Urinary concentrations of bisphenol analogues among school-aged children in South China: exposure characterization and risk assessment
Abstract
Human exposure to bisphenol analogues (BPs) and their health risks have raised increasing concern due to their potential toxicity. However, limited data are available on exposure levels and associated risks in school-aged children. In this study, we quantified 26 BPs in urine samples from 184 school-aged children living in representative rural and urban areas of South China, using a high-throughput, sensitive analytical method. The aim was to assess the co-exposure levels and potential non-carcinogenic risks. The detection frequency (DF) of the total concentration of 26 BPs (∑BPs) was 100.0%. Concentrations of ∑BPs ranged from 0.089 to 45.33 μg/L, with a median of 2.14 μg/L. The predominant compounds were bisphenol A (BPA, median 0.803 μg/L, DF 97.8%), 2,4’-bisphenol S (2,4-BPS, 0.068 μg/L, DF 93.5%), 4,4’-dihydroxytetraphenyl methane (BPBP, 0.041 μg/L, DF 66.3%), and bisphenol F (BPF, 0.033 μg/L, DF 61.4%), accounting for 77.2%, 6.5%, 4.0%, and 3.2% of ∑BPs, respectively. Both the concentration and DF of 2,4-BPS were much higher than those of bisphenol S (BPS, <0.003 μg/L, DF 48.9%), suggesting that 2,4-BPS was the predominant derivative. BPA concentrations were positively associated with family income levels, while 2,4-BPS concentrations were linked to plastic-bottled water consumption. BPF concentrations were associated with both instant noodle consumption frequency and maternal education level. The median estimated daily intakes of BPA, 2,4-BPS, and BPF were 0.035, 0.003, and 0.002 μg/kg-bw/day, respectively. Although non-carcinogenic risk assessments indicated minimal risk, revised tolerable daily intake values suggested potential health concerns for school-aged children. These findings highlight the need for continued monitoring of BPs in susceptible populations, and further research should focus on cumulative risks and exposure sources of BPs.
Keywords
INTRODUCTION
Bisphenol A (BPA), a key additive in the production of polycarbonate plastics and epoxy resins, is widely used in consumer products such as plastic toys, medical devices, electronic gadgets, and thermal paper receipts[1]. BPA is known to exert cardiovascular, reproductive, neurotoxic, and developmental toxicity, and it can also interfere with endocrine signalling[2-5]. Thus, many countries, including the United States, Canada, and France, have banned the use of BPA in polycarbonate containers and food packaging[6,7]. Following these restrictions, various BPA substitutes - such as bisphenol F (BPF), bisphenol S (BPS), bisphenol B (BPB), bisphenol AP (BPAP), bisphenol AF (BPAF), bisphenol Z (BPZ), 2,4’-bisphenol S (2,4-BPS), 4-hydroxy-4’-isopropoxydiphenylsulfone (BPS-IP), 4-allyloxy-4’-hydroxydiphenyl sulfone (BPS-MAE), and 4-benzyloxy 4'-hydroxydiphenyl sulfone (BPS-MPE) - have been increasingly introduced[2,8-10]. However, these alternatives are not necessarily safer, and their potential health risks and exposure pathways remain a major concern.
Despite being developed as replacements for BPA, many substitutes exhibit toxicological properties comparable to, or even exceeding, those of BPA[11,12]. For example, BPS and BPF show similar endocrine-disrupting activities in both in vitro and in vivo studies[3,13]. As derivatives of BPS, BPS-IP, BPS-MAE, and BPS-MPE may induce similar toxicological effects, and in some cases, more pronounced adverse outcomes than BPS itself[10]. Nevertheless, research on co-exposure levels, particularly for these emerging derivatives, and on the risk characterization of BPs in human biological matrices such as milk, amniotic fluid, urine, and serum remains limited.
Previous studies have detected BPs in various human matrices[14,15]. In humans, these compounds undergo rapid biotransformation, with renal excretion serving as the primary elimination pathway. Urine is therefore considered the most suitable biomonitoring matrix for population-level exposure assessment, as it is non-invasive and reflects cumulative exposure from all potential routes[16,17]. Epidemiological evidence further reveals a clear exposure gradient between rural and urban populations, with higher body burdens typically observed in urban residents[18]. This difference may be attributed to variations in industrial activity, lifestyle patterns, and environmental contamination. Additional factors such as the use of plastic food packaging, canned food consumption[1,19,20], dietary intake, drinking water, pharmaceuticals[21], thermal paper contact[2], and other lifestyle behaviors may also contribute to elevated urinary BPs. Comprehensive characterization of exposure sources serves as the scientific basis for designing targeted pollution control strategies.
Children are particularly vulnerable to environmental pollutants because of their developing physiological systems and unique exposure pathways. Growing evidence suggests that emerging BPA substitutes are associated with an increased risk of developmental impairments in children. Prenatal exposure to BPA and BPS has been linked to adverse birth outcomes[22,23], while BPS-IP has been associated with abnormal developmental effects[24]. Prenatal exposure to bis(3-allyl-4-hydroxyphenyl) sulfone (TGSH) has also been associated with preterm birth and reduced infant height[23]. However, data on the occurrence and health risks of emerging BPA substitutes in school-aged children remain scarce.
To address these knowledge gaps, this study simultaneously analyzed 26 BPs in urine samples collected from school-aged children living in representative rural and urban areas of South China. Estimated daily intake (EDI) values and corresponding health risks were assessed. The aim of this study was to evaluate BP exposure and related health risks in school-aged children, and to investigate the influence of demographic characteristics and lifestyle factors on exposure levels.
MATERIALS AND METHODS
Reagents and materials
A total of 26 BPs and their corresponding isotope-labeled internal standards [Supplementary Table 1] were purchased from TRC (Toronto, Canada), Cato Research Chemicals (Eugene, USA), AccuStandard Inc. (New Haven, USA), and Dr. Ehrenstorfer (Augsburg, Germany). MS-grade solvents (methanol, ethyl acetate, glacial acetic acid, and ammonium acetate) were obtained from Merck (Darmstadt, Germany). β-glucuronidase/sulfatase was obtained from CNW Technologies (Anpel, China). Distilled water (Watsons, China) was used to prepare the mobile phase and for laboratory procedures.
Study design and urine collection
Two representative sites in a certain city of South China, were selected: a rural agricultural region and a densely populated urban area. The sites are approximately 52 km apart, and residents of the two areas differ in lifestyle habits and socioeconomic status. Elementary school students aged 9-10 years were recruited in 2024, yielding 184 participants (107 males and 77 females). Informed consent was obtained from their guardians prior to enrollment. First morning urine samples (40 mL) were self-collected using urine containers, aliquoted, and stored at -80 °C until analysis. Height and body weight were measured, and participants completed a questionnaire (covering basic information, demographic characteristics, and lifestyle habits). The study was approved by the Ethics and Human Subject Committee of Guangdong Provincial Center for Disease Control and Prevention (W96-027E-202419).
