Extracellular vesicles from bacteria and fungi: mechanistic insights and implications for urinary tract infections
Abstract
Urinary tract infections (UTIs) pose a significant public health challenge, affecting approximately 407 million people worldwide and causing substantial morbidity and approximately 237,000 deaths. Bacteria and fungi represent the most frequent causative microbes, leading to symptoms such as low abdominal pain, fever, frequent urination, hematuria, sepsis, inflammation of the bladder and kidney, and even death. In recent years, extracellular vesicles (EVs) have emerged as critical mediators of UTI pathogenesis. EVs are lipid bilayer nanoscale particles that carry DNA, RNA, enzymes, and other biomolecules. They can facilitate microbial colonization, immune modulation and evasion, tissue invasion, and antimicrobial agent resistance. This review summarizes current knowledge on the role of bacterial and fungal-derived EVs in UTIs, their mechanisms of action, and their potential therapeutic implications.
Keywords
INTRODUCTION
Urinary tract infections (UTIs) remain a significant public health challenge worldwide. In 2019, approximately 407 million people were affected, resulting in approximately 237,000 deaths[1]. UTIs can be caused by highly diverse microbes, including fungi, bacteria, parasites, and viruses, and the symptoms can range from dysuria and low abdominal pain to fever, frequent urination, and hematuria[2]. Severe complications include sepsis, renal inflammation, and organ failure, as well as multi-organ dysfunction[3]. Of note, recurrent infection is widespread[4].
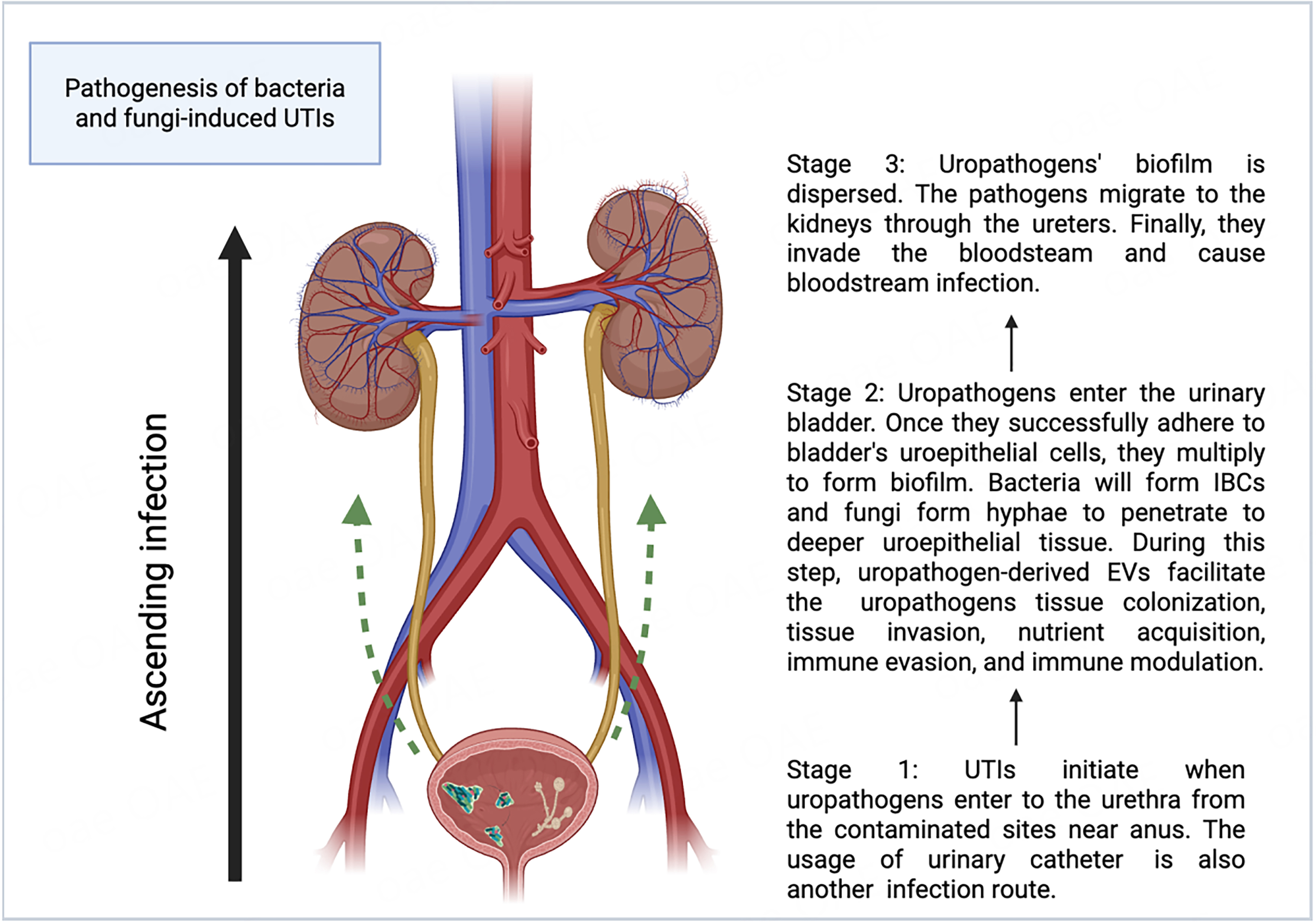
UTIs typically start when pathogens enter from the anus or a contaminated area and ascend the urethra to the urinary bladder[5]. Successful urethral colonization is a prerequisite for UTIs[6]. Pathogens utilize their flagella to migrate from the urethra to the urinary bladder[7]. Adhesins are secreted to counteract the urine flow and facilitate attachment to receptors on uroepithelial cells[8,9]. Urinary catheters provide an additional route for pathogen entry into the bladder[10]. Once inside the bladder, pathogens attach to the uroepithelial cells and begin to replicate[11,12]. In the meantime, uropathogens adopt various strategies to evade and modulate the host immune response[13-19]. Bacteria, such as uropathogenic Escherichia coli (UPEC), hide in and enter the uroepithelial cell cytosol to form intracellular bacterial communities (IBCs)[20]. Fungi, especially Candida albicans (C. albicans), form hyphae and secrete agglutinin-like sequence 3 protein (Als3) for the uroepithelial cell attachment, which is critical for biofilm formation and active penetration into the host’s deeper uroepithelial cells and further into the bloodstream[21,22]. Furthermore, uropathogens produce toxins and express proteins for survival in the bladder[14,23]. Upon successfully colonizing the urinary bladder, uropathogens can be dispersed from the biofilm and invade the kidneys through the ureters, ultimately causing bloodstream infection[12,24-26]. Figure 1 shows the pathogenesis of bacteria- and fungi-induced UTIs.
Figure 1. Pathogenesis of bacteria- and fungi-induced UTIs (Created in Biorender). UTIs can be divided into three stages. In the first stage, uropathogens invade and colonize the urethra[5-7]. In the second stage, they invade the urinary bladder and form biofilms[11,12,20-22], where uropathogen-derived extracellular vesicles (EVs) facilitate invasion, colonization, nutrient acquisition, and immune modulation and evasion[13-19,27-33]. The third stage involves biofilm dispersion and propagation of uropathogens to the kidneys via the ureters and eventually to the bloodstream, causing systemic infection[12,24-26]. Created in BioRender. Chau, C. (2025) ( https://BioRender.com/st81pck). UTIs: Urinary tract infections; EVs: extracellular vesicles; IBCs: intracellular bacterial communities.
