Tear-derived extracellular vesicles as diagnostic biomarkers for ocular and neurodegenerative diseases: opportunities and challenges
Abstract
In recent years, the prevalence of ocular diseases has increased considerably. However, timely diagnosis and treatment are hampered by the challenge of early detection since symptoms often appear in advanced stages. Emerging research highlights extracellular vesicles (EVs) as potential biomarkers for ocular diseases, with tear-derived EVs offering a minimally invasive source for early diagnosis. Tears play a crucial role in maintaining eye health and reflect the physiological state of the eye; thus, abnormalities in tear composition can provide valuable insight into inflammatory eye diseases. Studies have demonstrated the utility of tear-derived EVs in identifying biomarkers not only for inflammatory eye diseases but also for neurodegenerative disorders, as they carry molecular signatures (including proteins and various RNA species) reflective of their cells of origin. In this review, we discuss the potential of tear-derived EVs as biomarkers for early detection and monitoring of ocular and neurodegenerative diseases and highlight the importance of standardizing tear collection and EV isolation protocols to ensure reproducibility.
Keywords
INTRODUCTION
The incidence of ocular diseases has increased significantly over the past decade, affecting at least 2.2 billion individuals worldwide[1]. These conditions are particularly prevalent among middle-aged and elderly populations, causing vision impairment and, in many cases, irreversible blindness[2]. A key challenge in addressing ocular diseases is the delayed onset of symptoms and the difficulty in obtaining biopsy samples, which hampers early diagnosis and treatment. Conventional diagnostic methods require baseline assessments and, therefore, often detect only advanced or end-stage disease[3,4]. Moreover, many ocular disorders, such as glaucoma, age-related macular degeneration (AMD), and diabetic retinopathy, often remain asymptomatic until significant and irreversible damage has already occurred[4-6]. This silent progression limits the opportunity for clinicians to intervene while vision could still be preserved. Current diagnostic approaches, including fundoscopy, visual field testing, and optical coherence tomography (OCT), while highly valuable, have their own limitations[7,8]. Their sensitivity for detecting early molecular alterations remains low, and results can be influenced by operator expertise, patient compliance, and the availability of specialized equipment, which is not always accessible[9]. These constraints highlight the need for novel screening strategies that enable early detection of sight-threatening conditions and more effective monitoring of disease progression.
Extracellular vesicles (EVs) - cell-derived membranous structures - have been extensively studied for their role in mediating a plethora of intercellular activities. They are involved in numerous physiological and pathological processes, and EV-related molecules have shown considerable promise as novel biomarkers in several diseases in the last decade[10].
Within this field, tear fluid has emerged as an attractive source of EVs. Unlike blood or serum, which are highly viscous and contain large amounts of proteins and lipids that could hinder EV isolation[11], tears are easier to process and yield molecules at relatively high concentration and purity[12]. Studies have shown significant molecular overlap between plasma and tears, including shared proteins and microRNAs, while also identifying molecules unique to tears[13]. Notably, tear fluid not only reflects systemic alterations but also contains EVs derived directly from ocular cells, providing disease-specific molecular signatures of particular relevance for eye disorders[14].
In this review, we summarize the most recent literature on the use of EVs for the detection of ocular diseases, with a special focus on tear-derived extracellular vesicles as a minimally invasive source of biomarkers, including their potential relevance in neurodegenerative disorders.
EYE STRUCTURE AND OCULAR FLUIDS AS A SOURCE OF BIOMARKERS
The eye is a bilateral and spherical organ that houses the structures responsible for vision. It is situated within the orbit and consists of the eyeball and its accessory structures. The eyeball itself is composed of three layers[15]. The outer layer comprises the cornea, the transparent part of the eye that allows light to enter, and the sclera, the white part of the eye. The middle layer is the choroid, whose anterior portion is the iris, the colored part of the eye that contains the pupil, a circular aperture regulating the amount of light entering the eye. The inner layer consists of the retina and includes the optic nerve, which transfers visual information to the brain. Surrounding the eyeball lies the conjunctiva, a membrane that covers the sclera and lines the inner eyelids. The conjunctiva and the cornea are continuously bathed by the tear film, which is essential for the production and distribution of tears, thereby maintaining ocular lubrication and protection. The interior of the eyeball is filled with aqueous humor and vitreous humor, which, together with the tears, support the vitality of tissues and provide lubrication, nourishment, and protection essential for vision[16].
Vitreous humor (VH) is a transparent gel-like extracellular matrix composed mainly of water and a meshwork of fine collagen fibrils, hyaluronan molecules, lipids, and inorganic salts[17,18]. It fills the space between the lens and the retina (posterior chamber), providing shape, elasticity, and volume, and facilitating light transmission to the retina[19]. VH captures proteins that are either secreted locally by the retina or diffused from adjacent ocular tissues, which positions it as a valuable source for investigating biomarkers associated with vitreoretinal diseases, including diabetic retinopathy and age-related macular degeneration[20,21].
Aqueous humor (AH) is a clear fluid located in the anterior chamber of the eye, between the cornea and the lens. Although predominantly water, it also contains small quantities of sugars, proteins, vitamins, cytokines, growth factors, and other nutrients[22]. AH maintains intraocular pressure, nourishes the anterior segment of the eye, and removes metabolic waste[3]. It is secreted by the non-pigmented ciliary epithelium and drains through the trabecular meshwork or the uveoscleral pathway[23]. Accordingly, AH is considered an important source of biomarkers in glaucoma[24,25]. Given its interaction with vitreous humor, it also holds potential for biomarker discovery in retinal pathologies[26,27].
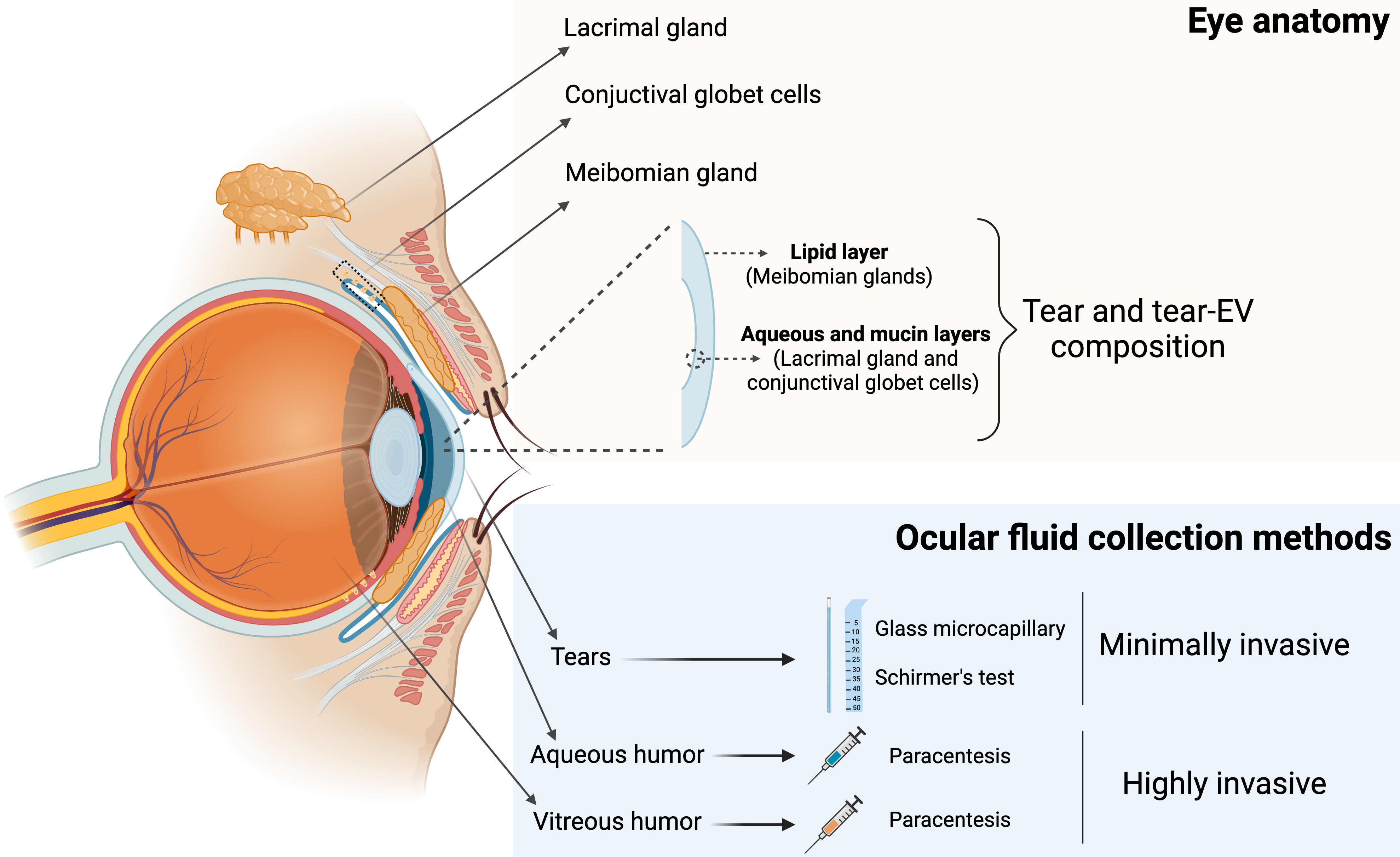
The tear film is a transparent fluid that covers the ocular surface. It is composed of three layers: an outer lipid layer, a middle aqueous layer, and an inner mucin layer[28]. The meibomian glands secrete most of the lipids in the tear film lipid layer, while the lacrimal glands and conjunctival goblet cells are mainly responsible for producing the aqueous and mucin components of the tear film, respectively[29] [Figure 1].
