A health conundrum of bisphenol A and its alternatives: charting a path beyond the structural analogue substitution pitfall
Abstract
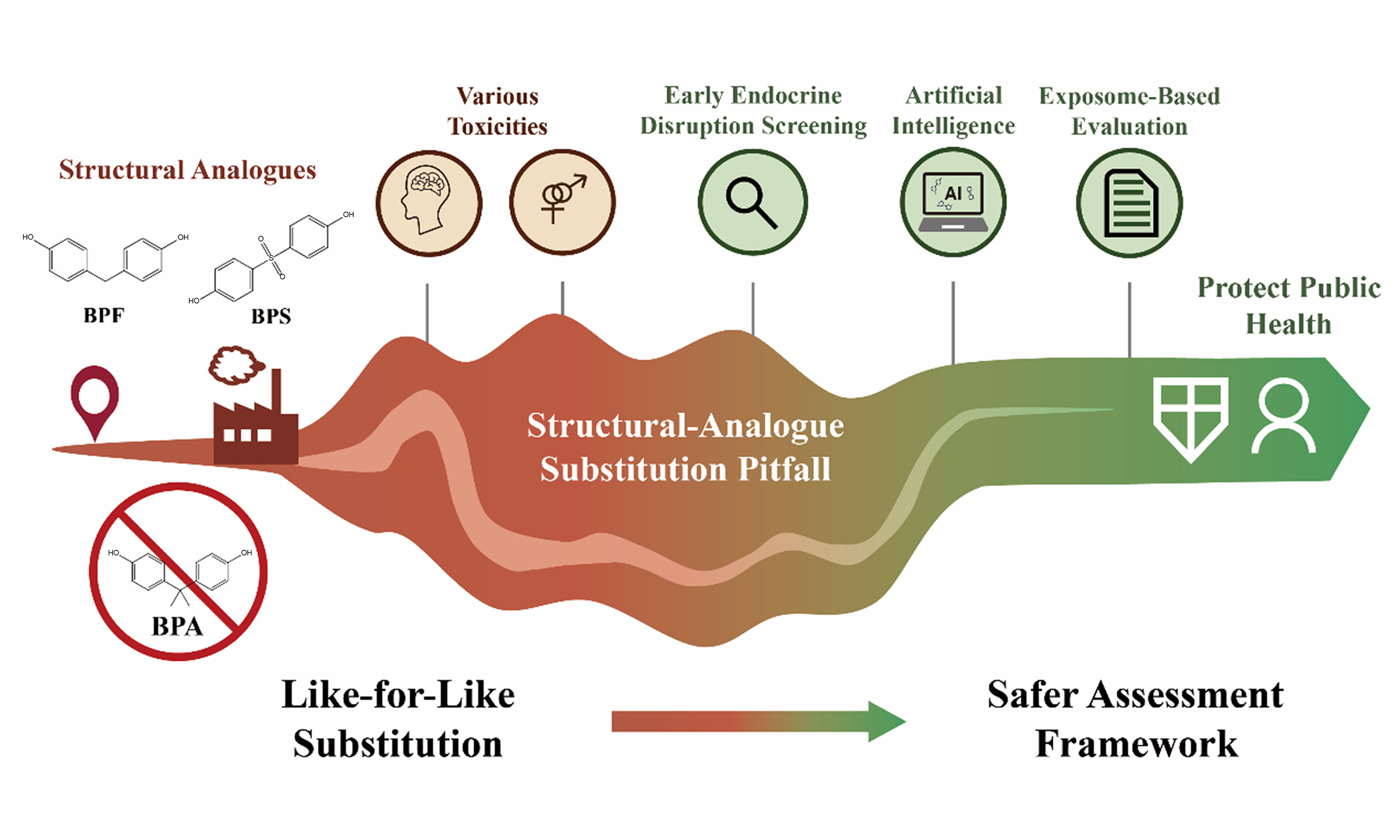
With the restriction of bisphenol A (BPA) in certain consumer products due to its endocrine-disrupting properties, structurally analogous alternatives such as bisphenol S (BPS) and bisphenol F (BPF) have rapidly entered the market. This shift has led to a wave of “regrettable substitutions” - chemicals that appear more environmentally friendly but may pose comparable or even unforeseen risks. Based on the latest toxicological evidence and population biomonitoring data, this perspective highlights that most mainstream BPA alternatives exhibit estrogenic/anti-androgenic activity, metabolic disruption potential, reproductive toxicity, and neurodevelopmental effects similar to those of BPA. Their widespread presence, environmental persistence, and hidden health hazards expose critical weaknesses in the current “like-for-like substitution” regulatory approach. To address this challenge, it is imperative to establish a safer assessment framework that integrates early endocrine disruption screening with exposome-based evaluation. Shifting from “hazard substitution” to a “functional safe-by-design” strategy is critical to circumvent the pitfalls of structural-analogue substitution, protect public health, and support sustainable development.