Treatment of UTIs currently relies primarily on antibiotics (e.g., trimethoprim sulfamethoxazole, ampicillin, and ciprofloxacin), antifungal drugs (e.g., fluconazole and flucytosine for Candida UTI), anti-parasitic drugs (e.g., praziquantel for treating urinary schistosomiasis), and anti-viral drugs (e.g., cidofovir for treating cystitis)[34-36]. However, the non-judicious use of these medications has led to the emergence of drug-resistant microorganisms, complicating the effective management of UTIs worldwide. This situation highlights the urgent need to study the drug-resistant mechanisms of uropathogens to identify new therapeutic targets, ultimately improving patient outcomes and addressing the challenge of drug-resistant UTIs[37,38].
Despite clear identification of causative agents in many UTIs, the mechanisms by which different microbes interact with the host and survive antimicrobial exposure still need further investigation. Recently, microbial-derived extracellular vesicles (EVs) have gained increasing attention. These lipid bilayer particles act as messengers between microbes and hosts by carrying metabolites, proteins, DNA, and RNA[39]. Compared to human-derived EVs, microbial-derived EVs differ substantially in biogenesis, composition, and function (except for fungi), which has attracted interest in their role during infections.
Regarding EV biogenesis, humans and fungi both generate exosomes via inward budding through the ESCRT (Endosomal Sorting Complex Required for Transport) pathway and ectosomes via outward budding[40-46]. The ESCRT pathway involves several components, including ESCRT-0, I, II, and associated proteins such as Alix[45-47]. First, ESCRT-0’s HRS (hepatocyte growth factor-regulated tyrosine kinase substrate) binds phosphatidylinositol-3-phosphate (PI3P), recruiting ESCRT-0 to the endosomal membrane[45-47]. The HRS domain subsequently attracts ESCRT-I through the TSG101 domain[45-47]. ESCRT-II, together with ESCRT-I, deforms the membrane to cause inward budding[45-47]. ESCRT-III will then undergo vesicle scission and promote the formation of intraluminal vesicles (ILVs) in the multivesicular bodies (MVB), which will then be released as exosomes[45-47]. However, for the outward budding, the ectosome is formed via the outward budding action of the cell membrane[45]. Of note, the possibility of the presence of other EVs biogenesis pathways of fungi that are different from that of humans may exist.
In contrast, gram-negative bacteria produce EVs (outer membrane vesicles and outer inner membrane vesicles) through outer membrane blebbing and explosive cell lysis[48,49]. In outer membrane blebbing, the EVs are formed through the blebbing of the bacterial outer membrane[48,50]. For explosive cell lysis, the membrane vesicles are formed from the lysis membrane fragments, as a result of stress-induced bacterial cell lysis[50,51]. Nonetheless, the EV production pathway of gram-positive bacteria is still uncertain, which leaves a research gap for scientists to study the components inside the bacterial cell that contribute to EV production[52].
Regarding the EVs’ composition and function, human-derived EVs deliver proteins, lipids, DNA, and RNA essential for maintaining homeostasis and involved in different pathological processes[48,53,54]. The main target of human-derived EVs is the human cell, for example, immune cells during the infection[55]. On the contrary, microbial-derived EVs carry proteins, lipids, DNA, and RNA that are important for facilitating their survival in the environment and invasion into the host[56,57]. It is of particular interest how these tiny vesicles can open a gateway to help microbes colonize and cause serious illness in human hosts, such as UTIs. Below, the differences between human and microbial-derived EVs (Fungi and bacteria) are shown in Table 1.
Similarities and differences of EVs derived from humans, fungi, and bacteria
| Humans | Fungi | Bacteria | References | |
| Reported EV size (nm) | 30-400 | 50-400 | 50-500 | [58-60] |
| Production route | Inward budding through ESCRT pathway and outward budding | Inward budding through ESCRT pathway and outward budding | Outer membrane blebbing and explosive cell lysis | [40-51] |
| Common EV markers | CD9, CD63, CD81, flottilin-1 | Not yet defined | Not yet defined | [61] |
| EV content | DNA, RNA, protein, lipid | DNA, RNA, protein, lipid | DNA, RNA, protein, lipid, peptidoglycan | [48,56,57] |
| Main function | Homeostasis maintenance and immune defense | Survival in environment, facilitation of host infection and invasion | Survival in environment, facilitation of host infection and invasion | [54-57] |
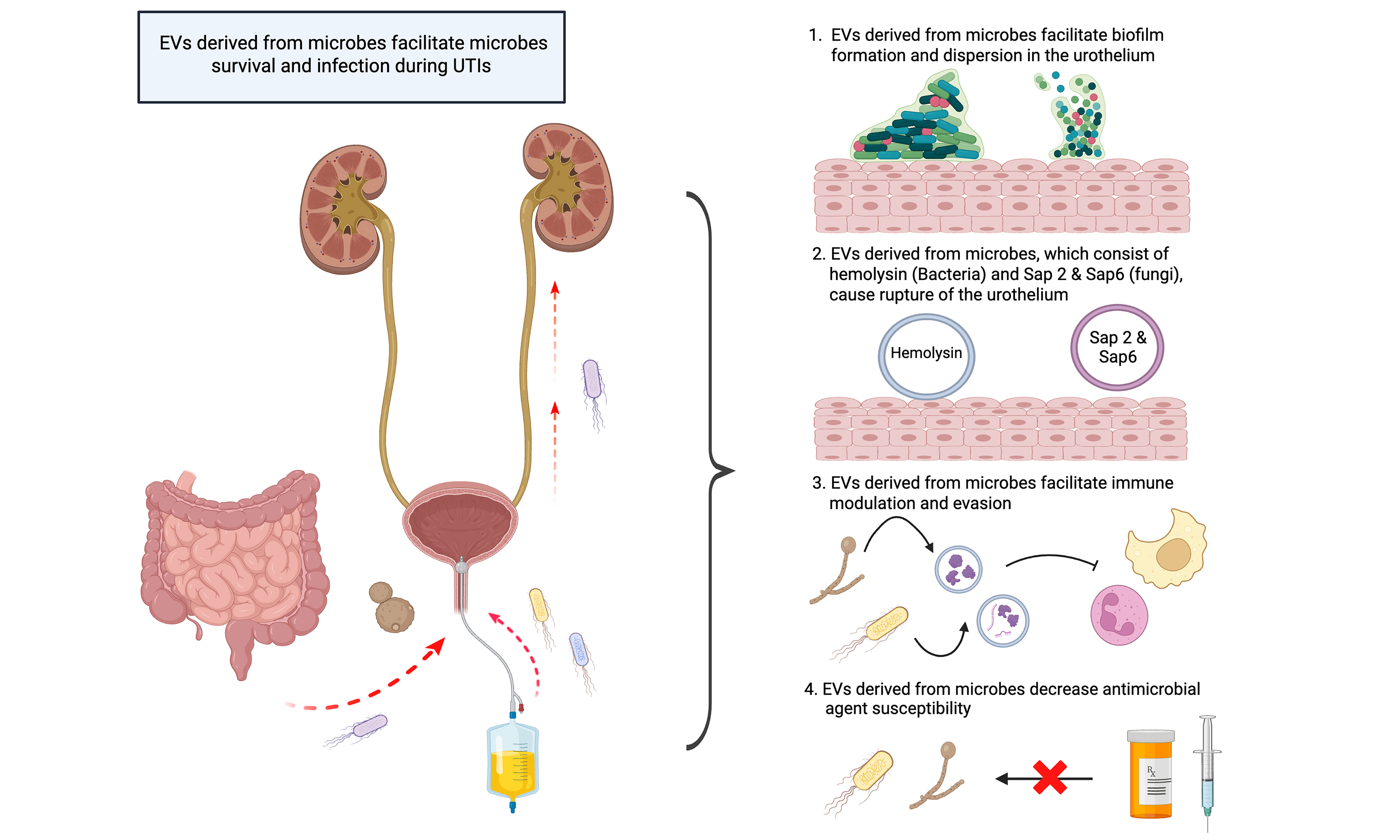
Besides, microbial-derived EVs are critical in enhancing microbes’ adherence to host tissue, biofilm formation, host immune response modulation, immune evasion, and decreasing antimicrobial agent susceptibility in the course of infection[62-65]. Few studies discussed how UTI pathogens interact with the host via EVs[27,29,66]. There is no doubt that EVs play a significant role during UTIs and minimize the effects of antimicrobial agents. In this review, we summarize the role of EVs derived from UTI-causing bacterial and fungal pathogens, thereby giving insight for microbiologists and clinicians to further study the UTI pathogen and host interaction. Additionally, Table 2 presents a brief summary to give readers an overview of the proteins or molecules in uropathogen-derived EVs that contribute to infection.
