Effects of gestational and lactational bisphenol AF exposure on female offspring reproductive function during two developmental stages
Abstract
Bisphenol AF (BPAF), a widely used substitute for bisphenol A (BPA), has been widely detected in pregnant women, raising concerns about its potential effects on reproductive health. However, studies on the reproductive toxicity of BPAF in mammals remain limited. This study aimed to investigate the reproductive toxicity and underlying mechanisms of BPAF exposure during gestation and lactation. Pregnant Sprague-Dawley rats were randomly assigned to four groups (n = 10/group) and administered BPAF by oral gavage (0, 2, 10, or 50 mg/kg/d in corn oil) from gestational day (GD) 0 to postnatal day (PND) 21. Female offspring [first filial generation (F1)] were examined at PND21 and PND70 for reproductive outcomes and steroidogenic alterations. Gestational and lactational BPAF exposure significantly reduced anogenital distance (AGD) at PND21, from 12.05 ± 0.59 mm in controls to 11.54 ± 0.50 mm in the 50 mg/kg group (P < 0.001). At PND21, serum estradiol (E2) levels were decreased in the 2 and 10 mg/kg BPAF groups compared with controls (P < 0.05). By PND70, all BPAF-exposed offspring showed reduced E2 and testosterone (T) levels (P < 0.05). In addition, BPAF exposure increased follicular atresia and downregulated the mRNA expression of key steroidogenic genes (StAR, CYP11A1, 17β-HSD, and CYP19A1; P < 0.05) at PND70. Our findings indicate that BPAF exposure during gestation and lactation impairs reproductive function in F1 females and may exert long-term effects through disruption of ovarian steroidogenesis. Further research is warranted to confirm these outcomes.
Keywords
INTRODUCTION
Bisphenol AF (BPAF), a structural analog and alternative to bisphenol A (BPA), has seen increasing application in the production of electronic materials, high-temperature composites, and other specialty polymers[1,2]. Due to its widespread use, BPAF has been detected in various environmental media, including soil, surface water, sludge, and dust[3-5]. It has also been found in human biological samples such as urine, serum, and breast milk[6-8]. Importantly, BPAF can be transferred via cord blood and lactation[9], posing a potential risk to fetuses and infants during the most sensitive early stages of development. The immature female reproductive system is particularly vulnerable to endocrine disruptors. Since BPAF shares a similar structure with BPA - already recognized as an endocrine disruptor - its potential reproductive toxicity in females, especially during pregnancy and lactation, as well as the underlying mechanisms, warrants closer investigation.
As an endocrine disruptor, BPAF may induce reproductive toxicity through several pathways, including oxidative stress, disruption of the hypothalamic-pituitary-gonadal (HPG) axis, and interference with steroidogenesis[10]. The ovary has been identified as a primary target organ for bisphenol compounds[11]. Given BPAF’s potent estrogenic activity[9,12-16] and the fact that ovarian granulosa cells are the primary site of estrogen receptor expression, it is plausible that BPAF contributes to reproductive dysfunction by altering granulosa cell-mediated steroidogenesis. This hypothesis provides the rationale for our current study. Disruption of steroid hormone production is a potential cause of abnormal reproductive development, infertility, and sterility[17]. Previous in vitro and zebrafish studies have shown that BPAF can interfere with sex steroid hormone synthesis, thereby affecting ovarian follicle development[18,19]. Cabaton et al.[19] reported that female mice exposed to BPAF exhibited a decrease in the cumulative number of pups. Moreover, maternal BPAF exposure significantly impaired key reproductive outcomes, including embryo implantation, ovarian weight, and the expression of steroidogenic genes in the ovary[20]. These findings suggest that disruption of steroid hormone synthesis may be a critical mechanism underlying the reproductive toxicity of BPAF. However, most existing studies have focused on maternal exposure or early postnatal effects in fish models, and very few have examined the long-term consequences of BPAF exposure in female mammals[9,19].
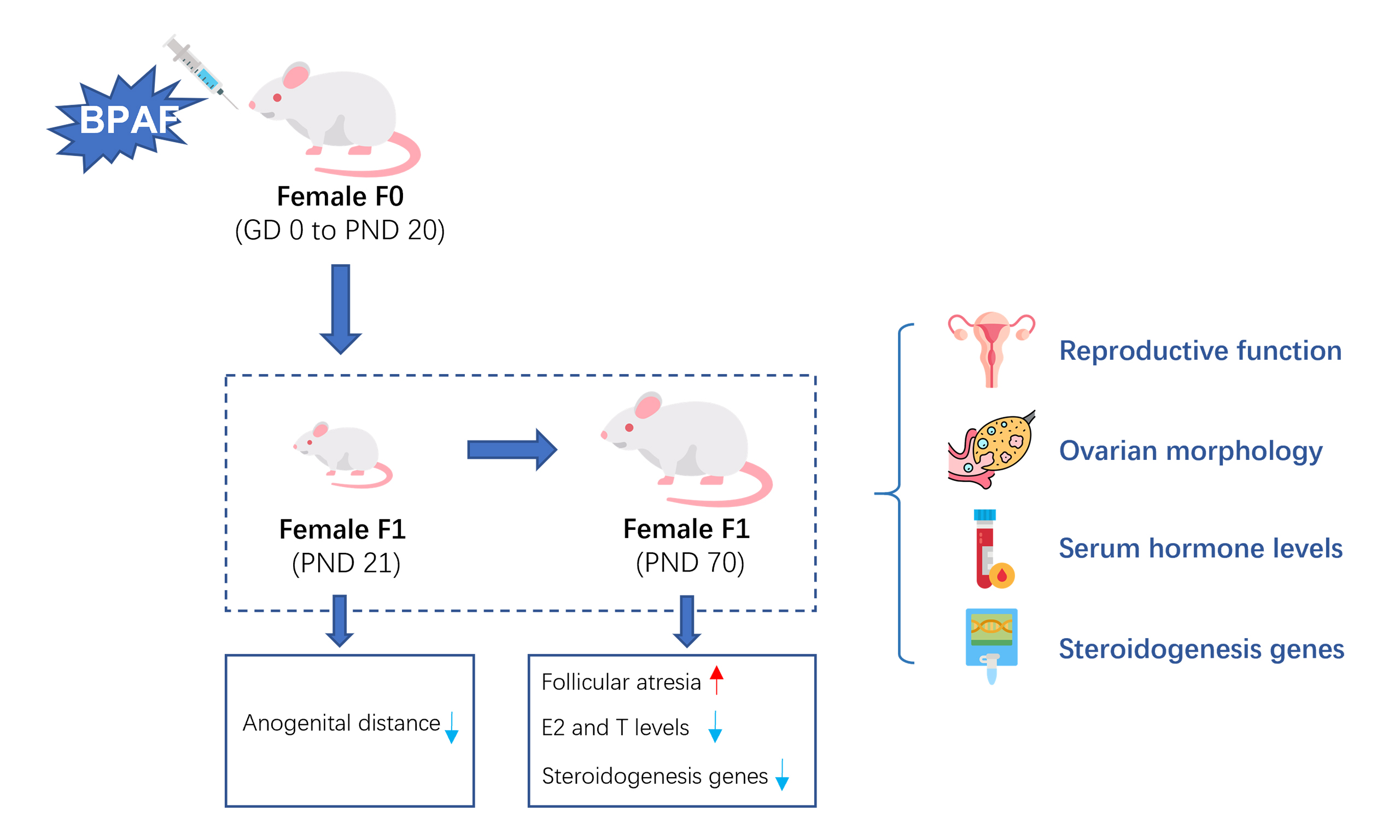
Since BPAF can be transferred through cord blood and breast milk, it is essential to explore its impact on offspring during pregnancy and lactation. Furthermore, prepuberty and adulthood represent distinct developmental stages: the former precedes rapid reproductive system development, while the latter marks sexual maturity. To comprehensively evaluate female reproductive toxicity during prepuberty [postnatal day (PND) 21] and adulthood (PND70), we exposed female rats to BPAF during gestation and lactation, followed their female offspring until PND70, and further explored the underlying mechanisms with a focus on steroid hormone synthesis.
EXPERIMENTAL
Chemicals
BPAF [257591, purity 97%, 2,2-bis(4-hydroxyphenyl) hexafluoropropane] was purchased from Sigma-Aldrich and dissolved in refined corn oil (Jinlongyu Pure Corn Oil, food-grade) by vortexing for 3 min, followed by sonication (40 kHz, 25 °C, 20 min). Fresh BPAF stock solutions (4 mg/mL in corn oil) were prepared weekly and stored at 4 °C.