Figure 1. Schematic representation of an anatomic eye with its associated glands, the three-layer composition of tear film, and different ocular fluid collection methods. Created with BioRender. Borràs, F. (2025) https://BioRender.com/cpfv79z. EV: Extracellular vesicle.
Tears play a crucial role in maintaining ocular health by providing hydration and lubrication to the mucosal surface, protecting against pathogens, clearing metabolic waste, and nourishing the underlying corneal and conjunctival epithelial cells[30]. They also serve as a valuable source of biomarkers for disorders of the anterior segment of the eye, such as dry eye syndrome and conjunctivitis[31,32].
Ocular fluids, produced by ocular glands and cells and by surrounding tissues and vasculature[16], may reflect the health and/or pathological state of the eye[33]. Moreover, ocular fluids are in direct contact with disease sites, which may be an additional advantage compared to detecting biomarkers in blood or plasma, where molecules may be highly diluted[30].
However, the use of aqueous and vitreous humor for biomarker discovery is limited by the invasiveness of the collection process [Figure 1].
Aqueous humor is obtained via anterior chamber paracentesis, and vitreous humor via vitreous aspiration, both procedures carrying significant risks, such as retinal tear or detachment[34]. Moreover, such invasive procedures are not ethically applicable to control subjects. In contrast, tear collection is minimally invasive [Table 1].
Summary of ocular fluid collection methodologies
| Ocular fluid | Methodology | Invasiveness | Difficulty | Patient discomfort |
| Vitreous humor | Vitreous aspiration | High | High | High |
| Aqueous humor | Retinal surgery process | High | High | High |
| Tears | Glass microcapillary | Low | Medium | Medium |
| Schirmer test | Low | Low | Low |
Tears are typically collected using glass microcapillaries[3] or the Schirmer type I tear test, which involves inserting a filter paper strip into the lower conjunctival fornix for 5 minutes[35]. Collection with microcapillaries requires specific skills from the operator (doctor or nurse), though it could be interrupted by blinking and usually yields a small sample volume. Schirmer test, by contrast, is simpler to perform and generally perceived by patients as significantly less invasive[36]. However, samples may be contaminated with epithelial cells from the sub-palpebral skin, a factor that should be carefully considered during analysis. Despite the specific advantages and limitations of each collection method, tear fluid stands out as a minimally invasive and promising source of EVs, holding significant potential for biomarker discovery and disease monitoring across a broad spectrum of conditions. For this reason, research on tears as a source of biomarkers has increased in recent years, surpassing interest in other ocular fluids. Accordingly, this review aims to summarize the current knowledge on tear-derived extracellular vesicles as a promising source of biomarkers for both ocular and neurodegenerative diseases.
BRIEFING ON EXTRACELLULAR VESICLES
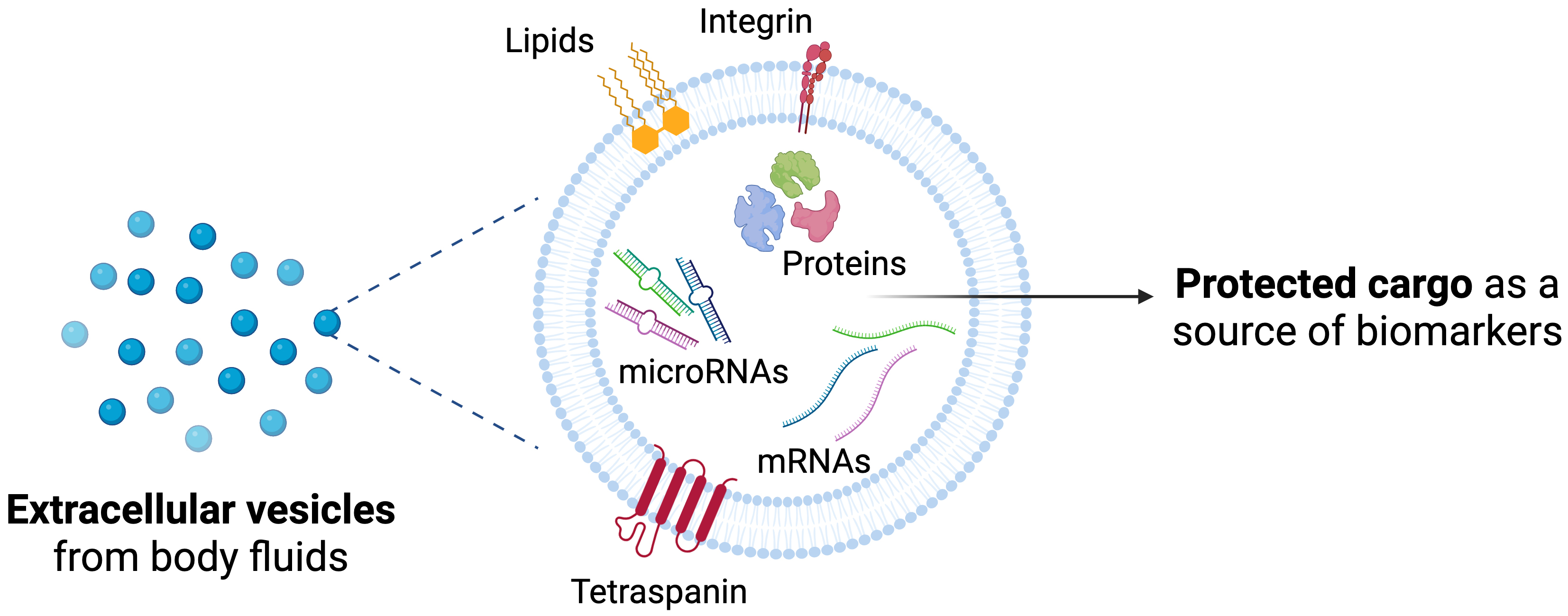
EVs are secreted by most cell types and are present in a wide range of body fluids, including ocular fluids, blood, urine, breast milk, cerebrospinal fluid, lymph, amniotic fluid, and saliva[37]. Their lipid bilayer membrane encapsulates and protects their molecular content from enzymatic degradation, preserving it as a source of both physiological and pathological information. This encapsulation also facilitates cargo transfer between cells, enabling intercellular communication[38]. The complex composition of EV cargo reflects the state of their cell of origin and can influence the function and phenotype of recipient cells. Moreover, it is reported that when a functional molecule is delivered by EVs, it may be more active than in its soluble form[38].