Summary of the role of identified proteins/molecules in uropathogenic bacterial and fungal-derived EVs
| Characteristics of UTI’s pathogen/roles during UTIs | Involved protein in bacterial-derived EVs | Involved protein/molecules in fungal-derived EVs | References |
| Colonization | AroB, AroG, AroK, MrpA | PHR1, XOG1, BGL2, CSH1, MP65, AMS1, Met6, TOS1, MNT1, CHT3, TRX2, SAP5, PET9 | [27,29-32,76-79,105,107,108] |
| Invasion to the host’s bloodstream | Hemolysin, EfeO, FepA | Sap2, RAS1 | [14,27,33,80,82,84-89,111,115-117] |
| Immune modulation/evasion | FimH, CNF1, LPS, Flagellin | Glucuronoxylomannan, Sap6 | [13-19,27,95-98,122,127-129] |
| Antimicrobial agent resistance | A-band liposaccharide, KCP protein, Aac6’-le-Aph2”-la, Aph3’-III, VanR-A, VanS-A, VanH-A, VanA, VanX-A, VanY-A, VanZ-A, VanS-B, ErmB | [30,31,65,87,99,101] | |
| Proposed protein that may contribute to antifungal agent resistance | xog-1-like, mp65-like and alcohol dehydrogenase 1 | [130-134] |
THE ROLE OF BACTERIA-DERIVED EVs IN BACTERIAL UTIs
Gram-negative bacteria, such as Escherichia coli and Klebsiella spp, are UTIs’ most common causative agents[67]. Although the human urinary tract has several intrinsic antimicrobial mechanisms, such as the secretion of glycoproteins that block microbial adherence to the uroepithelium, bacteria have a “secret strategy”, EVs, to overcome these defense mechanisms[68].
Bacteria-derived EVs facilitate movement and biofilm formation
Biofilm formation and motility are critical factors for bacterial colonization in urinary tissue and urinary catheters during UTIs[69,70]. Studies have shown that EVs derived from UPEC clinical isolate PMH can enhance motility and biofilm formation, potentially affecting the motility-to-biofilm transition pathway[29]. A key regulator molecule of this pathway, bis-(3’-5’)-cyclic dimeric guanosine monophosphate (c-di-GMP), is upregulated during biofilm formation; then, it interacts with the protein YcgR (commonly known as flagellar brake)[71,72]. The interaction between these molecules further leads to the direct contact of YcgR to the flagellar motor proteins FliG and MotA, thereby inhibiting the motility of the bacteria for biofilm formation[72]. Additionally, to facilitate the dispersion of bacteria’s biofilm in the urinary tract, a carbon storage regulator, CsrA, is gradually released during biofilm formation, whose primary function is to disaggregate the biofilm and facilitate the propagation of bacteria in the urinary tract[73,74]. However, further investigation is needed to confirm the role of EVs in the motility-to-biofilm transition pathway and to identify which components are affected by the bacterial-derived EVs. Previous research has demonstrated a strong relationship between the c-di-GMP level and bacterial EV synthesis, which in turn affects biofilm formation[75]. It would be interesting to determine whether EVs function as downstream effectors or regulators within this pathway. Alternatively, aromatic amino acid (AAA) synthesis proteins [3-dehydroquinate synthases (AroB), Phospho-2-dehydro-3-deoxyheptonate aldolase, Phe-sensitive (AroG), Phospho-2-dehydro-3-deoxyheptonate aldolase, Trp-sensitive (AroH), and shikimate kinase I (AroK)] have been identified in the EVs of E. coli[29]. A recent study reported a significant reduction of motility in E. coli mutants lacking AroB, AroG, and AroK, while the addition of EVs derived from the wild type successfully restored the motility of the AroB mutant. This finding implies that E. coli-derived EVs can transfer to other bacterial cells and facilitate their migration along the urinary tract[29,76]. In addition, EVs from another uropathogen, Proteus mirabilis, contain MR/P fimbriae structural component (MrpA), which is involved in the attachment of uroepithelial tissue[27,77-79]. Altogether, these findings indicate that bacterial EVs play an extensive role in promoting bacterial motility and tissue colonization during UTIs.
EVs facilitate persistent bacterial infection and invasion
Another well-studied human urinary tract defense mechanism is iron depletion. Iron serves as an essential enzyme cofactor in bacterial survival during colonization[80,81]. In humans, most iron is complexed with heme groups and found in the hemoglobin of erythrocytes[82]. Meanwhile, iron released into plasma is bound to transferrin, limiting bacterial access to free iron[82,83]. Despite this, bacteria can acquire iron from the host during infection. One strategy is to secrete hemolysin, which is believed to lyse host cells and release nutrients and minerals, such as iron, during UTIs[82,84-86]. A recent study has illustrated that the iron content in the urine of healthy individuals is lower than that of UTI patients[85]. This suggests a sign of iron extraction in the urinary system. In this process, EVs derived from uropathogens, such as Proteus mirabilis, UPEC, and Pseudomonas aeruginosa, are responsible for delivering hemolysin to the host cell and causing urinary tissue damage, including uroepithelium shedding and bladder hemorrhage[27,33,87,88]. Furthermore, the iron acquisition system components, such as ferrienterobactin receptors FepA and EfeO (an iron-binding protein), were found in the EVs derived from E. coli[27,33,89]. FepA is an outer membrane receptor that facilitates the transport of iron-siderophore complexes into bacterial cells[80]. Inside the bacterial cytoplasm, iron-enterobactin esterase releases iron from the siderophore complex by cleaving the enterobactin backbone[80]. It is possible that FepA in bacterial EVs can fuse the membranes of other bacterial cells and increase iron uptake. This concept is supported by a study that discovered the translocation of receptors from EVs to the cell membranes of other cells[90]. Additionally, EfeO is a membrane protein responsible for transporting ferric ions into bacterial cells[91]. It has also been found to play a role in maintaining iron homeostasis in the cells by facilitating the oxidation of excess ferrous ions to ferric ions[92]. Altogether, this highlights that EVs facilitate the invasion and survival of uropathogens in the nutrient-resource-limited urinary system after or during colonization.