Keywords
INTRODUCTION
Bisphenol A (BPA), a major monomer in polycarbonate plastics and epoxy resins, is widely used in food storage containers, thermal paper receipts, medical devices, and other consumer products[1-4]. However, extensive research has demonstrated that BPA poses risks as a typical environmental endocrine-disrupting chemical (EDC)[2,5]. Even at extremely low doses, BPA can increase the risk of reproductive disorders, metabolic diseases, and neurodevelopmental abnormalities by mimicking estrogen, antagonizing androgen, and disrupting thyroid hormone pathways[5-8]. These findings have prompted regulatory actions worldwide. For example, China, the United States, and the European Union (EU) have banned BPA in baby bottles and imposed strict migration limits for food-contact materials[9-12]. The EU first imposed a ban on BPA in baby bottles through “Directive 2011/8/EU” in 2011[13]. More recently, BPA was added to the SVHC list under “Regulation (EU) 2024/3190” and completely banned in food-contact materials in 2024[14].
Consequently, market demand for “BPA-Free” products has accelerated the adoption of structurally similar alternatives such as bisphenol S (BPS), bisphenol F (BPF), and bisphenol AF (BPAF). Currently, more than 20 bisphenol analogues are in commercial use[15]. In 2012, global annual BPA production exceeded 6 million tons[16], and worldwide consumption reached approximately 7.7 million tons by 2015[17]. That same year, the U.S. Environmental Protection Agency (USEPA) identified eight manufacturers and/or importers of BPS, reporting combined annual volumes between 454 and 4,540 tons[18]. Data from the European Chemicals Agency (ECHA) for the European Economic Area (EEA) in 2014 showed BPS production or import levels of 1,000-10,000 tons, while BPF had not yet been registered[18]. By 2023, BPS volumes had risen to 10,000-100,000 tons, whereas BPF volumes were reported at 10-100 tons[19]. BPAF was also registered by 2023, with production or import levels estimated at 100-1,000 tons[19].
While these alternatives meet industrial performance requirements due to their similar physicochemical properties, their shared bisphenolic structure confers comparable potential for biological disruption. Their rapidly increasing global production and environmental prevalence are of particular concern. These compounds are now detected ubiquitously in water, dust, soil, sediments, organisms, and even the human body[20-22]. For example, a recent study found BPS in over 70% of urine samples (n = 1,157) collected from residents of 26 provincial capitals in China[22]. Globally, human biomonitoring data also confirm widespread exposure. In Australia, urinary and wastewater levels of BPA declined between 2012 and 2017, whereas BPS concentrations increased[23]. Similar trends - declining BPA exposure alongside increasing BPS and BPF levels - have been documented in North America. The geometric mean urinary concentration of BPA decreased from 2.07 μg/L in 2010 to 0.36 μg/L in 2014, while BPS detection frequency rose above 70% with steadily increasing average concentrations[24]. Comparable rising trends for alternative bisphenols were also observed in European populations, with urinary concentrations in 2018 significantly higher than in 2015[25].
Notably, a recent study derived reference doses (RfDs) of 0.37 and 8.09 ng/kg-bw/day for BPS and BPF, respectively, using Benchmark Dose Software (BMDS) to calculate the lower confidence limit of the benchmark dose (BMDL) based on epidemiological evidence of declining male semen quality[26]. These RfDs are orders of magnitude lower than the BPA RfD of 50 μg/kg-bw/day established by the USEPA in 1988[27] and the temporary tolerable daily intake (t-TDI) of 4 μg/kg-bw/day set by the European Food Safety Authority (EFSA) in 2015[28]. In 2023, EFSA[29] revised the tolerable daily intake (TDI) for BPA to 0.2 ng/kg-bw/day, placing the derived RfD for BPF within the same order of magnitude as BPA.
