Characterization of select salon chemical exposures among Black and Latina U.S. hairdressers serving women of color
Abstract
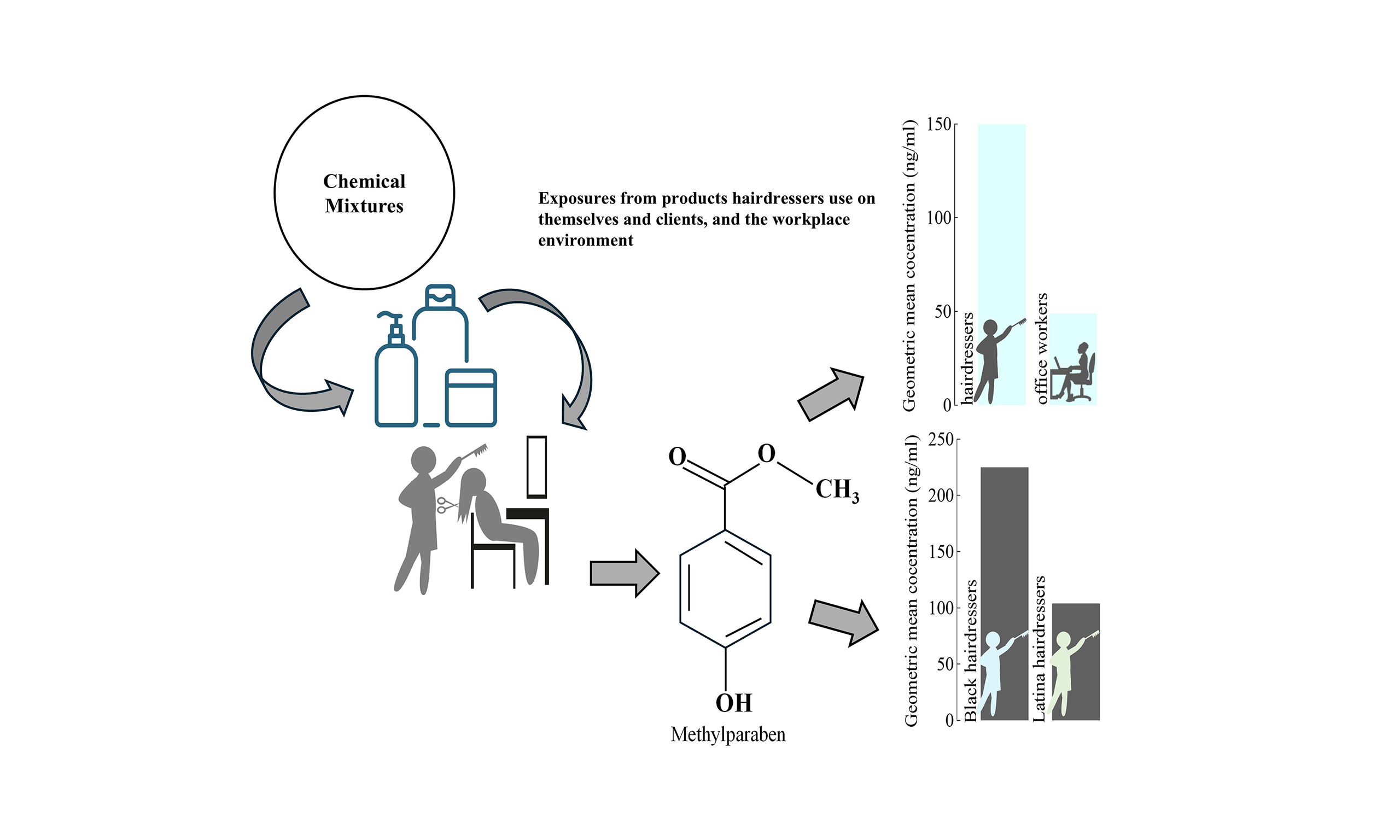
Hairdressers are continually exposed to chemicals, including many endocrine-disrupting chemicals (EDCs), yet few studies have assessed these exposures among U.S. hairdressers despite the potential health risks. We quantified concentrations of five biomarkers - four EDC exposure biomarkers [2-naphthol, methylparaben (MeP), ethylparaben, and propylparaben] and capsaicin - in post-shift urine samples from 23 female hairdressers of color (Black/Latina) serving a primarily ethnic clientele in the Maryland/Washington DC metropolitan area. Results from hairdressers were compared to those from 17 female office workers of similar race/ethnicity and to a representative sample of 431 similarly aged women in the U.S. general population. We also assessed exposure determinants for highly detected biomarkers among hairdressers. Overall, hairdressers had higher biomarker concentrations than office workers and women in the U.S. general population. Geometric mean (GM) concentrations of 2-naphthol and MeP were 2-3 times higher in hairdressers than in office workers (2-naphthol:17.4 vs. 7.59 ng/mL; MeP: 150 vs. 48.9 ng/mL; both P < 0.01), and 1.5-2.5 times higher than in U.S. women (2-naphthol: 15.5 vs. 6.31 ng/mL; MeP: 134 vs. 87.0 ng/mL). Hairdressers serving predominantly Black clientele had higher biomarker concentrations than those serving a predominantly Latinx clientele. Select salon services and products (e.g., chemical straightening/relaxing, semipermanent hair color, hair extensions, hairspray) were associated with higher 2-naphthol and MeP concentrations, while hair bleach use and braiding were associated with lower concentrations. Mask use during chemical-intensive services was associated with reduced MeP concentrations (GM: 117 vs. 159 ng/mL). Larger studies are needed to comprehensively assess exposures and reduce health risks for this workforce.
Keywords
INTRODUCTION
Over 90% of the more than 700,000 hairdressers in the U.S. are women, many of whom are of reproductive age and low socioeconomic status[1]. These women may face unique health risks from exposure to chemicals present in and emitted from hair products they work with daily. Many of these chemicals are known or suspected endocrine-disrupting chemicals (EDCs)[2,3], and exposure to some of these EDCs, including during the prenatal period, has been linked to increased risks of adverse reproductive[4-6], respiratory[7-9], and neurodevelopmental effects[10-12]. As a result, these exposures represent a potential health risk for women and children, as many hairdressers work during the preconception and prenatal periods. Despite these risks, few studies have quantified salon exposures through biomonitoring[7,13-15].
Globally, biomonitoring studies on hairdressers have detected and quantified exposure to select chemicals, including phthalates[7,13], volatile organic compounds (VOCs)[14,15], parabens[16], phenols[17], polycyclic aromatic hydrocarbons (PAHs)[18], and aromatic amines[19], confirming workplace exposure to these chemicals among hairdressers, albeit primarily among Caucasian and Asian hairdressers. However, biomonitoring has not been widely used to assess exposures among hairdressers in the U.S. To our knowledge, only two U.S. studies conducted by our team have examined hairdressers’ exposures using biomonitoring[13,14]. We previously measured nine phthalate[13] and 28 VOC[14] biomarkers in urine among 23 female hairdressers of color and 17 office workers and found that hairdressers had higher biomarker concentrations than office workers. Our previous work also showed a differential exposure burden between hairdressers of color who serviced Black and Latinx clients, suggesting that the unique products and services offered (e.g., chemical straightening/relaxing) in salons may also influence exposures[13,14]. Altogether, findings from these studies underscore the need to expand biomonitoring efforts among U.S. hairdressers to characterize exposures that could translate to occupational health disparities and to inform exposure mitigation efforts.
The limited chemical ingredient disclosure requirements in professional salon products pose unique challenges when conducting exposure assessments in this workforce. The U.S. Food and Drug Administration (FDA) regulates cosmetics used in professional settings, including hair salons, under the 2022 Modernization of Cosmetics Regulation Act (MoCRA)[20]. As of 2025, individual chemical ingredients are not subject to pre-market approval under MoCRA. Manufacturers must report ingredients to the FDA, although they are not required to disclose them to consumers. While the FDA’s MoCRA law mandates that fragrance allergens be listed in professional products, the rule is yet to be fully enacted, and as of August 2025, a fragrance allergen list is unavailable[20]. Most prior research on chemical exposures among hairdressers targeted a predetermined list of chemicals based on anticipated exposure from product ingredient lists. While this targeted approach is time- and cost-efficient, it is insufficient for capturing all chemical exposures of concern among hairdressers via biomonitoring, and secondary compounds that could be of concern but unknown at the onset of the study because they may be generated during product use. Ultimately, these challenges constrain efforts to expand our understanding of workplace exposures and their associated health risks among U.S. hairdressers.
We addressed several of these gaps by conducting a two-phased pilot study where we first utilized a novel suspect screening method to identify chemicals that may be present in the urine of hairdressers as described previously[21], and then used a targeted approach to quantify detected chemicals with identities determined to be present in a higher abundance in the urine of hairdressers compared to office workers - an occupational group expected to have lower exposures; herein, we present our results of the targeted analysis. While our previous studies on hairdressers of color focused on phthalates and VOCs, the current study applied an integrated suspect screening method with targeted analysis, enabling the identification and quantification of a wider range of chemical exposures.