Bacteria-derived EVs promote immune modulation and evasion
Immune evasion is also significant in maintaining the persistent colonization and invasion of uropathogenic bacteria. Although few studies have described the relationship between bacteria-derived EVs and immune evasion during UTIs, some general mechanisms that explain the interaction between bacteria-derived EVs and the host immune system are relevant. For example, Tomasek et al. have discovered that the FimH protein, a component of type 1 pili in UPEC, can bind with CD14 expressed on dendritic cells and inhibit the dendritic cell migration to lymph nodes[13]. This is achieved by overactivation of the integrins and nuclear factor of activated T-cells pathway, thus suppressing the immune response[13]. In addition,
Bacteria-derived EVs promote antibiotic resistance
Bacteria-derived EVs play a critical role in inducing antibiotic resistance. For instance, EVs from a uropathogen, Pseudomonas aeruginosa, increased the minimum inhibitory concentration (MIC) of gentamicin 4-fold, and A-band LPS was present only in gentamicin-induced EVs but not in natural EVs[87]. This may be the critical component contributing to drug resistance. Another uropathogen, carbapenem-resistant Klebsiella pneumoniae, has been shown to secrete EVs to hydrolyze meropenem, and the authors speculate that the K. pneumoniae carbapenemase (KPC) protein in EVs contributes to the hydrolysis effect in meropenem[99]. Furthermore, carbapenem-resistant hypervirulent K. pneumoniae was shown to secrete EVs to deliver the drug-resistant and virulence plasmid to the less virulent K. pneumoniae, showcasing that EVs can facilitate horizontal gene transfer between the same species and protect bacterial plasmids from degradation[100]. In addition, EVs derived from Enterococcus faceium were shown to consist of proteins that are associated with resistance to aminoglycoside (Aac6’-le-Aph2”-la, Aph3’-III), glycopeptide (VanR-A, VanS-A, VanH-A, VanA, VanX-A, VanY-A, VanZ-A, VanS-B), and macrolide (ErmB), suggesting the significant role of uropathogenic bacteria-derived EVs in promoting antibiotic resistance[101].
ROLE OF FUNGAL-DERIVED EVs IN FUNGAL UTIs
Fungi utilize EVs to strive for persistent survival and propagation in the urinary system. A notable species is C. albicans[102]. There are two major infection pathways: entry of uropathogenic fungi from the bloodstream into the urinary system, or ascension from the urethra or a site near the urethra into the upper urinary tract[103]. Adhesion is the first stage in these pathways.
EVs facilitate adhesion and colonization in the urinary system via biofilm formation and dispersion
Fungi-induced UTIs, particularly C. albicans, can commonly be found in chronically catheterized patients in hospitals due to the ability of the fungus to form hyphae and biofilm in the urinary tract and urinary catheter[9,104]. EVs derived from fungi play a vital role in this process. Recently, a study showed that the composition of EVs derived from C. albicans is highly similar to the matrix material for biofilm formation[30,105]. Later, three enzymes, Glucan 1,3-beta-glucosidase (XOG1), cell wall 1,3-beta-glucosyltransferase (BGL2), and glycosylphosphatidylinsitol-anchored beta(1,3)-glucanosyltransferase (PHR1), in the EVs from C. albicans were identified to be essential for delivering the beta-1,3 glucan (key components of biofilm) to the biofilm matrix and contributing to the mature biofilm mass[30,31]. Notably, EVs derived from C. albicans contain several proteins [e.g., PHR1, cell surface hydrophobicity protein 1 (CSH1), 65-kilodalton mannoprotein (MP65), XOG1, α-mannosidase (AMS1), homocysteine methyltransferase (MET6), circularly permuted 1,3-beta-glucanase (TOS1), glycolipid 2-alpha-mannosyltransferase (MNT1), chitinase 3 (CHT3), thioredoxin 2 (TRX2), candidapepsin-5 (SAP5), ADP/ATP carrier protein 2 (PET9)] involved in the endosomal sorting complexes required for transport (ESCRT) pathway. These cargo proteins, which can either promote the biofilm adhesion or biofilm dispersion, are selectively packaged into EVs of C. albicans[32]. This facilitates the adaptation of the uropathogenic fungus in the dynamic urinary system and the propagation of the fungus in the host, since it can adjust and control its behavior based on different environmental condition changes, for example, nutrient availability, the presence of immune cells and flow conditions, in the urinary system[106]. Apart from C. albicans, EVs of the other uropathogenic fungi, Candida parapsilosis (C. parapsilosis), and Candida tropicalis (C. tropicalis) comprise the cell wall mannoprotein MP65[107,108]. This protein is responsible for adhesion on the plastic surface and is essential for germ tube development during hyphae formation[107,108]. This phenomenon increases the risk of catheter-associated UTIs, particularly because some urinary catheters are made of plastic[109].
EVs derived from uropathogenic fungi promote invasion into the bloodstream in the urinary system
In addition to the characteristics of biofilm dispersion discussed previously, EVs derived from the fungus promote the yeast-to-hyphae transition in a study[28]. The transition process (or the dimorphism) mainly depends on the presence of nutrients and chemicals during the infection, such as glucose and trichloroacetic acid in the serum, the quorum-sensing molecules (e.g., farnesol), and the activation of the gene RAS-like protein 1 (RAS1)[110-112]. Research has shown that C. albicans uptake with EVs derived from the C. albicans hyphae state can promote more hyphae and pseudohyphae formation when compared with the EVs derived from the yeast form of C. albicans during their growth, and there is a depression of the CHT2 gene (endochitinase)[28]. The CHT2 gene was abundant in the yeast form of the fungus but not in its hyphae state[113]. Notably, hyphae are a significant virulence factor of fungi during UTIs, as they can penetrate the urinary endothelial cells and further into the bloodstream[102]. However, it remains unclear whether the EVs of C. albicans can promote both pseudohyphae and hyphae formation. Another study shows a contradictory result, in which they found that EVs from the yeast state of C. albicans favor the formation of pseudohyphae only, not hyphae[114]. The conflicting results may stem from different growing conditions or the state of the fungus when the EVs are obtained[28]. This assumption can be further supported by a study of Martínez-
EVs derived from uropathogenic fungi cause immune modulation and evasion
The interaction between the immune systems of the hosts and fungi during UTIs has not been investigated extensively. Limited and non-UTI-related studies have suggested that EVs can act as double-edged swords in this case[57]. A recent non-UTIs study illustrated that EVs derived from the hyphae state of C. albicans caused cytotoxic effects on THP-1 macrophages[40]. However, other studies reported that EVs from
EVs from uropathogenic fungi promote antifungal drug resistance
