3D-printed conductive hydrogels for flexible electrochemical energy storage: mechanisms, fabrication, and applications
Abstract
Conductive hydrogels have emerged as crucial components for sophisticated flexible energy storage devices, such as batteries and supercapacitors, because of their customizable microstructures, mechanical versatility, and integrated electronic/ionic conductivity. Conventional fabrication methods face persistent challenges in balancing electrical performance with mechanical durability and constructing complex three-dimensional (3D) geometries. Three-dimensional-Printed addresses these limitations by enabling precise spatial control over material deposition and structural design. This review comprehensively analyses three critical aspects of 3D-printed conductive hydrogels: (1) Fundamental conduction mechanisms in electronic, ionic, and composite hydrogels, focusing on material optimization through nanoscale dispersion control and dynamic network design; (2) Advanced manufacturing methods including photopolymerization and direct ink writing, analyzing critical parameters including rheological behavior, printing resolution, and structural-functional synergy; (3) Groundbreaking applications in flexible energy storage, particularly supercapacitors with geometrically enhanced electrodes and batteries featuring self-healing zinc anodes. Persistent challenges in material compatibility, scalability trade-offs between resolution and speed, and interfacial stability are critically assessed. Future research directions focus on multifunctional ink development, multiscale structural engineering, and reliability optimization to enable customized, commercially viable flexible energy storage technologies.
Keywords
INTRODUCTION
In recent years, electrochemical energy storage devices, such as batteries and supercapacitors (SCs), have been extensively employed as primary power sources for everyday applications[1,2]. Hydrogels, characterized by water-filled cross-linked hydrophilic polymer networks[3], have become a very promising substitute for traditional electrolytes. Their exceptional water retention capacity and inherent softness make them
Traditional template-based fabrication methods have limited the hydrogel electrolyte preparation
Comparison between 3D printing and traditional methods for hydrogel fabrication
| Conventional methods | 3D printing | Ref. | |
| Process principle | Depending on the mold, formed by casting or pressing | Based on a digital model, materials are deposited layer by layer | [11] |
| Forming freedom | Difficult to prepare complex structures due to mold limitations | Complex 3D structures can be designed | [12,13] |
| Material utilization rate | May generate excess waste | Allocation on demand, utilization rate > 90% | [14] |
| Resolution | Limited by molds (typically > 100 μm); difficult to achieve micro-scale features | High precision, 50-510 µm | [11] |
| Cost | Mold development costs are high, suitable for mass production | High equipment investment, but flexible for single-piece/small-batch production | [15] |
| Multi-material integration capabilities | Challenging; requires post-assembly; limited material compatibility | Printable multi-material composite structures | [16-18] |
In recent years, hydrogels have shown great promise as feedstocks for 3D printing, facilitating the fabrication of fiber scaffolds with micro- and submicron-scale resolution. Advanced 3D printing inks and printable hydrogel formulations have been the focus of increasing research efforts. For instance, Zhu et al.[19] looked into the basic ideas behind 3D printing inks along with the developments and difficulties of flexible electrochemical energy storage devices such as SCs and batteries. Additionally, silk fibroin-based hydrogels have been developed, showing considerable potential as 3D printing materials for various applications[20]. Moreover, an effective gelation strategy has been developed using dual-addition-induced physical cross-linking, enhancing the extrusion-based 3D printing performance of Poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate) (PEDOT: PSS) hydrogels[21]. These creative studies demonstrate how well hydrogel materials and 3D printing technologies work together.
The field of flexible energy storage devices utilizing 3D-printed hydrogels represents a highly promising frontier for research and innovation, as illustrated in Figure 1. Numerous studies have delved into the advancements, applications, and transformative potential of this emerging field. These cutting-edge advanced fabrication technologies are revolutionizing the production of 3D-structured electrochemical energy storage devices, bridging the gap between the macro and nanoscales[28]. Significant gains in energy and power densities are made possible by the unmatched ability of 3D printing technology to accurately regulate the spatial geometry and architecture of devices[29]. Furthermore, the additive nature of 3D printing enables the creation of complex designs through a streamlined and cost-effective process[30]. Evolving from rapid prototyping, 3D printing is an advanced fabrication technique that constructs 3D objects with intricate geometries directly from programmed designs through layer-by-layer material deposition[31]. Leveraging its unique ability to manipulate materials in both spatial and temporal dimensions,
In light of the rapid advancements in novel hydrogel materials, innovative hydrogel ink formulation techniques, and state-of-the-art 3D printing technologies, a comprehensive and critical review is both timely and imperative. This review systematically consolidates and critically analyzes the latest advancements in 3D-printed hydrogels specifically designed for flexible electrochemical energy storage devices. Initially, we provide an in-depth summary of the classification, conduction mechanisms, and intrinsic characteristics of conductive hydrogels. Following this, we delve into the integration of 3D printing technologies in hydrogel fabrication, highlighting the categorization of fabrication methods, fundamental principles, and unique material characteristics. Furthermore, we investigate the applications of 3D-printed hydrogels in flexible energy storage systems, with a particular focus on recent advancements and technological breakthroughs. Furthermore, the applications of 3D-printed hydrogels in flexible energy storage systems, with a particular emphasis on recent advancements and technological breakthroughs, were systematically summarized. Finally, we provide a critical evaluation of the impact of 3D printing on electrochemical performance, exploring its compatibility with hydrogels, strategies for enhancing electrochemical properties, and the ongoing challenges that remain in the field. This review aims to facilitate further innovation in flexible electrochemical energy storage devices, expanding the potential of 3D-printable hydrogel materials and fabrication methodologies while positioning them as pivotal candidates for the next generation of flexible energy storage technologies.
FUNDAMENTALS OF CONDUCTIVE HYDROGELS
Powering wearable electronics demands flexible energy storage systems capable of enduring continuous bending, stretching, and twisting. Among these, SCs and batteries emerge as the most promising candidates, necessitating a balance between electrochemical stability and mechanical robustness[33]. Hydrogels are soft, moisture-rich materials with a 3D porous network structure, enabling them to absorb and retain substantial water content while preserving their semi-solid integrity [Figure 2A-C][34,35]. The incorporation of conductive materials into the hydrogel matrix transforms hydrogels into conductive materials. An extensive variety of conductive agents, such as conductive polymers[36], metallic nanoparticles/nanowires[37], liquid metals (LMs)[38], carbon-based nanomaterials[39], and ionic liquids[40], have been integrated into hydrogel matrices to engineer conductive hydrogels, as illustrated in Figure 2D and E[41]. Typically, the conductivity of hydrogels is improved through the incorporation of increased amounts of conductive materials or the doping of conductive polymers. Based on their conduction mechanisms, these enhanced hydrogels are categorized into electronically conductive hydrogels, ionically conductive hydrogels, and composite conductive hydrogels. Conductive hydrogels synergize the distinctive benefits of conductive materials and hydrogels, offering outstanding electronic properties, adjustable mechanical flexibility, and facile processability[42].
Figure 2. Classification of different conductive hydrogels, including (A) electronically conductive hydrogels[35], (B) ion conductive hydrogels[35], and (C) composite conductive hydrogels[35]. Molecular structures of (D) matrix polymers[41] and (E) conductive fillers commonly utilized in conductive hydrogel formulations[41].
Electronically conductive hydrogels
Electronically conductive hydrogels represent a unique category of hydrogel materials that enable electrical conductivity via electron transport mechanisms, predominantly driven by the free electrons of metals or the delocalized π-electrons of conjugated systems. The conductive fillers integrated into these hydrogels are generally classified into metal-based, carbon-based, and conductive polymer-based, etc.[43]. Metal-based conductive hydrogels seamlessly integrate the exceptional electrical conductivity of metals with the inherent flexibility of hydrogels. Typical metal fillers include gold and silver nanoparticles or nanowires, and LMs. While metal nanoparticles offer excellent conductive properties, their propensity to aggregate within the hydrogel matrix poses challenges. Furthermore, the mechanical disparity between rigid metallic fillers and the soft, elastomeric hydrogel matrix often results in stress concentration at the hydrogel-filler interface, thereby restricting scalability and undermining long-term durability[44]. Additionally, LMs represent a novel class of alloys that uniquely combine the superior electrical conductivity of traditional metals with the remarkable fluidity characteristic of liquids at or near ambient conditions[45]. Soft LMs endow hydrogels with exceptional conductivity and deformability. However, challenges such as poor interfacial compatibility between LMs and the polymer matrix hinder mechanical performance, while the susceptibility of LMs to oxidation further limits their long-term stability[46]. Toward this, Wu et al.[47] addressed these challenges by stabilizing LM emulsions via strong electrostatic interactions. This enabled the polymerization of acrylic acid (AA) within the stabilized emulsions, yielding a LM-polyacrylic acid (PAA) hydrogel with exceptional conductivity (1.54 S m-1), superior tensile strength, notable elongation at break, self-adhesion, and rapid self-healing capabilities.
Carbon-based materials, including graphene (GN)[48] and carbon nanotubes (CNTs)[49], offer remarkable advantages, such as high surface area, exceptional electron mobility, and outstanding mechanical flexibility[50]. However, the inherent hydrophobicity of GN and CNTs often results in aggregation within aqueous media, posing significant challenges to achieving uniform dispersion[51]. To overcome this challenge, Zheng et al.[52] developed a highly stretchy, durable, and self-healing hydrogel created by
Figure 3. (A) Preparation of the TOCNF-GN/PAA composite hydrogels[52]; (B) Demonstration of the excellent self-healing ability, stretchability, and flexibility of the hydrogels[52]; (C) Compressive stress-strain curve of the PAM-co-HEA hydrogel[53]; (D) EIS spectra of the hydrogels with varying CNT content[53]; (E) Photographs of the hydrogel under bending, knotting, twisting, and stretching conditions[53]; (F) Photographs of the LM/CNT hydrogel applied to the finger pulp and wrist[54]; (G) Photographs of the LM/CNT hydrogel before and after stretching[54]; (H) Schematic of the synthesis process for anisotropic bamboo template (ABT) and polypyrrole (PPy) bamboo-based/polyacrylamide hydrogel (PBPH)[61]; (I) Schematic synthesis of the composite hydrogels[63]; (J) Conductivity of the PAM/OM composite hydrogels[63]. TOCNFs-GN/PAA: 2,2,6,6-tetramethylpiperidine-1-oxyl radical-oxidized cellulose nanofibers-GN nanocomposites into PAA; PAM-co-HEA: polyacrylamide-co-hydroxyethyl acrylate; PAA: polyacrylic acid; CNT: carbon nanotube; LM: liquid metal; OM: oxidized MXene; EIS: electrochemical impedance spectroscopy.
Conductive polymer-based hydrogels typically incorporate conductive polymers such as polypyrrole (PPy)[55], polyaniline (PANI)[56], and poly(3,4-ethylenedioxythiophene) (PEDOT)[57]. Unlike other conductive fillers, CPHs maintain structural uniformity between the hydrogel matrix and the conductive material, eliminating the interfacial issues typical in organic-inorganic composites. This normally results in more consistent and adaptable electronic conductivity[58]. However, these hydrogels often suffer from low mechanical strength, primarily due to the inherent brittleness of conductive polymers, which makes it challenging to incorporate toughening agents without compromising the conductive network[59]. Zhou et al.[60] introduced a dual-continuous CPH (BC-CPH) that integrates high conductivity (> 11 S cm-1), superior fracture toughness (> 3,300 J m-2), and exceptional stretchability (> 400%). This hydrogel is appropriate for sophisticated fabrication processes such as 3D printing since it is made from
Since the successful exploration of 2D transition metal carbides/nitrides (MXene) in 2011, they have garnered significant attention in research, with their integration into hydrogel systems notably enhancing performance[62]. However, the abundance of polar groups on MXenes leads to their tendency to aggregate during hydrogel formation. To address this, Zhang et al.[63] employed oxidation treatment to prepare oxidized MXene (OM) to adjust its nanostructure. The oxidation products were embedded within the MXene layers, improving their dispersion in the hydrogel matrix. A composite hydrogel was synthesized by combining OM with PAM [Figure 3I]. The nanostructural modification of MXene improved its dispersion in the composite hydrogel, leading to the PAM/OM (POM) hydrogel exhibiting superior conductivity, enhanced transparency, mechanical strength, and sensitivity compared to the PAM/MXene (PM) hydrogel [Figure 3J]. A high loading of conductive fillers is essential for establishing efficient conductive networks. However, their poor hydrophilicity and strong interactions with the hydrogel matrix often lead to aggregation, undermining the mechanical integrity of the composite[64,65]. Therefore, striking an optimal balance between conductivity and mechanical performance remains a formidable challenge.
Ion conductive hydrogels
Ion-conductive hydrogels (ICHs) are typically synthesized by integrating salt solutions[66], polyelectrolytes[67], or ionic liquids[68] into the hydrogel matrix. The 3D network of the hydrogel provides channels to facilitate ion transport, endowing the material with enhanced conductivity. Because of their superior ionic conductivity and chemical durability, ICHs are extensively used in flexible electronics, electrochemical energy storage, catalytic transistors, and other applications[69]. However, their brittle mechanical properties and limited moldability still seriously hinder their broader applications[70]. To address this, Cui et al.[71] demonstrated the synergistic effects of cations and anions in toughening the mechanical properties of ICHs, offering new perspectives for network design and functional optimization. In addition, Yao et al.[72] developed a multifunctional ICH using a phenylboronic acid-ionic liquid (PBA-IL)-based system, enhancing its application potential. This semi-interpenetrating network hydrogel was synthesized by integrating cellulose nanofibrils (CNF) into a PBA-IL/acrylamide cross-linked network through a straightforward one-step process [Figure 4A]. Ingeniously, the dynamic borate ester bonds, along with hydrogen and electrostatic interactions within the cross-linked network, imparted the hydrogels with remarkable stretchability (1,810 ± 38%), self-healing efficiency (92 ± 2%), and adhesion [Figure 4B]. The incorporation of CNF effectively synergized the enhancement of both mechanical strength and ionic conductivity.
