FAIREHR: a novel online research registry platform to advance global environmental and occupational health research
Abstract
The FAIREHR (Findable, Accessible, Interoperable, Reusable Environmental and Health Registry) platform is a state-of-the-art online registry for prospective harmonization of human biomonitoring (HBM). It was developed by the HBM working group of the Europe Regional Chapter of the International Society of Exposure Science (ISES Europe) and is supported by the HBM Global Network. FAIREHR is designed to harmonize HBM metadata and support the implementation of the FAIR (Findable, Accessible, Interoperable and Reusable) Guiding Principles throughout HBM studies or programs. The registry enables preregistration of HBM by capturing key metadata on study design, metadata management, and planned methods before participant recruitment. This process enhances transparency and reproducibility in environmental and occupational health research. FAIREHR includes both study-level and program-level metadata. Its harmonized metadata template facilitates the storage of results (measurement data) in repositories such as IPCHEM and PEH. Here we outline the unique features of the FAIREHR platform, emphasizing its role in increasing research visibility, improving metadata comparability and harmonization, and strengthening the exchange of information. By supporting the effective use of HBM data, FAIREHR is expected to yield significant benefits for researchers, policymakers, and the broader fields of environmental and occupational health.
Keywords
INTRODUCTION
The growing complexity of environmental and occupational health issues requires a comprehensive approach to effectively manage, utilize, compare, and interpret research data. Human biomonitoring (HBM) plays a crucial role in exposure science, environmental epidemiology, and occupational health by providing integrative information on internal chemical exposure[1]. However, the integration and reuse of HBM data are often hampered by inconsistencies in metadata collection and reporting standards[2]. The FAIREHR (Findable, Accessible, Interoperable, Reusable Environmental and Health Registry) platform, developed by the Europe Regional Chapter of the International Society of Exposure Science (ISES Europe) HBM working group and supported by HBM Global Network, aims to address these challenges by improving the FAIRification of HBM data. Research data encompass the information, observations, or measurements collected or generated during a study, forming the basis for analysis, interpretation, and scientific conclusions.
FAIREHR is among the first protocol registries to apply FAIR principles in environmental and occupational health research. It ensures that valuable metadata on chemical exposures, environmental impacts, and health outcomes are systematically organized and made readily available to researchers, healthcare professionals, and policymakers[3].
Mission and vision of the FAIREHR platform
FAIREHR is an independent, open platform dedicated to advancing environmental and occupational health research through prospective harmonization. It fosters a collaborative environment for improved data management, transparency, and accessibility. Rather than focusing solely on presenting final results, FAIREHR emphasizes enhancing the quality and comparability of HBM study or program from the outset.
Researchers worldwide can register their HBM protocols on the FAIREHR platform, which supports the research community from project conception to completion. This ensures the creation of reusable, high-quality metadata and provides visibility throughout the research lifecycle. The platform hosts study and program metadata and links to final results. Its harmonized metadata template/schema enables compatibility with several existing tools or platforms available for data exchange and calculation using HBM data, such as Personal Exposure and Health (PEH) Data Platform (https://hbm.vito.be/peh-data-platform) and Information Platform for Chemical Monitoring (IPCHEM - https://ipchem.jrc.ec.europa.eu/), tand the Monte Carlo Risk Assessment (MCRA) platform (https://www.rivm.nl/en/food-safety/chemicals-in-food/monte-carlo-risk-assessment-mcra). By consolidating metadata on chemical exposure in a secure and FAIR-compliant central platform, FAIREHR enables policymakers and stakeholders to identify relevant research more efficiently.
