An overview of aqueous zinc-ion batteries based on conversion-type cathodes
Abstract
The scarcity of lithium resources and the unsafety of organic electrolytes limit the further application of lithium-ion batteries (LIBs) in electric vehicles and grid-scale energy storage. Aqueous zinc-ion batteries (AZIBs) are potential complements for LIBs for large-scale grid energy storage because of their abundant resources, environmental friendliness, intrinsic safety and low cost. However, current AZIBs are mainly based on intercalation-type cathodes and their energy densities are not competitive with LIBs. Fortunately, conversion-type cathodes, with higher specific capacity and lower price, endow AZIBs with excellent potential for practical applications. In this review, the mechanism of energy storage and the progress in developing AZIBs based on conversion-type cathodes are summarized. Perspectives on critical scientific issues and the potential developmental directions of AZIBs are also proposed.
Keywords
INTRODUCTION
Considering that the current energy and environmental crisis is becoming increasingly prominent, it is imperative to establish new low-carbon and eco-friendly energy systems. Due to the intermittent characteristics of renewable energy resources, such as solar, wind and tidal energy, advanced energy storage technology has become an essential element for establishing new energy systems in the future. Lithium-ion batteries (LIBs), as ubiquitous advanced secondary batteries, have been successfully applied to mobile communications, consumer electronics, electric vehicles and numerous other fields. Nevertheless, commercial LIBs generally employ organic electrolytes, such as ethers or esters. The flammability, pollution and expensiveness of organic electrolytes hinder the further application of LIBs and therefore require us to seek safer and cleaner low-cost alternatives.
Given the benefit of water-based electrolytes, aqueous batteries (ABs) have the merits of high safety and low cost and toxicity. Furthermore, their insensitivity to the environment allows them to be manufactured in air atmospheres, which can improve the production efficiency and reduce the manufacturing costs simultaneously. In addition, the high ionic conductivity and good interfacial compatibility of aqueous electrolytes endow ABs with fast reaction kinetics and outstanding rate capability[1]. Based on the aforementioned advantages, the development of high-performance ABs has become a global scientific research area for energy storage. In recent years, alkali metal (Li[2], Na[3] and K[4])-ion batteries and multivalent metal (Mg[5], Ca[6], Zn[7] and Al[8])-ion batteries have drawn increasing attention. In particular, aqueous zinc-ion batteries (AZIBs) have considerable practical potential because of their natural abundance, eco-friendliness, non-toxicity, high theoretical specific capacity (5855 Ah L-1 and 820 Ah kg-1) and moderate redox potential (-0.763 V vs. a standard hydrogen electrode) of zinc metal. Even more importantly, the high hydrogen evolution overpotential of metallic zinc can significantly reduce the rate of hydrogen evolution, which enables zinc metal to be used directly as an anode in ABs[9].
The most studied AZIBs usually employ zinc foil as an anode, ZnSO4, Zn(OTF)2 and other weakly acidic solutions as electrolytes and manganese-based, vanadium-based, Prussian blue analogs, organics and other materials as cathodes. These cathode materials generally complete the process of energy storage through the intercalation of zinc ions[10,11]. Despite many advantages, the energy densities of AZIBs are still far behind those of commercial LIBs, owing to the narrow electrochemical window of water-based electrolytes and the limited specific capacity of intercalation-type cathodes, thereby impeding the industrialization of AZIBs. Hence, there is an urgent need to develop AZIBs with higher energy densities.
In contrast to intercalation-type cathodes, conversion-type cathodes accomplish the process of energy storage via the “conversion reaction” of zinc ions, which tends to realize a higher specific capacity and energy density[12]. Furthermore, the conversion-type cathode materials commonly used in AZIBs, such as S, I2 and Br2, have significant competitiveness over their counterparts for intercalation-type cathodes in light of their more abundant resources and lower cost. In fact, conversion-type cathodes have been studied extensively in other battery systems, such as LIBs and sodium-ion batteries, and several reviews have presented the reaction mechanism and research progress of the conversion reaction in different batteries[13-18]. To date, there have been some studies and reports on AZIBs based on conversion reactions, but the systematic overview and in-depth discussion of these studies are still lacking. It is noteworthy that some traditional intercalation-type electrode materials also show a conversion reaction mechanism in AZIBs. For example, the conversion reactions of manganese oxide based on near-neutral[19], acid[20] and acid-alkaline dual electrolytes[21] have all been reported. In commonly used weak acid electrolytes, some researchers even confirmed that the reaction mechanism of MnO2 is based on the coupling effect of intercalation and conversion reactions[22,23]. The reaction mechanism of manganese oxide cathodes has always been complicated and still faces significant controversy and is therefore not discussed in this review.
Herein, we review and discuss the conversion reactions in AZIBs reported in recent years. Firstly, the reaction mechanism and electrode materials in AZIBs based on conversion-type cathodes are briefly introduced, and the key issues hampering the development of these batteries are proposed. Secondly, the optimization strategies and potential research directions of Zn-S, Zn-I2 and Zn-Br2 batteries are discussed in sequence, as shown in Figure 1. Finally, the development prospects of AZIBs based on conversion reactions are also highlighted.
REACTION MECHANISM AND KEY ISSUES
Conversion-type cathode materials mainly include halogen elements and their compounds, as well as chalcogen elements and their compounds. The conversion reactions of cathodes in AZIBs can be categorized into the following two types[17]:
First type:
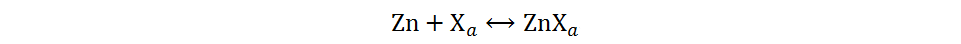
Second type:
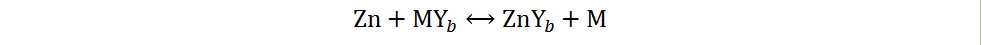
The first type is a chemical conversion reaction, where X is usually I, Br, O, S, Se or Te. The second type is a real conversion reaction, where M is typically a transition metal (e.g., Cu or Co) and Y may also be, e.g., O, S or I. The conversion-type cathode materials currently reported in AZIBs generally undergo the first type of reactions, especially the chemical conversion reactions where O2, S, I2 and Br2 are the cathodes. In consideration of the special mechanism and extensive research of Zn-O2 batteries, the discussion of this review focuses on Zn-S, Zn-I2 and Zn-Br2 batteries.
The redox reaction of sulfur has been extensively studied in Li-S and Na-S batteries, owing to the abundant resources, low cost and non-toxicity of elemental sulfur[13]. Different sulfur cathodes in Zn-S batteries have different reaction mechanisms in AZIBs. Due to the high affinity between sulfur and zinc metal, as well as the low solubility of zinc sulfide, the battery composed of a cathode of elemental sulfur, an anode of zinc metal and a single mild electrolyte mainly undergoes a two-electron solid-solid redox reaction[33]. During the discharge process, the anode of Zn metal loses electrons and oxidizes to Zn2+, while the cathode of elemental S obtains electrons and combines with Zn2+ to form ZnS. The reaction mechanism can be briefly generalized as follows:
Anode:
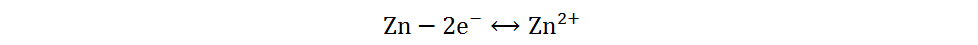
Cathode:
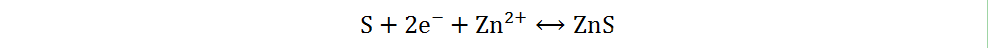
Overall:
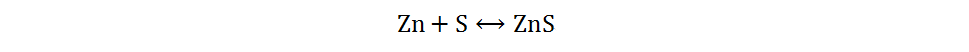
Because of its high abundance, low cost and multivalent characteristic, iodine is a cathode material for AZIBs with excellent application potential[34]. Current Zn-I2 batteries include redox flow and static batteries. Redox flow batteries have a unique structure, of which the electrolyte circulation system is independent of the electrode system. By adjusting the volume or concentration of electrolytes, a megawatt-hour (MWh) capacity can be achieved[35]. The electrolytes of Zn-I2 redox flow batteries are composed of Zn2+-rich anolytes and I−-rich catholytes, and the energy storage process is generally completed by I3−/I− redox reactions based on the low solubility of iodine in water[36]. However, due to the complex structures, high cost and low volumetric energy densities of redox flow batteries, static Zn-I2 batteries have received more attention and will be primarily discussed in the following section. Static Zn-I2 batteries mainly consist of a zinc metal anode, iodine-containing cathode and mild electrolyte, in which the conversion reaction of two electrons occurs based on the I2/I− redox couple. During the discharge process, the metallic Zn loses electrons and oxidizes to Zn2+, while the I2 at the cathode gains electrons and reduces to I−. Simultaneously, the polyiodide ions may be gradually formed resulting from the reaction of I2 and I−. This reaction process is summarized as follows[37]:
Anode:
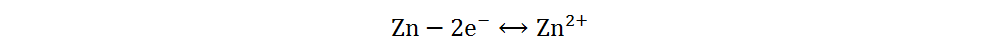
Cathode:
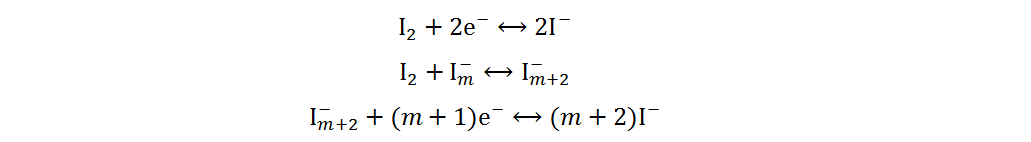
Overall:
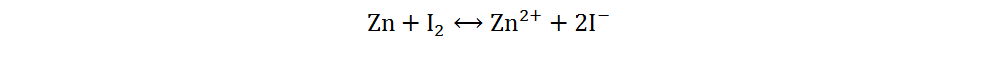
In addition to the traditional two-electron redox reaction, Zn-I2 batteries based on a four-electron redox reaction have been reported recently[38,39]. Specifically, I+ was successfully activated and stabilized by the electrolyte containing Cl−, which enables the batteries to experience two conversion reactions (I+/I2 and I2/I−). Moreover, there is no polyiodide ion detected in the discharge process, so the reaction process can be summarized as follows:
Anode:
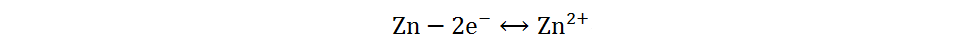
Cathode:
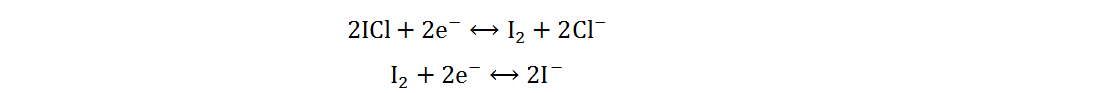
Overall:
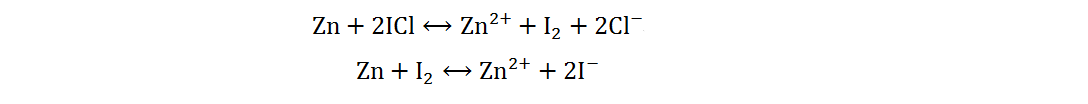
Current Zn-Br2 batteries are mainly concentrated in the field of redox flow batteries as a result of elemental bromine being liquid at room temperature. To overcome the disadvantages of redox flow batteries, such as complex structure and insufficient energy density, static Zn-Br2 batteries have gradually attracted the interest of researchers around the world[40]. Bromine and iodine are both halogen elements, so the reaction mechanism of static Zn-Br2 batteries is similar to that of Zn-I2 batteries and can be summarized by the following equations:
Anode:
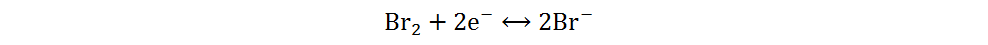
Cathode:
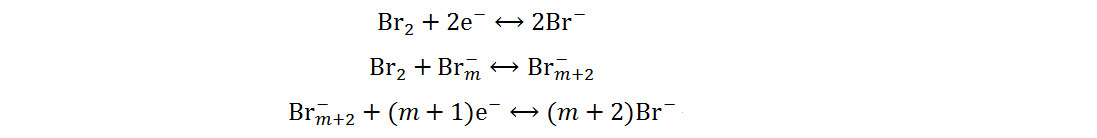
Overall:
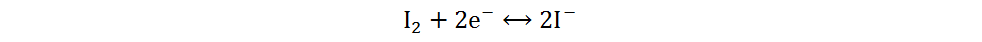
Zn-S, Zn-I and Zn-Br batteries have different theoretical capacities and discharge potentials due to the differences in atomic masses and electrochemical potentials of the three elements[18]. Among them, Zn-S batteries have the highest theoretical capacity of 1675 mAh g-1 and the lowest discharge plateau of ~0.5-0.7 V. Zn-Br2 batteries have a medium theoretical capacity of 335 mAh g-1 and a higher discharge plateau of ~1.7 V. The theoretical capacity and discharge plateau of Zn-I2 batteries are different with different redox couples. They have the lowest theoretical capacity of 211 mAh g-1 and a medium discharge plateau of ~1.1-1.4 V under the two-electron redox reaction but have a much higher theoretical capacity of 422 mAh g-1 and two discharge plateaus of ~1.7 and ~1.3 V under the four-electron redox reaction. The actual capacities and energy densities of these three battery types are notably different, owing to the distinction in electrode materials, electrolytes, current densities and calculation methods. The specific capacities of Zn-I2 and Zn-Br2 batteries are close to their theoretical capacities and even exceed the theoretical capacities derived from the contribution of matrix materials. The theoretical capacity of Zn-S batteries is very high, but the actual capacities have significant room for improvement compared with the theoretical capacity. Given the differences in the thermodynamic state of the three elements and the solubility of their compounds, the reaction mechanisms of Zn-S, Zn-I2 and Zn-Br2 batteries are solid-solid, solid-liquid and liquid-liquid reactions, respectively. As a consequence, there are significant contrasts in the kinetic performance, reaction reversibility and cycle stability of these batteries. Performance comparison of these battery types is presented in Table 1.
Performance comparison of AZIBs with conversion-type cathodes. The specific capacity is based on composites of active materials and hosts
| Cathode | Electrolyte | Plateau (V) | Specific capacity (mAh g-1) | Capacity retention (cycles) | Energy density (Wh kg-1) | Ref. |
| KB-S | 1 M ZnCl2 | 0.7 | 1236 (0.05 A g-1) | 33.6%/4 (0.05 A g-1) | 1083.3 (S based) | [41] |
| SeS5.76@3D-NPCF | 3 M ZnSO4 + 0.1 wt.% I2 | 0.71 | 611 (0.2 A g-1) | 75%/500 (4 A g-1) | 867.6 (SeS5.76 based) | [24] |
| SeS2@PCS | 1 M ZnSO4 + 0.1 wt.% I2 | 0.74 | 675 (0.1 A g-1) | 85%/1000 (5 A g-1) | 772 (SeS2 based) | [25] |
| S@C | gelatin/ZnSO4; gelatin/CuSO4 | 1.15 | 1471 (0.1 A g-1) | 78%/100 (0.5 A g-1) | 2372 (S based) | [42] |
| LF-PLSD | 1 M Zn(TFSI)2 | 0.7 | N/A | 54%/700 (1 A g-1) | 724.7 (cathode based) | [43] |
| S@CNTs | 1 M Zn(Ac)2 + 0.05 wt.% I2 | 0.5 | 496 (0.1 A g-1) | 85%/50 (1 A g-1) | 502 (S based) | [44] |
| S/KB | 0.5 M ZnSO4; 0.5 M CuSO4 | 1.15 | N/A | 77.5%/110 (1 A g-1) | 547 (Cu2S/KB based) | [26] |
| I2/ACF | 1 M ZnSO4 | 1.22 | 97.6 (0.5C) | 90%/3000 (2C) | N/A | [45] |
| I2/ACC | 1 M ZnSO4 | 1.2 | N/A | 90%/1500 (5C) | 151 (I and Zn based) | [46] |
| 3D-NSGF | 0.5 M ZnSO4; 0.1 M ZnI2 + 0.01 M I2 | 1.35 | N/A | 81%/500 (0.2 A g-1) | N/A | [47] |
| I2@C | 2 M Zn(CF3SO3)2 | 1.16 | N/A | 75%/9000 (5 A g-1) | 237.4 (I2 based) | [48] |
| CC | 0.5 M ZnSO4 + F77; 1 M KI + 0.1 M I2 + F77 | 1.2 | N/A | 94.3%/500 (1 C) | N/A | [49] |
| KB | 0.5 M ZnSO4 + 1 M LiI + 0.1 M I2 | 1.23 | N/A | 84.6%/6000 (1.92 A g-1) | N/A | [29] |
| I2-NPC | 1 M ZnSO4 | 1.37 | 180 (0.2 C) | 80.9%/10000 (10 C) | N/A | [50] |
| PANI-I2 | 2 M ZnSO4 | 1.2 | 71.3 (0.3 A g-1) | 79%/700 (1.5 A g-1) | N/A | [51] |
| I2-Nb2CTX | 1 M ZnSO4 | 1.3 | 164 (1 A g-1) | 80%/23000 (6 A g-1) | 259.3 (1 A g-1) | [52] |
| Co[Co1/4Fe3/4(CN)6]/I2 | 2 M ZnSO4 | 1.27 | N/A | 80.2%/2000 (4 A g-1) | 305.5 (0.1 A g-1) | [27] |
| CC | 0.5 M ZnSO4 + F77; MOG-I | 1.35 | N/A | 95.8%/1500 (1 C) | N/A | [53] |
| I2@AC | 1 M Zn(Ac)2 hydrogel | 1.25 | N/A | 66.8%/2000 (2 A g-1) | N/A | [28] |
| Ti3C2I2 MXene | 2 M ZnCl2 + 1 M KCl | 1.65+1.3 | 165.6 (0.5 A g-1) | 80%/2800 (3 A g-1) | 467 (Ti3C2I2 based) | [39] |
| PAC-I | 19 M ZnCl2 + 19 M LiCl + 8 M ACN | 1.7+1.18 | 136.6 (0.4 A g-1) | 82%/6000 (2 A g-1) | 750 (I based) | [38] |
| Br-Ti3C2TX | 1 M Zn(OTT)2 | 1.75 | 97 (0.5 A g-1) | 81%/1000 (2 A g-1) | 259 (Br based) | [31] |
| CMK-3 | 0.5 M ZnBr2 + 0.2 M TPABr | 1.70 | N/A | 63%/11000 (5 A g-1) | 142 (cathode based) | [32] |
| PCP | 2 M KOH + 0.02 M Zn(Ac)2; 1 M KBr + Br2+ 0.5 M H2SO4 | 2.1 | N/A | 93%/2000 (1 A g-1) | 780 (Br based) | [54] |
Although AZIBs based on conversion reactions show many advantages, they still face many challenges before their successful application. In order to promote the development and application of these batteries, the following key issues must be resolved[23,36,55,56]:
(1) Thermodynamic instability: Iodine and sulfur can be easily sublimed and bromine is liquid at room temperature, which not only limits the manufacturing methods of the electrodes but also results in difficulties for the accurate calculation of the mass loading of active materials.