Antifungal drug resistance is an urgent public health problem, and EVs derived from fungi are believed to contribute to this issue[32,65]. A study shows that back-addition of nano to microgram of EVs secreted from a uropathogenic fungus, C. auris, increased the MIC to amphotericin B (AMB) 16-fold, which the authors propose that the presence of high quantities of alcohol dehydrogenase 1 (Adh1), XOG1, and MP65-like (mannoprotein-65) protein in EVs may contribute to this resistant effect[65]. Adh1, an enzyme that manipulates alcohol production, is associated with biofilm formation and fluconazole resistance[130]. The efflux pump is the major mechanism of fluconazole resistance[131]. Researchers propose that Adh1 overexpression activates the glycolytic pathway, which increases adenosine triphosphate (ATP) production to support the efflux pump activity[131]. However, the relationship between AMB resistance and Adh1 is waiting to be established. Apart from that, the xog-1 protein is heavily involved in forming biofilm[30,31]. It has been previously shown that its expression in C. albicans was upregulated with the AMB treatment[132]. Moreover, MP65 is a beta-glucanase and its deletion mutant lost the ability to form biofilm[133,134]. Thus, it is important to determine whether this protein contributes to AMB and other antifungal drug resistance. Furthermore, another study has demonstrated that adding EVs to the C. tropicalis culture increased the thickness of the biofilm and its metabolic activity under the treatment of fluconazole and caspofungin[135]. Still, the fungal viability was only increased in the caspofungin group[135]. Additionally, an antifungal drug, turbinmicin, which inhibits the vesicle trafficking pathway in fungi, impaired C. albicans’ EVs delivery to the biofilm matrix and increased the fluconazole susceptibility in the [(sodium 3′-[1- (phenylaminocarbonyl)- 3,4- tetrazolium]-bis (4-methoxy6-nitro) benzene sulfonic acid hydrate)] (XTT) assay[136,137]. The vesicle trafficking pathway inhibition by turbinmicin is due to the binding of a protein, Sec14 (yeast phosphatidylinositol transfer protein), and the hindrance of the accumulation of Snc1 (Synaptobrevin homolog 1) in the buds of the plasma membrane[137]. However, the addition of C. albicans-derived EVs restored the resistant
FUTURE DIRECTION AND CHALLENGES OF EV RESEARCH IN THE FIELD OF UTIs
It is still crucial to explore the role of EVs and the content derived from uropathogens during UTIs. Many research gaps or directions remain to be discovered. Until this stage, almost all studies focused on the protein content inside EVs derived from uropathogens instead of lipids, RNA, and DNA. In the future, researchers can study DNA and RNA content in EVs, and discover if there are any urinary EV biomarkers specific to certain types of uropathogens during UTIs. Moreover, they can investigate the potential molecules or chemicals that can act as EV inhibitors. One method is to focus more on the EVs’ biogenesis in microbes, especially gram-positive bacteria. Recent studies have proposed that the disruption of the peptidoglycan layer caused by the action of prophage promotes EV formation in gram-positive bacteria[138,139]. Thus, it can be a direction for the scientists to find which key components control the above pathway and inhibit the EV formation. In addition, testing for any synergistic antimicrobial effects when using the antimicrobial drug in conjunction with the EV inhibitors is also an important research direction. However, the EV inhibitor candidates should mainly target the microbes rather than the human cells. If no such inhibitor is found, a more feasible method is to develop a small interfering RNA drug that targets the pathogenic genes’ expression corresponding to their protein production. Finding a common and consensus target in EVs of bacteria and fungi is better for overcoming multi-species UTIs.
Nevertheless, some challenges exist when scientists and clinicians explore the above research direction. The challenge primarily lies in identifying specific biomarkers, as there is no standardized method to isolate and purify the EVs. At this stage, there are lots of EV isolation and purification methods available, for example, size-exclusion chromatography (qEV columns, a product from Izon Science Limited for performing Size exclusion chromatography), precipitation (ExoQuick and ExoQuick Ultra), iodixanol gradient ultracentrifugation (Optiprep), and affinity-based capture method[140,141]. However, different EV isolation and purification methods will co-isolate the impurities, for example, Tamm Horsfall protein (THP) in urine, which entraps EVs and interferes with RNA extraction and miRNA quantification[142-144]. Also, studies have shown that THP is a highly glycosylated protein, which can mask the signal of other glycosylated proteins during mass spectrometry proteomic analysis[145]. Furthermore, different centrifugation methods and parameters will affect the RNA concentration[146]. This may cause bias when examining the abundance of RNA biomarkers in urine during UTIs. Notably, unlike EVs derived from human cells, the distinct biomarker of EVs from many pathogens has not yet been discovered and confirmed, although some studies propose biomarkers for specific pathogens, for example, Sur7 in EVs of C. albicans and the Hsp70 domain in nine fungal species[147,148]. This further complicates the identification procedure for EV sources. Collectively, more research efforts should be made to tackle the above challenges.
CONCLUSION
In summary, EVs derived from bacteria and fungi play a significant role in almost every aspect of the pathogenesis of UTIs, including colonization, invasion, immune modulation, immune evasion, and host environmental modulation. They carry different biomolecules that facilitate their persistence, survival, and infection in the urinary system. In addition, given the increasing recognition of the defensive roles of host-derived EVs during infection, scientists should deeply investigate whether there are any potential biomarkers or therapeutic targets for UTIs, rather than focusing solely on pathogen-derived EVs. With a greater understanding of the roles and mechanisms of EVs derived from uropathogens and their hosts, microbiologists and clinicians may identify new therapeutic directions, thereby mitigating the global problem of drug-resistant UTIs.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception of the review: Chow FWN, Lee CL, Chau ECT
Wrote the majority of the manuscript: Chau ECT
Wrote the abstract and assisted with the literature search: Chau ECT, Hau PT, Murillo M
Review and editing: Chow FWN, Lee CL, Tsang CC, Tam EWT, Seto SW
Supervision: Chow FWN, Lee CL
Availability of data and materials
Not applicable.
AI and AI-assisted tools statement
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Chow FWN is a Junior Editorial Board member of the journal Extracellular Vesicles and Circulating Nucleic Acids. Chow FWN was not involved in any steps of the editorial process for this manuscript, including reviewer selection, manuscript handling, or decision making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Zeng Z, Zhan J, Zhang K, Chen H, Cheng S. Global, regional, and national burden of urinary tract infections from 1990 to 2019: an analysis of the global burden of disease study 2019. World J Urol. 2022;40:755-63.
2. National Health Service. Urinary tract infections (UTIs). Available from: https://www.nhs.uk/conditions/urinary-tract-infections-utis/. [Last accessed on 12 Nov 2025].
3. Pietrucha-Dilanchian P, Hooton TM. Diagnosis, treatment, and prevention of urinary tract infection. Microbiol Spectr. 2016;4:10.1128/microbiolspec.uti-0021-2015.
4. Osamwonyi B, Foley C. Management of recurrent urinary tract infections in adults. Surgery. 2017;35:299-305.
5. Rana DS, Sharma V, Sheershwal A. Understanding host-pathogen interactions in urinary tract infections and advancements in diagnostic methods. Urol Sci. 2025;36:61-75.
6. Klein RD, Hultgren SJ. Urinary tract infections: microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat Rev Microbiol. 2020;18:211-26.
7. Idrees MM, Saeed A. Genetic and molecular mechanisms of multidrug-resistance in uropathogens and novel therapeutic combat. In: Ahmed S, Chandra Ojha S, Najam-ul-haq M, Younus M, Hashmi MZ, Editors. Biochemistry of drug resistance. Cham: Springer International Publishing; 2021. pp. 505-38.
8. Zalewska-Piątek B, Olszewski M, Lipniacki T, et al. A shear stress micromodel of urinary tract infection by the Escherichia coli producing Dr adhesin. PLoS Pathog. 2020;16:e1008247.
9. La Bella AA, Andersen MJ, Gervais NC, et al. The catheterized bladder environment promotes Efg1- and Als1-dependent Candida albicans infection. Sci Adv. 2023;9:eade7689.
10. Werneburg GT. Catheter-associated urinary tract infections: current challenges and future prospects. Res Rep Urol. 2022;14:109-33.
12. Nett JE, Brooks EG, Cabezas-Olcoz J, et al. Rat indwelling urinary catheter model of Candida albicans biofilm infection. Infect Immun. 2014;82:4931-40.