This evidence raises a critical question: Is the shift away from BPA inadvertently exposing the public to an even more insidious and complex set of health risks?
TOXICOLOGICAL EVIDENCE ON BPA ALTERNATIVES
As BPA alternatives have entered commercial use, accumulating toxicological evidence demonstrates their ability to induce multiple adverse health effects[11,30]. Moreover, emerging findings are dismantling the “BPA-Free” safety illusion, revealing that bisphenol substitutes retain the core endocrine-disrupting mechanisms of BPA[31,32]. In vitro and animal studies consistently demonstrate that bisphenol compounds effectively bind to and activate estrogen receptors (ERα/β)[11,33,34]. For example, long-term BPAF exposure in rats resulted in uterine fibrosis in vivo[35]. Exposure during critical developmental windows (e.g., gestation, lactation) has been shown to cause uterine malformations and accelerate ovarian follicular atresia[11,36,37]. In parallel, their antagonistic effects on thyroid hormone receptors (TR) can disrupt metabolic homeostasis and neurodevelopment, with potential transgenerational health consequences[11,38,39].
These bisphenol alternatives have been linked to concerning metabolic, immunotoxic, neurodevelopmental, and reproductive effects[2,5,26,40,41], which act as silent drivers of chronic diseases and may cause long-lasting harm to humans. For instance, BPS exposure exacerbates the progression of intestinal inflammatory diseases[42], while BPF disrupts cholesterol and testosterone regulation through PPARα activation[43]. Epidemiological data further reveal that BPS exposure is significantly associated with type II diabetes mellitus[44], and BPF exposure correlates with reduced sperm quality[45,46]. Far from being inert, these alternatives emerge as potent environmental triggers of metabolic dysfunction with long-term adverse effects on human health. For example, Jiang et al. and Geiger et al. reported that prenatal exposure to BPA and its alternatives has lasting effects on neurodevelopment by two years of age[47,48].
Furthermore, real-world human exposures typically involve complex mixtures of bisphenol analogues and/or other EDCs (e.g., phthalates, perfluoroalkyl substances)[1,5]. A recent study on co-exposure to bisphenols and other EDCs - including phthalates and parabens - in the follicular fluid of women undergoing assisted reproductive technologies revealed that these mixtures primarily influence hormone levels through combined effects, rather than individual compounds[49]. Increasing mixture concentrations were inversely associated with estradiol levels on the hCG trigger day and with reduced basal progesterone, with BPS and bisphenol P (BPP) identified as key contributors[49]. Notably, BPS and BPF exhibit synergistic effects when co-exposed with each other, with BPA, or with other harmful environmental factors, contradicting simple dose-additive predictions[41,50,51]. These findings demonstrate that current “safe threshold” assessment models, which are based on single-chemical evaluations, systematically underestimate the combined health risks of mixed exposures. In real-world environments, such mixture effects act as a magnifying lens, altering or amplifying the risks posed by individual chemicals.
THE “LIKE-TO-LIKE SUBSTITUTION” STRATEGY APPEARS FLAWED
The risks posed by bisphenol alternatives stem from structural shortcomings in current regulatory science paradigms. Early frameworks relied primarily on structure-activity relationship assessments and a narrow set of toxicity endpoints (e.g., acute toxicity, mutagenicity) when evaluating alternatives to BPA[52]. However, such approaches fail to capture the defining risks of EDCs, including low-dose effects, delayed latency periods, and non-monotonic dose-response relationships. The assumption that BPS, BPF, and related analogues are “safer BPA alternatives” represents a dangerous oversimplification that disregards the mechanistic complexity of endocrine disruption and highlights the inherent flaws in this “like-to-like substitution” logic.
Current EDC testing guidelines endorsed by major regulatory bodies (e.g., OECD) still rely largely on animal studies, which are expensive, time-consuming, and low-throughput, despite the introduction of some in vitro methods[53,54]. These methods remain inadequate for addressing the testing and evaluation demands of the growing number of new chemicals. Importantly, existing protocols have notable limitations: they cannot fully meet the requirements of endocrine disruptor testing, nor do they simulate the long-term consequences of exposures during critical developmental windows in humans. This has created a widening gap between advances in scientific understanding of bisphenol alternatives and the regulatory testing frameworks intended to evaluate them.