The primary objectives of this study were to: (1) quantify five biomarkers of exposure among hairdressers identified via a novel suspect screening approach; (2) assess differences in workplace exposures between hairdressers and female office workers; (3) compare urinary biomarker concentrations in hairdressers to those reported among women in the U.S. general population; and (4) examine exposure determinants among hairdressers. By addressing these objectives, we aim to provide a more comprehensive understanding of chemical exposures among hairdressers of color, an understudied and vulnerable occupational group.
EXPERIMENTAL
Participant recruitment
Our recruitment strategy and study design have been described in detail elsewhere[13,14,22]. Briefly, we recruited 23 female hairdressers between December 2018 and May 2019 from six salons in Maryland and the Washington, D.C. metropolitan area. Three salons catered primarily to Black/African American clients (“Black salons”) and three to Latinx clients (“Dominican salons). Black salons specialized in services such as hair relaxing and braiding for their Black/African American clients, while Dominican salons offered the “Dominican Blowout” (hair washing, setting rollers, blow-drying, and flat ironing)[14] as well as some services offered in Black salons. We limited our study to women of color based on emerging evidence that using specialty hair care products, such as hair relaxers marketed solely to this demographic, may result in elevated chemical exposures in this occupational subgroup[3,23-26].
We also recruited a comparison group of 17 female office workers working in a university setting. Hairdressers and office workers were eligible to enroll in the study if they were at least 18 years of age and willing to complete two interviewer-administered questionnaires in English or Spanish, provide a urine sample, and complete other study protocols. Hairdressers were additionally required to work mainly in a hair salon for at least one year prior to enrollment in the study to ensure they had a sustainable clientele base and provided a wide range of hair services. The University of Maryland Institutional Review Board reviewed and approved all study protocols, and each participant provided written informed consent before enrolling in the study.
Data and biospecimen sample collection
Detailed data and biospecimen sample collection activities have been described in detail elsewhere[13,14,22]. Briefly, bilingual study staff administered two questionnaires to participants in their preferred language (English or Spanish). An initial baseline 72-item questionnaire was used to collect participant demographics (e.g., age, race/ethnicity, level of education, income, and smoking status), general health history information (e.g., reproductive health, respiratory conditions and symptoms), personal and workplace behaviors [e.g., use of personal protective equipment (PPE), services provided and hair products used in a typical work week, number of hours in the work week, etc.], and information on personal care products (PCPs) used in the previous 24-48 h. This time frame was selected based on the relatively short half-lives of many chemicals in PCPs, as urinary biomarkers quantified in samples reflect recent exposures. In addition, on the day of biospecimen collection, we administered a post-shift questionnaire to collect information about the products used and services rendered that day. Office workers were exempted from salon-specific inquiries, such as PPE use.
All participants provided post-shift spot urine samples on the same day the baseline and post-shift questionnaires were administered. We determined the study salon visit day (i.e., the day of the week) for hairdressers based on their availability, with a preference for a day with a high clientele volume. We collected urine samples in metal- and phthalate-free polypropylene collection containers and aliquoted the samples into 2 mL cryovials. Participants were instructed to wear powder-free gloves during urine sample collection to minimize the risk of contamination from products used on their hands. Study staff transported urine samples within an hour of collection to the laboratory in a portable cooler with ice packs and stored them at -80 °C until ready for laboratory analysis. Prior to storage, we measured the specific gravity of urine samples using a handheld refractometer pen (ATAGOTM3741, Tokyo, Japan) to account for urine dilution in the analyses[27,28]. Specific gravity-corrected biomarker concentrations were calculated as reported previously[13,14].
Laboratory analysis of urine samples
We analyzed urine samples using a two-phase strategy. First, we analyzed urine specimens via a suspect screening workflow previously described[21]. Briefly, 250 μL of participant urine was incubated with β-glucuronidase (from H. pomatia, type H-2, ≥ 85,000 U/mL; Sigma-Aldrich, St. Louis, MO, USA), followed by protein precipitation, and supernatants were analyzed via liquid chromatography-high resolution mass spectrometry (LC-HRMS). Raw data were processed using computational tools to detect and tentatively identify compounds that were present in hairdressers’ urine at higher abundances compared to office workers. Then, the identities of the compounds were confirmed by comparing them to reference standards when commercially available. We confirmed the identities of five compounds: 2-naphthol, methylparaben (MeP), ethylparaben (EtP), propylparaben (PrP), and capsaicin. Four of the compounds (capsaicin excluded) are EDCs. Capsaicin is an ingredient derived from hot peppers and uniquely used in hair growth products to stimulate hair follicles and treat various hair growth-related conditions, including alopecia areata [Supplementary Table 1][29-34]. This ingredient has been reported to act as an irritant and sensitizer in other occupational settings[35-37].
For the second phase, the focus of the present study, we quantified the urinary concentrations of the five chemicals described above. New aliquots of the participants’ urine were analyzed using the same sample preparation and instrumental method previously described[21], except that mass spectrometric data were acquired using Full Scan mode. A matrix-matched calibration curve was prepared for each compound and analyzed with the participant samples. A detailed description of the preparation of the calibration curves and verification of the quantification results is provided in the Supplementary Materials. Data were processed using Xcalibur QuanBrowser (v4.1, Thermo Scientific, Waltham, MA, USA). Linear ranges for 2-naphthol, capsaicin, EtP, MeP, and PrP were 2-501, 3.7-252.7, 50-1,000, 15-1,014, and 100-1,000 ng/mL, respectively.
Statistical analysis
We calculated descriptive statistics (i.e., frequencies, means, standard deviations, and range) to summarize study population characteristics and compared demographic differences between hairdressers and office workers and between hairdressers by salon type (Black vs. Dominican salons). To further characterize and describe work practices in salons, we also compared differences in select workplace practices, such as the services provided across hairdressers by salon type. We compared differences in select demographic characteristics between hairdressers and office workers and among hairdressers by using Chi-square or Fisher’s exact tests for categorical variables (e.g., race, educational attainment, income, smoking status) and Wilcoxon Mann-Whitney tests for continuous variables (e.g., age, number of clients per week, number of hours worked per day).
We summarized detection frequency (DF), geometric mean (GM), percentiles (p25, p50 and p75), and the range of urinary biomarker concentrations (ng/mL). We evaluated biomarker concentrations as specific gravity-corrected concentrations, and for those below the limit of quantification (LOQ), we assigned a value of LOQ/√2[38,39]. Analyses of biomarkers were stratified by occupation (i.e., hairdressers vs. office workers) and salon type (i.e., Black vs. Dominican salons). We modeled less frequently detected biomarkers (DF < 60%) as dichotomous variables (i.e., detect vs. non-detect) and used Chi-square or Fisher’s exact tests to test for differences in biomarker concentrations between hairdressers and office workers, as well as among hairdressers, for these less frequently detected biomarkers. Similarly, we used the Wilcoxon Mann-Whitney tests to explore similar comparisons for frequently detected chemicals (DF ≥ 60%) using continuous biomarker concentrations. In addition, we compared summary statistics (i.e., DF, GM, percentiles, range) of biomarker concentrations between hairdressers and a representative sample of 431 women of a similar age (22-58) and race/ethnicity (Non-Hispanic Black and Hispanic) from the U.S. general population participating in the National Health and Nutrition Examination Survey (NHANES) 2015-2016 cycle[40]. For these comparisons, we used biomarker concentrations uncorrected for dilution, as dilution correction methods in NHANES differ from those in the present study (i.e., creatinine correction vs. specific gravity correction).
We further examined the relationship between urinary biomarker concentrations and services rendered (e.g., chemical straightening/relaxer, Brazilian Keratin Treatment), hair products used (hair spray, leave-in conditioner), and PPE (e.g., masks) worn in a typical workweek. Specifically, we used dot plots to compare specific gravity-corrected biomarker concentrations based on whether (i.e., Yes/No) participants provided the service, used the product, or wore PPE in a typical week. We restricted these comparisons to questions with at least a 30% response rate per response category[41] and biomarkers with DFs ≥ 60% (only 2-naphthol and MeP met this criterion and were retained for these analyses). We did not perform statistical tests to compare 2-naphthol and MeP concentrations by services rendered, products used, or PPE worn due to the small sample size (i.e., based on the number of hairdressers who answered “yes” or “no”). We conducted all analyses in Stata 15.0 software (Stata Corp, College Station, TX) and generated figures using GraphPad Prism 9 Software (San Diego, CA). Statistical significance was set at P < 0.05 for all analyses.