13. Tomasek K, Leithner A, Glatzova I, Lukesch MS, Guet CC, Sixt M. Type 1 piliated uropathogenic Escherichia coli hijack the host immune response by binding to CD14. Elife. 2022;11:e78995.
14. Martínez-López R, Hernáez ML, Redondo E, et al. Candida albicans hyphal extracellular vesicles are different from yeast ones, carrying an active proteasome complex and showing a different role in host immune response. Microbiol Spectr. 2022;10:e0069822.
15. Huang Y, Li S, Teng Y, et al. Glucuronoxylomannan (GXM) modulates macrophage proliferation and apoptosis through the STAT1 signaling pathway. Cell Biol Int. 2025;49:317-28.
16. Yauch LE, Lam JS, Levitz SM. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog. 2006;2:e120.
17. Rodrigues ML, Nakayasu ES, Oliveira DL, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58-67.
18. Kouokam JC, Wai SN, Fällman M, et al. Active cytotoxic necrotizing factor 1 associated with outer membrane vesicles from uropathogenic Escherichia coli. Infect Immun. 2006;74:2022-30.
19. Yang H, Li Q, Wang C, et al. Cytotoxic necrotizing factor 1 downregulates CD36 transcription in macrophages to induce inflammation during acute urinary tract infections. Front Immunol. 2018;9:1987.
20. Pang Y, Cheng Z, Zhang S, et al. Bladder epithelial cell phosphate transporter inhibition protects mice against uropathogenic Escherichia coli infection. Cell Rep. 2022;39:110698.
21. Coady A, Ramos AR, Olson J, Nizet V, Patras KA. Tamm-horsfall protein protects the urinary tract against Candida albicans. Infect Immun. 2018;86:e00451-18.
22. Ponde NO, Lortal L, Ramage G, Naglik JR, Richardson JP.
23. Derakhshan S, Ahmadi S, Ahmadi E, Nasseri S, Aghaei A. Characterization of Escherichia coli isolated from urinary tract infection and association between virulence expression and antimicrobial susceptibility. BMC Microbiol. 2022;22:89.
24. Timm MR, Russell SK, Hultgren SJ. Urinary tract infections: pathogenesis, host susceptibility and emerging therapeutics. Nat Rev Microbiol. 2025;23:72-86.
25. Nhu NTK, Ravi C, Schembri MA. Uropathogenic Escherichia coli biofilms. Microbiol Aust. 2023;44:109-12.
26. Uppuluri P, Chaturvedi AK, Srinivasan A, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6:e1000828.
27. González MJ, Navarro N, Cruz E, et al. First report on the physicochemical and proteomic characterization of Proteus mirabilis outer membrane vesicles under urine-mimicking growth conditions: comparative analysis with Escherichia coli. Front Microbiol. 2024;15:1493859.
28. Bitencourt TA, Hatanaka O, Pessoni AM, et al. Fungal extracellular vesicles are involved in intraspecies intracellular communication. mBio. 2022;13:e03272-21.
29. Liu L, Law COK, Nie Q, et al. Comparative analysis of outer membrane vesicles from uropathogenic Escherichia coli reveal the role of aromatic amino acids synthesis proteins in motility. Int J Med Microbiol. 2023;313:151573.
30. Zarnowski R, Sanchez H, Covelli AS, et al.
31. Taff HT, Nett JE, Zarnowski R, et al. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog. 2012;8:e1002848.
32. Zarnowski R, Noll A, Chevrette MG, et al. Coordination of fungal biofilm development by extracellular vesicle cargo. Nat Commun. 2021;12:6235.
33. Hong J, Dauros-Singorenko P, Whitcombe A, et al. Analysis of the Escherichia coli extracellular vesicle proteome identifies markers of purity and culture conditions. J Extracell Vesicles. 2019;8:1632099.
34. Fisher JF, Sobel JD, Kauffman CA, Newman CA.
35. Kramer CV, Zhang F, Sinclair D, Olliaro PL. Drugs for treating urinary schistosomiasis. Cochrane Database Syst Rev. 2014;2014:CD000053.
36. Gorczynska E, Turkiewicz D, Rybka K, et al. Incidence, clinical outcome, and management of virus-induced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:797-804.
37. Ahmed SS, Shariq A, Alsalloom AA, Babikir IH, Alhomoud BN. Uropathogens and their antimicrobial resistance patterns: relationship with urinary tract infections. Int J Health Sci. 2019;13:48-55.
38. Belete MA, Saravanan M. A systematic review on drug resistant urinary tract infection among pregnant women in developing countries in Africa and Asia; 2005-2016. Infect Drug Resist. 2020;13:1465-77.
39. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977.
40. Martínez-López R, Hernáez ML, Redondo E, et al.
41. Colombo M, Moita C, van Niel G, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553-65.
42. Liu J, Hu X. Fungal extracellular vesicle-mediated regulation: from virulence factor to clinical application. Front Microbiol. 2023;14:1205477.
44. Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47:11-25.
45. Fang Y, Wang Z, Liu X, Tyler BM. Biogenesis and biological functions of extracellular vesicles in cellular and organismal communication with microbes. Front Microbiol. 2022;13:817844.
46. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-89.
47. Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337-62.
48. Amabebe E, Kumar A, Tatiparthy M, Kammala AK, Taylor BD, Menon R. Cargo exchange between human and bacterial extracellular vesicles in gestational tissues: a new paradigm in communication and immune development. Extracell Vesicles Circ Nucl Acids. 2024;5:297-328.
49. Baeza N, Delgado L, Comas J, Mercade E. Phage-mediated explosive cell lysis induces the formation of a different type of O-IMV in Shewanella vesiculosa M7(T). Front Microbiol. 2021;12:713669.
50. Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17:13-24.
51. Turnbull L, Toyofuku M, Hynen AL, et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016;7:11220.
52. Briaud P, Carroll RK. Extracellular vesicle biogenesis and functions in gram-positive bacteria. Infect Immun. 2020;88:e00433-20.
53. Albino D, Falcione M, Uboldi V, et al. Circulating extracellular vesicles release oncogenic miR-424 in experimental models and patients with aggressive prostate cancer. Commun Biol. 2021;4:119.
54. Kumar MA, Baba SK, Sadida HQ, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9:27.
55. Hazrati A, Soudi S, Malekpour K, et al. Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications. Biomark Res. 2022;10:30.
56. Jan AT. Outer membrane vesicles (OMVs) of gram-negative bacteria: a perspective update. Front Microbiol. 2017;8:1053.
57. Lai Y, Jiang B, Hou F, et al. The emerging role of extracellular vesicles in fungi: a double-edged sword. Front Microbiol. 2023;14:1216895.
58. Bos J, Cisneros LH, Mazel D. Real-time tracking of bacterial membrane vesicles reveals enhanced membrane traffic upon antibiotic exposure. Science Advances. 2021;7:eabd1033.
59. Kwaku GN, Jensen KN, Simaku P, et al. Extracellular vesicles from diverse fungal pathogens induce species-specific and endocytosis-dependent immunomodulation. PLoS Pathog. 2025;21:e1012879.
60. Eitan E, Green J, Bodogai M, et al. Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci Rep. 2017;7:1342.
61. Ekström K, Crescitelli R, Pétursson HI, Johansson J, Lässer C, Olofsson Bagge R. Characterization of surface markers on extracellular vesicles isolated from lymphatic exudate from patients with breast cancer. BMC Cancer. 2022;22:50.
62. Hoyer LL, Cota E.
63. Spencer N, Yeruva L. Role of bacterial infections in extracellular vesicles release and impact on immune response. Biomed J. 2021;44:157-64.
