0.01, ***P < 0.001, and ****P < 0.0001. Reproduced from Ref.[86] with permission from © 2024 Published by Elsevier B.V.
In experiments with 4 T1 breast tumor-bearing mice, combining nanoassemblies with laser irradiation resulted in smaller tumors and stronger antitumor effects, with negligible growth during treatment. The area under the drug-time curve (AUC) for the nanoassemblies was significantly increased, indicating prolonged circulation time and greater accumulation at the tumor site. Importantly, these nanoassemblies reduced toxicity associated with Ce6 and organic solvents while maintaining overall safety; liver and kidney function indexes remained normal, and H&E staining revealed no significant tissue damage. Breast cancer remains the leading cause of malignancy-related deaths in women[155,156]. These nanoassemblies present an efficient and safe platform for tumor therapy[86].
The synergistic effect of PPT and Ce6 in nanoassemblies enhances the antitumor effect through multiple mechanisms. Specifically, the following points are key: (1) Enhanced cellular uptake: PPT/Ce6 NPs improve cellular uptake of drugs; (2) Synergistic generation of ROS: under laser irradiation, PPT and Ce6 work together to produce a number of ROS. This synergistic effect resulted in an increase in ROS levels within tumor cells, which enhanced the killing effect of the cells; (3) Pharmacokinetic advantages: compared with PPT/Ce6 solution, the area under the drug time curve (AUC0-24) of nanoassemblies is significantly increased (2.6 times), and the half-life is extended (1.8 times); and (4) In vivo antitumor activity: compared with PPT alone or PPT/Ce6 mixtures, the nanoassemblies exhibited stronger tumor inhibition under laser irradiation, with the smallest tumor volume and the lowest tumor loading rate. The PPT/Ce6 nanoassemblies circulate in the blood for a longer time, increasing the probability of their accumulation at tumor sites. In summary, the synergistic effect of PPT and Ce6 in nanoassemblies significantly enhances the antitumor effect by enhancing cell uptake, enhancing ROS production, achieving self-sensitive release, and improving pharmacokinetic properties.
It is worth noting that the size of NPs significantly affects their behavior in the body after intravenous injection, influencing blood circulation, accumulation, and retention in tumors. Particles larger than
Computer-aided, noncovalent NPs for chemo-photodynamic combination therapy
To achieve the optimal synergistic ratio of drugs in carrier-free supramolecular nanoassemblies, it is crucial to consider both the physicochemical properties of the drugs and their interactions within the nano-assembly[158]. Computational simulations allow researchers to investigate atomic-level interactions among molecular components[159,160]. These tools can analyze various parameters, including drug interactions, to simulate different combinations and their potential outcomes[161,162]. By employing these methods, researchers can identify those combinations that maximize synergistic effects while ensuring stability and effective drug delivery by uncovering the driving forces behind NP formation.
The development of self-delivery NPs made from PPT and Pyrrolidine derivative (pyropheophorbide-α (PPa), a modified form of chlorophyll) offers a promising strategy for synergistic cancer therapy.
Figure 6. Illustration of the preparation process and chem-PDT synergistically enhanced cancer therapy of PPT/PPa NPs with computer-aided design. (A) The computer-aided design of PPT/PPa NPs; (B) Combination therapy of PPT/PPa NPs under 660 nm laser irradiation; (C) In vivo synergistic anti-tumor efficacy of PPT/PPa NPs under 660 nm laser treatment. *P < 0.05, and **P < 0.01. Reproduced from Ref.[87] with permission from © 2020 Published by Elsevier B.V.
PEG chains are commonly attached to liposomes, providing "stealth" properties[163,164]. This modification creates a hydrophilic barrier that enhances liposome stability and reduces protein adsorption, preventing rapid clearance by the reticuloendothelial system[165,166]. The average diameter of PPT/PPa NPs is about
Advanced computational modeling has further accelerated NP optimization by elucidating structure-function relationships, informing design improvements for enhanced therapeutic performance. These collective results advocate for progressing PPT/PPa NPs toward clinical translation in oncology. It should be emphasized that the self-assembled PPT/PPa NPs maintain structural integrity through noncovalent interactions, which may destabilize under physiological conditions. Such instability could lead to premature payload release during systemic circulation, potentially diminishing tumor-specific drug accumulation while increasing off-target toxicity risks. Current research focuses on developing stabilization strategies, including surface functionalization with amphiphilic polymers that reinforce NP integrity without impeding tumor penetration capabilities.
Natural product-empowered, GSH-responsive, covalent NPs for tumor chemotherapy
Natural products have been critical to the development of multi-drug regimens currently used in cancer chemotherapy and are used to treat a variety of malignancies[167-173]. Meanwhile, the use of natural products as adjuvants or sensitizers for chemotherapy represents a promising strategy in cancer treatment[174,175].
Figure 7. Schematic illustration of a BL-193 prodrug nanoassembly that elicits strong antitumor responses while minimizing off-target toxicity. (A) Preparation of PSSF/BL-193 NAs and its action mechanism; (B) Molecular docking simulation of PSSF and BL-193; (C) Cytotoxicity of PPT Sol or PPT/BL-193 Sol against 4T1 cells; (D) In vivo antitumor evaluation, ****P < 0.0001. Reproduced from Ref.[90] with permission from © 2024 Elsevier B.V.
The study began with the observation that BL-193, when administered at a non-cytotoxic dose, significantly enhanced the sensitivity of breast cancer cells to PPT. BL-193 alone demonstrated minimal cytotoxicity, failing to induce substantial cellular death even at a concentration of 2 μM. Meanwhile, BL-193 can be safely utilized at higher concentrations without negatively affecting normal cell viability, rendering it an appealing candidate for combination therapy. The introduction of BL-193 at a non-cytotoxic concentration of 0.50 μM significantly enhanced PPT's cytotoxic effects on 4T1 breast cancer cells, indicating a synergistic effect with PPT. In non-cancer cell lines, mouse embryonic fibroblasts (3T3 cells) were used to assess the cytotoxicity of prodrug strategies. Prodrug PSSF exhibited significantly lower cytotoxicity than PPT, with its IC50 value increasing by over 40 times. Next, Lin et al. conducted a comprehensive study on various dose ratios (1:0 to 1:9) of PSSF combined with BL-193, using a one-step nanoprecipitation method to enhance therapeutic efficacy[90]. The 1:5 molar ratio showed the strongest cytotoxic synergy in 4T1 cancer cells, achieving an IC50 value of 0.233 μM, which is 1.73 times lower than that of PSSF alone. For comparison, self-assembled PSSF NAs without BL-193 were prepared as controls. Both PSSF NAs (diameter = 87.60 nm, PDI = 0.13,
BL-193 alone exhibited minimal cytotoxicity to 4T1 cells, but PSSF/BL-193 NAs (IC50 = 0.382 μM) showed more powerful cytotoxicity than PSSF NAs (IC50 = 1.120 μM), indicating that BL-193 enhances tumor-specific chemotherapy sensitization. Additionally, the cytotoxicity of PSSF NAs (IC50 > 2.0 μM) or PSSF/BL-193 NAs (IC50 > 2.0 μM) in normal 3T3 cells was lower than that of PPT solution
This study combined the prodrug nanoassembly technology with a natural sensitizer (BL-193) to form a new chemotherapy regimen. This combination not only reduces the toxicity of prodrug, but also improves its therapeutic efficiency, especially in dealing with the delayed and insufficient activation of the prodrug. Although BL-193 as a chemotherapy sensitizer can open the window of ultra-low dose chemotherapy, in practical applications, accurately optimizing the ratio of PSSF and BL-193 to achieve optimal efficacy remains a challenge. Continued investigation into the specific mechanisms of action of BL-193 and its interactions with PPT will be critical.
Overall, carrier-free NP-based DDSs show promise in enhancing drug efficacy and reducing side effects. However, their clinical translation faces challenges, such as limited effectiveness compared to established formulations[176,177]. Researchers have developed several strategies to address these issues. Additive and polymer modification[178,179]: Adding small amounts of additives or modifying NPs with polymers can improve stability and extend in vivo circulation time, thus enhancing drug delivery efficiency. Introduction of targeting agents[180,181]: Attaching targeting molecules to NPs directs them to specific tumor sites, increasing local drug concentration while minimizing damage to healthy tissues. Responsiveness to linkers and external stimuli[182,183]: Utilizing responsive linkers (e.g., amide bonds, ester bonds) or external stimuli (e.g., heat, light) allows for site-specific drug release at tumors, accelerating therapeutic effects. Adjustment of physicochemical properties[184,185]: Optimizing the morphology, size, charge, or surface chemistry of NPs can enhance their in vivo behavior. Additionally, further investigation is needed on how additive and polymer modifications influence NP safety and the selection and design of targeting agents for improved specificity and effectiveness. By continuously optimizing carrier-free NP design and performance, it is hoped that current limitations will be overcome for broader clinical application.
LIPOSOMES NANOMEDICINES
The pioneering work by Alec D. Bangham in the development of liposomes in the mid-20th century has significantly shaped the landscape of modern medicine, offering innovative solutions for targeted drug delivery and personalized medical interventions[186]. This enduring legacy continues to inspire ongoing research and innovation in the dynamic field of nanomedicine[187-190]. Liposome therapy, characterized by its remarkable biocompatibility, degradability, and low immunogenicity, has significantly transformed the landscape of cancer treatment [Figure 8][191,192]. On the one hand, liposomes consist of one or more layers of concentric phospholipid bilayers that encase inner aqueous compartments. These nanocarriers markedly improve the dissolution properties and chemical integrity of various substances spanning low-molecular-weight compounds, nucleic acids, and protein-based molecules[193,194]. This architectural design preserves the integrity and bioactivity of therapeutic compounds during systemic circulation until reaching pathological sites[195]. Surface modifications employing diverse targeting moieties such as antibodies, aptamers, folate derivatives, and peptide conjugates demonstrate enhanced tumor specificity[196-200]. Such molecular engineering improves cellular uptake efficiency and enables precision targeting through biological recognition mechanisms. Engineered release kinetics further allow temporal regulation of pharmaceutical agents, enabling prolonged therapeutic exposure through controlled liberation mechanisms[201-203]. The amphiphilic nature of these vesicles enhances compound bioavailability while achieving spatial-temporal delivery control with reduced adverse reactions[204,205]. Collectively, these nanoscale systems provide a versatile platform for oncological interventions through coordinated mechanisms of molecular stabilization, tissue-selective transport, and sustained release patterns. These characteristics establish lipid-based vesicles as sophisticated delivery vehicles capable of transporting bioactive compounds to malignant lesions with improved therapeutic precision. The unique amphiphilic structure permits PPT encapsulation within their aqueous core or incorporation into phospholipid membranes, forming a protective shield against destabilizing environmental factors while maintaining pharmacological activity.
Hypoxia-responsive dual-drug liposomes for combined tumor chemotherapy and PDT
The efficacy of PDT is considerably compromised by tumor hypoxia, which results from the consumption of oxygen during the therapeutic process[206,207]. To improve the outcomes of PDT, various strategies have been developed to alleviate hypoxic conditions within tumors. These measures include supplemental oxygen, use of oxygen generators, downward adjustment of oxygen consumption and use of low-oxygen microenvironments[208-211]. Utilizing hemoglobin as a carrier can effectively deliver oxygen to tumor sites. Hemoglobin has a high affinity for oxygen and can release it in the low pH environments typical of tumor tissues, thereby increasing local oxygen concentrations during PDT[212-215]. Perfluorocarbons are another promising strategy for oxygen delivery; they can dissolve large amounts of oxygen and facilitate its transport to hypoxic areas within tumors, enhancing the efficacy of PDT[216-219]. Catalase can decompose hydrogen peroxide (H2O2) into water and oxygen, providing an endogenous source of oxygen within the tumor microenvironment. By administering catalase, oxygen levels can be elevated, supporting the ROS generation necessary for effective PDT[220-223]. Certain metal compounds have been explored for their ability to generate oxygen. Transition metal-based NPs, for instance, initiate oxygen-generating reactions to counteract hypoxic conditions in PDT settings[224,225]. Approaches focusing on minimizing oxygen consumption of tumor cells may preserve elevated oxygen concentration. Such methods could involve disrupting oxygen-dependent biochemical processes, thereby creating optimal conditions for effective PDT implementation. Hypoxia-activated prodrug development embodies a novel strategy for targeted drug delivery within oxygen-deprived tumor microenvironments[226-229]. Mitigating tumor hypoxia remains paramount for enhancing PDT treatment outcomes. The aforementioned techniques - including oxygen enrichment methods and hypoxia-targeted prodrug applications - offer viable pathways for optimizing PDT effectiveness. Continued research will be essential for optimizing PDT protocols and maximizing their clinical effectiveness in treating cancer.
Prodrug engineering has emerged as an innovative pharmaceutical strategy to enhance drug delivery parameters[230,231]. Yu et al. developed a dual-drug liposome using the "turn enemy into friend" strategy to enhance synergistic chemotherapy and PDT [Figure 9][92]. They synthesized an anoxic-responsive prodrug (PAG) and incorporated it with the photosensitizer Ce6 in the liposome. By employing an active loading method based on a metal ion gradient, PAG and Ce6 were optimally loaded in a favorable synergistic ratio. In mouse models, PAG/Ce6 liposomes demonstrated notable antitumor efficacy by effectively inhibiting tumor growth without causing significant toxicity to normal tissues[92]. The study highlighted the excellent biosafety profile of PAG/Ce6 liposomes; there were no significant changes in body weight during treatment, nor was there considerable damage observed in major organs upon histological examination. In summary, Yu et al. present a novel dual-agent liposome platform capable of efficiently releasing hypoxia-responsive prodrugs and photosensitizers in synergy for more effective tumor therapy[92].
Figure 9. Schematic of PAG/Ce6 LPs for synergistic cancer therapy. (A) Preparation of PAG/Ce6 LPs and its action mechanism; (B) In vivo pharmacodynamics evaluation of PAG/Ce6 LPs, **P < 0.01, ***P < 0.001. Reproduced from Ref.[92] with permission from © 2024 Elsevier B.V.
PAG/Ce6 LPs kill tumor cells through several mechanisms: (1) Production of ROS: Under laser irradiation, PAG/Ce6 LPs generate a significant amount of ROS, which directly induces tumor cell death; (2) Depletion of intracellular GSH: PAG/Ce6 LPs consume GSH in tumor cells, disrupting the REDOX balance and triggering apoptosis; (3) Exacerbating the hypoxic microenvironment: the action of PAG/Ce6 LPs intensifies the hypoxic conditions within tumor cells, promoting the release of antitumor drug PPT and enhancing chemotherapy effectiveness. These mechanisms interact synergistically to improve therapeutic outcomes.
The "adversary to ally" tactic repurposes tumor hypoxia (traditionally viewed as a detrimental factor) into a beneficial element that amplifies pharmaceutical activation. This methodology integrates oxygen-sensitive prodrug formulations with light-activated compounds, capitalizing on the tumor's low-oxygen microenvironment to optimize treatment outcomes[92]. Researchers demonstrated successful tumor cell eradication by developing hypoxia-activated polyphenolic prodrugs (PAG) combined with photosensitizing agents (such as Ce6), jointly encapsulated within lipid NPs (PAG/Ce6 LPs). This innovative approach presents three key therapeutic benefits: (1) Optimized pharmaceutical activation: The prodrug system specifically triggers drug release under oxygen-deprived conditions; (2) Multimodal tumor elimination: Cancer cell destruction occurs through dual mechanisms involving ROS production and GSH depletion; (3) Enhanced pharmacokinetic performance: The nanoliposome delivery system prolongs systemic circulation duration while promoting tumor-specific aggregation, thereby maximizing therapeutic potential against malignancies. However, challenges remain: (1) Heterogeneity of tumor hypoxia: varying degrees of hypoxia may lead to inconsistent prodrug activation efficiency; (2) Phototoxicity: While liposomes maintain fluorescence quenching during circulation, photosensitizer activation at tumors may harm surrounding normal tissues; (3) Barriers to clinical translation: Despite their potential, hypoxia-activated prodrugs face limitations in clinical studies with no FDA-approved drugs yet.
MicroRNA-424-based, cationic lipids for combined chemotherapy and immunotherapy
The effectiveness of single-drug chemotherapy is limited by the complexity of the cancer ecosystem[232-234]. Wang et al. proposed a NP system for simultaneous chemotherapy and gene therapy, developing a cationic lipid bilayer NP system (miR-424@PPCNs) for co-delivery of microRNA-424 (miR-424) and PPT as a multimodal anticancer treatment [Figure 10][95]. The combination enhances the sensitivity of non-small cell lung cancer cells to chemotherapy through multiple mechanisms. Specifically, miR-424 directly targets PD-L1 mRNA, reducing PD-L1 expression, an immune checkpoint protein that allows tumor cells to evade immune attacks. By decreasing PD-L1 production, miR-424 promotes T cell attack on tumor cells, enhancing the immune response[235]. MiR-424 is a novel therapeutic target and prognostic factor in malignancies[236]. This multimodal strategy results in higher efficacy with the miR-424@PPCN compared to using miR-424 or PPT alone.
Figure 10. Preparation of miR-424@PPCN complexes and their mechanism of action on tumor cells. (A) Preparation of miR-424@PPCN complexes; (B) Action mechanism of miR-424@PPCN complexes; (C) In vivo antitumor effects of miR-424@PPCN complexes in tumor-bearing mice, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Reproduced from Ref.[95] with permission from © 2022 American Chemical Society.
In the control group, tumor volume rapidly increased to 2030 mm3, while mice treated with the miR-424@PPCN complex showed only 129 mm3, indicating a significant inhibitory effect[95]. Additionally, mice receiving the miR-424@PPCN treatment had significantly longer survival compared to controls. The combination of miR-424 and PPT notably influenced tumor growth. The miR-424@PPCN achieved a mean tumor inhibition rate of 91%, surpassing that of miR-424 (42%) or PPCNs alone (67%)[95]. This suggests that miR-424 enhances the inhibitory effects of PPT on tumor cells by potentially reversing chemotherapy resistance and promoting apoptosis. Although the study mentions the combined therapeutic mechanism of miR-424 and PPCNs, it does not deeply explore the specific molecular mechanisms and signaling pathways, potentially leading to an incomplete understanding of their therapeutic effects.
Bioresponsive liposome nanomedicines
In general, combination therapy surpasses monotherapy by enhancing efficacy and safety[237-239]. This strategy has significantly improved clinical outcomes for tumor patients, including response rates and overall survival[240,241]. Ling et al. investigate the application of bio-responsive NPs in antitumor immunity, specifically examining how these NPs can enhance antitumor immune responses through the integration of anti-angiogenic therapies and chemotherapy regimens [Figure 11][96]. Firstly, by introducing an alkanoyloxymethyl ester linkage between PPT and cholesterol, an esterase-cleavable PPT prodrug (Chol-Podo) was afforded. Then, two bio-responsive NPs were constructed: Podo-NP (prodrug containing PPT) and CbP-NP (prodrug containing carboplatin). By administering sequential treatment to tumors, these NPs promote an antitumor T-cell response. Notably, Podo-NP suppresses tumor angiogenesis through curtailing endothelial cell proliferation and motility, impeding neovascularization while reinforcing preexisting vasculature. Complementarily, CbP-NP augments therapeutic outcomes by disrupting tumor cell cycle progression and triggering programmed cell death. Concurrently, CD40 agonists prime antigen-presenting cells to present tumor-associated antigens derived from apoptotic cells, effectively counteracting immunosuppressive microenvironmental conditions[242,243]. This sequential administration approach combining angiogenesis inhibitors with chemotherapeutic agents and immunomodulators demonstrated significant suppression of primary tumor growth and metastatic progression in murine lung carcinoma models. Both nanocarriers display optimal hydrodynamic diameters (93 nm for Podo-NP vs. 66 nm for CbP-NP) with narrow polydispersity indices (0.2 and 0.1, respectively), ensuring colloidal stability and therapeutic reliability. Stability assessments revealed both formulations maintained structural integrity at
Figure 11. Sequential administration of esterase-responsive Podo-NP, redox-sensitive CbP-NP, and a CD40 agonist enhances the antitumor T cell response. (A) Bioresponsive Podo-NP and CbP-NP; (B) Improving immunotherapy outcomes with sequential antiangiogenesis and chemotherapy; (C) Proposed Podo prodrug bioconversion via esterase-catalyzed cascade hydrolysis. Reproduced from Ref.[96] with permission from © 2020 American Chemical Society.
CD40 is present on several APCs, and agonistic CD40 antibodies (αCD40) elicit broad immunostimulatory responses[244]. CD40 agonists demonstrate substantial potential in amplifying immunotherapy outcomes through multifaceted modulation of the tumor microenvironment and immune cell dynamics[245-247]. Key pathways involve dendritic cell activation, tumor niche remodeling, immune cell recruitment enhancement, and macrophage polarization control. Such activation drives the differentiation of naïve T cells into tumor-targeting cytotoxic effectors while potentiating systemic antitumor immunity[96]. The immunomodulatory effects extend to converting suppressive tumor microenvironments into immune-permissive states. Combined regimens with angiogenesis inhibitors and chemotherapeutic drugs reveal synergistic effects, where CD40 agonists mitigate immunosuppression by altering T cell composition - suppressing regulatory T cell populations while expanding both CD4+ helper and CD8+ cytotoxic T cell infiltration. Marked increases in tumor-infiltrating CD4+ and CD8+ T lymphocyte populations post-treatment[96], indicating enhanced immune cell trafficking to tumor sites that strengthens localized antitumor responses.
In different treatment groups, changes in tumor growth inhibition (TGI) rate reflect the effectiveness of each treatment strategy. Specifically, the TGI of Etop + Carb, CbP-NP, and Podo-NP alone was 44.7%, 48.1%, and 31.0%, respectively[96]. Although these treatments have some tumor inhibition, the effect is relatively limited. However, when treated with Podo-NP in combination with CbP-NP, TGI was significantly increased to 70.6%. Furthermore, with the addition of αCD40, TGI was further increased to 89.9%[96]. The strategy of combining Podo-NP and CbP-NP significantly enhances the antitumor effect, which is further enhanced by the addition of αCD40. In different treatment groups, measurements of tumor weight showed that the Podo-NP + CbP-NP + αCD40 group had significantly lower tumor weight than the other groups (0.08 ± 0.01 g), indicating the effectiveness of the combination therapy in inhibiting tumor growth. Collectively, the observed alterations in TGI rates and extended survival durations provide robust evidence for the therapeutic efficacy of the combination therapy approach.
Furthermore, the sequential therapeutic approach exhibits multiple benefits compared to single-agent treatments regarding tumor suppression effectiveness. These include amplified immune cell penetration, diminished immunosuppressive tumor microenvironment, and facilitated recruitment of M1-polarized macrophages. Experimental groups displayed marked increases in tumor-infiltrating CD3ε+CD4+ helper T lymphocytes (Ths) and CD3ε+CD8a+ cytotoxic T cells (Tcs) post sequential treatment. Quantitative analysis revealed the infiltration levels climbing to 24.4% in the triple combination group, in stark contrast to the 5.9% observed in phosphate-buffered saline (PBS)-treated counterparts. Tcs infiltration rates similarly demonstrated substantial elevation at 31.6%, vs. a minimal 2.0% in control groups. These metrics confirm the regimen's capacity to boost immune cell accumulation at neoplastic sites. The protocol effectively suppressed immunosuppressive Treg populations, with triple therapy groups showing 33.2% Treg levels compared to 93.2% in PBS controls[96]. Sequential administration notably elevated CD86 expression (M1 macrophage marker) to 52.8%, dramatically exceeding the 2.7% baseline in PBS groups. Conversely, CD206 expression (M2 macrophage indicator) plummeted to 6.1% vs. 57.5% in controls, indicating successful macrophage repolarization toward antitumor phenotypes. Collectively, the Podo-NP/CbP-NP/αCD40 regimen presents a multifaceted solution for immunologically inert tumors such as LL/2 Lewis lung carcinoma[96]. By first addressing vascular abnormalities before modulating the tumor microenvironment and triggering comprehensive immune activation, this methodology shows promise for enhancing clinical outcomes in comparable malignancies, highlighting its value in multimodal therapeutic strategies.
Liposomal biomaterials offer promising opportunities for tumor treatment[248,249]. As a nanodrug delivery system, liposomes effectively transport chemotherapy agents, genes, antibiotics, and other therapeutic compounds[250-254]. Modifying the structures of liposomes can enhance drug delivery and targeted therapy while minimizing adverse effects. Current research highlights the potential of liposome-based nanomedicine in cancer; however, systematic studies are necessary to determine maximum tolerated doses, elucidate drug properties, and analyze metabolites. Furthermore, the complexity inherent in lipid nano-delivery systems presents challenges for mass production; clinical applications necessitate scalable manufacturing techniques that are both cost-effective and reliable while ensuring long-term storage and sterilization capabilities.