(2) Low conductivity: The intrinsic low electrical conductivity of sulfur, iodine and bromine is adverse to the kinetics of the redox reaction. In particular, in Zn-S batteries, the electrode reaction shows large voltage hysteresis and poor reversibility.
(3) Low loading: Given the thermodynamic instability and low conductivity, sulfur, iodine and bromine often need to be made into composites with porous conductive carbonaceous materials or other matrix materials. The mass loading and utilization rate of active materials are important factors that restrict the increase in energy densities of batteries.
(4) Shuttle effect: Polysulfides, polyiodides, polybromides and elemental bromine dissolved in aqueous electrolytes tend to diffuse to the zinc anode and cause electrode corrosion, which reduces the Coulombic efficiency and aggravates the self-discharge behavior of batteries.
(5) Corrosion and dendrites: Hydrogen evolution corrosion, by-products and dendrite growth at the interface between aqueous electrolytes and the zinc anode contribute to the continuous consumption of active materials and electrolytes, which drastically shorten the cycle life of batteries.
OPTIMIZATION STRATEGIES
To address these issues, researchers have carried out extensive studies of cathode design, electrolyte optimization and separator engineering. Since one strategy often has an effect on multiple problems simultaneously and the primary issues of S, I2 and Br2 are distinct to some extent, and we elaborate the research advances for Zn-S, Zn-I2 and Zn-Br2 batteries, respectively.
Aqueous Zn-S batteries
Sulfur is a typical insulator with a conductivity of 5 × 10-28 S m-1. Conductive carbon materials are usually used as the hosts of sulfur to improve the kinetics and reversibility of the electrode reaction. Ketjenblack[26,41], activated carbon[26], carbon nanotubes[44], honeycomb carbon[42,57], phosphorus-doped carbon sheets[25] and nitrogen and phosphorus co-doped carbon foam[24] have all been reported to be used in the preparation of sulfur/carbon composite electrodes. Luo et al.[41]prepared a Ketjenblack/sulfur (KB-S) composite with a sulfur content of ~74.1% [Figure 2A] and assembled a Zn-S battery with the electrolytes of 1 M ZnCl2, ZnSO4, Zn(Ac)2 and Zn(OTF)2, respectively. It was found that the battery with 1 M ZnCl2 delivered the highest discharge plateau of ~0.7 V and a specific capacity of 1668 mAh g-1, leading to an outstanding energy density of 1083.3 Wh kg-1. Unfortunately, this battery had a voltage hysteresis of 0.8 V during cycling and the specific capacity decayed to 33.6% of the initial capacity after four cycles. Although increasing the electrolyte concentration to 30 M could improve the cycle performance of the battery, it also resulted in a lower specific capacity and greater voltage hysteresis.
Figure 2. A: SEM images, elemental mapping, N2 adsorption-desorption isotherms and pore size distribution of KB-S composite. B: Thermogravimetry (TG) curves, X-Ray Diffraction (XRD) patterns and N2 adsorption-desorption isotherms of S@CNTs. C: Optical photographs, electronic conductivities and XRD patterns of S-Se solid solutions. Figures reproduced with permission from Luo et al.[41], Li et al.[44] and Li et al.[24].
Li et al.[44] prepared sulfur/carbon nanotube composites (S@CNTs) with different sulfur contents [Figure 2B] by a melt infiltration method and studied the effect of sulfur content on the performance of a Zn-S battery. The results showed that as the sulfur content increased from 30 to 60 wt.%, the discharge specific capacity decreased from 1088 to 659 mAh g-1 and the voltage hysteresis increased from 0.72 to 0.82 V. In summary, the composite with 50 wt.% sulfur (S@CNTs-50) provides the best balance between the specific capacity of 1105 mAh g-1 and the discharge plateau of 0.5 V at 0.1 A g-1.
In addition to conductive carbon materials, selenium with a higher conductivity of 1 × 10-3 S m-1 can form an infinite solid solution with sulfur, thereby significantly improving the conductivity of electrodes. Li et al.[24] studied the electrical conductivity of three sulfur-selenium solid solutions (SeS14, SeS5.76 and SeS2.46) and demonstrated that with increasing selenium content, the electrical conductivity of the three solid solutions increased to 4 × 10-22, 3 × 10-9 and 2 × 10-8 S m-1, respectively [Figure 2C]. In order to further improve the conductivity of the electrodes, a 3D nitrogen-phosphorus co-doped carbon foam (3D-NPCF) was used as the substrate of the sulfur-selenium solid solutions. The authors analyzed the electron density, band structure and binding energy of different solid solutions through Density Functional Theory (DFT) calculations. It was found that Se doping can increase the electron cloud density and reduce the band gap of S atoms, thereby increasing the conductivity of the electrodes and reducing the overpotential of batteries. Furthermore, it can also reduce the breaking energy and binding energy of chemical bonds to enhance the electrode reaction kinetics.
To further enhance the redox kinetics of electrodes, iodine has been proposed as a redox mediator to increase the utilization of sulfur and reduce the overpotential of batteries. Li et al.[44] used S@CNTs-50 as a cathode material and studied the performance of batteries with different electrolytes [1 M Zn(OTF)2, 1 M ZnSO4, 1 M Zn(Ac)2 and 1 M Zn(Ac)2 + 0.05 wt.% I2]. The results showed that the battery assembled with the electrolyte of 1 M Zn(Ac)2 with 0.05 wt.% I2 exhibits the lowest voltage hysteresis of 0.72 V and the highest discharge specific capacity of 1105 mAh g-1 [Figure 3A], with the energy density reaching 502 Wh kg-1 based on S. In addition, the additive I2 can also stabilize the plating and stripping behavior of the zinc anode, so that the battery has a capacity retention of 85% after 50 cycles at 1 A g-1.
Figure 3. A: Charge and discharge curves of S@CNTs-50 cathode in different electrolytes. B: Charge and discharge curves of SeS2@PCS in different electrolytes and calculated Eads values of different ligands by DFT. C: Schematic diagram of the structure and the working mechanism of Zn-S batteries with a redox-ion charge-carrier electrode. D: Schematic diagram of the working mechanism of a cascade battery in the discharge process. Figures reproduced with permission from Li et al.[44], Li et al.[25], Wu et al.[26] and Dai et al.[42,57]. DFT: Density functional theory.
Li et al.[25] constructed a Zn-S battery with a sulfur-selenium solid solution (SeS2@PCS) electrode and a 1 M ZnSO4 electrolyte with 0.1 wt.% I2. The effect of I2 was explored by DFT calculations. During the discharge process, the coordination effect of SeS2 and the iodine species can enhance the hydrophilicity and reduce the reaction barrier, which enables SeS2 to capture zinc ions at a higher potential, thus improving the reaction kinetics and discharge potential of Zn-S batteries. During the charging process, ZnS and ZnSe also have coordination effects with iodine species, and I3− can oxidize S2− and Se2− spontaneously, thereby accelerating the oxidation process and reducing electrochemical polarization [Figure 3B]. Thus, the battery has a small voltage hysteresis of 0.41 V, a specific capacity of 1107 mAh g-1, an energy density of 772 Wh kg-1 based on SeS2 and a capacity retention of 85% after 1000 cycles at 5 A g-1.
The above-mentioned Zn-S batteries are all based on a two-electron redox reaction. Different from the typical two-electron reaction, Wu et al.[26] reported a Zn-S battery with a four-electron electrode reaction realized by using Cu2+ as the redox-ion charge carrier [Figure 3C]. The battery was assembled with a KB-S cathode composite, a zinc foil anode, a 0.5 M CuSO4 catholyte, a 0.5 M ZnSO4 anolyte and an anion exchange membrane separator. It was shown that the active material undergoes the reaction process of S↔CuS↔Cu2S in the discharge/charge process. Therefore, the battery has a discharge plateau of 1.15 V and a specific capacity of 2000 mAh g-1 at 50 mA g-1, resulting in an energy density of 547 Wh kg-1 based on the cathode and 255 Wh kg-1 based on the cathode and anode.