Figure 4. (A) Fabrication mechanism and dynamic networks of the PAM/PBA-IL/CNF hydrogel[72]; (B) Adhesive properties of the PAM/PBA-IL/CNF hydrogel[72]; (C) A flexible Zn ion battery with “SEU” characters punctured, capable of powering the
To improve their ionic conductivity, water-based hydrogels are usually swelled with saline solutions. However, this often hinders their intimate interaction with electrodes and diminishes their mechanical characteristics. To address this, Xia et al.[73] developed a zinc-ion conductive hydrogel electrolyte by incorporating a PAM network with a pseudo-polyurethane structure. At ambient temperature, this hydrogel electrolyte demonstrated an ionic conductivity of 22.4 mS cm-1, a high Zn2+ transference number of 0.923, and can power a hygrometer [Figure 4C]. In addition to their mechanical properties, hydrogels face limitations in operating across a broad temperature range due to issues such as severe dehydration, deionization, and freezing, etc.[74]. For instance, Zhu et al.[67] developed a self-assembled 3D polysaccharide network with different contents of phytic acid (PA) to improve the mechanical properties of conductive hydrogels, enhancing freeze resistance and water retention. PA acted as a multifunctional cross-linker, forming a robust network through ion-dipole interactions and hydrogen bonding, addressing the brittleness and low conductivity of glycerol-solvent hydrogels. The hydrogel exhibited impressive mechanical performance with a maximum stress of 2.26 MPa, 0.95 MPa modulus, and 3.59 S m-1 conductivity [Figure 4D]. Moreover, it demonstrated a wide operational temperature range down to -48 °C and maintained flexibility for 7 days.
In addition, constructing ICHs with ionic group-containing polymers is a promising strategy. Notably, zwitterionic hydrogels excel in ion transport dynamics, offering high migration numbers and conductivity through their rapid anion-cation channels[75]. Liu et al.[76] developed self-adhesive hydrogel electrolytes (PAD@SC) by copolymerizing AA with the zwitterionic monomer sulfonated betaine. The incorporation of bio-based lignosulfonate and nanocellulose facilitated the reconstruction of a robust non-covalent hydrogen bond network, significantly enhancing mechanical properties. The resulting PAD@SC hydrogel exhibited a remarkably high Zn2+ transference number (tZn2+ = 0.87) and ionic conductivity of 43.2 mS cm-1 [Figure 4E].
In addition to utilizing salt solutions and ionic group-containing polymers, ionic liquids, which are
Electro-ionic composite conductive hydrogel
Besides electronic and ICHs, some studies have integrated both types to develop hydrogels with dual transport capabilities. For instance, Wan et al.[79] incorporated MXene and ionic liquids into a gelatin/PAM precursor solution. The flexible strain sensors, based on a PAM/gelatin/ILs/MXene/glycerol (PGIMG) hydrogel, utilized a dual electronic-ionic conductive network created through electrohydrodynamic printing and in-situ photopolymerization. Ionic liquids provided ionic conduction, while MXene nanosheets formed an interpenetrating network with gelatin and PAM, enhancing both mechanical and sensing performance. The PGIMG hydrogel exhibited a tensile strength of 0.21 MPa at 602.82% strain, high sensitivity (sensitivity factor = 4.17), and high conductivity of 1.636 × 10-3 S cm-1. Moreover, the glycerol further imparted excellent freeze resistance (at -60 °C) and water retention [Figure 4G]. In addition, Zhao et al.[80] also developed a novel dual-network (DN) ICH system, combining poly(vinyl alcohol) (PMP DN ICH), MXene, and poly(ionic liquid) using freeze-thaw cycles and ionizing radiation. The ICH demonstrated remarkable ionic conductivity (63.89 mS cm-1 at 25 °C), exceptional thermal stability (-60 °C to 80 °C), long-term durability (30 days at ambient temperature), and notable antioxidant and antibacterial properties, alongside superior mechanical strength and adhesion. The PMP DN ICH electrolyte demonstrated remarkable environmental adaptability when utilized in solid-state SCs, achieving an excellent areal capacitance of 253.38 mF cm-2 at
In summary, composite conductive hydrogels often demonstrate conductivity several orders of magnitude higher than that of ICHs[81]. The integration of conductive fillers within the hydrogel matrix, coupled with advanced fabrication techniques and design strategies tailored to specific applications, plays a pivotal role in enhancing their conductivity, mechanical robustness, and other performance attributes.
Conductive hydrogels combine the unique advantages of conductive materials and water, giving them excellent electronic properties, adjustable mechanical flexibility, and easy processing characteristics. And many researchers are constantly optimizing the performance of conductive hydrogels and expanding the application scenarios of conductive hydrogels. However, due to their inherent properties, they still face many challenges in terms of conductivity, mechanical strength, stability and processing adaptability, such as the problem that hydrogels are easy to lose water in the air, insufficient chemical stability under actual working conditions such as alkalinity/acidity/high temperature, and the conflict between conductivity and mechanical properties. These problems seriously restrict their development. In order to solve the above problems, it is urgent to carry out systematic research in material design, structural optimization, and process control.
3D PRINTING TECHNOLOGY IN HYDROGEL PREPARATION
The technology, 3D printing, commonly known as additive manufacturing, is a process that constructs objects layer by layer from digital models. It enables the rapid, highly precise fabrication of complex structures, with exact control over design and production facilitated by advanced computer-aided
Preparation methods
The 3D printing technology has rapidly advanced in recent years, driven by its mold-free nature and versatile capabilities for customized structural design. Depending on material deposition methods, curing processes, and bonding mechanisms, there are seven different types of 3D printing: (i) Material extrusion [e.g., direct ink writing (DIW), fused deposition modeling (FDM)]; (ii) Material jetting [e.g., inkjet printing (IJP)]; (iii) Photopolymerization [e.g., stereolithography (SLA), digital light processing (DLP)]; (iv) Powder Bed Fusion [e.g., selective laser melting (SLM), selective laser sintering (SLS)]; (v) Sheet Lamination [e.g., laminated object manufacturing (LOM)], etc. Each of these 3D printing methods possesses unique characteristics and advantages, such as material options, speed, resolution, and cost, making them appropriate for a range of uses[83,84]. The successful 3D printing of conductive hydrogels has been well demonstrated by recent investigations employing cutting-edge methods including DIW, DLP, SLA, and FDM, with significant applications in areas such as flexible energy and electronics, biosensors, and soft robotics[35]. As shown in Table 2, a thorough analysis of the most recent developments in the 3D printing of conductive hydrogels was carried out in this section.
Summary of 3D printing technologies for conductive hydrogel fabrication
| 3D printing technology | Materials | Layer thickness (μm) | Advantages | Disadvantages | Ref. |
| DIW | Ceramics, composites, polymers, tissues | 50-300 | Fast, adaptable, and devoid of support materials | Low resolution, postprocess | [85-87] |
| FDM | Thermosets, glass, metals | 50-200 | Fast, affordable, simple, versatile | Poor mechanical strength, limited materials | [88,89] |
| IJP | Ceramics, metals, polymers | 1-200 | Fast printing, large-scale printing | Low resolution, poor adhesion between layers | [90] |
| SLA | UV-curing resins or photopolymers with ceramics & nanocomposites | 1-50 | Microscale resolution, high structural fidelity | Limited supplies, sluggish printing, and expensive | [91,92] |
| DLP | Photosensitive resin, photopolymers | 1-50 | Microscale resolution, high structural fidelity | Limited supplies, sluggish printing, and expensive | [81,93,94] |
DIW
DIW, an extrusion-based 3D printing technique, employs a three-step fabrication process: (1) formulation of a viscoelastic ink system exhibiting well-defined yield stress properties; (2) controlled extrusion of the ink through micron-scale nozzles under precisely regulated pressure to generate continuous filaments; and (3) layer-by-layer deposition of 3D architectures guided by CAD/CAM systems [Figure 5A]. The manufacturing process involves coordinated motion control, where the printhead executes programmed trajectories in the XY plane while the build platform undergoes controlled displacement along the Z-axis, enabling the sequential construction of complex 3D structures[95].
Figure 5. Schematic overview of the light- and ink-based 3D printing techniques. (A) DIW printing[22,27]; (B) FDM printing[22,27]; (C) DLP printing[23,27]; (D) SLA printing[25,27]; (E) Inkjet printing[26,27]. DIW: Direct ink writing; FDM: fused deposition modeling; DLP: digital light processing; SLA: selective laser sintering.
The printing resolution of this technology is primarily determined by three key factors: nozzle geometry, extrusion pressure, and the rheological properties of the ink. Among these, the rheological behavior of the ink plays a critical role in influencing printing outcomes, with essential parameters including yield stress
DIW technology exhibits significant advantages in the fabrication of functional materials. First, its material utilization efficiency exceeds 95%, markedly outperforming traditional subtractive manufacturing methods. Second, it enables the construction of composite systems incorporating diverse functional components, such as nano-conductive particles, carbon-based fibers, and ionic hydrogels. Third, the equipment costs associated with DIW are approximately 30%-50% lower than those of other 3D printing technologies. By precisely regulating the viscoelastic properties of precursor solutions and controlling curing kinetics, DIW facilitates the fabrication of functional hydrogels with complex 3D topological structures[14]. Nevertheless, a major technical bottleneck remains: the development of functional inks that can simultaneously satisfy stringent criteria, including high solid content (> 20 wt%), well-controlled rheological behavior (viscosity in the range of 106-108 cP), and stable extrusion performance[95].
There have been recent developments in the creation of functional inks. Yu et al.[96] innovatively constructed a CPH system based on a PVA matrix. By integrating PEDOT: PSS conductive networks and ionic liquid plasticizers, they successfully formulated a multifunctional, 3D-printable ink. Benefiting from its unique structural design, the system demonstrates significantly enhanced performance: an electrical conductivity of 1.2 S·m-1 (two orders of magnitude greater than traditional hydrogels), a reduced interfacial impedance of
FDM
One of the most popular commercial 3D printing technologies is FDM, which is preferred due to its low cost, patent advantages, fast printing speed, and large-scale manufacturing capabilities. In order for FDM to work, thermoplastic filaments must be heated to a semi-molten state, extruded through a fine nozzle, and rapidly solidified on the build platform to achieve layer-by-layer construction of 3D structures[97] [Figure 5B]. Common materials for FDM include polylactic acid (PLA), acrylonitrile butadiene styrene (ABS), polycarbonate (PC), polyamide (PAM), and thermoplastic polyurethane (TPU), all of which must be fabricated into filaments of specific diameters. Although the limited material selection restricts FDM applications in fields such as battery electrode manufacturing, this challenge can be addressed by embedding active materials into thermoplastic matrices to produce composite filaments, thus broadening the application scope. In addition, FDM can be employed to print substrates, which can subsequently be functionalized by post-processing techniques such as electrode or electrolyte deposition. However, compared with DIW, FDM has limited throughput, less flexibility for integrating several materials, and a poorer printing resolution (usually between 50 and 200 μm). Unlike DIW, which relies on the preparation of printable inks, FDM fundamentally depends on the development and processing of composite thermoplastic filaments[95].
Photopolymerization
Photopolymerization-based 3D printing technologies mainly include SLA and DLP, both commonly employing photopolymerizable hydrogels as printing materials. In this approach, UV light initiates the polymerization of photosensitive polymers to form patterns in designated areas, enabling the layer-by-layer construction of 3D structures. SLA achieves complex geometries through point-by-point laser scanning, whereas DLP cures an entire layer simultaneously via digital projection. Despite the high cost, limited mechanical strength, and shrinkage risk associated with photopolymerizable hydrogels, SLA and DLP exhibit significant advantages in fabricating flexible electrochemical energy storage devices with intricate geometries, offering high resolution, rapid printing speeds, the elimination of support structures, and excellent surface quality. Moreover, photopolymer resins suitable for SLA are generally compatible with DLP technology[35].
DLP is an advanced 3D printing technology that constructs 3D structures through the layer-by-layer
SLA is a photopolymerization-based 3D printing technology that uses UV laser to focus on the surface of a photosensitive resin, which solidifies layer by layer through a precise photochemical curing process to form a 3D model [Figure 5D]. This technique relies on the efficient combination of prepolymers and photoinitiators to ensure rapid curing of the resin under laser exposure, forming a cross-linked network. SLA offers high flexibility in resolution, enabling the production of intricate and detailed structures. However, its application is limited by material selection and cost factors. Despite these limitations, SLA remains highly favored due to its nozzle-free design, high precision, and versatility in industries such as automotive, aerospace, and biotechnology[95]. Using riboflavin as the photoinitiator and poly(ethylene glycol) dimethacrylate (PEGDMA) as the encapsulating polymer network, Karakurt et al.[98] created a unique water-soluble resin chemistry for SLA 3D printing. A viable route for the pharmaceutical industry to use non-toxic 3D printing materials for regulated and customizable drug release systems is provided by the system, which shows 3D-printed hydrogels with a variety of distinct geometries and their corresponding ascorbic acid release rates.
IJP
IJP, a non-contact extrusion-based technique [Figure 5E], utilizes nozzle-ejected droplets that are rapidly deposited onto substrates to fabricate pre-designed patterns. IJP technology primarily utilizes two distinct droplet generation methods: drop-on-demand (DOD) and continuous inkjet (CIJ) systems. In CIJ printing, a pressurized liquid jet exiting the nozzle undergoes controlled hydrodynamic instability, resulting in the formation of a monodisperse droplet stream. Selective electrostatic charging of individual droplets enables precise trajectory control while facilitating ink recovery and recirculation. However, exposure to the surrounding environment during recycling may lead to contamination of the ink. In DOD inkjet systems, discrete droplet ejection is achieved through precisely controlled pressure pulses generated either by piezoelectric actuator deformation or thermally induced vapor bubble nucleation. The DOD mode is more cost-effective and can produce smaller ink droplets (CIJ droplets range from 20-50 μm, while DOD droplets are typically around 100 μm), which is highly advantageous for 3D printing manufacturing[97].