POLYMERIC MICELLES
Nanomicelles form through self-assembly of amphiphilic molecules such as block copolymers in aqueous environments[255]. This structure provides excellent solubilization capabilities that substantially improve PPT's solubility. A nanomicelle's core consists of hydrophobic chain segments for encapsulating PPT, while its shell comprises hydrophilic chain segments that ensure good solubility and stability in water. The rational design of targeted micelles involves four endogenous targeting strategies [Figure 12[255]]: (1) Modifying micelles with specific fragments to enhance drug delivery; (2) Developing stimulus-responsive micelles that release drugs in response to endogenous stimuli such as ROS; (3) Attaching ligands to micelles for high-affinity recognition of overexpressed receptors at target sites, improving tissue accumulation; (4) Surface modifications on micelles to evade immune detection within cells, enhancing targeting efficacy and therapeutic outcomes.
Figure 12. Designing polymeric micelles for targeted drug delivery. Reproduced from Ref.[255] with permission from © 2024 Elsevier Ltd.
Active targeting incorporates specific antibodies, ligands, and other moieties into NP carriers by utilizing receptors that are highly expressed in tumor tissues or unique to these environments[256,257]. This process leverages the precise binding interactions between receptors and ligands, as well as the efficient engagement between antibodies and antigens, facilitating targeted drug delivery. Commonly used targeting ligands include polysaccharides (such as hyaluronic acid (HA) and mannose), small molecular compounds (such as biotin), peptides (e.g., arginine-glysine-aspartic acid (RGD) peptides), and proteins (including antibodies and transferrin [Tf])[258-264].
pH-sensitive, hyaluronan-targeted polymeric micelles
Tumor tissue has a weakly acidic environment, with a pH of 6.5 to 6.8, compared to the normal tissue pH of about 7.4[265]. This difference is significant as tumor cells proliferate rapidly and often face inadequate blood supply, leading to hypoxia[266]. Consequently, some tumor cells rely on glycolysis for energy, increasing lactic acid levels and contributing to the acidity[267]. Intracellular compartments such as endosomes and lysosomes have even lower pH values (4.5 to 5.5)[268]. Utilizing the pH gradient between tumor and normal tissues enables the development of pH-sensitive NP DDS that remain stable in circulation while reducing drug leakage[269,270]. In response to acidic conditions in tumors, these systems can release antitumor drugs directly at the site, enhancing local drug concentration and improving therapeutic efficacy. At low pH, these linkages become unstable and undergo hydrolytic cleavage, resulting in drug release. Common acid-labile bonds include ester, amide, hydrazone, acetal or ketone groups, and borate esters[271-274]. Another pH-sensitive DDS utilizes proton donor or acceptor groups, such as imidazole and acrylic moieties, to modify nanomedicine carriers[275,276]. This modification results in drug carriers that can effectively respond to fluctuations in environmental pH levels. These protonic groups possess specific pKa values that determine their hydrophilic or hydrophobic state depending on the solution pH. When the pH exceeds the pKa of the proton group, the segment containing it becomes hydrophobic, promoting polymer self-assembly into micelles. As the pH gradually decreases, an increasing number of protonation sites become protonated; consequently, the hydrophilicity of the micelles is enhanced. This alteration leads to instability or dissociation of the micelles, resulting in drug release.
Li et al. developed a novel pH-sensitive HA-targeted prodrug micelle, named HA-CO-O-PPT, to enhance chemotherapy efficacy [Figure 13][99]. The pH-sensitive prodrug is synthesized by conjugating the hydrophobic antitumor agent PPT with hydrophilic HA via a one-step esterification reaction. In an aqueous environment, the prodrug self-assembles into nano-spherical micelles (HP micelles), exhibiting excellent serum stability and blood compatibility. Under simulated physiological conditions (pH = 7.4), HP micelles maintain their particle size even in media containing 10% fetal bovine serum (FBS), indicating structural integrity during circulation. They demonstrate a pH-responsive drug release mechanism in acidic tumor microenvironments, achieving an 81.2% drug release at pH 5.0. Additionally, these micelles facilitate targeted drug delivery and controlled release through efficient cellular uptake (over 97%)[99]. Hemolysis tests showed a hemolysis ratio of less than 5%, confirming excellent blood compatibility and minimal blood damage when used in vivo, thereby reducing side effect risks.
Figure 13. Illustration of the fabrication of pH-responsive HP micelles for the controlled and targeted delivery of PPT, enhancing cancer therapy. Reproduced from Ref.[99] with permission from © 2022 Elsevier B.V.
In vivo experiments showed that MCF-7 cells implanted in Balb/c mice exhibited stronger fluorescence signals from fluorescein isothiocyanate (FITC)-labeled HP micelles compared to the control group[99]. For antitumor activity, MCF-7 tumor-bearing Balb/c nude mice were divided into three groups and injected with normal saline, free PPT, or HP micelles (equivalent to a PPT dose of 15 mg/kg). Over 30 days of treatment, the saline group experienced significant tumor volume increase, while the free PPT group had a slight increase. In contrast, the HP micelles group showed no significant growth increase and achieved an 85% inhibition rate[99]. TdT-mediated dUTP-biotin nick end labeling (TUNEL) experiments revealed the highest tumor cell apoptosis rate in the HP micelles group, indicating superior therapeutic efficacy against tumor growth. Further investigation is needed on the combination of HP microcapsules with other antitumor drugs to enhance therapeutic effects and reduce drug resistance. Meanwhile, to facilitate clinical application, the following studies must be conducted: further investigation into the degradation mechanisms of targeted nano-delivery carriers in a simulated tumor microenvironment; and clarification of drug release sites, associated degradation processes, and the therapeutic effects of resulting degradation products.
Hyaluronan micelles targeting FR + CD44 overexpressing tumors
Small molecular substances such as folic acid (FA), anisamide, biotin, and phenylboric acid are favored as ligands for drug targeting vectors due to their stability, low cost, low toxicity, and ease of modification[277-280]. FA is the most widely used targeting ligand; it is a vitamin with a natural affinity for folate receptor (FR) protein and can be converted to tetrahydrofolic acid in the body to participate in normal metabolism[281]. FRs are highly expressed in tumor tissues, with levels in malignant cells being 20 to 200 times higher than in normal cells[282]. The expression of FRs correlates with the severity of malignancy and metastatic potential - metastatic tumors express more FRs than localized tumors[283]. Thus, FA is extensively utilized as an active targeting ligand for NPs in anticancer therapy. Zhang et al. have developed an innovative double-targeted nanodrug delivery system aimed at tumor cells that overexpress FRs and cluster determinant 44 (CD44)
Figure 14. Preparation and biocompatibility of FA-HA-SS-PPT and FA-HA-CC-PPT NPs. (A) Preparation of FA-HA-SS-PPT and FA-HA-CC-PPT NPs; (B) Body weight and tumor volume after treatment with vehicle, free PPT, etoposide, FA-HA, FA-HA-CC-PPT, FA-HA-SS-PPT, **P < 0.01, ***P < 0.001. Reproduced from Ref.[100] with permission from © 2025 Elsevier B.V.
In murine models, FA-HA-SS-PPT NPs achieved a TGI rate of 73.1%, surpassing both free PPT (43.5%) and FA-HA-CC-PPT NPs (41.5%)[100]. Biodistribution studies further indicated 2.3-fold greater tumor accumulation of FA-HA-SS-PPT compared to Rh-PPT, confirming enhanced tumor-targeting characteristics. This improved targeting mechanism and therapeutic outcome primarily stems from the NPs' dual-targeting molecular architecture.
The fabrication of FA-HA-SS-PPT presents intricate challenges due to its multi-stage synthesis process, which poses obstacles for industrial-scale manufacturing. Streamlining the production pathway by minimizing procedural stages and improving operational efficiency may significantly advance its scalability. While existing investigations have predominantly utilized in vitro analyses and murine models, expanding preclinical evaluations to diverse cancer subtypes remains crucial for validating FA-HA-SS-PPT's therapeutic potential and biosafety profile. Although dual-targeting approaches have been implemented, variations in tumor biology might influence treatment outcomes, highlighting the necessity for comprehensive exploration of the compound's versatility across heterogeneous malignancies.
While considerable progress has been made in developing targeted polymeric micelles, their translation from experimental research to clinical implementation remains fraught with difficulties. Multiple barriers persist, including manufacturing scalability, unresolved safety profiles, insufficient pharmacokinetic characterization, and ambiguous biodistribution patterns post-administration. The intricate biological milieu further complicates clinical adaptation, as nanocarrier-biological component interactions frequently demonstrate unanticipated behaviors that may modulate immune activation pathways and metabolic processing. Future investigations should focus on three key areas: first, analyzing structural parameters including nanocarrier dimensions and surface functionalization in relation to biomolecular corona formation; second, deciphering immune-modulatory mechanisms of polymeric assemblies; third, improving the reliability of in vitro screening platforms for assessing batch-to-batch reproducibility and therapeutic performance predictability. In addition, although FA-HA-SS-PPT NPs have good biocompatibility, it is still necessary to ensure their long-term safety and non-toxicity in clinical applications, especially during metabolism and excretion in the body. Conduct larger animal experiments to evaluate the efficacy and safety of FA-HA-SS-PPT NPs to provide data support for clinical trials.
OTHER PPT-BASED NANOPLATFORMS
Polymer-drug conjugates
Polymer-drug conjugates, commonly termed polymer prodrugs, spontaneously form nanostructured particles when exposed to aqueous environments, facilitating tumor accumulation through EPR mechanisms[284,285]. The covalent linkages bridging therapeutic agents with polymeric carriers are frequently designed to be sensitive to pathological conditions within tumor microenvironments. Such hybrid systems demonstrate enhanced stability and controlled release profiles in biological systems while preserving therapeutic efficacy[286,287]. These systems serve as effective strategies to address challenges linked to PPT, particularly regarding biodistribution and site-specific activation[288]. Glioblastoma (GBM) is a highly aggressive brain tumor that falls under the category of gliomas and primarily originates from astrocytes[289-291]. The key characteristics of GBM include rapid proliferation, aggressiveness, high recurrence rates, and resistance to treatment[292,293]. From a pathological perspective, GBM is typically associated with various genetic mutations and epigenetic alterations, such as abnormalities in the tumor protein P53 (TP53), epidermal growth factor receptor (EGFR), and phosphatase and tensin homolog deleted on chromosome ten (PTEN) genes, which facilitate the proliferation and survival of tumor cells[294-296]. Treatment challenges for GBM are significant. One major obstacle is blood-brain barrier (BBB) penetration: effective therapies must be able to cross the BBB to reach the tumor site; however, this process poses considerable difficulties. Additionally, tumor heterogeneity complicates treatment efforts since the diversity among GBM cells often renders single-agent therapies ineffective. Although small molecules, peptides or proteins have been identified that can target DDS to enhance their accumulation within GBMs, achieving deep tissue penetration remains problematic.
Xiang et al. proposed that enhancing drug penetration and therapeutic efficacy for GBM can be achieved through the design of nanodrug carriers that are responsive to the unique microenvironment of tumors[102]. This innovative approach in nanomedicine has the potential to demonstrate a remarkable capacity to effectively penetrate both the BBB and deeper layers within GBM tissues, thereby improving therapeutic outcomes while minimizing systemic toxicity. Consequently, Xiang et al. developed a novel polymer-drug conjugate known as GBEPPT (pGBEMA22-b-pSSPPT9), which responds specifically to overexpressed
Figure 15. Mitocytosis to improve intercellular transfer of GBEPPT for glioblastoma therapy. (A) Self-assembly and charge reversal processes; (B) Mitocytosis for deep penetration. Reproduced from Ref.[102] with permission from © 2024 Wiley-VCH GmbH.
The penetration patterns of GBEPPT and GEPPT across the BBB and their tumor distribution revealed marked variations. GBEPPT exhibited superior efficacy in traversing the BBB and achieving comprehensive intratumoral dispersion, while GEPPT remained predominantly localized in neoplastic periphery. Conversely, GEPPT's limited permeability appeared related to GBEPPT's unique mitochondrial modulation mechanism - GGT-activated mitocytic processes enhanced cellular internalization efficiency. Pharmacological disruption using nocodazole substantially impaired GBEPPT's infiltration depth without significantly altering GEPPT's distribution profile, confirming mitocytic mediation as the principal penetration enhancer. Microtubular networks, crucial for intracellular mitochondrial trafficking and peripheral network assembly during mitocytosis, facilitate both organelle positioning and damaged mitochondrial extrusion. This dynamic process ensures cellular homeostasis through coordinated transport mechanisms. Nocodazole's microtubule depolymerization effect specifically disrupts these mitochondrial motility patterns and network formation processes.
GBEPPT has demonstrated notable benefits in minimizing systemic toxicity during treatment. Through encapsulating the cytotoxic compound PPT within NP carriers, this formulation effectively alleviates the toxicological effects commonly linked to PPT administration while maintaining therapeutic efficacy. While administration of PPT alone may lead to severe side effects, including liver damage, the use of GBEPPT has not resulted in any notable abnormalities in major organs. GBEPPT can effectively cross the BBB and remain at the tumor site for a longer time, thus achieving a higher concentration of the drug within the tumor tissue and reducing the impact on normal tissue.
The above advantages have important implications for the future treatment of GBM: The success of GBEPPT shows that the use of nanocarriers can effectively improve the targeting and penetration of drugs, thereby improving the therapeutic effect and reducing side effects, which provides a reference for the development of other types of nano-medal DDS; As an alternative or complementary treatment, GBEPPT may provide new treatment options for patients who do not respond well to existing chemotherapy regimens; By combining different therapeutic mechanisms, GBEPPT may provide new ideas for the design of personalized treatment options, especially in the face of drug-resistant tumors. Together, GBEPPT's findings provide a new direction for the treatment of GBM, highlighting the potential of nanotechnology to improve drug efficacy and reduce toxicity.
Peptide-based nanoparticles
Peptides are bioactive compounds recognized for their exceptional biocompatibility, low toxicity, and non-immunogenic properties[297,298]. Polypeptide-mediated active targeted DDSs use small peptides of 2 to 50 amino acids to enhance drug delivery[299,300]. They possess the ability to selectively target specific receptors and are frequently employed as active targeting ligands in cancer chemotherapy DDSs. Certain peptide receptors exhibit significantly higher expression levels on tumor cells compared to normal cells. Consequently, some short peptides can function as guiding agents to enhance the active targeting of cancer cells by delivery vectors. E-selectin plays a crucial role in the tumor microenvironment by facilitating the adhesion of circulating tumor cells to vascular endothelium, particularly in inflamed areas with elevated
The PEG-Pep-PPT NPs had a size of 108.70 nm, a zeta potential of -6.41 mV, and a PDI of 0.295[103]. In vitro, the MCF-7 cell line showed significant TGI with the PEG-Pep-PPT. At a dose of 10 mg/kg, it achieved a tumor volume inhibition (TMI) rate of 76.97 %, surpassing both free PPT (67.22%) and VP-16 (64.79%). Specifically, the conjugate effectively inhibited tumor cell lines while exhibiting significantly lower cytotoxicity towards normal endothelial cells (HUVEC, 90.76% viability for PEG-Pep-PPT vs. 21.02% for PPT at 1 μM)[103]. In vivo, PEG-Pep-PPT significantly differed from PPT regarding mouse body weight changes. Mice treated with PPT at doses of 5 and 10 mg/kg lost substantial weight, decreasing by 14.67% and 8.96%, respectively. In contrast, mice receiving both dosages of PEG-Pep-PPT did not experience any weight loss[103]. This discrepancy highlights the safety profiles of the drugs; significant weight loss in PPT-treated groups indicates severe toxicity and side effects such as gastrointestinal reactions and bone marrow suppression - known limitations of PPT as an antitumor agent. Conversely, the lack of weight loss in PEG-Pep-PPT-treated mice suggests that this conjugate markedly reduces systemic toxicity compared to PPT, indicating it is safer for therapeutic use in antitumor treatments while potentially allowing for more effective dosing with fewer adverse effects.
The enhanced pharmacokinetic profile of PEG-Pep-PPT - characterized by prolonged circulation and higher blood concentration - likely contributes to its improved effectiveness in treating tumors compared to traditional PPT, which suffers from rapid clearance and associated side effects. Specifically, while the distribution half-life of PEG-Pep-PPT is shorter (0.122 vs. 11.444 h), its elimination half-life is much longer (49.977 vs. 11.446 h)[103]. Additionally, higher area under the curve (AUC) values indicate greater blood concentration, which may improve targeting and accumulation at the tumor site due to its E-selectin peptide ligand (CIELLQAR) designed for tumor vascular endothelial cells[103]. In summary, due to its amphiphilic structure, PEG-Pep-PODO can form relatively uniform NPs in an aqueous environment. At the in vitro cellular level, it shows cytotoxic effects similar to those of PPT against multiple tumor cell lines; however, its toxicity to normal endothelial cells is significantly lower than that of PPT, demonstrating a certain "toxicity reduction" effect. Meanwhile, PEG-Pep-PODO exhibits significant E-selectin targeting at the cellular level and can effectively inhibit E-selectin-mediated cell adhesion. Its superior antitumor and targeting effects were further verified in tumor-bearing mice at the in vivo level, with a tumor inhibition rate of as high as 76.97%. The in vivo safety, pharmacokinetic properties, and tumor vascular targeting of PEG-Pep-PODO are significantly better than those of PPT monomer, confirming its potential as a targeted antitumor drug against E-selectin. While the PPT-loaded PEGylated E-selectin peptide conjugate demonstrates significant potential in targeting tumors and minimizing side effects, challenges must be addressed in future research. For example, the synthesis of this conjugate entails multiple steps, including the formation of disulfide bonds and the coupling of various components. This complexity may present obstacles to scaling up production for clinical applications. Meanwhile, further investigation is needed to clarify the toxicity mechanisms of PEG-Pep-PODO in animal models for a comprehensive safety profile. Existing studies suggest that PEG-Pep-PODO's damaging effects are linked to apoptosis and cell cycle changes; however, specific cell signaling pathways mediating cytotoxicity have not been thoroughly examined. Additionally, it remains to be confirmed through further experiments whether the E-selectin peptide ligand formed upon binding with E-selectin can initiate a new mechanism of action following cysteine addition.
Host-guest drug delivery systems for combined chemotherapy, CDT, and PTT
Recently, the design of multifunctional NPs to improve PPT targeting and regulate its release through various stimulus-response mechanisms has garnered increasing interest from pharmaceutical researchers. Typically, a host-guest DDS is a host-guest complex comprising an artificial receptor and therapeutic agent that dissociates at the lesion site and releases the loaded therapeutic agent[305,306]. Several artificial macrocyclic receptors, including cyclodextrins, calixarenes, cucurbiturils, and pillararenes, have been used as drug carriers to construct host-guest DDS to deliver small molecule drugs as well as large biomolecules such as proteins[307-310]. These macrocyclic receptors have precise MWs, well-defined molecular structures, exact drug loading patterns, and quantifiable binding constants, providing theoretical feasibility to construct DDSs with good batch-to-batch reproducibility. Host-guest DDSs offer a range of notable benefits[311,312]. The preparation of macrocyclic structures is relatively uncomplicated, while their remarkable chemical resilience ensures minimal breakdown under physiological conditions. These carriers exhibit accurate molecular masses and clearly delineated architectures, substantially reducing inconsistencies between manufacturing batches. Furthermore, the modular design principle of host-guest components permits flexible integration with diverse pharmaceutical compounds. This modularity is particularly advantageous in the context of personalized medicine, as it enables the adaptation of drugs to meet the specific needs of individual patients while maintaining consistent host molecules. However, for practical application of host-guest DDSs, a macrocyclic carrier should meet the following demands: (1) high binding affinity for a therapeutic agent with selectivity to biologically coexisting interferents to prevent off-target leakage; (2) broad-spectrum encapsulation capability to make it appealing in personalized medicine, where the same carrier could be used for delivering different drugs alone or in combination; and (3) stimuli-responsiveness to fulfill controlled release targeting the assigned lesion.
PTT is a promising tumor treatment method that utilizes photothermal conversion materials to generate heat when exposed to an external near-infrared (NIR) laser (650-900 nm)[313,314]. This heat raises the temperature of tumor tissue, and if it exceeds 42 °C for a sufficient duration, it can cause significant damage or ablation of tumor cells. In addition to being non-invasive and highly controllable, research shows that the heat from photothermal materials can also inhibit tumor metastasis[315]. These advantages have led to considerable interest in PTT among researchers. Good photothermal materials must meet several key criteria: (i) high photothermal conversion efficiency and stability; (ii) excellent biocompatibility and long-term biosafety; (iii) ease of surface modification. These features reduce laser power density, enhance treatment effectiveness, provide versatility, improve in vivo safety, and ensure safe tumor therapy. However, limited water solubility and non-specific targeting hinder further clinical use. Consequently, researchers have developed nanocarrier-based photothermal materials.
Li et al. developed and synthesized a novel supramolecular host, acyclic cucurbit [n]uril (ACB-L), to target carbonic anhydrase IX (CAIX) receptors on cancer cells [Figure 17][105]. ACB-L self-assembled with ferrocenyl disulfide-linked PPT into multifunctional supramolecular NPs through a host-guest mechanism. These NPs target cancer cells precisely via sulfanilamide groups, allowing for targeted tumor therapy. CAIX is highly expressed in various cancer cells, particularly in anoxic microenvironments. ACB-L, a novel supramolecular host, effectively binds to CAIX receptors on cancer cell surfaces. Its benzene sulfonamide group enters the pocket cavity of CAIX and binds with Zn2+, enhancing its affinity for CAIX. This binding allows ACB-L to target tumor cells precisely. When combined with CAIX, ACB-L induces the Fenton reaction through Fe2+ in its structure. By targeting CAIX, ACB-L not only promotes ROS generation but also facilitates chemotherapy by releasing the attached drug PPT[105]. Additionally, NIR irradiation enhances oxidative stress and temperature within tumors while accelerating the Fenton reaction, achieving a triple therapeutic effect of chemotherapy, CDT, and PTT.
Figure 17. The diagram illustrates the preparation of supramolecular ACB-L/Fe-SS-PPT NPs and their roles in chemotherapy, CDT, and PTT. Reproduced from Ref.[105] with permission from © 2025 Elsevier B.V.
In vitro cytotoxicity assays demonstrated that the IC50 values of the NPs under NIR irradiation against HepG2 and HeLa cells were 2.90 and 2.92 μM, respectively, which are lower than those of free PPT by 1.44 and 3.08 times[105]. For A549 cells, a similar level of toxicity to PPT was observed, with IC50 values recorded at 8.85 and 6.16 μM, respectively. In contrast, the NPs exhibited reduced toxicity towards normal 293 T cells, presenting an IC50 value of 11.97 μM - significantly higher than that of free PPT by 2.62 times[105]. Notably, in CDT, excess GSH in tumor cells can neutralize •OH, increasing tumor cell resistance to oxidative stress and diminishing CDT effectiveness[105]. Due to the dynamic nature of host-guest recognition, preventing leaks in DDS is crucial. From a thermodynamic perspective, enhanced binding affinity is vital to resist dissociation from interference or dilution. Kinetically, increasing the energy barrier within these systems can effectively prolong dissociation time, minimizing leakage before reaching the target site. In addition, the absence of validation through animal experiments likely limits the effectiveness and safety of these NPs in practical applications. Therefore, without animal studies, it may be challenging to fully understand NPs' performance and potential side effects in vivo.
CONCLUSION
The role of conventional chemotherapy is being reevaluated, with PPT-based nanoplatforms expected to become a new paradigm in cancer treatment due to their ability to (i) integrate multiple therapeutic molecules for synergistic antitumor effects; (ii) combine various mechanisms of action - such as chemotherapy and PDT; and (iii) enhance antitumor efficacy while minimizing drug dosage and toxicity. Furthermore, they can (iv) improve drug targeting and tissue permeability, overcome biological barriers, and regulate drug release at specific sites. However, its clinical translation faces significant challenges. First, bridging the gap between animal research and human trials remains a major obstacle. It is crucial to determine if the antitumor efficacy seen in preclinical studies can be replicated in humans. Second, while the toxicity of PPT-based drugs has been studied during nanotherapeutic development, there is an urgent need to further investigate potential toxicity and biosafety from carrier materials and excipients, along with adverse effects from drug interactions. Finally, the complexity of manufacturing and scaling up nano-therapies limits their industrial viability. To improve clinical translation efforts, it is essential that PPT nanotherapeutics are designed for reliability and simplicity rather than unnecessary complexity or extravagance. For instance, PPT-based multifunctional nanoplatforms can be efficiently produced via supramolecular self-assembly rather than through complex chemical reactions. This method can overcome the limitations of small-molecule drugs, including poor targeting and low bioavailability, while circumventing the challenges related to nanomedicine preparation and their low biocompatibility. To move PPT-based nanoplatforms forward and ensure translational impact, several prospective strategies are necessary to fully realize the potential of PPT-based nanoplatforms, particularly concerning safety concerns and clinical efficacy.