RESULTS AND DISCUSSION
Population characteristics
Hairdressers vs. office workers
Most hairdressers (96%) were women of color (48% Hispanic/Latina and 48% Non-Hispanic Black), had at least a high school education or equivalent (83%), and were nonsmokers (83%) [Table 1]. Similarly, most office workers (82%) were also women of color (41% Hispanic/Latina and 41% Non-Hispanic Black) and nonsmokers (94%). While most office workers had a college education (71%), only 22% of hairdressers were college-educated. Most office workers earned an annual income of ≥ $30,000 USD (82%) annually. Hairdressers were slightly older than office workers, with a mean age of 40 vs. 34 years (P-value = 0.05). Reported use of cosmetics and PCPs in the past 24-48 h was not significantly different between hairdressers and office workers (P-value = 0.83) [Table 1]. Over half of the hairdressers (65%) reported providing chemical-intensive hair services, such as chemical straightening/relaxers and permanent hair dying, 1 to 3 times a week. Hairdressers also reported serving an average of 26 clients in a typical workweek (range: 7-50 clients/week) and working in salons for an average (SD) of 15 (9.5) years. Both work groups reported working a comparable number of hours during a typical workweek (hairdressers vs. office workers: 44 vs.
Study population characteristics (N = 40)
| Characteristic | Hairdressers from Black Salons (N = 11) | Hairdressers from Dominican Salons (N = 12) | P-valuea | All hairdressers (N = 23) | Office workers (N = 17) | P-valuea |
| n (%) | n (%) | |||||
| Race | ||||||
| Hispanic/Latina | 1 (9.1) | 10 (83.3) | < 0.01 | 11 (47.8) | 7 (41.2) | 0.52 |
| Non-Hispanic Black | 10 (90.9) | 1 (8.3) | 11 (47.8) | 7 (41.2) | ||
| Otherb | 0 (0) | 1 (8.3) | 1 (4.4) | 3 (17.6) | ||
| Highest education obtained | ||||||
| < High school | 0 (0) | 4 (33.3) | 0.21 | 4 (17.4) | 0 (0) | < 0.01 |
| High school or GED | 4 (36.4) | 2 (16.7) | 6 (26.1) | 1 (5.9) | ||
| Trade school | 4 (36.4) | 4 (33.3) | 8 (34.8) | 1 (5.9) | ||
| College | 3 (27.3) | 2 (16.7) | 5 (21.7) | 12 (70.6) | ||
| Other | 0 (0) | 0 (0) | 0 (0) | 3 (17.7) | ||
| Smoking status | ||||||
| No | 7 (63.6) | 12 (100.0) | 0.04 | 19 (82.6) | 16 (94.1) | 0.37 |
| Yes | 4 (36.4) | 0 (0) | 4 (17.4) | 1 (5.9) | ||
| Incomec | ||||||
| ≤ $30,000 | 5 (45.5) | 5 (62.5) | 0.59 | 10 (52.6) | 3 (17.7) | 0.10 |
| $30,001-$50,000 | 2 (18.2) | 2 (25.0) | 4 (21.1) | 3 (17.7) | ||
| $50,001-$75,000 | 1 (9.0) | 1 (12.5) | 2 (10.5) | 4 (23.5) | ||
| > $75,000 | 3 (27.3) | 0 (0) | 3 (15.8) | 7 (41.2) | ||
| PCP use in the past 48 h | ||||||
| < 5 Products | 0 (0) | 1 (8.3) | 0.50 | 1 (4.3) | 0 (0) | 0.83 |
| 5-10 Products | 4 (36.4) | 2 (16.7) | 6 (26.1) | 3 (17.6) | ||
| > 10 Products | 7 (63.6) | 9 (75.0) | 16 (69.6) | 14 (82.4) | ||
| Number of different chemical-intensive hair services provided per week | ||||||
| 1-3 Times | 40 (66.7) | 34 (63.0) | 0.60 | 74 (64.9) | n/a | |
| 4-7 Times | 16 (26.7) | 14 (26.0) | 30 (26.3) | |||
| ≥ 8 Times | 4 (6.6) | 6 (11.0) | 10 (8.80) | |||
| Number of natural hair services provided per week | ||||||
| 1-3 Times | 15 (51.7) | 9 (81.8) | 0.40 | 24 (60.0) | n/a | |
| 4-7 Times | 6 (20.7) | 1 (9.10) | 7 (17.5) | |||
| ≥ 8 Times | 8 (27.6) | 1 (9.10) | 9 (22.5) | |||
| Characteristic | Mean (SD) | P-valued | Mean (SD) | P-valued | ||
| Age (years) | 37.3 (10.2) | 42.8 (10.6) | 0.22 | 40.2 (10.6) | 33.6 (7.9) | 0.05 |
| Number of clients per week | 19.2 (8.8) | 32.7 (11.4) | < 0.01 | 26.2 (12.1) | n/a | |
| Number of years working in hair salons | 14.9 (9.4) | 15.3 (10.1) | 0.83 | 15.1 (9.5) | n/a | |
| Number of hours worked during the week | 46.2 (23.7) | 42.6 (13.4) | 0.69 | 44.3 (18.7) | 40.4 (10.4) | 0.73 |
Hairdressers by salon type
All hairdressers in Black salons reported having at least a high school diploma, compared to 67% of hairdressers in Dominican salons. We observed no significant differences in income between hairdressers working in Black and Dominican salons. Additionally, even though Black hairdressers reported providing more chemical-intensive hair services (i.e., services that are considered potentially harmful because they involve the use of several chemical preparations or treatments to permanently change the structure and texture of hair) and natural hair services (i.e., involving the provision of services to create “natural hairstyles” that do not alter hair chemically) than Dominican hairdressers, these differences were not statistically significant (P-value = 0.60 and P-value = 0.40, respectively). Hairdressers in Black salons also reported having fewer clients in a typical work week (mean: 19; range 7-40 clients/week) compared to those in Dominican salons (mean: 33; range 7-50 clients/week; and P < 0.01).
Urinary biomarker concentrations across populations
Biomarker concentrations in hairdressers vs. office workers
All five biomarkers were detected in hairdressers, but only 2-naphthol, capsaicin, and MeP were detected in urine samples provided by office workers [Table 2]. The biomarkers 2-naphthol and MeP were frequently detected (i.e., DF% ≥ 60) in both groups, with DFs ranging from 82% to 100%. Three biomarkers, 2-naphthol, capsaicin, and MeP, were detected in > 50% of hairdressers, while EtP and PrP were detected in only 22% and 30% of hairdressers, respectively. All quantified biomarkers were higher in hairdressers compared to office workers. For example, the GM concentrations of 2-naphthol and MeP were 2 and 3 times, respectively, higher in hairdressers (P < 0.01) compared to office workers (GM values- 2-naphthol: 17.4 vs. 7.59 ng/ml and MeP: 150 vs. 48.9 ng/mL; Table 2). The lack of significant differences in PCP usage between hairdressers and office workers suggests that other exposures, both within and outside the workplace, such as environmental factors (e.g., built environment such as flooring and paint types) and dietary habits[32,42-44], may also contribute to hairdressers’ chemical exposures.
Summary statistics for uncorrected and specific gravity-corrected urinary biomarker concentrations in ng/mL among study participants (n = 40, 2018-2019) and women in the U.S. general population (NHANES 2015-2016)a
| Biomarker | LOQ | Hairdressers (n = 23) | Office workers (n = 17) | P-valueb,c | NHANES participants (n = 431)d,e | ||||||||||
| DF% | GM | p50 (p25, p75) | Range | DF% | GM | p50 (p25, p75) | Range | LOD | DF% | GM | p50 (p25, p75) | Range | |||
| 2-Naphthol | 2.00 | 96 | 15.5 | 15.0 (6.80, 37.6) | < LOQ-393 | 88 | 0.09 | 100 | 6.31 | 6.34 (3.18, 13.6) | 0.12-397 | ||||
| 17.4 | 14.7 (8.29, 25.2) | < LOQ-282 | 7.59 | 5.93 (4.98, 13.2) | < LOQ-29.0 | < 0.01* | |||||||||
| Capsaicin | 3.70. | 57 | 14.9 | 7.40 (4.05, 72.0) | < LOQ-163 | 35 | - | - | - | - | - | ||||
| 16.0 | 15.4 (< LOQ, 76.1) | < LOQ-112 | < LOQ | < LOQ | < LOQ-41.4 | 0.23 | |||||||||
| EtP | 50.0 | 22 | < LOQ | < LOQ | < LOQ-376 | 0 | 1.00 | 59 | 3.33 | 1.60 (< LOD, 11.9) | < LOD-1,660 | ||||
| < LOQ | < LOQ | < LOQ-338 | < LOQ | < LOQ | < LOQ | 0.58 | |||||||||
| MeP | 50.0 | 100 | 134 | 109 (50.3, 304) | 16.9-2,570 | 82 | 1.00 | 99 | 87.0 | 90.8 (27.7, 302) | < LOD-16,000 | ||||
| 150 | 152 (52.7, 262) | 30.9-1,820 | 48.9 | 37.7 (20.8, 113) | < LOQ-464 | < 0.01* | |||||||||
| Propyl paraben | 100 | 30 | < LOQ | < LOQ | < LOQ-990 | 0 | 0.10 | 99 | 13.6 | 14.4 (3.50, 56.4) | < LOD-2,210 | ||||
| < LOQ | < LOQ | < LOQ-702 | < LOQ | < LOQ | < LOQ | 0.07 | |||||||||
Biomarker concentrations in hairdressers vs. women in the U.S. general population (NHANES 2015-2016) and other populations
Comparisons with women from the general U.S. population were limited to biomarkers measured in participants from the NHANES cycle (2015-2016) closest to when study samples were collected, including 2-naphthol and three parabens. We found that 2-naphthol was widely detected among hairdressers and similarly aged women of color participating in NHANES (96% vs. 100%, respectively); similar findings were observed for MeP (100% vs. 99%) [Table 2].