Dai et al.[42] constructed a flexible Zn-S battery according to this mechanism [Figure 3C]. Specifically, a flexible zinc anode was prepared by electroplating, and a flexible sulfur/carbon (S@C) cathode was prepared using honeycomb carbon as the host of sulfur. Gelatin/ZnSO4 and gelatin/CuSO4 were prepared for the anolyte and catholyte, respectively. The cathode of this flexible battery also undergoes the redox of S↔CuS↔Cu2S, and the battery exhibits a reversible specific capacity of 2016 mAh g-1 at 0.1 A g-1 and an outstanding energy density of 2372 Wh kg-1 based on S. Dai et al.[57] also reported another similar work, but interestingly, two flat discharge plateaus of 1.15 and 1.05 V appeared during the discharge process. In-situ XRD characterization confirmed that these two plateaus were attributed to the reaction mechanism of S↔CuS↔Cu2S of the Zn-S battery and Cu2+↔Cu/Cu2O of the Zn-Cu battery, respectively [Figure 3D]. As a result, the authors proposed the concept of a cascade battery. It is also found that Cu2S, the discharge product of the first step of the cascade battery, can act as a catalyst for the second step of charging and discharging, thus improving the reaction kinetics of the Zn-Cu battery. Benefitting from the sequentially coupling two-step discharge process, the battery delivers an excellent specific capacity of 48 mAh cm-2 and an energy density of 47.6 mWh cm-2.
In addition to the traditional elemental sulfur cathode, Zhao et al.[43] reported a polysulfide cathode (LF-PLSD) based on the stabilization of a liquid membrane and explored its electrochemical behavior and performance in dilute, highly concentrated and gel electrolytes. The results showed that this electrode with a 3D network structure has good interfacial compatibility with the electrolyte and excellent structural stability during cycling. The battery assembled with 1 M Zn(TFSI)2 has a specific capacity of 1146 mAh g-1 based on the cathode, and the energy density and power density are up to 724.7 Wh kg-1 and 432.5W kg-1, respectively.
Because of the difference in the reaction mechanism of sulfur in water-based and organic electrolytes, research into aqueous Zn-S batteries is still in its infancy[33]. Although Zn-S batteries have a theoretical specific capacity of 1675 mAh g-1, the actual capacity based on a single electrolyte is still far lower. Dual electrolyte systems based on redox-ion charge carriers can significantly increase the discharge capacity and energy density, but these batteries have a complicated structure design and require an expensive anion exchange membrane as the separator, which is not conducive to the industrialization of Zn-S batteries. In particular, limited by the low conductivity of sulfur and the solid-solid reaction mechanism of aqueous Zn-S batteries, the kinetics and reversibility of the electrode reaction of Zn-S batteries are generally poor, resulting in significant voltage hysteresis. As a consequence, the aqueous Zn-S batteries currently reported all exhibit poor voltage efficiency and cycle life. Achieving faster electrode reaction kinetics and better cycle stability through the reasonable design of electrodes and optimization of electrolytes are critical issues for the development of aqueous Zn-S batteries. More attempts should be made to develop suitable catalytic matrixes, explore viable heterostructure electrodes and find new electrolyte formulations.
Aqueous Zn-I2 batteries
Compared with sulfur, iodine has higher electrical conductivity (1 × 10-7 S m-1) and outstanding electrode reaction reversibility, so it is not only widely studied in redox flow batteries but has also drawn attention for static batteries. Although the solubility of iodine in water is negligible, the polyiodides derived from the combination of iodine and iodide can increase the solubility, leading to a shuttle effect similar to Li-S batteries, resulting in rapid capacity degradation and self-discharge behavior. With the aim of suppressing the dissolution and shuttle effect of iodine species, the solid-liquid electrode reaction of iodine is usually firmly confined in host materials. It has been reported that a variety of porous carbon materials can be used as the host of iodine in aqueous Zn-I2 batteries, including carbon fiber cloth, carbon fiber paper and active carbon.
Pan et al.[45] prepared an iodine/active carbon fiber cloth composite (I2/ACF) with different contents of iodine by the method of solution adsorption and studied the influence of different iodine mass and electrolyte volume on the performance of the battery [Figure 4A]. They showed that the smaller the ratio of electrolyte volume to iodine mass (E/I), the better the rate capability and cycle performance of the battery. This was ascribed to the lower E/I being beneficial to accelerating the balance of iodine between the adsorption on the porous carbon and the dissolution in the electrolyte, thereby reducing the loss of iodine in the cathode. DFT calculations further demonstrated that iodine species are preferentially adsorbed on the surface of carbon rather than dissolving in the electrolyte, which helps to suppress the shuttle effect of polyiodides and the self-discharge behavior of batteries.
Figure 4. A: Schematic illustration of the structure of a Zn-I2 battery with an I2/ACF cathode. B: Configurations of I2, ZnI2 and Zn(I3)2 by DFT calculations and schematic illustration of the reaction in the I2/CC cathode. C: Schematic diagram of NPC fabrication. D: Schematic diagram of I2-Nb2CTX fabrication. E: Schematic diagram of PBAs/I2 fabrication and reaction. F: Schematic illustration of PANI-I2 fabrication. G: Schematic illustration of the structure of the Zn-I2 battery with double-layered cathodes. Figures reproduced with permission from Pan et al.[45], Li et al.[58], Yu et al.[50], Ma et al.[27], Li et al.[ 52], Zeng et al.[51] and Lin et al.[59]. NPC: Nitrogen-doped porous carbon ; DFT: density functional theory.
Bai et al.[46] prepared an activated carbon cloth (ACC)/I2 composite cathode by infusing I2 into nanoporous ACC by heat treatment and investigated its reaction mechanism in a 1 M ZnSO4 electrolyte. Benefiting from the confinement effect of the porous structure of ACC on the iodine species, both Cyclic Voltammetry tests and Raman tests confirmed the direct conversion process of I2/I− without the formation of triiodide intermediates, thus greatly mitigating the self-discharge behavior and enhancing the cycle stability of the battery, which exhibited a specific capacity of 255 mAh g-1 at 0.5 C and a capacity retention of 90% after cycling 1500 cycles. Li et al.[58] reported a flexible Zn-I2 battery based on an iodine/microporous carbon cloth composite (I2/CC) and explored the effect of pore structure on the reaction mechanism of the battery via experiments and DFT calculations. The results showed that the microporous structure of the carbon cloth (pore size of ~0.63nm) could accommodate I2 and ZnI2 but not Zn(I3)2 (minimal diameter of ~0.95 nm) [Figure 4B]. Therefore, there were no polyiodides detected in the charging and discharging process of the battery. In contrast, a cathode material made of non-porous carbon KS6 and Super P could be detected the formation of polyiodides in the discharge process. Benefitting from the confinement effect of the microporous structure, a battery assembled with 2 M ZnSO4 exhibited a specific capacity of 355 mAh g-1 and an ultrahigh energy density of 400 Wh kg-1.
The confinement effect of non-polar carbon materials on iodine species is limited. In order to enhance the interaction between carbon materials and iodine species, heteroatom doping has been proven to be an effective method[47,50,60]. Yu et al.[50] prepared a nitrogen-doped porous carbon as the host of iodine by a simple pyrolysis method [Figure 4C] and demonstrated that heteroatom doping can enhance the adsorption capability of carbon for iodine species. In particular, the interaction between iodine and graphite N (N-Q) was the strongest compared with its counterparts of N-6, N-5 and N-O, which could significantly improve the rate capability and cycle stability. Therefore, a battery assembled with a 1 M ZnSO4 electrolyte delivered an operating voltage of 1.37 V, a specific capacity of 345.3 mAh g-1 and a capacity retention of 80.9% after 10000 cycles at 10 C. Lu et al.[47] prepared an N and S co-doped 3D graphene foam (3D-NSGF) and employed it as the scaffolds of both the cathode and anode of a Zn-I2 battery. Benefiting from the increased defects and surface polarity derived from the doping of N and S, the specific surface and mesopores of the 3D-NSGF with a honeycomb architecture showed a certain degree of improvement. Hence, this 3D-NSGF exhibited an excellent confinement effect on iodine and could improve the transfer efficiency of electrons and ions, boosting the electrochemical performance of the cathode and anode simultaneously.
In addition to porous carbon materials, other materials with continuous channels have also been reported as the substrate of iodine. Li et al.[52] fabricated I2/MXene composite electrode (I2-Nb2CTX) using electrochemical deposition to confine iodine in the layers of the MXene [Figure 4D]. Benefiting from the good conductivity of MXene and the adsorption to iodine species, the cathode could improve the redox kinetics and suppress the shuttle effect. The battery exhibited good rate capability and ultralong cycle stability with a power density of 23505 W kg-1, an energy density of 259.3 Wh kg-1 and a cyclic life of 23000 cycles. Ma et al.[27] also reported a composite electrode (PBAs/I2) based on a Prussian blue analogue Co[Co1/4Fe3/4(CN)6] [Figure 4E]. The ordered pore structure of the composite and the synergistic effect of Fe and Co were conducive to the confinement of iodine. Furthermore, the electrocatalysis of transition metals could also facilitate the transport of electrons and ions, improving the redox kinetics of the electrode. Therefore, the battery showed a specific capacity of 236.8 mAh g-1 and an energy density of 305 Wh kg-1 at 0.1 A g-1.