EVs are a heterogeneous nanosized population ranging from 30 to 1,000 nm, with different biogenesis mechanisms. Historically, they have been categorized into exosomes (originating from endosomal multivesicular bodies) and microvesicles (formed by membrane budding of the plasma membrane). Alternatively, EVs can be classified as small (< 200 nm) or large (> 200 nm) based on size. Yet, despite their specific biogenesis mechanisms, overlapping sizes and densities and shared molecular markers among different EV populations make their precise identification and separation challenging. Thus, the International Society for Extracellular Vesicles (ISEV) recommends the use of the generic term “EVs” while acknowledging operational extensions that include non-vesicular particles often co‐isolated with EVs, as outlined in MISEV2023 guidelines[39]. The molecular composition of EVs is diverse, encompassing proteins, lipids, carbohydrates, and genetic material. These components may be embedded within the vesicle membrane, constitute the internal cargo, or correlate with surface corona[40].
Beyond universally expressed markers such as tetraspanins, EVs also carry cell-type-specific signatures that reflect cellular status and phenotype, along with their stability in biological fluids and the protection of their cargo [Figure 2].
Figure 2. Extracellular vesicles as a source of biomarkers. Schematic view of protected cargo and membrane-associated molecules. Created in BioRender. Borràs, F. (2025) https://BioRender.com/cpfv79z.
Compared with whole cells, EVs offer enhanced stability, improved safety, increased tissue permeability, and lower tumorigenicity[41]. These properties allow EVs to cross biological barriers, such as the blood-brain and blood-retinal barriers, enabling the delivery of molecular information from different tissues[42]. These positioned EVs as promising candidates for defining potential biomarkers for the diagnosis and prognosis of various diseases[39,43,44].
TEAR AND TEAR-DERIVED EV COMPOSITION
The tear film has a volume of 3 to 10 μL and is secreted at a rate of 1 to 2 μL/min[45]. Its composition is primarily water (98,3%), with smaller fractions of salts (1%), proteins and glycoproteins (0.7%), and other minor constituents including metabolites[46]. Compared to blood and serum, tears contain significant amounts of proteins and other components, with lysozyme, lactoferrin, secretory IgA, and lipocalin being the four most abundant and well-characterized tear proteins[47].
The biochemical composition of tears varies with context and stimulus type. Four distinct tear types have been identified: basal, reflex, closed-eye, and emotional tears. Basal tears are continuously produced under normal conditions to maintain ocular surface homeostasis. Reflex tears are secreted in response to irritants and in higher volumes than basal tears to help clear foreign substances from the ocular surface. Closed-eye tears are produced during sleep to ensure overnight lubrication of the eye, while emotional tears are associated with intense emotional states[48]. Protein, lipid, and secretory IgA concentrations differ among tear types, with basal tears containing the highest levels of protein and lipid content[49]. Nevertheless, compared to blood and serum, tears contain much lower amounts of proteins and other components[50]. Key tear film proteins, including lactoferrin, lipocalin-1, lysozyme, and tear-specific prealbumin[47], remain relatively stable across the different tear types[51], but are largely absent from serum[52].
Tears also contain EVs, whose cargo content depends on the tear type. These EVs originate from the lacrimal glands, meibomian glands, goblet cells, and ocular surface epithelial cells[53].
Abnormalities in tear film, affecting the constituents or the volume, can rapidly result in serious eye surface dysfunction and ultimately impair corneal transparency[54]. Disease-specific changes in tear proteins and metabolites have been identified in diseases such as dry eye syndrome, diabetic retinopathy, age-related macular degeneration, and glaucoma[14,55-59]. In diabetic retinopathy, for instance, inflammation and blood-retinal barrier dysfunction may alter retinal vasculature, increasing barrier permeability and reducing junction protein levels, which leads to a higher proportion of serum proteins in the tear film[60]. Due to their inherent ability to cross biological barriers, EVs from blood may also reach the retina, modifying ocular fluid composition. Consequently, tear composition, and specifically tear-derived EV content, could reflect the (patho)physiological state of the eye, providing specific information about the tissues underneath the eye and serving as a potential tool for evaluating ocular health and disease.
TEAR COLLECTION AND TEAR-EV ISOLATION
In most studies using tears, the starting sample is reported as tear volume (µL) using a 10 µL capillary tube[61,62], or as the tear fluid migration length (mm) on a Schirmer strip after 5 min of sampling, since the distance traveled by the tears is proportional to their production[63]. However, there is currently no standardized protocol for tear sample handling, from collection to analysis, particularly regarding storage format, duration, and temperature. All these parameters are important for subsequent EV analyses, and should be reported as recommended for other biological fluids[64].
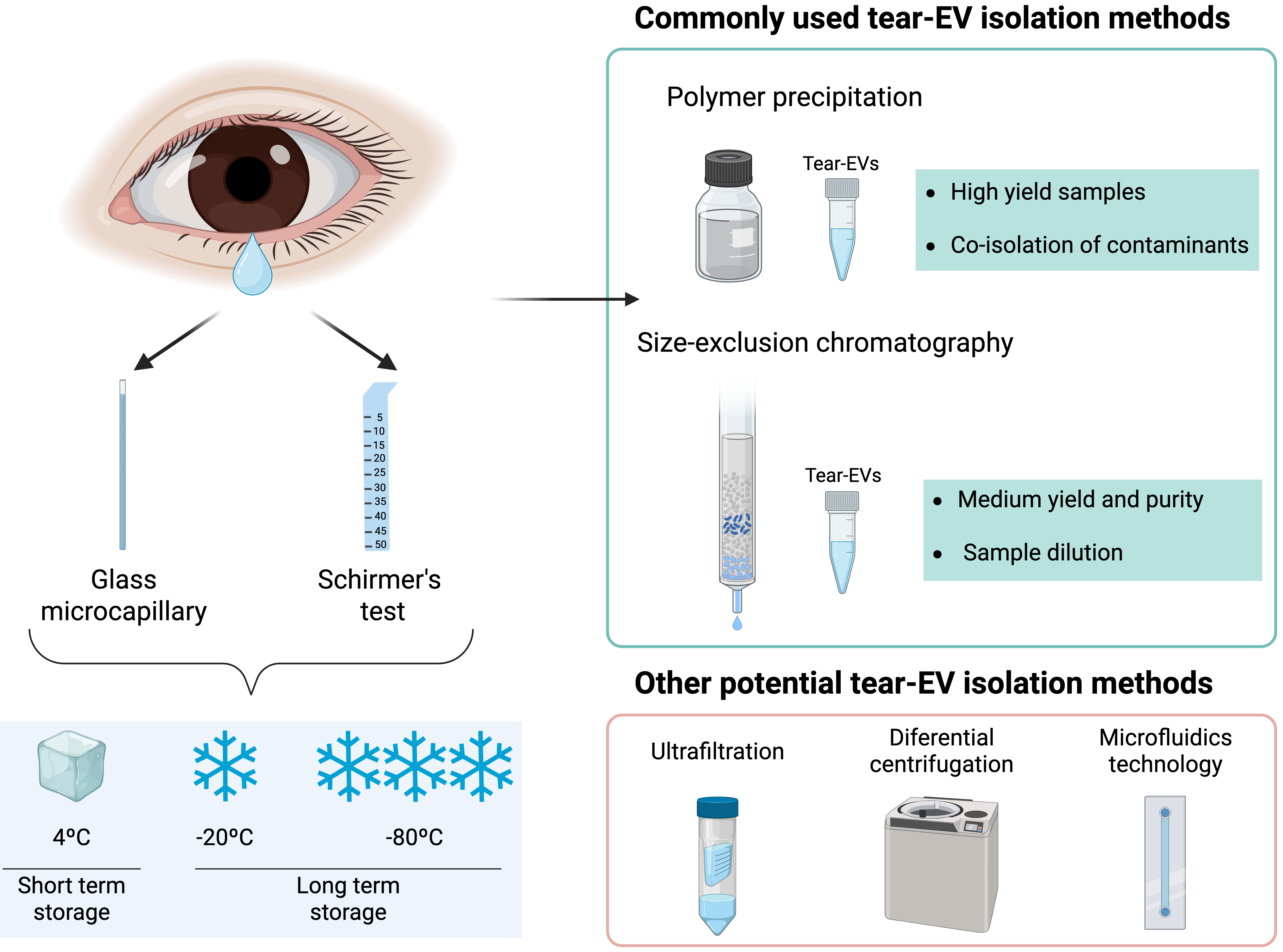
When using strips for tear sampling, it is important to avoid contact with the eyelid or the skin beneath the eye to minimize contamination by epithelial cells and their proteins, such as keratins[65]. Collected strips can be stored dry or submerged in a specific buffer (wet storage), such as phosphate-buffered saline or sodium chloride (NaCl), and maintained at room temperature, refrigerated (4 °C), or frozen (-20 and -80 °C) [Figure 3].