Animal maintenance
A total of 140 pathogen-free Sprague Dawley SD rats (60 males and 80 females, 7-week-old) were obtained from Zhejiang Vital River Laboratory Animal Technology Co., Ltd (SCXK; Zhejiang, China, 2019-0001). Rats were housed in the animal facility of the Shanghai Municipal Center for Disease Control and Prevention (SYXK; Shanghai, China, 2018-0031) under controlled conditions (20-25 °C, 12:12-h light/dark cycle, relative humidity 40%-70%). Animals had free access to a commercial diet (Shanghai Zhouyu Biotechnology Co., Ltd., Shanghai, China) and water. All experimental procedures were approved by the Animal Ethics Review Committee of the Shanghai Municipal Center for Disease Control and Prevention (Protocol number: 20220008).
Experimental design
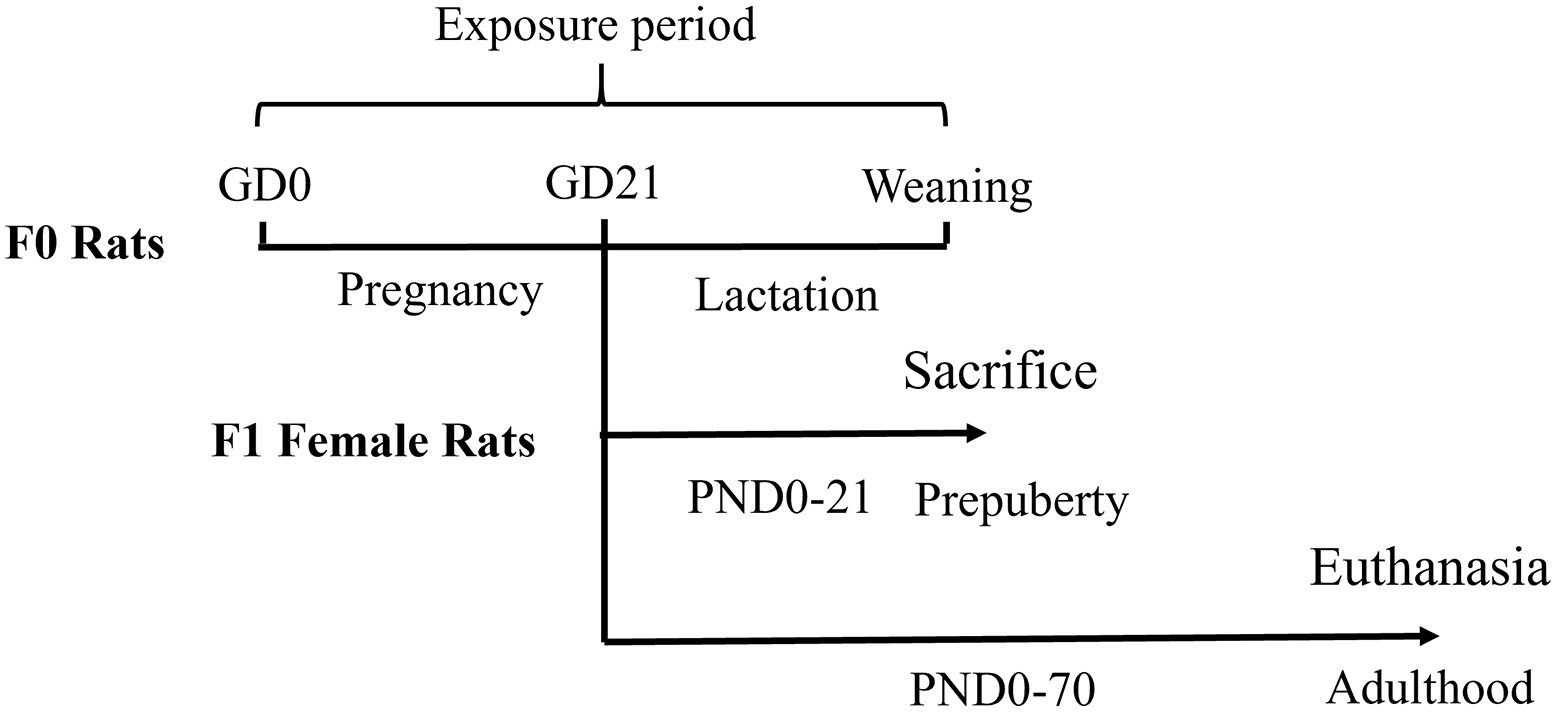
The experimental design is shown in Figure 1. All mating procedures were conducted during the light-to-dark transition (16:00-18:00), which corresponds to peak rodent sexual activity. Individual nulliparous females were placed with sexually experienced males (1:1 ratio) and monitored for vaginal smears. The day sperm-positive smears were detected was designated gestational day (GD) 0. A randomized complete block design was used. Forty confirmed-pregnant females were stratified by body weight and randomly assigned to four exposure groups (10 dams/group). Pregnant rats received daily oral gavage of BPAF (Sigma-Aldrich; 0, 2, 10, or 50 mg/kg body weight dissolved in corn oil) from GD0 to PND20. Maternal body weight was recorded every three days to adjust the dose (4 mL/kg by gavage). The dosing regimen was based on the provisional Tolerable Daily Intake (TDI) of 4 µg/kg established by the European Food Safety Authority (EFSA) for BPA[21]. Dose calculations followed the formula: Human Equivalent Dose (HED) × intraspecies uncertainty factor (UFH) × interspecies uncertainty factor (UFA) × toxicokinetic uncertainty factor (UFT)[22,23]. Based on this calculation, 2 mg/kg was selected as the lowest dose, with 5-fold intervals defining the medium (10 mg/kg) and high (50 mg/kg) doses. According to the steady-state plasma concentration formula[24] and parameters from a rat gavage model of BPAF[25], the 2 mg/kg dose corresponded to a plasma concentration of 0.872 ng/mL, comparable to reported human plasma levels of BPAF (0.44 ng/mL)[26].
At PND21 (prepuberty), parental generation (F0) females were euthanized, and one female pup from each litter was randomly selected for blood and organ collection (n = 8-10/group). The remaining first filial generation (F1) females received a standard diet without BPAF until PND70 (adulthood), when they were also euthanized for blood and organ sampling.
Pregnancy outcomes
At PND0, pregnancy outcomes including maternal weight gain, gestation length, litter size, sex ratio of pups, anogenital distance (AGD), and body weight of female pups were recorded. AGD was measured from the cranial edge of the anus to the caudal edge of the genital tubercle by an investigator blinded to group assignments.
Physical development and reproductive function
From PND0 to PND21, when rapid physical development occurs in F1 females, the following developmental milestones were recorded daily: ear detachment, eruption of incisor teeth, and eye opening. At PND21, AGD was measured in F1 females. Vaginal opening was monitored by daily visual inspection from PND25. At PND45, when puberty has stabilized, estrous cyclicity was assessed by daily vaginal smears for two consecutive weeks to capture at least two full estrous cycles.
Organ weights and histological examination
The liver, spleen, uterus, and ovaries of F1 females were collected at PND21 and PND70. Each organ was weighted, and the organ coefficient (organ-to-body weight ratio × 100%) was calculated. Ovaries were fixed in 10% neutral buffered formalin for 3 days, embedded in paraffin along their longest axis, and sectioned at 4 µm thickness at 30-µm intervals. Sections were stained with hematoxylin and eosin (H&E), and histopathological evaluation was performed in a blinded manner using fluorescence microscopy. Analyses included follicular development and abnormal morphological changes. Follicular atresia was defined according to standardized criteria: presence of apoptotic bodies at the antral periphery, cell debris within the antrum, pyknotic granulosa and/or theca cell nuclei, and detachment of granulosa cells from the basement membrane.