The materials reported above mainly absorb iodine through the physical interaction with iodine species. In addition to physical adsorption, chemical adsorption has also been reported to effectively confine iodine and suppress the shuttle effect of polyiodides. Polymer materials that have a good interaction with iodine usually show good adsorption to iodine species. In particular, polyvinylpyrrolidone (PVP) has been used as the substrate of iodine in Al-I2[61], Li-I2[62] and Mg-I2[63] batteries. However, the poor conductivity of PVP is not beneficial for the improvement of reaction kinetics, which hinders its further application. Zeng et al.[51] prepared an iodine-doped polyaniline cathode (PANI-I2) and verified that most of the iodine was chemically doped in the PANI chain by ex-situ Raman and XPS tests [Figure 4F]. During the cycle process, the polyiodides are confined to the polymer backbone through electrostatic interaction, which can effectively suppress the dissolution and shuttle effect of iodine species. Therefore, the battery assembled with 2 M ZnSO4 has a reversible capacity of 160 mAh g-1 and a capacity retention of 79% after 700 cycles at 1.5 A g-1. Lin et al.[59] constructed a double-layered cathode (CC-PPy-120) containing a conductive layer and an adsorption layer, a layer of polypyrrole (PPy) on the carbon cloth by in situ electropolymerization [Figure 4G]. While ensuring good electrical conductivity, this composite cathode could also reversibly absorb polyiodide ions through the electrostatic interaction between the PPy chain and the iodine species, thereby suppressing the shuttle effect and improving the Coulombic efficiency and cycle stability of the battery.
The optimization of the electrolyte also has a significant effect on alleviating the dissolution and shuttle effect of iodine species. The species[48] and concentration[64] of zinc salts, the pH of the electrolytes[65] and additives in the electrolytes[60,66] have all been reported to have a significant impact on the Coulombic efficiency, kinetic performance or cycle stability of aqueous Zn-I2 batteries. Li et al.[48] reported a Zn-I2 battery with 2 M Zn(CF3SO3)2 as the electrolyte. DFT calculations demonstrated that the absolute value of the binding energy of I2 and CF3SO32− is distinctly lower than that of SO42−, meaning that the interaction of I2 and CF3SO32− is more reversible, leading to a remarkable improvement in the utilization of iodine [Figure 5A]. In addition, the interaction of Zn(CF3SO3)2, ZnI2 and I2 can also form a dense SEI film on the surface of the Zn anode, which can protect the zinc anode and suppress the zinc dendrite growth. In consequence, this battery exhibited an excellent energy density of 237 Wh kg-1 and a capacity retention of 75% after cycling 9000 cycles at 5 A g-1. Wang et al.[60] designed a Zn-I2 battery with overcharge protection capability by adding 35 mg mL-1 of poly(2-vinylpyridine) (P2VP) to a 1 M ZnSO4 electrolyte. In the overcharged state, due to the increase in pH, P2VP undergoes a deprotonation effect accompanied by a rapid transition from a hydrophilic soluble state to a hydrophobic gel state [Figure 5B]. This contributes to a sharp increase in the solution resistance and the charge transfer resistance of the electrolyte, thereby reducing the overcharge capacity to 6% of the initial capacity to avoid the failure of the battery. When the pH of the electrolyte was recovered, P2VP could transform into a soluble state again, allowing the battery to resume normal operation.
Figure 5. A: Structures and binding energies of different complexes by DFT calculations and the initial charge and discharge curves in different electrolytes. B: Schematic illustration of self-protection function of a Zn-I2 battery and electrolyte resistances based on different concentrations of P2VP. C: Schematic diagram of Zn-I2 battery configuration with hydrogel electrolytes. D: Schematic illustration of the structure of alginate-based hydrogels and Zn-I2 battery with an alginate-based hydrogel electrolyte. E: Schematic diagram of Zn-I2 batteries with different separators and optical images of the resistance property against triiodide of different separators. Figures reproduced with permission from Li et al.[48], Wang et al.[60], Soni et al.[49], Shang et al.[28] and Yang et al.[29]. DFT: density functional theory.
Compared with liquid electrolytes, the reasonable design of gel electrolytes cannot only suppress the dissolution of iodine in cathodes but also mitigate the side reactions and dendrite growth on anodes. Sonigara et al.[49] used an amphiphilic block copolymer (PEOn-PPOm-PEOn) with 0.5 M ZnSO4 and 1 M KI + 0.1 M I2 to prepare a gel electrolyte and a gel catholyte, respectively [Figure 5C]. The unique core-shell structure of this electrolyte can confine anions to the PPO region but allows zinc ions to rapidly migrate by the interaction with PEO, which can not only suppress the shuttle effect of iodide species but also endow the electrolyte with excellent ionic conductivity (> 1 mS cm-1), thereby enabling the specific capacity of the battery to reach 228 mAh g-1 at 0.5 C. Based on this work, this team also reported an iodine-enriched porous metal-organic gel catholyte (MOG-I)[53]. The unique microporous structure of the MOG catholyte and the strong polarity of the central metal can effectively inhibit the diffusion of iodine species, which enables the battery to exhibit a specific capacity of 231.6 mAh g-1 at 0.5 C and a capacity retention of 98.5% after cycling for 1500 cycles at 1 C. Shang et al.[28] designed a hydrogel electrolyte based on sodium alginate [Figure 5D], which can suppress the shuttle effect of triiodide while rapidly transporting zinc ions, thereby alleviating the corrosion and dendrite growth of the zinc anode. A quasi-solid Zn-I2 battery assembled with this hydrogel delivered a specific capacity of 188 mAh g-1 at 0.2 A g-1 and a capacity retention of 66.8% after cycling 2000 cycles at 2 A g-1.
Another effective method for suppressing the dissolution and shuttle effect of iodine is through separator modification. Tangthuam et al.[67] synthesized a series of polyelectrolyte membranes using different ratios of carboxymethyl cellulose (CMC) and polyvinyl alcohol (PVA) and used them as the separators of Zn-I2 batteries. The results showed that with increasing CMC content, the polyelectrolyte membrane has a more compact morphology, a lower ion conductivity and a stronger blocking effect on polyiodides. However, unfortunately, all polyelectrolyte membranes can not completely suppress the shuttle effect of polyiodides. Yang et al.[29] prepared a multifunctional metal-organic framework membrane, which not only suppressed the migration of triiodide to the anode but also changed the solvation structure of zinc ions to depress the water activity [Figure 5E]. Based on this synergistic effect, the side reactions and dendrite growth of the anode could be significantly suppressed. This Zn-I2 battery delivered a specific capacity of 203 mAh g-1 and a capacity retention of 86.4% after cycling 6000 cycles at 1.92 A g-1.
Most current aqueous Zn-I2 batteries are based on the two-electron redox reaction of I2/I−, with a discharge plateau of ~1.3 V and a theoretical capacity of 211 mAh g-1. Iodine is a typical polyvalent element and the batteries based on it exhibit a much higher discharge voltage and specific capacity if the redox reaction of iodine based on high valence can be activated. Li et al.[39] prepared a halogenated MXene cathode (Ti3C2I2) via heat treatment and constructed a Zn-I2 battery with an electrolyte containing F− or Cl−. Under the interaction between I+ and F− or Cl− verified by in-situ Raman characterization, the I+/I2 redox is successfully activated and stabilized. In the optimized electrolyte of 2 M ZnCl2 with 1 M KCl, the battery exhibited two discharge plateaus of 1.65 and 1.30 V [Figure 6A], as well as good reaction reversibility and cycle stability, with a cycle life of 2800 cycles at 3 A g-1 and an energy density of 467 Wh kg-1 based on I and 280 Wh kg-1 based on Ti3C2I2. Zou et al.[38] employed an electrolyte of extremely concentrated ZnCl2 and LiCl with a diluent of acetonitrile (ACN) to successfully realize a voltage plateau at ~1.71 V of I+/I2 redox [Figure 6B]. When the molar ratio of the three materials was 19:19:8, the battery showed a specific capacity of 594 mAh g-1, an energy density of 750 Wh kg-1 and a capacity retention of 82% after cycling 6000 cycles at 2 A g-1.
Figure 6. A: CV and Galvanostatic charge-discharge (GCD) curves of Zn||Ti3C2I2 battery with different electrolytes. B: Charge and discharge curves of Zn-I2 batteries with different electrolytes and typical voltage-capacity curve of 19-19-8 system and the corresponding in-situ Raman spectrum. Figures reproduced with permission from Li et al.[39] and Zou et al.[38]. CV: Cyclic voltammetry.
In view of the good reaction reversibility of active materials and the capacity contribution of host materials, the actual discharge capacities are generally close to the theoretical capacity or even exceeds the theoretical capacity of aqueous Zn-I2 batteries, which has a long cycle life and considerable energy density. However, the energy densities of Zn-I2 batteries are usually calculated based on the active materials. The rational design of the cathode structure to increase the mass loading and utilization rate of iodine to accomplish better overall energy density plays a pivotal role in the practical application of Zn-I2 batteries. Another key factor restricting the improvement of the energy density of Zn-I2 batteries is their inferior discharge plateau of ~1.3 V. Although it has been reported that the average discharge voltage of the batteries can be increased by activating the I+/I2 redox, the employment of high-concentration electrolytes significantly raises the manufacturing cost of the batteries. Therefore, it is a more feasible method for developing new cathode substates or optimizing electrolytes to introduce a new redox reaction in a low-concentration electrolyte with the aim of achieving a higher operating voltage and energy density.
