Organophosphate esters in indoor and outdoor air in Birmingham, UK: implications for human exposure
Abstract
We report concentrations of organophosphate esters (OPEs) in air from living rooms, bedrooms, and offices in Birmingham, UK. To place concentrations in these commonly frequented indoor microenvironments in context, we also measured the same OPEs in air samples collected from an outdoor location on the University of Birmingham campus. Concentrations of tris(2-chloroethyl) phosphate (TCEP), tris(2-chloroisopropyl) phosphate (TCIPP), and tri-n-butyl phosphate (TnBP) in indoor air significantly exceeded (P > 0.05) those in outdoor air. In contrast, concentrations of tris(1,3-dichloroisopropyl) phosphate (TDCIPP), triphenyl phosphate (TPhP), and 2-ethylhexyl diphenyl phosphate (EHDPP) in indoor and outdoor air were statistically indistinguishable (P < 0.05). Comparison of estimates of human exposure via inhalation derived from our data with previous estimates of exposure via dust ingestion, diet, drinking water, and dermal contact with furniture reveals that inhalation is the most important contributor to aggregate UK adult exposure to TCIPP (85%  exposure) and TCEP (67%
exposure) and TCEP (67% 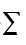 exposure). While average aggregate exposures of UK adults were well below health-based reference dose values, continued evaluation of human exposure is recommended as high-end exposures of some individuals (e.g., those inhabiting microenvironments containing concentrations of TCIPP at the high end of the range reported here) likely exceed substantially average exposures.
exposure). While average aggregate exposures of UK adults were well below health-based reference dose values, continued evaluation of human exposure is recommended as high-end exposures of some individuals (e.g., those inhabiting microenvironments containing concentrations of TCIPP at the high end of the range reported here) likely exceed substantially average exposures.
Keywords
INTRODUCTION
Organophosphate esters (OPEs) have and continue to find widespread use as additives to a wide range of domestic, commercial, and industrial products. For example, chlorinated OPEs such as tris(2-chloroethyl) phosphate (TCEP), tris(2-chloroisopropyl) phosphate (TCIPP), and tris(1,3-dichloroisopropyl) phosphate TDCIPP are added to polyurethane foam (PUF) used in domestic and office furniture, vehicle seating, as well as in building insulation, primarily to meet fire safety regulations[1]. In addition, other OPEs, including tri-n-butyl phosphate (TnBP), triphenyl phosphate (TPhP), and 2-ethylhexyl diphenyl phosphate (EHDPP), are widely used as plasticisers, for example, in plastic housing for electrical goods[1]. As in these applications, OPEs are not chemically bound to the matrix to which they are added; their release to air during use via volatilisation is relatively facile. As a consequence, there have been numerous reports of concentrations of OPEs in indoor dust in the UK and many other countries. In contrast, studies reporting concentrations of OPEs in indoor and outdoor air are relatively scarce, and to the best of our knowledge, no data on atmospheric concentrations of OPEs exist for the UK. Importantly, despite their widespread use, there have been a number of reports of potential adverse health effects of some OPEs (e.g., carcinogenicity and neurotoxicity) that have led some researchers and regulators to derive health-based reference dose (RfD) values[2-5]. Against this backdrop, this study provides the first report of concentrations of OPEs in UK indoor and outdoor air. These data are used to estimate human exposure via inhalation, and its importance relative to other exposure pathways and to RfD values, in so doing, testing the hypothesis that inhalation is an important pathway of human exposure to OPEs. In addition, this study provides insights into the relative impact of indoor sources of individual OPEs, and also establishes a baseline against which any current or future measures designed to reduce environmental contamination with OPEs may be evaluated.