Navigating biological barriers
Achieving optimal therapeutic outcomes with minimal adverse reactions represents the paramount objective for PPT-derived nanoplatform development. Nevertheless, the intricate tumor microenvironment and cellular-level impediments pose significant challenges to nanomedicine efficacy. Anticancer agent delivery mechanisms typically involve five sequential phases: systemic circulation, tumor-targeted localization, tissue infiltration, cellular uptake, and controlled payload liberation[316-320]. This sequence is crucial for intravenously administered nanomedicines to effectively reach the cytosol of tumor cells. Each step poses unique biological barriers; thus, conventional nanomedicines often struggle to overcome these challenges, leading to suboptimal therapeutic outcomes. Insufficient pharmacokinetic enhancement may be a key factor contributing to poor treatment outcomes, as complex biological barriers hinder the ability of drug carriers to improve pharmacokinetics and distribution. The mononuclear phagocytic system quickly eliminates foreign substances from circulation, reducing their half-life and bioavailability. One promising avenue to promote clinical transformation of PPT nanomaterials is to develop methods to increase the accumulation of nanocarriers in tumors. Among them, highly efficient adsorption-mediated endocytosis has been shown to be effective against tumor accumulation and nanomedicine penetration, independent of the action of EPR. Additionally, in tumors of various types, sizes, locations, and stages, each barrier - such as deep tissue-penetrating stimulation, the hypoxic tumor microenvironment, tumor penetration capabilities, resistance to apoptosis, immunogenicity, and drug resistance - exhibits differing levels of significance. Therefore, depending on the intended application, it is crucial to balance costs with synthesis challenges and potential practical advantages. For example, effectively delivering an adequate quantity of NPs to a palm-sized solid tumor requires a platform that possesses deep tissue penetration capabilities. In addition, when faced with the anoxic microenvironment of tumors, one strategy is to use preexisting species as a source of oxygen, while another involves precursors that generate oxygen. Additionally, hypoxia can be utilized to treat tumors by activating specific prodrugs. Therefore, careful design strategies must be employed to ensure these vectors maintain satisfactory stability in vivo without compromising spatio-temporal responsive release of therapeutics. Note, for example, that at most three key obstacles to overcome should be addressed, and then a reasonable combination of modules needed to overcome these obstacles should be considered.
Leveraging "combo" drug delivery system
Targeting the dynamic nature and drug resistance of cancer, monotherapies often fail to address its versatility. Combination therapies utilizing multiple treatment modalities can enhance anticancer efficacy synergistically while reducing the dosage of each drug, thus minimizing side effects[321-325]. Co-administering various therapeutic agents requires a delivery platform that normalizes pharmacokinetics and pharmacodynamics, prolongs circulation time, selectively accumulates at target sites, binds specifically to intended targets, and achieves controlled release. To achieve this goal, treatments should work synergistically across distinct yet interrelated carcinogenic signaling pathways. The nanoplatform enhances drug functionalities with specific cell targeting, imaging signal generation, and responsiveness to external triggers. "Combo" DDS enables innovative multi-dimensional therapies that integrate chemotherapy, gene therapy, PTT, PDT, and immunotherapy. This strategy shows promise in overcoming various forms of drug resistance. However, potential side effects linked to nanomaterials - such as oxidative stress, adverse inflammatory responses, and genotoxicity - must be carefully considered when designing multimodal treatment protocols. Meanwhile, the self-assembled nanostructures themselves could introduce new or different risks related to toxicity and immunogenicity. Specifically, it is crucial to further explore how these therapies synergize at the molecular level, particularly with respect to mechanisms such as free radical generation, tumor targeting, and immune modulation. Additionally, the design of combination drug delivery platforms must avoid introducing complexities in production or quality control procedures. Essential considerations such as chemical synthesis proficiency and industrial-scale manufacturing requirements - including batch consistency, scalability, material stability, economic viability, and self-assembly properties - require thorough attention during the development phase to ensure successful clinical translation.
Artificial intelligence enhances the clinical transition
The progression of nanomedicine represents an intricate and resource-intensive endeavor requiring coordinated efforts across scientific disciplines. Strategic interdisciplinary partnerships combined with systematic design methodologies are poised to expedite the clinical implementation of PPT-based therapies in forthcoming research cycles. Recent advancements in AI systems, especially sophisticated language processing architectures and creative artificial intelligence frameworks, present transformative opportunities for this domain[326-330]. Machine learning algorithms are reshaping therapeutic innovation through sophisticated analysis of multi-dimensional health data, precise biomarker discovery, and anomaly detection capabilities. Specifically, AI-powered algorithms dramatically improve image analysis capabilities by enhancing resolution parameters and recognizing subtle diagnostic patterns. Such technological progress enables precise patient categorization through radiographic evidence, elucidates pharmacokinetic profiles of nanoscale therapeutics, guides personalized intervention planning - including optimal administration schedules and targeted delivery mechanisms - while supporting comprehensive treatment evaluation. Nevertheless, substantial obstacles must be overcome to fully actualize these computational advantages. Primary challenges include developing novel solutions for limited data availability in PPT research. Establishing collaborative data ecosystems, implementing uniform experimental guidelines, and creating adaptive machine learning architectures will prove critical for refining therapeutic efficacy metrics and ensuring pharmaceutical safety standards. Additionally, integrating multimodal analytical techniques can unlock synergistic insights from heterogeneous biological datasets to propel nanomedical innovations. Contemporary methodologies frequently emphasize isolated data streams while overlooking complex systemic interactions within physiological networks. Consequently, designing next-generation AI platforms capable of constructing sophisticated digital twin simulations will deepen comprehension of oncological pathways, nanotherapeutic behaviors, and interpatient variability. For instance, machine learning models can be trained on multi-omics data (genomics, proteomics, metabolomics) from patient-derived xenografts to predict which tumor subtype responds best to a specific hypoxia-activated PPT prodrug.
Standardized protocols move the field forward
By establishing a standardized evaluation system framework and a long-term monitoring mechanism, the clinical transformation of PPT-based nanoplatforms can be facilitated, moving the field forward. Firstly, a standardized protocol should be established, including synthesis standards (clearly defining the synthesis methods of nanomaterials and the quality standards of raw materials used); characterization techniques (adopting unified characterization techniques to assess the physicochemical properties of nanomaterials); stability studies (ensuring that the drug does not degrade or become inactive under specified storage conditions, maintaining stability in terms of appearance, solubility, particle size and dispersion, and maintaining an effective concentration in the body); evaluation procedures (formulating a systematic safety and efficacy evaluation process to ensure the repeatability and reliability of clinical trials). Secondly, a complete regulatory framework should be established: carrier design (in the design of nanomedicine carriers, the impact on drug release rate, targeting ability and biodegradability should be considered); safety assessment (detailed assessment standards should be formulated, covering the synthesis process, physicochemical properties, biocompatibility and potential toxicity of nanomaterials); target specificity (assessing the selectivity of nanomedicines for specific target cells or tissues to reduce damage to normal cells). Finally, a long-term monitoring and evaluation framework should be improved: side effect monitoring (tracking the side effects of patients after using nanomedicines and their changes, and adjusting the treatment plan in a timely manner); long-term impact assessment (conducting long-term follow-up studies to evaluate the potential impact of PPT nanomedicines on health, such as immune response, organ function and tumor recurrence rate, etc.).
In summary, while translational hurdles continue to exist for PPT-based nanoplatforms in clinical applications, strategic emphasis must be placed on strengthening connections between preclinical research and human trials. Critical priorities involve comprehensive evaluation of carrier material toxicities, optimization of production protocols, and focused development of reliable, user-friendly systems with demonstrated therapeutic benefits. These innovations possess transformative potential for the field, ultimately enabling the successful transition of PPT-based nanoplatforms into clinical practice.
DECLARATIONS
Authors’ contributions
Writing-original draft, writing-review & editing, funding acquisition, supervision, conceptualization:
Writing-review & editing: Song, X.
Collecting the literature, Data curation: Sun, M.
Data curation: Zhang, R.
Conceptualization, Investigation, Writing-review & editing, Funding acquisition: Yang, L.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China [grant number 22309103], Natural Science Foundation of Shandong Province [grant numbers ZR2022MH162, ZR2022QE202], PhD Research Start-up Foundation of Qufu Normal University [grant numbers 614901, 615201], and the project of introduction and cultivation for young innovation talents in the colleges and universities of Shandong Province [grant number 614202].
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bray, F.; Laversanne, M.; Sung, H.; et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer. J. Clin. 2024, 74, 229-63.
2. Roerden, M.; Spranger, S. Cancer immune evasion, immunoediting and intratumour heterogeneity. Nat. Rev. Immunol. 2025, 25, 353-69.
4. Wang, J.; Liao, Z. Research progress of microrobots in tumor drug delivery. Food. Med. Homol. 2024, 1, 9420025.
6. Zhang, Y.; You, P.; Liu, R.; et al. Artificial intelligence in clinical trials of lung cancer: Current and future prospects. Intell. Oncol. 2025, 1, 34-51.
7. Crosby, D.; Bhatia, S.; Brindle, K. M.; et al. Early detection of cancer. Science 2022, 375, eaay9040.
8. Zhen, Z.; Yang, Y.; Feng, C.; Lin, L.; Ma, J.; Sun, Y. From data to decisions: big data and AI are shaping the future of radiotherapy and individualized treatment of nasopharyngeal carcinoma. Intell. Oncol. 2025, 1, 52-60.
9. Timofeev, O.; Giron, P.; Lawo, S.; Pichler, M.; Noeparast, M. ERK pathway agonism for cancer therapy: evidence, insights, and a target discovery framework. NPJ. Precis. Oncol. 2024, 8, 70.
10. Beretta, G. L.; Cassinelli, G.; Rossi, G.; et al. Novel insights into taxane pharmacology: an update on drug resistance mechanisms, immunomodulation and drug delivery strategies. Drug. Resist. Updat. 2025, 81, 101223.
11. Zhang, M.; Miao, Y.; Zhao, C.; et al. Fine-tuning the activation behaviors of ternary modular cabazitaxel prodrugs for efficient and on-target oral anti-cancer therapy. Asian. J. Pharm. Sci. 2024, 19, 100908.
13. Zheng, L.; Chang, R.; Liang, B.; et al. Overcoming drug resistance through extracellular vesicle-based drug delivery system in cancer treatment. Cancer. Drug. Resist. 2024, 7, 50.
14. Zhu, L.; Shen, Z.; Liu, X.; et al. Acid and phosphatase-triggered release and trapping of a prodrug on cancer cell enhance its chemotherapy. Biomaterials 2025, 320, 123254.
15. Zhang, Z.; Zeng, W.; Guo, N.; et al. A nanodrug loading indocyanine green and metformin dually alleviating tumor hypoxia for enhanced chemodynamic/sonodynamic therapy. J. Colloid. Interface. Sci. 2025, 680, 341-55.
16. Wang, Z.; Yang, L.; Li, Y.; et al. An activatable, carrier-free, triple-combination nanomedicine for ALK/EGFR-mutant non-small cell lung cancer highly permeable targeted chemotherapy. New. J. Chem. 2022, 46, 17673-7.
17. Wang, Z.; Song, X. Q.; Xu, W.; Lei, S.; Zhang, H.; Yang, L. Stand up to stand out: natural dietary polyphenols curcumin, resveratrol, and gossypol as potential therapeutic candidates against severe acute respiratory syndrome coronavirus 2 infection. Nutrients 2023, 15, 3885.
18. Wang, Z.; Yang, L. The therapeutic potential of natural dietary flavonoids against SARS-CoV-2 infection. Nutrients 2023, 15, 3443.
19. Takemoto, M.; Delghandi, S.; Abo, M.; et al. Covalent plant natural product that potentiates antitumor immunity. J. Am. Chem. Soc. 2025, 147, 2902-12.
20. Wang, Z.; Wang, N.; Yang, L.; Song, X. Q. Bioactive natural products in COVID-19 therapy. Front. Pharmacol. 2022, 13, 926507.
21. Wang, Z.; Yang, L.; Zhao, X. E. Co-crystallization and structure determination: an effective direction for anti-SARS-CoV-2 drug discovery. Comput. Struct. Biotechnol. J. 2021, 19, 4684-701.
22. Feng, T.; Ahmed, W.; Ahmed, T.; Chen, L. Nanoparticles derived from herbal preparations May represent a novel nucleic acid therapy. Interdiscip. Med. 2024, 2, e20230029.
23. Wang, Z. Advances in the asymmetric total synthesis of natural products using chiral secondary amine catalyzed reactions of α,β-unsaturated aldehydes. Molecules 2019, 24, 3412.
24. Wang, Z.; Yang, L. Chinese herbal medicine: fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 2021, 270, 113869.
25. Wang, Z.; Yang, L. Turning the tide: natural products and natural-product-inspired chemicals as potential counters to SARS-CoV-2 infection. Front. Pharmacol. 2020, 11, 1013.
26. Anand, U.; Biswas, P.; Kumar, V.; et al. Podophyllum hexandrum and its active constituents: novel radioprotectants. Biomed. Pharmacother. 2022, 146, 112555.
27. Liu, W.; Zhao, D.; Yin, D.; Duan, K.; Wang, Z. Plant origin source, content profile and bioactivity of podophyllotoxin as an important natural anticancer agent. Chem. Biodivers. 2025, 22, e202402375.
28. Guo, Y.; Lu, P.; He, J.; et al. Research progress and quality markers prediction analysis of Dysosma versipellis. Tradit. Med. Res. 2023, 8, 16.
29. Hao, M.; Xu, H. Chemistry and biology of podophyllotoxins: an update. Chemistry 2024, 30, e202302595.
30. Peer, L. A. Phytochemistry, pharmacological activities, and ethnomedicina l importance of the highly valuable endangered plant, Podophyllum hexandrum; A comprehensive review. Inter. J. Biol. Pharm. Allied. Sci. 2024, 13, 951-64.
31. Plotzker, R. E.; Vaidya, A.; Pokharel, U.; Stier, E. A. Sexually transmitted human papillomavirus: update in epidemiology, prevention, and management. Infect. Dis. Clin. North. Am. 2023, 37, 289-310.
32. Yang, C.; Xie, Q.; Zeng, X.; et al. Novel hybrids of podophyllotoxin and formononetin inhibit the growth, migration and invasion of lung cancer cells. Bioorg. Chem. 2019, 85, 445-54.
33. Zhang, W.; Liu, C.; Li, J.; et al. Target analysis and mechanism of podophyllotoxin in the treatment of triple-negative breast cancer. Front. Pharmacol. 2020, 11, 1211.
34. Xu, Y.; Tang, L.; Liu, Y.; et al. Dual-modified albumin-polymer nanocomplexes with enhanced in vivo stability for hepatocellular carcinoma therapy. Colloids. Surf. B. Biointerfaces. 2021, 201, 111642.
35. Zhang, L.; Zhang, Z.; Chen, F.; Chen, Y.; Lin, Y.; Wang, J. Aromatic heterocyclic esters of podophyllotoxin exert anti-MDR activity in human leukemia K562/ADR cells via ROS/MAPK signaling pathways. Eur. J. Med. Chem. 2016, 123, 226-35.
36. Lee, S. O.; Joo, S. H.; Kwak, A. W.; et al. Podophyllotoxin induces ROS-mediated apoptosis and cell cycle arrest in human colorectal cancer cells via p38 MAPK signaling. Biomol. Ther. 2021, 29, 658-66.
37. Zhao, W.; Shen, R.; Li, H. M.; Zhong, J. J.; Tang, Y. J. Podophyllotoxin derivatives-tubulin complex reveals a potential binding site of tubulin polymerization inhibitors in α-tubulin adjacent to colchicine site. Int. J. Biol. Macromol. 2024, 276, 133678.
38. Shah, Z.; Gohar, U. F.; Jamshed, I.; et al. Podophyllotoxin: history, recent advances and future prospects. Biomolecules 2021, 11, 603.
39. Ma, Y.; Chen, L.; Deng, L.; et al. Redox-responsive self-assembled podophyllotoxin twin drug nanoparticles for enhanced intracellular drug delivery. Biomed. Mater. 2023, 18, 045019.
40. Qu, Y.; Zhang, C.; Ma, X.; Gao, Y.; Liu, J.; Wu, L. Synthesis and biological evaluation of NQO1-activated prodrugs of podophyllotoxin as antitumor agents. Bioorg. Med. Chem. 2020, 28, 115821.
41. Shi, R. J.; Fan, H. Y.; Yu, X. H.; Tang, Y. L.; Jiang, J.; Liang, X. H. Advances of podophyllotoxin and its derivatives: patterns and mechanisms. Biochem. Pharmacol. 2022, 200, 115039.
42. Xu, Y.; He, Z.; Chen, L.; Wang, H. Podophyllotoxin derivatives targeting tubulin: an update (2017-2022). Drug. Discov. Today. 2023, 28, 103640.
43. Zhang, W.; Berthelet, J.; Michail, C.; et al. Human CREBBP acetyltransferase is impaired by etoposide quinone, an oxidative and leukemogenic metabolite of the anticancer drug etoposide through modification of redox-sensitive zinc-finger cysteine residues. Free. Radic. Biol. Med. 2021, 162, 27-37.
44. Motyka, S.; Jafernik, K.; Ekiert, H.; et al. Podophyllotoxin and its derivatives: potential anticancer agents of natural origin in cancer chemotherapy. Biomed. Pharmacother. 2023, 158, 114145.
45. Luo, Z.; Yin, F.; Wang, X.; Kong, L. Progress in approved drugs from natural product resources. Chin. J. Nat. Med. 2024, 22, 195-211.
46. Sinkule, J. A. Etoposide: a semisynthetic epipodophyllotoxin. Chemistry, pharmacology, pharmacokinetics, adverse effects and use as an antineoplastic agent. Pharmacotherapy 1984, 4, 61-73.
47. Mosijczuk, A. D.; Ruymann, F. B.; Mease, A. D.; Bernier, R. D. Anthracycline cardiomyopathy in childrenReport of two cases. Cancer 1979, 44, 1582-7.
48. Chauhan, A. S.; Chand, P.; Parashar, T. Lipid-based nanoparticles: strategy for targeted cancer therapy. BIOI 2025, 6.
49. Liao, S.; Li, X.; Lu, Y.; Luo, K. Nanomedicine in immunotherapy for non-small cell lung cancer: applications and perspectives. Small. Methods. 2025, 9, e2401783.
50. Kalayil, N.; Budar, A. A.; Dave, R. K. Nanofibers for drug delivery: design and fabrication strategies. BIOI 2024, 5.
51. Prasad, R.; Ghosh, A.; Patel, V.; et al. Voices of nanomedicine: blueprint guidelines for collaboration in addressing global unmet medical needs. ACS. Nano. 2025, 19, 2979-91.
52. Hadi, M. K.; Zeng, H.; Pantrangi, M.; Sangaraju, S.; Ran, F. Biocompatible polyethersulfone membrane modified by hydrophilic polymer decorated magnetic nanoparticles gilded by facile external magnetic field. Interdiscip. Med. 2024, 2, e20240004.
53. Hu, C.; He, X.; Gao, H.; Zhang, J. DELIVER: the core principles for the clinic translation of nanomedicines. Acta. Pharm. Sin. B. 2025, 15, 1196-8.
54. Liang, X.; Ding, L.; Ma, J.; et al. Enhanced mechanical strength and sustained drug release in carrier-free silver-coordinated anthraquinone natural antibacterial anti-inflammatory hydrogel for infectious wound healing. Adv. Healthc. Mater. 2024, 13, e2400841.
55. Miao, Y.; Wang, X.; Zhao, X.; Hu, Y.; Liu, X.; Deng, D. Co-assembly strategies of natural plant compounds for improving their bioavailability. Food. Med. Homol. 2025, 2, 9420022.
56. Huang, J.; Yang, J.; Yang, Y.; et al. Mitigating doxorubicin-induced cardiotoxicity and enhancing anti-tumor efficacy with a metformin-integrated self-assembled nanomedicine. Adv. Sci. 2025, 12, e2415227.
57. Sun, X.; Zhao, P.; Lin, J.; Chen, K.; Shen, J. Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer. Drug. Resist. 2023, 6, 390-415.
58. Wang, X.; Xu, Z.; Wang, J.; et al. A mitochondria-targeted biomimetic nanomedicine capable of reversing drug resistance in colorectal cancer through mitochondrial dysfunction. Adv. Sci. 2025, 12, e2410630.
59. Liu, R.; Zhao, R.; Yu, Z.; Liu, F.; Liu, C.; Wu, X. Metal-organic coordinated self-assembled nanomedicine for enhanced cuproptosis-mediated chemo-photo-chemodynamic synergistic therapy of non-small cell lung cancer. Chem. Eng. J. 2025, 509, 161305.
60. Tao, J.; Ning, W.; Lu, W.; et al. Smart self-transforming nano-systems for overcoming biological barrier and enhancing tumor treatment efficacy. J. Control. Release. 2025, 380, 85-107.
61. Germain, M.; Caputo, F.; Metcalfe, S.; et al. Delivering the power of nanomedicine to patients today. J. Control. Release. 2020, 326, 164-71.
62. Jarrett, T. R.; Pregelj, L.; Bell, C. A.; Fletcher, N. L.; Thurecht, K. J. Material trends and clinical costings in systematically identified CDER-approved nanomedicines. Adv. Ther. 2024, 7, 2400124.
63. Reddy, K. T. K.; Reddy, A. S. Recent breakthroughs in drug delivery systems for targeted cancer therapy: an overview. Cell. Mol. Biomed. Rep. 2025, 5, 13-27.
64. Wu, Z.; Chen, J.; Wang, B.; Wen, Q.; Fu, S. Nanoparticle-based drug delivery strategies for targeted therapy to hypoxic solid tumors. Chem. Eng. J. 2024, 502, 158081.
65. Thomas, M. R.; Badekila, A. K.; Pai, V.; et al. Navigating tumor microenvironment barriers with nanotherapeutic strategies for targeting metastasis. Adv. Healthc. Mater. 2025, 14, e2403107.
66. Miranda-Vera, C.; Hernández, ÁP.; García-García, P.; Díez, D.; García, P. A.; Castro, MÁ. Bioconjugation of podophyllotoxin and nanosystems: approaches for boosting its biopharmaceutical and antitumoral profile. Pharmaceuticals 2025, 18, 169.
67. Roy, A.; Zhao, Y.; Yang, Y.; Szeitz, A.; Klassen, T.; Li, S. D. Selective targeting and therapy of metastatic and multidrug resistant tumors using a long circulating podophyllotoxin nanoparticle. Biomaterials 2017, 137, 11-22.
68. Zhang, C.; Zuo, Y.; Zhang, T.; et al. Advances in nanoscale carrier-based approaches to reduce toxicity and enhance efficacy of podophyllotoxin. Acta. Mater. Med. 2023, 2, 430-48.
69. Yadav, R.; Chawra, H. S.; Dubey, G.; et al. Herbal based nanoparticles as a possible and potential treatment of cancer: a review. Explor. Target. Antitumor. Ther. 2025, 6, 1002285.
70. Florczak, A.; Grzechowiak, I.; Deptuch, T.; Kucharczyk, K.; Kaminska, A.; Dams-Kozlowska, H. Silk particles as carriers of therapeutic molecules for cancer treatment. Materials 2020, 13, 4946.
71. Appidi, T.; China, D.; Ștefan, G. R.; et al. Engineered multifunctional nanoparticles for enhanced radiation therapy: three-in-one approach for cancer treatment. Mol. Cancer. 2025, 24, 68.
72. Liu, H.; Zou, J.; Li, X.; Ge, Y.; He, W. Drug delivery for platinum therapeutics. J. Control. Release. 2025, 380, 503-23.
73. Tenchov, R.; Hughes, K. J.; Ganesan, M.; et al. Transforming medicine: cutting-edge applications of nanoscale materials in drug delivery. ACS. Nano. 2025, 19, 4011-38.
74. Dechbumroong, P.; Hu, R.; Keaswejjareansuk, W.; Namdee, K.; Liang, X. J. Recent advanced lipid-based nanomedicines for overcoming cancer resistance. Cancer. Drug. Resist. 2024, 7, 24.