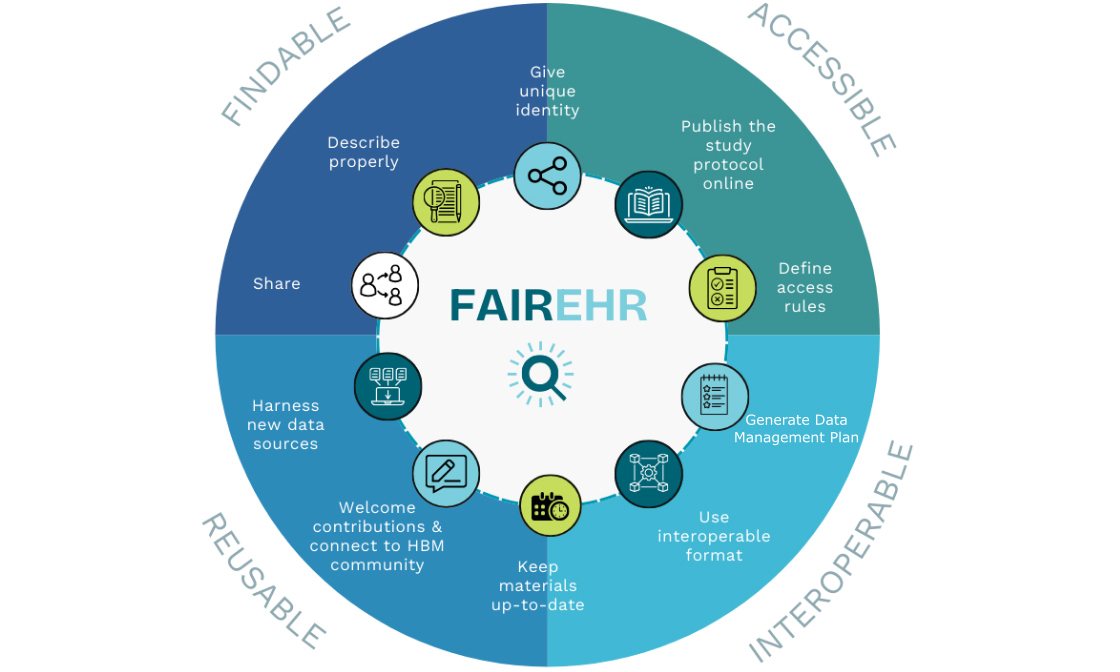
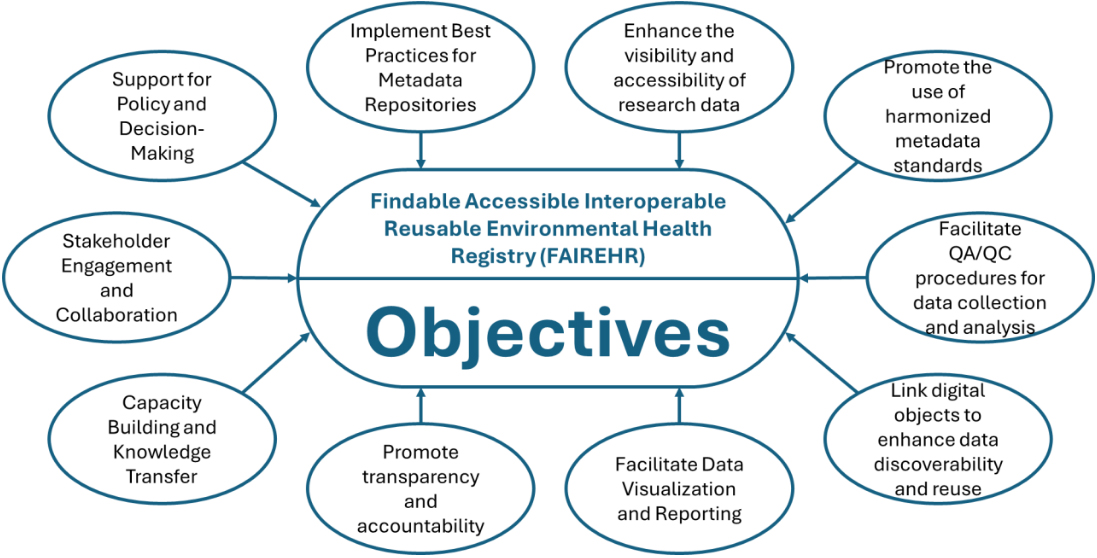
By providing a unified view of global research records, the FAIREHR platform strengthens information exchange in environmental and occupational health, improves machine discoverability, and facilitates data reuse. Serving as a bridge between science, policy, industry, and other stakeholders, FAIREHR fosters innovative initiatives to address emerging monitoring challenges. Ultimately, it empowers informed decision making by improving the quality of exposure data used in chemical risk assessment. Figure 1 summarizes the objectives of the FAIREHR platform.