Aqueous Zn-Br2 batteries
The liquid-liquid reaction mechanism of bromine endows it with faster reaction kinetics[55] and therefore attracts extensive research on redox flow batteries. However, for static batteries, liquid Br2 makes the structure design of batteries very challenging. Biswas et al.[68] employed the sedimentation effect by gravity of bromine to design a minimal architecture Zn-Br2 battery without a separator. The battery was constructed using 2 M ZnBr2 as the electrolyte, porous carbon foam on the bottom of the battery and carbon cloth on the top of the battery as the cathode and anode current collector, respectively [Figure 7A]. During the charging process, due to the density of Br2 being larger than that of the electrolyte, Br− is oxidized to Br2 and then gradually precipitates into the interior and on the surface of the porous carbon foam, while Zn2+ is reduced to Zn on the surface of the carbon cloth. If zinc dendrites are formed and contact the cathode, the Br2 on the surface of the porous carbon foam reacts with them and converts them into Zn2+ to be dissolved in the electrolyte, thereby preventing the battery from short-circuiting. Lee et al.[30] also reported a similar work. In order to enhance the ability of cathode to adsorb bromine, they assembled the battery employing nitrogen-doped microporous carbon on graphite felt as the cathode current collector [Figure 7B]. It was shown that nitrogen-doped microporous carbon exhibited excellent adsorption to bromides and polybromides, therefore significantly inhibiting the cross-diffusion and shuttle effect of bromine species. Therefore, the battery delivered excellent cycle stability with a VE value of 84% after 1000 cycles.
Figure 7. A: Schematic illustration and optical photographs of the minimal architecture Zn-Br2 battery without a separator. B: Schematic diagram of the structure and mechanism of the membraneless and flowless Zn-Br2 battery with an NGF electrode. C: Schematic diagram of fabrication of Br-Ti3C2TX and crystal structure of Br-Ti3C2TX and adsorption energy of Br species on Ti3C2TX and the charge-of-density patterns of Br species on Ti3C2TX. D: Schematic diagram of the structure of the Zn-Br2 battery with TPABr additive. E: Schematic illustration and electrochemical stability window of the Zn-Br2 battery with the alkaline-acid hybrid electrolytes. Figures reproduced with permission from Biswas et al.[68], Lee et al.[30], Li et al.[31], Gao et al.[32] and Yu et al.[54].
Although there is a higher requirement for the substrates of liquid bromine, the substrates currently reported are still porous carbon materials, such as porous carbon foam[68], nitrogen-doped microporous carbon[30], ordered mesoporous carbon CMK-3[32], porous carbon powder[54], VC/carbon textile[69] and so on. These carbon materials have an unsatisfactory confinement effect on bromine species. In addition to porous carbon materials, Li et al.[31] prepared a Br-containing MXene cathode (Br-Ti3C2TX) by electrodeposition [Figure 7C] and analyzed the interaction between bromine species and MXene by DFT calculations. The results showed that there was a good affinity and electron transfer ability between bromine species and MXene, which not only restricts the movement of bromine species, inhibiting the shuttle effect, but also improves the reaction kinetics, leading to good rate capability and cycle stability. The battery assembled with the electrolyte of 1 M Zn(OTF)2 exhibits a discharge plateau of 1.75 V, a specific capacity of 179.6 mAh g-1, an energy density of 259 Wh kg-1 based on Br and a capacity retention of 81% after 2000 cycles at 2 A g-1.
As well as the confinement of bromine through the structure design of cathodes, electrolyte optimization is another alternative method for confining bromine species. Gao et al.[32] added a surfactant of 0.2 M TPABr to the electrolyte of 0.5 M ZnBr2 to stabilize the bromine species and assembled a static Zn-Br2 battery with CMK-3 as the cathode [Figure 7D]. Benefitting from the strong complexing ability of TPA+ to bromine species, soluble tribromide can be converted into insoluble TPABr3, thereby greatly suppressing the shuttle effect of polybromide and the self-discharge behavior of the battery. In addition, TPA+ can impede the dendrite growth by adsorbing on the surface of the zinc anode during the charging process, thus improving the Coulombic efficiency and cycle life of the battery. Under the synergistic effect of the chemical adsorption of TPA+ additives and the physical adsorption of the CMK-3 cathode, the Zn-Br2 battery has a discharge voltage of 1.7 V and a specific capacity of 487 mAh g-1 at 0.1 A g-1. It can cycle stably for 11000 cycles with a capacity retention of 63% at 5 A g-1.
The high discharge voltage and theoretical capacity of aqueous Zn-Br2 batteries are conducive to increasing the energy density of batteries. However, the liquid bromine has severely impeded the research progress of Zn-Br2 static batteries. Due to the liquid-liquid reaction mechanism of Zn-Br2 batteries, many hosts that have a good confinement effect on iodine have limited effect on bromine. As a result, there is an urgent need to develop more effective matrix materials to achieve a good restraint effect on bromine species. The cathode materials currently reported are almost all based on the physical interaction between the host materials and bromine species. It may be an effective method to develop new hosts based on chemical interactions, such as polymer materials. In addition, reasonable electrolyte additives can also confine bromine species from another perspective, so multifunctional additives should also get more attention. The separator design can simultaneously mitigate the dissolution of cathodes and the corrosion and dendrite growth of anodes, but this has not been reported in Zn-Br2 batteries. This is also a development direction worthy of future study.
SUMMARY AND OUTLOOK
In conclusion, AZIBs, with the merits of low cost, high safety and environmental friendliness, are promising candidates for the next generation of large-scale grid energy storage[10]. Conversion-type cathode materials, of which the resources are abundant and the prices are low, not only with the structural advantage of storing multivalent ions, but also with a unique reaction mechanism to achieve high energy density, are ideal cathode materials for AZIBs[12]. This review introduced the electrode reaction mechanism and the latest research progress of conversion-type cathode materials in AZIBs and summarized the critical issues that affect the performance and application of AZIBs. Although the AZIBs based on the conversion reaction are still in their infancy, Li-S batteries have been developed adequately due to their excellent theoretical capacity (1675 mAh g-1) and energy density (2600 Wh kg-1 or 2800 Wh L-1). The critical challenges of Li-S batteries include low conductivity, structural instability, shuttle effect and sluggish redox kinetics, which result in poor rate capability and cycle stability[70]. In order to address these problems, tremendous efforts have been exerted, which involve cathode design, separator modification, electrolyte optimization and anode protection, and the corresponding electrode design principles have been summarized elaborately[71,72]. The valuable experience of Li-S batteries is worthy of learning, but it should be noted that the reaction mechanism of sulfur in organic electrolyte and aqueous electrolyte is different, so appropriate choices need to be made based on practical problems of electrodes when refering to Li-S batteries[33].
The conversion-type cathodes of AZIBs are usually composites composed of active and host materials. The energy density and cycle stability of batteries are affected by the species and content of active materials, the category and structure of substrates and the manufacturing process of the composites. In the face of different application scenarios, active materials must be selected appropriately in consideration of the discharge capacity, output voltage, thermal stability, electrical conductivity, density and other properties. In order to balance the effects of each factor, it is logical to combine two active materials by doping or other methods. The substrates have diverse functions, such as increasing electrical conductivity, confining active materials, alleviating volume expansion, acting as a current collector, and their structures should be designed properly according to the major problems of active materials. The polarity, conductivity, porosity distribution and spatial configuration of substrates are key factors that affect the content of active material and the efficiency of electrode reaction, and the modification of substrates can be made by integration, doping and other ways to achieve better performance. To date, the loading methods of active materials of conversion-type cathodes in AZIBs mainly involve solution adsorption, heat treatment and electrodeposition. In practical applications, the optimum loading process should be selected based on the characteristic of active materials and the substrates. In some cases, a cathode without active material can also be prepared by adding active materials into the electrolyte, such as non-iodine[59] and non-bromine electrodes[32].