Figure 3. Schematic representation of methodologies for tear collection and EV isolation. Created in BioRender. Borràs, F. (2025) https://BioRender.com/cpfv79z. EV: Extracellular vesicle.
According to the literature, wet storage results in less variability in tear fluid protein concentration compared to dry storage, while storage at -20 or -80 °C is preferable to room temperature or 4 °C[66]. When tears are collected using a microcapillary tube, they are typically frozen directly at -80 °C until analysis[61,62]. Regarding storage duration, long-term storage (> 4 months at -70 °C) has been reported to reduce total tear protein concentration[67], whereas a more recent study determined that there was no effect when tear samples were stored for up to 6 hours at 4 ºC before freezing at -80 ºC[68], with frozen samples remaining stable for 6 months before analysis.
Collected tear volumes are often limited, depending on the patient’s condition and disease[69]. Therefore, for EV studies, it is crucial to adopt EV isolation techniques that maximize purity while minimizing yield loss. Numerous EV-isolation methods are available[70], which can be classified into three groups based on sample purity and yield: high-yield but low-purity methods (ultrafiltration, polymer precipitation), intermediate methods balancing purity and yield (size exclusion chromatography, ultracentrifugation), and high-purity but low-yield methods (affinity isolation, microfluidics technology)[70].
The selection of the EV isolation method should consider the intended purpose of the experiment, as different methods may influence the characteristics of isolated EVs[71], potentially masking their function or hampering biomarker discovery.
Achieving an optimal balance between recovery and selective separation of EVs from co-isolates remains challenging, and the chosen method may differ depending on the source biological fluid and its composition. As for tear-derived EVs, most published studies employ polymer precipitation, with fewer using size exclusion chromatography, reflecting different experimental goals [Figure 3].
Polymer precipitation enables high-yield EV recovery through the use of hydrophilic polymers that decrease EV solubility, allowing for pellet formation[72], although it usually co-isolates contaminants and undesired components, affecting the screening process[73]. Given that tear fluid contains relatively low levels of proteins and cellular debris compared to other biological fluids, polymer precipitation could be considered an optimal method for tear-EV isolation. Commercial kits based on this method are straightforward, rapid, and widely applicable for biomarker discovery. A variety of kits exist for fluids such as urine, blood, or plasma, but no commercial kit has been specifically designed for tear-EV isolation, and researchers typically adapt protocols from kits intended for other fluids[74,75].
In contrast, size exclusion chromatography (SEC) achieves a balance between purity and yield by separating sample molecules based on size and weight[76]. Smaller molecules, such as free proteins, elute later than EVs, resulting in fractions enriched in different components. Although SEC achieves high purity, it may dilute EV samples, so starting volume and sample composition must be considered. To ensure sufficient volume and representative EV composition, many researchers pool tear samples from multiple donors for downstream analyses[77,78]. Regarding EV storage, standardized procedures are lacking, and studies report conflicting results. The most common method is storage at -80 °C, although repeated freeze-thaw cycles could affect EV functionality[79]. ISEV guidelines[80] acknowledge the influence of preservation on EVs and encourage further research to clarify the effects of storage conditions on EV recovery and function. Thus, more advancement is required to address volume limitations and standardize tear-EV sample handling, storage, and processing.
TEAR-DERIVED EVS AS POTENTIAL BIOMARKERS IN OCULAR DISEASE
Given their association with the ocular surface and proximity to disease sites, tears are considered a potential reservoir of protein, lipid, and molecular biomarkers for a variety of ocular conditions. Inflammatory eye diseases are among these pathologies, primarily encompassing degenerative conditions with inflammatory components[44]. The most prevalent of these diseases include glaucoma, dry eye syndrome, and diabetic retinopathy.
Glaucoma is a progressive optic neuropathy frequently associated with elevated intraocular pressure (IOP) resulting from impaired aqueous humor drainage. It affects the optic nerve and remains the leading cause of permanent blindness worldwide[81].
Dry eye is a multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film and accompanied by ocular symptoms. Key etiological factors include tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities[82].
Diabetic retinopathy, a common diabetes complication, arises from high blood sugar levels and is characterized by the growth of abnormal blood vessels in the retina. If inadequately controlled, complications of this pathology can lead to vision loss[83].
Tear-derived EVs were first identified only a few years ago. Initial studies provided a detailed characterization of tear-EVs, confirming their nature and examining their morphological and molecular features[84]. This is an emerging and rapidly expanding field, with recent studies increasingly focusing on tear-EVs as a source of biomarkers for ocular diseases[85] [Table 2].