Serum sample collection and hormone analysis
Blood samples from F1 females were collected at PND21 and PND70 and centrifuged at 2,000 rpm for
RNA extraction and ovarian qPCR
Total RNA was extracted from ovarian samples of F1 females at PND21 and PND70 (n = 6 per group) using the RNAeasy™ Animal RNA Isolation Kit (Beyotime Biotech Inc., China) with spin columns, according to the manufacturer’s instructions. RNA purity was assessed by spectrophotometry (Nanodrop 8000, Thermo, USA), with A260/A280 ratios close to 2.0, indicating high purity and minimal protein contamination. RNA was reverse-transcribed into cDNA using the PrimeScript RT reagent kit (Takara Bio Inc., Otsu, Japan). PCR cycling conditions were 37 °C for 15 min and 85 °C for 5 s, repeated for 40 cycles. Real-time quantitative PCR (qPCR) was conducted to assess the expression of genes related to steroid hormone biosynthesis, including STAR, 3β-HSD, CYP11A1, 17β3-HSD, CYP19A1, and CYP17A1. Primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai), with sequences provided in Supplementary Table 1. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as the endogenous control. Gene expression was quantified using the comparative Ct method (2-ΔΔCt) with efficiency correction, normalizing to GAPDH. Reference gene stability (CV < 5%) and amplification efficiency (90%-110%) were confirmed for all primer sets.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Normality was tested using the Shapiro-Wilk test
RESULTS AND DISCUSSION
Pregnancy outcomes and physical development
Pregnancy outcomes for F0 females, including gestational length, gestational weight gain, litter size, pup sex ratio, and body weights of F1 females, are presented in Table 1. Except for gestational length, which was significantly increased in the 50 mg/kg group compared with the control group, no other parameters differed significantly among treatment groups. Physiological development indicators, such as incisor eruption, ear detachment, and eye opening, are also shown in Table 1. Compared with the control group, significant decreases were observed in weight gain in the 2 mg/kg BPAF group at PND21, in ovarian wet weight and ovarian coefficient in the 50 mg/kg BPAF group at PND21, and in spleen and uterine coefficients in the 2 and 10 mg/kg BPAF groups, respectively, at PND70. No significant differences were detected for other physiological development indicators [Table 1, Supplementary Table 2].
Effects of BPAF exposure on pregnancy outcomes in F0 dams and physical development of F1 females
| Dose (mg/kg/d) | 0 | 2 | 10 | 50 | ||||
| P a | P b | P c | ||||||
| Gestational weight gain (GD 1 to GD 20) | 154.00 ± 31.56 | 165.50 ± 27.87 | 0.838 | 139.33 ± 62.62 | 0.871 | 134.88 ± 33.39 | 0.244 | |
| Gestational length | 22.67 ± 0.50 | 22.88 ± 0.64 | 0.240 | 22.75 ± 0.46 | 0.183 | 23.63 ± 0.92* | 0.016 | |
| Pregnant dams (pup numbers) | 9(111) | 10(127) | 0.861 | 8(108) | 0.609 | 8(89) | 0.581 | |
| Litter weight (g) | 87.09 ± 28.79 | 89.88 ± 30.89 | 0.832 | 96.23 ± 28.82 | 0.518 | 79.98 ± 23.13 | 0.574 | |
| Litter size | 12.33 ± 4.53 | 12.70 ± 4.42 | 0.861 | 13.50 ± 4.66 | 0.609 | 11.13 ± 4.26 | 0.581 | |
| Ratio of pup’s sexes | 0.96 ± 0.41 | 1.13 ± 0.60 | 0.475 | 1.31 ± 0.76 | 0.810 | 1.05 ± 0.50 | 0.926 | |
| Weights of female pups at PND1 (g) | 7.23 ± 0.82 | 7.31 ± 0.48 | 0.519 | 7.19 ± 0.67 | 0.762 | 7.22 ± 1.12 | 0.945 | |
| The ages of the ears detachment (d) | 2.11 ± 0.33 | 2.20 ± 0.35 | 0.620 | 2.50 ± 0.55 | 0.109 | 2.25 ± 0.46 | 0.485 | |
| Eruption of the incisor teeth (d) | 8.22 ± 2.11 | 7.30 ± 0.46 | 0.195 | 7.38 ± 0.52 | 0.287 | 7.75 ± 0.89 | 0.566 | |
| Opening eyes (d) | 13.33 ± 0.71 | 13.40 ± 0.76 | 0.839 | 13.25 ± 0.71 | 0.812 | 13.88 ± 0.64 | 0.120 | |
| Weight at PND21 (g) | 68.92 ± 4.16 | 65.63 ± 5.15** | 0.009 | 70.51 ± 3.82 | 0.257 | 67.93 ± 5.46 | 0.578 | |
| Weight gain from PND1 to PND21 (g) | 56.97 ± 3.56 | 54.24 ± 4.20** | 0.009 | 58.18 ± 3.18 | 0.304 | 55.77 ± 4.80 | 0.440 | |
| Organ coefficients at PND21 (g/100 g) | Liver | 3.35 ± 0.27 | 3.24 ± 0.33 | 0.289 | 3.37 ± 0.17 | 0.752 | 3.23 ± 0.31 | 0.289 |
| Spleen | 0.40 ± 0.09 | 0.36 ± 0.06 | 0.148 | 0.38 ± 0.08 | 0.642 | 0.36 ± 0.05 | 0.159 | |
| Uterus | 0.17 ± 0.11 | 0.16 ± 0.06 | 0.637 | 0.16 ± 0.06 | 0.701 | 0.14 ± 0.05 | 0.326 | |
| Ovaries | 0.09 ± 0.04 | 0.07 ± 0.03 | 0.177 | 0.07 ± 0.02 | 0.168 | 0.06 ± 0.02* | 0.040 | |
| Weight at PND70 (g) | 292.22 ± 42.83 | 266.50 ± 37.50 | 0.173 | 293.25 ± 31.55 | 0.956 | 294.50 ± 32.15 | 0.904 | |
| Organ coefficients at PND70 (g/100 g) | Liver | 3.02 ± 0.52 | 2.85 ± 0.17 | 0.354 | 2.95 ± 0.23 | 0.723 | 2.86 ± 0.19 | 0.424 |
| Spleen | 0.19 ± 0.02 | 0.23 ± 0.03** | 0.003 | 0.23 ± 0.03** | 0.003 | 0.20 ± 0.03 | 0.451 | |
| Uterus | 0.18 ± 0.02 | 0.26 ± 0.06** | 0.003 | 0.27 ± 0.07** | 0.003 | 0.22 ± 0.08 | 0.219 | |
| Ovaries | 0.08 ± 0.02 | 0.08 ± 0.02 | 0.910 | 0.08 ± 0.02 | 0.506 | 0.07 ± 0.01 | 0.059 | |
Reproductive function and morphology
AGD of F1 females were measured at PND0, PND21 and PND70 repeatedly. Compared to the control group, significantly reduction of AGD was found in all treatment groups (2, 10 and 50 mg/kg) at PND21, but not at PND0 and PND70. Additionally, no significant difference was found for vaginal opening and estrous cycle length among all the treated groups, although an increasing trend was observed [Table 2].