IJP technology has proven to be a simple and efficient method for printing a wide variety of materials, including metals, polymers, and ceramics, offering excellent spatial resolution and uniformity for creating diverse geometries. The success of high-quality pattern printing heavily relies on the use of printable inks with specific characteristics, such as viscosity, surface tension, shear yield stress, shear elasticity, and loss modulus. These inks typically consist of active materials, solvents [such as water, N-methyl-2-pyrrolidone (NMP), dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and ethanol], and functional additives to optimize fluid behavior. Stable droplet generation requires careful matching of ejection parameters (velocity, nozzle geometry) with the ink’s fluid characteristics to maintain optimal jetting performance[97].
ADVANCEMENTS IN 3D PRINTING FOR FLEXIBLE ELECTROCHEMICAL ENERGY STORAGE DEVICES
With the continuous growth in energy demand and the ongoing challenges in energy supply, efficient and functional flexible energy storage devices have become a major focus of current research. This is particularly true in fields such as wearable electronics and medical devices, where there is an increasing demand for flexible energy storage devices that offer fast charging, high energy density, power density, lightweight properties, and long-term stability. SCs and batteries, as typical representatives of flexible energy storage devices, have their performance significantly influenced by the properties of electrode and electrolyte materials. In this regard, hydrogels, with their exceptional electrical properties and mechanical performance, provide an ideal material foundation for the design of flexible electrochemical energy storage (FEES) devices[99]. The 3D printing technologies have recently enabled unprecedented expansion of hydrogel functionality and application scope. Through precise control over structure and function, 3D-printed hydrogels can achieve exceptional attributes such as stretchability, biocompatibility, sensitivity, adhesion, self-healing, and freeze resistance, thereby meeting the requirements of various application scenarios. This technology also increases the surface area, improving electrochemical reaction efficiency. This section delves into the advancements of 3D-printed hydrogels in SCs and batteries, highlighting their potential to create efficient, multifunctional FEES devices and exploring their broad prospects in the future of energy storage and conversion[83,84].
Supercapacitors
The rapid miniaturization, integration, and intelligence of electronic devices have significantly increased the demand for high-energy-density, exceptional power density, long cycle life, and customizable
3D printing of hydrogels for supercapacitor electrodes
Nowadays, electrode materials based on metal oxides, conductive polymers, and electroactive carbon-based materials are used mainly in the fabrication of flexible SCs. When compared to metal oxides, conductive polymers, including PANI, PPY, and PEDOT: PSS, provide better conductivity and flexibility. Moreover, they can achieve higher capacitance and energy density than carbon-based materials due to their Faradaic pseudocapacitance. Conductive polymers are therefore frequently seen in flexible SCs. While conventional conductive polymers offer numerous advantages, their mechanical flexibility is inherently constrained by rigid π-conjugated backbones. More critically, these materials require exogenous structural support due to insufficient freestanding mechanical stability when deployed as flexible SC electrodes. Unfortunately, these substrates lack electrochemical activity and occupy significant volume and mass, reducing the overall specific capacitance and energy density and adding extra costs while complicating integration. Additionally, due to the mismatch in Young’s modulus between the electrode materials and the substrate, the deformability of these SCs is severely limited by inevitable dislocations at the electrode-substrate interface. To construct high-capacity and highly deformable SCs, substrate-free SCs present a promising and feasible solution. The key challenge lies in developing independent electrode materials that exhibit both high conductivity and mechanical stretchability[21].
Unlike traditional conductive polymers, CPHs achieve self-supporting capabilities and maintain mechanical deformation without the need for an external substrate, thanks to the gelation process. This renders CPHs highly suitable for developing standalone SCs with high capacity and mechanical deformability. For example, Cheng et al.[21] proposed a dual-additive gelation strategy for fabricating high-performance PEDOT: PSS hydrogels with intrinsic conductivity [Figure 6A]. By physically cross-linking the conductive PEDOT nanofibrils, these hydrogels exhibited enhanced conductivity (≈3,000 S·m-1), low Young’s modulus
Figure 6. (A) Schematic illustration of the gelation mechanism for PEDOT: PSS hydrogels[21]; (B) Diagram of the extrusion-based 3D printing process[21]; (C) Cyclic stability and Ragone plot of supercapacitors based on PEDOT: PSS hydrogels[21]; (D) Photographs of the original, bent, and twisted supercapacitors[21]; (E) Wearable demonstrations of the supercapacitors based on PEDOT: PSS hydrogels connected in series[21]; (F) Electrical conductivity of PEDOT: PSS, PM gel composite, and Zn-PM gel composite[102]; (G) Cycling stability of Zn-PM ZHMSCs with different printing layers at 20 mA cm-2[102]. PEDOT: PSS: Poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate; ZHMSCs: zinc-ion hybrid micro-supercapacitors.
3D printing hydrogels for supercapacitor electrolytes
As a key component of SCs, electrolytes critically determine the operating voltage window, which in turn governs the achievable energy density. Therefore, precise design and adjustment of the electrolyte are essential. Currently, the stable operating voltage of aqueous electrolytes is typically limited by the thermodynamic decomposition voltage of water (1.23 V). Organic electrolytes (≥ 2 V) and ionic liquids
Mogli et al.[104] explored a photopolymerized hydrogel based on a semi-interpenetrating polymer network
Figure 7. (A) Schematic illustration of the composition and fabrication process of the ionically conductive PVA/AAc/NaCl hydrogel[104]; (B) Tensile curve of 3D-printed dumbbell sample (at a tensile test velocity of 10 mm min-1)[104]; (C) Images of the integrated device featuring two different configurations. At the top, a pouch-sealed supercapacitor and sensor connected via electrical terminals (left), alongside a close-up view of the sealed LIG-hydrogel supercapacitor (right). At the bottom, the supercapacitor is encapsulated directly on the top electrode of the sensor[104]; (D) Printed compression specimens (bulk, honeycomb, stars) and their STL models[104]; (E) Schematic illustration of the GPE printing setup and its key compositional elements[105]; (F) Optical images and false-colored cross-sectional SEM micrographs of the samples captured at various stages of the dip-coating process[105]; (G) Schematic of customizable external geometry and internal 3D interdigitated architecture of 3D-printed EDLSCs[105]. PVA: Polyvinyl alcohol; 3D: three-dimensional; LIG: laser-induced graphene; STL: standard tessellation language; GPE: gel polymer electrolyte; SEM: scanning electron microscope; EDLSCs: electric double layer supercapacitors.
Design of 3D-printed supercapacitor configurations
In-plane solid-state SCs are considered promising candidates for fully printed solid-state energy devices, offering advantages such as fast charge/discharge rates, high-frequency response, long cycle life, operational stability in ambient conditions, and shape-factor-free fabrication. To date, various 3D-printable electrode materials have been investigated primarily to enhance the energy density of vertically assembled SC devices. However, despite significant advancements in electrode materials, other component layers, such as metal current collectors and solid-state electrolytes, have seldom been reported as 3D-printable materials. Despite significant advances in 3D-printed electrode materials, realizing fully 3D-printed SC devices remains a considerable challenge. Fully 3D-printed SC devices have yet to be demonstrated in structures with arbitrary designs.
Chae et al.[108] developed a unique strategy for on-demand shaping of energy storage units through a 3D printing process involving nozzle-based metal current collectors, carbon electrodes, and ionic liquid electrolyte fluids. By combining 3D printing and 3D laser sintering techniques, they created Ni metal current collectors that can operate stably under high-voltage conditions (up to 3V). GN nanoplatelet-based electrode fluids and UV-curable ionic liquid-based electrolyte fluids were continuously printed onto the top of the 3D-structured current collectors. The results demonstrated that the fully 3D-printed micro SC devices exhibited energy densities ranging from 25.4 μWh cm-2 to 8.5 μWh cm-2 and power densities from 150 μWh cm-2 to 6,483 μW cm-2.
Batteries
To improve the overall performance of batteries, including critical metrics such as energy density, volumetric energy density, power density, cycle life, and safety, it is essential to design and fabricate different battery modules, including cathodes, anodes, electrolytes, and separators. We will look at the existing printed battery modules from an architectural standpoint in this part. A single battery module requires specific characteristics, such as mechanical strength, high electronics, and ionic conductivity. Selecting and combining suitable 3D printing technologies and materials is essential to ensure they meet these requirements. The development of novel functional nanomaterials for electrochemical energy storage into printable materials for particular 3D printing technologies is unquestionably crucial for the growth of batteries and other energy storage technologies, given the rapid increase in the quantity of these materials[95].
Rechargeable electrochemical batteries (REBs) play an essential role in energy storage and transmission by effectively converting electrical and chemical energy through electron and ion transfer in the electrodes.
3D printing hydrogels for battery electrodes
The emergence of flexible and stretchable electronics has created a need for stretchable electrodes that possess outstanding mechanical durability and consistent electrochemical performance. Numerous initiatives have been undertaken to achieve this goal. For example, Qu et al.[110] prepared stretchable metal (zinc or nickel)-coated polyurethane sponge electrodes by a two-step deposition method, and Yu et al.[111] prepared ultra-stretchable CNT composite electrodes by coating CNT films and active material powders on a bidirectional pre-strained polydimethylsiloxane substrate. Compared with these methods, 3D printing technology can quickly and efficiently generate complex patterns in a plane or 3D space, and through appropriate geometric patterns, inherently hard and brittle materials can be made compliant and deformable. In addition, many studies have prepared 3D-printed conductive inks with various properties and stability, which provides us with a simple and effective solution for manufacturing stretchable electrodes.
Shi et al.[112] developed a PAM-hemicellulose/EGaIn microdroplet hydrogel [Figure 8A] for 3D printing scaffolds [Figure 8B] and self-healing anodes for zinc-ion batteries (ZIBs, Figure 8C). The hydrogel, initiated by LM, is a dual covalent hydrogen bonding system [Figure 8D] with self-healing and
Figure 8. (A) Digital photos of the viscoelastic composite[112]; (B) 3D-printed hydrogel scaffold made of polyacrylamide, hemicellulose, and EGaIn microdroplets[112]; (C) The composite is used as the anode host in a Zn-ion battery[112]; (D) Schematic of the hydrogel containing covalent bonds and hydrogen bonds[112]; (E) The EGaIn LM microdroplets cause acrylamide monomers to undergo radical polymerization with the microdroplets[112]; (F) Diagram of the printed LTO/LFP electrodes in a complete pouch cell battery[113]; (G) A pouch cell with printed electrodes that illuminates an LED in its folded, twisted, bent, and flat configurations is demonstrated. There is a 1 cm scale bar[113]. 3D: Three-dimensional; LTO: Li4Ti5O12; LFP: LiFePO4; LED: light emitting diode.
3D printing hydrogels for battery electrolytes
Traditional template manufacturing methods can only form hydrogel electrolytes with simple 2D or 3D structures. This limitation restricts their applicability in designing batteries with complex shapes and structures. Three-dimensional printing technology can quickly produce customized complex 3D structures. This method has the advantages of low loss, low cost, high throughput, and customization. However, the rheological characteristics of the produced inks must meet specific specifications set by 3D printing technology[114]. At present, the precursors of hydrogel electrolytes are usually low-viscosity liquids and are not suitable for 3D printing. Adding a thickener is the most popular way to make the ink more viscous. The hydrogel electrolyte structure’s stability will be harmed by the addition of the thickener, which will also have a major impact on its mechanical and electrochemical characteristics. As a result, we must satisfy the viscosity requirements of 3D printing technology while also guaranteeing the hydrogel electrolyte’s mechanical and electrochemical qualities.
Lu et al.[3] designed a novel 3D-printable hydrogel electrolyte ink based on DN cross-linked PAM/hydroxypropyl methylcellulose (PHHE) [Figure 9A and B]. This PHHE material demonstrated exceptional electrochemical properties, including: (1) high ionic conductivity (31.72 mS cm-1), (2) remarkable cycling stability (maintaining performance over 600 h at 0.5 mA cm-2 current density) [Figure 9C], and (3) a broad electrochemical stability window (0-2.3 V). By employing this PHHE electrolyte in 3D printing fabrication, the researchers successfully developed flexible zinc-ion micro batteries (FZIMBs) that achieved both high areal capacity (6.45 mAh cm-2 at 0.5 mA cm-2 discharge current) [Figure 9D] and superior mechanical flexibility [Figure 9E and F]. These FZIMBs, integrated with pressure-sensing components, formed an interactive sensing system for practical use in flexible wearable devices. Poompiew et al.[115] fabricated a novel class of 3D-printable flexible hydrogel polymer electrolytes for ZIBs utilizing PAM as the hydrogel matrix. Through DLP 3D printing technology, the researchers engineered an optimized porous architecture specifically designed for ZIB applications. This advanced manufacturing approach enabled the direct fabrication of functional ZIB devices with tailored structural characteristics. The 40% porosity PAM hydrogel electrolyte produced via 3D DLP showed promising potential as a solid-state electrolyte for ZIBs, with excellent electrochemical performance [Figure 9G-I].
Figure 9. The FZIMBs’ electrochemical performance. (A) FZIMBs printing procedure[3]; (B) The FZIMBs’ optical pictures[3]; (C) Performance of cycling at 5 mA cm-2[3]; (D) GCD curve from 0.5 mA cm-2 to 5 mA cm-2[3]; (E) Voltage variation during the battery bending process, with embedded test images[3]; (F) Battery GCD curves at various bending angles at 3 mA cm-2[3]; Charge-discharge curves at (G) different current densities[115] and (H) at 0.1 A g-1 for 3D printed 40% porosity hydrogel electrolyte[115], and (I) battery with 40% porosity hydrogel electrolyte connected with LED light[115]. FZIMBs: Flexible zinc-ion micro batteries; 3D: three-dimensional; GCD: galvanostatic charge-discharge.
In summary, conductive hydrogels can be used as both electrodes and ionic electrolytes in energy storage systems, depending on their structural and compositional design. When used as electrodes, hydrogels are often embedded with conductive fillers and active materials to facilitate the transport of electrons and ions while maintaining mechanical flexibility. In contrast, hydrogel electrolytes mainly serve as ionic conductors and separators, providing a hydrated environment that enables ions to migrate between electrodes. For hydrogel-based electrodes, future research may focus on enhancing electronic conductivity and mechanical integrity. Strategies such as nanoconfinement of conductive networks (e.g., CNTs, MXenes), hierarchical porous structures, and dynamic cross-linking are expected to improve charge transfer, durability, and structural adaptability. In addition, integrating pseudocapacitive or redox-active units into the hydrogel matrix can significantly improve energy density. On the other hand, hydrogel electrolytes need to improve ionic conductivity, long-term hydration stability, and chemical compatibility with a variety of electrode materials. The performance gap between commercially available flexible devices and 3D printing devices is shown in Tables 3 and 4.