Overview of the FAIREHR platform
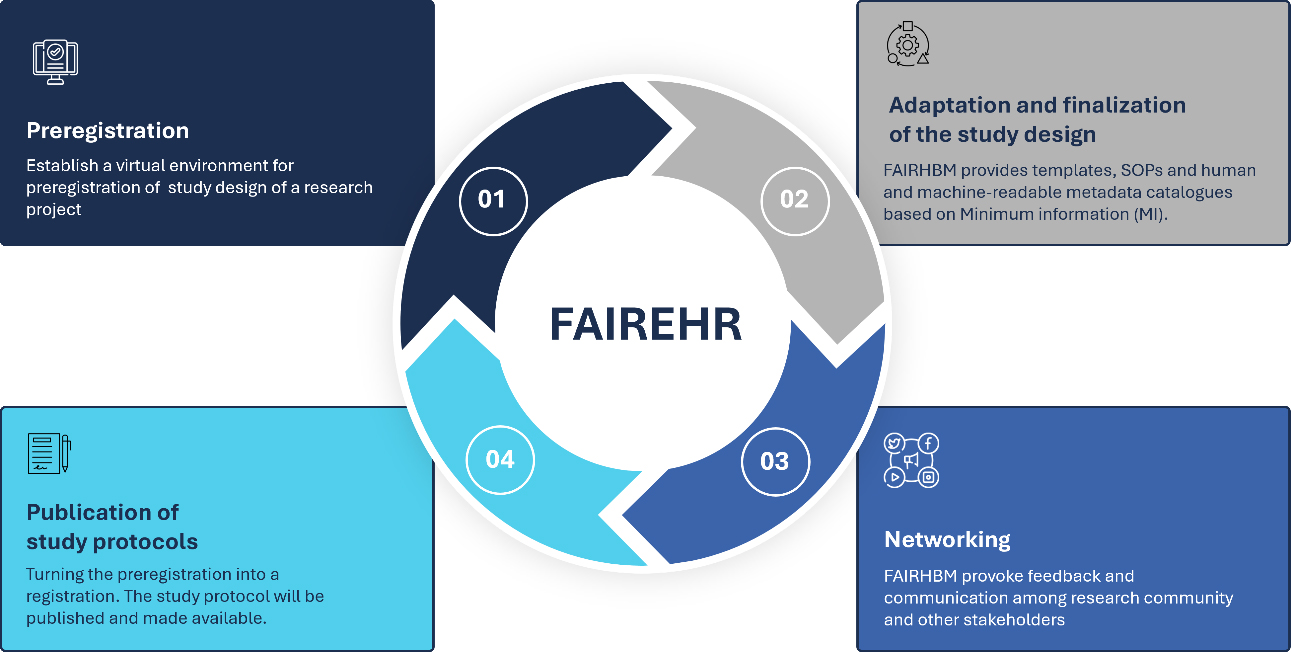
At its core, the FAIREHR platform enables researchers to preregister their studies by detailing key aspects of their research, including study design, (meta)data management strategies, and planned methodological approaches [Figure 2]. The registration questions are crafted and implemented based on the Minimum Information Requirements for HBM (MIR-HBM), which was created in collaboration with global stakeholders[4]. The MIR-HBM defines a set of criteria/parameters to guide the conceptualization, description, planning, and interpretation of HBM studies for exposure assessment. These criteria include (but are not limited to) population characteristics, sample and data collection procedures, potential exposure covariates, quality control measures, and data analysis strategies. Preregistration supports more consistent study design, enhances comparability across studies, reduces duplication of efforts, and facilitates the coordination of research (e.g., when similar studies are conducted across countries or groups). It also saves resources, builds trust in HBM studies and programs, combats publication bias, and fosters collaboration among researchers. Furthermore, the use of a harmonized metadata schema enhances the comparability and reusability of HBM data, thereby ensuring transparency and reproducibility in environmental and occupational health research. The platform will provide automated data quality assessment. Once registration is finalized, study protocols can be published through the platform after peer review, which increases visibility, promotes peer engagement, and supports reproducibility by documenting planned methods before results are available.