Sample pretreatment protocols
Urine samples (1.0 mL) were transferred into 15 mL polypropylene (PP) tubes, followed by the addition of 20 μL internal standard solution (50 ng/mL), 20 μL of β-glucuronidase/sulfatase enzyme, and 1.0 mL acetic acid-ammonium acetate buffer (1 mol/L, pH 5.0). The mixture was incubated at 37 °C overnight. Subsequently, 3.0 mL ethyl acetate was added, vigorously shaken for 20 min, and centrifuged at 4,700 rpm for 5 min. The supernatant was transferred to another 15 mL PP tube. A second extraction was performed with an additional 3 mL of ethyl acetate. The combined extracts were concentrated and reconstituted in 0.20 mL of 50% (v/v) methanol-water for instrumental analysis.
Instrumental analysis
Target compounds were identified and quantified using UPLC-MS/MS (Agilent 1290 UPLC, Sciex Triple Quad 6500+, USA). Chromatographic separation was performed on a Waters Acquity UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) maintained at 35 °C. Separation was achieved with a gradient of mobile phase A (0.5 mm ammonium acetate in water) and mobile phase B (methanol) at a constant flow rate of 0.35 mL/min. The gradient program was as follows: 0-0.5 min, 85% A; 0.5-10.0 min, decreasing from 85% to 5% A; 10.0-11.5 min, increasing from 5% to 85% A; held for 1.5 min. The total run time was 13 min, with an injection volume of 5.0 μL. Quantification was performed using scheduled multiple reaction monitoring (sMRM) in negative ion electrospray (ESI-) mode. The source temperature was 550 °C, with an ionization voltage of -4,500 V. Nebulizer gas (GS1) and auxiliary heating gas (GS2) pressures were both set at 55 psi. Mass spectroscopic parameters are shown inSupplementary Table 1.
Quality control
Procedure blanks and laboratory blanks were included during sampling and analysis. Method reliability was verified by assessing matrix-spike recoveries of individual analytes. Blank urine samples were spiked with standards (0.10, 1.0, and 5.0 ng/mL) and internal standards (1.0 ng/mL each). For each batch, a procedural blank, a matrix spiked sample (1.0 ng/mL), and a randomly selected duplicate sample were analyzed. Average blank concentrations were subtracted from ample measurements. Mean recoveries ranged from 61.6% to 117.5%, with relative standard deviations ≤ 11.0% [Supplementary Table 2]. Calibration curves showed excellent linearity over 0.020-20 μg/L, with correlation coefficients ≥ 0.9991.
The method detection limit (MDL) was defined as the lowest concentration distinguishable from the method blank with 99% confidence[25]. The method quantitation limit (MQL) was set at three times the MDL. MDLs ranged from 0.002 to 0.023 ng/mL, and MQLs from 0.005 to 0.070 ng/mL [Supplementary Table 1]. Results below the MDL were reported as not detected (ND).
Health risk assessment
EDI (μg/kg-bw/day) was calculated using the following equation[26,27]:
where C (μg/L) is the urinary concentration; R (L/day) is daily urine volume; f is the urinary excretion ratio (dimensionless); and BW (kg) is body weight.
Non-carcinogenic risks were assessed using the hazard quotient (HQ) and hazard index (HI)[26,27]:
where HQ (dimensionless) represents the risk from an individual chemical; HI (dimensionless) represents cumulative risk from multiple pollutants; and reference dose (RfD)(μg/kg-bw/day) is the reference dose.
R values were set at 1.299 L/day for boys and 1.258 L/day for girls aged 9–11 years[28]. The f value for BPs was set at 100%[26,29]. For BPA, the RfD and temporary tolerable daily intake (t-TDI) were 50 μg/kg-bw/day[30] and 4 μg/kg-bw/day[31], respectively. Notably, the recommended TDI was revised downward in 2023 to 0.2
Statistical analysis
IBM SPSS Statistics version 27.0 (IBM, USA) and Origin 2021 (OriginLab, USA) were used for statistical analyses and graph drawing, respectively. Concentrations below the MDL were replaced with MDL/√2[29,33], and those between the MDL and MQL were replaced with MQL/√2 for statistical analysis[28,34]. Normality of BP concentrations was assessed using the Kolmogorov-Smirnov test. Spearman correlation analysis was used to examine correlations among BPs. The Mann-Whitney U test compared exposures between two groups, while the Kruskal-Wallis H test was used for multiple-group comparisons. Multiple linear regression was performed to evaluate associations between urinary concentrations and lifestyle or demographic factors. Statistical significance was set at P < 0.05.
RESULTS AND DISCUSSION
Characteristics of environmental exposure among participants
The demographic characteristics and lifestyle habits of the participants are presented in Supplementary Table 3. All participants were 9-10 years old, with an average age of 9.5 years, and 58.2% were males. The mean body mass index (BMI) was 16.8 kg/m2.
Occurrence of urinary BPs
Urinary concentrations of BPs are shown in Table 1. The detection frequency (DF) of the total concentration of 26 BPs (∑BPs) was 100.0%, indicating widespread exposure among participants. BPA was detected in nearly all urine samples (DF = 97.8%), followed by 2,4-BPS (93.5%), BPM (80.4%), BPT (66.3%), 4,4’-dihydroxytetraphenyl methane (BPBP) (66.3%), BPP (62.5%), and BPF (61.4%). Compounds with DF exceeding 50% were considered the major BPs in this study. In contrast, BPS-MAE, BPB, MCBPA, TCBPA, BPS-MPE, BPTMC, 3,3’-DCBPA, and TBBPA were rarely detected (DF < 5%). Other compounds with DF below 50% were excluded from further statistical analysis.