Performance comparison between commercial flexible supercapacitors and 3D-printed conductive hydrogel supercapacitors
| Parameter | Commercial flexible supercapacitors | 3D-printed conductive hydrogel supercapacitors | Ref. |
| Areal capacitance (mF cm-2) | 500-2,600 | 100-600 | [116] |
| Energy density (mWh cm-2) | 0.1-0.5 | 0.1-2 | [116] |
| Power density (mW cm-2) | 1-50 | 0.1-58 | [116,117] |
| Cycle life (capacitance retention) | 10,000-40,000 cycles (88%-98%) | 5,000-10,000 cycles (83%-96%) | [116,118] |
| Bending angle (°) | 90°-180° | 150°-180° | [116,118,119] |
| Flexibility | Good | Excellent | [116,119] |
Performance comparison between commercial flexible batteries and 3D-printed conductive hydrogel batteries
| Parameter | Commercial flexible supercapacitors | 3D-printed conductive hydrogel supercapacitors | Ref. |
| Areal capacity (mAh·cm-2) | 0.05-23 | 0.5-10 | [3,120,121] |
| Energy density (mWh·cm-2) | 0.5-20 | 0.2-10 | [121,122] |
| Power density (mW·cm-2) | 1-10 | 2-8 | [102,121] |
| Cycle life (capacity retention) | 500-3,000 cycles (> 80%) | 100-2,000 cycles (> 80%) | [120-122] |
| Bending angle (°) | 90°-180° | 120°-180° | [3,123] |
| Flexibility | Good | Excellent | [124,125] |
THE EFFECT OF 3D-PRINTED HYDROGELS ON ELECTROCHEMICAL PERFORMANCE
The main reasons for the creative use of 3D printing technology in electrochemical energy storage are its special benefits in material integration and structural design. Compared to traditional manufacturing technologies, 3D printing can precisely construct electrode structures with customized geometric shapes, optimized pore distributions, and high specific surface areas. This precise control of microstructures plays a decisive role in enhancing ion transport kinetics and electronic conductivity networks. The synergistic effects between hydrogel materials and 3D printing technology are particularly notable: on one hand, hydrogels’ inherent high ionic conductivity, excellent mechanical flexibility, and biocompatibility provide a material foundation for improving electrochemical performance; on the other hand, 3D printing’s spatial precision control capability (with a resolution as high as 10-50 μm) enables the precise construction of complex porous networks and functional gradients.
Mass transfer kinetics optimization
Three-dimensional printing hydrogels significantly shortens ion diffusion paths and reduces tortuosity by controlling microstructures, thereby improving electrochemical reaction kinetics. Meng et al.[102] prepared Zn-poly(3,4-ethylenedioxythiophene): polystyrene sulfonate/MXene (Zn-PM) gel electrodes via 3D printing, featuring ultra-high mass loading (32.2 mg cm-1) and high shape fidelity. Even at ultra-high mass loading, the electrodes maintained efficient charge transfer efficiency and ion diffusion kinetics. The electrochemical characterization of the Zn-PM gel electrode demonstrated its exceptional rate performance (capacitance retention of 84% at 10 A g-1), high specific capacitance (261 F g-1 at 0.5 A g-1), and outstanding cycling stability (capacity retention near 100%). Using a straightforward PSS chain engineering technique that included the addition of thermally cross-linkable N-(hydroxymethyl)acrylamide segments, Yu et al.[126] created a PEDOT: PSS-based hydrogel that is highly conductive, naturally soft, durable, and stretchable. The hydrogel satisfies several performance requirements for realistic bioelectronic applications with its high conductivity (1,850 S m-1), high stretchability (> 50%), low Young’s modulus (4 MPa), and exceptional toughness (400 kJ m-3).
Customized geometric configuration
Three-dimensional printing hydrogels have broken through the structural limitations of traditional manufacturing, enabling the precise design of complex geometric configurations such as biomimetic hierarchical channels and periodic lattices, thereby optimizing the specific surface area of electrodes and charge transport pathways. Baur et al.[12] reported a method to controllably introduce open pores with diameters of tens of nanometers into hydrogels while maintaining substantial mechanical properties, with a compressive modulus greater than 100 kPa. Notably, these hydrogels are 3D printable, allowing for the adjustment of pore sizes within the hydrogel from tens of nanometers to the centimeter scale. This work leverages the 3D printability of the material to locally modify porosity while preserving mechanical performance, making the overall sample easy to handle.
Future prospects and roadmap for 3D-printed hydrogels in flexible electrochemical energy storage devices
Mechanical and functional synergy of composite hydrogels
Future 3D-printed hydrogels will break through the performance limits of single materials by achieving synergistic improvements in mechanical strength and electrochemical performance through nanocomposite and multilevel structure design. For example, incorporating ceramic nanoparticles (such as silica or barium titanate) into hydrogel matrices can simultaneously enhance mechanical strength (tensile strength exceeding 10 MPa) and ionic conductivity (maintained above 20 mS cm-1). Concurrently, the development of smart hydrogels with photoresponsive or magnetoresponsive properties will enable flexible electrochemical energy storage devices to possess dynamic functions such as self-healing and shape memory, thereby adapting to complex environmental demands.
From two-dimensional planes to three-dimensional functionalization
The introduction of 4D printing technology will enable hydrogel energy storage structures to adapt to their environment. By programming temperature/pH-responsive hydrogels, battery modules can autonomously dissipate heat at high temperatures or contract in shape at low temperatures, thereby enhancing device safety and efficiency. Bionic microstructure design (such as 15-35 μm porous structures regulated by
Extreme environment stability and energy density enhancement
To address the performance degradation of hydrogels under extreme temperatures, future efforts will focus on modifying them with antifreeze agents or hydrophobic groups to achieve an ion migration rate degradation of less than 20% at -20 °C. Additionally, the application of phase-change hydrogels will reduce the operating temperature of battery packs by over 8 °C, control cell temperature differences within 5 °C during fast-charging cycles, and extend device lifespan. In terms of energy density, hydrogels directly used as electrode materials can achieve a 95% capacity retention rate at a current density of 1 A g-1, driving the practical application of flexible batteries.
CONCLUSION AND OUTLOOK
In summary, 3D printing has become a revolutionary approach for constructing advanced electrochemical energy storage systems, offering advantages over traditional methods by streamlining production processes, enhancing reproducibility, and providing portability with integrated functionality. This review summarizes the fundamental properties of hydrogels, discusses key 3D printing strategies, and highlights their applications in SCs and batteries. Additionally, the impact of 3D printing hydrogel on electrochemistry has been discussed, highlighting its potential to revolutionize the field. However, the widespread adoption of 3D printing in future electrochemical energy storage systems remains hindered by several critical challenges, including the rational selection and optimization of electrode materials, achieving optimal electrode packing density, maintaining interface stability, and improving printing precision, speed, repeatability, and
Currently, the limited availability of 3D printable materials for electrochemical energy storage devices highlights the need to develop novel electrode materials, especially functional nanocomposites, in order to optimize material combinations and synthesis strategies. Establishing a comprehensive material property database and standardizing materials are essential for advancing high-performance 3D-printed energy storage devices. Additionally, 3D printing can create hierarchical pore structures that enhance ion and electron transport, but these structures reduce electrode density, limiting volumetric energy density. Therefore, new densification strategies such as interface chemistry, plasma etching, and strain engineering are necessary to optimize both volumetric and areal energy densities.
To advance the performance of 3D-printed electrochemical energy storage devices, efforts should focus on improving interfacial stability between components, optimizing printing accuracy, and increasing printing speed. Advances in interface engineering, coupled with the optimization of printing parameters and the adoption of multi-head or parallel printing techniques, will improve device efficiency and consistency. Furthermore, addressing issues such as parameter fluctuations and material heterogeneity through standardized protocols, strict quality control, and real-time monitoring will enhance reproducibility and stability. By overcoming these challenges, 3D printing technology can be fully optimized for large-scale, cost-effective production of energy storage devices.
DECLARATIONS
Authors’ contributions
Contributed equally to this work: Hu, Y.; Zhou, H.; Wang, N.
Data analysis and interpretation: Zhi. L.; Li, N.; Liu. Q.
Topic selection, manuscript review: Chen, Z.; Mo, F.
Editing and supervision: Chen, Z.; Liu, Q.; Mo, F.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2024A1515011694). The authors would also like to acknowledge financial support from the Natural Science Foundation of Top Talent of SZTU (No. GDRC202316) and the Shenzhen Key Technology Research and Development Program (No. KCXFZ20240903093914020). This work was also supported by the Shenzhen Municipality under the Stability Support Program of Shenzhen Colleges and Universities (Grant No. GXWD20220817150352006), Shenzhen Science and Technology Program (Grant No. RCBS20221008093222009), and Pingshan District Innovation Platform Project of Shenzhen Hi-tech Zone Development Special Plan (Grant No. 29853M-KCJ-2023-002-02).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Zhang, W.; Liu, H.; Zhang, X.; Li, X.; Zhang, G.; Cao, P. 3D printed micro-electrochemical energy storage devices: from design to integration. Adv. Funct. Mater. 2021, 31, 2104909.
2. Zhang, F.; Wei, M.; Viswanathan, V. V.; et al. 3D printing technologies for electrochemical energy storage. Nano. Energy. 2017, 40, 418-31.
3. Lu, Y.; Li, Z.; Wang, X.; et al. 3D printed dual network cross-linked hydrogel electrolytes for high area capacity flexible zinc ion micro-batteries. Chem. Eng. J. 2024, 490, 151523.
4. Nujud Badawi, M.; Kuniyil, M.; Bhatia, M.; et al. Recent advances in flexible/stretchable hydrogel electrolytes in energy storage devices. J. Energy. Storage. 2023, 73, 108810.
5. Devi, L. S.; Palathinkal, R. P.; Dasmahapatra, A. K. Preparation of cross-linked PANI/PVA conductive hydrogels for electrochemical energy storage and sensing applications. Polymer 2024, 293, 126673.
6. Tiwari, R.; Verma, D. K.; Kumar, D.; et al. Single-ion-conducting SPP-SPA blend hydrogel-based pseudo-solid polymeric electrolyte material for Na+-ion constructed energy storage devices. Energy. Fuels. 2022, 36, 6459-67.
7. Yi, F.; Meng, F.; Li, Y.; et al. Highly stretchable CNT Fiber/PAAm hydrogel composite simultaneously serving as strain sensor and supercapacitor. Compos. Part. B. Eng. 2020, 198, 108246.
8. Cheng, T.; Liu, Z. T.; Qu, J.; et al. High-performance organic-inorganic hybrid conductive hydrogels for stretchable elastic all-hydrogel supercapacitors and flexible self-powered integrated systems. Adv. Sci. 2024, 11, 2403358.
9. Zhou, C.; Wu, T.; Xie, X.; et al. Advances and challenges in conductive hydrogels: From properties to applications. Eur. Polym. J. 2022, 177, 111454.
10. Han, X.; Xiao, G.; Wang, Y.; et al. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A. 2020, 8, 23059-95.
11. Omidian, H.; Mfoafo, K. Three-dimensional printing strategies for enhanced hydrogel applications. Gels 2024, 10, 220.
12. Baur, E.; Hirsch, M.; Amstad, E. Porous 3D printable hydrogels. Adv. Mater. Technol. 2023, 8, 2201763.
13. Zu, G.; Meijer, M.; Mergel, O.; Zhang, H.; van Rijn, P. 3D-printable hierarchical nanogel-GelMA composite hydrogel system. Polymers 2021, 13, 2508.
14. Yin, X. Y.; Zhang, Y.; Cai, X.; et al. 3D printing of ionic conductors for high-sensitivity wearable sensors. Mater. Horiz. 2019, 6, 767-80.
15. Liu, H.; Yu, S.; Liu, B.; et al. Space-efficient 3D microalgae farming with optimized resource utilization for regenerative food. Adv. Mater. 2024, 36, 2401172.
16. Zhu, C.; Gemeda, H. B.; Duoss, E. B.; Spadaccini, C. M. Toward multiscale, multimaterial 3D printing. Adv. Mater. 2024, 36, 2314204.
17. Mu, H.; Wang, Z.; Xu, X.; et al. Heterogeneous growth of 3D printed polymer network for multi-material integration. Adv. Funct. Mater. 2025, 35, 2415638.
18. Huo, H.; Shen, J.; Wan, J.; et al. A tough and robust hydrogel constructed through carbon dots induced crystallization domains integrated orientation regulation. Nat. Commun. 2025, 16, 6221.
19. Zhu, Y.; Qin, J.; Shi, G.; et al. A focus review on 3D printing of wearable energy storage devices. Carbon. Energy. 2022, 4, 1242-61.
20. Pudkon, W.; Laomeephol, C.; Damrongsakkul, S.; Kanokpanont, S.; Ratanavaraporn, J. Comparative study of silk fibroin-based hydrogels and their potential as material for 3-dimensional (3D) printing. Molecules 2021, 26, 3887.
21. Cheng, T.; Wang, F.; Zhang, Y.; et al. 3D printable conductive polymer hydrogels with ultra-high conductivity and superior stretchability for free-standing elastic all-gel supercapacitors. Chem. Eng. J. 2022, 450, 138311.
22. Ravanbakhsh, H.; Karamzadeh, V.; Bao, G.; Mongeau, L.; Juncker, D.; Zhang, Y. S. Emerging technologies in multi-material bioprinting. Adv. Mater. 2021, 33, 2104730.
23. Khalaj, R.; Tabriz, A. G.; Junqueira, L. A.; Okereke, M. I.; Douroumis, D. 3D printed stents using fused deposition modelling. J. Drug. Deliv. Sci. Technol. 2024, 97, 105724.
