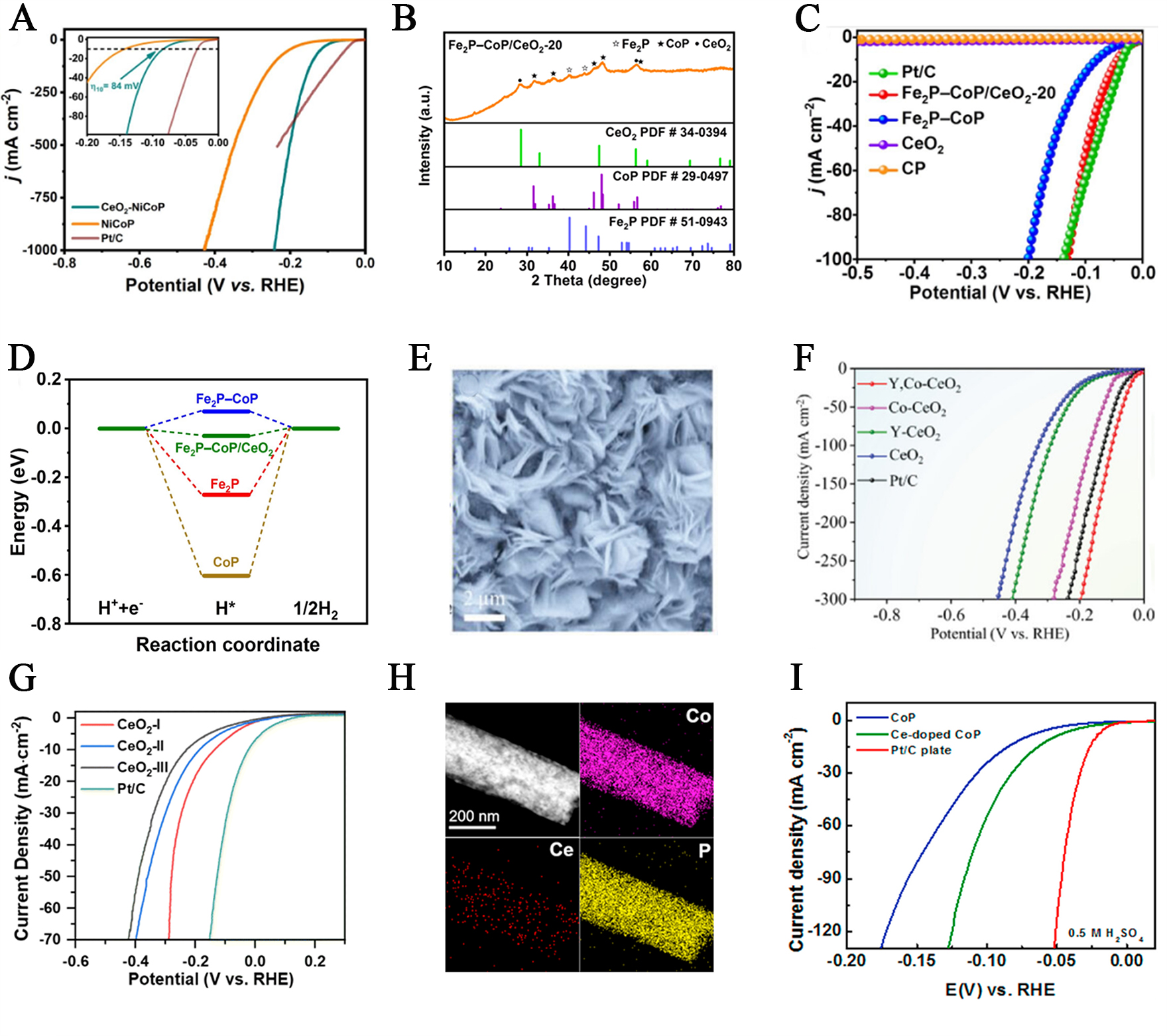
fig7
Figure 7. (A) Polarization curves and the corresponding Tafel plots of CeO2-NiCoP, NiCoP and commercial Pt/C catalysts. This figure is quoted with permission from Yu et al.[86]. (B) XRD pattern of Fe2P-CoP/CeO2-20; (C) LSV curves of Fe2P-CoP/CeO2; (D) ΔGH* profiles a on CoP, Fe2P, Fe2P-CoP, and Fe2P-CoP/CeO2. This figure is quoted with permission from Ding et al.[87]; (E) SEM image; (F) Illustration of the HER mechanism on Y, Co-CeO2. This figure is quoted with permission from Liu et al.[89]; (G) HER performance examined in 1.0 M KOH of CeO2-I, CeO2-II, and CeO2-III. This figure is quoted with permission from Liu et al.[90]; (H) DF-TEM image of Ce-doped CoP, and element mapping images of Co, Ce and P, respectively; (I) Polarization curves (with iR corrections) of CoP, Ce-doped CoP and Pt/C catalysts with a scan rate of 5 mV/s in 0.5 M H2SO4. This figure is quoted with permission from Gao et al.[91]. RHE: Reversible hydrogen electrode; XRD: X-ray diffraction; LSV: linear sweep voltammetry; SEM: scanning electron microscopy; LSV: linear sweep voltammetry; iR: internal resistance; DF-TEM: dark-field transmission electron microscopy.