Effects of gestational and lactational exposure to BPAF on anogenital distance, vaginal opening, and estrous cycle in female offspring
| Dose (mg/kg/d) | n | AGD (mm) | Age of vaginal opening (d) | Estrous cycle length (d) | ||
| PND0 | PND21 | PND70 | ||||
| 0 | 9 | 5.64 ± 0.46 | 12.05 ± 0.59 | 16.84 ± 0.68 | 31.33 ± 1.73 | 4.44 ± 0.73 |
| 2 | 10 | 5.73 ± 0.38 | 11.51 ± 0.67*** | 16.20 ± 1.88 | 31.70 ± 2.47 | 4.90 ± 1.20 |
| 10 | 8 | 5.67 ± 0.40 | 11.75 ± 0.54* | 17.17 ± 1.31 | 30.63 ± 4.24 | 4.88 ± 1.06 |
| 50 | 8 | 5.58 ± 0.45 | 11.54 ± 0.50* | 17.33 ± 1.20 | 32.38 ± 1.41 | 5.11 ± 0.74 |
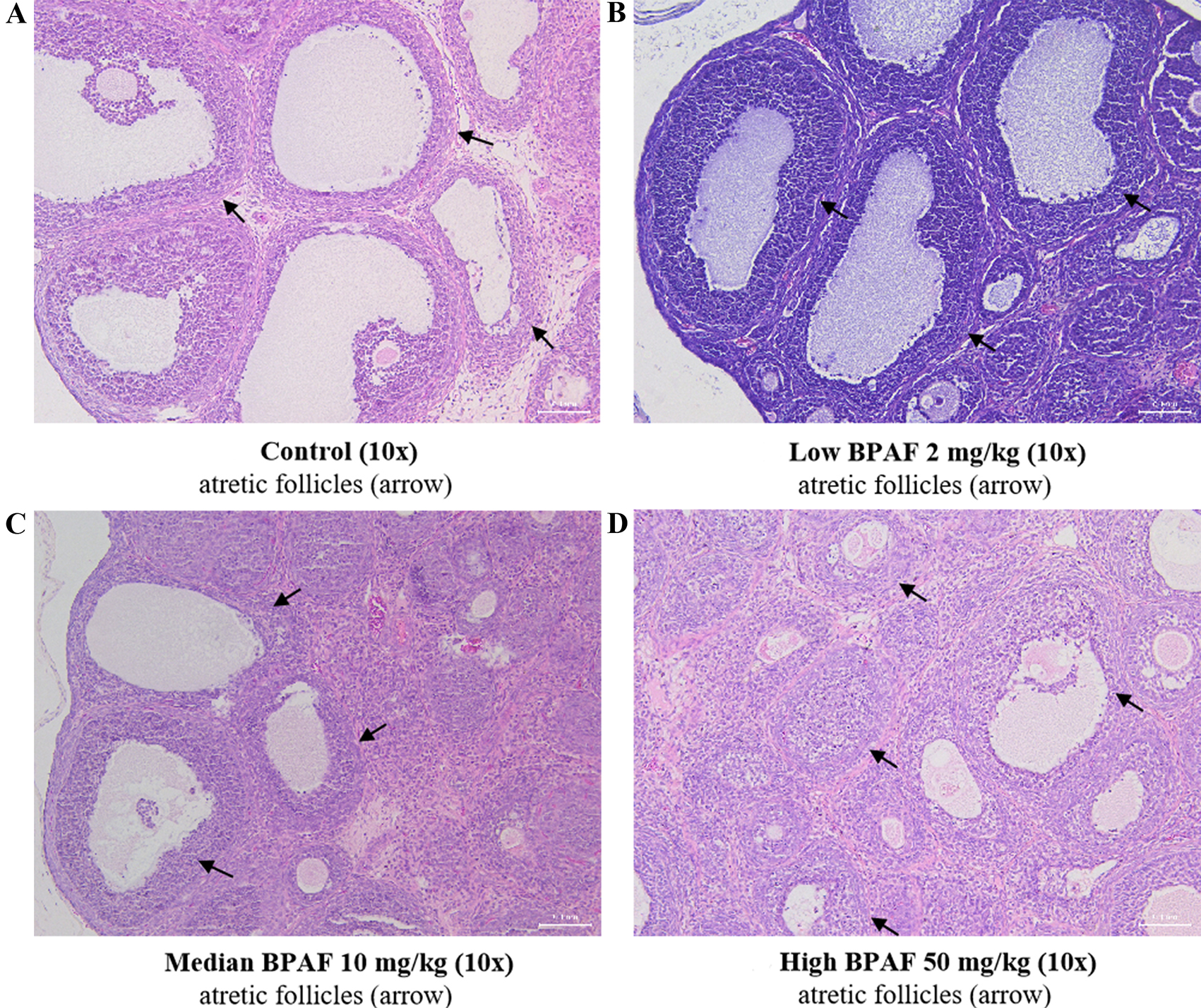
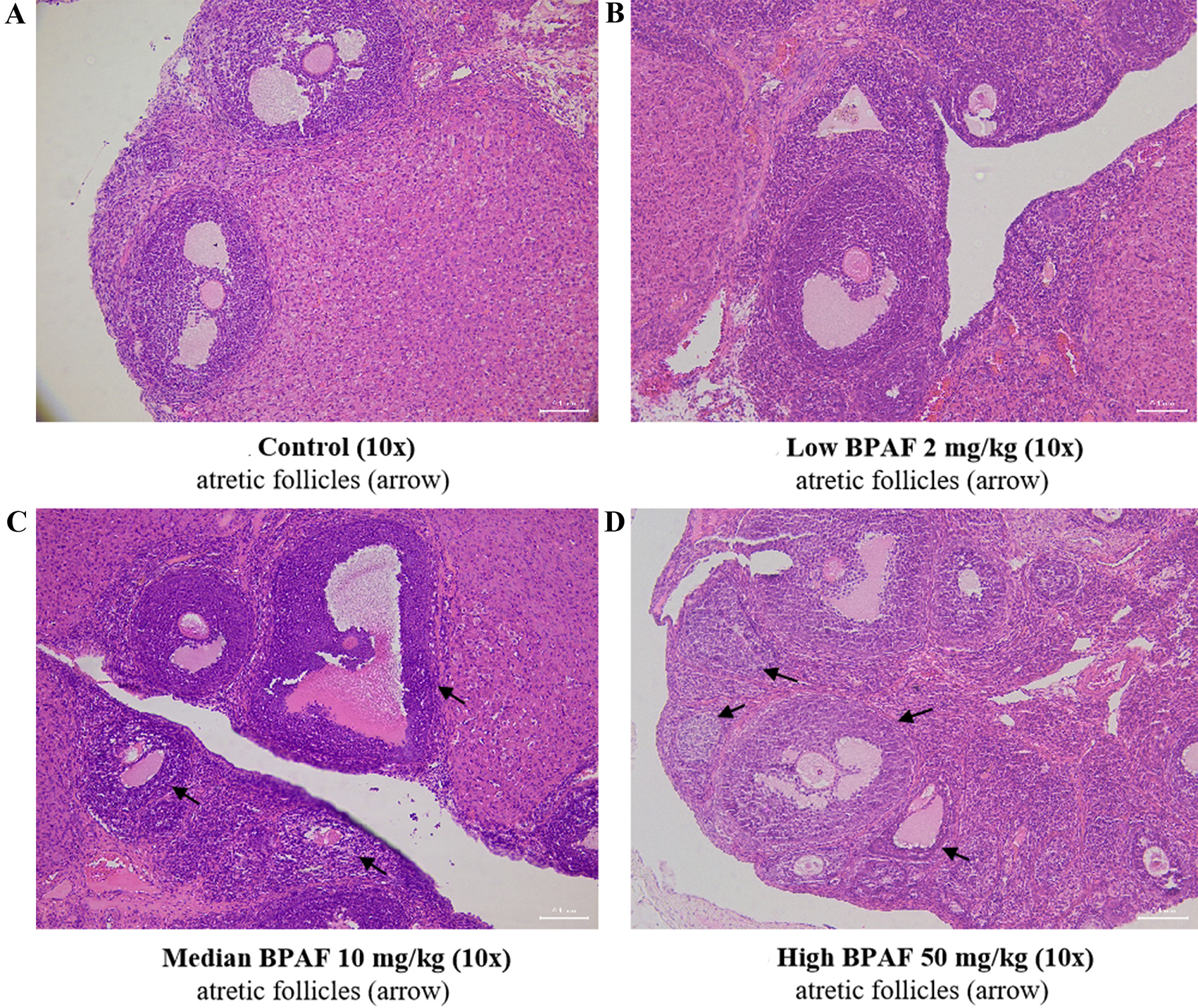
In addition, ovarian morphology was also examined [Figures 2 and 3]. At PND21, no difference in ovarian morphology was found significantly among all the BPAF treated groups when compared with the control group [Figure 2]. At PND70, a noticeable trend towards an increased incidence of follicular atresia was found only in the 50 mg/kg BPAF [Figure 3].