Additionally, detection rates for 2-napthol and MeP were also similar to those reported among Iranian hairdressers[16] and nonpregnant women in Japan and Sweden[45,46]. However, uncorrected GM concentrations for MeP and 2-naphthol were 1.5-2.5 times higher in hairdressers than in U.S. women (2-naphthol: 15.5 vs. 6.31 ng/mL; MeP: 134 vs. 87.0 ng/mL) [Table 2]. While median MeP concentrations were also higher in hairdressers than those reported among Iranian hairdressers and Swedish women[46], they were lower than those reported previously in Japanese women[45]. These exposure differences may be attributable, in part, to regulatory and product formulation differences between the U.S. and other nations. For instance, the FDA regulates PCPs sold in the U.S., although its legislative authority to mandate safety testing is limited[47]. As a result, the FDA has restricted the use of 11 chemical compounds in cosmetics[48], while Europe has restricted 1,328[49]. Policy differences may impact the detection and frequency of exposure to chemicals in PCPs based on the origin of the PCPs used or the location of the research study. Differences in PCP usage patterns and in product formulation content across these racially/ethnically diverse studies could also account for the differences in MeP concentrations observed[50-52].
EtP and PrP were detected in > 50% of U.S. women, while they were less frequently detected in hairdressers [Table 2]. The GM concentrations for EtP and PrP were < LOQ for hairdressers, preventing further comparisons. The overall lower detection rates observed in our study for EtP and PrP among hairdressers may reflect both lower exposures to EtP and PrP-containing products and methodological constraints. In particular, our analytical method employed higher LOQs (EtP: 50 ng/mL; PrP: 100 ng/mL) compared to much lower limits of detection (LODs) and LOQs reported in other studies (EtP: 0.01-1 ng/mL; PrP: 0.03-
Biomarker concentrations in hairdressers in Black vs. Dominican salons
Besides EtP, we detected all other biomarkers in > 50% of hairdressers working in Black salons [Table 3]. However, only 2-naphthol and MeP were detected in > 50% of hairdressers working in Dominican salons. Capsaicin and PrP were more widely detected in hairdressers working in Black salons than in Dominican salons, with a DF% of 100% compared to 17% (P-value = 0.02) for capsaicin and 55% compared to 8% for PrP. For frequently detected biomarkers among both work groups (2-napthol, MeP), GM concentrations among hairdressers working in Black hair salons were approximately two times higher than those working in Dominican salons (2-naphthol: 22.9 vs. 13.6 ng/mL; MeP: 225 vs. 115 ng/mL) [Table 3]; a similar pattern was observed when our team previously measured phthalates[13] and VOCs[14] in this study population.
Summary statistics for specific gravity-corrected urinary biomarker concentrations (ng/mL) among hairdressers by salon typea
| Biomarker | LOQ | Hairdressers from Black salons (n = 11) | Hairdressers from Dominican salons (n = 12) | P-valueb,c | ||||||
| DF% | GM | p50 (p25, p75) | Range | DF% | GM | p50 (p25, p75) | Range | |||
| 2-Naphthol | 2.00 | 92 | 22.9 | 19.7 (12.7, 39.6) | < LOQ-69.8 | 100 | 13.6 | 8.98 (6.80, 21.8) | 5.73-282 | 0.04 |
| Capsaicin | 3.70 | 100 | 27.4 | 59.3 (4.28, 91.9) | < LOQ-112 | 17 | < LOQ | < LOQ | < LOQ-18.5 | 0.02 |
| EtP | 50.0 | 36 | < LOQ | < LOQ | < LOQ-338 | 8 | < LOQ | < LOQ | < LOQ-50 | 0.35 |
| MeP | 15.0 | 100 | 225 | 228 (106, 520) | 30.9-1,820 | 100 | 104 | 115 (49.4, 178) | 43.9-467 | 0.06 |
| PrP | 100 | 55 | 152 | 129 (< LOQ, 326) | <LOQ-702 | 8 | < LOQ | < LOQ | < LOQ-334 | 0.05 |
The disparate detections and exposures noted among the hairdresser groups could be attributed to the influence of racial/ethnic PCP usage patterns consistent with observed significant differences in usage reported in the literature by race/ethnicity for several cosmetics, with Black women reporting more hair product use than women of other races[50,51]. For example, capsaicin is used in hair growth products to stimulate hair growth and treat numerous hair growth-related conditions, including alopecia areata[30-34]. Given that hair growth-related conditions like alopecia affect more African Americans than other racial groups[56,57], it is plausible that hairdressers working in Black salons utilize more hair products containing capsaicin to service their clients than those working in Dominican salons. While it is possible that dietary intake may contribute to measured capsaicin concentrations, we did not capture information on dietary intake among study participants; future studies should consider capturing such data to control for other non-occupational sources of capsaicin that could impact workplace exposures among hairdressers. Nonetheless, racial/ethnic patterns in hair product use can be a significant source of differential exposure risk to many chemicals for this worker population and should be considered in future studies. To further clarify potential sources of variation in biomarker levels between salon subgroups, future studies should consider obtaining detailed product inventories and ingredient analyses of hair and beauty products used in different salon types.
Urinary biomarker concentrations by services rendered and use of hair products and PPE in a typical workday
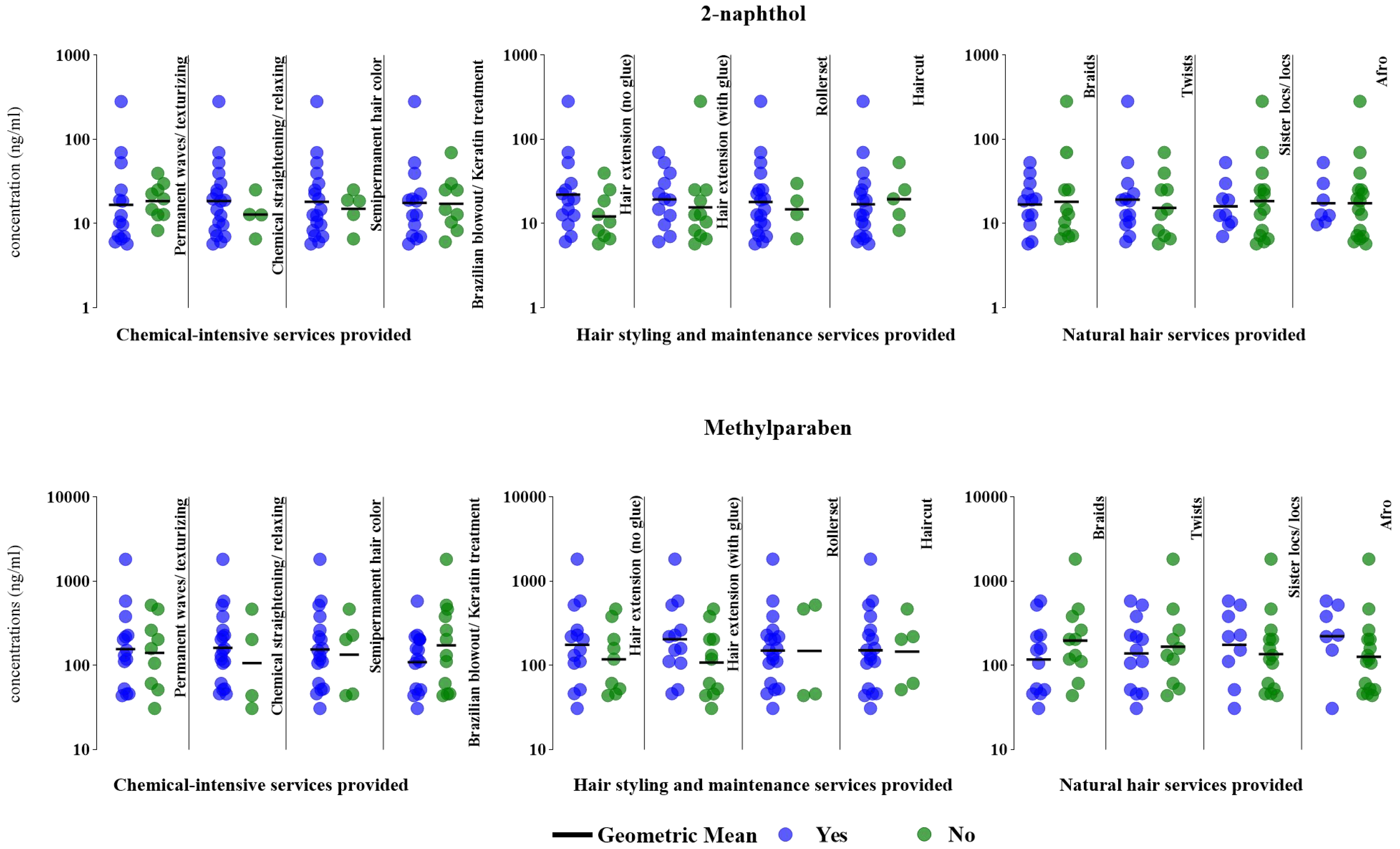
In general, we observed a wider distribution of 2-naphthol and MeP concentrations among hairdressers who offered chemical-intensive hair styling and maintenance services (e.g., permanent waves/texturizers, chemical straighteners/relaxers, extensions with and without adhesive, and roller sets) compared to those who did not offer these services (2-naphthol: 5.73-282 vs. 6.07-39.6 ng/mL) [Figure 1]. We observed similar patterns in this group of hairdressers in our prior work, where we found that certain VOCs[14] and phthalates[13] were elevated among hairdressers who performed certain chemical-intensive services compared to those who did not. In contrast, hairdressers who offered natural hair services (e.g., braiding, sister locs, and afros) had a narrower range of these concentrations compared to those who did not offer these services (2-naphthol: 5.73-53.0 vs. 5.73-282 ng/mL; MeP: 30.9-583 vs. 43.9-1,820 ng/mL).