MATERIALS AND METHODS
Sampling
Indoor air samples were collected between January and May 2016 from the following microenvironment categories: 23 bedrooms and 23 living rooms from the same houses within the West Midlands conurbation, UK, as well as 20 offices from the University of Birmingham. In addition, between January and July 2016,
The passive air samplers (PAS) used in this study to sample outdoor air comprised a polyurethane foam (PUF) disk (140 mm diameter, 12 m thickness, 360.6 cm2 surface area, 0.07 g·cm-3 density, PACS, Leicester, UK) sheltered by stainless steel housings (18 cm, bottom housing and 23 cm, top housing, respectively). Samplers used to collect indoor air were identical except for the omission of the bottom housing. Before use, shelters were cleaned carefully and solvent rinsed to remove potential contamination. PUF disks were washed in tap water, dried at room temperature, and pre-cleaned using pressurised liquid extraction (Dionex Europe, UK, ASE 350). PUF disks were treated with PCB 129 (50 ng) as a sampling evaluation standard (SES) prior to field deployment. The total sampling time was one month.
All indoor air samples were collected under normal room use conditions to reflect actual human exposure. Passive air sampling rates for PAS have been shown to differ depending on whether they are deployed indoors or outdoors for semivolatile organic compounds[6,7]. Indoor sampling rates were obtained by dividing the outdoor rates by two to allow for factors such as the lower air flow and the part-sheltered sampler configuration used indoors. The rates used are from a previous study[8] and are listed in Table 1.
Passive air sampling rates (m3·day-1) used in this study[8]
| OPE | TnBP | TCIPP | TCEP | TDCIPP | EHDPP | TPhP |
| Outdoor rate | 3.7 | 3.2 | 3.2 | 5.3 | 3.8 | 2.5 |
| Indoor rate | 1.8 | 1.6 | 1.6 | 2.7 | 1.9 | 1.3 |
Following collection, PUF disks were wrapped carefully inside hexane-cleaned aluminium foil, placed in sealed ziplockTM bags, and stored at -18˚C before analysis.
Chemicals used
The native compounds (TnBP, TCEP, TPHP, EHDPP, TDCIPP, TCIPP, and TPhP), D27-TnBP and D15-TPhP used as internal (or surrogate) standards and D10-anthracene and D12-benz[a]anthracene used as recovery determination (or syringe) standards were purchased from Wellington Laboratories (Canada) as stock solutions in toluene at 1 mg/mL.
Sample extraction and analysis
Prior to extraction, PUF disks were loaded into pre-cleaned 66 mL ASE 350 stainless steel extraction cells (Thermo Scientific, UK) and spiked with 150 ng D27TnBP and D15TPHP as internal (or surrogate) standards. The ASE cells were extracted with: hexane: ethyl acetate (3:4 v/v) (15 mL hexane: 20 mL ethyl acetate) at
Following extraction, the extract was evaporated to incipient dryness before reconstitution in 1 mL hexane and purification using Florisil chromatography. This was achieved using a Pasteur pipette filled with 1 g of pre-cleaned Florisil. The Florisil column was prewashed using 8 mL of methanol and then 4 mL of hexane. Following the addition of the sample extract, the column was eluted with 8 mL of hexane, which was then discarded. The OPEs were then eluted with 10 mL ethyl acetate and the eluate evaporated to incipient dryness before resolubilisation with 100 μL of iso-octane containing 100 ng D10-anthracene and
Instrumental analysis was carried out on an Agilent 5975 GC-MS. In accordance with a previous study in our group[9], the methodology followed was thus: the analysis was conducted on a 30 m DB-5 MS column (0.25 mm id, 0.25 μm film thickness). The carrier gas was helium, with a constant flow rate of 1.0 mL/min. Mass spectrometer temperatures used were: injector 290 °C under splitless conditions and MS solvent delay of 3.8 min. The ion source, quadrupole and interface temperatures were 230 °C, 150 °C and 300 °C, respectively. The GC temperature programme is as shown in fig 2.5, at the beginning 100 °C, hold for