Summary of diseases and methodologies for biomarker profiling in tear samples, including sample number, collection method, EV isolation and characterization, and biomarker discovery
| Aim | Methodology | Disease | Sample number | Tear collection method | EV isolation method | EV characterization | Biomarker discovery | References |
| Proteomic analysis | Liquid chromatography | Graves’ ophthalmopathy | 48 patients 16 controls | Schirmer strips | Polymer precipitation | NTA, TEM, western blot, Luminex, ELISA | IL-1, IL-18, Caspase-3, C4A, APOA-IV | [75] |
| Proteomic analysis | Liquid chromatography | Sjögren syndrome | 27 patients | Schirmer strips | Size exclusion chromatography | NTA, flow cytometry | CPNE1, CALM | [77] |
| Proteomic analysis | Liquid chromatography | Sjögren syndrome | 25 patients 10 controls | Schirmer strips | Size exclusion chromatography | NTA, flow cytometry | STOM, ANXA4, ANXA11 | [78] |
| Proteomic analysis | Liquid chromatography | Glaucoma | 16 patients 17 controls | Schirmer strips | Instrumental cell sorting | Flow cytometry | PML | [86] |
| Transcriptomic analysis | RNA-sequencing | Dry eye disease | 5 patients 5 controls | Washing ocular surface | Polymer precipitation | ELISA, TEM | miR-6506-5p, miR-6750-3p, miR-3669, miR-7853-5p, miR-4492 | [87] |
| Transcriptomic analysis | RNA-sequencing | Dry eye disease | 10 patients 2 groups | Schirmer strips | Polymer precipitation | NTA, TEM, flow cytometry, western blot | SCNM, mir-130b | [88] |
| Transcriptomic analysis | RNA-sequencing | Diabetic retinopathy | 19 patients 11 controls | Schirmer strips | Negative-pressure | NTA, TEM, western blot | miR-145-5p, miR-214-3p, miR-218-5p, and miR-9-5p | [89] |
In this sense, proteomic studies using liquid chromatography with tandem mass spectrometry analysis have been conducted to analyze tear-EV protein expression in diseases such as glaucoma, Graves’ ophthalmopathy, and primary Sjögren syndrome. Rossi et al. collected tears via the Schirmer test and isolated EVs using instrumental cell sorting based on specific EV markers. They reported PML protein activation in tear-derived EVs from glaucoma patients, which is involved in apoptosis and regulated, among others, by ACTG1, Actin, and lysozyme C[86]. Their analysis also revealed that EV cargo was enriched in lysozyme, one of the most expressed tear proteins, involved in the recruitment of neutrophils[90]. Regarding Graves’ ophthalmopathy, an autoimmune thyroid eye disease that affects the ocular surface, Shi et al. reported overexpression of IL-1 and IL-18 in tear EVs compared to healthy controls[75]. These cytokines are well-known mediators of inflammation and the aging process[91]. Tears were collected using the Schirmer test, and tear-EVs were subsequently isolated with a commercial kit based on precipitation. Aqrawi et al. studied tear-derived EVs from patients with primary Sjögren syndrome, an autoimmune disease affecting exocrine glands including salivary and lacrimal glands. They collected tears using the Schirmer test, and isolated EVs via size exclusion chromatography for proteomic analysis. They observed upregulated proteins in patients compared to healthy controls, involved in NF-α signaling and B cell survival, such as thioredoxin-dependent peroxide reductase (PRDX3), copine (CPNE1), and aconitate hydratase (ACO2)[77]. The authors also reported upregulated cellular processes related to retina homeostasis and central innate and adaptive immune responses in the patient group[78].
Transcriptomic analyses of tear-derived EVs from dry eye disease and diabetic retinopathy patients have also been performed via RNA sequencing. Pucker et al. collected tears by eye wash with PBS and isolated EVs using polyethylene glycol polymer precipitation. RNA sequencing of EV microRNAs revealed several upregulated microRNAs involved in inflammation (miR-127-5p, miR-1273h-3p, miR-1288-5p, miR-130b-5p, miR-139-3p, miR-1910-5p, miR-203b-5p, miR-22-5p, and miR-4632-3p)[87]. Similarly, Cross et al. analyzed tear-EV RNA profiles from two groups of dry eye patients, identifying differential expression of sodium channel modifier 1 (SCNM1) and immature miRNA-130b[88]. In this case, tears were collected via the Schirmer test, and EVs were isolated using a commercial precipitation kit. Finally, Hu et al.[89] studied tear-EV RNA expression in patients with diabetic retinopathy. Tears were collected by the Schirmer test, and EVs were isolated using a custom harmonic oscillation-based kit. They identified several dysregulated microRNAs, including miR-145-5p, related to NF-kB signaling and endothelial dysfunction[92]; miR-9-5p, implicated in angiogenesis and insulin secretion[93]; and miR-214-3p and miR-218-5p, strongly associated with insulin resistance[94].
These findings highlight the potential of tear-derived EVs in the diagnosis of inflammatory and autoimmune eye diseases. However, the use of tear EVs for biomarker discovery remains in its initial stages, and further research is needed to establish their clinical utility.
TEAR-EV POTENTIAL IN NEURODEGENERATIVE DISEASE BIOMARKER DISCOVERY
Tear fluid provides specific information about the tissues beneath the eye. However, systemic diseases can also influence tear proteome patterns. Consequently, analysis of tear film protein composition has emerged as a useful diagnostic approach not only for ocular conditions but also for systemic diseases[56,95]. Because the retina is an extension of the central nervous system[96], the brain-eye axis has attracted increasing attention in the study of neurodegenerative disorders such as multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease. Given that tears are a more accessible and less complex body fluid than serum or plasma and their collection is much less invasive[95] than that of aqueous or vitreous humor, there is a growing interest in their study as a source of biomarkers for neurodegenerative diseases. In particular, several studies have explored tear-derived EVs as promising biomarker candidates.
Pieragostino et al. found microglia- and neural-derived EVs in tears through multiple proteomic studies in patients with multiple sclerosis. The authors collected CSF and tears using lumbar puncture and Schirmer test, respectively, and isolated EVs from both fluids by cell sorting based on specific markers. Their results showed that EVs from the CSF and tears of multiple sclerosis patients shared similar protein profiles, with a 73.1% overlap, particularly in proteins involved in inflammation, angiogenesis, and immune response signaling[97]. In another study, Salvisberg et al. reported reduced levels of proline-rich protein 4 in tears, CSF, and serum of patients with multiple sclerosis[98]. Proline-rich protein 4, a major tear protein, has previously been associated with various ocular diseases including dry eye syndrome[31,99] and glaucoma[90]. These findings suggest that the protein cargo of tears, particularly within tear EVs, may reflect the pathophysiological state of the CSF.
Other investigations focused on the close relationship between the brain and the eye have explored the implications of tear fluid in Alzheimer’s disease. Lee et al. performed proteomic analyses and identified adenylyl cyclase-associated protein 1 as a potential tear biomarker for the disease[100]. This protein is essential for the proper functioning of cones, and altered levels may contribute to synaptic dysfunction[101]. This finding is consistent with another study reporting differential expression of adenylyl cyclase-associated protein 1 in serum-derived EVs from Alzheimer’s patients[102], reinforcing the predictive value of tear biomarkers in this neurodegenerative disease. Following this line, a pilot study in Parkinson’s disease revealed some disease-specific protein patterns in patients’ tear fluid. The authors found 21 proteins significantly increased and 9 downregulated compared to controls, most of them involved in immune response, lipid metabolism, and oxidative stress[103]. Notably, in line with the aforementioned study on multiple sclerosis[98], proline-rich protein 4 was among the downregulated proteins. These shared alterations across different biofluids point to molecular crosstalk among CSF, plasma, and tears, underscoring the diagnostic potential of tear-derived EVs in neurodegenerative disorders.
ADVANTAGES, LIMITATIONS, AND FUTURE PERSPECTIVES
As outlined throughout this review, the use of tears as a source of biomarkers has gained considerable attention in recent years, primarily due to their easy accessibility, non-invasive collection, and simple sampling procedures. Numerous studies have explored the potential of this biofluid to reveal key molecular pathways involved in the progression of various diseases[95]. These include ocular conditions such as dry eye disease, diabetic retinopathy, and glaucoma[49]. Tears are particularly valuable in this context, as they originate close to the site of pathology and specifically reflect the (patho)physiological state of the eye. They can mirror changes in inflammatory and pro-fibrotic markers induced by topical medications prescribed for diverse inflammatory eye diseases[104]. Recent reviews have summarized the key molecular mechanisms underlying glaucoma development[105], offering new perspectives for preventing ocular fibrosis in clinical practice[106]. On the other hand, tears can also serve as a valuable tool for detecting systemic disorders, including neurodegenerative diseases and certain cancers[107,108]. For instance, breast cancer-specific miR-21 and miR-200c have been identified in tear EVs from patients with metastatic disease, pointing to the broader diagnostic potential of tear-derived EVs[50].
The potential of tear-based biomarkers in different pathological contexts has been reviewed previously[109]. However, research on tear-EV-derived biomarkers for ocular, neurodegenerative diseases, and cancer is still scarce. Recent reviews have highlighted the diagnostic potential of EVs derived from different ocular fluids, including aqueous humor, vitreous humor, and tears[16,110,111]. In fact, although the collection of aqueous[112,113] and vitreous humor[114,115] is more invasive, these fluids may still have specific applications in ocular disease research. Nevertheless, the non-invasive collection of tear-EVs offers a clear advantage, providing a rapid, simple, and minimally invasive method for identifying biomarkers for early disease detection and monitoring.