Figure 2. Overview of the FAIREHR platform. FAIREHR: Findable, Accessible, Interoperable, Reusable Environmental and Health Registry.
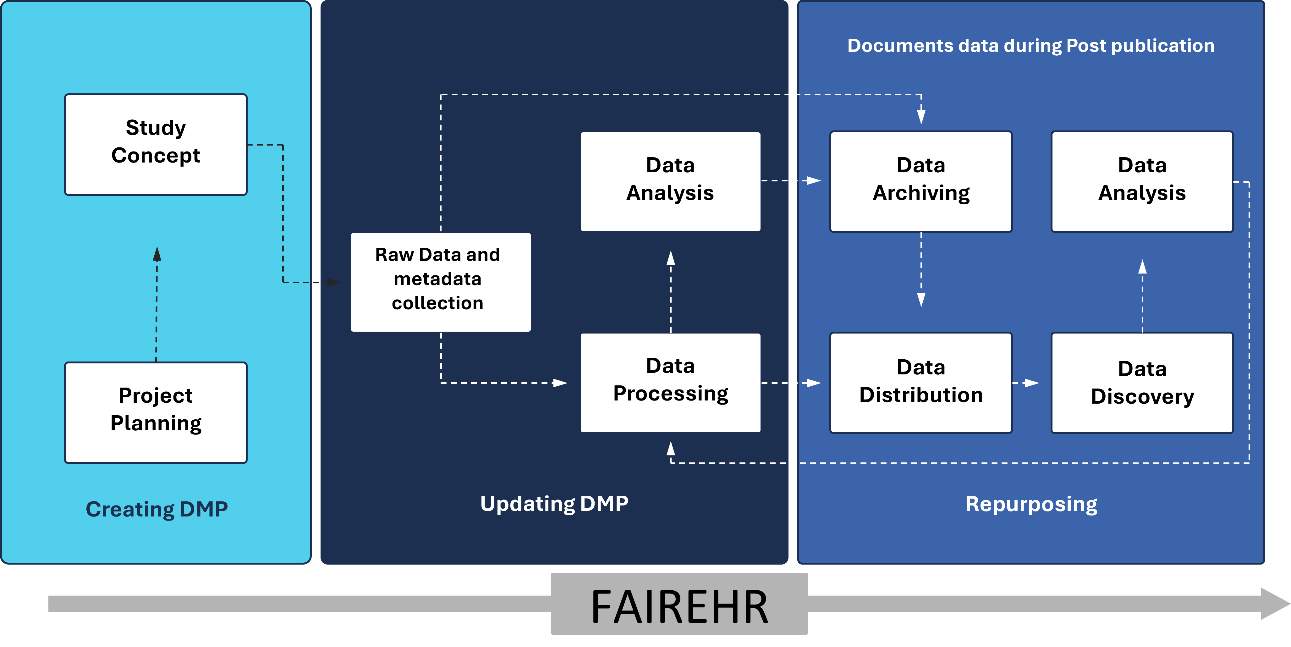
The FAIREHR platform also promotes the use of a Data Management Plan (DMP), as responsible research data management is a key component of good research practice that underpins scientific integrity and reproducibility[5]. The FAIREHR DMP [Figure 3] provides a comprehensive framework for managing metadata and data throughout the research lifecycle, starting from the study concept and project planning stages. It includes strategies for metadata archiving, ensuring long-term storage and accessibility, and making metadata available to relevant stakeholders. While FAIREHR serves as a repository for study and program metadata, it also provides links to research results. Additionally, the platform emphasizes the importance of metadata discovery, which facilitates efficient retrieval and reuse of research data in future projects. Finally, the DMP addresses the repurposing of research data and the maintenance of documentation after publication, thereby ensuring transparency and reproducibility. This structured approach ensures effective data management and enhances their utility for ongoing and future research endeavors.