Urinary concentrations and DF of BPs in school-aged children (N = 184) from South China (μg/L)
| Compounds | Median | GM | Mean ± SD | Range | P95 | DF (%) |
| BPA | 0.803 | 0.887 | 1.96 ± 3.86 | < MDL-43.4 | 6.88 | 97.8 |
| 2,4-BPS | 0.068 | 0.061 | 0.150 ± 0.284 | < MDL-2.27 | 0.469 | 93.5 |
| BPF | 0.033 | 0.039 | 0.559 ± 1.59 | < MDL-13.1 | 3.05 | 61.4 |
| BPBP | 0.041 | 0.022 | 0.059 ± 0.097 | < MDL-0.801 | 0.178 | 66.3 |
| BPM | 0.015 | 0.014 | 0.023 ± 0.058 | < MDL-0.497 | 0.091 | 80.4 |
| BPP | 0.010 | 0.010 | 0.015 ± 0.014 | < MDL-0.080 | 0.046 | 62.5 |
| BPT | 0.005 | 0.004 | 0.011 ± 0.059 | < MDL-0.670 | 0.016 | 66.3 |
| BPAP | 0.003 | 0.029 | 0.288 ± 0.434 | < MDL-1.95 | 1.22 | 46.2 |
| BPE | < MDL | 0.014 | 0.095 ± 0.179 | < MDL-1.02 | 0.567 | 30.4 |
| PHBB | < MDL | 0.008 | 0.018 ± 0.047 | < MDL-0.490 | 0.063 | 49.5 |
| BPS | < MDL | 0.005 | 0.022 ± 0.149 | < MDL-2.00 | 0.053 | 48.9 |
| BPFL | < MDL | 0.005 | 0.025 ± 0.047 | < MDL-0.209 | 0.141 | 27.7 |
| BPS-IP | < MDL | 0.005 | 0.026 ± 0.126 | < MDL-1.07 | 0.042 | 29.9 |
| BPAF | < MDL | 0.003 | 0.008 ± 0.048 | < MDL-0.652 | 0.019 | 47.8 |
| TGSH | < MDL | < MDL | 0.008 ± 0.024 | < MDL-0.283 | 0.024 | 17.9 |
| BPC | < MDL | < MDL | 0.045 ± 0.109 | < MDL-0.719 | 0.191 | 13.6 |
| BPZ | < MDL | < MDL | 0.005 ± 0.012 | < MDL-0.100 | 0.010 | 7.1 |
| Tric-BPA | < MDL | < MDL | 0.008 ± 0.036 | < MDL-0.424 | 0.009 | 5.4 |
| BPS-MAE | < MDL | < MDL | 0.004 ± 0.008 | < MDL-0.090 | 0.003 | 2.7 |
| BPB | < MDL | < MDL | 0.004 ± 0.007 | < MDL-0.071 | 0.003 | 4.9 |
| MCBPA | < MDL | < MDL | 0.004 ± 0.005 | < MDL-0.066 | 0.003 | 1.1 |
| TCBPA | < MDL | < MDL | < MDL | < MDL | < MDL | 0.0 |
| BPS-MPE | < MDL | < MDL | < MDL | < MDL | < MDL | 0.0 |
| BPTMC | < MDL | < MDL | < MDL | < MDL | < MDL | 0.0 |
| 3,3’-DCBPA | < MDL | < MDL | < MDL | < MDL | < MDL | 0.0 |
| TBBPA | < MDL | < MDL | < MDL | < MDL | < MDL | 0.0 |
| ∑2BP | 1.15 | 1.22 | 2.51 ± 4.23 | 0.008-43.8 | 8.79 | 100 |
| ∑BPs | 2.14 | 2.19 | 3.36 ± 4.35 | 0.089-45.3 | 9.52 | 100 |
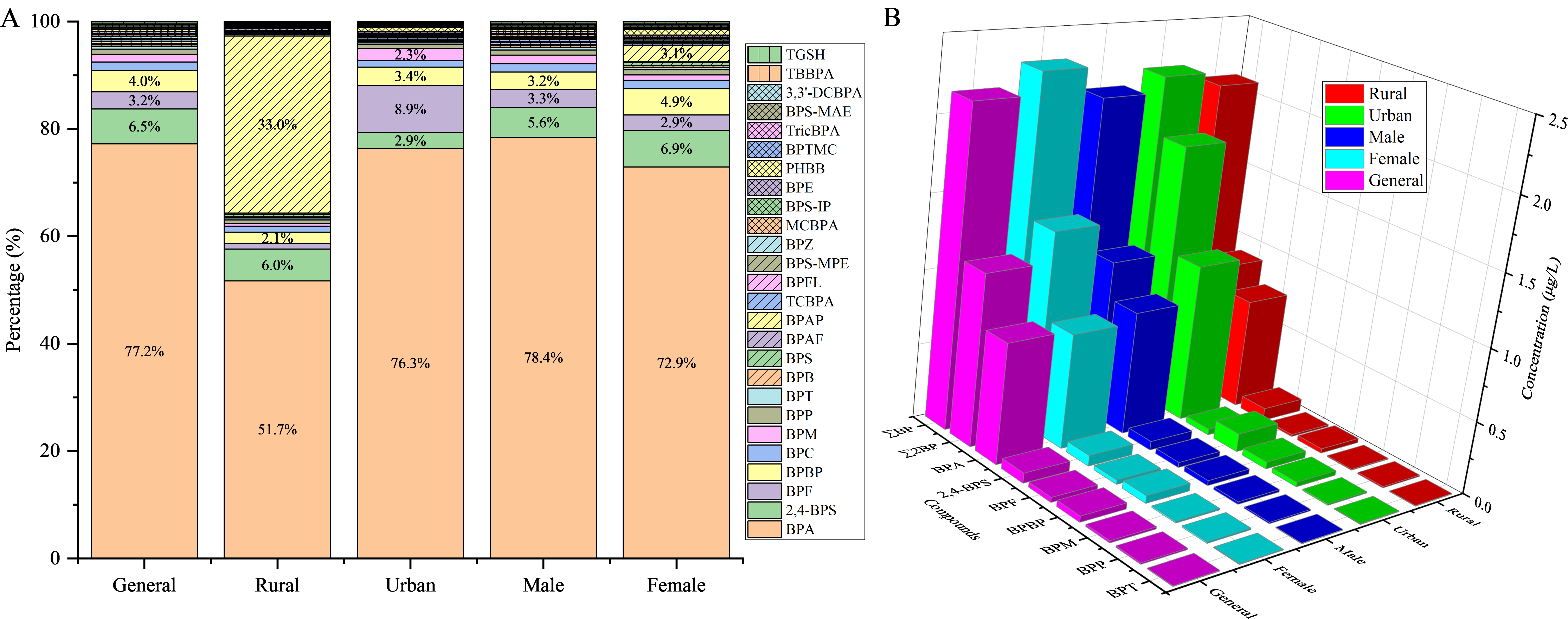
∑BPs ranged from 0.080 to 45.3 μg/L, with a median concentration of 2.14 μg/L. BPA was the predominant compound (median: 0.803 μg/L), followed by 2,4-BPS (0.068 μg/L), BPBP (0.041 μg/L), BPF (0.033 μg/L), BPM (0.015 μg/L), BPP (0.010 μg/L), and BPT (0.005 μg/L). The median concentrations of BPAP, BPE, and BPFL were close to their respective MDLs. However, their 95th percentile values reached 1.22, 0.567, and 0.141 μg/L, respectively, indicating that only a small fraction of participants were exposed to elevated levels.