Figure 1. Specific gravity-corrected urinary biomarker concentrations (ng/mL) based on hair services provided in a typical week (n = 23, 2018-2019). The figure compares 2-naphthol and MeP concentrations between hairdressers who provided (“yes”, denoted by blue dots) select chemical-intensive services, hair styling and maintenance services, and natural hair services and those who did not offer these services (“no”, denoted by green dots). MeP: Methylparaben.
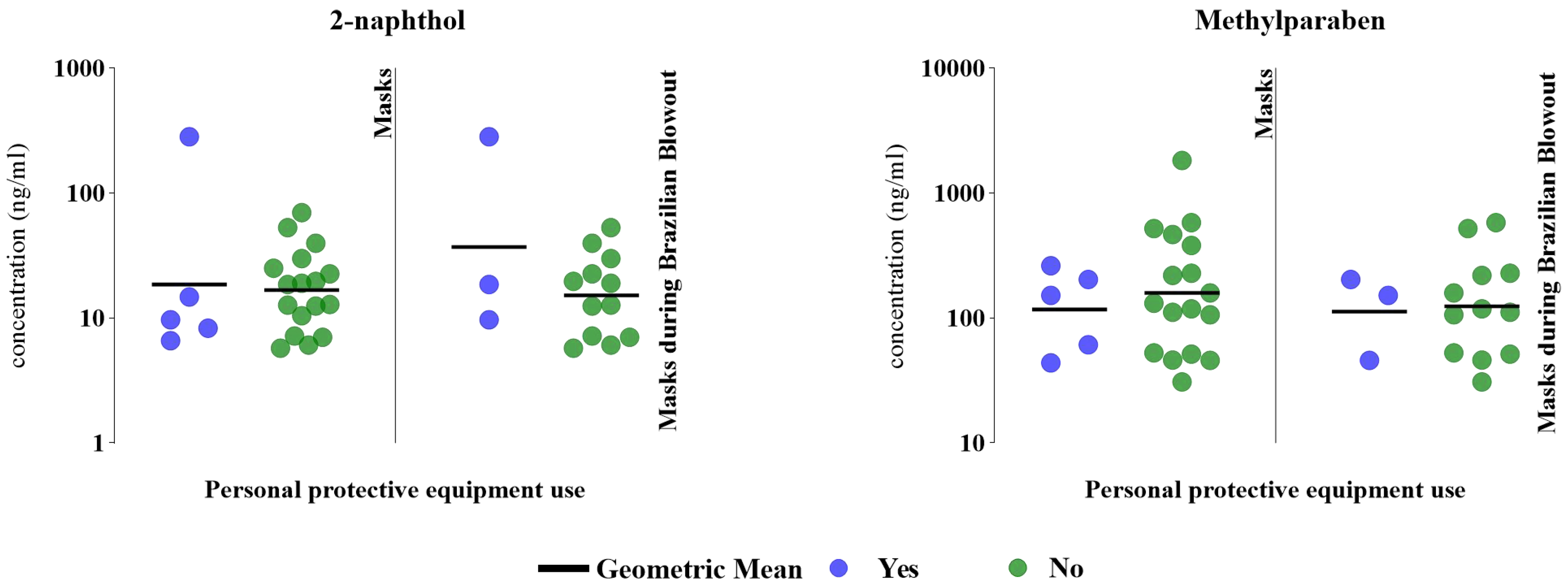
Additionally, hairdressers who used masks during chemical-intensive services, including Brazilian keratin treatment, had a wider distribution of 2-naphthol concentrations compared to those who did not report using masks (6.59-282 vs. 5.73-69.8 ng/mL). However, those who reported wearing a mask during chemical-intensive services had a narrower range of MeP concentrations compared to those who did not wear masks during these services (43.9-262 vs. 30.9-1,820 ng/mL) [Figure 2]. Although we did not collect detailed information on the type of masks worn (e.g., cloth, surgical, N95), precluding analysis of the effectiveness of specific mask types or levels of filtration, our findings are comparable with those of Arfaeinia et al., who found a significant inverse correlation (P < 0.05) between any mask use and urine paraben concentrations among Iranian hairdressers[16]. These results suggest that inhalation may be a significant route of paraben exposure among hairdressers, aligning with evidence that parabens are released in particle-phase emissions[58,59]. While the focus should be on eliminating sources of exposure, interventions focused on PPE to reduce chemical exposures in hairdressers should consider masks capable of filtering both liquid and solid-phase aerosols. Future investigations should explore the effectiveness of different mask types, usage patterns, and frequency, as well as the impact of formal workplace policies in salon environments, given the potential for masks to serve as a practical and testable protective measure. While the measurement of urinary biomarkers enabled assessment of internal dose by capturing cumulative exposure from all relevant routes, including inhalation, dermal absorption, and potential incidental ingestion[60], we could not discern the relative contributions of each exposure route. For example, low response rates regarding glove use precluded robust evaluation of protection against dermal exposures. Larger studies are needed to assess how PPE use across different routes influences chemical exposures in this workforce.
Figure 2. Specific gravity-corrected urinary biomarker concentrations (ng/mL) based on PPE used while performing chemical services (n = 22, 2018-2019). The figure compares 2-naphthol and MeP concentrations between hairdressers who wore masks while providing chemical services (“yes”, denoted by blue dots) and those who did not offer these services (“no”, denoted by green dots). Mask use during the provision of Brazilian blowout only includes hairdressers who provide the service (n = 15). PPE: Personal protective equipment; MeP: methylparaben.
Chemical-intensive services
Hairdressers who offered permanent waves/texturizers had comparable GM 2-naphthol concentrations (16.7 vs. 18.6 ng/mL) to those who did not [Figure 1]. Conversely, those who provided chemical straightening/relaxing (18.6 vs. 12.8 ng/mL) and semipermanent coloring (18.2 vs. 15.0 ng/mL) had slightly higher GM 2-naphthol concentrations than those who did not. Hairdressers who offered Brazilian keratin treatment had comparable GM 2-naphthol concentrations to those who reported not offering this service (17.6 vs. 17.2 ng/mL).
GM MeP concentrations were slightly higher among hairdressers who offered permanent wave/texturizing (157 vs. 141 ng/mL), chemical straightening/relaxing (161 vs. 107 ng/mL), and semipermanent coloring services (155 vs. 134 ng/mL) compared with those who did not provide these services [Figure 1]. Lower GM MeP concentrations were observed in hairdressers who provided Brazilian keratin treatment services than those who did not (109 vs. 246 ng/mL, respectively).
Hairstyling and maintenance services
Except for haircuts, hairdressers who offered select hairstyling and maintenance services had GM 2-naphthol concentrations that were 1.2-1.8 times higher than those who did not, including hair extensions with the use of adhesives (19.4 vs. 15.5 ng/mL), hair extensions without adhesive use (22.0 vs. 12.1 ng/mL), and setting the hair in rollers (18.1 vs. 14.8 ng/mL) [Figure 1].
Hairdressers who applied hair extensions with or without adhesives had 1.5-1.9 times higher GM MeP concentrations than those who did not offer hair extension services (with adhesive: 175 vs. 118 ng/mL; no adhesive: 203 vs. 108 ng/mL) [Figure 1]. We also observed comparable GM MeP concentrations among hairdressers who offered haircuts and those who did not (151 vs. 146 ng/mL).