Figure 3. Data and metadata management in human biomonitoring within the FAIREHR platform. FAIREHR: Findable, Accessible, Interoperable, Reusable Environmental and Health Registry; DMP: Data Management Plan.
Metadata refers to information about the data itself, such as how it was collected, processed, and analyzed, which is crucial for interpreting raw data. The HBM metadata schema (templates used to generate metadata records) implemented in the FAIREHR platform addresses a major challenge in environmental health research: the lack of standardized and comprehensive metadata collection and reporting. Insufficient standards often result in datasets with missing metadata, incomplete annotations, or inconsistent variables, all of which hinder the broader repurposing of research data. The metadata schema in FAIREHR captures elements related to: (1) quality assurance and control (QA/QC), including laboratory external QA and internal QC statements, as well as extended method validation; (2) sample integrity protocols; and (3) plans for inter-laboratory comparisons, supporting external QA through robust schemes such as G-EQUAS (https://app.g-equas.de/web/). Future updates will further enhance QA/QC tracking by aligning metadata fields with international standards. By following the guidance offered in the FAIREHR platform, researchers can generate harmonized data in formats that allow research findings to be more easily compared, integrated, and reused across disciplines. Ultimately, this enables HBM data to support more robust exposure assessments, leading to more accurate evaluations of human exposure to environmental or occupational pollutants.
Metadata security considerations
The FAIREHR platform ensures strong metadata security and integrity through Cyber Essentials Plus accreditation and the use of secure cloud-based storage solutions. All metadata transfers are encrypted with SSL at every stage. An efficient data indexing system enables rapid access to large datasets. The platform uses NET Core, providing high performance, scalability, and a secure operating environment. The platform’s servers are designed for high availability and secure operation, incorporating redundancy measures such as failover and load balancing. Regular cloud-based backups further safeguard data. The FAIREHR platform is fully compliant with the General Data Protection Regulation (GDPR), upholding the highest standards of privacy and data protection. All metadata associated with each dataset will be made openly accessible in the FAIREHR data repository under a standard open license (e.g., CC BY 4.0), ensuring discoverability and reusability in line with the FAIR principles. Metadata will describe the dataset, including methodology, variables, licensing, and access conditions, but will not contain any personally identifiable information, thereby maintaining GDPR compliance.
Future developments of the FAIREHR platform
The future development strategy of the FAIREHR platform revolves around enhancing its functionality, ensuring continued compliance with FAIR principles, upholding legal and ethical standards, and fostering wider adoption across the global research community.
The platform will include an automated system for harmonized chemical identification in research, enabling registrants to search for chemicals by CAS number or other identifiers. Users will be able to select chemicals and automatically retrieve related identifiers such as SMILE (Simplified Molecular Input Line Entry System), InChI (IUPAC International Chemical Identifier)[6,7], and key physicochemical properties. InChI, by enhancing the discoverability of chemical structures, directly supports the FAIR principles. The system will also link parent compounds to their metabolites, providing researchers with available metabolite lists and identifying the most suitable matrices for monitoring these compounds in the human body. By leveraging this framework, the platform will ensure that chemicals and materials used in environmental and health studies are consistently identified, classified, and connected to authoritative databases. This integration will strengthen data interoperability and accuracy, facilitating more efficient data sharing and analysis across different research initiatives. Incorporating resources such as the Norman Network database[8], which provides comprehensive information on environmental pollutants, will further align the FAIREHR platform with international standards, supporting cross-study comparisons and enabling better integration of data from various sources.