The median BPA concentration observed in this study (0.803 μg/L) was at a moderate level. It was higher than levels reported in children from Shanghai (0.090 μg/L), Guangzhou (0.480 μg/L), and Hangzhou (0.670 μg/L) in China[33,35,36], as well as in adults from Chengdu (0.686 μg/L), Nantong (0.765 μg/L), Shehong (0.409 μg/L), Guangzhou (0.624 μg/L), Wuxi (0.072 μg/L), and Taishun (0.081 μg/L) in China, 15 regional centers in Japan (0.40 μg/L), and Liège in Belgium (0.79 μg/L)[18,23,28,37-39] [Supplementary Table 4]. However, the median BPA concentration was lower than those reported in children from Jingyang (0.938 μg/L), Jiangsu (1.410 μg/L), Guangzhou (2.430 μg/L), Nanjing (4.870 μg/L) in China, as well as in Prekmufje, Solvenja (1.900 and 1.600 μg/L), Odense, Denmark (0.940 μg/L), and Poland (2.500 μg/L)[1,2,40-44]. It was also lower than levels reported in adults from Chengdu (1.13 μg/L), Qingyuan (3.000 μg/L), Guangzhou (3.880 μg/L), and 26 provincial capitals in China (1.070 μg/L)[26,27,29,45]. In contrast, the median concentration of BPF (0.033 μg/L) was one to two orders of magnitude lower than values reported in previous research, where BPF was often the second most abundant BP after BPA[1,40,43]. Similarly low concentrations were observed for six other BPs (BPS, BPAF, BPAP, BPB, BPP, and BPZ), suggesting potential regional differences in chemical use or environmental fate.
Interestingly, the DF of 2,4-BPS (93.5%) was substantially higher than that of BPS (48.9%). The median 2,4-BPS concentration (0.068 μg/L) was one order of magnitude higher than that of BPS, and its exposure levels were comparable to those of traditional BPA derivatives (BPF, BPBP, and BPM)[23]. In contrast, BPS and its derivatives (BPS-IP, BPS-MAE, and BPS-MPE) were detected in only a few samples. Previous studies have reported 2,4-BPS in the urine of children and workers from five occupational groups, with median levels (0.035-0.184 ng/mL) similar to those of BPS. Meanwhile, urinary levels of BPS-IP, BPS-MAE, and BPS-MPE in pregnant women from South China were much lower than those of BPS[23,46]. These results indicate that 2,4-BPS is the predominant BPS derivative in the study population. Given the increasing use of BPS derivatives in industrial and consumer products[9,10], their widespread detection in human urine raises concerns and warrants further investigation.
Overall, BP exposure levels in this study were moderate compared to those reported in other countries[2,18,23,26-28,43]. Differences across studies are likely influenced by variations in usage patterns and environmental pollution. Additionally, discrepancies may arise from differences in sampling period, sample type, analytical methods, and interindividual metabolic variation[26,47].
Composition profiles of urinary BPs and source implications
The composition profiles of urinary BPs are shown in Figure 1 and Supplementary Figure 1. Among school-aged children, BPA, 2,4-BPS, BPBP, and BPF were identified as the predominant pollutants, accounting for 77.2%, 6.5%, 4.0%, and 3.2% of ∑BPs, respectively. In contrast, the concentrations of BPP, BPM, and BPT were relatively low (ranging from < MDL to 0.0586 μg/L) and collectively contributed less than 2.0% of
Figure 1. Composition profiles (A) and median concentrations of major BPs (B) in urine. BP: Bisphenol analogue; BPA: bisphenol A; BPF: bisphenol F; BPBP: 4,4’-dihydroxytetraphenyl methane; BPS: bisphenol S; BPB: bisphenol B; 2,4-BPS: 2,4’-bisphenol S; BPAP: bisphenol AP; BPAF: bisphenol AF; BPZ: bisphenol Z; BPS-IP: 4-hydroxy-4’-isopropoxydiphenylsulfone; BPS-MAE: 4-allyloxy-4’-hydroxydiphenyl sulfone; BPS-MPE: 4-benzyloxy 4'-hydroxydiphenyl sulfone; ∑2BP: the total concentration of BPA and BPF; ∑BPs: the total concentration of 26 BPs.
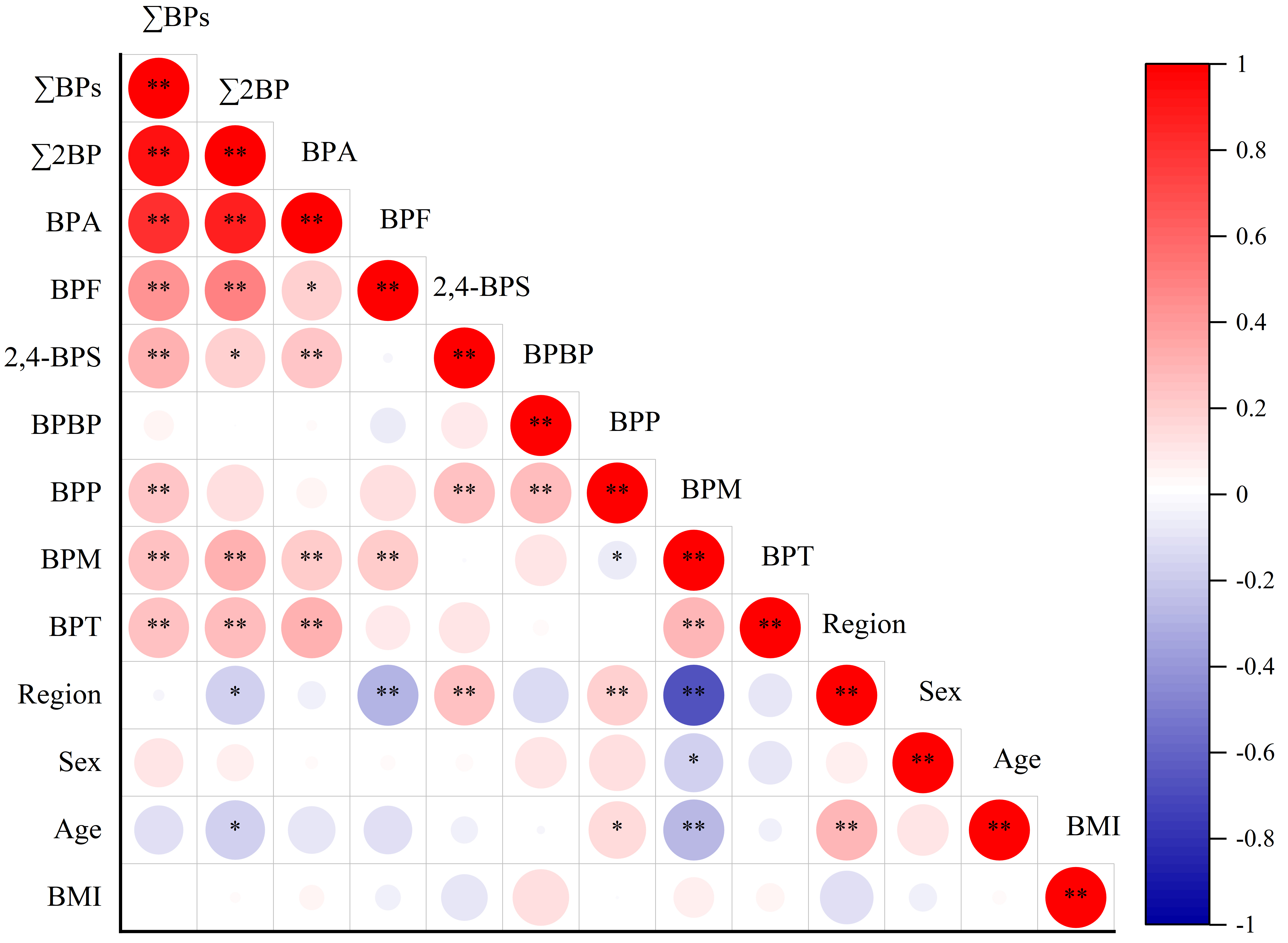
To investigate potential shared exposure sources, Spearman correlation analysis was performed to examine relationships among the urinary concentrations of seven major BPs (BPA, 2,4-BPS, BPM, BPT, BPBP, BPP, and BPF) [Supplementary Table 5 and Figure 2]. Significant correlations were observed among BPA, BPF, BPM, 2,4-BPS, BPT, the combined concentration of BPA and BPF (∑2BP), and ∑BPs (P < 0.05), with correlation coefficients ranging from 0.024 to 0.872. These findings suggest that these compounds may share multiple exposure sources. Previous studies have reported associations between elevated urinary BPA levels and the use of plastic food packaging and canned food consumption[1,19,20]. BPF is widely used in industrial and consumer applications, such as linings, adhesives, plastics, water pipes, dental sealants, oral prosthetic devices, and tissue substitutes[2]. Dietary intake may therefore represent exposure pathways for BPF in humans[21]. Meanwhile, BPM, 2,4-BPS, and BPT are commonly employed as BPA alternatives in consumer products including electronics, food packaging, can coatings, and thermal paper[10,49].