Figure 2. Effects of gestational and lactational BPAF exposure on ovarian histology of PND21 F1 females. (A) Control; (B) BPAF
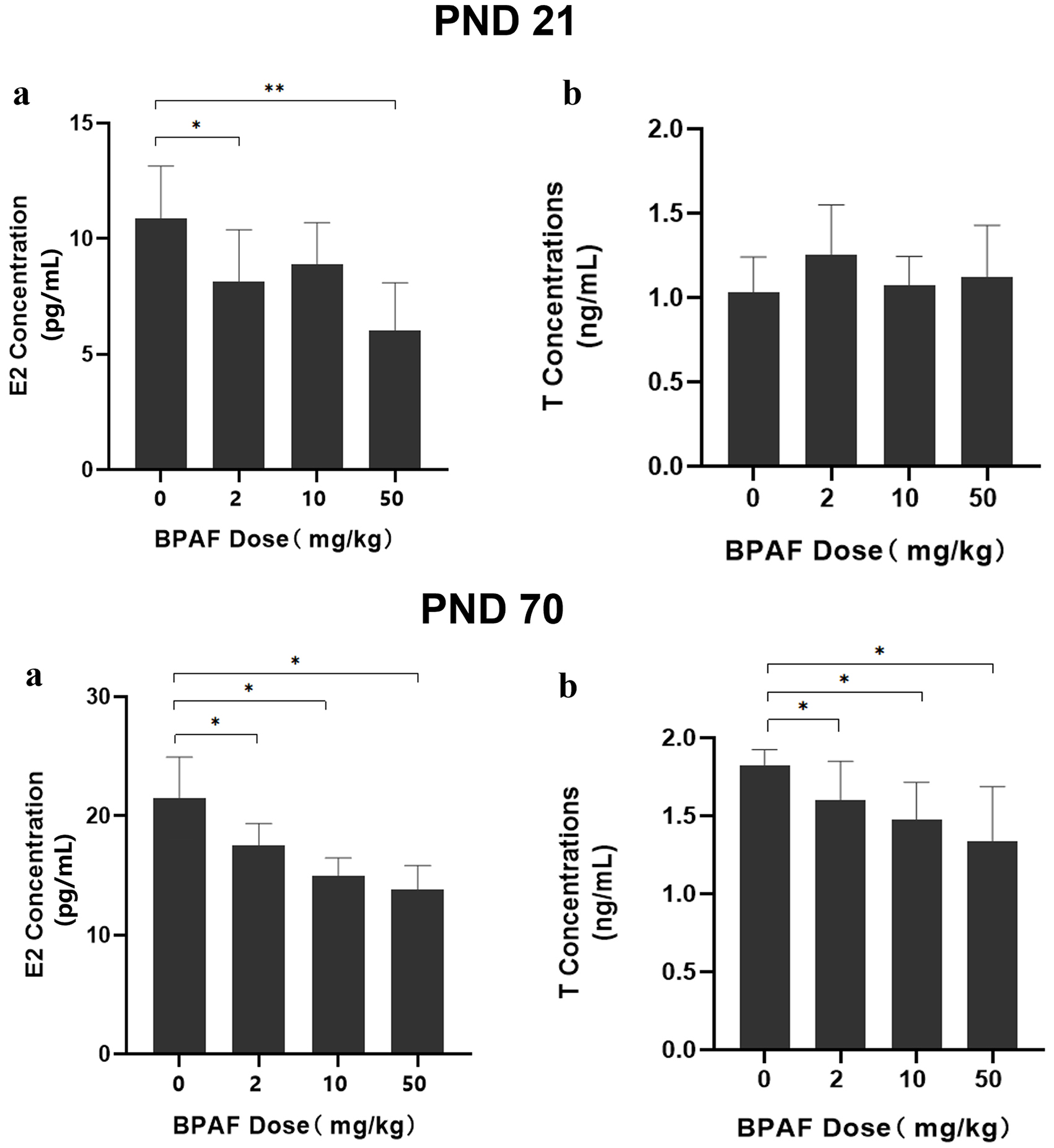
Serum hormone levels
Serum hormone levels (E2 and T) of F1 females were measured at PND21 and PND70 [Figure 4]. At PND21, significantly lower serum E2 levels were observed in the 2 mg/kg (8.15 ± 2.24 pg/mL, P < 0.05) and 10 mg/kg (6.02 ± 2.07 pg/mL, P < 0.01) BPAF-exposed groups compared with the control group (10.90 ± 2.24 pg/mL). At PND70, serum E2 levels exhibited a dose-dependent decrease across treatment groups: 21.48 ± 3.45 pg/mL (control), 17.51 ± 1.85 pg/mL (2 mg/kg), 14.18 ± 2.30 pg/mL (10 mg/kg), and 13.76 ± 2.06 pg/mL (50 mg/kg). Similarly, serum T levels were reduced in the BPAF-exposed groups compared with the control group: 1.83 ± 0.10 ng/mL (control) vs. 1.54 ± 0.21 ng/mL (2 mg/kg), 1.48 ± 0.24 ng/mL
mRNA expression of steroidogenesis-related genes
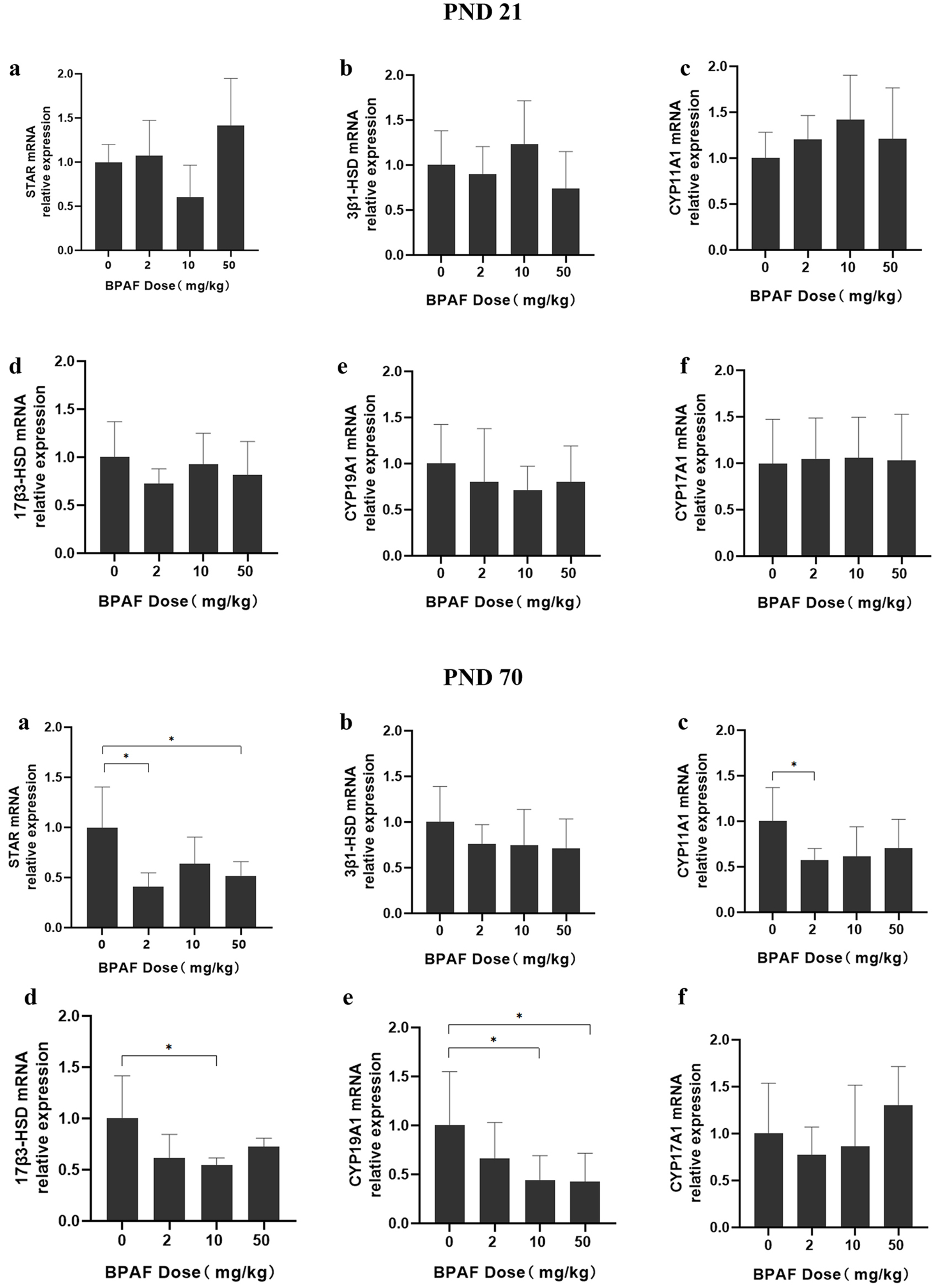
The mRNA expression of steroidogenesis-related genes (STAR, 3β-HSD, CYP11A1, 17β3-HSD, CYP19A1, and CYP17A1) in the ovaries of F1 females was measured [Figure 5]. At PND21, no significant differences were observed for any of the genes in prepuberty F1 females. However, at PND70, significantly decreased mRNA expression of several steroidogenesis-related genes (StAR, CYP11A1, 17β3-HSD, and CYP19A1) was detected in BPAF-treated groups compared with the control group. Specifically, reduced mRNA levels were observed for StAR in the 2 and 50 mg/kg BPAF groups, CYP11A1 in the 2 mg/kg BPAF group, 17β3-HSD in the 10 mg/kg/d BPAF group, and CYP19A1 in the 10 and 50 mg/kg/d BPAF groups.
Discussion
In this study, we found that exposure to BPAF during gestation and lactation may impair the reproductive system of F1 females, as evidenced by reduced AGD and serum sex hormone levels, increased follicular atresia, and decreased mRNA expression of genes involved in steroid hormone biosynthesis. Moreover, these changes varied across reproductive stages, with more pronounced effects observed at PND70, suggesting that early-life BPAF exposure may exert long-term effects on female reproduction.