Natural hair services
We observed comparable GM 2-naphthol concentrations among hairdressers who braided hair and those who provided sister locs/locs compared to those who did not offer these services (braiding: 16.9 vs. 18.1 ng/mL; sister locs/locs: 16.0 vs. 18.5 ng/mL) [Figure 1]. Hairdressers who provided twisting (i.e., a hairstyle created by intertwining two or three strands of hair) had slightly higher GM 2-naphthol concentrations than those who did not provide this service (19.2 vs. 15.4 ng/mL), while hairdressers who offered natural afro hair services and those who did not had the same GM 2-naphthol concentration (17.4 ng/mL).
GM MeP concentrations were lower for hairdressers who provided braiding (117 vs. 196 ng/mL) and twisting (139 vs. 166 ng/mL) compared to those who did not provide these services [Figure 1]. In contrast, GM MeP concentrations were 1.3-1.8 times higher in hairdressers who offered sister locs/locs (175 vs.
While our limited sample size may preclude an in-depth investigation of exposure differences between those providing “natural hairstyles” vs. those that do not, the provision of these services may not always result in lower exposures. Despite the perception of being safer alternatives to chemical-intensive hairstyles (e.g., hair relaxing or texturizing, Brazilian blowout or Keratin treatments), natural hairstyles often require the use of multiple hair products, including styling aids previously shown to contain several chemical ingredients of concern [Supplementary Table 1][2,61]. Thus, hairstylists providing “natural hairstyles” could still be exposed to potentially harmful chemical ingredients. Using replacement hair products deemed safer because of claims of being free of specific concerning chemical ingredients (e.g., paraben- and phthalate-free) may not always be feasible for hairdressers, as such products might still contain other harmful ingredients. These challenges persist regardless of whether they provide natural hairstyles or chemical-intensive services, due in part to the limited availability of safer alternatives[52,62], the lack of transparency in the ingredient disclosure[2], and consumer preferences or resistance to change[51]. When Collins et al. surveyed Californian women on their PCP usage patterns, they determined that compared to women of other races/ethnicities, Vietnamese and Latina women were less likely to avoid specific ingredients such as parabens in their PCPs[51]. Comparatively, Black women were more inclined to use imported products to suit their needs. Thus, future research should investigate exposures among hairdressers and clients seeking natural hairstyles, as they may still be exposed to chemicals of concern. Given these challenges, culturally tailored interventions may be more suitable for mitigating chemical exposures among hairdressers based on their clientele.
Product usage
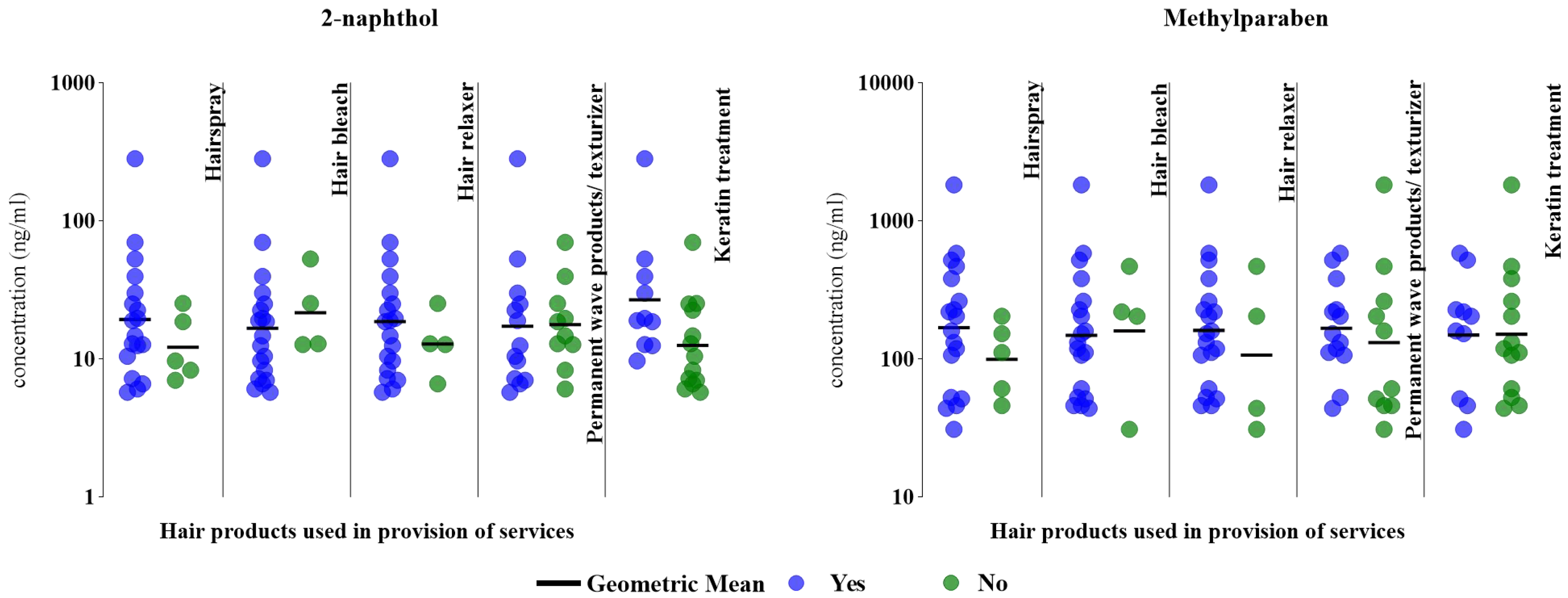
Select products were associated with higher biomarker concentrations for 2-naphthol [Figure 3]. Specifically, we observed 1.5-2.1 times higher GM 2-naphthol concentrations in hairdressers who reported using chemical straightener/relaxer (18.6 vs. 12.8 ng/mL), hairspray (19.3 vs. 12.1 ng/mL), and Brazilian Keratin Treatment products (26.8 vs. 12.5 ng/mL) to service their clients than hairdressers who did not use these products. Those who reported using hair bleach had lower GM 2-naphthol concentrations than those who did not (16.7 vs. 21.6 ng/mL).
Figure 3. Specific gravity-corrected urinary biomarker concentrations (ng/mL) based on hair salon products used in a typical week (n = 23, 2018-2019). The figure compares 2-naphthol and MeP concentrations between hairdressers who used (“yes”, denoted by blue dots) select hair products, compared to those who did not use these products (“no”, denoted by green dots). MeP: Methylparaben.
The GM MeP concentrations were 1.3-1.7 times higher in hairdressers who reported using hairspray (168 vs. 99.4 ng/mL), chemical straightener/relaxer (161 vs. 107 ng/mL), and permanent waves/texturizer products (167 vs. 131 ng/mL) [Figure 3]. Similar to 2-naphthol, GM MeP concentrations were slightly lower among hairdressers who reported using hair bleach than those who did not (148 vs. 159 ng/mL). GM MeP biomarker concentrations for hairdressers who reported using Brazilian Keratin Treatment products were comparable to those who did not report using these products (149 vs. 151 ng/mL) [Figure 3].
Study limitations and strengths
Our study had some limitations, including the small sample size and cross-sectional design. The collection of only post-shift urine samples limited the assessment of exposure variability (both within and across participants) and temporal trends, so we may have missed crucial exposure windows due to the short half-lives of these biomarkers[63-65]. Our design could not differentiate occupational from non-workplace exposures; however, reported PCP use outside work did not differ significantly between hairdressers and office workers. Future studies should consider collecting both pre- and post-shift urine samples to better distinguish occupational exposures from background sources. Dietary sources of exposure (e.g., capsaicin intake) were not accounted for, nor did we gather detailed data on mask and glove use or salon PPE policies, limiting evaluation of their influence on chemical exposures. Although office workers served as a comparison group, the absence of Caucasian hairdressers or those serving a non-ethnic or mixed clientele limits generalizability to hairdressers working predominantly with women of color. Finally, our study did not account for seasonal variations in product use or ventilation, which can be affected by factors such as air conditioning or closed windows. Seasonal shifts may also influence styling practices and product choices, thereby impacting chemical exposures[61]. Future studies should examine these seasonal patterns to enhance the accuracy and applicability of findings.
Despite the limitations, our study has several notable strengths. Given the complexity and diversity of chemicals present in hair and beauty products, conducting comprehensive product inventories and ingredient analyses can be challenging. However, in the present study, a suspect screening analytical approach was integrated, providing the opportunity to identify both known and previously unrecognized chemicals, which can enhance understanding of exposure sources. Future studies should consider combining prioritized product inventories with suspect ingredient screening to more comprehensively elucidate sources of exposure disparities in this workforce.