Figure 2. Spearman correlation analysis of region, sex, age, BMI, and urinary BP concentrations. *P < 0.05 (two-tailed); **P < 0.01 (two-tailed). BMI: Body mass index; BP: bisphenol analogue; BPA: bisphenol A; BPF: bisphenol F; BPBP: 4,4’-dihydroxytetraphenyl methane; 2,4-BPS: 2,4’-bisphenol S; ∑2BP: the total concentration of BPA and BPF; ∑BPs: the total concentration of 26 BPs; BPF: bisphenol F; BPS: bisphenol S.
Interestingly, significant correlations were observed between BPS and its derivatives (2,4-BPS and BPS-IP, P < 0.05), likely reflecting their common use in product applications[46]. Similar associations have been reported in previous studies[23,46]. Multiple BPS derivatives have been detected in various matrices, including paper products[50], urine samples from the general population[23,46], and human milk[14]. The widespread detection of BPS and its derivatives, particularly 2,4-BPS and BPS-IP, in school-aged children is concerning, given the currently limited toxicity data for these compounds and the possibility of interactive effects.
Factors influencing urinary BPs
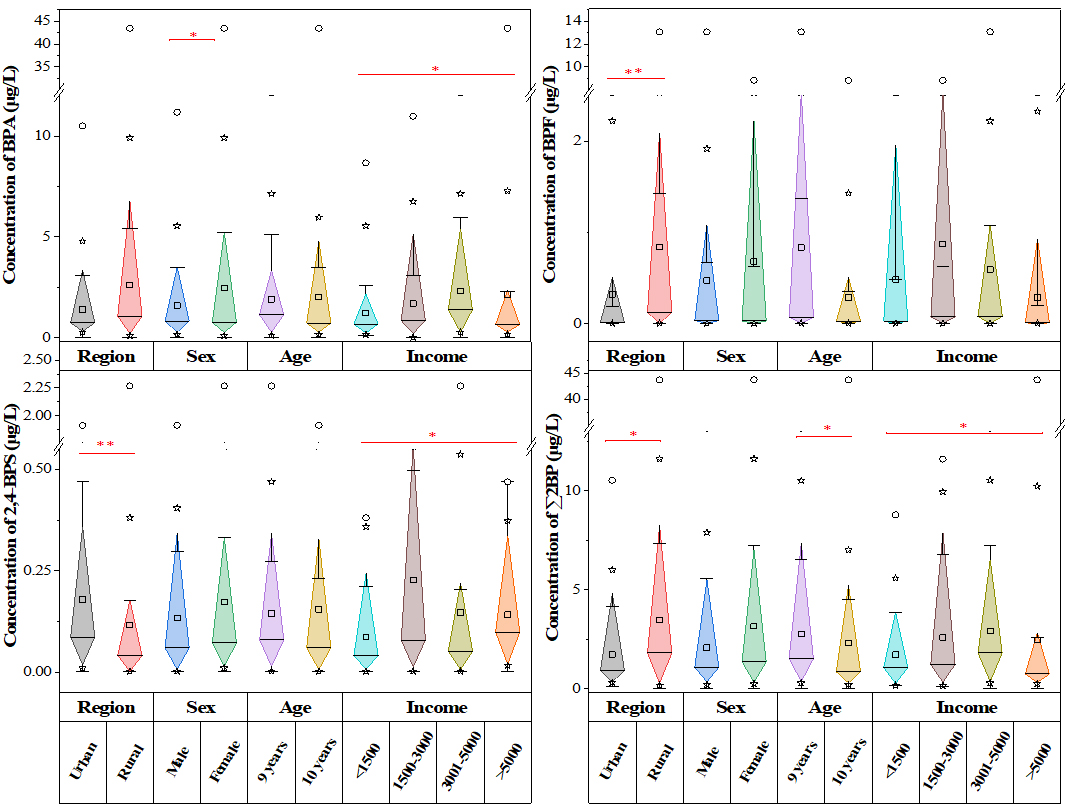
As demonstrated in Figure 3 and Supplementary Table 6, most major BPs with DF > 50% showed statistically significant associations with demographic characteristics and lifestyle habits (P < 0.05). The concentrations of BPA, ∑2BP, and ∑BP were positively associated with family income. This threshold effect aligns with previous findings on income-related exposure disparities, whereby affluent populations may reduce BPA and BPF exposure through conscious consumption choices[28]. The ∑2BP was significantly associated with region, age, and family income. BPA and BPF collectively accounted for 83.7% of the ∑BP burden, underscoring their predominant contribution to overall exposure. The exposure patterns observed in this study corroborate earlier findings, particularly the dominance of BPA and BPF across both rural and urban populations, confirming the pervasiveness of these contaminants[18,51]. Our results also suggested that milk tea consumption was significantly associated with higher BPA levels (P < 0.05) [Supplementary Figure 2], potentially due to BPA migration from polystyrene cup liners under high-temperature conditions. However, BPA concentrations were not significantly associated with other hypothesized exposure pathways (e.g., plastic-bottled water or instant noodles) or with participant characteristics such as region, sex, age, BMI, and maternal education.
Figure 3. Comparison of urinary BP concentrations across demographic characteristics. *P < 0.05 (two-tailed); **P < 0.01 (two-tailed). BP: Bisphenol analogue; BPA: bisphenol A; BPF: bisphenol F; 2,4-BPS: 2,4’-bisphenol S; ∑2BP: the total concentration of BPA and BPF.