75. Aalhate, M.; Mahajan, S.; Dhuri, A.; Singh, P. K. Biohybrid nano-platforms manifesting effective cancer therapy: fabrication, characterization, challenges and clinical perspective. Adv. Colloid. Interface. Sci. 2025, 335, 103331.
76. Yang, Y.; Long, K.; Chu, Y.; Lu, H.; Wang, W.; Zhan, C. Photoresponsive drug delivery systems: challenges and progress. Adv. Funct. Mater. 2024, 34, 2402975.
77. Puccetti, M.; Pariano, M.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Biologics, theranostics, and personalized medicine in drug delivery systems. Pharmacol. Res. 2024, 201, 107086.
78. Hu, S.; Zhao, R.; Shen, Y.; Lyu, B. Revolutionizing drug delivery: the power of stimulus-responsive nanoscale systems. Chem. Eng. J. 2024, 496, 154265.
79. Wang, Z.; Yang, L. Natural-product-based, carrier-free, noncovalent nanoparticles for tumor chemo-photodynamic combination therapy. Pharmacol. Res. 2024, 203, 107150.
80. Beach, M. A.; Nayanathara, U.; Gao, Y.; et al. Polymeric nanoparticles for drug delivery. Chem. Rev. 2024, 124, 5505-616.
81. Zhou, H.; Yang, Z.; Jin, G.; et al. Polymeric nanoparticles simultaneously delivering paclitaxel prodrug and combretastatin A4 with exceptionally high drug loading for cancer combination therapy. Nano. Lett. 2025, 25, 3479-88.
82. Meng, F.; He, Y.; Zhao, J.; et al. Timely administration of drug combination improves chemoimmunotherapy of an immune-cold tumor. J. Control. Release. 2025, 381, 113579.
83. Sharma, R.; Yadav, V.; Jha, S.; Dighe, S.; Jain, S. Unveiling the potential of ursolic acid modified hyaluronate nanoparticles for combination drug therapy in triple negative breast cancer. Carbohydr. Polym. 2024, 338, 122196.
84. Chen, H.; Xing, C.; Lei, H.; et al. ROS-driven supramolecular nanoparticles exhibiting efficient drug delivery for chemo/Chemodynamic combination therapy for Cancer treatment. J. Control. Release. 2024, 368, 637-49.
85. Li, K.; Li, J.; Li, Z.; Men, L.; Zuo, H.; Gong, X. Cisplatin-based combination therapies: Their efficacy with a focus on ginsenosides co-administration. Pharmacol. Res. 2024, 203, 107175.
86. Zhang, X.; Lou, X.; Qiao, H.; et al. Supramolecular self-sensitized dual-drug nanoassemblies potentiating chemo-photodynamic therapy for effective cancer treatment. Int. J. Pharm. 2024, 662, 124496.
87. Wang, Q.; Sun, M.; Li, C.; et al. A computer-aided chem-photodynamic drugs self-delivery system for synergistically enhanced cancer therapy. Asian. J. Pharm. Sci. 2021, 16, 203-12.
88. Xiong, H.; Du, C.; Ye, J.; et al. Therapeutic co-assemblies for synergistic NSCLC treatment through dual topoisomerase I and tubulin inhibitors. J. Control. Release. 2025, 377, 485-94.
89. Wang, X.; Wang, Y.; Yu, J.; et al. Reduction-hypersensitive podophyllotoxin prodrug self-assembled nanoparticles for cancer treatment. Pharmaceutics 2023, 15, 784.
90. Lin, Z.; Wang, Y.; Li, W.; et al. A natural compound-empowered podophyllotoxin prodrug nanoassembly magnifies efficacy-toxicity benefits in cancer chemotherapy. Asian. J. Pharm. Sci. 2024, 19, 100892.
91. Liu, T.; Xia, F.; Zheng, Y.; et al. Steric hindrance-engineered redox-responsive disulfide-bridged homodimeric prodrug nanoassemblies for spatiotemporally balanced cancer chemotherapy. J. Med. Chem. 2025, 68, 11916-27.
92. Yu, J.; Zhang, B.; Li, J.; et al. “Transforming enemy into friend” strategy-based stimuli responsive dual-drug liposomes for synergistic chemo-photodynamic therapy. Chem. Eng. J. 2024, 487, 150526.
93. Cheng, X.; Yu, P.; Zhou, X.; et al. Enhanced tumor homing of pathogen-mimicking liposomes driven by R848 stimulation: A new platform for synergistic oncology therapy. Acta. Pharm. Sin. B. 2022, 12, 924-38.
94. Wang, R.; Zhao, Y.; Huang, Z.; et al. Self-assembly of podophyllotoxin-loaded lipid bilayer nanoparticles for highly effective chemotherapy and immunotherapy via downregulation of programmed cell death ligand 1 production. ACS. Nano. 2022, 16, 3943-54.
95. Wang, R.; Xuan, Y.; Zhao, Y.; et al. Cationic nanoparticulate system for codelivery of MicroRNA-424 and podophyllotoxin as a multimodal anticancer therapy. Mol. Pharm. 2022, 19, 2092-104.
96. Ling, X.; Jiang, X.; Li, Y.; et al. Sequential treatment of bioresponsive nanoparticles elicits antiangiogenesis and apoptosis and synergizes with a CD40 agonist for antitumor immunity. ACS. Nano. 2021, 15, 765-80.
97. Zhang, L.; Li, R.; Zhang, H.; Suo, X.; Guo, B. DSPE-mPEG2000-modified podophyllotoxin long-circulating liposomes for targeted delivery: their preparation, characterization, and evaluation. Curr. Drug. Deliv. 2025, 22, 1481-92.
98. Li, M.; Zhao, Y.; Sun, J.; et al. pH/reduction dual-responsive hyaluronic acid-podophyllotoxin prodrug micelles for tumor targeted delivery. Carbohydr. Polym. 2022, 288, 119402.
99. Li, M.; Zhang, L.; Xuan, Y.; et al. pH-sensitive hyaluronic acid-targeted prodrug micelles constructed via a one-step reaction for enhanced chemotherapy. Int. J. Biol. Macromol. 2022, 206, 489-500.
100. Zhang, C.; Chen, Y.; Zuo, Y.; et al. Dual targeting of FR+CD44 overexpressing tumors by self-assembled nanoparticles quantitatively conjugating folic acid-hyaluronic acid to the GSH-sensitively modified podophyllotoxin. Chem. Eng. J. 2025, 505, 159276.
101. Liu, M.; Zhang, Z. X.; Wang, J. H.; et al. Immunomodulatory and anti-ovarian cancer effects of novel astragalus polysaccharide micelles loaded with podophyllotoxin. Int. J. Biol. Macromol. 2025, 290, 138960.
102. Xiang, Y.; Wang, B.; Yang, W.; et al. Mitocytosis mediated by an enzyme-activable mitochondrion-disturbing polymer-drug conjugate enhances active penetration in glioblastoma therapy. Adv. Mater. 2024, 36, e2311500.
103. Xiang, C.; Fu, Y.; Hao, T.; et al. Podophyllotoxin-loaded PEGylated E-selectin peptide conjugate targeted cancer site to enhance tumor inhibition and reduce side effect. Eur. J. Med. Chem. 2023, 260, 115780.
104. Zhang, W.; Liu, S.; Hou, Y.; et al. Functional nanoplatform for modulating cellular forces to enhance antitumor immunity via mechanotransduction. J. Control. Release. 2025, 379, 850-65.
105. Li, Y.; Wang, L.; Xiao, F.; Yin, T.; Chu, Z.; Yang, B. Targeted cancer cell supramolecular nanoparticles self-assembled from acyclic cucurbit[n]urils for combined chemo/chemodynamic/photothermal therapy. Colloid. Surface. A. 2025, 709, 136124.
106. Jia, Y.; Sun, C.; Chen, T.; et al. Recent advance in phytonanomedicine and mineral nanomedicine delivery system of the treatment for acute myeloid leukemia. J. Nanobiotechnol. 2023, 21, 240.
107. Fu, Z.; Zhou, D.; Liu, Z.; Ni, D. GSH-responsive nanomedicine for disease smart imaging and therapy. Coordin. Chem. Rev. 2025, 533, 216554.
108. Zhang, X.; Duan, Q.; Zhuang, J.; Huang, X. Synthetic biology-based strategy for nanomedicine design. Small. Methods. 2025, 9, e2401969.
109. Zhu, L.; Zhong, W.; Meng, X.; et al. Polymeric nanocarriers delivery systems in ischemic stroke for targeted therapeutic strategies. J. Nanobiotechnol. 2024, 22, 424.
110. Song, K.; Ming, J.; Tao, B.; et al. Emerging glucose oxidase-delivering nanomedicines for enhanced tumor therapy. J. Control. Release. 2025, 381, 113580.
111. Fan, M.; Zheng, X.; Cheng, W.; et al. Reinforcing carrier-free photothermal nanodrugs through flavonol-driven assembly. Adv. Funct. Mater. 2024, 34, 2402455.
112. Ren, S.; Zhang, M.; Cai, C.; et al. A carrier-free ultrasound-responsive polyphenol nanonetworks with enhanced sonodynamic-immunotherapy for synergistic therapy of breast cancer. Biomaterials 2025, 317, 123109.
113. Seo, M.; Jeong, Y.; Seo, B.; et al. Lenalidomide-utilizing self-assembled immunogenic cell death-inducing heparin/doxorubicin nanocomplex for anticancer immunotherapy. Nano. Today. 2025, 62, 102677.
114. Wang, Z.; Xu, W.; Lei, S.; et al. A computer-aided, carrier-free drug delivery system with enhanced cytotoxicity and biocompatibility: a universal model for multifunctional lung cancer therapy. Colloids. Surf. B. Biointerfaces. 2025, 250, 114557.
115. Wang, H.; Lu, X.; Fan, J.; et al. A carrier-free DNA nanoplatform for efficient three-in-one tumor therapy in vivo. Nano. Today. 2025, 62, 102734.
116. Fang, F.; Chen, X. Carrier-free nanodrugs: from bench to bedside. ACS. Nano. 2024, 18, 23827-41.
117. Sun, Y.; Wang, S.; Liu, J.; et al. Tailoring modification modules of paclitaxel prodrug nanoassemblies to manipulate efficacy and tolerance. Nano. Today. 2024, 56, 102275.
118. Cao, Y.; Zhao, X.; Miao, Y.; Wang, X.; Deng, D. How the versatile self-assembly in drug delivery system to afford multimodal cancer therapy? Adv. Healthc. Mater. 2025, 14, e2403715.
119. Li, S.; Jiang, S.; Rahman, M. S. U.; et al. Pre-induced ICD membrane-coated carrier-free nanoparticles for the personalized lung cancer immunotherapy. Small. Methods. 2023, 7, e2201569.
120. Hao, Y.; Li, Y.; Zang, W.; et al. Self-assembled doxorubicin prodrug riding on the albumin express train enable tumor targeting and bio-activation. J. Colloid. Interface. Sci. 2025, 684, 97-108.
121. Kuang, Y.; Li, Z.; Chen, H.; Wang, X.; Wen, Y.; Chen, J. Advances in self-assembled nanotechnology in tumor therapy. Colloids. Surf. B. Biointerfaces. 2024, 237, 113838.
122. Liu, L.; Zhang, X. Carrier-free nanomedicines for cancer treatment. Prog. Mater. Sci. 2022, 125, 100919.
123. An, J.; Zhang, Z.; Zhang, J.; Zhang, L.; Liang, G. Research progress in tumor therapy of carrier-free nanodrug. Biomed. Pharmacother. 2024, 178, 117258.
124. Ji, H.; Wang, W.; Qiao, O.; Hao, X. Review of carrier-free self-assembly of anticancer nanodrugs. ACS. Appl. Nano. Mater. 2024, 7, 4564-87.
125. Behera, A.; Padhi, S. Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: a review. Environ. Chem. Lett. 2020, 18, 1557-67.
126. Zhang, X.; Chen, Y.; Li, X.; et al. Carrier-free self-assembled nanomedicine based on celastrol and galactose for targeting therapy of hepatocellular carcinoma via inducing ferroptosis. Eur. J. Med. Chem. 2024, 267, 116183.
127. Jia, W.; Yang, M.; Zhang, W.; Xu, W.; Zhang, Y. Carrier-free self-assembled nanomedicines for promoting apoptosis and inhibiting proliferation in hepatocellular carcinoma. ACS. Biomater. Sci. Eng. 2024, 10, 4347-58.
128. Pei, Z.; Hu, S.; Wei, H.; et al. Multifunctional carrier-free nanodrugs for enhanced delivery and efficacy of hydrophobic antitumor drugs. Chin. Chem. Lett. 2025, 110981.
129. Karaosmanoglu, S.; Zhou, M.; Shi, B.; Zhang, X.; Williams, G. R.; Chen, X. Carrier-free nanodrugs for safe and effective cancer treatment. J. Control. Release. 2021, 329, 805-32.
130. Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Lan, M.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557.
131. Li, H.; Zang, W.; Mi, Z.; et al. Tailoring carrier-free nanocombo of small-molecule prodrug for combinational cancer therapy. J. Control. Release. 2022, 352, 256-75.
132. Yang, L.; Zhang, Y.; Lai, Y.; et al. A computer-aided, heterodimer-based "triadic" carrier-free drug delivery platform to mitigate multidrug resistance in lung cancer and enhance efficiency. J. Colloid. Interface. Sci. 2025, 677, 523-40.
133. Tong, L. W.; Le, J. Q.; Song, X. H.; et al. Synergistic anti-tumor effect of dual drug co-assembled nanoparticles based on ursolic acid and sorafenib. Colloids. Surf. B. Biointerfaces. 2024, 234, 113724.
134. Feng, X.; Brown, C. M.; Wang, H.; et al. Carrier-free chemo-phototherapeutic nanomedicines with endo/lysosomal escape function enhance the therapeutic effect of drug molecules in tumors. J. Mater. Chem. B. 2024, 12, 6703-15.
135. Cheng, G.; Wang, H.; Zhang, C.; et al. Multifunctional nano-photosensitizer: a carrier-free aggregation-induced emission nanoparticle with efficient photosensitization and pH-responsibility. Chem. Eng. J. 2020, 390, 124447.
136. Liu, Y.; Long, K.; Wang, T.; Kang, W.; Wang, W. Carrier-free nanodrugs for stemness inhibition-enhanced photodynamic therapy. Aggregate 2023, 4, e284.
137. Xue, Y.; Chen, K.; Chen, Y.; et al. Engineering diselenide-IR780 homodimeric nanoassemblies with enhanced photodynamic and immunotherapeutic effects for triple-negative breast cancer treatment. ACS. Nano. 2023, 17, 22553-70.
138. Du, X.; Hou, Y.; Huang, J.; et al. Cytosolic delivery of the immunological adjuvant Poly I:C and cytotoxic drug crystals via a carrier-free strategy significantly amplifies immune response. Acta. Pharm. Sin. B. 2021, 11, 3272-85.
139. Kong, L.; Huang, P.; Yuan, F.; et al. A metal-free bionic nanozyme for efficient inhibition of cancer recurrence and metastasis following photothermal therapy. Chin. Chem. Lett. 2025, 36, 111030.
140. Wang, Z.; Sun, X.; Sun, M.; Wang, C.; Yang, L. Game changers: blockbuster small-molecule drugs approved by the FDA in 2024. Pharmaceuticals 2025, 18, 729.
141. Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017, 117, 13566-638.
142. Wang, Y.; Chen, W.; Mo, Z.; He, Y.; Nie, K. Therapeutic potential of the citrus flavonoid hesperidin and its aglycone hesperetin against chemotherapy-induced toxicity. Tradit. Med. Res. 2025, 10, 22.
144. Besse, B.; Pons-Tostivint, E.; Park, K.; et al. Biomarker-directed targeted therapy plus durvalumab in advanced non-small-cell lung cancer: a phase 2 umbrella trial. Nat. Med. 2024, 30, 716-29.
145. Chong, L. M.; Tng, D. J. H.; Tan, L. L. Y.; Chua, M. L. K.; Zhang, Y. Recent advances in radiation therapy and photodynamic therapy. Appl. Phys. Rev. 2021, 8, 041322.
146. Yang, H.; Liu, R.; Xu, Y.; Qian, L.; Dai, Z. Photosensitizer nanoparticles boost photodynamic therapy for pancreatic cancer treatment. Nanomicro. Lett. 2021, 13, 35.
147. Chen, J.; Xu, X.; Wang, K.; et al. Hypoxia-activated liposomes enable synergistic photodynamic therapy for oral cancer. Adv. Healthc. Mater. 2025, 14, e2404395.
148. Dong, P.; Hu, J.; Yu, S.; et al. A Mitochondrial oxidative stress amplifier to overcome hypoxia resistance for enhanced photodynamic therapy. Small. Methods. 2021, 5, e2100581.
149. Xiong, J.; Wang, X.; Kim, J.; et al. Tumor-activated prodrug with synergistic anti-stemness chemical and photodynamic therapies. Adv. Funct. Mater. 2024, 34, 2312590.
150. Zhang, H.; Li, J.; Li, Y.; et al. Modified oxygen metabolism toward “sunlight-friendly” photodynamic therapy. Adv. Funct. Mater. 2025, 35, 2414817.
151. Pham, T. C.; Nguyen, V. N.; Choi, Y.; Lee, S.; Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021, 121, 13454-619.
152. Kolarikova, M.; Hosikova, B.; Dilenko, H.; et al. Photodynamic therapy: innovative approaches for antibacterial and anticancer treatments. Med. Res. Rev. 2023, 43, 717-74.
153. Mishchenko, T. A.; Balalaeva, I. V.; Vedunova, M. V.; Krysko, D. V. Ferroptosis and photodynamic therapy synergism: enhancing anticancer treatment. Trends. Cancer. 2021, 7, 484-7.
154. Zhang, X.; Zhang, X.; Chen, S.; et al. Glutathione-depleting polyprodrug nanoparticle for enhanced photodynamic therapy and cascaded locoregional chemotherapy. J. Colloid. Interface. Sci. 2024, 670, 279-87.
155. Shen, H.; Zhang, Y.; Wang, P.; et al. 2,3,5,4’-tetrahydroxystilbene-2-O-b-D-glucoside modulates CHEK2 and CCND1 alternative splicing to inhibit MCF-7 cells proliferation. Tradit. Med. Res. 2024, 9, 4.
156. Andreeva, O. E.; Sorokin, D. V.; Vinokurova, S. V.; et al. Breast cancer cell resistance to hormonal and targeted therapeutics is correlated with the inactivation of the NR6A1 axis. Cancer. Drug. Resist. 2024, 7, 48.
157. Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-dependent in vivo transport of nanoparticles: implications for delivery, targeting, and clearance. ACS. Nano. 2023, 17, 20825-49.
158. Li, Z.; Zhang, Z.; Ma, L.; et al. Combining multiple photosensitizer modules into one supramolecular system for synergetic enhanced photodynamic therapy. Angew. Chem. Int. Ed. 2024, 63, e202400049.
159. Han, L.; Liang, S.; Mu, W.; et al. Amphiphilic small molecular mates match hydrophobic drugs to form nanoassemblies based on drug-mate strategy. Asian. J. Pharm. Sci. 2022, 17, 129-38.
160. Shan, X.; Cai, Y.; Zhu, B.; et al. Computer-aided design of self-assembled nanoparticles to enhance cancer chemoimmunotherapy via dual-modulation strategy. Adv. Healthc. Mater. 2025, 14, e2404261.
161. Yang, Y.; Zuo, S.; Zhang, J.; et al. Prodrug nanoassemblies bridged by Mono-/Di-/Tri-sulfide bonds: exploration is for going further. Nano. Today. 2022, 44, 101480.
162. Xu, X.; Liu, A.; Liu, S.; et al. Application of molecular dynamics simulation in self-assembled cancer nanomedicine. Biomater. Res. 2023, 27, 39.
163. Zheng, N.; Xie, D.; Wang, C.; et al. Water-soluble, zwitterionic poly-photosensitizers as carrier-free, photosensitizer-self-delivery system for in vivo photodynamic therapy. ACS. Appl. Mater. Interfaces. 2019, 11, 44007-17.
164. Wei, W.; Zhang, X.; Chen, X.; Zhou, M.; Xu, R.; Zhang, X. Smart surface coating of drug nanoparticles with cross-linkable polyethylene glycol for bio-responsive and highly efficient drug delivery. Nanoscale 2016, 8, 8118-25.
165. Li, Y.; Lin, J.; Cai, Z.; et al. Tumor microenvironment-activated self-recognizing nanodrug through directly tailored assembly of small-molecules for targeted synergistic chemotherapy. J. Control. Release. 2020, 321, 222-35.
166. Xue, K.; Wei, F.; Lin, J.; et al. Tumor acidity-responsive carrier-free nanodrugs based on targeting activation via ICG-templated assembly for NIR-II imaging-guided photothermal-chemotherapy. Biomater. Sci. 2021, 9, 1008-19.
167. Xu, H.; Gao, W.; Pan, D.; et al. Puerarin modulation of CENPA affects downstream PLK1 and CCNB1 expression to inhibit bladder cancer cell proliferation. Tradit. Med. Res. 2024, 9, 52.
168. Yang, L.; Lei, S.; Xu, W.; et al. Rising above: exploring the therapeutic potential of natural product-based compounds in human cancer treatment. Tradit. Med. Res. 2025, 10, 18.
169. Chen, Y.; Chen, S.; Chen, K.; Ji, L.; Cui, S. Magnolol and 5-fluorouracil synergy inhibition of metastasis of cervical cancer cells by targeting PI3K/AKT/mTOR and EMT pathways. Chin. Herb. Med. 2024, 16, 94-105.
170. Wang, X.; Miao, Y.; Zhao, X.; Liu, X.; Hu, Y.; Deng, D. Perspectives on organ-on-a-chip technology for natural products evaluation. Food. Med. Homol. 2024, 1, 9420013.
171. Liu, T.; Yang, L.; Dong, C.; Qi, D.; Ji, B.; Gao, Q. Spectrum-effect relationship between components and antitumor activity of Lonicerae Japonicae Flos based on orthogonal partial least squares regression. Sci. Tradit. Chin. Med. 2024, 2, 138-47.
172. Cheng, Y.; Han, L.; Tang, Y.; Wang, Q. Xiaoyaosan inhibits liver metastases of colorectal cancer in vivo by regulating neutrophil extracellular traps formation. Tradit. Med. Res. 2025, 10, 46.
173. Zhang, J.; Zhou, J.; Wang, W.; et al. Quercetin exerts anti-breast cancer effect by inducing cellular senescence. Tradit. Med. Res. 2025, 10, 62.
174. Teng, C.; Chen, J. W.; Shen, L. S.; Chen, S.; Chen, G. Q. Research advances in natural sesquiterpene lactones: overcoming cancer drug resistance through modulation of key signaling pathways. Cancer. Drug. Resist. 2025, 8, 13.
175. Wang, S.; Dong, S.; Dong, Q.; Lin, W.; Dong, M.; Liu, D. Natural product-induced oxidative stress-synergistic anti-tumor effects of chemotherapeutic agents. Tradit. Med. Res. 2024, 9, 14.
176. Park, J.; Sun, B.; Yeo, Y. Albumin-coated nanocrystals for carrier-free delivery of paclitaxel. J. Control. Release. 2017, 263, 90-101.
177. Mohankumar, M.; Fernandes, S.; Cavalieri, F.; Cortez-Jugo, C.; Caruso, F. Ultrasound-driven coassembly of anticancer drugs into carrier-free particles. ACS. Nano. 2025, 19, 13366-80.
178. Wang, H.; Qiao, C.; Guan, Q.; Wei, M.; Li, Z. Nanoparticle-mediated synergistic anticancer effect of ferroptosis and photodynamic therapy: novel insights and perspectives. Asian. J. Pharm. Sci. 2023, 18, 100829.
179. Yang, S. B.; Lee, D. N.; Lee, J. H.; et al. Design and evaluation of a carrier-free prodrug-based palmitic-DEVD-doxorubicin conjugate for targeted cancer therapy. Bioconjug. Chem. 2023, 34, 333-44.
180. Meng, F.; Ren, S.; Wang, G.; et al. Carrier-free nanoparticles via coassembly of paclitaxel and gambogic acid with folate-functionalized albumin for targeted tumor treatment. ACS. Appl. Nano. Mater. 2024, 7, 26941-51.
181. Liu, Y.; Luo, Y.; Gao, Y.; et al. Carrier-free biomimetic organic nanoparticles with super-high drug loading for targeted NIR-II excitable triple-modal bioimaging and phototheranostics. Small 2024, 20, e2406003.