With regard to ovarian steroid hormone synthesis, our results suggest several possible mechanistic pathways. BPAF exposure may downregulate mRNA expression of key genes involved in steroid hormone synthesis, including STAR, CYP11A1, 17β3-HSD, and CYP19A1, leading to decreased T and E2 levels. It is well exhibited that sex steroid hormone plays essential roles in follicle development and maintenance of regular estrous cycle[30]. For example, reduced E2 levels may impair follicular development[31], lower pregnancy and implantation rates[32,33], while low serum T levels may be associated with reduced AGD[34,35]. Similarly, previous research reported that BPA exposure decreases AGD in female rats[36]. Epidemiological evidence also links shorter AGD with increased risks of endometriosis, polycystic ovary syndrome (PCOS), and hypospadias[37-39]. In our study, we observed shortened AGD, lower serum E2 and T levels, and increased follicular atresia, supporting the hypothesis that disrupted steroid hormone synthesis is an important mechanism underlying BPAF-induced reproductive toxicity. Interestingly, the shortened AGD at PND21 that later recovered by PND70 might reflect the transient nature of this change following BPAF exposure ceased at weaning (PND21). Gradual recovery of AGD to normal levels by PND70 has also been reported in other studies[40]. These findings suggest that the reproductive system possesses compensatory capacity against EED exposure, although long-term consequences may persist. In addition, the discrepancy between unchanged vaginal opening/estrous cycles and altered hormone/gene expression profiles may indicate differential sensitivity among biomarkers, as well as differences in sampling time (e.g., serum T and E2 were measured at PND21 and PND70, while AGD is primarily established during mid-to-late gestation in rats[41]). Other unidentified factors may also contribute.
Relatively few studies have examined BPAF’s effects on steroidogenesis in vivo. Consistent with our results, several studies have shown that BPAF alters steroid hormone levels by regulating expression of steroidogenic genes[2,9,42-48]. For instance, LU S et al.[20] reported that BPAF (3 and 30 mg/kg/d) during early gestation significantly downregulated ovarian StAR, CYP11A, 17β3-HSD, and CYP19A1 expression and decreased serum progesterone and E2 levels in mice. Another study showed that BPAF (100 mg/kg/d) exposure during gestation and lactation increased serum T levels in F1 male rats[9]. Our study provides additional evidence that even relatively low-dose BPAF exposure (2 mg/kg/d) during gestation and lactation can disrupt steroid hormone synthesis and cause reproductive toxicity in female offspring. Collectively, our findings indicate that BPAF exerts reproductive toxicity similar to BPA, with both compounds disrupting steroid hormone synthesis. Previous studies have demonstrated that gestational and lactational exposure to BPA or BPAF reduces E2 and T levels and significantly suppresses mRNA expression of STAR, CYP11A1, and CYP19A1[47,49,50]. Importantly, a comparative study demonstrated that BPAF produced stronger inhibitory effects on STAR, CYP11A1, and progesterone levels than BPA at equivalent doses (900 ppm)[51]. In vitro studies further indicate that BPAF may have higher binding affinity for estrogen receptors (ERs) than BPA[12,14]. Taken together, these findings suggest that although BPAF has been marketed as a substitute for BPA, it may in fact exert stronger endocrine-disrupting effects and greater toxicity. Therefore, BPAF should not be considered a safe replacement for BPA, and its long-term reproductive effects warrant closer attention.
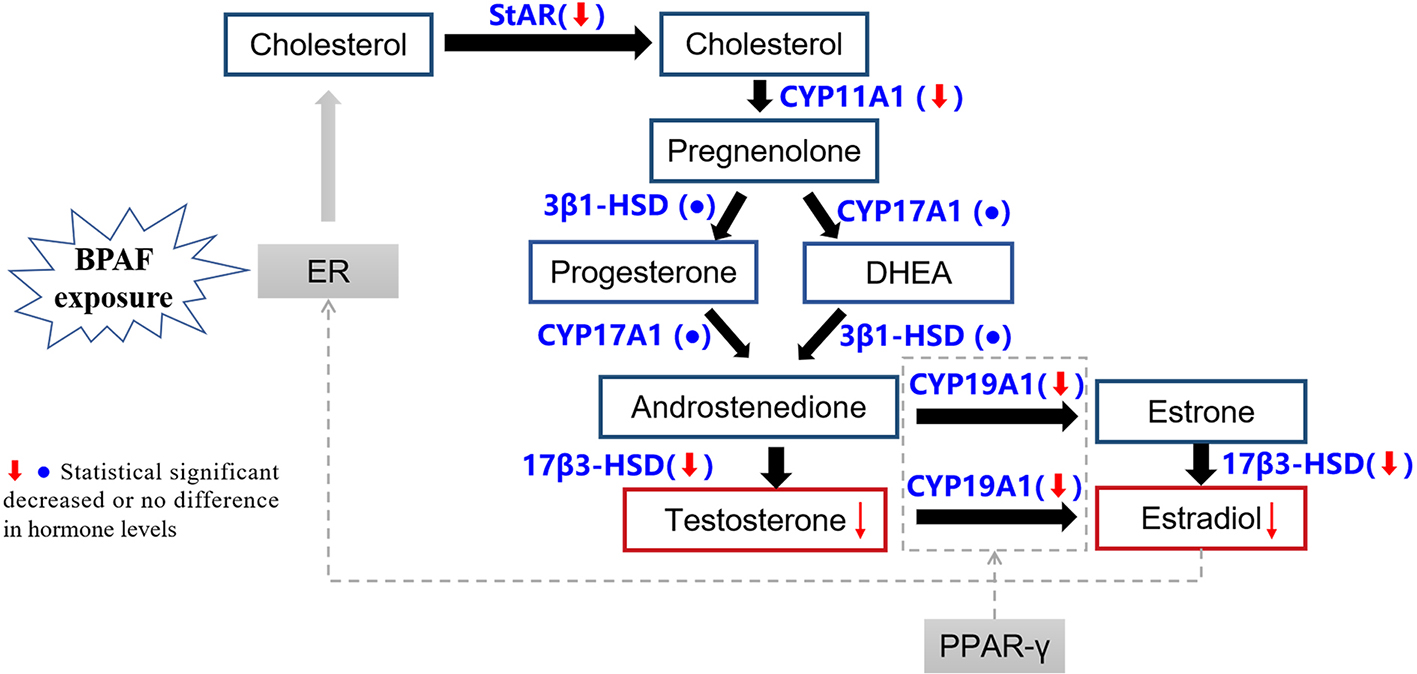
Figure 6 illustrates the possible pathways through which BPAF exposure may affect ovarian steroid hormone synthesis in adult female offspring. Our results suggested that gestational and lactational BPAF exposure reduced E2 and T levels and decreased ovarian mRNA expression of STAR, CYP11A1, 17β3-HSD, and CYP19A1 in F1 females (PND70). We therefore hypothesize that BPAF disrupts steroidogenesis by downregulating the expression of these key genes. In Figure 6, blue text indicates potential molecular targets of BPAF-induced reproductive toxicity identified in previous studies, while red boxes highlight E2 and T as key hormones affected. Based on the literature, we also included possible regulatory factors (gray boxes). For example, BPA can bind to ERα and upregulate ERα expression, thereby interfering with the steroidogenesis pathway and ultimately altering hormone levels. This imbalance may, in turn, modulate ER expression through negative feedback regulation[28]. Additionally, recent evidence suggests that peroxisome proliferator-activated receptor γ (PPAR-γ) may participate in CYP19A1 transcriptional activation, thereby influencing steroid biosynthesis[52].
Figure 6. The key pathways of the impact of BPAF exposure on ovarian steroid hormone synthesis of adult female offspring based on the present study and literature. BPAF: Bisphenol AF; PND: postnatal day; PPAR-γ: peroxisome proliferator-activated receptor γ; ER: estrogen receptor.
In this study, we compared outcomes at two reproductive stages and found that effects on reproductive function and steroidogenesis were more pronounced at PND70 than at PND21. For example, altered expression of STAR, CYP11A1, 17β3-HSD, and CYP19A1 was only observed at PND70, while sex hormone levels and ovarian histology also showed stronger changes at this stage. Similar findings have been reported in studies of BPA and BPAF exposure, with effects generally more evident after reproductive maturation than at puberty[53,54]. For example, one study showed that oral BPA exposure (0.5, 20, and 50 μg/kg) from GD11 until birth did not affect the timing of vaginal opening but increased the proportion of dead pups at three months in female offspring[55]. Another study exposing F0 CD-1 mice to BPAF (5 mg/kg) between GD10 and GD17 found significant alterations in mammary gland morphology only in late adulthood (PND56)[56]. A plausible explanation is that sexual organs remain immature during prepuberty[9,56,57]. At this stage, hormone levels are relatively low and stable, steroid hormone synthesis is not yet fully established, and hormonal feedback mechanisms are relatively simple - factors that may mask immediate effects of environmental pollutants[58]. Nonetheless, early subtle changes can lead to long-term reproductive toxicity, as demonstrated by alterations in morphology and hormone levels in adulthood[9,56,57,59]. Although our study was limited to observations up to PND70 in the F1 generation, these results provide valuable insight into the potential reproductive and transgenerational consequences of BPAF exposure. Altered sex hormone levels may impair ovarian function and reduce fertility, consistent with previous studies showing that other endocrine-disrupting pollutants such as polystyrene nanoplastics[60], cadmium[61], and phthalate mixtures[62] also impair F1 reproductive capacity following gestational exposure. Furthermore, steroidogenic disruption in F1 females may dysregulate gene expression in the second filial generation (F2)[61], potentially leading to reduced primordial follicles in F2 females[62]. These findings highlight the need for further research into the transgenerational effects of BPAF exposure.