Significant spatial disparities in exposure levels were observed between rural and urban school-aged children for BPF, 2,4-BPS, BPP, BPM, and ∑2BP (P < 0.01), whereas no significant difference was found for BPBP and BPT (P > 0.05). As shown in Supplementary Table 7, urinary BPF concentrations were significantly associated with both instant noodle consumption frequency and maternal education level. Similarly, 2,4-BPS levels were significantly associated with plastic-bottled water consumption and family income (P < 0.05). These results suggest that dietary sources, particularly plastic-bottled water, milk tea, and instant noodles, as well as lifestyle behaviors, might collectively contribute to BP exposure.
Human health risk assessment
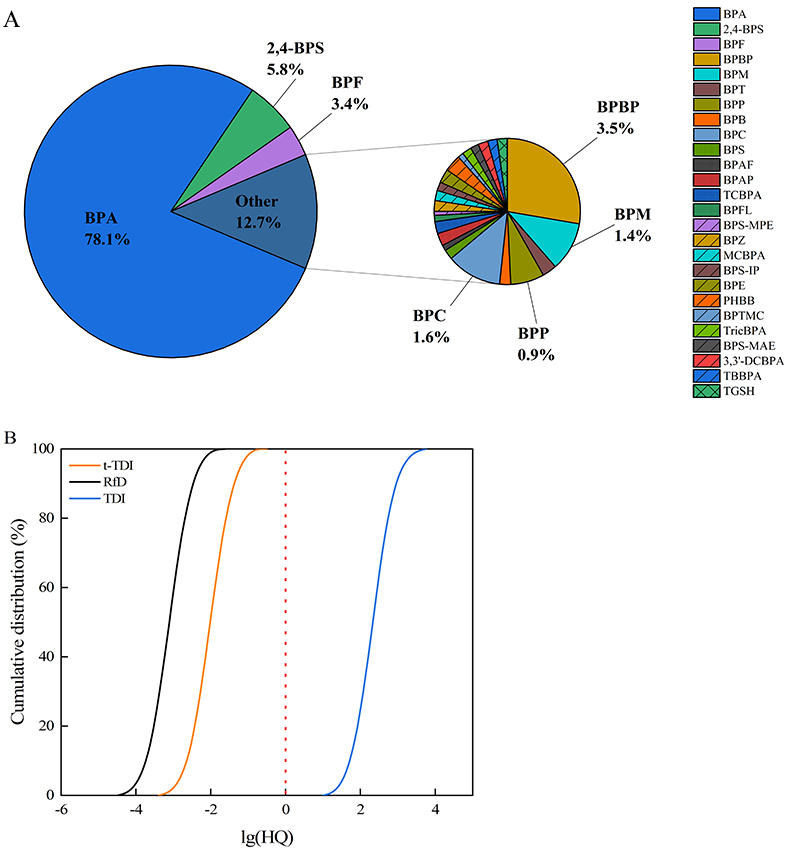
The EDI values of BPs are shown in Table 2. Median EDI values for BPA, 2,4-BPS, and BPF were 0.035, 0.003, and 0.002 μg/kg-bw/day, accounting for 78.1%, 5.8%, and 3.4% of the total BP burden, respectively [Figure 4]. The median BPA EDI value in this study was comparable to that reported for the general population across 26 Chinese cities (0.036 μg/kg-bw/day)[26], but lower than values reported for children from Jingyang (0.0662 μg/kg-bw/day)[42], Nanjing (0.478 μg/kg-bw/day)[2], Guangzhou (0.0538
Figure 4. Contributions of median EDI values of BPs in urine (A) and Monte Carlo simulation of BPA exposure based on RfD, t-TDI, and TDI (B). EDI: Estimated daily intake; BP: bisphenol analogue; TDI: tolerable daily intake; BPA: bisphenol A; BPF: bisphenol F; BPBP: 4,4’-dihydroxytetraphenyl methane; BPS: bisphenol S; BPB: bisphenol B; 2,4-BPS: 2,4’-bisphenol S; BPAP: bisphenol AP; BPAF: bisphenol AF; BPZ: bisphenol Z; BPS-IP: 4-hydroxy-4’-isopropoxydiphenylsulfone; BPS-MAE: 4-allyloxy-4’-hydroxydiphenyl sulfone; BPS-MPE: 4-benzyloxy 4'-hydroxydiphenyl sulfone; ∑2BP: the total concentration of BPA and BPF; ∑BPs: the total concentration of 26 BPs.
EDI of BPs in urine (μg/kg-bw/day)
| GM | Median | Mean ± SD | Range | P25 | P75 | P95 | |
| BPA | 0.035 | 0.035 | 0.080 ± 0.166 | 0-1.93 | 0.017 | 0.083 | 0.298 |
| 2,4-BPS | 0.002 | 0.003 | 0.006 ± 0.013 | 0-0.113 | 0 | 0.007 | 0.022 |
| BPF | 0.002 | 0.002 | 0.024 ± 0.068 | 0-0.563 | 0 | 0.012 | 0.124 |
| BPBP | 0.001 | 0.002 | 0.004 ± 0.019 | 0-0.183 | 0 | 0.003 | 0.007 |
| BPM | 0.001 | 0.001 | 0.001 ± 0.003 | 0-0.032 | 0 | 0.001 | 0.004 |
| BPP | 0 | 0 | 0.001 ± 0.001 | 0-0.003 | 0 | 0.001 | 0.002 |
| BPT | 0 | 0 | 0.001 ± 0.003 | 0-0.034 | 0 | 0 | 0.001 |
| ∑2BP | 0.049 | 0.045 | 0.103 ± 0.182 | 0-1.95 | 0.020 | 0.122 | 0.374 |
| ∑BPs | 0.088 | 0.080 | 0.139 ± 0.188 | 0.003-2.02 | 0.050 | 0.169 | 0.416 |
HQ values were calculated to assess non-carcinogenic risks of BPA exposure [Supplementary Table 8]. Using the RfD of 50 μg/kg-bw/day, HQ values were well below unity (median: 0.0007; 95th percentile: 0.005), suggesting minimal risk. Similar results were obtained with the t-TDI of 4 μg/kg-bw/day (median: 0.008; 95th percentile: 0.062). Monte Carlo simulations confirmed negligible non-carcinogenic risk under both RfD and t-TDI thresholds. However, under the revised 2023 TDI (0.2 ng/kg-bw/day), the median and 95th percentile HQs were 165.0 and 1239.7, respectively. The margin of safety decreased substantially when applying this benchmark [Figure 4].
Strengths and limitations
This study provides new insights into BP exposure levels and potential health risks among school-aged children. Using a novel, high-throughput, and sensitive analytical method, we conducted the first comprehensive assessment of 26 urinary BPs in this population. This dataset enables systematic evaluation of co-exposure patterns to multiple BPs and their associated non-carcinogenic risks. Several limitations should be considered when interpreting our findings. First, due to the short biological half-lives of BPs, urine concentrations primarily reflect short-term exposure. Second, the study did not include a comprehensive assessment of exposure sources, such as dietary intake, in the questionnaires. Third, health risks associated with bisphenols other than BPA were not evaluated, potentially leading to an underestimation of the overall non-carcinogenic risk.