QA/QC
Recoveries of the sampling evaluation standard (PCB-129) ranged between 30 and 73% (arithmetic mean 52%). Concentrations were not corrected for any losses during sampling. Recoveries of the internal (surrogate) standards were: D27-TnBP (average = 91%; range = 42%-135%) and D15-TPhP (average = 94%; range = 44%-137%). During method validation, 5 replicate aliquots of NIST SRM2585 (organics in indoor dust) were analysed, with 1 further measurement made for every 20 samples. In the absence of certified or indicative concentrations for our target OPEs, we compared our data with previous reports for this SRM in Table 2. This comparison reveals our method to be precise (relative standard deviations between 3% and 15% for individual OPEs) and to agree well with previous studies. None of our target compounds were detected in field blanks (n = 16) for air samples. These consisted of a pre-cleaned PUF disk, placed in the sampling housing in a sampling location, which was immediately removed before sampling could occur and then analysed as a sample. Limits of quantification for individual OPEs in indoor air were:
Concentrations (µg/g) of target OPEs in SRM2585 in this and previous studies
| OPE/parameter | TnBP | TCEP | TCIPP | TDCIPP | EHDPP | TPHP | Reference |
| Arithmetic mean | 0.18 | 0.70 | 0.82 | 2.00 | - | 0.99 | [3] |
| Standard deviation | 0.02 | 0.17 | 0.10 | 0.26 | - | 0.07 | |
| Arithmetic mean | 0.19 | 0.84 | 0.88 | 2.30 | 1.30 | 1.10 | [11] |
| Standard deviation | 0.02 | 0.06 | 0.14 | 0.28 | 0.12 | 0.10 | |
| Arithmetic mean | 0.29 | 0.81 | 0.75 | 2.50 | 1.23 | 0.89 | [12] |
| Standard deviation | 0.01 | 0.04 | 0.02 | 0.01 | 0.02 | 0.04 | |
| Arithmetic mean | 0.23 | 1.07 | 1.06 | 2.02 | 1.06 | 1.09 | This study |
| Standard deviation | 0.03 | 0.12 | 0.11 | 0.15 | 0.09 | 0.09 |
Estimation of exposure to OPEs via inhalation
To estimate UK adult human exposure to our target OPEs, we assumed a daily inhalation rate of 15 m3·day-1 and that adults spend an average of 4.5% and 22.3% of their time outdoors and in offices over the course of a week[10]. In the absence of data on concentrations of OPEs in the air from other non-domestic indoor microenvironment categories, we have assumed that UK adults spend the remainder of their time at home, equally distributed between living rooms and bedrooms (73.2%). We have then multiplied the daily volume of air inhaled at home, in offices, and outdoors by the average OPE concentrations in those microenvironments, taking the average of the concentrations in living rooms and bedrooms to represent those in homes.
Statistical analysis
Descriptive statistics were calculated using Microsoft Excel (MS Office 2011), with ANOVA (with Tukey post hoc test) and Kolmogorov-Smirnov testing of data distribution conducted using SPSS for Windows v22. As visual inspection revealed that our data did not follow a normal distribution, we log-transformed our data before subjecting it to ANOVA.
RESULTS AND DISCUSSION
Concentrations of OPEs in UK indoor and outdoor air
Concentrations of our target OPEs in indoor air from bedrooms, living rooms, and offices, and in outdoor air from EROS are summarised in Table 3. In all three indoor microenvironment categories examined, average concentrations fell in the order: TCIPP > TCEP > TnBP > TPHP > EHDPP > TDCIPP. This order was only slightly different for outdoor air, specifically: TCIPP > TCEP > TPHP > TnBP > EHDPP > TDCIPP. These orders partly reflect the relative magnitude of emissions of our target OPEs, but also their vapour pressures, as our PUF disk PAS primarily collect the vapour phase. Vapour pressures for our target OPEs fall in the order TnBP > TCEP > TCIPP > TDCIPP > TPhP > EHDPP[13,14].