Despite these advantages, several technical limitations must be considered. The first challenge lies in the method of tear collection. Currently, the most widely used techniques - microcapillary tubes and Schirmer strips - are both minimally invasive and easy to implement. However, microcapillary collection can be hindered by involuntary blinking or low tear volume[3], while the application of anesthesia may have an impact on tear composition. When performed with topical anesthesia, the Schirmer test measures basal tear production under unstimulated conditions; without anesthesia, however, it induces irritation and reflex tearing, thus measuring both basal and reflex tear secretion[69]. Consequently, the collection method itself may influence tear composition. For example, two of the studies discussed in this review, which conducted transcriptomic analyses of tear-derived EVs in patients with dry eye syndrome[87,88], despite using the same EV isolation methodology, employed different tear collection techniques and reported distinct results. While both studies included a limited number of patients, the findings suggest that the sampling method may significantly affect outcomes. Further comparative analyses are therefore warranted to clarify the extent of these differences[88,116].
Once collected, an equally critical step is the selection of an appropriate EV isolation method. Size exclusion chromatography, either alone or combined with ultrafiltration, or ultracentrifugation is commonly considered a gold-standard approach in EV research[80]. However, the limited yield obtained from tear samples represents a significant bottleneck in tear-EV analysis. Notably, tears contain significantly less protein than serum or plasma, which may facilitate cleaner EV isolation. On the other hand, SEC may further dilute samples, resulting in the loss of valuable EV fractions. To address this, some researchers pool tear samples from multiple individuals before applying SEC[77,78]. This approach increases sample volume and reduces inter‐ or intra‐subject background noise[36], but at the cost of sample identity and potentially meaningful individual variability. Alternatively, commercial precipitation-based kits are frequently used because they provide higher yields, although with reduced purity[75,88,89]. As with other EV studies, the choice of isolation method should ultimately depend on the specific objectives of the research.
Another source of variability across studies arises from storage conditions for tear samples and/or isolated EVs. Most researchers store tear samples at 4 °C for short-term use, followed by freezing at -80 °C for long-term storage, either before or after EV isolation. However, standardized protocols for EV storage in low-volume fluids such as tears are lacking, and key details including temperature, storage duration, and buffer composition are often omitted from published studies. Establishing methodological standards and reporting all preanalytical details will be critical to improving reproducibility in future research.
CONCLUSIONS
Significant progress has been made in recent years in exploiting tear-EV characteristics as biomarkers. Nonetheless, further efforts are required to standardize and fully report tear-EV collection and isolation methods to ensure reproducibility across laboratories. In this context, the adoption of Standard PREanalytical Code (SPREC) parameters[117] for codifying and documenting preanalytical conditions in biofluid specimens may help in assessing EV parameters relevant to clinical practice. Together with adherence to ISEV guidelines for reporting EV research[39], these measures will advance tear-derived EV research as a promising frontier in predictive and diagnostic biomarker discovery, particularly for ocular and neurodegenerative diseases.
DECLARATIONS
Acknowledgments
Figures were created with Biorender.com. Graphic Abstract: https://BioRender.com/o3r915t.
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Sanroque-Muñoz M
Drafted the work and revised it critically for important intellectual content: Sanroque-Muñoz M
Approved the final version to be published: Sanroque-Muñoz M, Garcia SG, Pan L, Clos-Sansalvador M, Font-Morón M, Botella-Garcia J, Loscos-Arenas J, Borras FE
Agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Sanroque-Muñoz M, Garcia SG, Pan L, Clos-Sansalvador M, Font-Morón M, Botella-Garcia J, Loscos-Arenas J, Borras FE
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported in part by the Instituto Carlos III project PI22/00688, integrated within the National R + D + I and funded by the ISCIII and the European Regional Development Funds. This work was partially funded by the CERTERA project (CERT22/00052) from the Instituto de Salud Carlos III (ISCIII), co-funded by the European Union - NextGenerationEU funds. Garcia SG was supported by the Catalan Health department (“Departament de Salut”) through a PERIS-PIF-Salut grant (SLT017/ 20/000158); Pan L is funded by the UAB-CSC collaborative program. Botella-Garcia J received a grant from the Catalan Society of Ophthalmology. The REMAR group is recognized as a consolidated research group by AGAUR (SGR-GRC-00187) and is funded through the Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD24/0004/0005), supported by the European Union - NextGenerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR). Borras FE is a senior researcher at the Germans Trias i Pujol Health Science Research Institute, supported by the Health Department of the Catalan Government (Generalitat de Catalunya).
Conflicts of interest
The authors declare that there are no conflicts of interest related to this work. The funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. World Health Organization. Blindness and vision impairment. World Health Organization (Geneva, Switzerland), August 10, 2023. Available from https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment [accessed 28 September 2025].
2. Fricke TR, Tahhan N, Resnikoff S, et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018;125:1492-9.
3. Beykin G, Norcia AM, Srinivasan VJ, Dubra A, Goldberg JL. Discovery and clinical translation of novel glaucoma biomarkers. Prog Retin Eye Res. 2021;80:100875.
4. Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12:1322-5.
5. Bhedasgaonkar S, Nadkarni SU. Extent of glaucomatous damage on the first presentation. Oman J Ophthalmol. 2023;16:227-32.
6. Schwartz R, Loewenstein A. Early detection of age related macular degeneration: current status. Int J Retina Vitreous. 2015;1:20.
7. Krishnadas R. The many challenges in automated glaucoma diagnosis based on fundus imaging. Indian J Ophthalmol. 2021;69:2566-7.
8. Tan O, Chen A, Li Y, et al. Prospective evaluation of optical coherence tomography for disease detection in the Casey mobile eye clinic. Exp Biol Med. 2021;246:2214-21.
9. Cicinelli MV, Marmamula S, Khanna RC. Comprehensive eye care - issues, challenges, and way forward. Indian J Ophthalmol. 2020;68:316-23.
10. Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23:369-82.
11. Momen-Heravi F, Balaj L, Alian S, et al. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 2012;3:162.
12. Posa A, Bräuer L, Schicht M, Garreis F, Beileke S, Paulsen F. Schirmer strip vs. capillary tube method: non-invasive methods of obtaining proteins from tear fluid. Ann Anat. 2013;195:137-42.
13. Ravishankar P, Daily A. Tears as the next diagnostic biofluid: a comparative study between ocular fluid and blood. Applied Sciences. 2022;12:2884.
14. Torok Z, Peto T, Csosz E, et al. Tear fluid proteomics multimarkers for diabetic retinopathy screening. BMC Ophthalmol. 2013;13:40.
15. Rehman I, Hazhirkarzar B, Patel BC. Anatomy, head and neck, eye. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.
16. Shiju TM, Yuan A. Extracellular vesicle biomarkers in ocular fluids associated with ophthalmic diseases. Exp Eye Res. 2024;241:109831.
17. Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19:323-44.
18. Angi M, Kalirai H, Coupland SE, Damato BE, Semeraro F, Romano MR. Proteomic analyses of the vitreous humour. Mediators Inflamm. 2012;2012:148039.
19. Zong Y, Gao QY, Hui YN. Vitreous function and intervention of it with vitrectomy and other modalities. Int J Ophthalmol. 2022;15:857-67.
20. Koss MJ, Hoffmann J, Nguyen N, et al. Proteomics of vitreous humor of patients with exudative age-related macular degeneration. PLoS One. 2014;9:e96895.
21. Hiller JK, Sandås EM, Rootwelt H, et al. Metabolomic biomarkers in vitreous humor: unveiling the molecular landscape of diabetic retinopathy progression. Int J Retina Vitreous. 2025;11:58.
22. Yu M, Xie F, Liu X, et al. Proteomic study of aqueous humor and its application in the treatment of neovascular glaucoma. Front Mol Biosci. 2020;7:587677.