Aqueous electrolytes are pivotal to AZIBs, and both mass and charge transfer need to be completed with the aid of electrolytes. The electrolytes reported in AZIBs based on conversion reactions mainly fall into three categories: low-concentration liquid electrolytes; high-concentration liquid electrolytes; hydrogel electrolytes. Among them, the high ionic conductivity of low-concentration liquid electrolytes leads to excellent reaction kinetics and rate capability of batteries, but abundant water can exacerbate the dissolution of active materials in cathode and the hydrogen evolution corrosion on anode. Due to its unique solvation structure, high-concentration liquid electrolytes can reduce the activity of water to mitigate the side reactions at both the cathode and anode, but the viscosity of electrolyte will increase and the ionic conductivity will decrease. The hydrogel electrolyte consists of zinc salt solutions and polymer matrixes. Not only does it have high ionic conductivity, but it also restricts the movement of water molecules and optimizes the migration of zinc ions, thereby suppressing the dendrite growth of anodes. Different electrolytes will endow batteries with different Coulombic efficiencies and cycle performance. We should choose the appropriate electrolyte according to the characteristics of electrode materials to achieve the optimal performance of batteries. We now propose some potential research directions and expect they will be helpful for researchers in this field:
(1) Investigation of new cathode materials. At present, most of the conversion-type cathode materials reported in AZIBs are elemental materials based on chemical conversion reactions such as I2, Br2, S, Se or Te. The compound materials based on true conversion reactions are rarely reported[73,74]. Hao et al.[75] studied the working mechanism of CuI, Cu2S and Cu2O in AZIBs, and the results showed that they all undergo direct conversion reactions. Multivalent transition metal compounds (Cu2+, Co2+ or Ni2+), which are expected to achieve higher specific capacity and output voltage, need more effort.
(2) Adjustment to the mass loading of active materials. In order to address the problems of thermodynamic instability, high solubility and low conductivity of active materials, it is usually necessary to firmly load active materials in matrix materials. The specific capacities and energy densities reported in the literature are mainly calculated based on the mass of active materials. However, the improvement of the overall energy densities of batteries is affected greatly by the mass loading of active materials. Generally, raising the content of active materials has a great contribution to the increase of the energy density of batteries. However, it is noteworthy that the utilization rate of active materials loaded in matrixes by the method of simple physical absorption tends to decline as the mass loading increases[34]. Therefore, the structure of the substrates should be rationally designed to achieve the optimal content of active materials.
(3) Development of multifunctional separators. The separator plays a vital role in the AZIBs based on the conversion reaction. The reasonable design of separators not only inhibits the dissolution and shuttling of active materials but also suppresses the corrosion and dendrite growth of anodes. Nevertheless, expensive glass fiber separators are still the general choice for AZIBs. The development of stable and inexpensive multifunctional separators, in spite of a promising and challenging task, requires more endeavor.
(4) Focusing attention on zinc anodes. The hydrogen evolution corrosion, by-products and dendrite growth of the zinc anode are inherent problems of AZIBs. These problems lead to continuous consumption of electrolytes and passivation of electrodes, resulting in low Coulombic efficiency, rapid capacity decay and short cycle life of AZIBs. The reasonable regulation of electrolytes is promising to address these problems, as well as the thermodynamic instability of cathodes, which should be paid more attention.
(5) Exploration of flexible zinc-ion batteries. AZIBs are considered as competitive candidates for flexible batteries due to their high safety, low toxicity and simple manufacture. Hydrogel electrolytes have been widely used to prepare flexible zinc-ion batteries because their outstanding ionic conductivity stemmed from aqueous electrolytes and mechanical property derived from polymer electrolytes[76]. In AZIBs based on conversion reaction, the hydrogel electrolyte can also suppress the active material dissolution of cathodes and the dendrite growth of anodes simultaneously through rational structural design, thereby significantly improving the Coulombic efficiency and cycle stability of batteries. However, as a matter of fact, there are only a couple of reports of flexible zinc-ion batteries based on conversion reactions[42,49,53,57], and therefore more work needs to be executed to provide deeper insights.
DECLARATIONS
AcknowledgmentsThis work was financially supported by Shaanxi Yanchang Petroleum CO., Ltd (18529), Yiwu Research Institute of Fudan University (21557), the National Science Foundation of China (22075048), and the Shanghai International Collaboration Research Project (19520713900).
Authors’ contributionsConceptualization, data curation, writing: Kang J
Conceptualization, data curation, editing: Zhao Z
Data curation, revise: Li H
Discussion, revise: Meng Y, Hu B
Editing, review, funding acquisition, supervision: Lu H
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by Shaanxi Yanchang Petroleum CO., Ltd (18529), Yiwu Research Institute of Fudan University (21557), the National Science Foundation of China (22075048), and the Shanghai International Collaboration Research Project (19520713900).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright©The Author(s) 2022.
REFERENCES
1. Chao D, Zhou W, Xie F, et al. Roadmap for advanced aqueous batteries: from design of materials to applications. Sci Adv 2020;6:eaba4098.
2. Jaumaux P, Yang X, Zhang B, et al. Localized Water-in-salt electrolyte for aqueous lithium-ion batteries. Angew Chem Int Ed Engl 2021;60:19965-73.
3. Suo L, Borodin O, Wang Y, et al. “Water-in-Salt” Electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting. Adv Energy Mater 2017;7:1701189.
4. Liu T, Liu K, Wang J, et al. Achievement of a polymer-free KAc gel electrolyte for advanced aqueous K-ion battery. Energy Storage Materials 2021;41:133-40.
5. Tang Y, Li X, Lv H, et al. High-energy aqueous magnesium hybrid full batteries enabled by carrier-hosting potential compensation. Angew Chem Int Ed Engl 2021;60:5443-52.
6. Tang X, Zhou D, Zhang B, et al. A universal strategy towards high-energy aqueous multivalent-ion batteries. Nat Commun 2021;12:2857.
7. Zhao Z, Wang R, Peng C, et al. Horizontally arranged zinc platelet electrodeposits modulated by fluorinated covalent organic framework film for high-rate and durable aqueous zinc ion batteries. Nat Commun 2021;12:6606.
8. Dong T, Ng KL, Wang Y, Voznyy O, Azimi G. Solid electrolyte interphase engineering for aqueous aluminum metal batteries: a critical evaluation. Adv Energy Mater 2021;11:2100077.
9. Yuan L, Hao J, Kao C, et al. Regulation methods for the Zn/electrolyte interphase and the effectiveness evaluation in aqueous Zn-ion batteries. Energy Environ Sci 2021;14:5669-89.
10. Song J, Xu K, Liu N, Reed D, Li X. Crossroads in the renaissance of rechargeable aqueous zinc batteries. Materials Today 2021;45:191-212.
11. Fang G, Zhou J, Pan A, Liang S. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett 2018;3:2480-501.
12. Wang H, Chen S, Fu C, et al. Recent advances in conversion-type electrode materials for post lithium-ion batteries. ACS Materials Lett 2021;3:956-77.
13. Wang Y, Li X, Wang W, et al. Chalcogen cathode and its conversion electrochemistry in rechargeable Li/Na batteries. Sci China Chem 2020;63:1402-15.
14. Zhang Z, Dong S, Cui Z, et al. Rechargeable magnesium batteries using conversion-type cathodes: a perspective and minireview. Small Methods 2018;2:1800020.
15. Kim J, Kim H, Kang K. Conversion-based cathode materials for rechargeable sodium batteries. Adv Energy Mater 2018;8:1702646.
16. Xin S, Chang Z, Zhang X, Guo Y. Progress of rechargeable lithium metal batteries based on conversion reactions. National Science Review 2017;4:54-70.
17. Wu F, Yushin G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ Sci 2017;10:435-59.
18. Kraytsberg A, Ein-eli Y. A critical review-promises and barriers of conversion electrodes for Li-ion batteries. J Solid State Electrochem 2017;21:1907-23.
19. Liang G, Mo F, Li H, et al. A universal principle to design reversible aqueous batteries based on deposition-dissolution mechanism. Adv Energy Mater 2019;9:1901838.
20. Chao D, Zhou W, Ye C, et al. An electrolytic Zn-MnO2 battery for high-voltage and scalable energy storage. Angew Chem Int Ed Engl 2019;58:7823-8.
21. Zhong C, Liu B, Ding J, et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc-manganese dioxide batteries. Nat Energy 2020;5:440-9.
22. Yang H, Zhou W, Chen D, et al. The origin of capacity fluctuation and rescue of dead Mn-based Zn-ion batteries: a Mn-based competitive capacity evolution protocol. Energy Environ Sci 2022;15:1106-18.
23. Zhong Z, Li J, Li L, et al. Improving performance of zinc-manganese battery via efficient deposition/dissolution chemistry. Energy Storage Materials 2022;46:165-74.
24. Li W, Ma Y, Li P, et al. Synergistic effect between s and se enhancing the electrochemical behavior of Se. Adv Funct Mater ;31:2101237.
25. Li W, Jing X, Ma Y, et al. Phosphorus-doped carbon sheets decorated with SeS2 as a cathode for aqueous Zn-SeS2 battery. Chemical Engineering Journal 2021;420:129920.
26. Wu X, Markir A, Ma L, et al. A four-electron sulfur electrode hosting a Cu2+ /Cu+ redox charge carrier. Angew Chem Int Ed Engl 2019;58:12640-5.
27. Ma L, Ying Y, Chen S, et al. Electrocatalytic iodine reduction reaction enabled by aqueous Zinc-Iodine battery with improved power and energy densities. Angew Chem Int Ed Engl 2021;60:3791-8.
28. Shang W, Zhu J, Liu Y, et al. Establishing high-performance quasi-solid Zn/I2 batteries with alginate-based hydrogel electrolytes. ACS Appl Mater Interfaces 2021;13:24756-64.
29. Yang H, Qiao Y, Chang Z, Deng H, He P, Zhou H. A metal-organic framework as a multifunctional ionic sieve membrane for long-life aqueous zinc-iodide batteries. Adv Mater 2020;32:e2004240.