182. Yang, L.; Xu, J.; Xie, Z.; Song, F.; Wang, X.; Tang, R. Carrier-free prodrug nanoparticles based on dasatinib and cisplatin for efficient antitumor in vivo. Asian. J. Pharm. Sci. 2021, 16, 762-71.
183. Fan, Z.; Wang, Y.; Xiang, S.; et al. Dual-self-recognizing, stimulus-responsive and carrier-free methotrexate-mannose conjugate nanoparticles with highly synergistic chemotherapeutic effects. J. Mater. Chem. B. 2020, 8, 1922-34.
184. Hu, L.; Dong, G.; Li, X.; Li, S.; Lv, Y. A free-radical initiator-based carrier-free smart nanobomb for targeted synergistic therapy of hypoxic breast cancer. RSC. Adv. 2025, 15, 3098-109.
185. Dong, X.; Liu, H.; Liu, H.; Zhang, X.; Deng, X. Carrier-free nanomedicines: mechanisms of formation and biomedical applications. Giant 2024, 18, 100256.
186. Allen, T. M.; Cullis, P. R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug. Deliv. Rev. 2013, 65, 36-48.
187. Cheng, Z.; Huang, H.; Yin, M.; Liu, H. Applications of liposomes and lipid nanoparticles in cancer therapy: current advances and prospects. Exp. Hematol. Oncol. 2025, 14, 11.
188. Menachem, R.; Nudelman, I.; Vorontsova, A.; et al. Bone marrow-targeted liposomes loaded with bortezomib overcome multiple myeloma resistance. ACS. Nano. 2025, 19, 11684-701.
189. Liu, Y.; Xie, Y.; Chen, Y.; et al. A protease-cleavable liposome for co-delivery of anti-PD-L1 and doxorubicin for colon cancer therapy in mice. Nat. Commun. 2025, 16, 2854.
190. Xia, J.; Gan, Z.; Zhang, J.; et al. Geometric-aware deep learning enables discovery of bifunctional ligand-based liposomes for tumor targeting therapy. Nano. Today. 2025, 61, 102668.
191. Ghosh, R.; De, M. Liposome-based antibacterial delivery: an emergent approach to combat bacterial infections. ACS. Omega. 2023, 8, 35442-51.
192. Wu, S.; Lu, J. Liposome-enabled nanomaterials for muscle regeneration. Small. Methods. 2025, 9, e2402154.
193. Trivedi, J.; Yasir, M.; Maurya, R. K.; Tripathi, A. S. Aptamer-based theranostics in oncology: design strategies and limitations. BIOI 2024, 5.
194. Zhao, Y.; Qin, J.; Yu, D.; et al. Polymer-locking fusogenic liposomes for glioblastoma-targeted siRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2024, 19, 1869-79.
195. Wang, G.; Lu, H.; Pan, Y.; Qi, Y.; Huang, Y. Ultrasound-sensitive targeted liposomes as a gene delivery system for the synergistic treatment of hepatocellular carcinoma. Small 2024, 20, e2406182.
196. Bhattacherjee, A.; Daskhan, G. C.; Bains, A.; et al. Increasing phagocytosis of microglia by targeting CD33 with liposomes displaying glycan ligands. J. Control. Release. 2021, 338, 680-93.
197. Ren, X.; Xue, R.; Luo, Y.; et al. Programmable melanoma-targeted radio-immunotherapy via fusogenic liposomes functionalized with multivariate-gated aptamer assemblies. Nat. Commun. 2024, 15, 5035.
198. Di, J.; Xie, F.; Xu, Y. When liposomes met antibodies: drug delivery and beyond. Adv. Drug. Deliv. Rev. 2020, 154-5, 151-62.
199. Park, Y. I.; Kwon, S. H.; Lee, G.; et al. pH-sensitive multi-drug liposomes targeting folate receptor β for efficient treatment of non-small cell lung cancer. J. Control. Release. 2021, 330, 1-14.
200. Yu, Y.; Zu, C.; He, D.; et al. pH-dependent reversibly activatable cell-penetrating peptides improve the antitumor effect of artemisinin-loaded liposomes. J. Colloid. Interface. Sci. 2021, 586, 391-403.
201. Lee, Y.; Song, S.; Yang, S.; et al. Photo-induced crosslinked and anti-PD-L1 peptide incorporated liposomes to promote PD-L1 multivalent binding for effective immune checkpoint blockade therapy. Acta. Pharm. Sin. B. 2024, 14, 1428-40.
202. Wang, C.; Lan, X.; Zhu, L.; et al. Construction strategy of functionalized liposomes and multidimensional application. Small 2024, 20, e2309031.
203. Rahman, M. M.; Wang, J.; Wang, G.; et al. Chimeric nanobody-decorated liposomes by self-assembly. Nat. Nanotechnol. 2024, 19, 818-24.
204. Jourdain, M. A.; Eyer, J. Recent advances in liposomes and peptide-based therapeutics for glioblastoma treatment. J. Control. Release. 2024, 376, 732-52.
205. Yang, Y.; Chu, Y.; Li, C.; et al. Brain-targeted drug delivery by in vivo functionalized liposome with stable D-peptide ligand. J. Control. Release. 2024, 373, 240-51.
206. Zhang, Y.; Zhu, J.; Sun, H.; Li, J. Modulation of tumor hypoxia and redox microenvironment using nanomedicines for enhanced cancer photodynamic therapy. Appl. Mater. Today. 2022, 29, 101687.
207. Wang, C.; Liang, C.; Hao, Y.; et al. Photodynamic creation of artificial tumor microenvironments to collectively facilitate hypoxia-activated chemotherapy delivered by coagulation-targeting liposomes. Chem. Eng. J. 2021, 414, 128731.
208. Yan, K.; Mu, C.; Zhang, C.; et al. Pt nanoenzyme decorated yolk-shell nanoplatform as an oxygen generator for enhanced multi-modality imaging-guided phototherapy. J. Colloid. Interface. Sci. 2022, 616, 759-68.
209. Ren, C.; Xu, X.; Yan, D.; et al. Dual-action nanoplatform with a synergetic strategy to promote oxygen accumulation for enhanced photodynamic therapy against hypoxic tumors. Acta. Biomater. 2022, 146, 465-77.
210. Sun, Y.; Zhao, D.; Wang, G.; et al. Recent progress of hypoxia-modulated multifunctional nanomedicines to enhance photodynamic therapy: opportunities, challenges, and future development. Acta. Pharm. Sin. B. 2020, 10, 1382-96.
211. Zhao, L. P.; Zheng, R. R.; Chen, H. Q.; et al. Self-delivery nanomedicine for O2-economized photodynamic tumor therapy. Nano. Lett. 2020, 20, 2062-71.
212. Xu, T.; Ma, Y.; Yuan, Q.; et al. Enhanced ferroptosis by oxygen-boosted phototherapy based on a 2-in-1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS. Nano. 2020, 14, 3414-25.
213. Wang, Y.; Li, N.; Qu, L.; et al. Hemoglobin nanoclusters-mediated regulation of KPNA4 in hypoxic tumor microenvironment enhances photodynamic therapy in hepatocellular carcinoma. J. Nanobiotechnol. 2024, 22, 473.
214. Lee, H. S.; Yoo, S.; Lee, S. M.; et al. Hypoxia-alleviating hemoglobin nanoclusters for sensitizing chemo-photodynamic therapy of cervical cancer. Chem. Eng. J. 2023, 457, 141224.
215. Han, W.; Ding, J.; Qiao, B.; et al. Self-sustained biophotocatalytic nano-organelle reactors with programmable DNA switches for combating tumor metastasis. Adv. Mater. 2025, 37, e2415030.
216. Li, J.; Xue, Y.; Tian, J.; et al. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr. Polym. 2020, 237, 116119.
217. Zhang, Y.; Liao, Y.; Tang, Q.; Lin, J.; Huang, P. Biomimetic nanoemulsion for synergistic photodynamic-immunotherapy against hypoxic breast tumor. Angew. Chem. Int. Ed. 2021, 60, 10647-53.
218. Zhang, S.; Li, Z.; Wang, Q.; et al. An NIR-II photothermally triggered "oxygen bomb" for hypoxic tumor programmed cascade therapy. Adv. Mater. 2022, 34, e2201978.
219. Zhang, S.; Yang, N.; Sun, S.; et al. Dually fluorinated unimolecular micelles for stable oxygen-carrying and enhanced photosensitive efficiency to boost photodynamic therapy against hypoxic tumors. Acta. Biomater. 2025, 193, 406-16.
220. Zhang, X.; Lyu, Y.; Li, J.; Yang, X.; Lan, Z.; Chen, Z. Bimetallic nanozymes-integrated parachute-like Au2Pt@PMO@ICG janus nanomotor with dual propulsion for enhanced tumor penetration and synergistic PTT/PDT/CDT cancer therapy. Adv. Funct. Mater. 2024, 34, 2406059.
221. Hao, R.; Zhang, G.; Zhang, J.; Zeng, L. Ultrasmall Au/Pt-loaded biocompatible albumin nanospheres to enhance photodynamic/catalytic therapy via triple amplification of glucose-oxidase/catalase/peroxidase. J. Colloid. Interface. Sci. 2024, 654, 212-23.
222. He, M.; Cheng, Z.; Wang, Z.; et al. Controllable regulation of Ag2S quantum-dot-mediated protein nanoassemblies for imaging-guided synergistic PDT/PTT/chemotherapy against hypoxic tumor. Adv. Healthc. Mater. 2023, 12, e2300752.
223. Wang, T.; Liu, T.; Li, Z.; Wu, D.; Zhao, X.; Zeng, L. Ultrasmall gold-encapsulated mesoporous platinum to promote photodynamic/catalytic therapy through cascade enzyme-like reactions. J. Colloid. Interface. Sci. 2025, 680, 117-28.
224. Song, C.; Wu, F.; Yao, S.; et al. DNA Damage-sensitized metal phenolic nanosynergists potentiate low-power phototherapy for osteosarcoma therapy. J. Colloid. Interface. Sci. 2024, 674, 1025-36.
225. Zhen, W.; Kang, D. W.; Fan, Y.; et al. Simultaneous protonation and metalation of a porphyrin covalent organic framework enhance photodynamic therapy. J. Am. Chem. Soc. 2024, 146, 16609-18.
226. Zhang, J.; Han, X.; Chen, P.; et al. Photodynamic therapy-accelerated hypoxia-responsive prodrug release for synergistic photo-chemotherapy of melanoma cancer based on noncovalent interactions. ACS. Materials. Lett. 2025, 7, 770-9.
227. Jiang, M.; Liu, Y.; Dong, Y.; Wang, K.; Yuan, Y. Bioorthogonal chemistry and illumination controlled programmed size-changeable nanomedicine for synergistic photodynamic and hypoxia-activated therapy. Biomaterials 2022, 284, 121480.
228. Jia, Z.; Gao, Y.; Ni, J.; et al. A hybrid metal-organic framework nanomedicine-mediated photodynamic therapy and hypoxia-activated cancer chemotherapy. J. Colloid. Interface. Sci. 2023, 629, 379-90.
229. Tang, Y.; Pan, T.; Pang, E.; et al. Thienothiophene-benzopyran derivative and AQ4N-assembled liposomes for near-infrared II fluorescence imaging-guided phototherapy, chemotherapy, and immune activation. Small 2025, 21, e2407680.
230. Wang, Z.; Yang, L. Broad-spectrum prodrugs with anti-SARS-CoV-2 activities: strategies, benefits, and challenges. J. Med. Virol. 2022, 94, 1373-90.
231. Wang, Z.; Yang, L.; Song, X. Q. Oral GS-441524 derivatives: next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase. Front. Immunol. 2022, 13, 1015355.
232. Wang, X.; Zhang, M.; Cui, L.; et al. Inhibitory effect of saffron on head and neck squamous cell carcinoma via targeting of ESR1 and CCND1 by its active compound crocetin. Tradit. Med. Res. 2024, 9, 39.
233. Chen, L.; Liu, H.; Zheng, Z.; et al. MicroRNA-32-5p inhibits metastasis by directly targeting VPS4B and increases sensitivity to dihydroartemisinin in neuroblastoma. Sci. Tradit. Chin. Med. 2024, 2, 202-13.
234. Guo, T.; Zhou, H.; Chen, F.; Gu, Y.; Li, L.; Cheng, H. A review on the pathogenesis theory of cancerous toxin from the viewpoint of system theory. Sci. Tradit. Chin. Med. 2024, 2, 187-93.
235. Li, L.; Yang, L.; Cheng, S.; et al. Lung adenocarcinoma-intrinsic GBE1 signaling inhibits anti-tumor immunity. Mol. Cancer. 2019, 18, 108.
236. Dastmalchi, N.; Baradaran, B.; Banan, Khojasteh. S. M.; Hosseinpourfeizi, M.; Safaralizadeh, R. miR-424: a novel potential therapeutic target and prognostic factor in malignancies. Cell. Biol. Int. 2021, 45, 720-30.
237. Li, J.; Yi, H.; Fu, Y.; et al. Biodegradable iridium coordinated nanodrugs potentiate photodynamic therapy and immunotherapy of lung cancer. J. Colloid. Interface. Sci. 2025, 680, 9-24.
238. Shi, X.; Bao, X.; Li, Y.; Yin, C. Theanine combined with cisplatin inhibits the proliferation and metastasis of TNBC cells through Akt signaling pathway. Tradit. Med. Res. 2023, 8, 25.
239. Yang, L.; Wang, Z. Natural products, alone or in combination with FDA-approved drugs, to treat COVID-19 and lung cancer. Biomedicines 2021, 9, 689.
240. Li, Q.; Liu, X.; Yan, C.; et al. Polysaccharide-based stimulus-responsive nanomedicines for combination cancer immunotherapy. Small 2023, 19, e2206211.
241. Zhang, Y.; Zhou, J.; Chen, X.; et al. Modulating tumor-stromal crosstalk via a redox-responsive nanomedicine for combination tumor therapy. J. Control. Release. 2023, 356, 525-41.
242. Zhao, Y. D.; An, H. W.; Mamuti, M.; et al. Reprogramming hypoxic tumor-associated macrophages by nanoglycoclusters for boosted cancer immunotherapy. Adv. Mater. 2023, 35, e2211332.
243. Luo, Y. L.; Liang, L. F.; Gan, Y. J.; et al. An all-in-one nanomedicine consisting of CRISPR-Cas9 and an autoantigen peptide for restoring specific immune tolerance. ACS. Appl. Mater. Interfaces. 2020, 12, 48259-71.
244. van, Hooren. L.; Vaccaro, A.; Ramachandran, M.; et al. Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma. Nat. Commun. 2021, 12, 4127.
245. Broos, S.; Sandin, L. C.; Apel, J.; et al. Synergistic augmentation of CD40-mediated activation of antigen-presenting cells by amphiphilic poly(γ-glutamic acid) nanoparticles. Biomaterials 2012, 33, 6230-9.
246. Correa, S.; Meany, E. L.; Gale, E. C.; et al. Injectable nanoparticle-based hydrogels enable the safe and effective deployment of immunostimulatory CD40 agonist antibodies. Adv. Sci. 2022, 9, e2103677.
247. Gaur, V.; Tyagi, W.; Das, S.; Ganguly, S.; Bhattacharyya, J. CD40 agonist engineered immunosomes modulated tumor microenvironment and showed pro-immunogenic response, reduced toxicity, and tumor free survival in mice bearing glioblastoma. Biomaterials 2024, 311, 122688.
248. Cui, Y.; Xia, H.; Liu, Q.; et al. A tumor-activatable liposomal nanoprobe for selective visualization of metastatic lymph nodes. Adv. Healthc. Mater. 2024, 13, e2401935.
249. Lee, J. S. F.; Cohen, R. M.; Khan, R. A.; et al. Paving the way for affordable and equitable liposomal amphotericin B access worldwide. Lancet. Glob. Health. 2024, 12, e1552-9.
250. Zhao, Z.; Wang, W.; Wang, G.; et al. Dual peptides-modified cationic liposomes for enhanced Lung cancer gene therapy by a gap junction regulating strategy. J. Nanobiotechnol. 2023, 21, 473.
251. Liu, X.; Zhao, Z.; Xu, X.; et al. Mobilizing STING pathway via a cationic liposome to enhance doxorubicin-induced antitumor immunity. Adv. Funct. Mater. 2025, 35, 2416406.
252. Wang, D.; Cao, Y.; Yang, G.; et al. Self-targeted Co-delivery of an antibiotic and a cancer-chemotherapeutic from synthetic liposomes for the treatment of infected tumors. Adv. Funct. Mater. 2023, 33, 2215153.
253. Han, K.; Cho, Y. S.; Moon, J. J. Antibiotic nanoparticles boost antitumor immunity. Nat. Biotechnol. 2024, 42, 1187-8.
254. Li, X.; Liu, W.; Yan, Z.; et al. Modulating autophagy in the tumor microenvironment with a salinomycin-loaded liposome hybrid nanovesicle system for tumor immunotherapy. Nano. Today. 2025, 61, 102644.
255. Zheng, Y.; Oz, Y.; Gu, Y.; et al. Rational design of polymeric micelles for targeted therapeutic delivery. Nano. Today. 2024, 55, 102147.
256. Chen, Z.; Kankala, R. K.; Long, L.; Xie, S.; Chen, A.; Zou, L. Current understanding of passive and active targeting nanomedicines to enhance tumor accumulation. Coordin. Chem. Rev. 2023, 481, 215051.
257. Jia, W.; Li, R.; Zou, F.; et al. Decorating delivery vehicles using hyaluronic acid oligosaccharides enables active targeting toward cancer and minimizes adverse effect of chemotherapeutics. Adv. Healthc. Mater. 2024, 13, e2402158.
258. Paula MC, Carvalho SG, Silvestre ALP, Dos Santos AM, Meneguin AB, Chorilli M. The role of hyaluronic acid in the design and functionalization of nanoparticles for the treatment of colorectal cancer. Carbohydr. Polym. 2023, 320, 121257.
259. Gao, Y.; Qiu, W.; Liang, M.; et al. Active targeting redox-responsive mannosylated prodrug nanocolloids promote tumor recognition and cell internalization for enhanced colon cancer chemotherapy. Acta. Biomater. 2022, 147, 299-313.
260. Chen, M. M.; Tang, X.; Li, J. J.; et al. Active targeting tumor therapy using host-guest drug delivery system based on biotin functionalized azocalix[4]arene. J. Control. Release. 2024, 368, 691-702.
261. Li, Y.; Dang, G.; Rizwan, Younis. M.; et al. Peptide functionalized actively targeted MoS2 nanospheres for fluorescence imaging-guided controllable pH-responsive drug delivery and collaborative chemo/photodynamic therapy. J. Colloid. Interface. Sci. 2023, 639, 302-13.
262. Lu, J.; Xu, X.; Sun, X.; Du, Y. Protein and peptide-based renal targeted drug delivery systems. J. Control. Release. 2024, 366, 65-84.
263. Li, J.; Zeng, H.; You, Y.; et al. Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin. J. Nanobiotechnology. 2021, 19, 289.
264. Lin, M.; Cai, Y.; Chen, G.; et al. A hierarchical tumor-targeting strategy for eliciting potent antitumor immunity against triple negative breast cancer. Biomaterials 2023, 296, 122067.
265. Ding, G. B.; Zhu, C.; Wang, Q.; et al. Molecularly engineered tumor acidity-responsive plant toxin gelonin for safe and efficient cancer therapy. Bioact. Mater. 2022, 18, 42-55.
266. Zhang, P.; Chen, D.; Li, L.; Sun, K. Charge reversal nano-systems for tumor therapy. J. Nanobiotechnol. 2022, 20, 31.
267. Ding, H.; Tan, P.; Fu, S.; et al. Preparation and application of pH-responsive drug delivery systems. J. Control. Release. 2022, 348, 206-38.
268. Schützmann, M. P.; Hasecke, F.; Bachmann, S.; et al. Endo-lysosomal Aβ concentration and pH trigger formation of Aβ oligomers that potently induce Tau missorting. Nat. Commun. 2021, 12, 4634.
269. Du, Y.; Yan, X.; Wu, W.; et al. Magnetic and pH dual responsive injectable magnetic zeolitic imidazole framework based colloidal gel for interventional treatment on hepatocellular carcinoma. Chem. Eng. J. 2024, 497, 154180.
270. Liu, X.; Wang, X.; Zang, D.; et al. pH-responsive oxygen self-sufficient smart nanoplatform for enhanced tumor chemotherapy and photodynamic therapy. J. Colloid. Interface. Sci. 2024, 675, 1080-90.
271. Ma, N.; Kwon, M. H.; Palanisamy, S.; et al. A novel sulfated mannan-carboxymethyl-5-fluorouracil-folic acid conjugates for targeted anticancer drug delivery. Carbohydr. Polym. 2023, 304, 120454.
272. Yang, L.; Li, H.; Luo, A.; et al. Macrophage membrane-camouflaged pH-sensitive nanoparticles for targeted therapy of oral squamous cell carcinoma. J. Nanobiotechnol. 2024, 22, 168.
273. Hou, G.; Qian, J.; Guo, M.; et al. Hydrazide-manganese coordinated multifunctional nanoplatform for potentiating immunotherapy in hepatocellular carcinoma. J. Colloid. Interface. Sci. 2022, 628, 968-83.
274. Xiao, W.; Geng, R.; Bi, D.; et al. pH/H2O2 cascade-responsive nanoparticles of lipid-like prodrugs through dynamic-covalent and coordination interactions for chemotherapy. Small 2024, 20, e2308790.
275. Hu, Q.; Xu, L.; Huang, X.; et al. Polydopamine-modified zeolite imidazole framework drug delivery system for photothermal chemotherapy of hepatocellular carcinoma. Biomacromolecules 2023, 24, 5964-76.
276. Weng, J.; Huang, Z.; Pu, X.; et al. Preparation of polyethylene glycol-polyacrylic acid block copolymer micelles with pH/hypoxic dual-responsive for tumor chemoradiotherapy. Colloids. Surf. B. Biointerfaces. 2020, 191, 110943.
277. Qu, D.; Jiao, M.; Lin, H.; et al. Anisamide-functionalized pH-responsive amphiphilic chitosan-based paclitaxel micelles for sigma-1 receptor targeted prostate cancer treatment. Carbohydr. Polym. 2020, 229, 115498.
278. Ma, J.; Fang, L.; Sun, Z.; et al. Folate-PEG-PROTAC micelles for enhancing tumor-specific targeting proteolysis in vivo. Adv. Healthc. Mater. 2024, 13, e2400109.
279. Huang, C.; Xie, T.; Liu, Y.; et al. A Sodium alginate-based multifunctional nanoplatform for synergistic chemo-immunotherapy of hepatocellular carcinoma. Adv. Mater. 2023, 35, e2301352.
280. Xu, C.; Xu, J.; Zheng, Y.; et al. Active-targeting and acid-sensitive pluronic prodrug micelles for efficiently overcoming MDR in breast cancer. J. Mater. Chem. B. 2020, 8, 2726-37.
281. Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349-59.
282. Sudimack, J.; Lee, R. J. Targeted drug delivery via the folate receptor. Adv. Drug. Deliv. Rev. 2000, 41, 147-62.
283. Shakeri-zadeh, A.; Rezaeyan, A.; Sarikhani, A.; et al. Folate receptor-targeted nanoprobes for molecular imaging of cancer: friend or foe? Nano. Today. 2021, 39, 101173.
284. Sheehy, T. L.; Kwiatkowski, A. J.; Arora, K.; et al. STING-activating polymer-drug conjugates for cancer immunotherapy. ACS. Cent. Sci. 2024, 10, 1765-81.
285. Gao, P.; Ha-Duong, T.; Nicolas, J. Coarse-grained model-assisted design of polymer prodrug nanoparticles with enhanced cytotoxicity: a combined theoretical and experimental study. Angew. Chem. Int. Ed. 2024, 63, e202316056.
286. Li, Y.; Feng, S.; Dai, P.; et al. Tailored Trojan horse nanocarriers for enhanced redox-responsive drug delivery. J. Control. Release. 2022, 342, 201-9.
287. Tokura, D.; Konarita, K.; Suzuki, M.; et al. Active control of pharmacokinetics using light-responsive polymer-drug conjugates for boron neutron capture therapy. J. Control. Release. 2024, 371, 445-54.
288. Yu, J.; Ha, W.; Chen, J.; Shi, Y. pH-Responsive supramolecular hydrogels for codelivery of hydrophobic and hydrophilic anticancer drugs. RSC. Adv. 2014, 4, 58982-9.
289. Wang, X.; Hao, X.; Zhang, Y.; et al. Bioinspired adaptive microdrugs enhance the chemotherapy of malignant glioma: beyond their nanodrugs. Adv. Mater. 2024, 36, e2405165.