Statistical summary of concentrations (ng·m-3) of OPEs in outdoor air and indoor air from homes and offices in Birmingham, UK, with range of concentrations reported in recent studies elsewhere
| Microenvironment category | Statistical parameter | TnBP | TCIPP | TCEP | TDCIPP | EHDPP | TPhP |
| Living Rooms, n = 23, this study | Average Standard deviation Median Minimum Maximum | 4.5 4.5 3.2 0.4 20 | 880 580 850 140 2,000 | 44 27 39 13 120 | 0.25 0.22 0.19 0.04 0.82 | 0.68 0.38 0.55 0.09 3.0 | 1.7 2.0 0.95 0.51 8.2 |
| Bedrooms, n = 23, this study | Average Standard deviation Median Minimum Maximum | 4.2 3.7 3.6 0.15 15 | 630 710 490 110 3,300 | 33 21 28 2.4 83 | 0.19 0.36 0.11 0.04 1.8 | 0.43 0.42 0.24 0.02 1.6 | 1.4 1.8 0.65 0.10 9.1 |
| Offices, n = 20, this study | Average Standard deviation Median Minimum Maximum | 2.8 3.7 1.3 0.53 15 | 640 500 500 62 2,000 | 41 22 34 16 94 | 0.23 0.11 0.22 0.04 0.73 | 0.75 1.3 0.28 0.09 5.3 | 1.4 1.3 1.1 0.27 6.2 |
| Outdoor, n = 7, this study | Average Standard deviation Median Minimum Maximum | 0.49 0.05 0.48 0.42 0.55 | 60 9.0 56 52 78 | 13 2.7 12 9.5 16 | 0.17 0.17 0.15 0.09 0.47 | 0.62 0.63 0.43 0.40 1.5 | 2.0 0.25 2.0 1.6 2.3 |
| Indoor, Spain[15] | Range | ND-4.6 | ND-390 | ND-6.3 | ND-1.7 | ND-7.0 | ND-2.3 |
| Indoor, USA[16] | Range | ND-29 | ND-290 | ND-19 | ND-45 | ND-9.0 | ND-44 |
| Indoor, USA[17] | Range | 8.9-33 | 7.1-44 | 1.8-10 | 0.016-9.7 | 0.25-11 | 0.34-1.0 |
| Indoor, Canada, Czechia, USA[18] | Range | ND-460 | 0.28-4200 | ND-150 | ND-6.5 | ND-67 | ND-3.3 |
| Indoor, Sweden[19] | Range | 0.96-4.3 | 84-1,900 | 6.7-20 | 0.16-0.67 | 0.085-0.64 | 0.92-2.1 |
| Indoor, India[20] | Range | 0.023-0.17 | 0.02-0.37 | 0.002-0.19 | 0.00005-0.002 | 0.02-0.15 | 0.0003-0.035 |
| Outdoor, Sweden[19] | Range | ND-0.50 | ND-5.9 | ND-2.4 | ND-0.24 | ND-0.76 | ND-0.19 |
| Outdoor, Svalbard[21] | Range | 0.067-0.54 | 0.018-0.24 | 0.016-0.39 | ND-0.13 | ND-0.11 | 0.003-0.23 |
| Outdoor, Turkey[22] | Range | - | 0.10-4.9 | ND-0.14 | ND | - | 0.05-0.32 |
| Outdoor, Canada/USA[23] | Range | ND-0.74 | ND-0.53 | ND-6.5 | ND-0.17 | ND-0.15 | ND-0.12 |
In the absence of previous data for the UK, Table 3 also places our data in context against concentrations reported in recent studies of indoor and outdoor air in other countries. Concentrations in indoor air in our study are within the range of those reported elsewhere recently in other parts of the world[15-20], although UK concentrations of TCIPP and TCEP are at the high end of the global range. In contrast, the maximum concentrations reported in outdoor air in our study exceed the range reported elsewhere[19,21-23] for all target OPEs except TnBP. This apparent discrepancy may simply reflect the limited number of other outdoor studies (n = 4) used for comparison, but other possible explanations include: a greater air exchange rate (more colloquially greater “leakiness”) of UK buildings, and greater outdoor emissions of OPEs near our EROS sampling site. In addition, we note that of the four outdoor studies used here for comparison, one used similar PUF disk PAS to this study, one polyethylene PAS, another XAD-2 PAS, and the other active air samplers. The influence of such different air sampling methods on measured concentrations of OPEs is not known and a comparative study examining this is a research priority. Moreover, further measurements of OPEs in both indoor and outdoor air across the globe are recommended.