23. Buffault J, Labbé A, Hamard P, Brignole-Baudouin F, Baudouin C. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. J Fr Ophtalmol. 2020;43:e217-30.
24. Kaeslin MA, Killer HE, Fuhrer CA, Zeleny N, Huber AR, Neutzner A. Changes to the aqueous humor proteome during glaucoma. PLoS One. 2016;11:e0165314.
25. Fernández-Vega Cueto A, Álvarez L, García M, et al. Candidate glaucoma biomarkers: from proteins to metabolites, and the pitfalls to clinical applications. Biology. 2021;10:763.
26. Huang K, Schofield C, Nguy T, et al. Proteomics approach identifies aqueous humor biomarkers in retinal diseases. Commun Med. 2025;5:134.
27. Jin Y, Liu J, Zhang X, et al. Stage-dependent proteomic alterations in aqueous humor of diabetic retinopathy patients based on data-independent acquisition and parallel reaction monitoring. J Transl Med. 2025;23:476.
28. Khanna RK, Catanese S, Emond P, Corcia P, Blasco H, Pisella PJ. Metabolomics and lipidomics approaches in human tears: a systematic review. Surv Ophthalmol. 2022;67:1229-43.
29. Sheppard JD, Nichols KK. Dry eye disease associated with meibomian gland dysfunction: focus on tear film characteristics and the therapeutic landscape. ophthalmol ther. 2023;12:1397-418.
30. Zhou L, Beuerman RW. The power of tears: how tear proteomics research could revolutionize the clinic. Expert Rev Proteomics. 2017;14:189-91.
31. Aluru SV, Agarwal S, Srinivasan B, et al. Lacrimal proline rich 4 (LPRR4) protein in the tear fluid is a potential biomarker of dry eye syndrome. PLoS One. 2012;7:e51979.
32. Bao J, Tian L, Meng Y, et al. Total IgE in tears accurately reflects the severity and predicts the prognosis of seasonal allergic conjunctivitis. Clin Transl Allergy. 2022;12:e12139.
33. Liu J, Jiang F, Jiang Y, et al. Roles of exosomes in ocular diseases. Int J Nanomedicine. 2020;15:10519-38.
34. Kapnisis K, Doormaal MV, Ross Ethier C. Modeling aqueous humor collection from the human eye. J Biomech. 2009;42:2454-7.
35. Fan Z, Hu Y, Chen L, et al. Multiplatform tear proteomic profiling reveals novel non-invasive biomarkers for diabetic retinopathy. Eye. 2024;38:1509-17.
36. Ponzini E, Santambrogio C, De Palma A, Mauri P, Tavazzi S, Grandori R. Mass spectrometry-based tear proteomics for noninvasive biomarker discovery. Mass Spectrom Rev. 2022;41:842-60.
37. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89.
38. Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
39. Welsh JA, Goberdhan DCI, O’Driscoll L, et al; MISEV Consortium. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404.
40. Radeghieri A, Bergese P. The biomolecular corona of extracellular nanoparticles holds new promises for advancing clinical molecular diagnostics. Expert Rev Mol Diagn. 2023;23:471-4.
41. García-Bernal D, García-Arranz M, Yáñez RM, et al. The current status of mesenchymal stromal cells: controversies, unresolved issues and some promising solutions to improve their therapeutic efficacy. Front Cell Dev Biol. 2021;9:650664.
42. Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics. 2021;13:122.
43. Fais S, O’Driscoll L, Borras FE, et al. Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano. 2016;10:3886-99.
44. Habibi A, Zarei-Behjani Z, Falamarzi K, et al. Extracellular vesicles as a new horizon in the diagnosis and treatment of inflammatory eye diseases: a narrative review of the literature. Front Immunol. 2023;14:1097456.
46. Anitua E, Muruzabal F, Tayebba A, et al. Autologous serum and plasma rich in growth factors in ophthalmology: preclinical and clinical studies. Acta Ophthalmol. 2015;93:e605-14.
47. Zhou L, Zhao SZ, Koh SK, et al. In-depth analysis of the human tear proteome. J Proteomics. 2012;75:3877-85.
48. Tham ML, Mahmud A, Abdullah M, et al. Tear samples for protein extraction: comparative analysis of schirmer’s test strip and microcapillary tube methods. Cureus. 2023;15:e50972.
49. Fu R, Klinngam W, Heur M, Edman MC, Hamm-Alvarez SF. Tear proteases and protease inhibitors: potential biomarkers and disease drivers in ocular surface disease. Eye Contact Lens. 2020;46 Suppl 2:S70-83.
50. Inubushi S, Kawaguchi H, Mizumoto S, et al. Oncogenic miRNAs identified in tear exosomes from metastatic breast cancer patients. Anticancer Res. 2020;40:3091-6.
51. Dartt DA, Willcox MD. Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp Eye Res. 2013;117:1-3.
52. Janssen PT, van Bijsterveld OP. Origin and biosynthesis of human tear fluid proteins. Invest Ophthalmol Vis Sci. 1983;24:623-30.
53. Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. 2012;31:527-50.
54. Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clin Chim Acta. 2006;369:17-28.
55. Ghaffariyeh A, Honarpisheh N, Shakiba Y, et al. Brain-derived neurotrophic factor in patients with normal-tension glaucoma. Optometry. 2009;80:635-8.
56. Thun Und Hohenstein-Blaul N, Funke S, Grus FH. Tears as a source of biomarkers for ocular and systemic diseases. Exp Eye Res. 2013;117:126-37.
57. Matheis N, Grus FH, Breitenfeld M, et al. Proteomics differentiate between thyroid-associated orbitopathy and dry eye syndrome. Invest Ophthalmol Vis Sci. 2015;56:2649-56.
58. Aass C, Norheim I, Eriksen EF, Thorsby PM, Pepaj M. Single unit filter-aided method for fast proteomic analysis of tear fluid. Anal Biochem. 2015;480:1-5.
59. Winiarczyk M, Kaarniranta K, Winiarczyk S, Adaszek Ł, Winiarczyk D, Mackiewicz J. Tear film proteome in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2018;256:1127-39.
60. Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: breaking the barrier. Pathophysiology. 2017;24:229-41.
61. Soria J, Acera A, Merayo-LLoves J, et al. Tear proteome analysis in ocular surface diseases using label-free LC-MS/MS and multiplexed-microarray biomarker validation. Sci Rep. 2017;7:17478.
62. Burgos-Blasco B, Güemes-Villahoz N, Santiago JL, et al. Hypercytokinemia in COVID-19: tear cytokine profile in hospitalized COVID-19 patients. Exp Eye Res. 2020;200:108253.
63. Holly FJ, Lamberts DW, Esquivel ED. Kinetics of capillary tear flow in the Schirmer strip. Curr Eye Res. 1982;2:57-70.
64. Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013:2.
65. Bruszel B, Tóth-Molnár E, Janáky T, Szabó Z. Sources of variance in human tear proteomic samples: statistical evaluation, quality control, normalization, and biological insight. Int J Mol Sci. 2024;25:1559.
66. Gijs M, Arumugam S, van de Sande N, et al. Pre-analytical sample handling effects on tear fluid protein levels. Sci Rep. 2023;13:1317.
67. Sitaramamma T, Shivaji S, Rao GN. Effect of storage on protein concentration of tear samples. Curr Eye Res. 1998;17:1027-35.
68. Chiang JCB, Krishnan AV, Goldstein D, Markoulli M. The impact of post-tear collection storage on tear film substance P concentration. Curr Eye Res. 2022;47:1116-20.
69. Brott NR, Zeppieri M, Ronquillo Y. Schirmer Test. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024.
70. Clos-Sansalvador M, Monguió-Tortajada M, Roura S, Franquesa M, Borràs FE. Commonly used methods for extracellular vesicles’ enrichment: implications in downstream analyses and use. Eur J Cell Biol. 2022;101:151227.
71. Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36:301-12.
72. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347.
73. Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031.
74. Amorim M, Martins B, Caramelo F, et al. Putative biomarkers in tears for diabetic retinopathy diagnosis. Front Med. 2022;9:873483.
75. Shi TT, Zhao RX, Xin Z, et al. Tear-derived exosomal biomarkers of Graves’ ophthalmopathy. Front Immunol. 2022;13:1088606.
76. Grubisic Z, Rempp P, Benoit H. A universal calibration for gel permeation chromatography. J Polym Sci B Polym Lett. 1967;5:753-9.
77. Aqrawi LA, Galtung HK, Vestad B, et al. Identification of potential saliva and tear biomarkers in primary Sjögren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res Ther. 2017;19:14.
78. Aqrawi LA, Galtung HK, Guerreiro EM, et al. Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res Ther. 2019;21:181.
79. Gelibter S, Marostica G, Mandelli A, et al. The impact of storage on extracellular vesicles: a systematic study. J Extracell Vesicles. 2022;11:e12162.
80. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
81. Asif M, Asif A, Rahman UA, et al. Incidence of glaucoma in type 2 diabetes patients treated with GLP-1 receptor agonists: a systematic review and meta-analysis. Endocrinol Diabetes Metab. 2025;8:e70059.
82. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276-83.
83. Dai X, Hui X, Xi M. Critical factors driving diabetic retinopathy pathogenesis and a promising interventional strategy. Biomed Pharmacother. 2025;189:118106.
84. Grigor’eva AE, Tamkovich SN, Eremina AV, et al. Characteristics of exosomes andmicroparticles discovered in human tears. Biomed Khim. 2016;62:99-106.
85. Manai F, Smedowski A, Kaarniranta K, Comincini S, Amadio M. Extracellular vesicles in degenerative retinal diseases: a new therapeutic paradigm. J Control Release. 2024;365:448-68.
86. Rossi C, Cicalini I, Cufaro MC, et al. Multi-omics approach for studying tears in treatment-naïve glaucoma patients. Int J Mol Sci. 2019;20:4029.
87. Pucker AD, Ngo W, Postnikoff CK, Fortinberry H, Nichols JJ. Tear film miRNAs and their association with human dry eye disease. Curr Eye Res. 2022;47:1479-87.
88. Cross T, Øvstebø R, Brusletto BS, et al. RNA profiles of tear fluid extracellular vesicles in patients with dry eye-related symptoms. Int J Mol Sci. 2023;24:15390.
89. Hu L, Zhang T, Ma H, et al. Discovering the secret of diseases by incorporated tear exosomes analysis via rapid-isolation system: iTEARS. ACS Nano. 2022;16:11720-32.
90. Pieragostino D, Agnifili L, Fasanella V, et al. Shotgun proteomics reveals specific modulated protein patterns in tears of patients with primary open angle glaucoma naïve to therapy. Mol Biosyst. 2013;9:1108-16.
91. Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S-55S.
92. Xiao J, Xu Z. Roles of noncoding RNAs in diabetic retinopathy: mechanisms and therapeutic implications. Life Sci. 2024;357:123092.
93. Suire S, Lécureuil C, Anderson KE, et al. GPCR activation of Ras and PI3Kc in neutrophils depends on PLCb2/b3 and the RasGEF RasGRP4. EMBO J. 2012;31:3118-29.
94. Bresciani E, Saletti C, Squillace N, et al. miRNA-218 targets lipin-1 and glucose transporter type 4 genes in 3T3-L1 cells treated with lopinavir/ritonavir. Front Pharmacol. 2019;10:461.
95. Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 2016;7:15.
96. Kaštelan S, Braš M, Pjevač N, et al. Tear biomarkers and Alzheimer’s disease. Int J Mol Sci. 2023;24:13429.
97. Pieragostino D, Lanuti P, Cicalini I, et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J Proteomics. 2019;204:103403.
98. Salvisberg C, Tajouri N, Hainard A, Burkhard PR, Lalive PH, Turck N. Exploring the human tear fluid: discovery of new biomarkers in multiple sclerosis. Proteomics Clin Appl. 2014;8:185-94.
99. Boehm N, Funke S, Wiegand M, Wehrwein N, Pfeiffer N, Grus FH. Alterations in the tear proteome of dry eye patients - a matter of the clinical phenotype. Invest Ophthalmol Vis Sci. 2013;54:2385-92.
100. Lee S, Kim E, Moon CE, et al. Amplified fluorogenic immunoassay for early diagnosis and monitoring of Alzheimer’s disease from tear fluid. Nat Commun. 2023;14:8153.
101. Schneider F, Duong TA, Metz I, et al. Mutual functional dependence of cyclase-associated protein 1 (CAP1) and cofilin1 in neuronal actin dynamics and growth cone function. Prog Neurobiol. 2021;202:102050.
102. Zhong J, Ren X, Liu W, et al. Discovery of novel markers for identifying cognitive decline using neuron-derived exosomes. Front Aging Neurosci. 2021;13:696944.
103. Boerger M, Funke S, Leha A, et al. Proteomic analysis of tear fluid reveals disease-specific patterns in patients with Parkinson’s disease - a pilot study. Parkinsonism Relat Disord. 2019;63:3-9.
104. Park HY, Kim JH, Lee KM, Park CK. Effect of prostaglandin analogues on tear proteomics and expression of cytokines and matrix metalloproteinases in the conjunctiva and cornea. Exp Eye Res. 2012;94:13-21.
105. Bugara K, Pacwa A, Smedowski A. Molecular pathways in experimental glaucoma models. Front Neurosci. 2024;18:1363170.
106. Mallone F, Costi R, Marenco M, et al. Understanding drivers of ocular fibrosis: current and future therapeutic perspectives. Int J Mol Sci. 2021;22:11748.
107. Fotovat-Ahmadi N, Siddiqui O, Ong J, et al. The ocular surface tear film as a biomarker for systemic health. Ocul Surf. 2025;37:283-300.
108. Sampani K, Ness S, Tuz-Zahra F, et al. Neurodegenerative biomarkers in different chambers of the eye relative to plasma: an agreement validation study. Alzheimers Res Ther. 2024;16:192.
109. Tamhane M, Cabrera-Ghayouri S, Abelian G, Viswanath V. Review of biomarkers in ocular matrices: challenges and opportunities. Pharm Res. 2019;36:40.
110. Su Y, Chen M, Xu W, Gu P, Fan X. Advances in extracellular-vesicles-based diagnostic and therapeutic approaches for ocular diseases. ACS Nano. 2024;18:22793-828.
111. Phan N, Li Y, Yang M, Liu F. Tear fluid derived extracellular vesicles for new biomarker discovery. Ocul Surf. 2025;37:314-22.
112. Wen K, Fu M, Li Y, et al. The effect of age on aqueous humor of humans with high myopia. Mol Vis 2024;30:137-49.
113. Metayer C, Kodjikian L, Nguyen AM, et al. Interest of regular assays of aqueous humor interleukin-10 levels in monitoring of vitreoretinal lymphoma. Retina. 2024;44:1807-13.
114. Asakage M, Usui Y, Komatsu H, et al. Comprehensive microRNA analyses using vitreous humor of ocular sarcoidosis. Graefes Arch Clin Exp Ophthalmol. 2025;263:501-26.
115. Balikov DA, Brown NA, Elner VM, Wubben TJ, Rao RC, Demirci H. Posterior uveitis in ocular-involving chronic lymphocytic leukemia and the utility of negative MYD88 L265P testing in the diagnosis. Ocul Oncol Pathol. 2024;10:103-13.
116. Rentka A, Koroskenyi K, Harsfalvi J, et al. Evaluation of commonly used tear sampling methods and their relevance in subsequent biochemical analysis. Ann Clin Biochem. 2017;54:521-9.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].