A key strength of our study is the use of relatively low BPAF doses, while most previous studies[9,42] examined much higher doses that may not accurately represent environmentally relevant human exposure. Another advantage is that we examined reproductive development at multiple stages in F1 females. Thus, compared with similar studies, our investigation provides a more continuous and comprehensive assessment of the reproductive toxicity of BPAF exposure.
Study limitations
Our study has several limitations. First, we primarily explored the mechanism of BPAF-induced reproductive and developmental toxicity from the perspective of hormone synthesis; however, other pathways may also contribute to its reproductive toxicity. Second, the study did not include validation of protein-level changes (e.g., by Western blot) to confirm transcriptional findings, which should be addressed in further research. Third, as BPAF concentrations in biological samples (e.g., serum or tissues) from experimental animals were not measured, internal exposure doses could not be determined. This limitation may affect the accuracy of exposure assessment. Future studies should quantify internal exposure levels to verify mechanistic pathways and improve the precision of risk assessment. Additionally, we did not quantify BPAF metabolites. Nevertheless, previous research has demonstrated that BPAF glucuronides (BPAF-G) - the primary metabolites in Sprague-Dawley (SD) rats after BPAF exposure[63] - show negligible estrogenic activity[64]. This evidence supports the validity of our findings despite the lack of metabolite measurements.
CONCLUSIONS
This study suggests that exposure to BPAF during gestation and lactation adversely affects reproductive function in female offspring, potentially through disruption of ovarian steroid hormone biosynthesis. The specific pathways and molecular targets underlying these toxic effects remain unclear, and further studies are needed to elucidate the mechanisms and assess long-term reproductive outcomes.
DECLARATIONS
Authors’ contributions
Writing - original draft: Cao, Y. J.; Chen, M. Y.; Hong, X. Y.; Gao, Y.
Writing - review & editing: Cao, Y. J.; Zhang, Y.; Shi, R.; Ma, M. Y.; Xiao, P.; Tian, Y.; Hong, X. Y.; Gao, Y.
Methodology, Resources: Ding, Y.; Liu, X.; Sun, Y. L.
Visualization, Formal analysis: Cao, Y. J.; Chen, M. Y.
Conceptualization: Cao, Y. J.; Ding, Y.
Data curation: Cao, Y. J.; Ding, Y.; Chen, M. Y.
Supervision: Xiao, P.; Tian, Y.; Hong, X. Y.; Gao, Y.
Availability of data and materials
Information related to this article can be found in the Supplementary Materials. Further data are available from the corresponding authors upon reasonable request.
Financial support and sponsorship
This work was supported by the Key Discipline Project of the Three-Year Action Plan (2023-2025) for Public Health System Construction in Shanghai (GWVI-11.1-41, GWVI-4).
Conflicts of interest
Gao, Y. is a Guest Editor of Journal of Environmental Exposure Assessment. Gao, Y. was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
All the procedures performed in this study were approved by the Animal Ethics Review Committee of the Shanghai Municipal Center for Disease Control and Prevention (Protocol number: 20220008).
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Liu, J.; Zhang, L.; Lu, G.; Jiang, R.; Yan, Z.; Li, Y. Occurrence, toxicity and ecological risk of bisphenol A analogues in aquatic environment - a review. Ecotoxicol. Environ. Saf. 2021, 208, 111481.
2. Yue, H.; Yang, X.; Wu, X.; Tian, Y.; Xu, P.; Sang, N. Identification of risk for ovarian disease enhanced by BPB or BPAF exposure. Environ. Pollut. 2023, 319, 120980.
3. Liao, C.; Liu, F.; Guo, Y.; et al. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ. Sci. Technol. 2012, 46, 9138-45.
4. Song, S.; Ruan, T.; Wang, T.; Liu, R.; Jiang, G. Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China. Environ. Sci. Technol. 2012, 46, 13136-43.
5. Ji, X.; Li, J.; Wang, W.; et al. Altered mammary gland development and pro-tumorigenic changes in young female mice following prenatal BPAF exposure. Environ. Res. 2025, 264, 120371.
6. Zhang, B.; He, Y.; Zhu, H.; et al. Concentrations of bisphenol A and its alternatives in paired maternal-fetal urine, serum and amniotic fluid from an e-waste dismantling area in China. Environ. Int. 2020, 136, 105407.
7. Li, A.; Zhuang, T.; Shi, W.; et al. Serum concentration of bisphenol analogues in pregnant women in China. Sci. Total. Environ. 2020, 707, 136100.
8. Pan, Y.; Deng, M.; Li, J.; et al. Occurrence and maternal transfer of multiple bisphenols, including an emerging derivative with unexpectedly high concentrations, in the human maternal-fetal-placental unit. Environ. Sci. Technol. 2020, 54, 3476-86.
9. Li, J.; Sheng, N.; Cui, R.; et al. Gestational and lactational exposure to bisphenol AF in maternal rats increases testosterone levels in 23-day-old male offspring. Chemosphere 2016, 163, 552-61.
10. Alexander, M. V.; Ayyar, A.; Gannon, A. W.; et al. The biological effects of bisphenol AF in reproduction and development: what do we know so far? Reprod. Toxicol. 2025, 132, 108857.
11. Głód, P.; Smoleniec, J.; Marynowicz, W.; Gogola-Mruk, J.; Ptak, A. The ovary as a target organ for new generation bisphenols toxicity. Toxics 2025, 13, 164.
12. Lopez-Rodriguez, D.; Franssen, D.; Bakker, J.; Lomniczi, A.; Parent, A. S. Cellular and molecular features of EDC exposure: consequences for the GnRH network. Nat. Rev. Endocrinol. 2021, 17, 83-96.
13. Li, Y.; Perera, L.; Coons, L. A.; et al. Differential in vitro biological action, coregulator interactions, and molecular dynamic analysis of bisphenol A (BPA), BPAF, and BPS ligand-ERα complexes. Environ. Health. Perspect. 2018, 126, 017012.
14. Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C. R. Acute toxicity, teratogenic, and estrogenic effects of bisphenol a and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 2017, 51, 12796-805.
15. Tišler, T.; Krel, A.; Gerželj, U.; Erjavec, B.; Dolenc, M. S.; Pintar, A. Hazard identification and risk characterization of bisphenols A, F and AF to aquatic organisms. Environ. Pollut. 2016, 212, 472-9.
16. Feng, Y.; Jiao, Z.; Shi, J.; Li, M.; Guo, Q.; Shao, B. Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere 2016, 147, 9-19.
17. Zhan, W.; Tang, W.; Shen, X.; Xu, H.; Zhang, J. Exposure to bisphenol A and its analogs and polycystic ovarian syndrome in women of childbearing age: a multicenter case-control study. Chemosphere 2023, 313, 137463.
18. Shi, J.; Jiao, Z.; Zheng, S.; et al. Long-term effects of bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere 2015, 128, 252-7.
19. Cabaton, N. J.; Wadia, P. R.; Rubin, B. S.; et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ. Health. Perspect. 2011, 119, 547-52.
20. Lu, S.; Liu, M.; Liu, H.; et al. Gestational exposure to bisphenol AF causes endocrine disorder of corpus luteum by altering ovarian SIRT-1/Nrf2/NF-kB expressions and macrophage proangiogenic function in mice. Biochem. Pharmacol. 2024, 220, 115954.
21. Hessel, E. V.; Ezendam, J.; van, Broekhuizen. F. A.; et al. Assessment of recent developmental immunotoxicity studies with bisphenol A in the context of the 2015 EFSA t-TDI. Reprod. Toxicol. 2016, 65, 448-56.
22. Wignall, J. A.; Shapiro, A. J.; Wright, F. A.; et al. Standardizing benchmark dose calculations to improve science-based decisions in human health assessments. Environ. Health. Perspect. 2014, 122, 499-505.
23. Thompson, C. M.; Fitch, S. E.; Ring, C.; Rish, W.; Cullen, J. M.; Haws, L. C. Development of an oral reference dose for the perfluorinated compound GenX. J. Appl. Toxicol. 2019, 39, 1267-82.
24. Curry, S. H.; Whelpton, R. Drug disposition and pharmacokinetics: principles and applications for medicine, toxicology and biotechnology, 2nd ed.; John Wiley & Sons Ltd., 2022.