The platform will also assign persistent identifiers (e.g., DOIs) to (meta)datasets, ensure metadata compliance with DataCite standards, and enhance searchability by indexing metadata both internally and in external repositories. Data accessibility will be supported through open protocols such as REST APIs (interfaces that enable secure information exchange between computer systems over the internet), while long-term metadata availability will be safeguarded. For interoperability, the FAIREHR platform will adopt open standards and integrate established ontologies such as Chemical Entities of Biological Interest (ChEBI - https://www.ebi.ac.uk/chebi/beta/), Ontology for Biomedical Investigations (OBI - https://obi-ontology.org), and Exposure Ontology (ExO)[9]. Ontologies provide structured vocabularies with hierarchical and qualified relationships (object properties), which are essential for building a FAIR metadata schema[5]. For annotating biological concepts, phenotypic traits, and experimental variables, the platform will implement I-ADOPT ((https://i-adopt.github.io/)), a Research Data Alliance (RAD) recommendation. Additionally, FAIREHR will employ web standards such as the Resource Description Framework (RDF)[10] to link and process metadata in machine-readable formats. The platform will also provide detailed provenance tracking and transparent data usage licenses, while exploring tools such as FAIR Data Point[11] for machine-readable metadata and or RO-Crate[12] for bundling datasets with rich metadata and provenance. Together, these measures will enhance the discoverability, usability, and reproducibility of environmental and health data, ensuring long-term sustainability and broad accessibility for future research. Relevant metabolomics and exposure-related databases will also be mapped and linked to the registry.
The FAIREHR platform will further introduce open metrics and cataloging use cases, providing transparent, measurable, and practical demonstrations of its value and applicability. A help desk will also be integrated to assist users with technical issues and other inquiries.
As the platform evolves, the integration of advanced analytical tools such as artificial intelligence (AI) and machine learning is becoming increasingly important. These tools can analyze large and complex datasets, uncover hidden patterns, detect anomalies in biomarker data, impute missing values, and generate knowledge graphs and insights not easily derived from traditional methods.
To support users, FAIREHR will include a dedicated training component to build competencies in HBM data management and reuse. Through structured modules and hands-on exercises, the training will cover topics such as FAIR principles, ethical and legal considerations, metadata standards, and interoperability of environmental and health datasets. This program is designed to benefit researchers, public health professionals, and data stewards, particularly in low- and middle-income countries (LMICs), by promoting best practices for generating, curating, harmonizing, and responsibly sharing HBM data. In doing so, it will foster data quality, transparency, and global collaboration.
To facilitate adoption in LMICs, active engagement of research teams in these regions will be critical. Their participation will guide the co-development and contextualization of platform functionalities to align with local priorities, regulatory environments, and research infrastructures. In the medium term, LMIC partners will help refine metadata standards, governance frameworks, and ethical protocols tailored to diverse sociocultural and institutional contexts. Over the long term, they will serve as regional nodes for capacity building, data stewardship, and collaborative research. This distributed model will promote the widespread adoption of FAIR data principles and ensure equitable access to FAIREHR.
To further strengthen harmonization, future versions of FAIREHR will provide references to validated analytical methods (e.g., GC-MS/MS, LC-MS/MS) for measuring environmental pollutants in biological matrices such as blood, urine, serum, and tissues, based on existing international guidelines. The platform will also offer access to chemical-specific Biomonitoring and Surveillance of Chemical Exposure in Occupational Settings (BASIC) Guides (https://www.fairehr.com/BASICGuides). These guides provide structured recommendations for conducting biomonitoring programs for various chemicals, including the identification of relevant biomarkers, procedures for collecting, handling, and analyzing biological samples, and guidance on results communication.
As part of its launch phase, expected in September 2025, FAIREHR will support pilot registrations from multiple academic and governmental HBM studies and programs. Preregistration of environmental and health research studies on the platform will enable scientists, regulators, policymakers (at EU, national, and regional levels), life sciences companies, occupational hygienists, publishers, journals, professional associations, Contract Research Organizations, meta-researchers, and funding bodies to track and identify planned, ongoing, and completed studies. Ultimately, the FAIREHR platform will make high-quality open metadata widely available, supporting more comparable and interpretable studies that can inform environmental and occupational health policies and regulatory decisions.