Moreover, the safety thresholds of RfD and TDI for bisphenol alternatives are generally derived from risk assessments of individual substances in isolation[11,26]. These thresholds are central to human health risk evaluation. Yet in reality, human exposure typically involves complex mixtures of multiple bisphenol analogues, other EDCs, and environmental stressors. The absence of robust frameworks for mixture risk assessment, combined with the lack of testing requirements, perpetuates a critical paradox: although the risks from individual substances may appear to fall below “safe” thresholds, their combined effects can far exceed biological tolerance. Addressing the health impacts of mixed exposures remains a major challenge, compounded by the severe scarcity of toxicity data for binary mixtures and, more critically, multicomponent pollutant mixtures.
Finally, chemical substitutions are often driven by market forces and implemented rapidly, whereas comprehensive risk characterization and regulatory decision making typically require many years, if not longer. This “substitution-first, assessment-later” approach enables potentially hazardous substances to be widely adopted in industrial applications long before adequate safety data are available, leading to their release into the environment and eventual human exposure. The rapid increase in the BPS market following BPA restrictions provides clear evidence of this systemic failure.
DEVELOPING AN INNOVATIVE PARADIGM FOR ENVIRONMENTAL HEALTH RISK ASSESSMENT AND MANAGEMENT
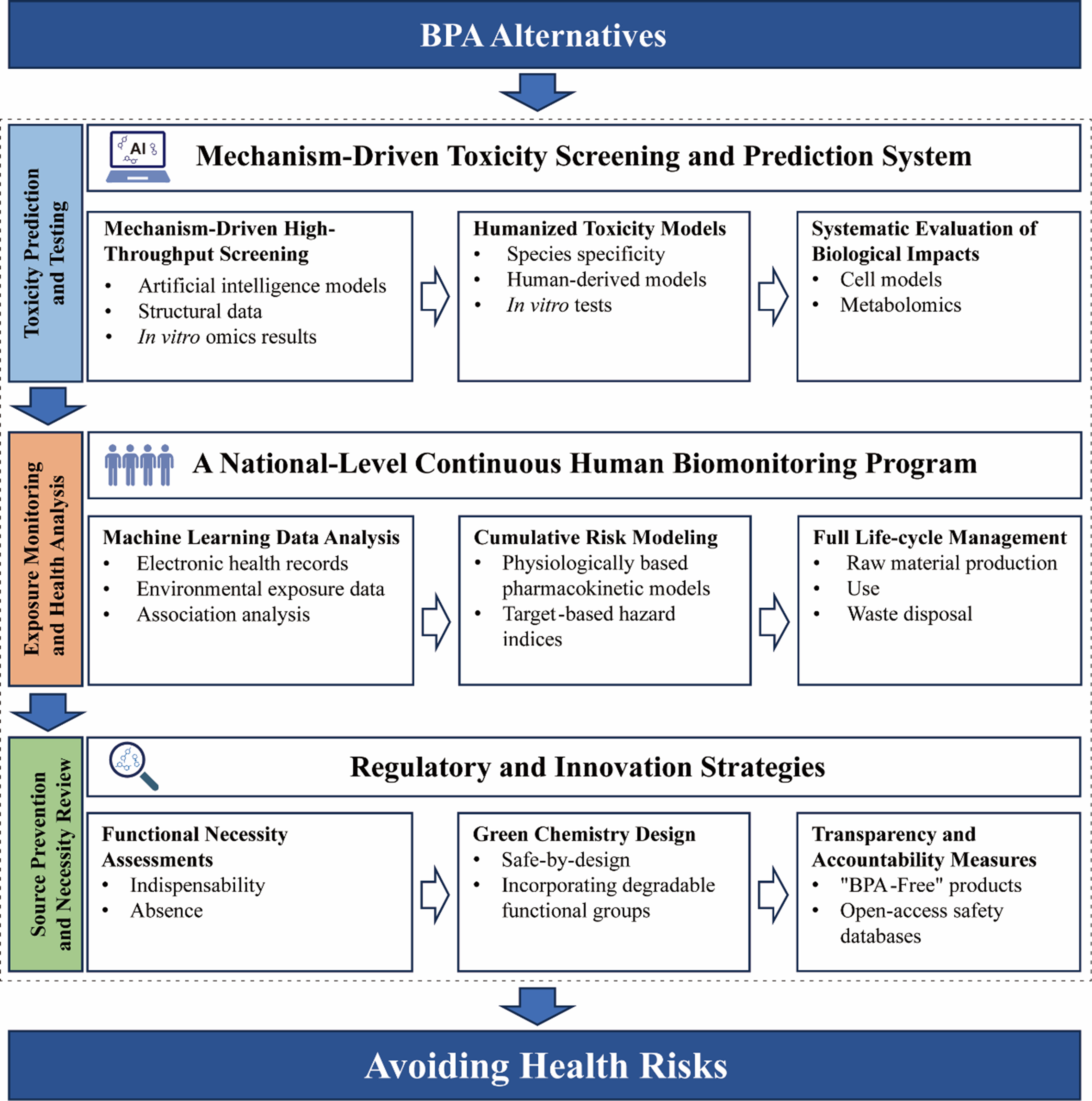
To avoid introducing new risks from chemical substitutes, a fundamental upgrade in the toxicity testing strategy for these alternatives is urgently needed, with a focus on developing mechanism-based predictive systems [Figure 1]. First, novel BPA alternatives must undergo mechanism-driven high-throughput screening that targets nuclear receptors (ER/AR, etc.), hormone-metabolizing enzymes, and key signaling pathways. These assays should integrate artificial intelligence models that combine structural data with in vitro omics results to provide early warnings. Second, priority should be given to replacing traditional animal testing with humanized toxicity models, such as in vitro systems built from human-derived cells, tissues, or organoids[31,42]. These approaches can more accurately capture relevant biological interactions and reduce errors caused by cross-species differences. Finally, systematic evaluations of how alternatives affect critical biological nodes should be performed using cell models and metabolomics to identify potential chronic disease risk factors.
Globally, human biomonitoring of bisphenols remains highly limited, both in national surveillance and large-scale regional studies[22]. Establishing a continuous, nationwide biomonitoring program is essential to track exposure profiles of bisphenol analogues, especially in vulnerable groups such as pregnant women and children. By integrating electronic health records with environmental exposure data, machine learning can be used to analyze associations between bisphenol analogue mixtures and outcomes related to neurodevelopment, reproductive toxicity, and metabolic