Following the log transformation of all concentrations, we subjected our data to ANOVA. This revealed that concentrations of TDCIPP, EHDPP, and TPHP in the four microenvironment categories were statistically indistinguishable (P > 0.05), suggesting that the sources of these OPEs are not indoor applications. In contrast, for TCIPP, TCEP, and TnBP, concentrations in all three indoor microenvironment categories examined significantly exceeded (P < 0.05) those detected in outdoor air. This implies that the principal emission sources of these compounds are to be found indoors. This effect is especially marked for TCIPP, for which average concentrations in living rooms, bedrooms, offices, and outdoor air were: 880, 630, 640, and 60 ng·m-3, respectively. However, no significant differences were observed between concentrations of TCIPP, TCEP, and TnBP in living rooms, bedrooms, and offices, suggesting no major differences in sources of these OPEs in these microenvironment categories.
Contribution of inhalation to UK human exposure to OPEs
Table 4 shows our estimates of inhalation exposure, expressed as ng/kg body weight (bw) per day, assuming a UK adult weight of 70 kg. To place these in context, we also provide previously reported estimates of average UK adult exposure to the same OPEs via indoor dust ingestion[9], diet[24], and drinking water[25]. While dermal exposure via contact with furniture fabrics has not been studied for all of our target OPEs, we have included estimates of such dermal exposure to TCIPP, TCEP, and TDCIPP for UK adults in Table 4 for context[26]. Based on these exposure estimates, Table 4 also provides estimates of overall (or aggregate) exposure (i.e., the sum of all pathways) and compares these with published health-based reference dose (RfD) values[2-5]. All summed exposure estimates in Table 4 are well below any of the RfD values, with even the smallest margin of exposure (for TCIPP) being ~20. Notwithstanding the reassurance that comparison of our central estimates of adult exposure provides, it is important to highlight that high-end exposures of young UK children will exceed those in Table 4. Specifically, young children are known to be exposed far more than adults relative to their body weight, and some individuals will be exposed to OPE concentrations at the high end of the range. For example, an adult exposed to the maximum concentrations in this study will be exposed to 510 ng/kg bw/day of TCIPP via inhalation alone.
Estimated UK adult exposure (ng/kg bw/day) to OPEs via inhalation and other pathways and comparison with health-based reference dose (RfD) values
| Pathway/OPE | TnBP | TCIPP | TCEP | TDCIPP | EHDPP | TPHP |
| Inhalation (this study) | 0.82 | 150 | 8.2 | 0.05 | 0.13 | 0.34 |
| Dust ingestion[9] | 0.01 | 0.92 | 0.03 | 0.07 | 0.09 | 0.13 |
| Diet[24] | 1.1 | 4.2 | 2.3 | 3.7 | 14 | 10 |
| Drinking water[25] | 0.011 | 0.06 | 0.06 | 0.002 | 0.006 | 0.02 |
| Dermal[26] | - | 20.4 | 1.7 | 3 | - | - |
| Sum | 1.9 | 176 | 12 | 6.8 | 14 | 10 |
| RfD[2] | 10,000 | 10,000 | 7,000 | 20,000 | - | - |
| RfD[3] | 2,400 | 8,000 | 2,200 | 1,500 | 600 | 7,000 |
| RfD[4] | 24,000 | 80,000 | 22,000 | 15,000 | - | 70,000 |
| RfD[5] | - | 3,600 | - | - | - | - |
Figure 1 expresses the percentage contribution of each pathway to overall adult exposure to each target OPE. While there will be considerable variation between individuals depending on factors such as their diet and OPE concentrations in air and dust in the microenvironments they frequent, the central estimates, on which Figure 1 is based, suggest that inhalation is a substantial contributor to the overall UK adult exposure to TCIPP (85%  exposure), TCEP (67%
exposure), TCEP (67%  exposure), and TnBP (42%
exposure), and TnBP (42%  exposure). A more detailed assessment of the magnitude of this exposure pathway is recommended, including the acquisition of data on concentrations in commonly frequented indoor microenvironments not included in our current study, such as school classrooms and cars. Measurements in cars may be important for TDCIPP in particular, given that previous reports of OPEs in UK indoor dust have shown car dust to be significantly more contaminated with TDCIPP than dust from homes, offices, and school classrooms[9].