290. Qin, N.; Yang, S.; Gao, S.; Zhu, T. Celastrol inhibits inflammatory factors expression in glioblastoma. Tradit. Med. Res. 2024, 9, 31.
291. Wang, R.; Zhang, X.; Huang, J.; et al. Bio-fabricated nanodrugs with chemo-immunotherapy to inhibit glioma proliferation and recurrence. J. Control. Release. 2023, 354, 572-87.
292. Yan, G.; Wang, Y.; Chen, J.; et al. Advances in drug development for targeted therapies for glioblastoma. Med. Res. Rev. 2020, 40, 1950-72.
293. Cui, J.; Wang, X.; Li, J.; et al. Immune exosomes loading self-assembled nanomicelles traverse the blood-brain barrier for chemo-immunotherapy against glioblastoma. ACS. Nano. 2023, 17, 1464-84.
294. Hua, Y.; Cao, P.; Wang, W.; et al. Suit the remedy to the case: an activatable DNA damage initiator for postsurgical therapy of glioblastoma with TP53 mutations. ACS. Mater. Lett. 2024, 6, 1870-82.
295. Gao, M.; Fu, Y.; Zhou, W.; et al. EGFR activates a TAZ-driven oncogenic program in glioblastoma. Cancer. Res. 2021, 81, 3580-92.
296. Choi, S. W.; Lee, Y.; Shin, K.; et al. Mutation-specific non-canonical pathway of PTEN as a distinct therapeutic target for glioblastoma. Cell. Death. Dis. 2021, 12, 374.
297. Wu, B.; Yang, X.; Kong, N.; Liang, J.; Li, S.; Wang, H. Engineering modular peptide nanoparticles for ferroptosis-enhanced tumor immunotherapy. Angew. Chem. Int. Ed. 2025, 64, e202421703.
298. Liang, Y.; Lei, R.; Tan, J.; et al. HER2-targeting peptide drug conjugate with better penetrability for effective breast cancer therapy. BIOI 2023, 4.
299. Zhou, Y.; Li, Q.; Wu, Y.; et al. Molecularly stimuli-responsive self-assembled peptide nanoparticles for targeted imaging and therapy. ACS. Nano. 2023, 17, 8004-25.
300. Qiu, J.; Tomeh, M. A.; Jin, Y.; Zhang, B.; Zhao, X. Microfluidic formulation of anticancer peptide loaded ZIF-8 nanoparticles for the treatment of breast cancer. J. Colloid. Interface. Sci. 2023, 642, 810-9.
301. Xu, X.; Chen, M.; Lou, X.; et al. Sialic acid-modified mesoporous polydopamine induces tumor vessel normalization to enhance photodynamic therapy by inhibiting VE-cadherin internalization. Chem. Eng. J. 2021, 414, 128743.
302. Du, Y.; Liu, D.; Sun, M.; et al. Multifunctional Gd-CuS loaded UCST polymeric micelles for MR/PA imaging-guided chemo-photothermal tumor treatment. Nano. Res. 2022, 15, 2288-99.
303. Lu, Y.; Lin, B.; Chai, S.; et al. A pyroptosis-enhanced leucocyte-hitchhiking liposomal nanoplatform for potentiated immunotherapy of hepatocellular carcinoma. Mater. Today. Nano. 2024, 27, 100492.