25. Waidyanatha, S.; Black, S. R.; Aillon, K.; et al. Toxicokinetics and bioavailability of bisphenol AF following oral administration in rodents: a dose, species, and sex comparison. Toxicol. Appl. Pharmacol. 2019, 373, 39-47.
26. Jin, H.; Zhu, J.; Chen, Z.; Hong, Y.; Cai, Z. Occurrence and partitioning of bisphenol analogues in adults’ blood from China. Environ. Sci. Technol. 2018, 52, 812-20.
27. Yusuf, A. N. M.; Amri, M. F.; Ugusman, A.; Hamid, A. A.; Mokhtar, M. H. Supraphysiological dose of testosterone impairs the expression and distribution of sex steroid receptors during endometrial receptivity development in female sprague-dawley rats. Int. J. Mol. Sci. 2024, 25, 10202.
28. Mishra, A.; Dewangan, G.; Dhakad, M. S.; et al. Exploration of the transfluthrin effects on fertility and pregnancy outcomes: an in-vivo study in rat. Pestic. Biochem. Physiol. 2025, 207, 106220.
29. Areloegbe, S. E.; Obong, N. N.; Badejogbin, O. C.; et al. Probiotics ameliorates hypothalamic amenorrhea in a rat model of PCOS. Metab. Brain. Dis. 2025, 40, 145.
30. Shoorei, H.; Seify, M.; Talebi, S. F.; Majidpoor, J.; Dehaghi, Y. K.; Shokoohi, M. Different types of bisphenols alter ovarian steroidogenesis: special attention to BPA. Heliyon 2023, 9, e16848.
31. Zamani, P.; Hemati, Z.; Kelishadi, R.; Kolahdozan, S.; Dianatinasab, M.; Keikha, M. Association between anogenital distance as a noninvasive index in the diagnosis and prognosis of reproductive disorder: a systematic review. Int. J. Reprod. Biomed. 2023, 21, 599-618.
32. Parisi, F.; Fenizia, C.; Introini, A.; et al. The pathophysiological role of estrogens in the initial stages of pregnancy: molecular mechanisms and clinical implications for pregnancy outcome from the periconceptional period to end of the first trimester. Hum. Reprod. Update. 2023, 29, 699-720.
33. Panagopoulos, P.; Mavrogianni, D.; Christodoulaki, C.; et al. Effects of endocrine disrupting compounds on female fertility. Best. Pract. Res. Clin. Obstet. Gynaecol. 2023, 88, 102347.
34. Dean, A.; Sharpe, R. M. Clinical review: anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J. Clin. Endocrinol. Metab. 2013, 98, 2230-8.
35. Mendiola, J.; Sánchez-Ferrer, M. L.; Jiménez-Velázquez, R.; et al. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum. Reprod. 2016, 31, 2377-83.
36. Christiansen, S.; Axelstad, M.; Boberg, J.; Vinggaard, A. M.; Pedersen, G. A.; Hass, U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 2014, 147, 477-87.
37. Pan, Z.; Zhu, F.; Zhou, K. A systematic review of anogenital distance and gynecological disorders: endometriosis and polycystic ovary syndrome. Front. Endocrinol. 2021, 12, 696879.
38. Hernández-Peñalver, A. I.; Sánchez-Ferrer, M. L.; Mendiola, J.; et al. Assessment of anogenital distance as a diagnostic tool in polycystic ovary syndrome. Reprod. Biomed. Online. 2018, 37, 741-9.
39. Takatani, T.; Takatani, R.; Eguchi, A.; et al.; Japan Environment and Children’s Study Group. Association between maternal blood or cord blood metal concentrations and catch-up growth in children born small for gestational age: an analysis by the Japan environment and children’s study. Environ. Health. 2024, 23, 18.
40. Hua, X. G.; Hu, R.; Hu, C. Y.; Li, F. L.; Jiang, W.; Zhang, X. J. Associations between hypospadias, cryptorchidism and anogenital distance: systematic review and meta-analysis. Andrologia 2018, 50, e13152.
41. Welsh, M.; Saunders, P. T.; Fisken, M.; et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Invest. 2008, 118, 1479-90.
42. Tian, F.; Li, Q.; Shi, L.; et al. In utero bisphenol AF exposure causes fetal Leydig cell dysfunction and induces multinucleated gonocytes by generating oxidative stress and reducing the SIRT1/PGC1α signals. Toxicol. Appl. Pharmacol. 2022, 447, 116069.
43. Mlynarcikova A, Scsukova S. Bisphenol analogs AF, S and F: effects on functional characteristics of porcine granulosa cells. Reprod. Toxicol. 2021, 103, 18-27.
44. Ronen-fuhrmann, T. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 1998, 139, 303-15.
45. Yang, X.; Liu, Y.; Li, J.; et al. Exposure to bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ. Toxicol. 2016, 31, 285-94.
46. Casarini, L.; Paradiso, E.; Lazzaretti, C.; et al. Regulation of antral follicular growth by an interplay between gonadotropins and their receptors. J. Assist. Reprod. Genet. 2022, 39, 893-904.
47. Zhou, W.; Liu, J.; Liao, L.; Han, S.; Liu, J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell. Endocrinol. 2008, 283, 12-8.
48. Kwintkiewicz, J.; Nishi, Y.; Yanase, T.; Giudice, L. C. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ. Health. Perspect. 2010, 118, 400-6.
49. Peretz, J.; Gupta, R. K.; Singh, J.; Hernández-Ochoa, I.; Flaws, J. A. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol. Sci. 2011, 119, 209-17.
50. Mlynarcíková, A.; Kolena, J.; Ficková, M.; Scsuková, S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol. Cell. Endocrinol. 2005, 244, 57-62.
51. Jones, R. L.; Lang, S. A.; Kendziorski, J. A.; Greene, A. D.; Burns, K. A. Use of a mouse model of experimentally induced endometriosis to evaluate and compare the effects of bisphenol A and bisphenol AF exposure. Environ. Health. Perspect. 2018, 126, 127004.
52. Hara, S.; Takahashi, T.; Igarashi, H.; et al. Peroxisome proliferator-activated receptor-γ agonists prevent tumor necrosis factor-α-mediated inhibition of FSH-induced follicle development and estradiol production in a preantral follicle culture system. J. Mamm. Ova. Res. 2014, 31, 2-11.
53. Li, Y.; Xiong, Y.; Lv, L.; Li, X.; Qin, Z. Effects of low-dose bisphenol AF on mammal testis development via complex mechanisms: alterations are detectable in both infancy and adulthood. Arch. Toxicol. 2022, 96, 3373-83.
54. Tucker, D. K.; Hayes, Bouknight. S.; Brar, S. S.; Kissling, G. E.; Fenton, S. E. Evaluation of prenatal exposure to bisphenol analogues on development and long-term health of the mammary gland in female mice. Environ. Health. Perspect. 2018, 126, 087003.
55. Wang, W.; Hafner, K. S.; Flaws, J. A.
56. Guo, J.; Wu, J.; He, Q.; Zhang, M.; Li, H.; Liu, Y. The potential role of ppars in the fetal origins of adult disease. Cells 2022, 11, 3474.
57. Vabre, P.; Gatimel, N.; Moreau, J.; et al. Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data. Environ. Health. 2017, 16, 37.
58. Naulé, L.; Maione, L.; Kaiser, U. B. Puberty, a sensitive window of hypothalamic development and plasticity. Endocrinology 2021, 162.
59. Silva, B. S.; Bertasso, I. M.; Pietrobon, C. B.; et al. Effects of maternal bisphenol A on behavior, sex steroid and thyroid hormones levels in the adult rat offspring. Life. Sci. 2019, 218, 253-64.
60. Xue, Y.; Cheng, X.; Ma, Z. Q.; et al. Polystyrene nanoplastics induce apoptosis, autophagy, and steroidogenesis disruption in granulosa cells to reduce oocyte quality and fertility by inhibiting the PI3K/AKT pathway in female mice. J. Nanobiotechnology. 2024, 22, 460.
61. Li, Z.; Li, T.; Leng, Y.; et al. Hormonal changes and folliculogenesis in female offspring of rats exposed to cadmium during gestation and lactation. Environ. Pollut. 2018, 238, 336-47.
62. Gonsioroski, A. V.; Aquino, A. M.; Alonso-Costa, L. G.; Barbisan, L. F.; Scarano, W. R.; Flaws, J. A. Multigenerational effects of an environmentally relevant phthalate mixture on reproductive parameters and ovarian miRNA expression in female rats. Toxicol. Sci. 2022, 189, 91-106.
63. Li, M.; Yang, Y.; Yang, Y.; et al. Biotransformation of bisphenol AF to its major glucuronide metabolite reduces estrogenic activity. PLoS. One. 2013, 8, e83170.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].