CONCLUSIONS
BPA was the predominant urinary bisphenol in school-aged children, followed by BPF and 2,4-BPS. Overall urinary BP concentrations were lower than those reported in earlier epidemiological studies. BP levels were significantly associated with geographic region, socioeconomic status, and dietary patterns. Although conventional risk assessments suggested safe exposure levels, reevaluation using the updated TDI benchmark revealed potential risks, highlighting the necessity for refined regulatory strategies. These findings underscore the importance of monitoring multiple bisphenols rather than individual compounds in children. Further research is warranted to assess the combined health risks of co-exposure to multiple BPs and to clarify their primary exposure pathways.
DECLARATIONS
Authors’ contributions
Methodology, data analysis, and draft preparation: Zhu, P.
Data analysis and draft preparation: Guo, L. C.
Methodology and data analysis: Su, G.; Deng, J; Cao, J.
Sampling and methodology: Zhong, X.; Lu, L.; Li, M.
Design, review, and editing: Yu, S.; Long, C.; Huang, S.
Availability of data and materials
Supplementary data associated with this article can be found in Supplementary Materials. Further data are available from the corresponding author upon reasonable request.
Financial support and sponsorship
The study was supported by the NHC Key Laboratory of Food Safety Risk Assessment [China National Center for Food Safety Risk Assessment, 202405], Guangdong Provincial Natural Science Foundation [2020A1515010655], Guangdong Provincial Medical Research Foundation [A2024235 and B2023036], and Talent Project of Center for Disease Prevention and Control of Guangdong Province (2024D344).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The epidemiological investigation was endorsed by the Ethics and Human Subject Committee of Guangdong Provincial Center for Disease Control and Prevention (W96-027E-202419). Informed consent was obtained for experimentation with human subjects.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Tkalec, Ž.; Kosjek, T.; Snoj, Tratnik. J.; et al. Exposure of Slovenian children and adolescents to bisphenols, parabens and triclosan: urinary levels, exposure patterns, determinants of exposure and susceptibility. Environ. Int. 2021, 146, 106172.
2. Fan, D.; Liang, M.; Guo, M.; et al. Exposure of preschool-aged children to highly-concerned bisphenol analogues in Nanjing, East China. Ecotoxicol. Environ. Saf. 2022, 234, 113397.
3. Martínez, MÁ.; Salas-Huetos, A.; Fernández, de. la. Puente. M.; et al. Exploring the association between urinary bisphenol A, S, and F levels and semen quality parameters: findings from Led-Fertyl cross-sectional study. Environ. Res. 2024, 263, 120086.
4. Qiu, W.; Chen, B.; Greer, J. B.; et al. Transcriptomic responses of bisphenol s predict involvement of immune function in the cardiotoxicity of early life-stage zebrafish (Danio rerio). Environ. Sci. Technol. 2020, 54, 2869-77.
5. Wang, P. W.; Huang, Y. F.; Wang, C. H.; Fang, L. J.; Chen, M. L. Prenatal to preschool exposure of nonylphenol and bisphenol A exposure and neurodevelopment in young children. Pediatr. Neonatol. 2024, 65, 76-84.
6. T. E. Commission directive 2011/8/EU of 28 January 2011 amending directive 2002/72/EC as regards the restriction of use of bisphenol a in plastic infant feeding bottlesText with EEA relevance. Off. J. Eur. Union. 2011, Union 26, 11-14.
7. Government of Canada Order amending schedule I to the hazardous products act 669 (Bisphenol A), Part II, 144,7. Available at: 670 http://www.chemicalsubstanceschimiques. gc.ca/challeng-defi/batch-lot-2/bisphenol671a/bpa-risk_hazard-eng.php (accessed on 2025-9-15).
8. Pelch, K.; Wignall, J. A.; Goldstone, A. E.; et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 2019, 424, 152235.
9. Miller, G. Z.; Pitzzu, D. T.; Sargent, M. C.; Gearhart, J. Bisphenols and alternative developers in thermal paper receipts from the U.S. market assessed by Fourier transform infrared spectroscopy. Environ. Pollut. 2023, 335, 122232.
10. Wu, N.; He, Y.; Sun, Z.; et al. The environmental occurrence, human exposure, and toxicity of novel bisphenol S derivatives: A review. Ecotoxicol. Environ. Saf. 2025, 296, 118182.
11. Algonaiman, R.; Almutairi, A. S.; Al, Zhrani. M. M.; Barakat, H. Effects of prenatal exposure to bisphenol A substitutes, bisphenol S and bisphenol F, on offspring’s health: evidence from epidemiological and experimental studies. Biomolecules 2023, 13, 1616.
12. Tucker, D. K.; Hayes, Bouknight. S.; Brar, S. S.; Kissling, G. E.; Fenton, S. E. Evaluation of prenatal exposure to bisphenol analogues on development and long-term health of the mammary gland in female mice. Environ. Health. Perspect. 2018, 126, 087003.
13. Lee, S.; Kim, C.; Shin, H.; Kho, Y.; Choi, K. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere 2019, 221, 115-23.
14. Luo, D.; Pan, Y.; Zeng, L.; Du, B.; Li, J.; Mei, S. Occurrence of multiple bisphenol S derivatives in breast milk from chinese lactating women and implications for exposure in breast-fed infants. Environ. Sci. Technol. Lett. 2021, 8, 176-82.
15. Zhang, B.; He, Y.; Zhu, H.; et al. Concentrations of bisphenol A and its alternatives in paired maternal-fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environ. Int. 2020, 136, 105407.
16. Gys, C.; Bastiaensen, M.; Malarvannan, G.; Ait, Bamai. Y.; Araki, A.; Covaci, A. Short-term variability of bisphenols in spot, morning void and 24-hour urine samples. Environ. Pollut. 2021, 268, 115747.
17. Zhang, H.; Quan, Q.; Zhang, M.; et al. Occurrence of bisphenol A and its alternatives in paired urine and indoor dust from Chinese university students: implications for human exposure. Chemosphere 2020, 247, 125987.
18. Wei, X.; Hu, Y.; Zhu, Q.; Gao, J.; Liao, C.; Jiang, G. Co-exposure and health risks of several typical endocrine disrupting chemicals in general population in eastern China. Environ. Res. 2022, 204, 112366.
19. Peng, C. Y.; Tsai, E. M.; Kao, T. H.; et al. Canned food intake and urinary bisphenol a concentrations: a randomized crossover intervention study. Environ. Sci. Pollut. Res. Int. 2019, 26, 27999-8009.
20. Sessa, F.; Polito, R.; Monda, V.; et al. effects of a plastic-free lifestyle on urinary bisphenol A levels in school-aged children of southern Italy: a pilot study. Front. Public. Health. 2021, 9, 626070.