This study is also the first to characterize exposure to 2-naphthol, capsaicin, EtP, MeP, and PrP among hairdressers of color (Black/Latina) primarily serving a clientele of color using biomonitoring methods in the U.S. Our study is one of only two studies to characterize hairdressers’ paraben exposures[16]. To our knowledge, no other study has assessed exposure to 2-naphthol and capsaicin among hairdressers. Additionally, we had two comparison groups - office workers and a representative sample of women from the U.S. general population - which enhanced our understanding of chemical exposures among hairdressers and populations considered less exposed. We also showed that Black hairdressers have higher concentrations of 2-naphthol, capsaicin, EtP, MeP, and PrP compared to Latina hairdressers, underscoring the need to expand our understanding of how racial/ethnic product usage and hair service patterns might drive risks associated with exposures and associated health disparities among hairdressers. Another strength of our study is that we examined exposure to two EDCs (i.e., 2-naphthol and MeP) among hairdressers in the context of the product types used, services offered, and work behaviors, including PPE use. As a result, we identified potential modifiable exposure factors that should be explored further to inform future larger studies aimed at informing interventions to reduce exposures in this worker group.
CONCLUSIONS
In summary, our findings suggest that hairdressers of color, who primarily serve women of color, experience disparate chemical exposures, including to select EDCs, potentially due in part to the services rendered and products used. Differential exposures to select EDCs measured may contribute to exposure and health disparities. This raises concerns as one-third of this primarily female, low-income workforce consists of women of color[1]. These women may already be overburdened by chemical and non-chemical stressors outside the workplace. Our work expands on EDC exposure assessment efforts among U.S. hairdressers and highlights potential exposure disparities that could lead to health inequities in this at-risk population and should be studied further. Additionally, our findings are of great public health relevance as many hairdressers are women of reproductive age and often work during pregnancy, posing an additional health risk to their children. However, our study’s limited sample size and focus on hairdressers in the United States must be considered when interpreting these results. While the findings provide important preliminary insights, larger studies with a racially/ethnically diverse group of hairdressers are needed for more in-depth exposure characterization of salon exposures and to help identify modifiable exposure determinants that can be tested in culturally relevant interventions to protect worker health.
DECLARATIONS
Acknowledgments
The authors gratefully acknowledge Centro de Apoyo Familiar and the UMD H.A.I.R. network for their help in recruiting hair salons, hairdressers, local community church leaders, and student volunteers. This research was supported by a grant from the U.S. Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, to the Johns Hopkins Education and Research Center for Occupational Safety and Health (award number T42 OH0008428).
Authors’ contributions
Conceptualization, data acquisition, data curation, data cleaning, formal analysis, methodology, visualization, writing - original draft, review and editing: Kavi, L. K.
Data cleaning, methodology, laboratory analyses, writing - review and editing: Newmeyer, M. N.
Outreach and human subject recruitment: Pool, W.; Randolph, K.
Project administration, writing - review and editing: Louis, L. M.
Conceptualization, resources, supervision, writing - review and editing, generation of study instruments, methodology: Rule, A. M.
Conceptualization, resources, supervision, methodology, writing - review and editing: Prasse, C.
Conceptualization, funding and data acquisition, investigation, methodology, project administration, generation of study instruments, resources, supervision of field work, data visualization, writing - assisting with writing the initial draft, review and editing: Quirós-Alcalá, L.
Availability of data and materials
The raw data supporting the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This research was supported by a Pilot grant from the U.S. Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health, to the Johns Hopkins Education and Research Center for Occupational Safety and Health (award number T42 OH0008428). Kavi, L. K. was supported by the Ann G. Wylie Dissertation Fellowship from the University of Maryland Graduate School.
Conflicts of interest
Pool, W. is a director at CAF, a community-based organization, and Randolph, K. is a salon owner and community leader; both contributed to participant recruitment and engagement in this study and are members of the community advisory group. These roles and affiliations did not influence the conduct, analysis, or interpretation of the research and do not constitute a conflict of interest. All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The University of Maryland’s Institutional Review Board reviewed and approved all study protocols (1076658-21; November 11, 2022). Informed consent was obtained from all study participants.
Consent for publication
Not applicable
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. U.S. Bureau of Labor Statistics. Labor force statistics from the current population survey. Household data annual averages. 11. Employed persons by detailed occupation, sex, race and hispanic or latino ethnicity. https://www.bls.gov/cps/cpsaat11.htm. (accessed 14 Oct 2025).
2. Helm, J. S.; Nishioka, M.; Brody, J. G.; Rudel, R. A.; Dodson, R. E. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ. Res. 2018, 165, 448-58.
3. James-Todd, T.; Connolly, L.; Preston, E. V.; et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 476-86.
4. Zhang, Y.; Mustieles, V.; Williams, P. L.; et al. Parental preconception exposure to phenol and phthalate mixtures and the risk of preterm birth. Environ. Int. 2021, 151, 106440.
5. Land, K. L.; Miller, F. G.; Fugate, A. C.; Hannon, P. R. The effects of endocrine-disrupting chemicals on ovarian- and ovulation-related fertility outcomes. Mol. Reprod. Dev. 2022, 89, 608-31.
6. Pan, J.; Liu, P.; Yu, X.; Zhang, Z.; Liu, J. The adverse role of endocrine disrupting chemicals in the reproductive system. Front. Endocrinol. 2023, 14, 1324993.
7. Kolena, B.; Petrovičová, I.; Šidlovská, M.; et al. Occupational phthalate exposure and health outcomes among hairdressing apprentices. Hum. Exp. Toxicol. 2017, 36, 1100-12.
8. Molinari, F.; Franco, G. A.; Tranchida, N.; Di Paola, R.; Cordaro, M. Molecular mechanism of action of endocrine-disrupting chemicals on the respiratory system. Int. J. Mol. Sci. 2024, 25, 12540.
9. Deng, C.; Jiang, Y.; Lin, Y.; et al. Potential effects of endocrine-disrupting chemicals on preserved ratio impaired spirometry revealed by five different approaches. Ecotoxicol. Environ. Saf. 2025, 302, 118701.
10. Özel, F.; Rüegg, J. Exposure to endocrine-disrupting chemicals and implications for neurodevelopment. Dev. Med. Child. Neurol. 2023, 65, 1005-11.
11. Yesildemir, O.; Celik, M. N. Association between pre- and postnatal exposure to endocrine-disrupting chemicals and birth and neurodevelopmental outcomes: an extensive review. Clin. Exp. Pediatr. 2024, 67, 328-46.
12. O’Shaughnessy, K. L.; Fischer, F.; Zenclussen, A. C. Perinatal exposure to endocrine disrupting chemicals and neurodevelopment: how articles of daily use influence the development of our children. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101568.
13. Boyle, M. D.; Kavi, L. K.; Louis, L. M.; et al. Occupational exposures to phthalates among Black and Latina U.S. hairdressers serving an ethnically diverse clientele: a pilot study. Environ. Sci. Technol. 2021, 55, 8128-38.
14. Louis, L. M.; Kavi, L. K.; Boyle, M.; et al. Biomonitoring of volatile organic compounds (VOCs) among hairdressers in salons primarily serving women of color: a pilot study. Environ. Int. 2021, 154, 106655.
15. Moradi, M.; Hopke, P.; Hadei, M.; et al. Exposure to BTEX in beauty salons: biomonitoring, urinary excretion, clinical symptoms, and health risk assessments. Environ. Monit. Assess. 2019, 191, 286.
16. Arfaeinia, H.; Ramavandi, B.; Yousefzadeh, S.; et al. Urinary level of un-metabolized parabens in women working in beauty salons. Environ. Res. 2021, 200, 111771.
17. Porras, S. P.; Hartonen, M.; Ylinen, K.; Tornaeus, J.; Tuomi, T.; Santonen, T. Environmental and occupational exposure to resorcinol in Finland. Toxicol. Lett. 2018, 298, 125-33.
18. Arfaeinia, H.; Dobaradaran, S.; Mahmoodi, M.; Farjadfard, S.; Tahmasbizadeh, M.; Fazlzadeh, M. Urinary profile of PAHs and related compounds in women working in beauty salons. Sci. Total. Environ. 2022, 851, 158281.
19. Gube, M.; Heinrich, K.; Dewes, P.; Brand, P.; Kraus, T.; Schettgen, T. Internal exposure of hairdressers to permanent hair dyes: a biomonitoring study using urinary aromatic diamines as biomarkers of exposure. Int. Arch. Occup. Environ. Health. 2011, 84, 287-92.
20. U.S. Food and Drug Administration (FDA). Modernization of Cosmetics Regulation Act of 2022 (MoCRA). https://www.fda.gov/cosmetics/cosmetics-laws-regulations/modernization-cosmetics-regulation-act-2022-mocra. (accessed 14 Oct 2025).
21. Newmeyer, M. N.; Quirós-Alcalá, L.; Kavi, L. K.; Louis, L. M.; Prasse, C. Implementing a suspect screening method to assess occupational chemical exposures among US-based hairdressers serving an ethnically diverse clientele: a pilot study. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 566-74.