exposure). A more detailed assessment of the magnitude of this exposure pathway is recommended, including the acquisition of data on concentrations in commonly frequented indoor microenvironments not included in our current study, such as school classrooms and cars. Measurements in cars may be important for TDCIPP in particular, given that previous reports of OPEs in UK indoor dust have shown car dust to be significantly more contaminated with TDCIPP than dust from homes, offices, and school classrooms[9].
Figure 1. Contribution of different pathways to overall UK adult exposure to individual OPEs (N.B. dermal exposure estimates only available for TCIPP, TCEP, and TDCIPP). TnBP: Tri-n-butyl phosphate; TCEP: Tris(2-chloroethyl) phosphate; TCIPP: Tris(2-chloroisopropyl) phosphate; TDCIPP: Tris(1,3-dichloroisopropyl) phosphate; EHDPP: 2-ethylhexyl diphenyl phosphate; TPHP: Triphenyl phosphate.
CONCLUSIONS
Concentrations of TnBP, TCIPP, and TCEP in UK indoor air from homes and offices were significantly higher than in outdoor air, implying indoor uses are the principal source. Conversely, concentrations of TDCIPP, EHDPP, and TPHP in indoor air were statistically indistinguishable from those outdoors, implying outdoor applications of these OPEs. While concentrations in UK homes and offices are within the global range reported previously, those outdoors, except TnBP, are higher in the UK than what has been reported elsewhere. Further measurements are recommended to elucidate the position of UK outdoor levels in the global context. Comparison with previous estimates of UK adult exposure via other pathways reveals inhalation to be the main contributor to overall exposure to TCIPP and TCEP, and a major contributor to TnBP. While average UK adult exposures are well below RfD values, caution is advised as an adult exposed to the maximum indoor air concentration of TCIPP recorded in this study would be exposed via inhalation alone, at a level within an order of magnitude of one reported RfD value.
DECLARATIONS
AcknowledgementsThis work is dedicated to the memory of Dr Yessica Ortiz. The work reported here is taken from the PhD thesis of Yessica Carrizales Ortiz. Ortiz, Yessica " Factors influencing human exposure assessment of organophosphorus flame retardants (OPFRS) via indoor dust ingestion” PhD thesis, University of Birmingham, 2020. https://etheses.bham.ac.uk//id/eprint/8071/.
Authors’ contributionsWriting - original draft, data curation, experimental work: Ortiz Y
Conceptulisation, project administration, writing - review & editing: Harrad S
Availability of data and materialsThese are as provided in the manuscript.
Financial support and sponsorshipYessica Ortiz was sponsored by the provision of a PhD scholarship by PROMEP, Mexico.
Conflicts of interestAll authors declare that there are no conflicts of interest.
Ethical approval and consent to participateThis study received ethical approval from the University of Birmingham - reference ERN_13-1470.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012;88:1119-53.
2. USEPA, Mid Atlantic risk assessment, regional screening levels (RSLs)-generic Tables. Available from: http://www.epa.gov/region9/superfund/prg [Last accessed on 28 Jul 2023].
3. Van den Eede N, Dirtu AC, Neels H, Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int 2011;37:454-61.
4. Ali N, Dirtu AC, Van den Eede N, et al. Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere 2012;88:1276-82.
5. Saito I, Onuki A, Seto H. Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air 2007;17:28-36.
6. Newton S, Sellström U, Harrad S, Yu G, de Wit CA. Comparisons of indoor active and passive air sampling methods for emerging and legacy halogenated flame retardants in Beijing, China offices. Emerging Contaminants 2016;2:80-8.