24. Lim, K. S.; Galarraga, J. H.; Cui, X.; Lindberg, G. C. J.; Burdick, J. A.; Woodfield, T. B. F. Fundamentals and applications of photo-cross-linking in bioprinting. Chem. Rev. 2020, 120, 10662-94.
25. Hwang, H. H.; Zhu, W.; Victorine, G.; Lawrence, N.; Chen, S. 3D-printing of functional biomedical microdevices via light- and extrusion-based approaches. Small. Methods. 2018, 2, 1700277.
26. Malda, J.; Visser, J.; Melchels, F. P.; et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011-28.
27. Ge, G.; Wang, Q.; Zhang, Y.; Alshareef, H. N.; Dong, X. 3D printing of hydrogels for stretchable ionotronic devices. Adv. Funct. Mater. 2021, 31, 2107437.
28. Chang, P.; Mei, H.; Zhou, S.; et al. 3D printed electrochemical energy storage devices. J. Mater. Chem. A. 2019, 7, 4230-58.
29. Zeng, L.; Li, P.; Yao, Y.; Niu, B.; Niu, S.; Xu, B. Recent progresses of 3D printing technologies for structural energy storage devices. Mater. Today. Nano. 2020, 12, 100094.
30. Jabbar Khan, A.; Mateen, A.; Khan, S.; et al. 3D printed micro-electrochemical energy storage devices. Batteries. Supercaps. 2023, 6, e202300190.
31. Fonseca, N.; Thummalapalli, S. V.; Jambhulkar, S.; et al. 3D printing-enabled design and manufacturing strategies for batteries: a review. Small 2023, 19, 2302718.
33. Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220-40.
34. Yuan, Y.; Zhang, Q.; Lin, S.; Li, J. Water: the soul of hydrogels. Prog. Mater. Sci. 2025, 148, 101378.
35. Liang, X.; Zhang, M.; Chong, C. M.; et al. Recent advances in the 3D printing of conductive hydrogels for sensor applications: a review. Polymers 2024, 16, 2131.
36. He, H.; Chen, Y.; Pu, A.; et al. Strong and high-conductivity hydrogels with all-polymer nanofibrous networks for applications as high-capacitance flexible electrodes. npj. Flex. Electron. 2024, 8, 56.
37. Lu, B.; Cheng, H.; Qu, L. Inorganic hydrogel based on low-dimensional nanomaterials. ACS. Nano. 2024, 18, 2730-49.
38. Zhao, K.; Zhao, Y.; Xu, J.; Qian, R.; Yu, Z.; Ye, C. Stretchable, adhesive and self-healing conductive hydrogels based on PEDOT:PSS-stabilized liquid metals for human motion detection. Chem. Eng. J. 2024, 494, 152971.
39. Yue, J.; Li, C.; Ji, X.; et al. Highly tough and conductive hydrogel based on defect-patched reduction graphene oxide for high-performance self-powered flexible sensing micro-system. Chem. Eng. J. 2023, 466, 143358.
40. Zhou, Y.; Fei, X.; Tian, J.; Xu, L.; Li, Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J. Colloid. Interface. Sci. 2022, 606, 192-203.
41. Peng, H.; Wang, D.; Zhang, F.; et al. Improvements and challenges of hydrogel polymer electrolytes for advanced zinc anodes in aqueous zinc-ion batteries. ACS. Nano. 2024, 18, 21779-803.
42. Li, G.; Li, C.; Li, G.; et al. Development of conductive hydrogels for fabricating flexible strain sensors. Small 2022, 18, 2101518.
43. Zhu, T.; Ni, Y.; Biesold, G. M.; et al. Recent advances in conductive hydrogels: classifications, properties, and applications. Chem. Soc. Rev. 2023, 52, 473-509.
44. Xu, Y.; Tan, C.; He, Y.; Luo, B.; Liu, M. Chitin nanocrystals stabilized liquid metal for highly stretchable and anti-freeze hydrogels as flexible strain sensor. Carbohydr. Polym. 2024, 328, 121728.
45. Zheng, Y.; Liu, H.; Yan, L.; Yang, H.; Dai, L.; Si, C. Lignin-based encapsulation of liquid metal particles for flexible and high-efficiently recyclable electronics. Adv. Funct. Mater. 2024, 34, 2310653.
46. Zhao, D.; Wang, L.; Fang, K.; Luo, J.; Zhou, X.; Jiang, K. Fabrication of lignocellulose/liquid metal-based conductive eutectic hydrogel composite for strain sensors. Int. J. Biol. Macromol. 2024, 273, 133013.
47. Wu, S.; Wang, B.; Chen, D.; et al. Highly sensitive and self-healing conductive hydrogels fabricated from cationic cellulose nanofiber-dispersed liquid metal for strain sensors. Sci. China. Mater. 2023, 66, 1923-33.
48. Tian, L.; Liu, T.; Jiang, Y.; He, B.; Hao, H. Multifunctional hydrogel sensor with Tough, self-healing capabilities and highly sensitive for motion monitoring and wound healing. Chem. Eng. J. 2024, 497, 154890.
49. Li, W.; Tao, L. Q.; Kang, M. C.; et al. Tunable mechanical, self-healing hydrogels driven by sodium alginate and modified carbon nanotubes for health monitoring. Carbohydr. Polym. 2022, 295, 119854.
50. Park, J.; Jeon, N.; Lee, S.; Choe, G.; Lee, E.; Lee, J. Y. Conductive hydrogel constructs with three-dimensionally connected graphene networks for biomedical applications. Chem. Eng. J. 2022, 446, 137344.
51. Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly stretchable and self-healing strain sensors based on nanocellulose-supported graphene dispersed in electro-conductive hydrogels. Nanomaterials 2019, 9, 937.
52. Zheng, C.; Lu, K.; Lu, Y.; et al. A stretchable, self-healing conductive hydrogels based on nanocellulose supported graphene towards wearable monitoring of human motion. Carbohydr. Polym. 2020, 250, 116905.
53. Xie, T.; Ou, F.; Ning, C.; et al. Dual-network carboxymethyl chitosan conductive hydrogels for multifunctional sensors and high-performance triboelectric nanogenerators. Carbohydr. Polym. 2024, 333, 121960.
54. Sun, M.; Li, P.; Qin, H.; et al. Liquid metal/CNTs hydrogel-based transparent strain sensor for wireless health monitoring of aquatic animals. Chem. Eng. J. 2023, 454, 140459.
55. Zheng, H.; Chen, M.; Sun, Y.; Zuo, B. Self-healing, wet-adhesion silk fibroin conductive hydrogel as a wearable strain sensor for underwater applications. Chem. Eng. J. 2022, 446, 136931.
56. Xu, M.; Zhu, J.; Xie, J.; Mao, Y.; Hu, W. Dynamically cross-linked, self-healable, and stretchable all-hydrogel supercapacitor with extraordinary energy density and real-time pressure sensing. Small 2024, 20, 2305448.
57. Zhang, M.; Wang, Y.; Liu, K.; et al. Strong, conductive, and freezing-tolerant polyacrylamide/PEDOT:PSS/cellulose nanofibrils hydrogels for wearable strain sensors. Carbohydr. Polym. 2023, 305, 120567.
58. Luo, J.; Song, T.; Han, T.; et al. Multifunctioning of carboxylic-cellulose nanocrystals on the reinforcement of compressive strength and conductivity for acrylic-based hydrogel. Carbohydr. Polym. 2024, 327, 121685.
59. He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 2023, 14, 759.
60. Zhou, T.; Yuk, H.; Hu, F.; et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895-902.
61. Bai, T.; He, X.; Yan, J.; et al. Anisotropic bamboo-polypyrrole-polyacrylamide composite hydrogels for high-performance integrated rigid supercapacitors. Chem. Eng. J. 2025, 508, 161064.
62. Zhou, C.; Zhao, X.; Xiong, Y.; et al. A review of etching methods of MXene and applications of MXene conductive hydrogels. Eur. Polym. J. 2022, 167, 111063.
63. Zhang, Y.; Zou, J.; Wang, S.; et al. Tailoring nanostructured MXene to adjust its dispersibility in conductive hydrogel for self-powered sensors. Compos. Part. B. Eng. 2024, 272, 111191.
64. Wang, X.; Zheng, S.; Xiong, J.; et al. Stretch-induced conductivity enhancement in highly conductive and tough hydrogels. Adv. Mater. 2024, 36, 2313845.
65. Li, N.; Wang, X.; Liu, Y.; et al. Ultrastretchable, self-adhesive and conductive MXene nanocomposite hydrogel for body-surface temperature distinguishing and electrophysiological signal monitoring. Chem. Eng. J. 2024, 483, 149303.
66. Wang, C.; Yang, B.; Xiang, R.; Ji, J.; Wu, Y.; Tan, S. High-saline-enabled hydrophobic homogeneous cross-linking for extremely soft, tough, and stretchable conductive hydrogels as high-sensitive strain sensors. ACS. Nano. 2023, 17, 23194-206.
67. Zhu, W.; Zhang, Y.; Huang, S.; et al. Self-assembly polysaccharide network regulated hydrogel sensors with toughness, anti-freezing, conductivity and wide working conditions. Chem. Eng. J. 2024, 497, 154409.
68. Yang, J.; Chang, L.; Ma, C.; Cao, Z.; Liu, H. Highly electrically conductive flexible ionogels by drop-casting ionic liquid/PEDOT:PSS composite liquids onto hydrogel networks. Macromol. Rapid. Commun. 2022, 43, 2100557.
69. Ge, X.; Guo, Y.; Gong, C.; et al. High-conductivity, low-impedance, and high-biological-adaptability ionic conductive hydrogels for Ear-EEG acquisition. ACS. Appl. Polym. Mater. 2023, 5, 8151-8.
70. Guo, Y.; Feng, S.; Gao, W.; et al. Attapulgite-reinforced robust and ionic conductive composite hydrogels for digital light processing 3D printing. Adv. Funct. Mater. 2024, 34, 2408775.
71. Cui, W.; Zheng, Y.; Zhu, R.; et al. Strong tough conductive hydrogels via the synergy of ion-induced cross-linking and salting-out. Adv. Funct. Mater. 2022, 32, 2204823.
72. Yao, X.; Zhang, S.; Qian, L.; et al. Super Stretchable, self-healing, adhesive ionic conductive hydrogels based on tailor-made ionic liquid for high-performance strain sensors. Adv. Funct. Mater. 2022, 32, 2204565.
73. Xia, H.; Xu, G.; Cao, X.; et al. Single-ion-conducting hydrogel electrolytes based on slide-ring pseudo-polyrotaxane for ultralong-cycling flexible zinc-ion batteries. Adv. Mater. 2023, 35, 2301996.
74. Yiming, B.; Han, Y.; Han, Z.; et al. A mechanically robust and versatile liquid-free ionic conductive elastomer. Adv. Mater. 2021, 33, 2006111.
75. Diao, W.; Wu, L.; Ma, X.; et al. Reversibly highly stretchable and self-healable zwitterion-containing polyelectrolyte hydrogel with high ionic conductivity for high-performance flexible and cold-resistant supercapacitor. J. Appl. Polym. Sci. 2020, 137, 48995.
76. Liu, J.; Wang, F.; Jiang, W.; et al. Polyzwitterionic hydrogel electrolytes via ultrafast autocatalytic gelation process for flexible Zn-ion hybrid supercapacitors. Chem. Eng. J. 2024, 483, 149360.
77. Ji, R.; Yan, S.; Zhu, Z.; et al. Ureido-ionic liquid mediated conductive hydrogel: superior integrated properties for advanced biosensing applications. Adv. Sci. 2024, 11, 2401869.
78. Zhang, Z.; Sang, M.; Pan, Y.; et al. Ionic liquid-reinforced transparent, stretchable, conductive organic ionic gel with ultra-high sensory capability and ultra-robust impact-resistance. Chem. Eng. J. 2024, 496, 154227.
79. Wan, Y.; Zhang, L.; Wu, T.; Tang, C.; Song, H.; Cao, Q. High-performance and frost-resistance MXene co-ionic liquid conductive hydrogel printed by electrohydrodynamic for flexible strain sensor. J. Colloid. Interface. Sci. 2024, 669, 688-98.
80. Zhao, W.; Zhou, H.; Li, W.; Chen, M.; Zhou, M.; Zhao, L. An environment-tolerant ion-conducting double-network composite hydrogel for high-performance flexible electronic devices. Nano. Micro. Lett. 2024, 16, 99.
81. Song, C.; Zhao, Q.; Xie, T.; et al. DLP 3D printing of electrically conductive hybrid hydrogels via polymerization-induced phase separation and subsequent in situ assembly of polypyrrole. J. Mater. Chem. A. 2024, 12, 5348-56.
82. Lodhi, S. K.; Gill, A. Y.; Hussain, I. 3D printing techniques: transforming manufacturing with precision and sustainability. ijmdsa 2024, 3, 129-38.
83. Browne, M. P.; Redondo, E.; Pumera, M. 3D printing for electrochemical energy applications. Chem. Rev. 2020, 120, 2783-810.
84. Li, M.; Zhou, S.; Cheng, L.; et al. 3D printed supercapacitor: techniques, materials, designs, and applications. Adv. Funct. Mater. 2023, 33, 2208034.
85. Baniasadi, H.; Abidnejad, R.; Fazeli, M.; et al. Innovations in hydrogel-based manufacturing: a comprehensive review of direct ink writing technique for biomedical applications. Adv. Colloid. Interface. Sci. 2024, 324, 103095.
86. Polychronopoulos, N. D.; Brouzgou, A. Direct ink writing for electrochemical device fabrication: a review of 3D-printed electrodes and ink rheology. Catalysts 2024, 14, 110.
87. Bhardwaj, D.; Singhmar, R.; Garg, M.; et al. Designing advanced hydrogel inks with direct ink writing based 3D printability for engineered biostructures. Eur. Polym. J. 2024, 205, 112736.
88. Jiang, J.; Lou, J.; Hu, G. Effect of support on printed properties in fused deposition modelling processes. Virt. Phys. Prototyp. 2019, 14, 308-15.