22. Shao, Y.; Kavi, L.; Boyle, M.; et al. Real-time air monitoring of occupational exposures to particulate matter among hairdressers in Maryland: a pilot study. Indoor. Air. 2021, 31, 1144-53.
23. James-Todd, T.; Senie, R.; Terry, M. B. Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. J. Immigr. Minor. Health. 2012, 14, 506-11.
24. Zota, A. R.; Shamasunder, B. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am. J. Obstet. Gynecol. 2017, 217, 418.e1-6.
25. Chan, M.; Parikh, S.; Shyr, D.; Shamasunder, B.; Adamkiewicz, G.; James-Todd, T. Evaluating neighborhood-level differences in hair product safety by Environmental Working Group Ratings among Retailers in Boston, Massachusetts. Environ. Health. Perspect. 2023, 131, 97002.
26. Beins, K.; Friedman, A.; Lin, H.; Edwards, K. Higher hazards persist in personal care products marketed to Black Women, report reveals. 2025. https://www.ewg.org/research/higher-hazards-persist-personal-care-products-marketed-black-women-report-reveals. (accessed 14 Oct 2025).
27. Sauvé, J. F.; Lévesque, M.; Huard, M.; et al. Creatinine and specific gravity normalization in biological monitoring of occupational exposures. J. Occup. Environ. Hyg. 2015, 12, 123-9.
28. Busgang, S. A.; Andra, S. S.; Curtin, P.; et al. A cross-validation based approach for estimating specific gravity in elementary-school aged children using a nonlinear model. Environ. Res. 2023, 217, 114793.
29. Mao, Y.; Xu, Z.; Song, J.; Xie, Y.; Mei, X.; Shi, W. Efficacy of a mixed preparation containing piperine, capsaicin and curcumin in the treatment of alopecia areata. J. Cosmet. Dermatol. 2022, 21, 4510-4.
30. Harada, N.; Okajima, K.; Arai, M.; Kurihara, H.; Nakagata, N. Administration of capsaicin and isoflavone promotes hair growth by increasing insulin-like growth factor-I production in mice and in humans with alopecia. Growth. Horm. IGF. Res. 2007, 17, 408-15.
31. Hosking, A. M.; Juhasz, M.; Atanaskova Mesinkovska, N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin. Appendage. Disord. 2019, 5, 72-89.
32. PubChem. Capsaicin. https://pubchem.ncbi.nlm.nih.gov/compound/1548943. (accessed 14 Oct 2025).
33. Consumer Product Information Database. What’s in it: health effects of consumer products. https://www.whatsinproducts.com/pages/index/1. (accessed 14 Oct 2025).
34. Environmental Working Group. EWG Skin Deep® Cosmetics Database. EWG Database. https://www.ewg.org/skindeep/. (accessed 14 Oct 2025).
35. Saljoughian, M. Capsaicin: risks and benefits. US. Pharm. 2009, 34, HS-17-JS-18. https://www.uspharmacist.com/article/capsaicin-risks-and-benefits. (accessed 14 Oct 2025).
36. Giménez-Arnau, A.; Gilaberte, M.; Serra-Baldrich, E.; Pujol-Vallverdú, R. FS09.6 Capsaicin contact dermatitis. Contact. Dermatitis. 2004, 50, 156-7.
37. Chang, A.; Rosani, A.; Quick, J. Capsaicin. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025. http://www.ncbi.nlm.nih.gov/books/NBK459168/. (accessed 14 Oct 2025).
38. Croghan, W.; Egeghy, P. Methods of dealing with values below the limit of detection using SAS. 2003. https://api.semanticscholar.org/CorpusID:12446551. (accessed 14 Oct 2025).
39. Lubin, J. H.; Colt, J. S.; Camann, D.; et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ. Health. Perspect. 2004, 112, 1691-6.
40. Centers for Disease Control and Prevention (CDC). National Report on Human Exposure to Environmental Chemicals. https://www.cdc.gov/exposurereport/. (accessed 14 Oct 2025).
41. Baruch, Y.; Holtom, B. C. Survey response rate levels and trends in organizational research. Hum. Relat. 2008, 61, 1139-60. https://www.researchgate.net/publication/228079609_Survey_Response_Rate_Levels_and_Trends_in_Organizational_Research. (accessed 14 Oct 2025).
42. Lee, A.; Choi, S.; Park, N. Y.; et al. Effects of dietary sources and personal care products on paraben exposure in young Korean adults: a crossover intervention study. Chemosphere 2025, 374, 144209.
43. PubChem. 2-Naphthol. https://pubchem.ncbi.nlm.nih.gov/compound/8663. (accessed 14 Oct 2025).
44. Monteagudo, C.; Robles-Aguilera, V.; Salcedo-Bellido, I.; et al. Dietary exposure to parabens and body mass index in an adolescent Spanish population. Environ. Res. 2021, 201, 111548.
45. Nishihama, Y.; Yoshinaga, J.; Iida, A.; et al. Association between paraben exposure and menstrual cycle in female university students in Japan. Reprod. Toxicol. 2016, 63, 107-13.
46. Larsson, K.; Ljung Björklund, K.; Palm, B.; et al. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ. Int. 2014, 73, 323-33.
47. U.S. Food and Drug Administration (FDA). FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. 2022. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. (accessed 14 Oct 2025).
48. U.S. Food and Drug Administration (FDA). Prohibited & restricted ingredients in cosmetics. 2022. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/prohibited-restricted-ingredients-cosmetics. (accessed 14 Oct 2025).
49. European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast) (Text with EEA relevance). 2009. pp. 59-209. http://data.europa.eu/eli/reg/2009/1223/oj/eng. (accessed 14 Oct 2025).
50. Dodson, R. E.; Cardona, B.; Zota, A. R.; Robinson Flint, J.; Navarro, S.; Shamasunder, B. Personal care product use among diverse women in California: taking stock study. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 487-502.
51. Collins, H. N.; Johnson, P. I.; Calderon, N. M.; et al. Differences in personal care product use by race/ethnicity among women in California: implications for chemical exposures. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 292-300.
52. Barrett, E. S.; Wadie, K.; Getz, K.; Greenberg, P.; Moore, T.; Llanos, A. A. M. Evaluating personal care product use by Environmental Working Group hazard scores in relation to consumers’ sociodemographic characteristics, purchasing behaviors, and product safety perceptions. J. Expo. Sci. Environ. Epidemiol. 2025.
53. Pycke, B. F.; Geer, L. A.; Dalloul, M.; Abulafia, O.; Halden, R. U. Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ. Int. 2015, 84, 193-200.
54. Chang, C. H.; Wang, P. W.; Liang, H. W.; et al. The sex-specific association between maternal paraben exposure and size at birth. Int. J. Hyg. Environ. Health. 2019, 222, 955-64.
55. Wu, C.; Xia, W.; Li, Y.; et al. Repeated measurements of paraben exposure during pregnancy in relation to fetal and early childhood growth. Environ. Sci. Technol. 2019, 53, 422-33.
56. Lee, H.; Jung, S. J.; Patel, A. B.; Thompson, J. M.; Qureshi, A.; Cho, E. Racial characteristics of alopecia areata in the United States. J. Am. Acad. Dermatol. 2020, 83, 1064-70.
57. Sy, N.; Mastacouris, N.; Strunk, A.; Garg, A. Overall and racial and ethnic subgroup prevalences of alopecia areata, alopecia totalis, and alopecia universalis. JAMA. Dermatol. 2023, 159, 419-23.
58. Chen, J.; Hartmann, E. M.; Kline, J.; Van Den Wymelenberg, K.; Halden, R. U. Assessment of human exposure to triclocarban, triclosan and five parabens in U.S. indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry. J. Hazard. Mater. 2018, 360, 623-30.
59. Chen, M. H.; Yu, B.; Zhang, Z. F.; Ma, W. L. Occurrence of parabens in outdoor environments: implications for human exposure assessment. Environ. Pollut. 2021, 282, 117058.
60. Hopf, N. B.; Rousselle, C.; Poddalgoda, D.; et al. A harmonized occupational biomonitoring approach. Environ. Int. 2024, 191, 108990.
61. Douglas, A.; Onalaja, A. A.; Taylor, S. C. Hair care products used by women of African descent: review of ingredients. Cutis 2020, 105, 183-8.
62. Environmental Working Group (EWG). Big market for Black cosmetics, but less-hazardous choices limited. 2016. https://www.ewg.org/research/big-market-black-cosmetics-less-hazardous-choices-limited. (accessed 14 Oct 2025).
63. Nguyen, H. T.; Isobe, T.; Iwai-Shimada, M.; et al. Urinary concentrations and elimination half-lives of parabens, benzophenones, bisphenol and triclosan in Japanese young adults. Chemosphere 2024, 349, 140920.
64. Choi, J. W.; Kim, M.; Song, G.; et al. Toxicokinetic analyses of naphthalene, fluorene, phenanthrene, and pyrene in humans after single oral administration. Sci. Total. Environ. 2023, 870, 161899.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].