30. Lee JH, Byun Y, Jeong GH, et al. High-energy efficiency membraneless flowless Zn-Br battery: utilizing the electrochemical-chemical growth of polybromides. Adv Mater 2019;31:e1904524.
31. Li X, Li N, Huang Z, et al. Confining aqueous Zn-Br halide redox chemistry by Ti3C2TX MXene. ACS Nano 2021;15:1718-26.
32. Gao L, Li Z, Zou Y, et al. A high-performance aqueous Zinc-Bromine static battery. iScience 2020;23:101348.
33. Liu J, Zhou W, Zhao R, et al. Sulfur-based aqueous batteries: electrochemistry and strategies. J Am Chem Soc 2021;143:15475-89.
34. Xing M, Zhao Z, Zhang Y, Zhao J, Cui G, Dai J. Advances and issues in developing metal-iodine batteries. Materials Today Energy 2020;18:100534.
35. Pei Z, Zhu Z, Sun D, et al. Review of the I-/I3- redox chemistry in Zn-iodine redox flow batteries. Materials Research Bulletin 2021;141:111347.
36. Ma J, Liu M, He Y, Zhang J. Iodine Redox Chemistry in Rechargeable Batteries. Angew Chem Int Ed Engl 2021;60:12636-47.
37. Li P, Li C, Guo X, Li X, Zhi C. Metal-Iodine and Metal-Bromine batteries: a review. BCSJ 2021;94:2036-42.
38. Zou Y, Liu T, Du Q, et al. A four-electron Zn-I2 aqueous battery enabled by reversible I-/I2/I+ conversion. Nat Commun 2021;12:170.
39. Li X, Li M, Huang Z, et al. Activating the I 0 /I + redox couple in an aqueous I 2 -Zn battery to achieve a high voltage plateau. Energy Environ Sci 2021;14:407-13.
40. Dai C, Hu L, Jin X, Zhao Y, Qu L. The emerging of aqueous Zinc-Based dual electrolytic batteries. Small 2021;17:e2008043.
41. Luo LW, Zhang C, Wu X, et al. A Zn-S aqueous primary battery with high energy and flat discharge plateau. Chem Commun (Camb) 2021;57:9918-21.
42. Dai C, Jin X, Ma H, et al. Maximizing energy storage of flexible aqueous batteries through decoupling charge carriers. Adv Energy Mater 2021;11:2003982.
43. Zhao Y, Wang D, Li X, et al. Initiating a reversible aqueous Zn/Sulfur battery through a “liquid film”. Adv Mater 2020;32:e2003070.
44. Li W, Wang K, Jiang K. A low cost aqueous Zn-S battery realizing ultrahigh energy density. Adv Sci (Weinh) 2020;7:2000761.
45. Pan H, Li B, Mei D, et al. Controlling solid-liquid conversion reactions for a highly reversible aqueous Zin-Iodine battery. ACS Energy Lett 2017;2:2674-80.
46. Bai C, Cai F, Wang L, Guo S, Liu X, Yuan Z. A sustainable aqueous Zn-I2 battery. Nano Res 2018;11:3548-54.
47. Lu K, Zhang H, Song B, et al. Sulfur and nitrogen enriched graphene foam scaffolds for aqueous rechargeable zinc-iodine battery. Electrochimica Acta 2019;296:755-61.
48. Li W, Wang K, Jiang K. A high energy efficiency and long life aqueous Zn-I 2 battery. J Mater Chem A 2020;8:3785-94.
49. Sonigara KK, Zhao J, Machhi HK, Cui G, Soni SS. Self-assembled solid-state gel catholyte combating iodide diffusion and self-discharge for a stable flexible aqueous Zn-I 2 battery. Adv Energy Mater 2020;10:2001997.
50. Yu D, Kumar A, Nguyen TA, Nazir MT, Yasin G. High-voltage and ultrastable aqueous Zinc-Iodine battery enabled by N-Doped carbon materials: revealing the contributions of nitrogen configurations. ACS Sustainable Chem Eng 2020;8:13769-76.
51. Zeng X, Meng X, Jiang W, et al. Anchoring polyiodide to conductive polymers as cathode for high-performance aqueous Zinc-Iodine batteries. ACS Sustainable Chem Eng 2020;8:14280-5.
52. Li X, Li N, Huang Z, et al. Enhanced redox kinetics and duration of aqueous I2 /I- conversion chemistry by MXene confinement. Adv Mater 2021;33:e2006897.
53. Machhi HK, Sonigara KK, Bariya SN, Soni HP, Soni SS. Hierarchically porous metal-organic gel hosting catholyte for limiting iodine diffusion and self-discharge control in sustainable Aqueous Zinc-I2 Batteries. ACS Appl Mater Interfaces 2021;13:21426-35.
54. Yu F, Pang L, Wang X, et al. Aqueous alkaline-acid hybrid electrolyte for zinc-bromine battery with 3V voltage window. Energy Storage Materials 2019;19:56-61.
55. Chen S, Zhang J. Redox reactions of halogens for reversible electrochemical energy storage. Dalton Trans 2020;49:9929-34.
56. Yang Y, Liang S, Zhou J. Progress and prospect of the zinc-iodine battery. Current Opinion in Electrochemistry 2021;30:100761.
57. Dai C, Hu L, Jin X, et al. A cascade battery: coupling two sequential electrochemical reactions in a single battery. Adv Mater 2021;33:e2105480.
58. Li Y, Liu L, Li H, Cheng F, Chen J. Rechargeable aqueous zinc-iodine batteries: pore confining mechanism and flexible device application. Chem Commun (Camb) 2018;54:6792-5.
59. Lin D, Rao D, Chiovoloni S, et al. Prototypical study of double-layered cathodes for aqueous rechargeable static Zn-I2 batteries. Nano Lett 2021;21:4129-35.
60. Wang F, Tseng J, Liu Z, et al. A stimulus-responsive Zinc-Iodine battery with smart overcharge self-protection function. Adv Mater 2020;32:e2000287.
61. Tian H, Zhang S, Meng Z, He W, Han W. Rechargeable Aluminum/Iodine Battery Redox Chemistry in Ionic Liquid Electrolyte. ACS Energy Lett 2017;2:1170-6.
62. Meng Z, Tian H, Zhang S, et al. Polyiodide-Shuttle Restricting Polymer Cathode for Rechargeable Lithium/Iodine Battery with Ultralong Cycle Life. ACS Appl Mater Interfaces 2018;10:17933-41.
63. Zhang Y, Tao D, Xu F, Li T. A low-cost and high-performance rechargeable magnesium battery based on povidone iodine cathode. Chemical Engineering Journal 2022;427:131592.
64. Hong JJ, Zhu L, Chen C, et al. A Dual plating battery with the Iodine/[ZnIx (OH2 )4-x ]2-x cathode. Angew Chem Int Ed Engl 2019;58:15910-5.
65. Wang J, Qiu H, Zhao Z, et al. Anti-corrosive hybrid electrolytes for rechargeable aqueous Zinc batteries. Chem Res Chin Univ 2021;37:328-34.
66. Yan M, Dong N, Zhao X, Sun Y, Pan H. Tailoring the Stability and Kinetics of Zn Anodes through Trace Organic Polymer Additives in Dilute Aqueous Electrolyte. ACS Energy Lett 2021;6:3236-43.
67. Tangthuam P, Pimoei J, Mohamad AA, et al. Carboxymethyl cellulose-based polyelectrolyte as cationic exchange membrane for zinc-iodine batteries. Heliyon 2020;6:e05391.
68. Biswas S, Senju A, Mohr R, et al. Minimal architecture zinc–bromine battery for low cost electrochemical energy storage. Energy Environ Sci 2017;10:114-20.
69. Liu B, Wang S, Wang Z, et al. Novel 3D Nanoporous Zn-Cu alloy as long-life anode toward high-voltage double electrolyte aqueous Zinc-Ion batteries. Small 2020;16:e2001323.
70. Li Y, Guo S. Material design and structure optimization for rechargeable lithium-sulfur batteries. Matter 2021;4:1142-88.
71. Chen Y, Wang T, Tian H, Su D, Zhang Q, Wang G. Advances in Lithium-Sulfur Batteries: from academic research to commercial viability. Adv Mater 2021;33:e2003666.
72. Li H, Li Y, Zhang L. Designing principles of advanced sulfur cathodes toward practical lithium-sulfur batteries. SusMat 2022;2:34-64.
73. Chen Z, Mo F, Wang T, et al. Zinc/selenium conversion battery: a system highly compatible with both organic and aqueous electrolytes. Energy Environ Sci 2021;14:2441-50.
74. Chen Z, Yang Q, Mo F, et al. Aqueous Zinc-Tellurium batteries with ultraflat discharge plateau and high volumetric capacity. Adv Mater 2020;32:e2001469.
75. Hao J, Yuan L, Johannessen B, et al. Studying the Conversion Mechanism to Broaden Cathode Options in Aqueous Zinc-Ion Batteries. Angew Chem Int Ed Engl 2021;60:25114-21.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.


























Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].