health. This integrated approach will support evidence-based chemical regulation grounded in real-world exposure data. Furthermore, physiologically based pharmacokinetic modeling and target-based hazard index methods should be developed to quantify cumulative risks of bisphenol analogue mixtures that share common or similar mechanisms (e.g., estrogenic activation). Full lifecycle management is also necessary, including the establishment of emission inventories for bisphenol analogues spanning raw material production to waste disposal, alongside strengthened wastewater treatment standards for the removal of trace EDCs.
For both regulatory authorities and industry, source prevention and necessity review should be guided by “functional necessity” assessments, which require verification of both the indispensability of a substitute and the absence of safer alternatives. Green chemistry design must be actively promoted by encouraging the development of “safe-by-design” alternatives - for example, avoiding bisphenol-based structures, incorporating degradable functional groups, and accelerating the commercialization of non-bisphenol alternatives such as epoxy resins. Additionally, mandatory labeling of substitute chemicals in “BPA-Free” products and the establishment of open-access safety databases are critical to preventing misleading marketing. Through coordinated scientific regulation and green innovation, the pitfalls of substitution can be fundamentally avoided, despite the significant challenges ahead.
CONCLUSIONS
The emergence of BPS, BPF, and other BPA alternatives, together with mounting evidence of their health risks, underscores the short-sightedness and inherent flaws of current “hazard substitution” strategies. Rather than escaping the threats posed by BPA, we may instead have created additional entry points into a structurally similar labyrinth. This dilemma highlights the failure of existing chemical risk management paradigms to keep pace with advances in endocrine disruption science and the complex reality of mixed exposures. The urgent priority now is to move beyond the passive mindset of “chemical substitution” and actively embrace a “functional safe-by-design” paradigm. This requires the use of advanced in vitro models and modern computational tools to assess endocrine-disrupting potential before new BPA alternatives enter the market; the application of exposomics to reveal real-world mixed exposure scenarios; the implementation of full lifecycle management for alternative material flows; and the development of policy incentives to drive industry toward inherently safer designs. Only through such measures can we avoid the paradox of addressing one recognized environmental and public health issue while generating a series of unknown, and potentially more serious, problems. Protecting human and environmental health demands both forward-looking vision and transformative action. We must end the futile cycle within the bisphenol labyrinth and forge a new path toward genuinely safe and sustainable solutions. By doing so, we can overcome the pitfalls of substitution and advance toward a new era of health-conscious design for BPA alternatives, despite the significant challenges ahead.