304. Li, C.; Liu, Q.; Han, L.; et al. The eATP/P2×7R axis drives quantum dot-nanoparticle induced neutrophil recruitment in the pulmonary microcirculation. Adv. Sci. 2024, 11, e2404661.
305. Li, Y.; Liu, F.; Abdiryim, T.; Liu, X. Cyclodextrin-derived materials: from design to promising applications in water treatment. Coordin. Chem. Rev. 2024, 502, 215613.
306. Hu, X. Y.; Gao, J.; Chen, F. Y.; Guo, D. S. A host-guest drug delivery nanosystem for supramolecular chemotherapy. J. Control. Release. 2020, 324, 124-33.
307. Bai, Y.; Pan, Y.; An, N.; et al. Host-guest interactions based supramolecular complexes self-assemblies for amplified chemodynamic therapy with H2O2 elevation and GSH consumption properties. Chin. Chem. Lett. 2023, 34, 107552.
308. Li, H.; Yang, X.; Sun, M. Self-strengthened cascade-explosive nanogel using host-guest interaction strategy for synergistic tumor treatment. Chin. Chem. Lett. 2025, 36, 110651.
309. Liu, Z.; Lin, W.; Liu, Y. Macrocyclic supramolecular assemblies based on hyaluronic acid and their biological applications. Acc. Chem. Res. 2022, 55, 3417-29.
310. Mousazadeh, H.; Bonabi, E.; Zarghami, N. Stimulus-responsive drug/gene delivery system based on polyethylenimine cyclodextrin nanoparticles for potential cancer therapy. Carbohydr. Polym. 2022, 276, 118747.
311. Hu, X.; Fu, R.; Guo, D. Hypoxia-responsive host-guest drug delivery system. Acc. Mater. Res. 2023, 4, 925-38.
312. Guan, W.; Chen, J.; Liu, J.; et al. Macrocycles-assembled AIE supramolecular polymer networks. Coordin. Chem. Rev. 2024, 507, 215717.
313. Gao, P.; Wang, H.; Cheng, Y. Strategies for efficient photothermal therapy at mild temperatures: Progresses and challenges. Chin. Chem. Lett. 2022, 33, 575-86.
314. Chen, P.; Wang, X.; Zhu, C.; Guo, T.; Wang, C.; Ying, L. Targeted delivery of quinoxaline-based semiconducting polymers for tumor photothermal therapy. ACS. Appl. Mater. Interfaces. 2024, 16, 38377-86.
315. Liu, C.; Chang, Q.; Fan, X.; et al. Rational construction of CQDs-based targeted multifunctional nanoplatform for synergistic chemo-photothermal tumor therapy. J. Colloid. Interface. Sci. 2025, 677, 79-90.
316. Li, P.; Wang, D.; Hu, J.; Yang, X. The role of imaging in targeted delivery of nanomedicine for cancer therapy. Adv. Drug. Deliv. Rev. 2022, 189, 114447.
317. Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv. Mater. 2017, 29, 1606628.
318. Wang, X.; Zhang, M.; Li, Y.; Cong, H.; Yu, B.; Shen, Y. Research status of dendrimer micelles in tumor therapy for drug delivery. Small 2023, 19, e2304006.