DECLARATIONS
Acknowledgments
The authors thank all members of the ISES Europe HBM Working Group and the HBM Global Network for their contributions and support in developing FAIREHR.
Authors’ contributions
Made substantial contributions to the conceptualization and design of the FAIREHR platform: All authors
Lead the drafting of this short communication: Galea KS, Zare Jeddi M, Rashid S
Made substantial contributions to the development and technical implementation of the online FAIREHR platform: Rashid S, Brooker F, Zare Jeddi M
Commented on drafts of this short communication, reviewed and agreed with the contents of the final manuscript: All authors.
Availability of data and materials
The FAIREHR platform can be accessed by the following link: https://fairehr.com/.
Financial support and sponsorship
The development of the online FAIREHR platform was supported by ECETOC (European Centre for Ecotoxicology and Toxicology of Chemicals).
Conflicts of interest
IOM (Karen S. Galea, Finlay Brooker, Shahzad Rashid) received funding from ECETOC to develop the FAIREHR online platform. Stuart Harrad is Editor-in-Chief of Journal of Environmental Exposure Assessment. Maryam Zare Jeddi is Associate Editor of the journal and Guest Editor for this Special Issue. Yu Ait Bamai, Radu-Corneliu Duca, and Yankai Xia are Editorial Board members of the journal. Konstantinos M. Kasiotis is Guest Editor for this Special Issue. None of the above were involved in any steps of the editorial processing of this manuscript, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Zare, Jeddi. M.; Hopf, N. B.; Louro, H.; et al. Developing human biomonitoring as a 21st century toolbox within the European exposure science strategy 2020-2030. Environ. Int. 2022, 168, 107476.
2. Zare, Jeddi. M.; Virgolino, A.; Fantke, P.; et al. A human biomonitoring (HBM) Global Registry Framework: Further advancement of HBM research following the FAIR principles. Int. J. Hyg. Environ. Health. 2021, 238, 113826.
3. Zare, Jeddi. M.; Galea, K. S.; Viegas, S.; et al. FAIR environmental and health registry (FAIREHR)- supporting the science to policy interface and life science research, development and innovation. Front. Toxicol. 2023, 5, 1116707.
4. Zare, Jeddi. M.; Galea, K. S.; Ashley-Martin, J.; et al. Guidance on minimum information requirements (MIR) from designing to reporting human biomonitoring (HBM). Environ. Int. 2025, 202, 109601.
5. Cunha-Oliveira, T.; Ioannidis, J. P. A.; Oliveira, P. J. Best practices for data management and sharing in experimental biomedical research. Physiol. Rev. 2024, 104, 1387-408.
6. Herres-Pawlis, S.; Blanke, G.; Brammer, J.; et al. Making the InChI FAIR and sustainable by moving to open-source on GitHub. Version: 2. ChemRxiv [Preprint] 2024. [16 P].
7. Glüge, J.; Mcneill, K.; Scheringer, M. Getting the SMILES right: identifying inconsistent chemical identities in the ECHA database, PubChem and the CompTox Chemicals Dashboard. Environ. Sci:. Adv. 2023, 2, 612-21.
8. Mohammed Taha, H.; Aalizadeh, R.; Alygizakis, N.; et al. The NORMAN Suspect List Exchange (NORMAN-SLE): facilitating European and worldwide collaboration on suspect screening in high resolution mass spectrometry. Environ. Sci. Eur. 2022, 34, 104.
9. Mattingly, C. J.; McKone, T. E.; Callahan, M. A.; Blake, J. A.; Hubal, E. A. Providing the missing link: the exposure science ontology ExO. Environ. Sci. Technol. 2012, 46, 3046-53.
10. W3C. World Wide Web Consortium. Resource description framework (RDF): Concepts and abstract syntax. Available from: https://www.w3.org/TR/rdf-concepts/ 2014 (accessed on 2025-8-25).
11. Silva Santos LOB, Burger K, Kaliyaperumal R, Wilkinson MD. FAIR Data Point: A FAIR-oriented approach for metadata publication. Data. Intell. 2023, 5, 163-83.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].