DECLARATIONS
Authors’ contributions
Data collection, drawing and drafting: Yang, X.
Design, writing - review and editing: Yu, Y.
Availability of data and materials
Not applicable.
Financial support and sponsorship
The study was supported by the National Key R&D Program of China (2024YFC3713205) and Guangdong-Hong Kong-Macao Joint Laboratory for Contaminants Exposure and Health (2020B1212030008).
Conflicts of interest
Yu, Y. is an Editorial Board member of Journal of Environmental Exposure Assessment. Yu, Y. is also a Guest Editor for the Special Issue The Impact of Bisphenol Exposure on Human Health. Yu, Y. was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. Yang, X. declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Chou, W. C.; Gaynor, J. W.; Graham, E. M.; et al. A machine learning-based clustering analysis to explore bisphenol A and phthalate exposure from medical devices in infants with congenital heart defects. Environ. Health. Perspect. 2025, 133, 67016.
2. Costa, H. E.; Cairrao, E. Effect of bisphenol A on the neurological system: a review update. Arch. Toxicol. 2024, 98, 1-73.
3. Li, B.; Zhao, X.; Ding, Y.; Zhang, Y. Network toxicology and molecular docking to investigate the mechanism of bisphenol A toxicity in human diabetic cardiomyopathy. Ecotoxicol. Environ. Saf. 2025, 299, 118301.
4. Liu, J.; Martin, J. W. Prolonged exposure to bisphenol A from single dermal contact events. Environ. Sci. Technol. 2017, 51, 9940-9.
5. Braun, J. M. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161-73.
6. Fang, H.; Tong, W.; Branham, W. S.; et al. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem. Res. Toxicol. 2003, 16, 1338-58.
7. Rochester, J. R. Bisphenol A and human health: a review of the literature. Reprod. Toxicol. 2013, 42, 132-55.
8. Moriyama, K.; Tagami, T.; Akamizu, T.; et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185-90.
9. National Standard of the People’s Republic of China. GB 38995-2020. Infant and toddler bottles and pacifiers. 2020. https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=39161D96403B68B05A675DBCA1564254. (accessed 23 Sep 2025).
10. National Standard of the People’s Republic of China. GB 4806.6-2016. National Food Safety Standard - plastic resin for food contact. 2016. http://www.foods-test.com/GB4806.6-2016-kw.pdf. (accessed 23 Sep 2025).
11. Mhaouty-Kodja, S.; Zalko, D.; Tait, S.; et al. A critical review to identify data gaps and improve risk assessment of bisphenol A alternatives for human health. Crit. Rev. Toxicol. 2024, 54, 696-753.
12. van den Brand, A. D.; Hessel, E. V. S.; Rijk, R.; et al. A prioritization strategy for functional alternatives to bisphenol A in food contact materials. Crit. Rev. Toxicol. 2024, 54, 291-314.
13. European Union. Commission Directive 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of Bisphenol A in plastic infant feeding bottles Text with EEA relevance. 2011. http://data.europa.eu/eli/dir/2011/8/oj. (accessed 23 Sep 2025).
14. European Union. Commission Regulation (EU) 2024/3190 of 19 December 2024 on the use of bisphenol A (BPA) and other bisphenols and bisphenol derivatives with harmonised classification for specific hazardous properties in certain materials and articles intended to come into contact with food, amending Regulation (EU) No 10/2011 and repealing Regulation (EU) 2018/213. 2024. http://data.europa.eu/eli/reg/2024/3190/oj. (accessed 23 Sep 2025).
15. Zhou, J.; Chen, X. H.; Zhang, D. D.; Jin, M. C.; Zhuang, L.; Du, Y. Determination of multiple bisphenol analogues and their metabolites in human serum by liquid chromatography tandem mass spectrometry. Environ. Pollut. 2022, 312, 120092.
