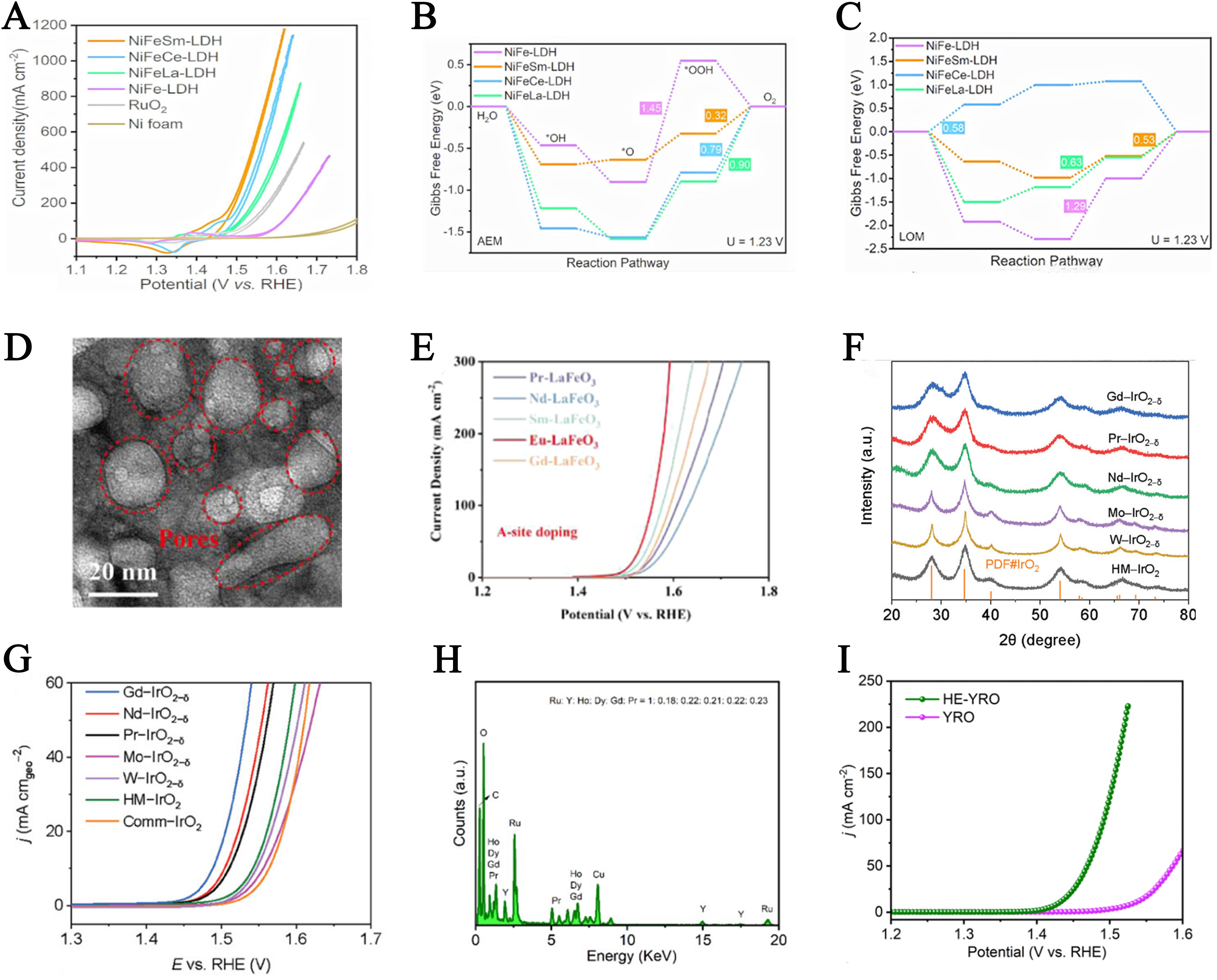
fig14
Figure 14. (A) CV curves in O2-saturated 1 M KOH solution at a scan rate of 2 mV/s with 85% iR correction; (B) AEM and (C) LOM pathways for NiFe-LDH, NiFeSm-LDH, NiFeCe-LDH and NiFeLa-LDH. This figure is quoted with permission from Wang et al.[125]; (D) TEM image from the LaFeO3 MPONs; (E) LSV polarization curves of LaFeO3 particles and LaFeO3 MPONs. This figure is quoted with permission from Wang et al.[126]; (F) XRD patterns of A-IrO2-𝛿 (A = Gd, Pr, Nd, Mo, W) and HM-IrO2; (G) OER performance of the prepared electrocatalysts in 0.5 M H2SO4 LSV curves. This figure is quoted with permission from Wu et al.[127]; (H) EDS spectrum of HE-YRO. The signals of Cu and C in the spectrum originated from the copper grid coated by carbon membrane; (I) Polarization curves of HE-YRO and YRO catalysts. This figure is quoted with permission from Zhang et al.[128]. RHE: Reversible hydrogen electrode; CV: cyclic voltammetry; iR: internal resistance; AEM: adsorbate evolution mechanism; LOM: lattice oxygen mechanism; LDH: layered double hydroxide; TEM: transmission electron microscopy; MPON: mesoporous perovskite oxide nanosheet; LSV: linear sweep voltammetry; XRD: X-ray diffraction; OER: oxygen evolution reaction; HM: home-made; EDS: energy-dispersive X-ray spectroscopy. HE-YRO: high-entropy (Y0.2Ho0.2Dy0.2Gd0.2Pr0.2)2Ru2O7.