89. Cano-vicent, A.; Tambuwala, M. M.; Hassan, S. S.; et al. Fused deposition modelling: current status, methodology, applications and future prospects. Addit. Manuf. 2021, 47, 102378.
90. Wu, Y.; Zhang, Y.; Yan, M.; et al. Research progress on the application of inkjet printing technology combined with hydrogels. Appl. Mater. Today. 2024, 36, 102036.
91. Sun, Y.; Cui, J.; Feng, S.; et al. Projection stereolithography 3D printing high-conductive hydrogel for flexible passive wireless sensing. Adv. Mater. 2024, 36, 2400103.
92. Odent, J.; Wallin, T. J.; Pan, W.; Kruemplestaedter, K.; Shepherd, R. F.; Giannelis, E. P. Highly elastic, transparent, and conductive 3D-printed ionic composite hydrogels. Adv. Funct. Mater. 2017, 27, 1701807.
93. Han, Y.; Sun, M.; Lu, X.; et al. A 3D printable gelatin methacryloyl/chitosan hydrogel assembled with conductive PEDOT for neural tissue engineering. Compos. Part. B. Eng. 2024, 273, 111241.
94. Lee, Y. J.; Ajiteru, O.; Lee, J. S.; et al. Highly conductive, stretchable, and biocompatible graphene oxide biocomposite hydrogel for advanced tissue engineering. Biofabrication 2024, 16, 045032.
95. Lyu, Z.; Lim, G. J.; Koh, J. J.; et al. Design and manufacture of 3D-printed batteries. Joule 2021, 5, 89-114.
96. Yu, J.; Wan, R.; Tian, F.; et al. 3D printing of robust high-performance conducting polymer hydrogel-based electrical bioadhesive interface for soft bioelectronics. Small 2024, 20, 2308778.
97. Yan, X.; Tong, Y.; Wang, X.; Hou, F.; Liang, J. Extrusion-based 3D-printed supercapacitors: recent progress and challenges. Energy. Environ. Mater. 2022, 5, 800-22.
98. Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: a controlled release study. Int. J. Pharm. 2020, 584, 119428.
99. Gusain, R.; Kumar, N.; Ray, S. S. 3D-printed hydrogels and aerogels for water treatment and energy storage applications. ChemistrySelect 2023, 8, e202300738.
100. Xiao, B. H.; Xiao, K.; Li, J. X.; Xiao, C. F.; Cao, S.; Liu, Z. Q. Flexible electrochemical energy storage devices and related applications: recent progress and challenges. Chem. Sci. 2024, 15, 11229-66.
101. Mevada, C.; Tissari, J.; Parihar, V. S.; et al. A 3D-printed fully biocompatible supercapacitor. J. Mater. Chem. A. 2024, 12, 24357-69.