16. Pivnenko, K.; Laner, D.; Astrup, T. F. Dynamics of bisphenol A (BPA) and bisphenol S (BPS) in the European paper cycle: need for concern? Resour. Conserv. Recycl. 2018, 133, 278-87.
17. Lehmler, H. J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: the national health and nutrition examination survey 2013-2014. ACS. Omega. 2018, 3, 6523-32.
19. ECHA. REACH - Registration, Evaluation, Authorisation and Restriction of Chemicals Regulation. 2023. https://echa.europa.eu/information-on-chemicals/registered-substances. (accessed 23 Sep 2025).
20. Catenza, C. J.; Farooq, A.; Shubear, N. S.; Donkor, K. K. A targeted review on fate, occurrence, risk and health implications of bisphenol analogues. Chemosphere 2021, 268, 129273.
21. Wu, L. H.; Zhang, X. M.; Wang, F.; et al. Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci. Total. Environ. 2018, 615, 87-98.
22. Huang, S.; Wang, D.; Qi, Z.; Long, C.; Li, G.; Yu, Y. A large-scale nationwide study of urinary phenols in the Chinese population. Sci. Total. Environ. 2023, 894, 164850.
23. Tang, S.; He, C.; Thai, P. K.; et al. Urinary concentrations of bisphenols in the Australian population and their association with the per capita mass loads in wastewater. Environ. Sci. Technol. 2020, 54, 10141-8.
24. Ye, X.; Wong, L. Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A. M. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000-2014. Environ. Sci. Technol. 2015, 49, 11834-9.
25. Gyllenhammar, I.; Glynn, A.; Jönsson, B. A.; et al. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs? Environ. Res. 2017, 153, 48-54.
26. Cao, J.; Ma, W.; Gao, Y.; Long, C.; Yu, Y. Derivation of the oral reference dose (RfD) for bisphenol S and bisphenol F based on epidemiological and experimental studies. Ecotoxicol. Environ. Saf. 2025, 293, 118045.
27. United States Environmental Protection Agency. Integrated Risk Information System (IRIS), Chemical Assessment Summary. Reference dose for oral exposure (RfD) (Bisphenol A; CASRN 80-05-7). 1988. https://iris.epa.gov/static/pdfs/0356_summary.pdf. (accessed 23 Sep 2025).
28. EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. Part II - toxicological assessment and risk characterization. EFSA. J. 2015, 13, 3978.
29. EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré C, Barat Baviera JM, Bolognesi C, et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA. J. 2023, 21, e06857.
30. Lee, S.; Liu, X.; Takeda, S.; Choi, K. Genotoxic potentials and related mechanisms of bisphenol A and other bisphenol compounds: a comparison study employing chicken DT40 cells. Chemosphere 2013, 93, 434-40.
31. Abdulla, A.; Yan, H.; Chen, S.; et al. A multichannel microfluidic device for revealing the neurotoxic effects of Bisphenol S on cerebral organoids under low-dose constant exposure. Biosens. Bioelectron. 2025, 267, 116754.
32. Zhang, Q.; Li, M.; Wang, P.; Lin, X.; Lai, K. P.; Ding, Z. Integrated analysis reveals the immunotoxicity mechanism of BPs on human lymphocytes. Chem. Biol. Interact. 2024, 399, 111148.
33. Zhang, X.; Zhang, X.; Zhang, Z.; et al. Bisphenol S causes excessive estrogen synthesis by activating FSHR and the downstream cAMP/PKA signaling pathway. Commun. Biol. 2024, 7, 844.
34. Hassan, A. A.; Abdelgayed, S. S.; Mansour, S. Z. Liver and ovarian toxicities boosted by bisphenol and gamma radiation in female albino rats. Hum. Exp. Toxicol. 2024, 43, 9603271231219264.
35. Liu, J.; Yu, L.; Castro, L.; et al. Induction of fibrosis following exposure to bisphenol A and its analogues in 3D human uterine leiomyoma cultures. J. Hazard. Mater. 2024, 476, 134772.
36. Chouchene, L.; Boughammoura, S.; Ben Rhouma, M.; et al. Effect of thyroid disruption on ovarian development following maternal exposure to Bisphenol S. Environ. Sci. Pollut. Res. Int. 2024, 31, 52596-614.