21. Huang, T.; Danaher, L. A.; Brüschweiler, B. J.; Kass, G. E. N.; Merten, C. Naturally occurring bisphenol F in plants used in traditional medicine. Arch. Toxicol. 2019, 93, 1485-90.
22. Aung, M. T.; Ferguson, K. K.; Cantonwine, D. E.; McElrath, T. F.; Meeker, J. D. Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens. Environ. Res. 2019, 169, 131-8.
23. Pan, Y.; Jia, C.; Zhu, Z.; et al. Occurrence and health risks of multiple emerging bisphenol S analogues in pregnant women from South China. J. Hazard. Mater. 2024, 478, 135431.
24. Crump, D.; Chiu, S.; Williams, K. L. Bis-(3-allyl-4-hydroxyphenyl) sulfone decreases embryonic viability and alters hepatic mRNA expression at two distinct developmental stages in chicken embryos exposed via egg injection. Environ. Toxicol. Chem. 2018, 37, 530-7.
25. United States Environmental Protection Agency Definition and procedure for the determination of the method detection limit, Revision 2. 2016. https://www.epa.gov/sites/default/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf (accessed 2025-9-22).
26. Huang, S.; Wang, D.; Qi, Z.; Long, C.; Li, G.; Yu, Y. A large-scale nationwide study of urinary phenols in the Chinese population. Sci. Total. Environ. 2023, 894, 164850.
27. Li, X.; Zhong, Y.; He, W.; et al. Co-exposure and health risks of parabens, bisphenols, triclosan, phthalate metabolites and hydroxyl polycyclic aromatic hydrocarbons based on simultaneous detection in urine samples from Guangzhou, south China. Environ. Pollut. 2021, 272, 115990.
28. Xu, L.; Hu, Y.; Zhu, Q.; Liao, C.; Jiang, G. Several typical endocrine-disrupting chemicals in human urine from general population in China: regional and demographic-related differences in exposure risk. J. Hazard. Mater. 2022, 424, 127489.
29. Pei, Z.; Zhang, L.; Bao, Y.; Li, J.; Zhuo, Q. The negative impacts of bisphenols on thyroid function in adults with bisphenol A exposure level exceeding the tolerable daily intake. Ecotoxicol. Environ. Saf. 2025, 290, 117790.
30. USEPA Integrated risk information system (IRIS), bisphenol A. (United States Environmental Protection Agency), 1988. http://iris.epa.gov/AdvancedSearch/?keyword=80-05-7 (accessed 2025-9-22).
31. Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on the risks to public health related to the presence of bisphenol a (BPA) in foodstuffs: part II-oxicological assessment and risk characterisation. EFSA. J. 2015, 13, 3978.
32. Lambré, C.; Barat, Baviera. J. M.; et al.; EFSA Panel on Food Contact Materials. Re-evaluation of the risks to public health re lated to the presence of bisphenol a (BPA) in foodstuffs. EFSA. J. 2023, 21, e06857.
33. Su, H.; Xi, J.; Miao, M.; et al. Bisphenol analogs exposure in 4-year-old children and their intelligence quotient at 6 years: a prospective cohort study. Environ. Res. 2025, 276, 121528.
34. Zhu, Q.; Jia, J.; Wang, Y.; et al. Spatial distribution of parabens, triclocarban, triclosan, bisphenols, and tetrabromobisphenol A and its alternatives in municipal sewage sludges in China. Sci. Total. Environ. 2019, 679, 61-9.
35. Cai, W.; Yan, Q.; Deng, Y.; Guo, Y. The correlation of bisphenol A exposure on inflammatory cytokines in preschool children. Cytokine 2025, 186, 156835.
36. Ji, Y.; Tian, Y.; Pan, Y.; et al. Exposure and potential risks of thirteen endocrine- disrupting chemicals in pharmaceuticals and personal care products for breastfed infants in China. Environ. Int. 2024, 192, 109032.
37. Pirard, C.; Charlier, C. Urinary levels of parabens, phthalate metabolites, bisphenol A and plasticizer alternatives in a Belgian population: time trend or impact of an awareness campaign? Environ. Res. 2022, 214, 113852.
38. Suwannarin, N.; Nishihama, Y.; Isobe, T.; Nakayama, S. F.; Japan Environment and Children’s Study Group. Urinary concentrations of environmental phenol among pregnant women in the Japan Environment and Children’s Study. Environ. Int. 2024, 183, 108373.
39. Tagne-Fotso, R.; Riou, M.; Saoudi, A.; et al. Exposure to bisphenol A in European women from 2007 to 2014 using human biomonitoring data - The European Joint Programme HBM4EU. Environ. Int. 2024, 190, 108912.
40. Garí, M.; Bury, D.; Moos, R. K.; et al. Urinary concentrations of BPA and analogous bisphenols (BPF and BPS) among school children from Poland: exposure and risk assessment in the REPRO_PL Cohort. Expo. Health. 2025, 17, 191-200.
41. Guo, J.; Zhang, J.; Wu, C.; et al. Urinary bisphenol A concentrations and adiposity measures at age 7 years in a prospective birth cohort. Chemosphere 2020, 251, 126340.
42. Hua, L.; Liu, W.; Liu, Y.; et al. Occurrence and profile characteristics of environmental phenols in human urine from a rural area in Northwestern China. Environ. Pollut. 2022, 315, 120405.
43. Sigvaldsen, A.; Frederiksen, H.; Højsager, F. D.; et al. Prenatal and childhood exposure to bisphenols and bone mineral density in 7-year-old children from the Odense Child Cohort. Int. J. Hyg. Environ. Health. 2024, 260, 114408.
44. Yang, Y.; Shi, Y.; Chen, D.; Chen, H.; Liu, X. Bisphenol A and its analogues in paired urine and house dust from South China and implications for children’s exposure. Chemosphere 2022, 294, 133701.
45. Zhang, T.; Xue, J.; Gao, C. Z.; et al. Urinary concentrations of bisphenols and their association with biomarkers of oxidative stress in people living near E-waste recycling facilities in China. Environ. Sci. Technol. 2016, 50, 4045-53.
46. Pan, Y.; Zhu, J.; Zhu, Z.; et al. Occurrence of multiple bisphenol S analogues in children from Shantou, China. Environ. Int. 2023, 174, 107926.
47. LaKind, J. S.; Pollock, T.; Naiman, D. Q.; Kim, S.; Nagasawa, A.; Clarke, J. Factors affecting interpretation of national biomonitoring data from multiple countries: BPA as a case study. Environ. Res. 2019, 173, 318-29.
48. Lehmler, H. J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. Adults and children: the national health and nutrition examination survey 2013-2014. ACS. Omega. 2018, 3, 6523-32.
49. Chen, D.; Kannan, K.; Tan, H.; et al. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-a review. Environ. Sci. Technol. 2016, 50, 5438-53.
50. Yang, Y.; Yang, Y.; Zhang, J.; Shao, B.; Yin, J. Assessment of bisphenol A alternatives in paper products from the Chinese market and their dermal exposure in the general population. Environ. Pollut. 2019, 244, 238-46.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].