320. Wang, Y.; Li, H.; Niu, G.; et al. Boosting sono-immunotherapy of prostate carcinoma through amplifying domino-effect of mitochondrial oxidative stress using biodegradable cascade-targeting nanocomposites. ACS. Nano. 2024, 18, 5828-46.
321. Chiang, M. R.; Hsu, C. W.; Pan, W. C.; et al. Reprogramming dysfunctional dendritic cells by a versatile catalytic dual oxide antigen-captured nanosponge for remotely enhancing lung metastasis immunotherapy. ACS. Nano. 2025, 19, 2117-35.
322. Huynh, T. M. H.; Huang, P. X.; Wang, K. L.; et al. Reprogramming immunodeficiency in lung metastases via PD-L1 siRNA delivery and antigen capture of nanosponge-mediated dendritic cell modulation. ACS. Nano. 2025, 19, 25134-53.
323. Huang, L.; Li, Y.; Du, Y.; et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat. Commun. 2019, 10, 4871.
324. Sun, H.; Yu, T.; Li, X.; et al. Second near-infrared photothermal-amplified immunotherapy using photoactivatable composite nanostimulators. J. Nanobiotechnol. 2021, 19, 433.
325. Jana, D.; He, B.; Chen, Y.; Liu, J.; Zhao, Y. A defect-engineered nanozyme for targeted NIR-II photothermal immunotherapy of cancer. Adv. Mater. 2024, 36, e2206401.
326. Tan, P.; Chen, X.; Zhang, H.; Wei, Q.; Luo, K. Artificial intelligence aids in development of nanomedicines for cancer management. Semin. Cancer. Biol. 2023, 89, 61-75.
327. Xie, H.; Jia, Y.; Liu, S. Integration of artificial intelligence in clinical laboratory medicine: advancements and challenges. Interdiscip. Med. 2024, 2, e20230056.
328. Xu, B. Redefining the future of cancer care: intelligent oncology unveiled. Intell. Oncol. 2025, 1, 31-3.
329. Zhang, C.; Yuan, Y.; Xia, Q.; et al. Machine learning-driven prediction, preparation, and evaluation of functional nanomedicines via drug-drug self-assembly. Adv. Sci. 2025, 12, e2415902.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data







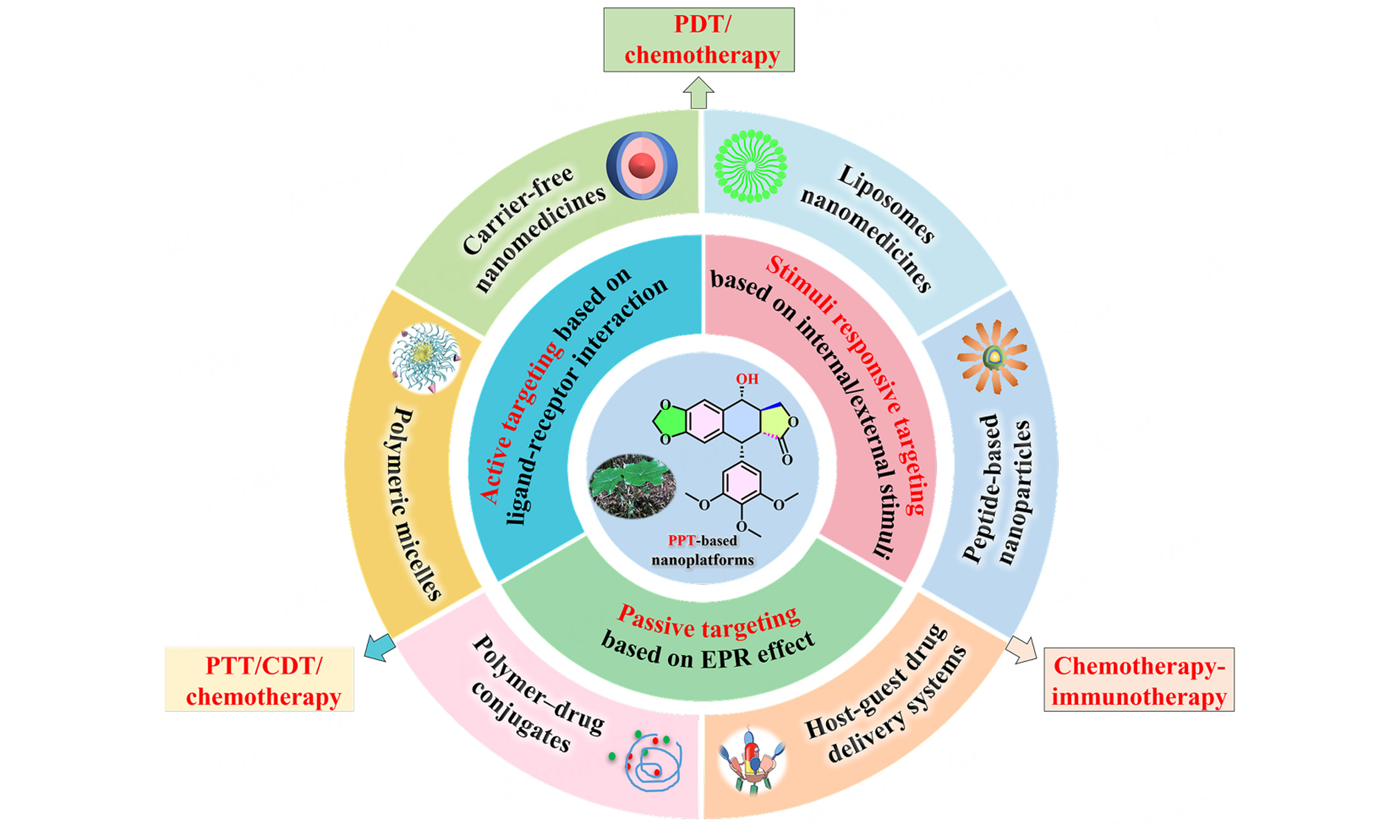
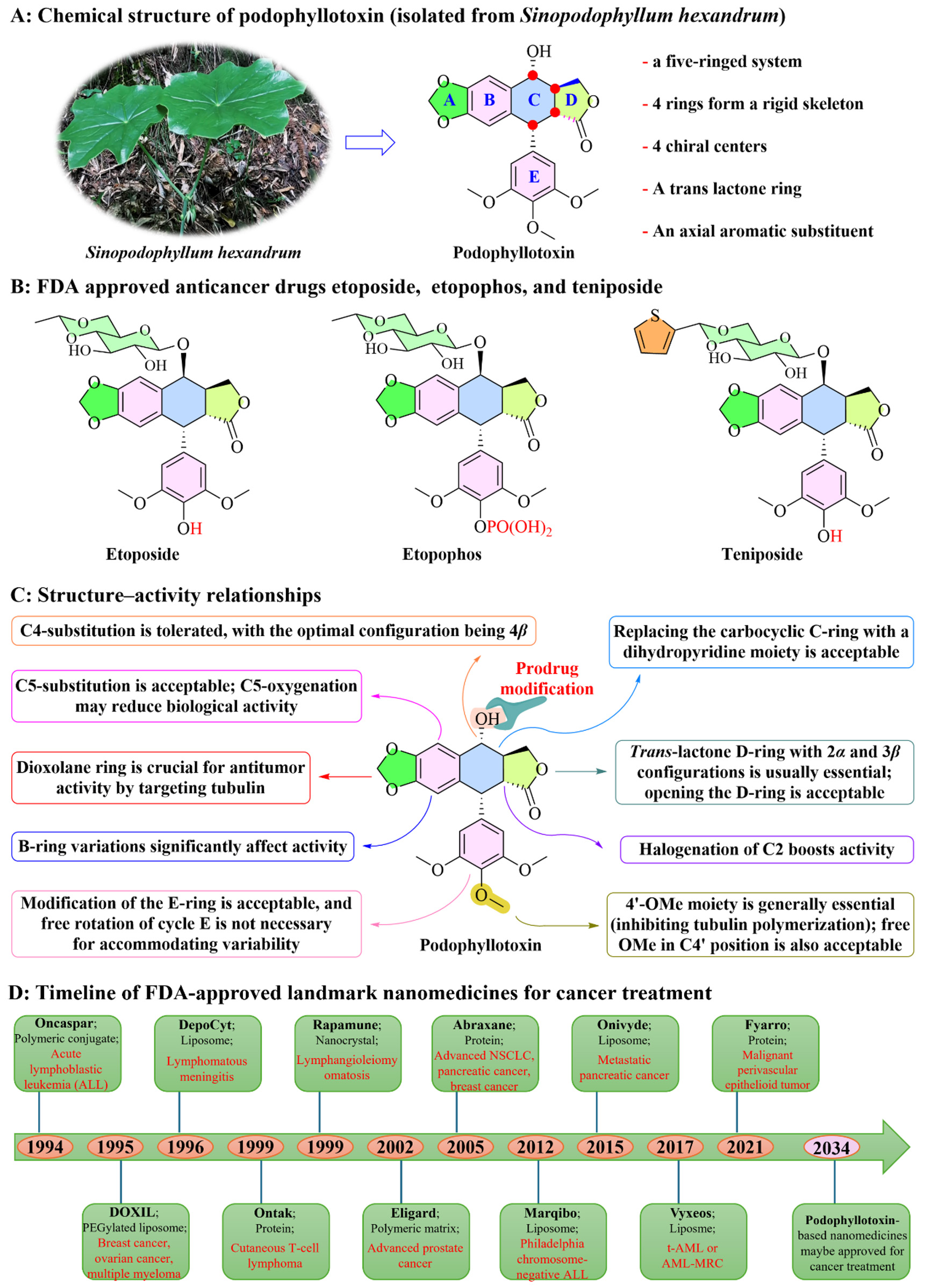
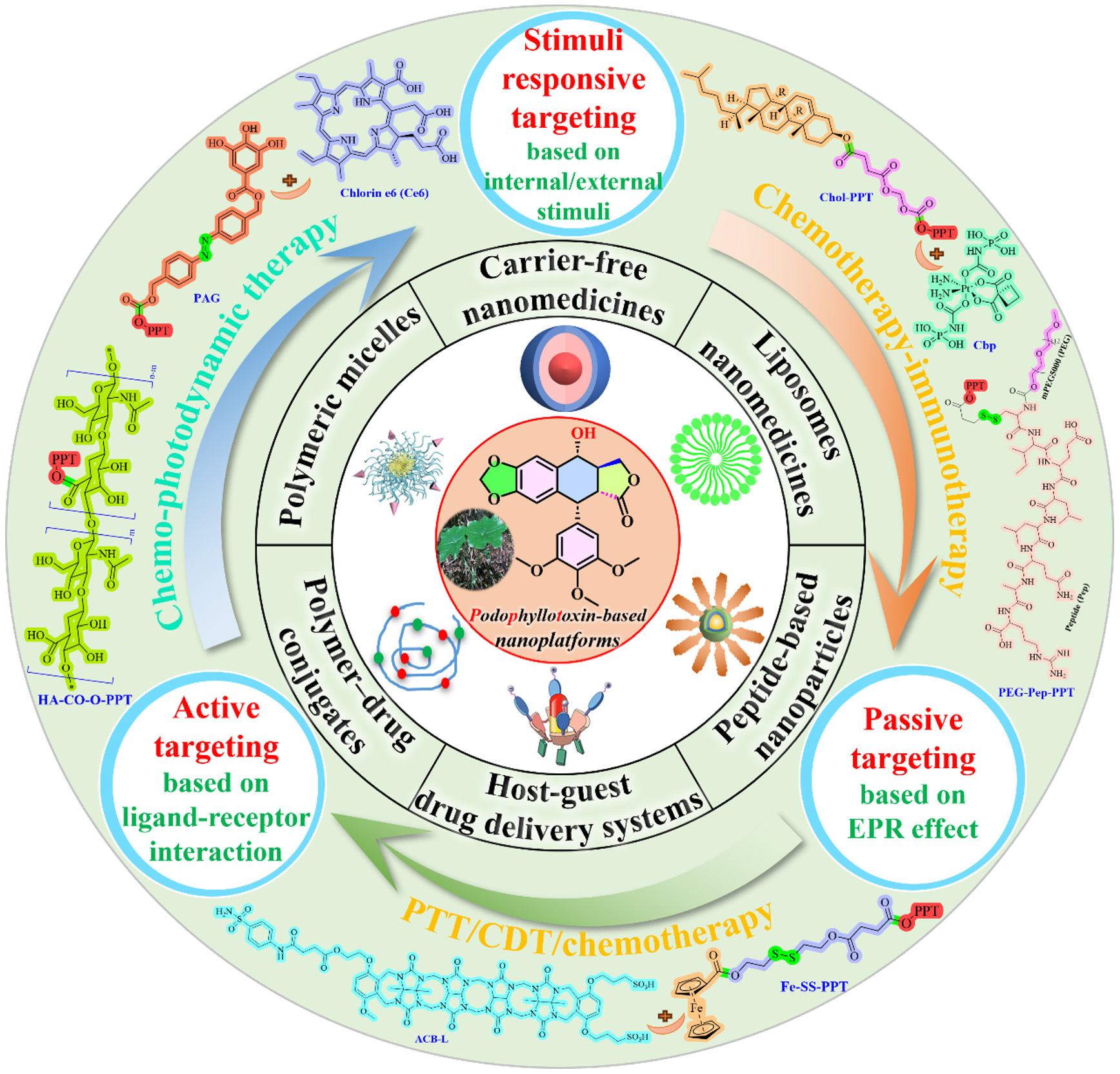
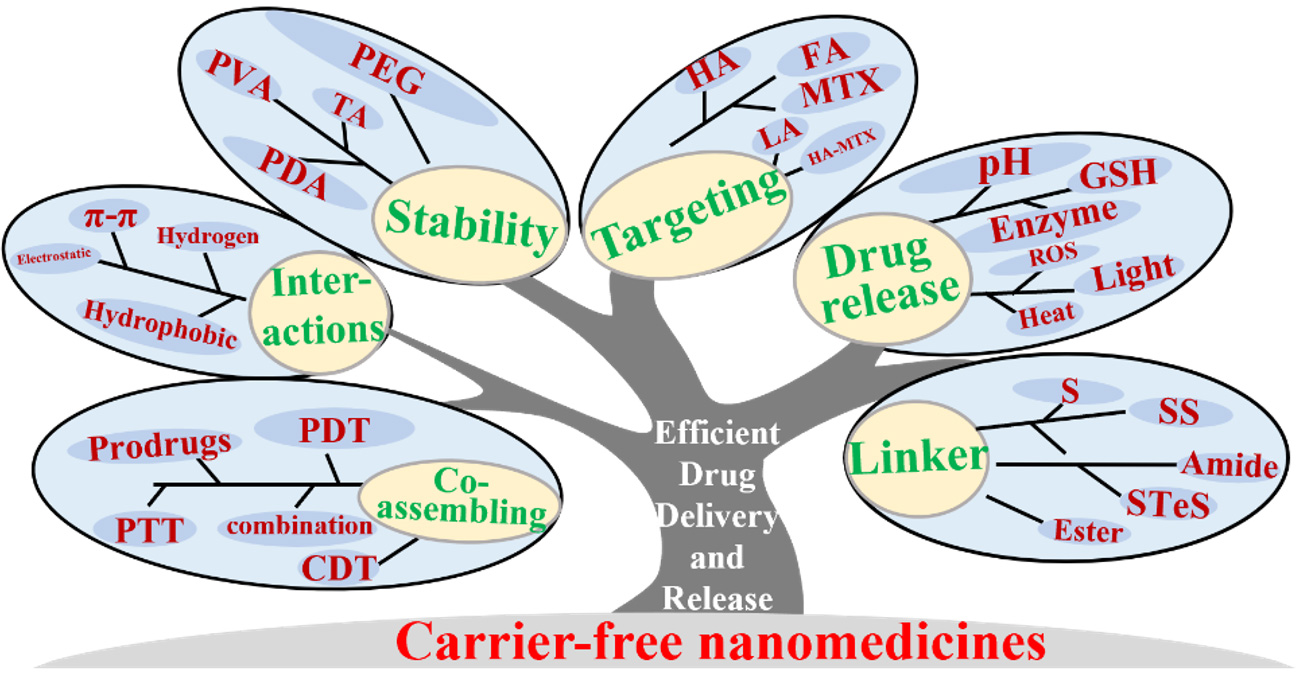
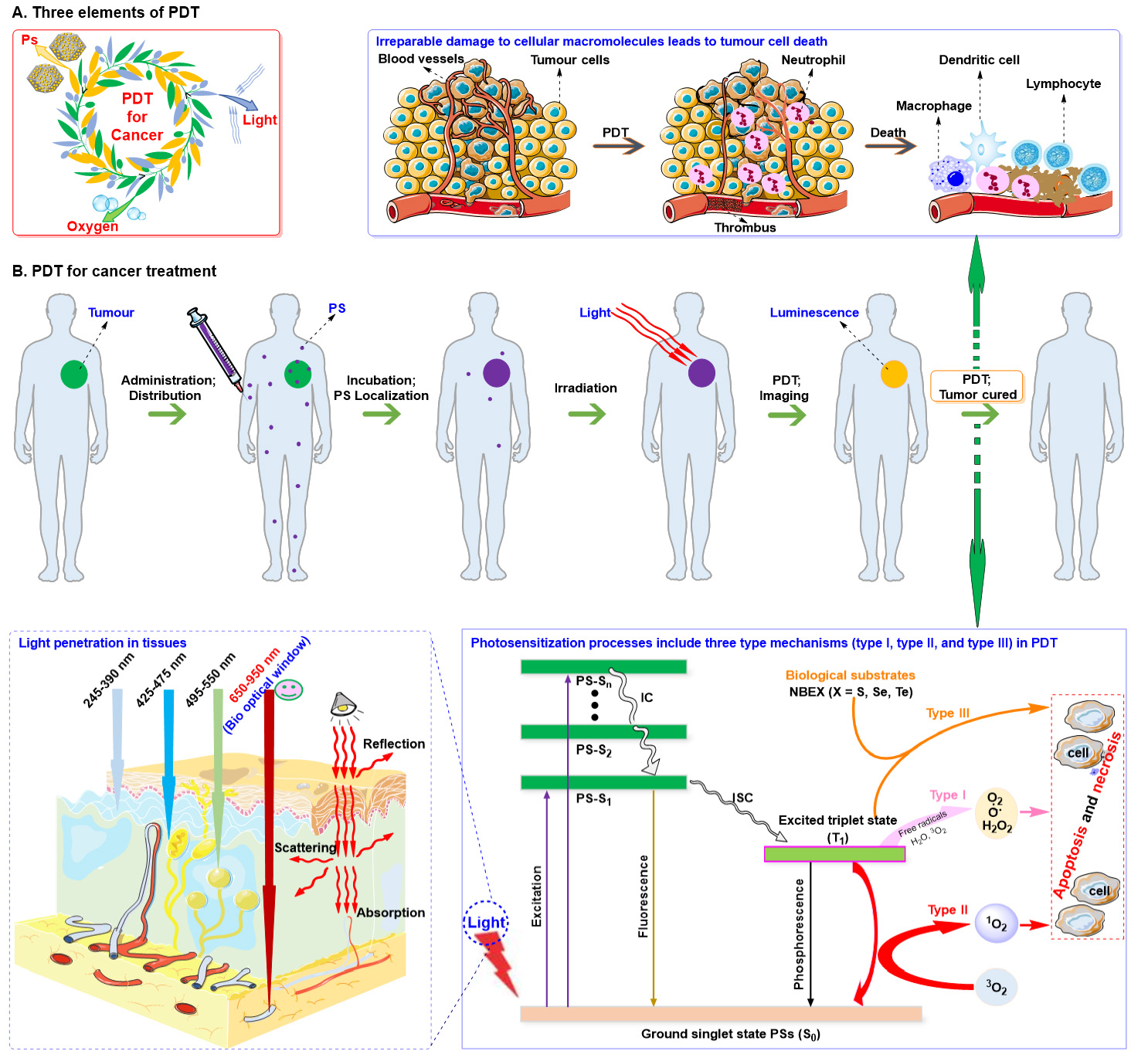
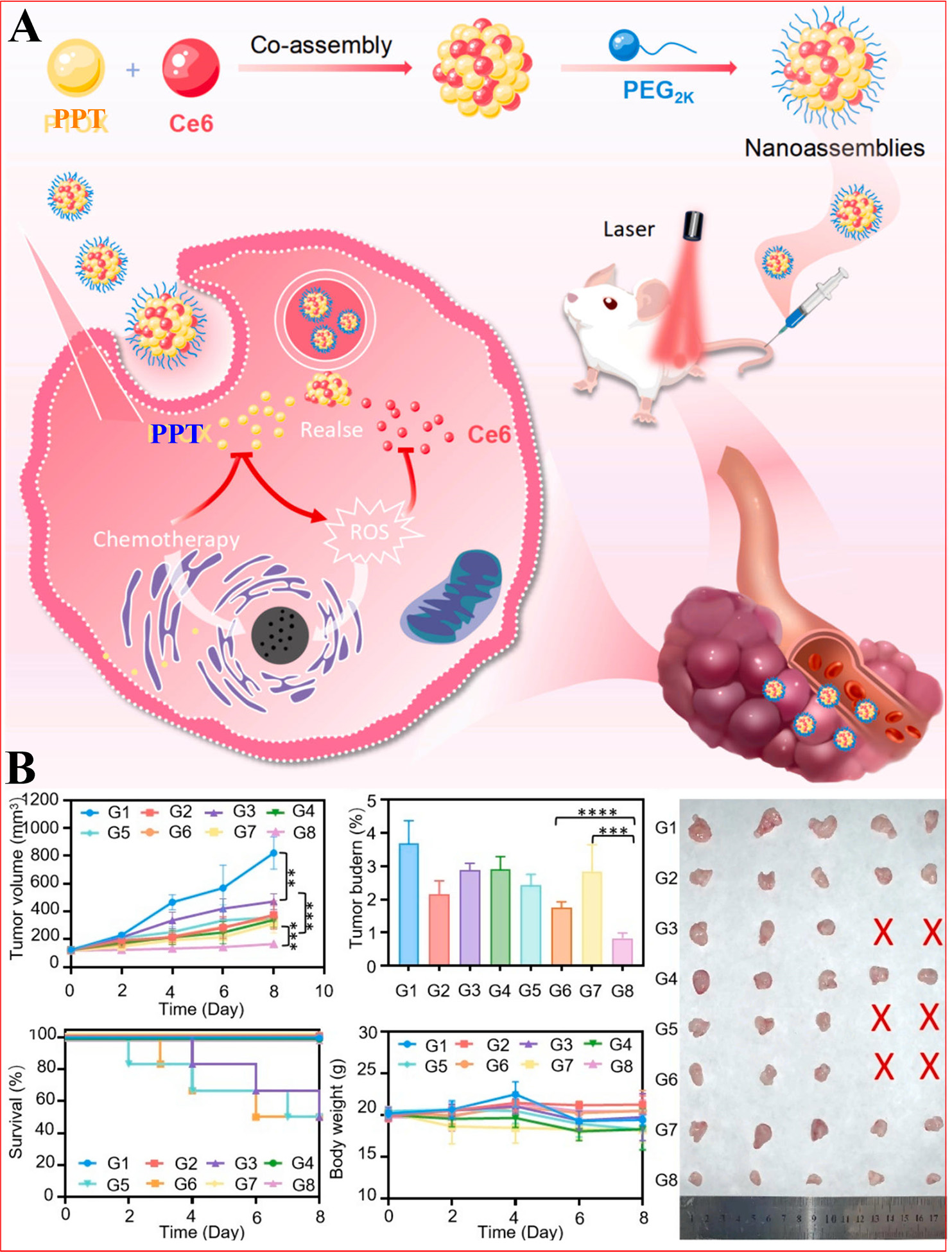
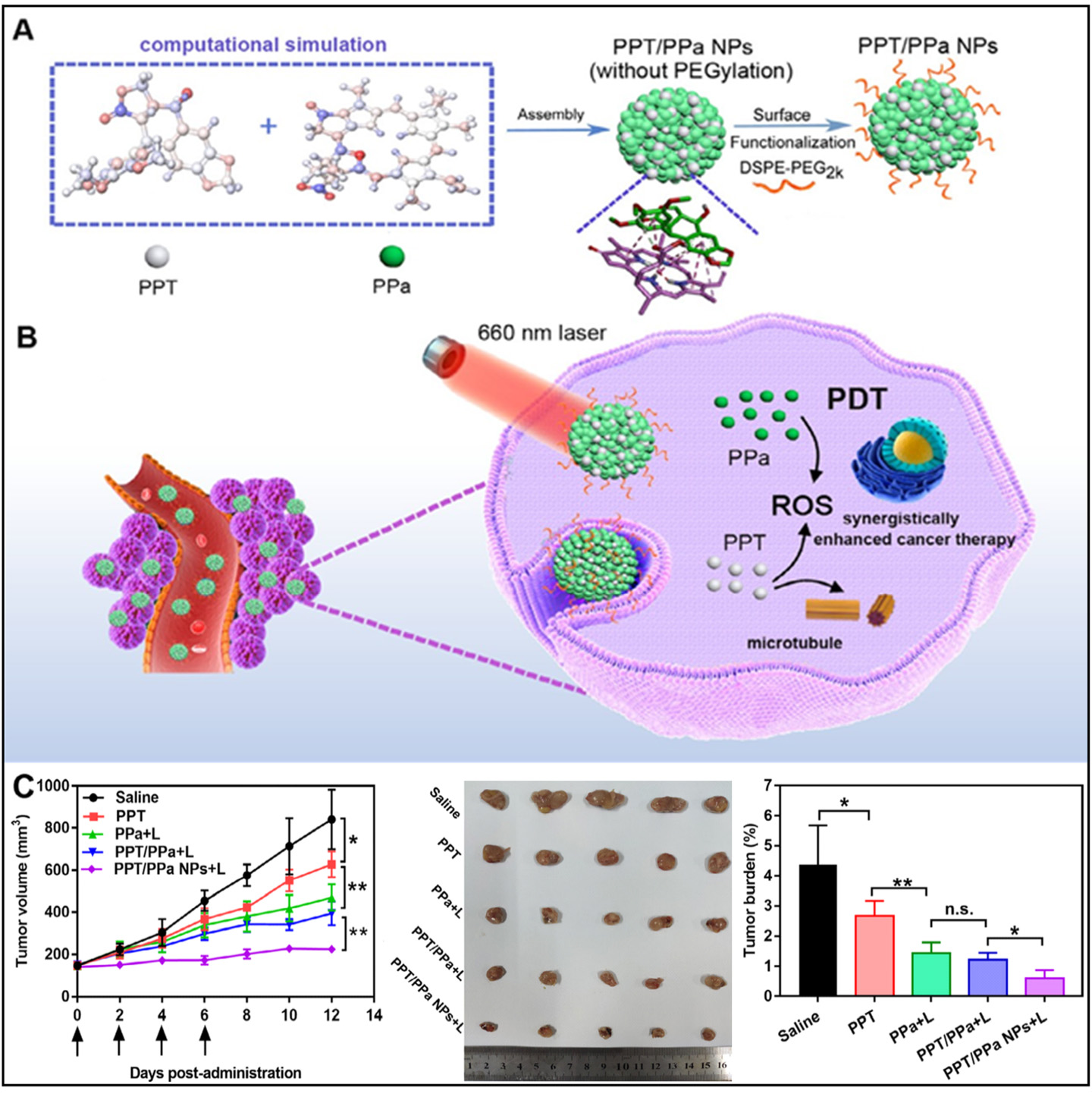
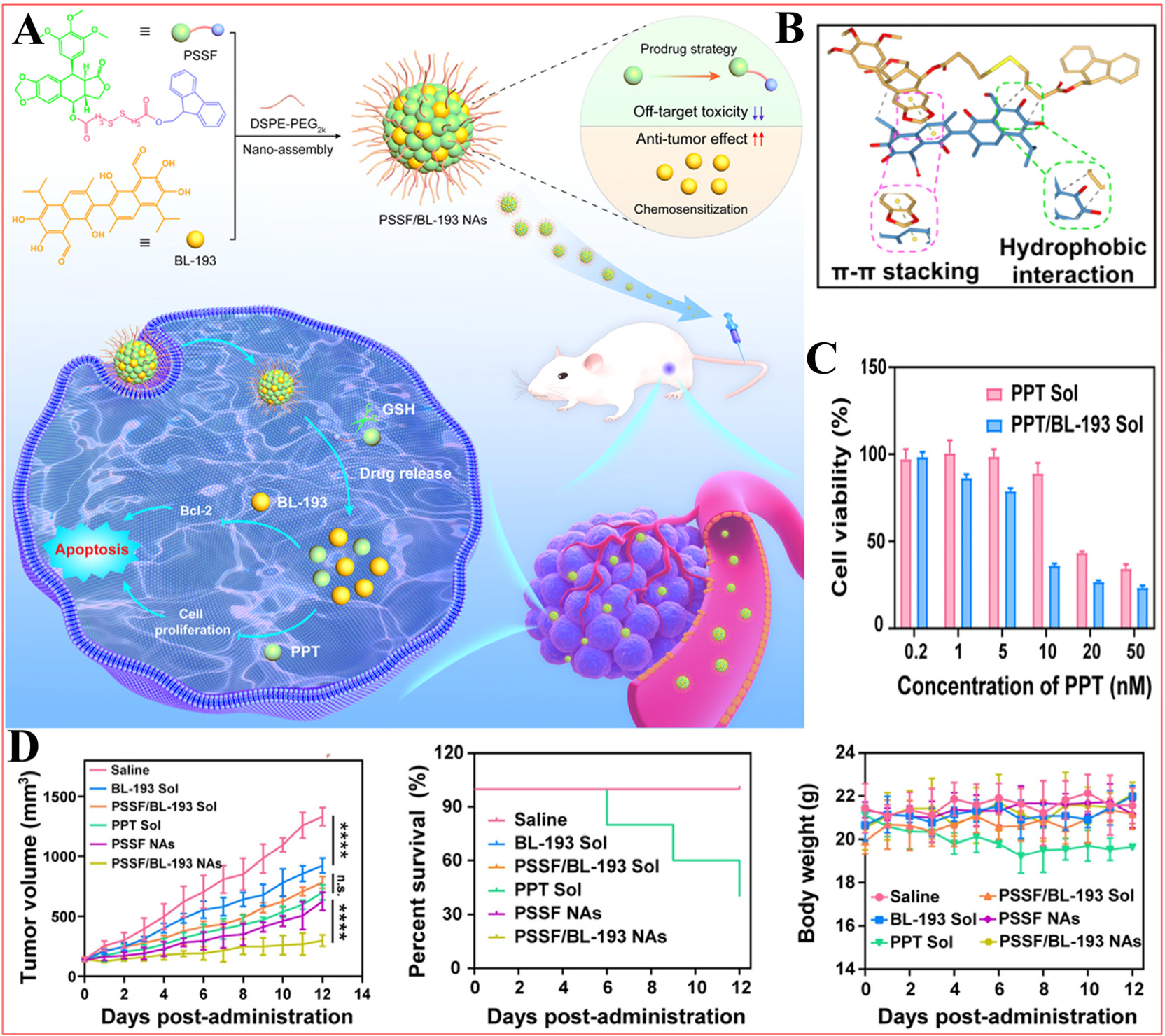
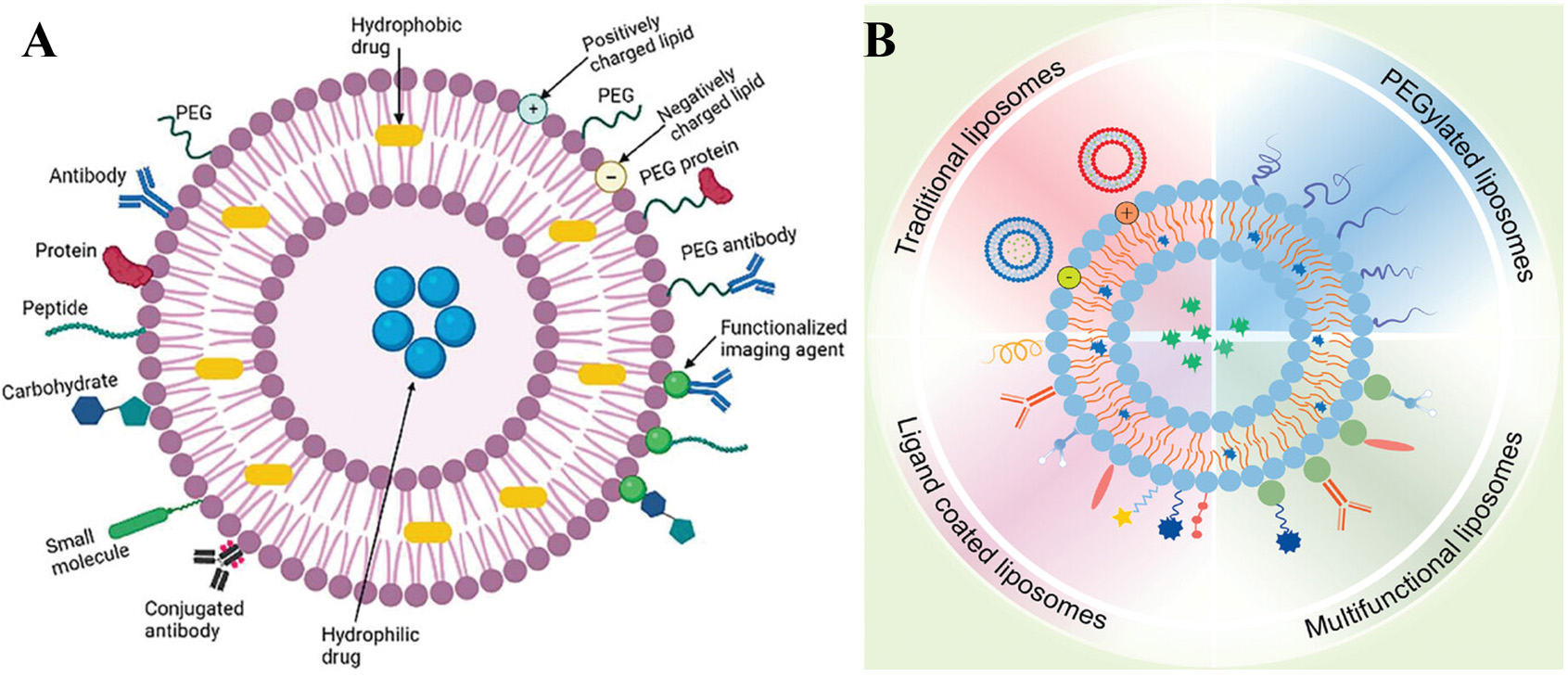
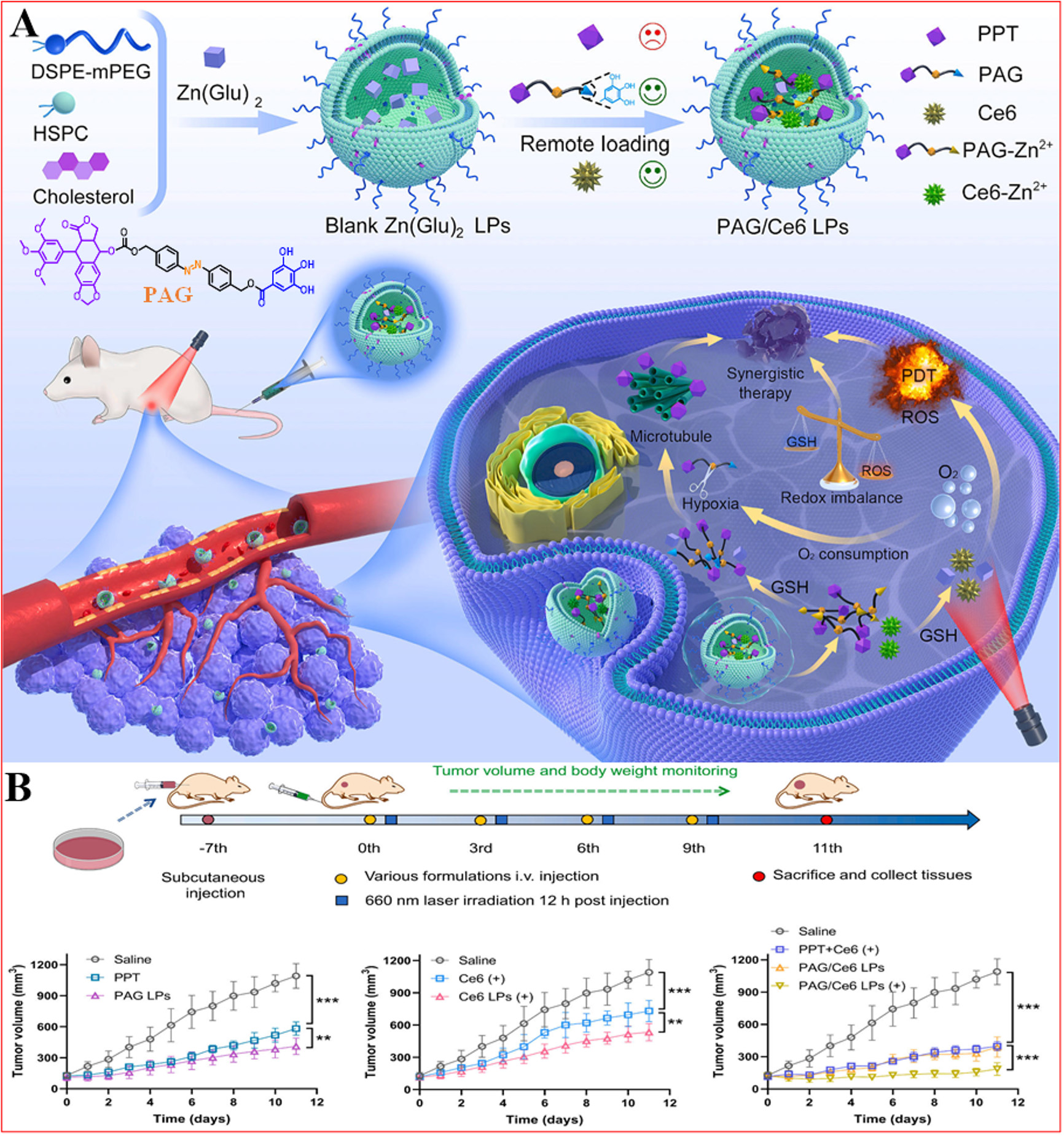
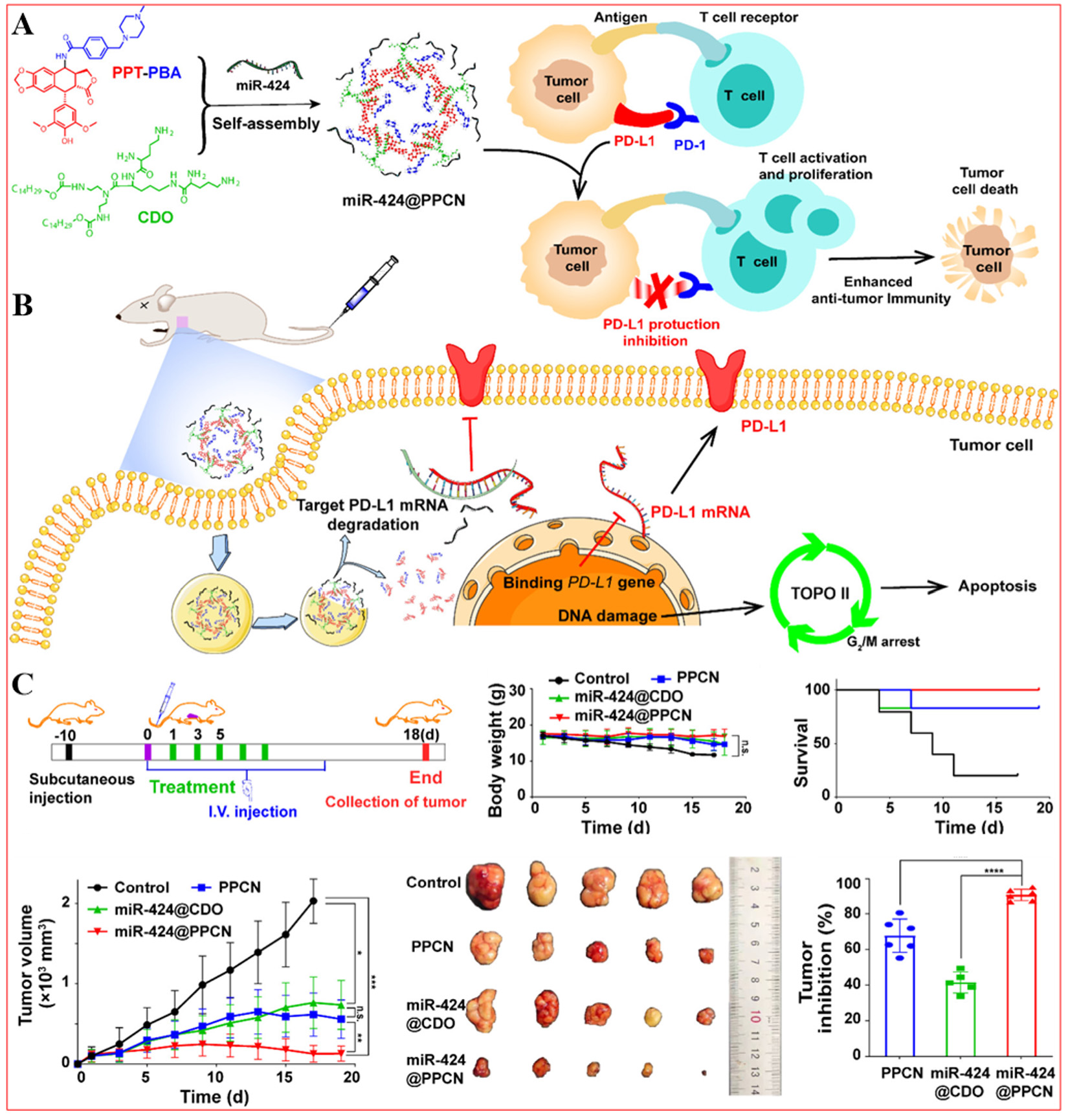
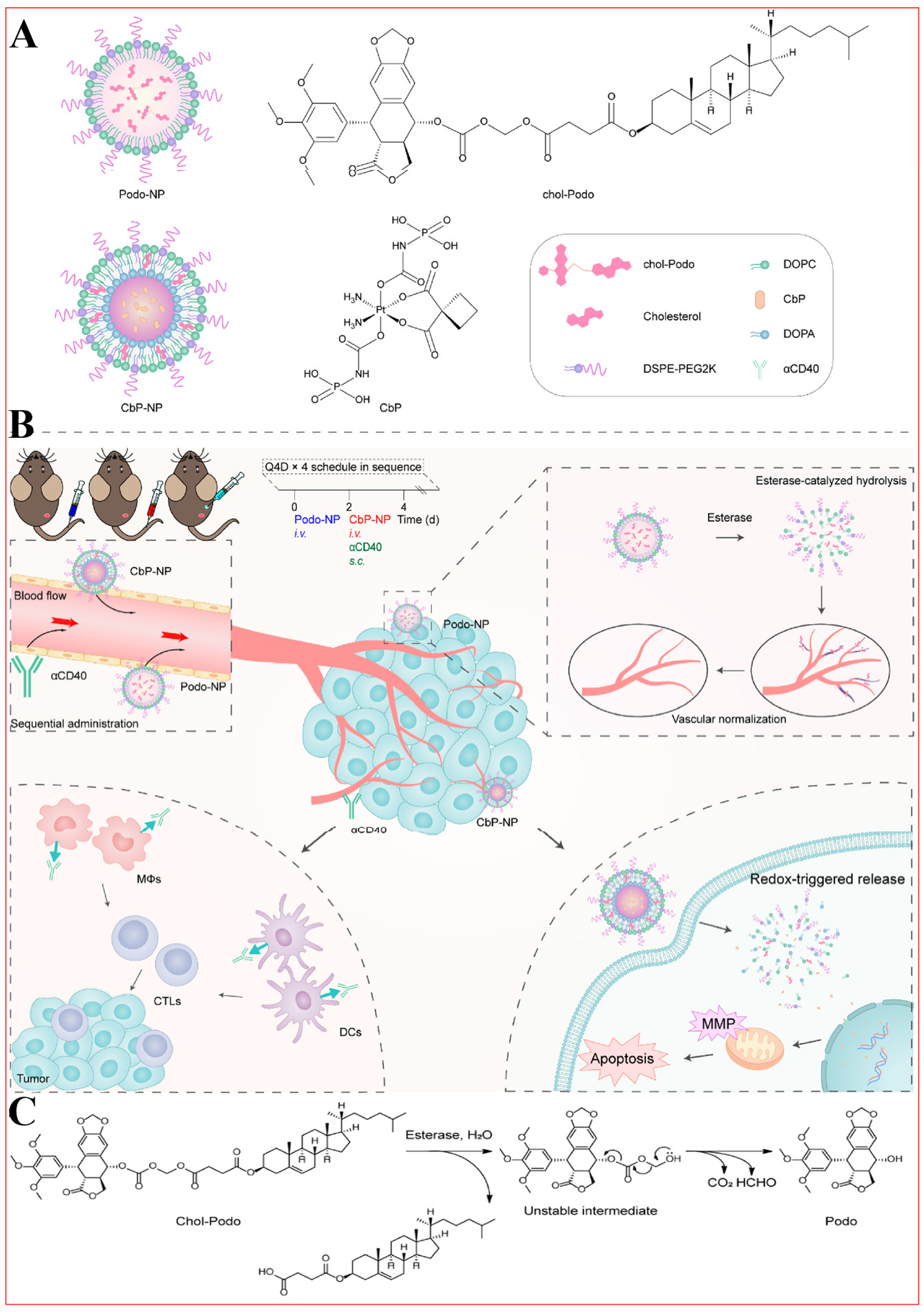
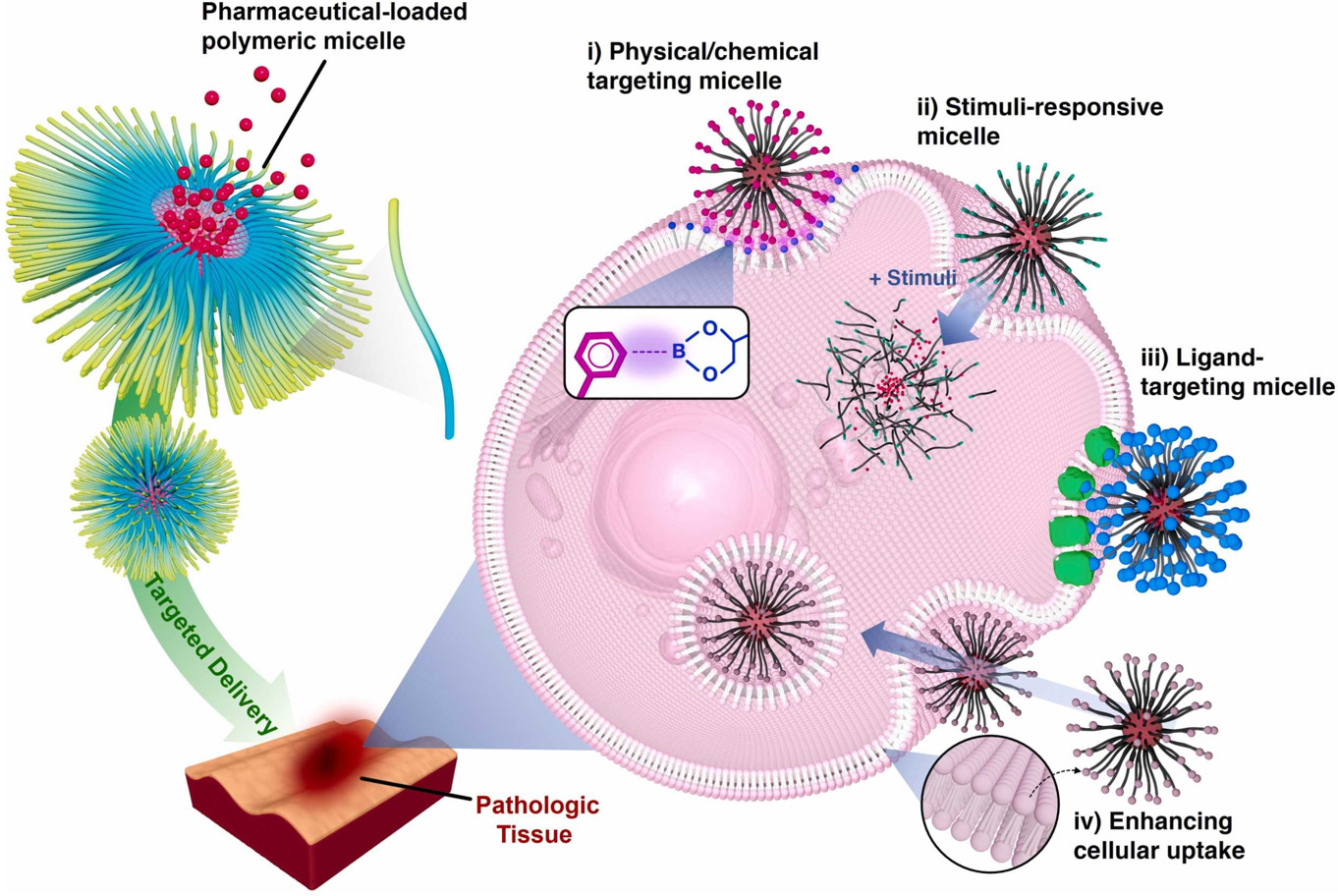
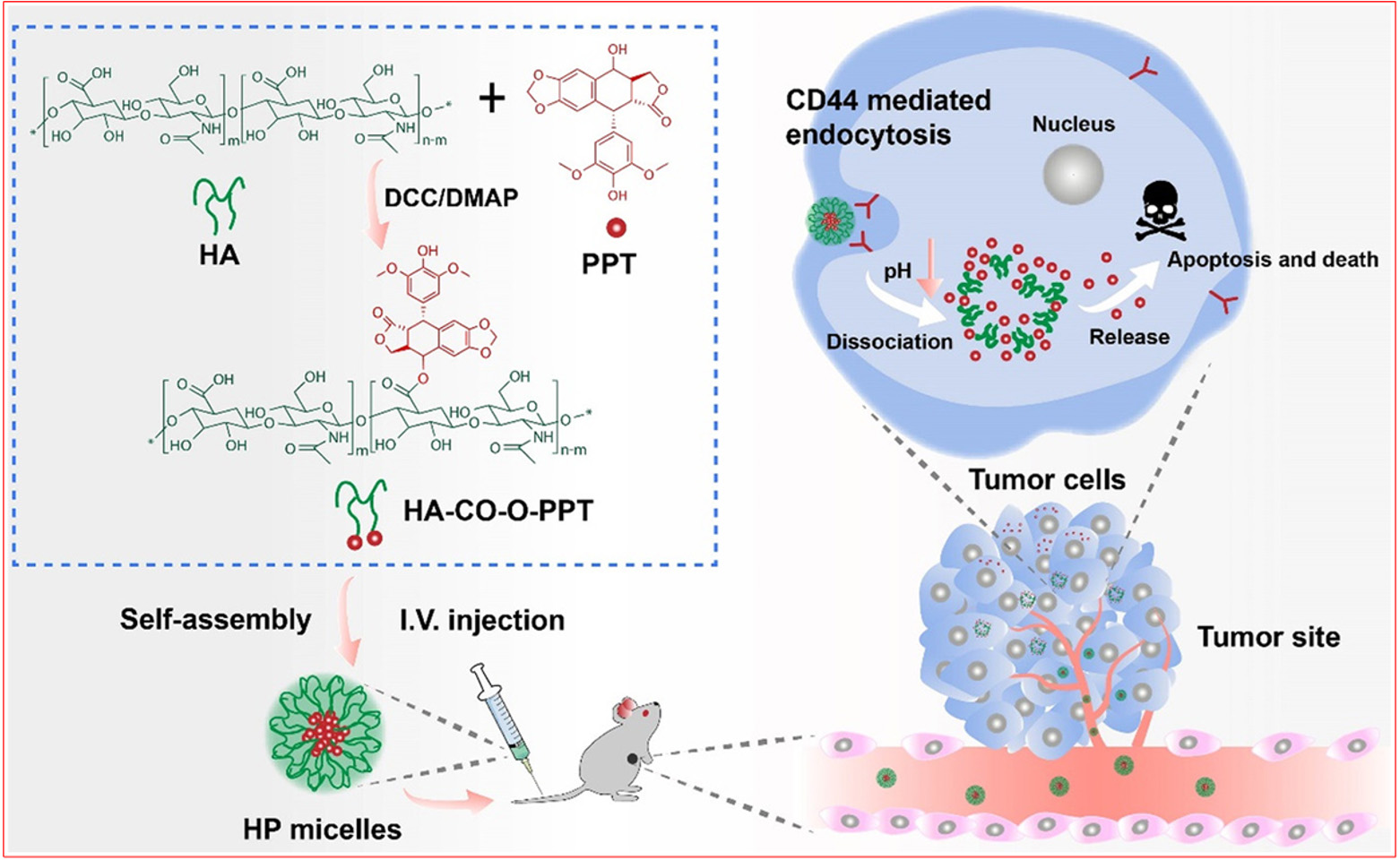
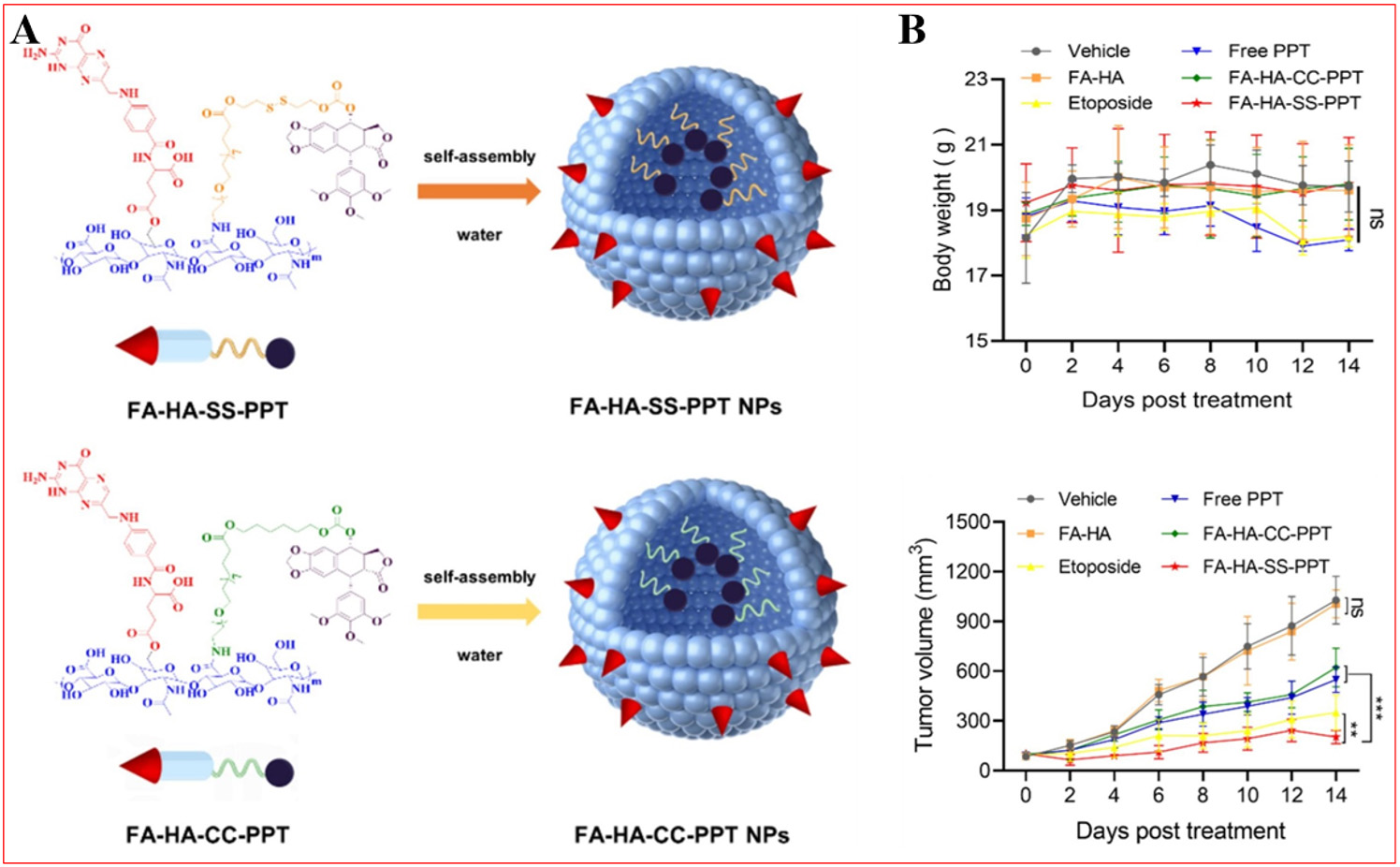
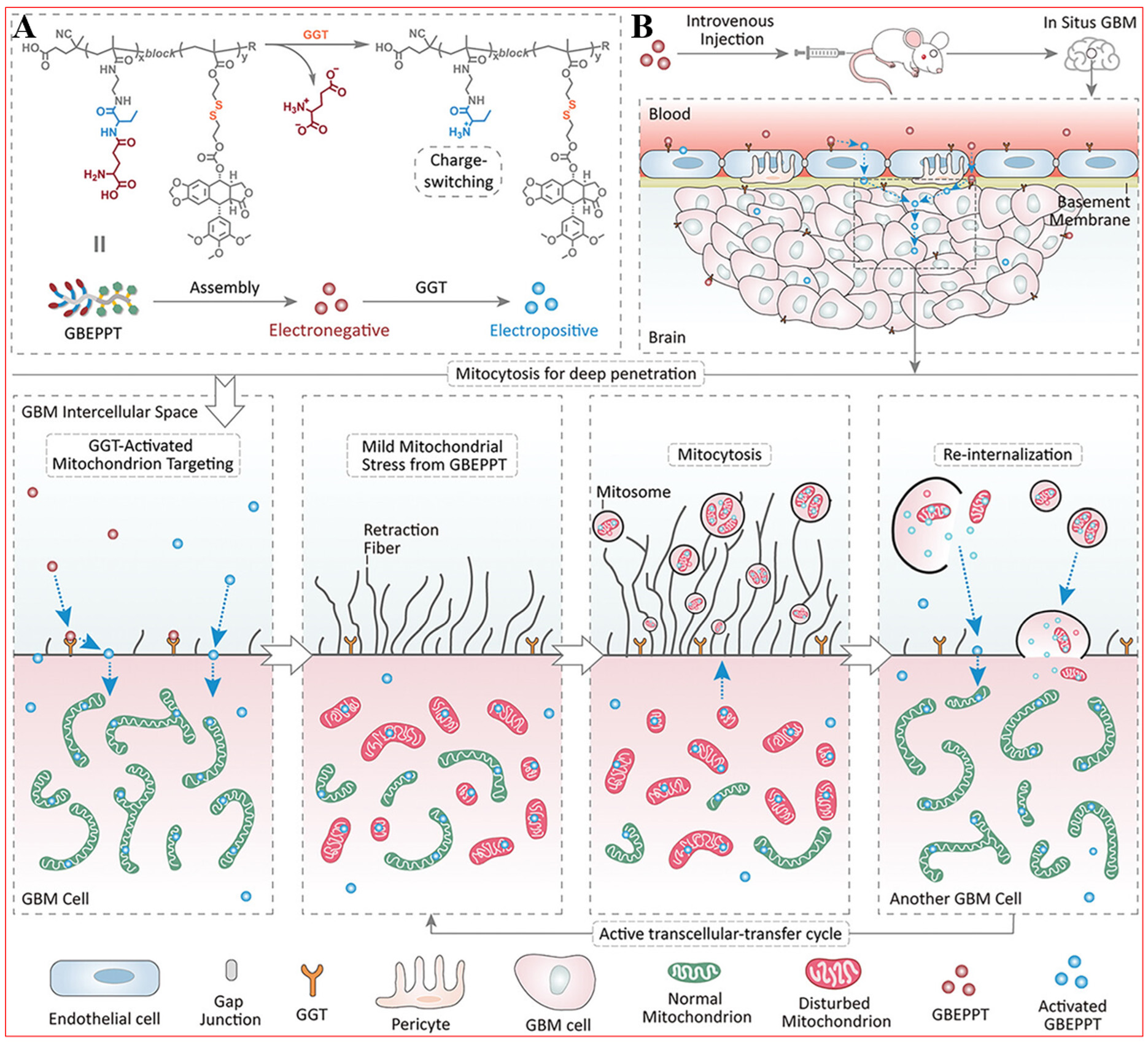
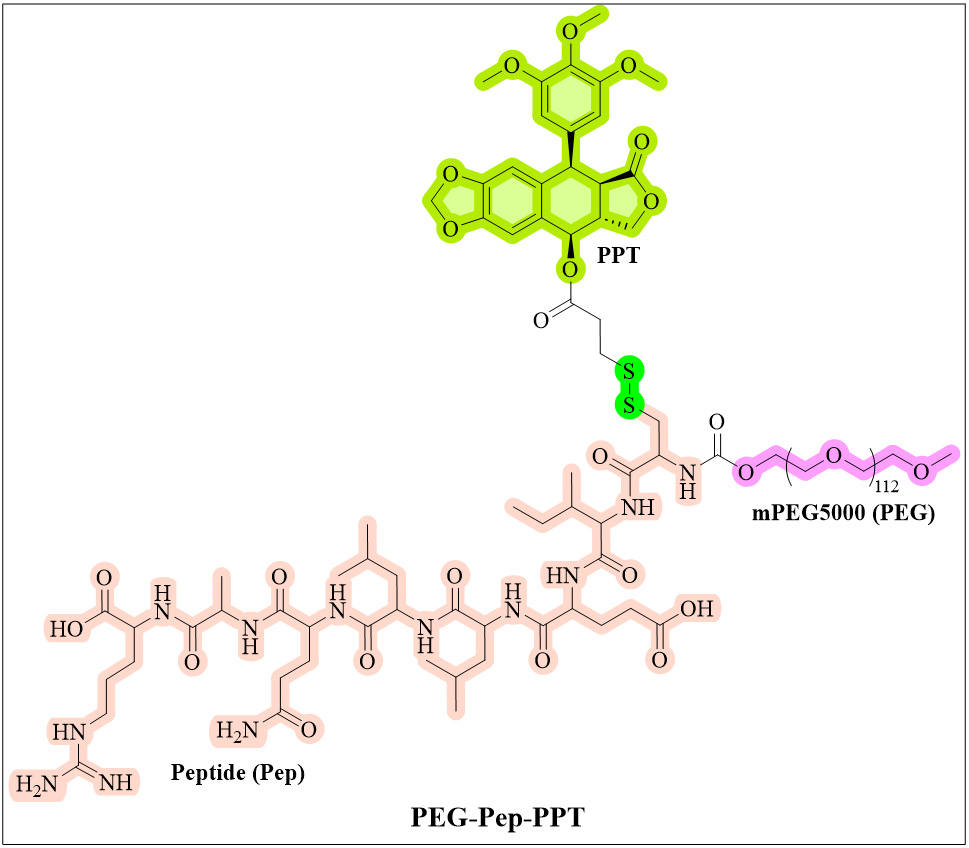
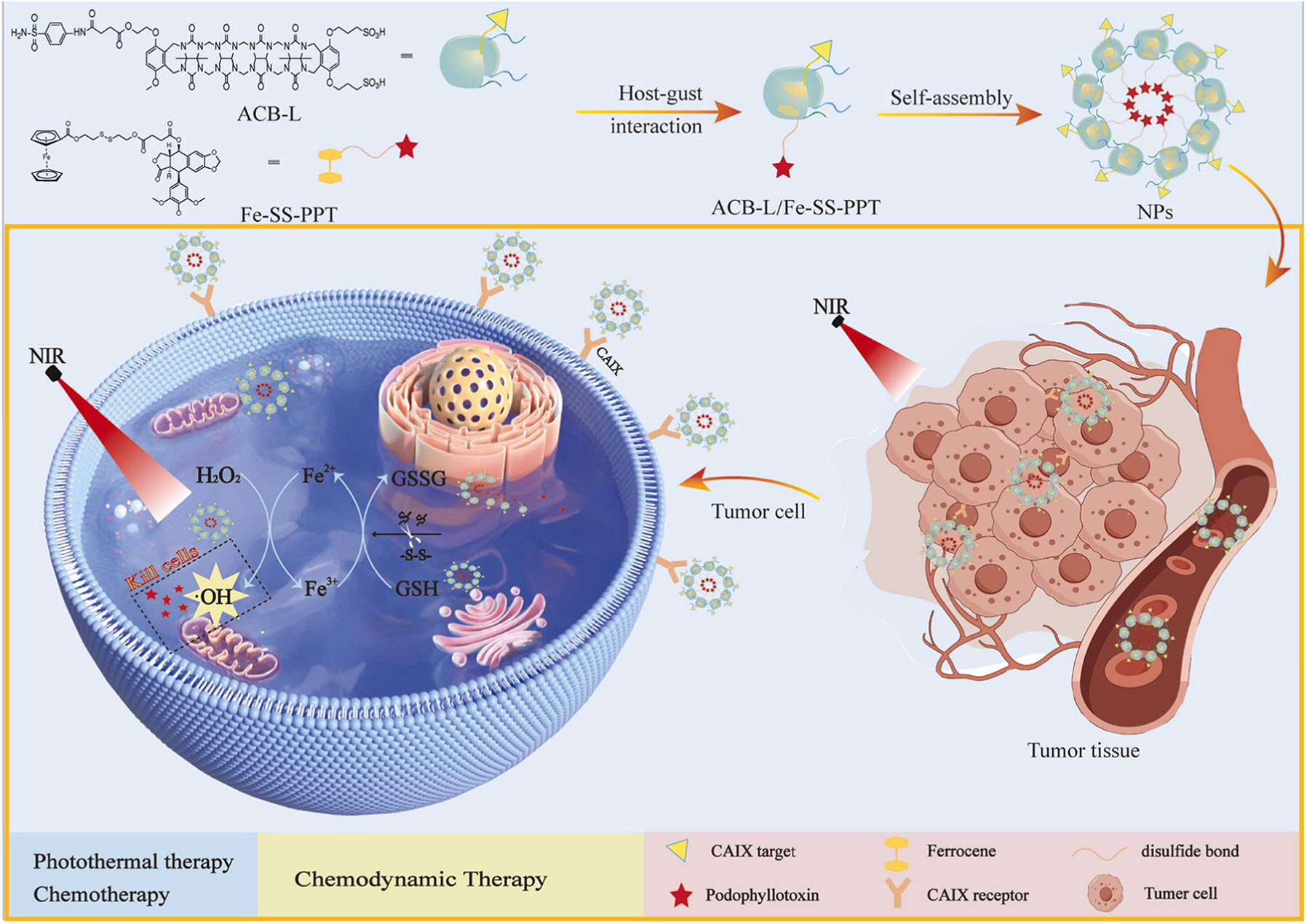












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].