37. Sudhakaran, G.; Kesavan, D.; Ranjan Nayak, S. P. R.; et al. Bisphenol A-induced ovarian damage countered by luteolin: experiments in in vitro CHO cells and in vivo PCOS phenotype zebrafish. Tissue. Cell. 2024, 91, 102532.
38. Guo, C.; Lv, L.; Chen, X.; et al. Low-dose bisphenol AF exerts slight effects on glycolipid metabolism but causes metabolic disorders under the stress of Western diet in mice. Environ. Pollut. 2025, 369, 125861.
39. Hasan, A. K. M. M.; Martyniuk, C. J.; Niyogi, S.; Chivers, D. P. A comprehensive review on the neurobehavioural effects of bisphenol compounds and the underlying molecular mechanisms in zebrafish (Danio rerio). Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2025, 296, 110228.
40. Alharbi, H. F.; Algonaiman, R.; Alduwayghiri, R.; et al. Exposure to bisphenol A substitutes, bisphenol S and bisphenol F, and its association with developing obesity and diabetes mellitus: a narrative review. Int. J. Environ. Res. Public. Health. 2022, 19, 15918.
41. Su, X.; Kai, L.; Han, X.; et al. Equipotent bisphenol S and bisphenol F with widely differing modes of action exhibit additive effects in immunotoxicity: insights based on intrinsic immunity, apoptosis and regeneration, and oxidative stress. Sci. Total. Environ. 2025, 977, 179405.
42. Wang, J.; Niu, G.; Mai, H.; et al. The protective role of 3-Indoleglyoxylic acid in bisphenol S-induced intestinal barrier dysfunction via mitochondrial ROS-Mediated IL-17/CXCL10/TNF-α signaling. Environ. Int. 2025, 199, 109477.
43. Gao, Z.; Liu, S.; Tan, L.; et al. Testicular toxicity of bisphenol compounds: homeostasis disruption of cholesterol/testosterone via PPARα activation. Sci. Total. Environ. 2022, 836, 155628.
44. Duan, Y.; Yao, Y.; Wang, B.; et al. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: a case-control study. Environ. Pollut. 2018, 243, 1719-26.
45. Ghayda, R. A.; Williams, P. L.; Chavarro, J. E.; et al. Urinary bisphenol S concentrations: potential predictors of and associations with semen quality parameters among men attending a fertility center. Environ. Int. 2019, 131, 105050.
46. Chen, P. P.; Liu, C.; Zhang, M.; et al. Associations between urinary bisphenol A and its analogues and semen quality: a cross-sectional study among Chinese men from an infertility clinic. Environ. Int. 2022, 161, 107132.
47. Jiang, Y.; Li, J.; Xu, S.; et al. Prenatal exposure to bisphenol A and its alternatives and child neurodevelopment at 2 years. J. Hazard. Mater. 2020, 388, 121774.
48. Geiger, S. D.; Musaad, S.; Hill, J.; Aguiar, A.; Schantz, S. Sex-specific associations between urinary bisphenols concentrations during pregnancy and problematic child behaviors at age 2 years. Neurotoxicol. Teratol. 2023, 96, 107152.
49. Dou, X.; Li, X.; Huang, S.; et al. Co-exposure of phthalates, bisphenols, parabens, and polycyclic aromatic hydrocarbons in follicular fluid of women undergoing assisted reproductive technologies and the associations with hormone levels. J. Environ. Expo. Assess. 2025, 4, 14.
50. Li, J.; Wang, Y.; Li, N.; et al. Toxic effects of bisphenol A and bisphenol S on Chlorella Pyrenoidosa under single and combined action. Int. J. Environ. Res. Public. Health. 2022, 19, 4245.
51. Song, J.; Meng, Q.; Song, H.; et al. Combined toxicity of pristine or artificially aged tire wear particles and bisphenols to Tigriopus japonicus. Chemosphere 2024, 363, 142894.
52. Chen, M. Y.; Ike, M.; Fujita, M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ. Toxicol. 2002, 17, 80-6.
53. OECD. Revised guidance document 150 on standardized test guidelines for evaluating chemicals for endocrine disruption. 2018. https://www.oecd.org/en/publications/guidance-document-on-standardised-test-guidelines-for-evaluating-chemicals-for-endocrine-disruption-2nd-edition_9789264304741-en.html. (accessed 23 Sep 2025).
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].