64. Chen N, Li Y, Liang X, et al. Bacterial extracellular vesicle: a non-negligible component in biofilm life cycle and challenges in biofilm treatments. Biofilm. 2024;8:100216.
65. Chan W, Chow FW, Tsang CC, et al. Induction of amphotericin B resistance in susceptible Candida auris by extracellular vesicles. Emerg Microbes Infect. 2022;11:1900-9.
66. Wang Z, Jiang Z, Zhang Y, et al. Exosomes derived from bladder epithelial cells infected with uropathogenic Escherichia coli increase the severity of urinary tract infections (UTIs) by impairing macrophage function. PLoS Pathog. 2024;20:e1011926.
67. Farajnia S, Alikhani MY, Ghotaslou R, Naghili B, Nakhlband A. Causative agents and antimicrobial susceptibilities of urinary tract infections in the northwest of Iran. Int J Infect Dis. 2009;13:140-4.
69. Soto SM. Importance of biofilms in urinary tract infections: new therapeutic approaches. Advances in Biology. 2014;2014:1-13.
70. Schulze A, Mitterer F, Pombo JP, Schild S. Biofilms by bacterial human pathogens: clinical relevance - development, composition and regulation - therapeutical strategies. Microb Cell. 2021;8:28-56.
71. Amikam D, Galperin MY. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics. 2006;22:3-6.
72. Han Q, Wang SF, Qian XX, et al. Flagellar brake protein YcgR interacts with motor proteins MotA and FliG to regulate the flagellar rotation speed and direction. Front Microbiol. 2023;14:1159974.
73. Jackson DW, Suzuki K, OakfordL
74. Khan F, Tabassum N, Pham DTN, Oloketuyi SF, Kim YM. Molecules involved in motility regulation in Escherichia coli cells: a review. Biofouling. 2020;36:889-908.
75. Hu XM, Peng L, Wu J, Wu G, Liang X, Yang JL. Bacterial c-di-GMP signaling gene affects mussel larval metamorphosis through outer membrane vesicles and lipopolysaccharides. NPJ Biofilms Microbiomes. 2024;10:38.
76. Baba T, Ara T, Hasegawa M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006.0008.
77. Rocha SP, Pelayo JS, Elias WP. Fimbriae of uropathogenic Proteus mirabilis. FEMS Immunol Med Microbiol. 2007;51:1-7.
78. Rózalski A, Sidorczyk Z, Kotełko K. Potential virulence factors of Proteus bacilli. Microbiol Mol Biol Rev. 1997;61:65-89.
79. Coker C, Poore CA, Li X, Mobley HL. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2000;2:1497-505.
80. Mey AR, Gómez-Garzón C, Payne SM. Iron transport and metabolism in Escherichia, Shigella, and Salmonella. EcoSal Plus. 2021;9:eESP-0034.
81. Klebba PE, Newton SMC, Six DA, et al. Iron acquisition systems of gram-negative bacterial pathogens define TonB-dependent pathways to novel antibiotics. Chem Rev. 2021;121:5193-239.
83. Sun Y, Wang X, Gong Q, et al. Extraintestinal pathogenic Escherichia coli utilizes surface-located elongation factor G to acquire iron from holo-transferrin. Microbiol Spectr. 2022;10:e01662-21.
84. Wiles TJ, Kulesus RR, Mulvey MA. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp Mol Pathol. 2008;85:11-9.
85. Subashchandrabose S, Hazen TH, Brumbaugh AR, et al. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A. 2014;111:18327-32.
86. Subashchandrabose S, Mobley HL. Back to the metal age: battle for metals at the host-pathogen interface during urinary tract infection. Metallomics. 2015;7:935-42.
87. Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998-4008.
88. Smith YC, Rasmussen SB, Grande KK, Conran RM, O’Brien AD. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect Immun. 2008;76:2978-90.
89. Nakatsuji S, Okumura K, Takase R, Watanabe D, Mikami B, Hashimoto W. Crystal structures of EfeB and EfeO in a bacterial siderophore-independent iron transport system. Biochem Biophys Res Commun. 2022;594:124-30.
90. Papareddy P, Tapken I, Kroh K, et al. The role of extracellular vesicle fusion with target cells in triggering systemic inflammation. Nat Commun. 2024;15:1150.
91. Banerjee S, Chanakira MN, Hall J, Kerkan A, Dasgupta S, Martin DW. A review on bacterial redox dependent iron transporters and their evolutionary relationship. J Inorg Biochem. 2022;229:111721.
92. Miethke M, Monteferrante CG, Marahiel MA, van Dijl JM. The Bacillus subtilis EfeUOB transporter is essential for high-affinity acquisition of ferrous and ferric iron. Biochim Biophys Acta. 2013;1833:2267-78.
93. Dadi BR, Abebe T, Zhang L, Mihret A, Abebe W, Amogne W. Distribution of virulence genes and phylogenetics of uropathogenic Escherichia coli among urinary tract infection patients in Addis Ababa, Ethiopia. BMC Infect Dis. 2020;20:108.
94. Deo P, Chow SH, Han ML, et al. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat Microbiol. 2020;5:1418-27.
95. Zhao G, Jones MK. Role of bacterial extracellular vesicles in manipulating infection. Infect Immun. 2023;91:e0043922.
96. Chen S, Lei Q, Zou X, Ma D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front Immunol. 2023;14:1157813.
97. Yang J, Hwang I, Lee E, et al. Bacterial outer membrane vesicle-mediated cytosolic delivery of flagellin triggers host NLRC4 canonical inflammasome signaling. Front Immunol. 2020;11:581165.
98. Vanaja SK, Russo AJ, Behl B, et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell. 2016;165:1106-19.
99. Yao L, Wei B, Wang Y, et al. A critical role of outer membrane vesicles in antibiotic resistance in carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2023;22:95.
100. Li P, Luo W, Xiang TX, et al. Horizontal gene transfer via OMVs co-carrying virulence and antimicrobial-resistant genes is a novel way for the dissemination of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front Microbiol. 2022;13:945972.
101. Wagner T, Joshi B, Janice J, et al. Enterococcus faecium produces membrane vesicles containing virulence factors and antimicrobial resistance related proteins. J Proteomics. 2018;187:28-38.
102. Fisher JF, Kavanagh K, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infection: pathogenesis. Clin Infect Dis. 2011;52:S437-51.
103. Fisher JF. Candida urinary tract infections--epidemiology, pathogenesis, diagnosis, and treatment: executive summary. Clin Infect Dis. 2011;52:S429-32.
104. Goetz LL, Howard M, Cipher D, Revankar SG. Occurrence of candiduria in a population of chronically catheterized patients with spinal cord injury. Spinal Cord. 2010;48:51-4.
105. Nett JE, Sanchez H, Cain MT, Andes DR. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J Infect Dis. 2010;202:171-5.
106. Nett JE, Andes DR. Fungal biofilms: in vivo models for discovery of anti-biofilm drugs. Microbiology Spectrum. 2015;3:10.1128/microbiolspec.mb-0008-2014.
107. Sandini S, La Valle R, De Bernardis F, Macrì C, Cassone A. The 65 kDa mannoprotein gene of Candida albicans encodes a putative beta-glucanase adhesin required for hyphal morphogenesis and experimental pathogenicity. Cell Microbiol. 2007;9:1223-38.
108. Karkowska-Kuleta J, Kulig K, Karnas E, et al. Characteristics of extracellular vesicles released by the pathogenic yeast-like fungi Candida glabrata, Candida parapsilosis and Candida tropicalis. Cells. 2020;9:1722.
