fig17
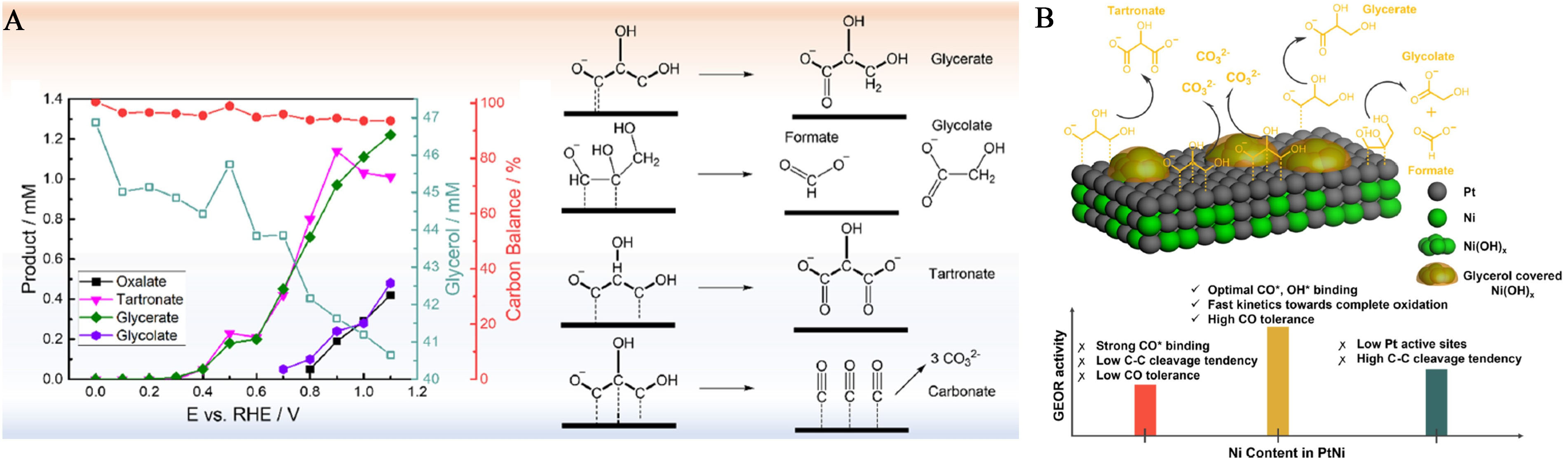
Figure 17. (A) Products formed at different potentials with the Pt/C catalyst detected by online sampling coupled with HPLC. Each potential was held for 10 min, and 200 μL of electrolyte containing glycerol and oxidation products were withdrawn at the end of chronoamperometry. Formate should also be produced during the reaction. However, its HPLC signal is embedded in the glycerol peak and thus cannot be quantified; (B) Illustration of the Electronic Effect in PtNi Electrocatalysts (Green: Ni, gray: Pt) Under Potential less than 1 V (vs. RHE)[184]. This figure is quoted with permission from Luo et al. HPLC: High-performance liquid chromatography; RHE: reversible hydrogen electrode.