102. Meng, J.; Tan, Z.; Zong, W.; et al. 3D-printed ultrahigh-conductivity polymer gel electrodes with high mass loading for thickness-independent zinc-ion hybrid micro-supercapacitors. Adv. Funct. Mater. 2025, e10541.
103. Zhu, Y.; Zhang, Q.; Ma, J.; et al. Three-dimensional (3D)-printed MXene high-voltage aqueous micro-supercapacitors with ultrahigh areal energy density and low-temperature tolerance. Carbon. Energy. 2024, 6, e481.
104. Mogli, G.; Reina, M.; Chiappone, A.; et al. Self-powered integrated tactile sensing system based on ultrastretchable, self-healing and 3D printable ionic conductive hydrogel. Adv. Funct. Mater. 2024, 34, 2307133.
105. Liu, D.; Wang, Z.; Qian, Q.; et al. Customizable supercapacitors via 3D printed gel electrolyte. Adv. Funct. Mater. 2023, 33, 2214301.
106. Kil, H. J.; Kim, S. R.; Park, J. W. A self-charging supercapacitor for a patch-type glucose sensor. ACS. Appl. Mater. Interfaces. 2022, 14, 3838-48.
107. Pazhamalai, P.; Krishnamoorthy, K.; Manoharan, S.; Mariappan, V. K.; Kim, S. Monolithic integration of MoS2 quantum sheets on solid electrolyte for self-charging supercapacitor power cell governed by piezo-ionic effect. Sustain. Mater. Technol. 2022, 33, e00459.
108. Chae, C.; Kim, Y.; Lee, S. S.; et al. All-3D-printed solid-state microsupercapacitors. Energy. Storage. Mater. 2021, 40, 1-9.
109. Zhang, M.; Mei, H.; Chang, P.; et al. 3D printing of structured electrodes for rechargeable batteries. J. Mater. Chem. A. 2020, 8, 10670-94.
110. Qu, S.; Liu, B.; Wu, J.; et al. Kirigami-inspired flexible and stretchable zinc-air battery based on metal-coated sponge electrodes. ACS. Appl. Mater. Interfaces. 2020, 12, 54833-41.
111. Yu, Y.; Luo, Y.; Wu, H.; et al. Ultrastretchable carbon nanotube composite electrodes for flexible lithium-ion batteries. Nanoscale 2018, 10, 19972-8.
112. Shi, G.; Peng, X.; Zeng, J.; et al. A liquid metal microdroplets initialized hemicellulose composite for 3D printing anode host in Zn-ion battery. Adv. Mater. 2023, 35, 2300109.
113. Bao, Y.; Liu, Y.; Kuang, Y.; Fang, D.; Li, T. 3D-printed highly deformable electrodes for flexible lithium ion batteries. Energy. Storage. Mater. 2020, 33, 55-61.
114. Chen, Z.; Zhao, D.; Liu, B.; et al. 3D printing of multifunctional hydrogels. Adv. Funct. Mater. 2019, 29, 1900971.
115. Poompiew, N.; Jirawatanaporn, N.; Okhawilai, M.; et al. 3D-printed polyacrylamide-based hydrogel polymer electrolytes for flexible zinc-ion battery. Electrochim. Acta. 2023, 466, 143076.
