fig11
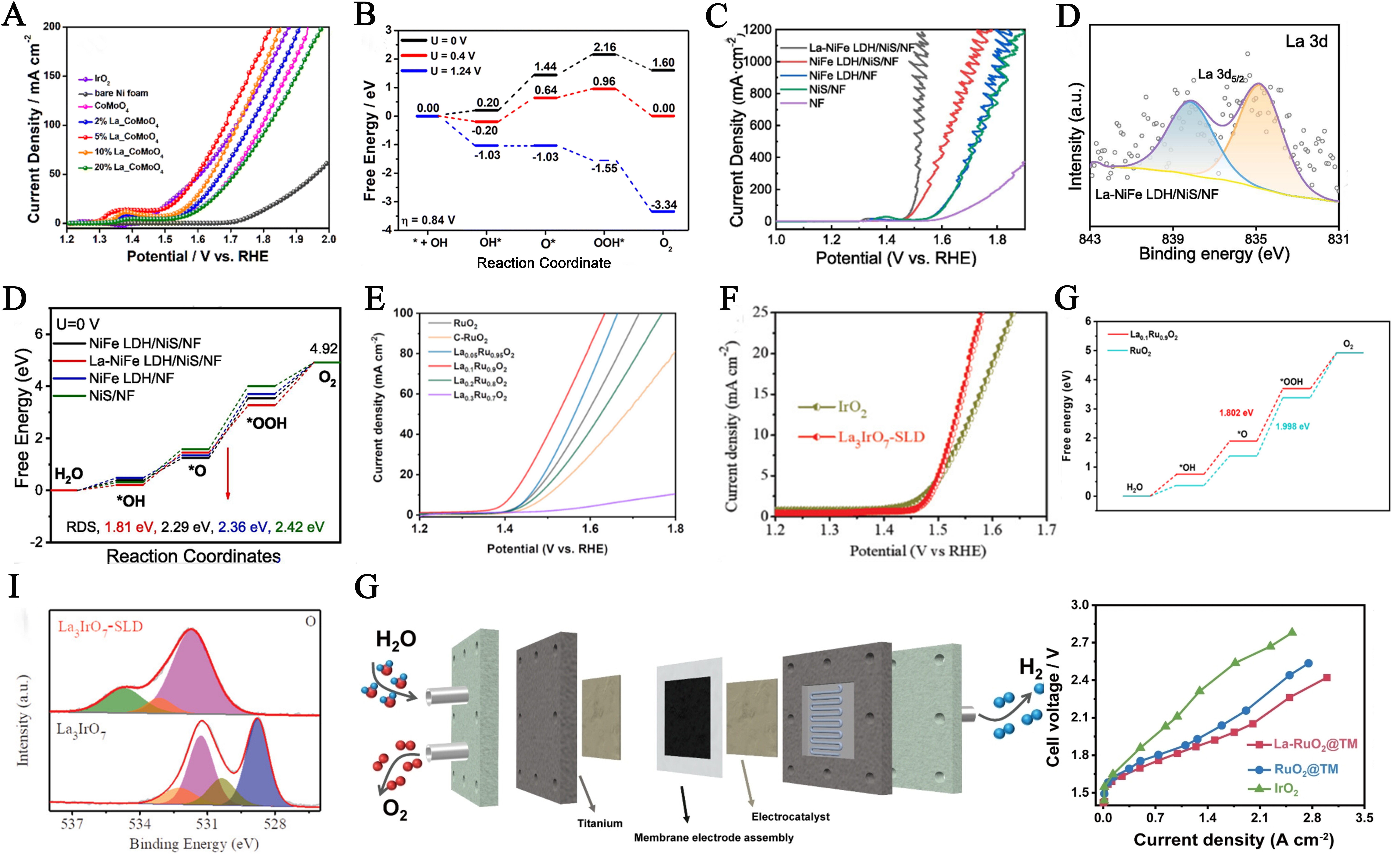
Figure 11. (A) LSV responses of different La-doped CoMoO4 catalysts toward the OER in 1 M KOH; (B) Free energy plots of OER intermediates on the Co-site in the 5% La-CoMoO4 system under different thermodynamic potentials in alkaline media. This figure is quoted with permission from Arumugam et al.[106]; (C) XPS spectrum of La-NiFe LDH/NiS/NF La 3d; (D) Polarization curves of different electrodes; (E) Free energy diagram. This figure is quoted with permission from Song et al.[107]; (F) LSV curves at 10 mA cm-2 and current densities at 1.6 V of commercial RuO2, RuO2, and LaxRu1-xO2; (G) Reaction free energy of the acidic OER on RuO2 and La0.1Ru0.9O2. This figure is quoted with permission from Li et al.[111]; (H) XPS spectra of O 1s for La3IrO7 and La3IrO7-SLD; (I) Polarization curves of La3IrO7-SLD and commercial IrO2 at a scan rate of 5 mV s-1. This figure is quoted with permission from Chen et al.[112]; (J) Schematic of the PEM electrolyzer device and I-V curves of PEM electrolyzer using La-RuO2. This figure is quoted with permission from Zhang et al.[113]. RHE: Reversible hydrogen electrode; NF: nickel foam; LSV: linear sweep voltammetry; OER: oxygen evolution reaction; XPS: X-ray photoelectron spectroscopy; PEM: proton exchange membrane; SLD: surface La-defective.