7. Zhang X, Wong C, Lei YD, Wania F. Influence of sampler configuration on the uptake kinetics of a passive air sampler. Environ Sci Technol 2012;46:397-403.
8. Abdollahi A, Eng A, Jantunen LM, et al. Characterization of polyurethane foam (PUF) and sorbent impregnated PUF (SIP) disk passive air samplers for measuring organophosphate flame retardants. Chemosphere 2017;167:212-9.
9. Brommer S, Harrad S. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ Int 2015;83:202-7.
10. Harrad S, Hazrati S, Ibarra C. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: implications for human exposure. Environ Sci Technol 2006;40:4633-8.
11. Bergh C, Luongo G, Wise S, Ostman C. Organophosphate and phthalate esters in standard reference material 2585 organic contaminants in house dust. Anal Bioanal Chem 2012;402:51-9.
12. Brandsma SH, de Boer J, van Velzen MJ, Leonards PE. Organophosphorus flame retardants (PFRs) and plasticizers in house and car dust and the influence of electronic equipment. Chemosphere 2014;116:3-9.
13. Brommer S, Jantunen LM, Bidleman TF, Harrad S, Diamond ML. Determination of vapor pressures for organophosphate esters. J Chem Eng Data 2014;59:1441-7.
14. Bergman A, Rydén A, Law RJ, et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ Int 2012;49:57-82.
15. Esplugas R, Rovira J, Mari M, et al. Emerging and legacy flame retardants in indoor air and dust samples of Tarragona Province (Catalonia, Spain). Sci Total Environ 2022;806:150494.
16. Kim UJ, Wang Y, Li W, Kannan K. Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ Int 2019;125:342-9.
17. Stubbings WA, Schreder ED, Thomas MB, Romanak K, Venier M, Salamova A. Exposure to brominated and organophosphate ester flame retardants in U.S. childcare environments: effect of removal of flame-retarded nap mats on indoor levels. Environ Pollut 2018;238:1056-68.
18. Vykoukalová M, Venier M, Vojta Š, et al. Organophosphate esters flame retardants in the indoor environment. Environ Int 2017;106:97-104.
19. Wong F, de Wit CA, Newton SR. Concentrations and variability of organophosphate esters, halogenated flame retardants, and polybrominated diphenyl ethers in indoor and outdoor air in Stockholm, Sweden. Environ Pollut 2018;240:514-22.
20. Yadav IC, Devi NL, Kumar A, Li J, Zhang G. Airborne brominated, chlorinated and organophosphate ester flame retardants inside the buildings of the Indian state of Bihar: Exploration of source and human exposure. Ecotoxicol Environ Saf 2020;191:110212.
21. Li Y, Xiong S, Hao Y, et al. Organophosphate esters in Arctic air from 2011 to 2019: Concentrations, temporal trends, and potential sources. J Hazard Mater 2022;434:128872.
22. Kurt-Karakus P, Alegria H, Birgul A, Gungormus E, Jantunen L. Organophosphate ester (OPEs) flame retardants and plasticizers in air and soil from a highly industrialized city in Turkey. Sci Total Environ 2018;625:555-65.
23. Ma Y, Vojta S, Becanova J, et al. Spatial distribution and air-water exchange of organophosphate esters in the lower Great Lakes. Environ Pollut 2021;286:117349.
24. Gbadamosi MR, Abdallah MA, Harrad S. Organophosphate esters in UK diet; exposure and risk assessment. Sci Total Environ 2022;849:158368.
25. Gbadamosi MR, Al-omran LS, Abdallah MA, Harrad S. Concentrations of organophosphate esters in drinking water from the United Kingdom: implications for human exposure. Emerging Contaminants 2023;9:100203.
26. Abdallah MA-E, Harrad S. Dermal uptake of chlorinated organophosphate flame retardants via contact with furniture fabrics; implications for human exposure. Environ Res 2022;209:112847.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].