116. Dubal, D. P.; Kim, J. G.; Kim, Y.; Holze, R.; Lokhande, C. D.; Kim, W. B. Supercapacitors based on flexible substrates: an overview. Energy. Technol. 2014, 2, 325-41.
117. Li, T.; Saadatnia, Z.; Chen, T.; Chen, J. X. M.; Shi, H. T. H.; Naguib, H. E. Facile material extrusion of 3D wearable conductive-polymer micro-super-capacitors. Addit. Manuf. 2023, 74, 103714.
118. Li, L.; Meng, J.; Bao, X.; et al. Direct-Ink-Write 3D printing of programmable micro-supercapacitors from MXene-regulating conducting polymer inks. Adv. Energy. Mater. 2023, 13, 2203683.
119. Yang, J.; Cao, Q.; Tang, X.; et al. 3D-printed highly stretchable conducting polymer electrodes for flexible supercapacitors. J. Mater. Chem. A. 2021, 9, 19649-58.
120. Sun, C.; Liu, S.; Shi, X.; Lai, C.; Liang, J.; Chen, Y. 3D printing nanocomposite gel-based thick electrode enabling both high areal capacity and rate performance for lithium-ion battery. Chem. Eng. J. 2020, 381, 122641.
121. Gaikwad, A. M.; Arias, A. C.; Steingart, D. A. Recent progress on printed flexible batteries: mechanical challenges, printing technologies, and future prospects. Energy. Tech. 2015, 3, 305-28.
122. Ma, J.; Zheng, S.; Fu, Y.; Wang, X.; Qin, J.; Wu, Z. S. The status and challenging perspectives of 3D-printed micro-batteries. Chem. Sci. 2024, 15, 5451-81.
123. Mao, L.; Meng, Q.; Ahmad, A.; Wei, Z. Mechanical analyses and structural design requirements for flexible energy storage devices. Adv. Energy. Mater. 2017, 7, 1700535.
124. Lu, Y.; Wang, Z.; Li, M.; et al. 3D printed flexible zinc ion micro-batteries with high areal capacity toward practical application. Adv. Funct. Mater. 2024, 34, 2310966.
125. Zhao, Y.; Guo, J. Development of flexible Li-ion batteries for flexible electronics. InfoMat 2020, 2, 866-78.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data





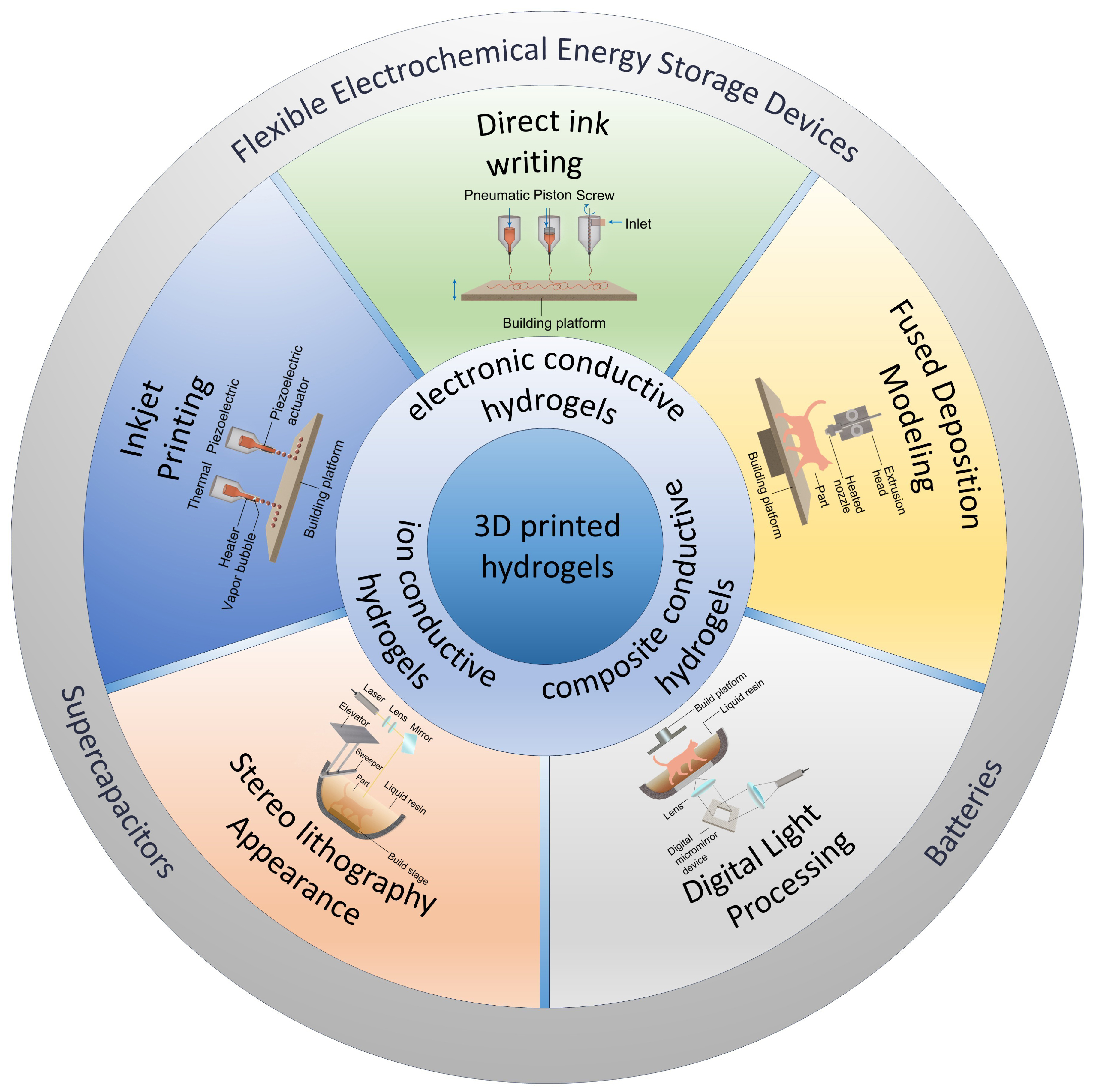
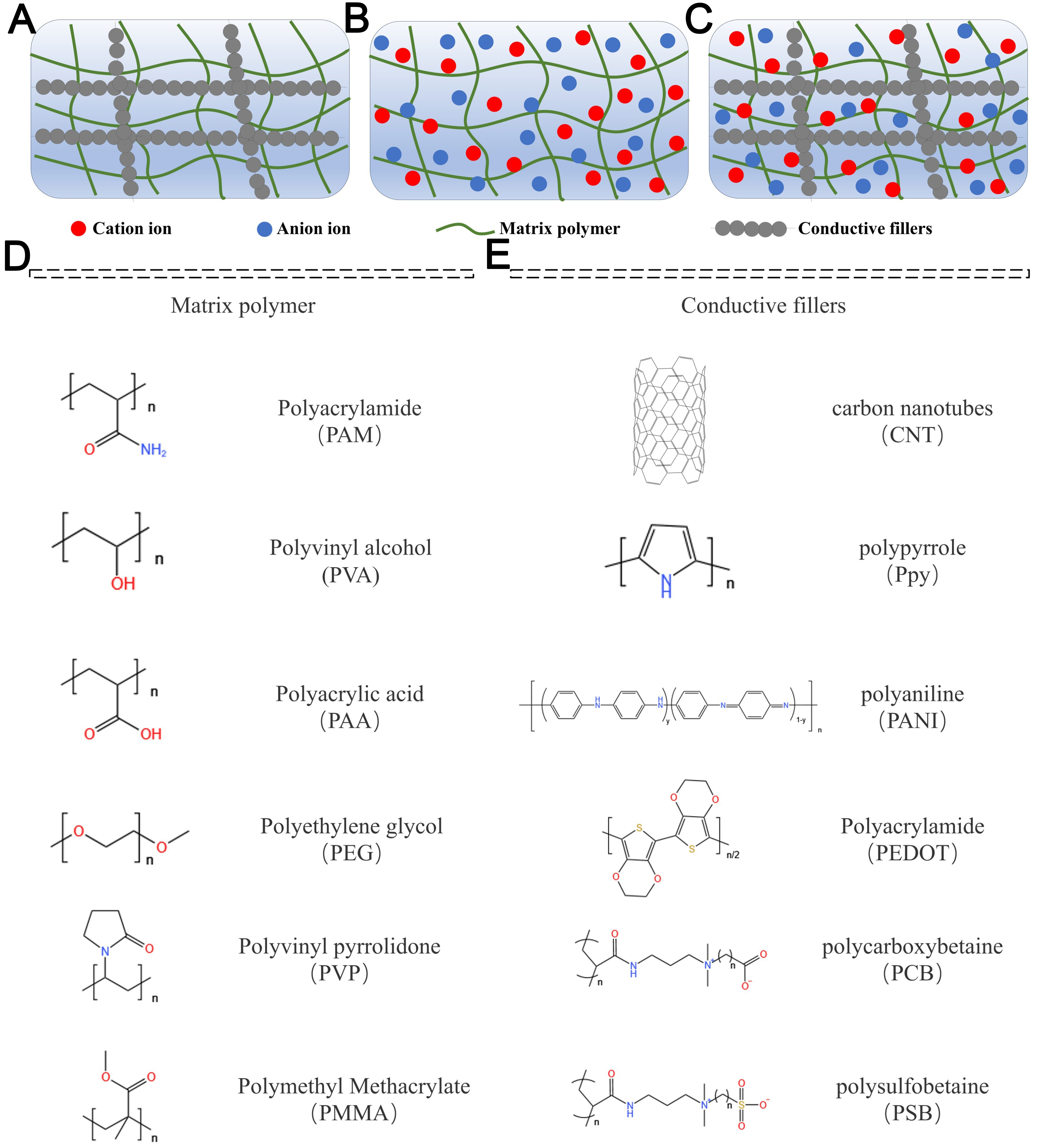
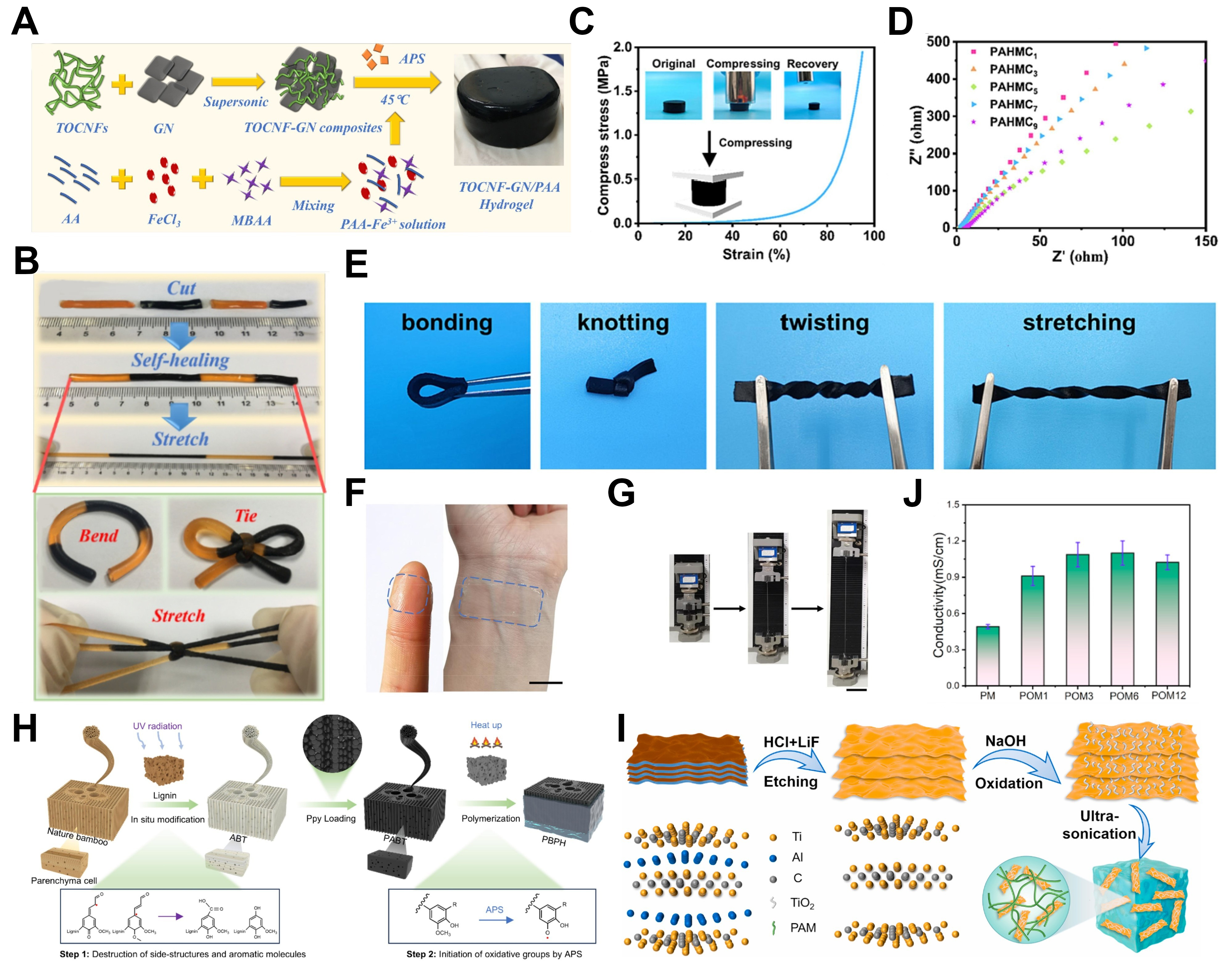
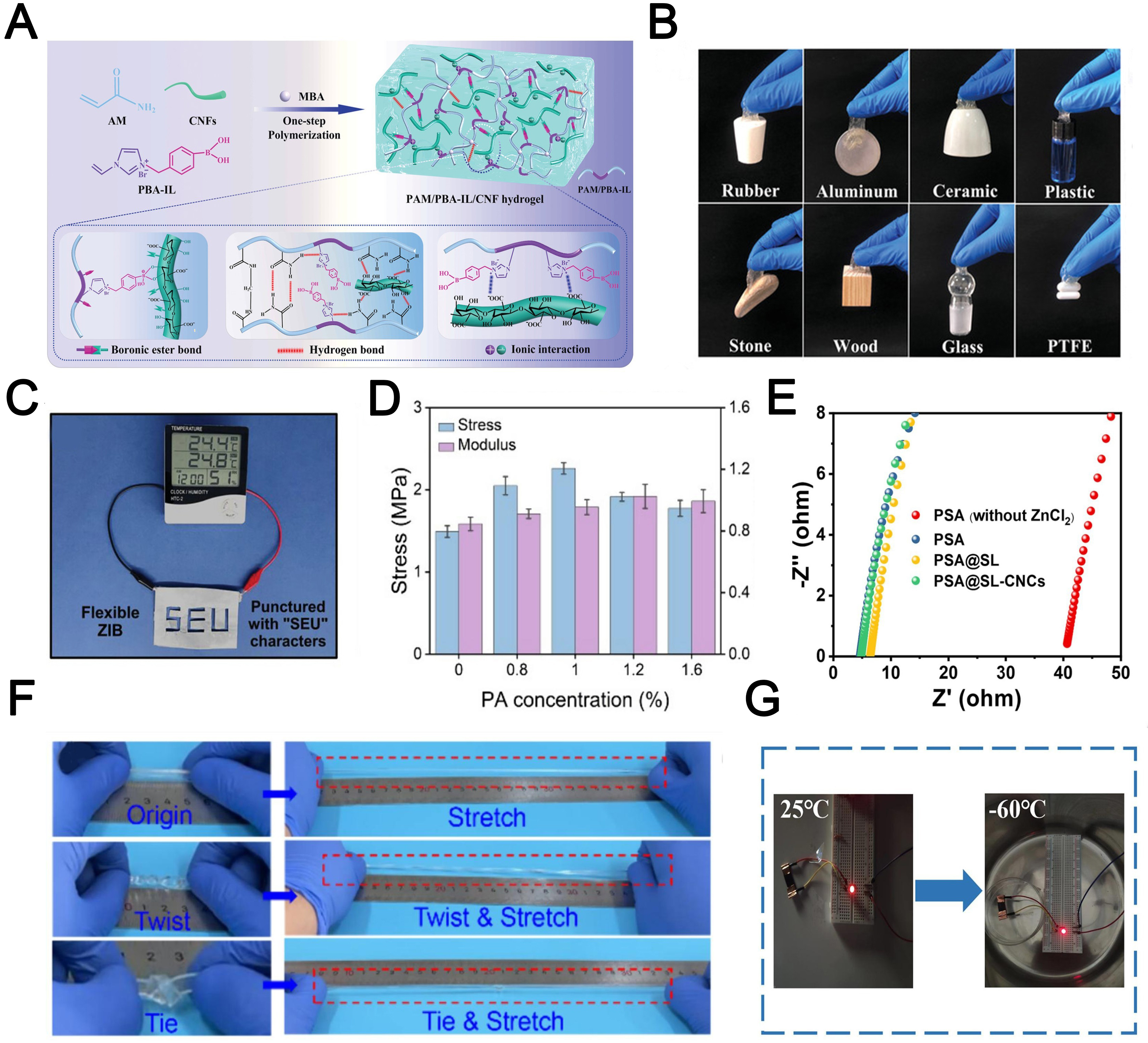
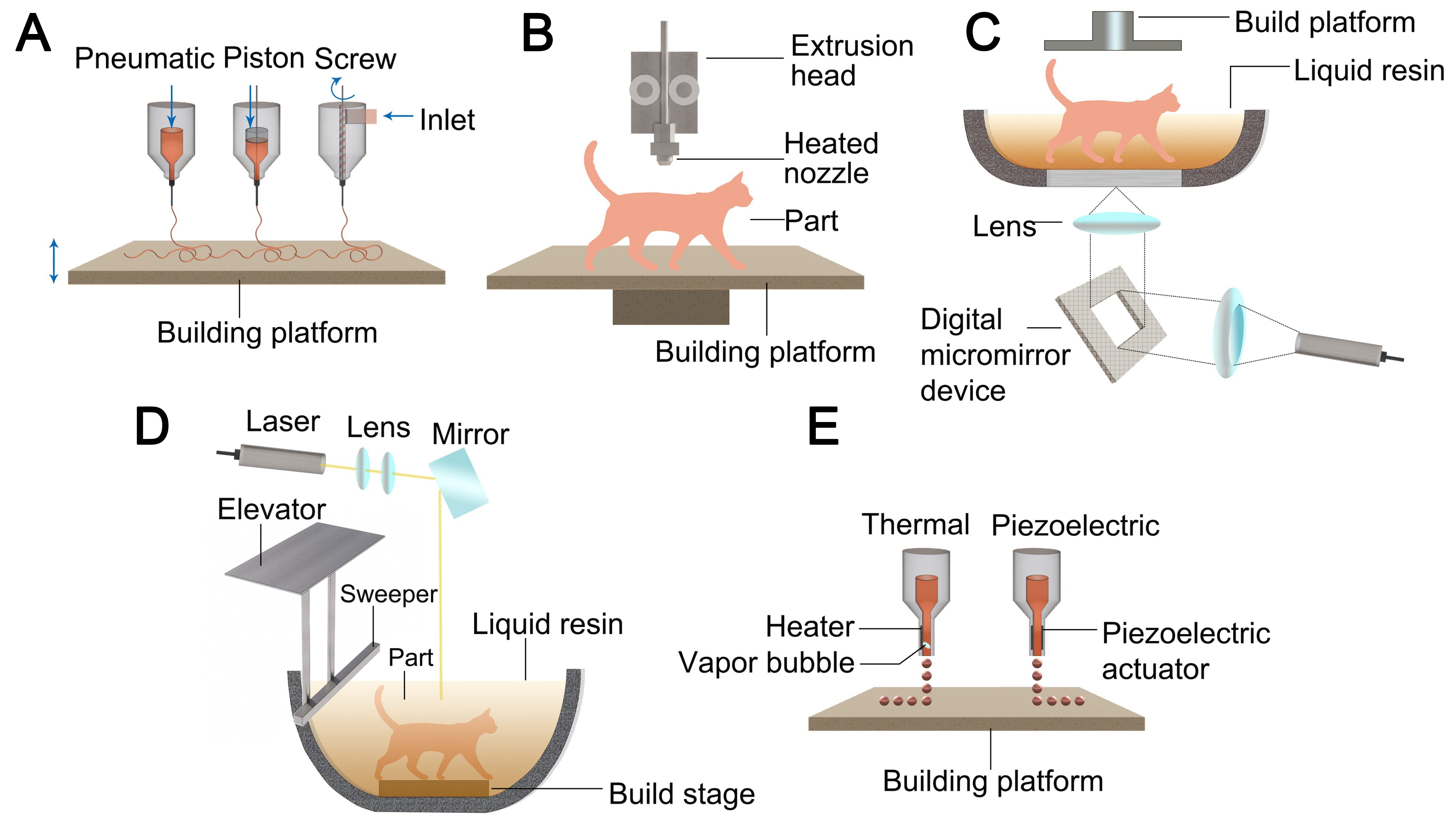
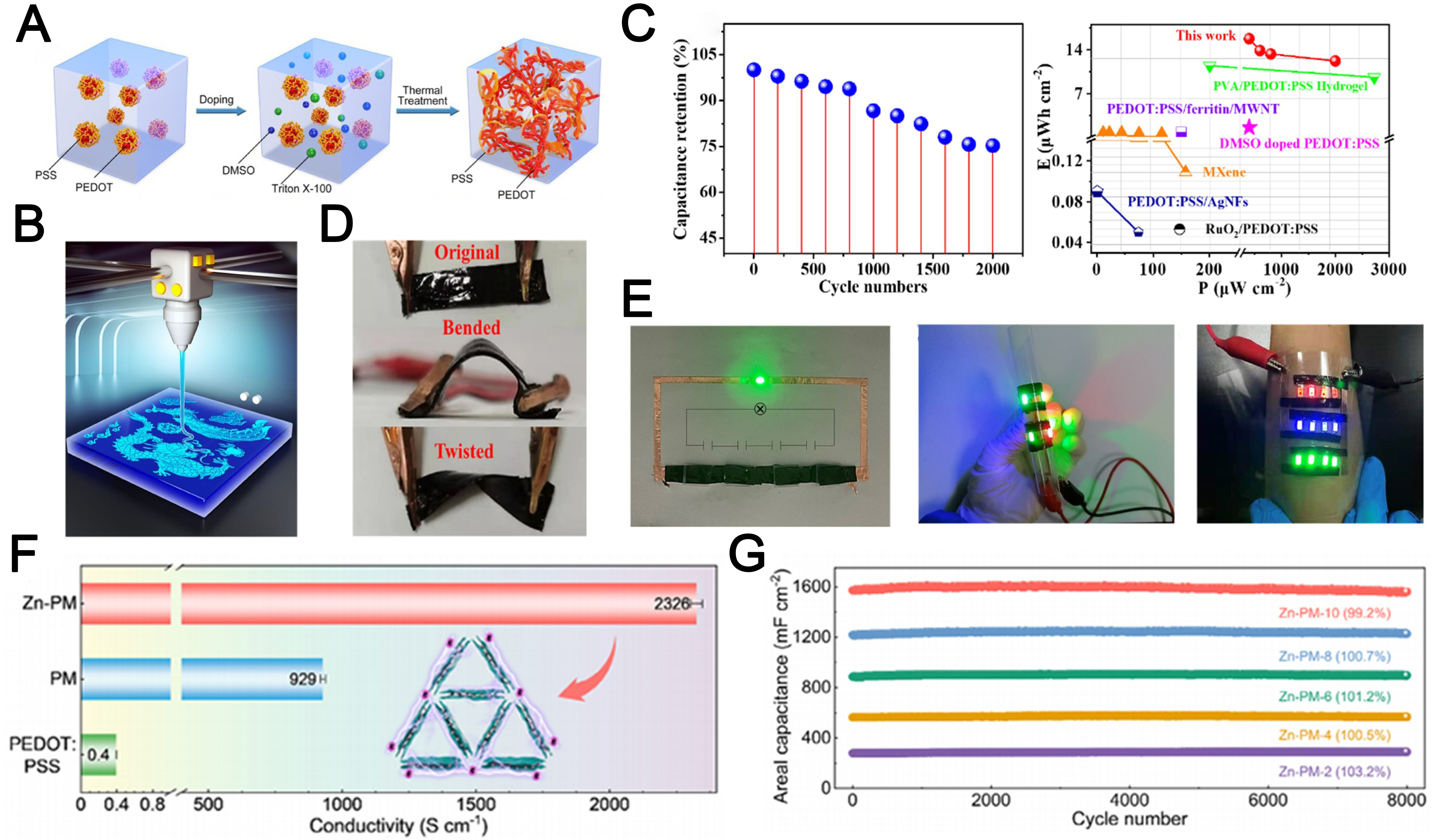
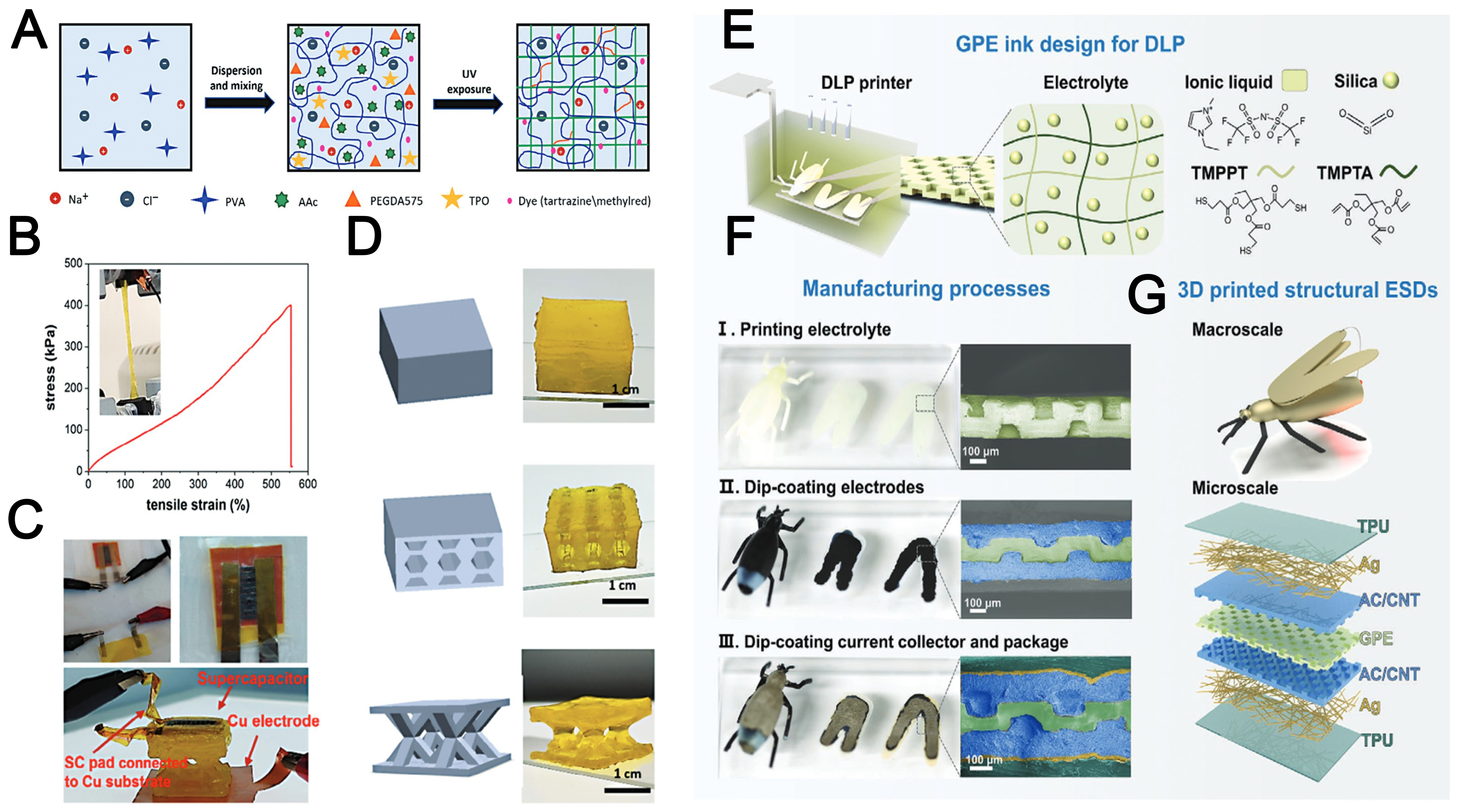
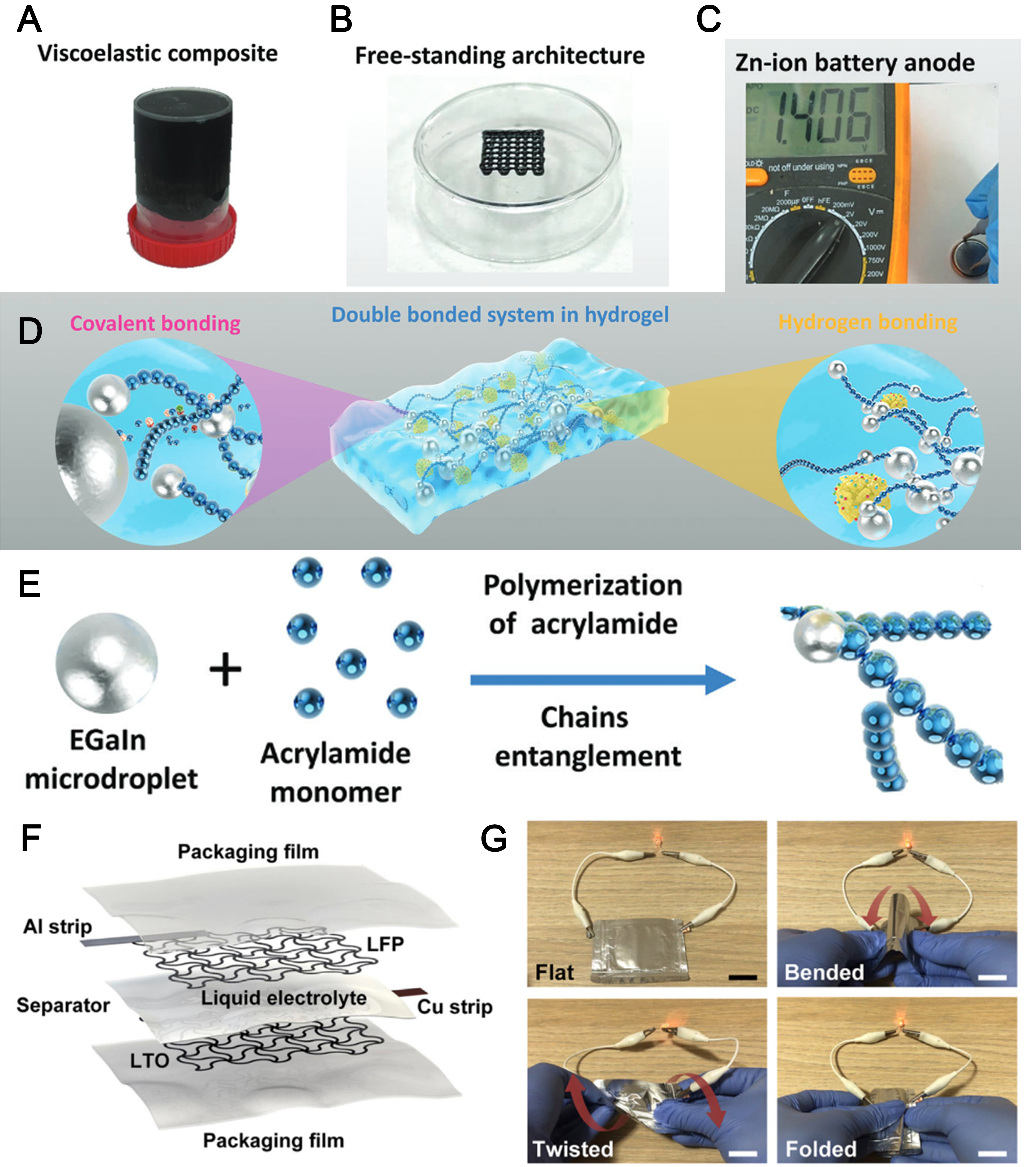
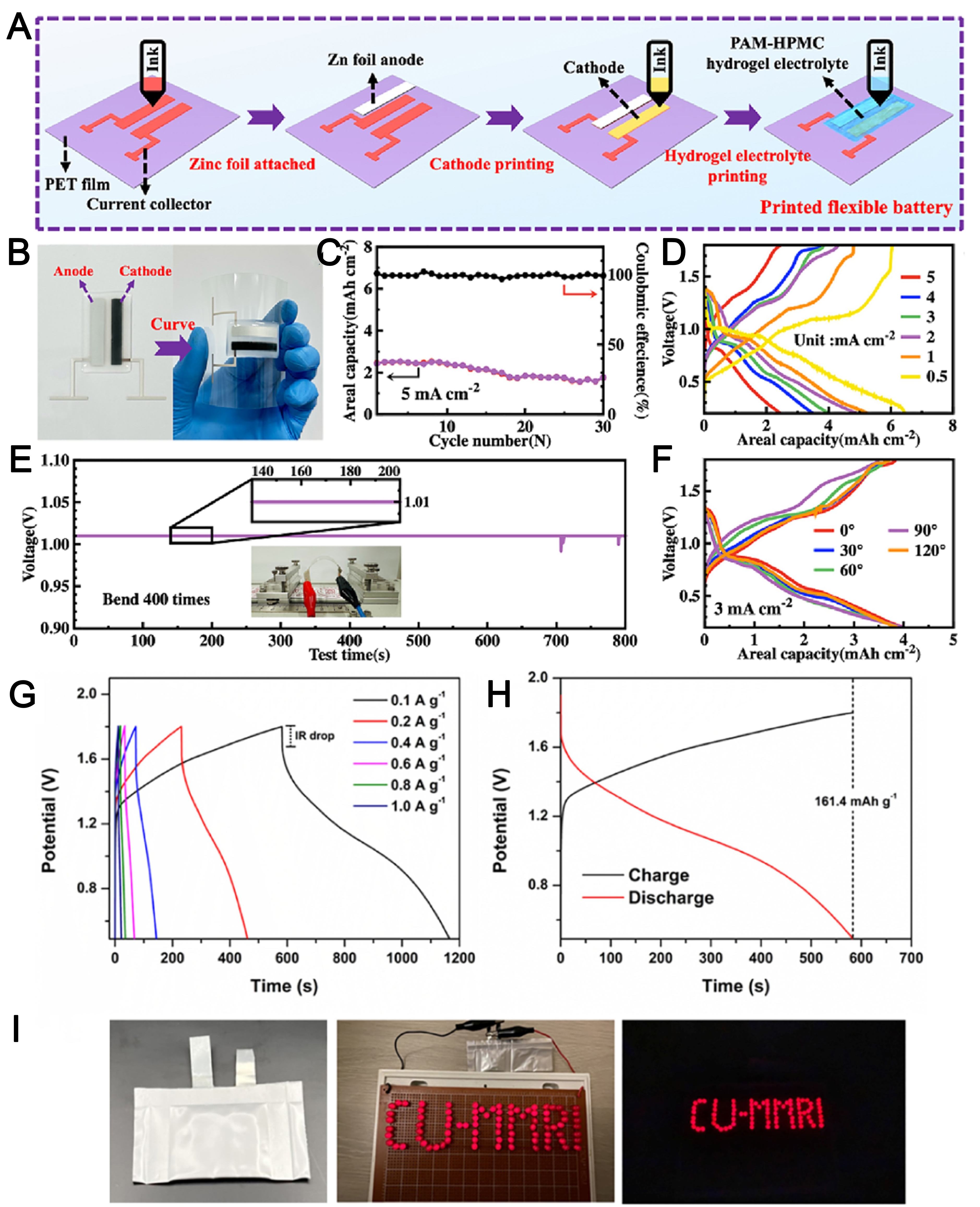












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].