109. Slettengren M, Mohanty S, Kamolvit W, van der Linden J, Brauner A. Making medical devices safer: impact of plastic and silicone oil on microbial biofilm formation. J Hosp Infect. 2020;106:155-62.
110. Hudson DA, Sciascia QL, Sanders RJ, et al. Identification of the dialysable serum inducer of germ-tube formation in Candida albicans. Microbiology. 2004;150:3041-9.
111. Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339-46.
112. Hornby JM, Jensen EC, Lisec AD, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67:2982-92.
113. McCreath KJ, Specht CA, Robbins PW. Molecular cloning and characterization of chitinase genes from Candida albicans. Proc Natl Acad Sci U S A. 1995;92:2544-8.
114. Honorato L, de Araujo JFD, Ellis CC, et al. Extracellular vesicles regulate biofilm formation and yeast-to-hypha differentiation in Candida albicans. mBio. 2022;13:e0030122.
115. Jothi R, Hari Prasath N, Gowrishankar S, Pandian SK. Bacterial quorum-sensing molecules as promising natural inhibitors of Candida albicans virulence dimorphism: an in silico and in vitro study. Front Cell Infect Microbiol. 2021;11:781790.
116. Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol. 2008;67:47-62.
117. Karkowska-Kuleta J, Kulig K, Bras G, et al.
118. Ibrahim AS, Filler SG, Sanglard D, Edwards JE, Hube B. Secreted aspartyl proteinases and interactions of Candida albicans with human endothelial cells. Infect Immun. 1998;66:3003-5.
119. Chen YT, Lin CY, Tsai PW, Yang CY, Hsieh WP, Lan CY. Rhb1 regulates the expression of secreted aspartic protease 2 through the TOR signaling pathway in Candida albicans. Eukaryot Cell. 2012;11:168-82.
120. Zamith-Miranda D, Heyman HM, Couvillion SP, et al. Comparative molecular and immunoregulatory analysis of extracellular vesicles from Candida albicans and Candida auris. mSystems. 2021;6:e0082221.
121. Vargas G, Rocha JD, Oliveira DL, et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol. 2015;17:389-407.
122. Zawrotniak M, Satala D, Juszczak M, Bras G, Rapala-Kozik M. Candida albicans aspartyl protease (Sap6) inhibits neutrophil function via a “Trojan horse” mechanism. Sci Rep. 2025;15:6946.
123. Brown Harding H, Kwaku GN, Reardon CM, et al. Candida albicans extracellular vesicles trigger type I IFN signalling via cGAS and STING. Nat Microbiol. 2024;9:95-107.
124. Chen T, Feng Y, Sun W, et al. The nucleotide receptor STING translocates to the phagosomes to negatively regulate anti-fungal immunity. Immunity. 2023;56:1727-42.e6.
125. Cheng Z, Dai T, He X, et al. The interactions between cGAS-STING pathway and pathogens. Signal Transduct Target Ther. 2020;5:91.
126. Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548-69.
127. Monari C, Retini C, Casadevall A, et al. Differences in outcome of the interaction between Cryptococcus neoformans glucuronoxylomannan and human monocytes and neutrophils. Eur J Immunol. 2003;33:1041-51.
128. Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun. 2010;78:1601-9.
129. Weiss ZF, DiCarlo JE, Basta DW, et al. Hidden in plain sight: urinary Cryptococcus neoformans missed by routine diagnostics in a patient with acute leukemia. Ann Clin Microbiol Antimicrob. 2022;21:49.
130. Wang Z, Zhang Q, Zhang H, Lu Y. Roles of alcohol dehydrogenase 1 in the biological activities of Candida albicans. Crit Rev Microbiol. 2025;51:484-98.
131. Rybak JM, Muñoz JF, Barker KS, et al. Mutations in TAC1B: a novel genetic determinant of clinical fluconazole resistance in Candida auris. mBio. 2020;11:e00365-20.
132. Li P, Seneviratne CJ, Alpi E, Vizcaino JA, Jin L. Delicate metabolic control and coordinated stress response critically determine antifungal tolerance of Candida albicans biofilm persisters. Antimicrobial Agents and Chemotherapy. 2015;59:6101-12.
133. Arita GS, Faria DR, Capoci IRG, Kioshima ES, Bonfim-Mendonça PS, Svidzinski TIE. Cell wall associated proteins involved in filamentation with impact on the virulence of Candida albicans. Microbiol Res. 2022;258:126996.
134. Sandini S, Stringaro A, Arancia S, et al. The MP65 gene is required for cell wall integrity, adherence to epithelial cells and biofilm formation in Candida albicans. BMC Microbiol. 2011;11:106.
135. Kulig K, Karnas E, Woznicka O, et al. Insight into the properties and immunoregulatory effect of extracellular vesicles produced by Candida glabrata, Candida parapsilosis, and Candida tropicalis biofilms. Front Cell Infect Microbiol. 2022;12:879237.
136. Zhao M, Zhang F, Zarnowski R, et al. Turbinmicin inhibits Candida biofilm growth by disrupting fungal vesicle-mediated trafficking. J Clin Invest. 2021;131:145123.
137. Zhang F, Zhao M, Braun DR, et al. A marine microbiome antifungal targets urgent-threat drug-resistant fungi. Science. 2020;370:974-8.
138. Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, et al. Prophage-triggered membrane vesicle formation through peptidoglycan damage in. Nat Commun. 2017;8:481.
139. Andreoni F, Toyofuku M, Menzi C, et al. Antibiotics stimulate formation of vesicles in Staphylococcus aureus in both phage-dependent and -independent fashions and via different routes. Antimicrob Agents Chemother. 2019;63:e01439-18.
140. Allelein S, Medina-Perez P, Lopes ALH, et al. Potential and challenges of specifically isolating extracellular vesicles from heterogeneous populations. Sci Rep. 2021;11:11585.
141. Clos-Sansalvador M, Monguió-Tortajada M, Roura S, Franquesa M, Borràs FE. Commonly used methods for extracellular vesicles’ enrichment: implications in downstream analyses and use. Eur J Cell Biol. 2022;101:151227.
142. Musante L, Bontha SV, La Salvia S, et al. Rigorous characterization of urinary extracellular vesicles (uEVs) in the low centrifugation pellet - a neglected source for uEVs. Sci Rep. 2020;10:3701.
143. Erdbrügger U, Blijdorp CJ, Bijnsdorp IV, et al. Urinary extracellular vesicles: a position paper by the urine task force of the international society for extracellular vesicles. J Extracell Vesicles. 2021;10:e12093.
144. Correll VL, Otto JJ, Risi CM, et al. Optimization of small extracellular vesicle isolation from expressed prostatic secretions in urine for in-depth proteomic analysis. J Extracell Vesicles. 2022;11:e12184.
145. Brown CJ, Gaunitz S, Wang Z, et al. Glycoproteomic analysis of human urinary exosomes. Anal Chem. 2020;92:14357-65.
146. Teixeira-Marques A, Monteiro-Reis S, Montezuma D, et al. Improved recovery of urinary small extracellular vesicles by differential ultracentrifugation. Sci Rep. 2024;14:12267.
147. Dawson CS, Garcia-Ceron D, Rajapaksha H, Faou P, Bleackley MR, Anderson MA. Protein markers for Candida albicans EVs include claudin-like Sur7 family proteins. J Extracell Vesicles. 2020;9:1750810.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].