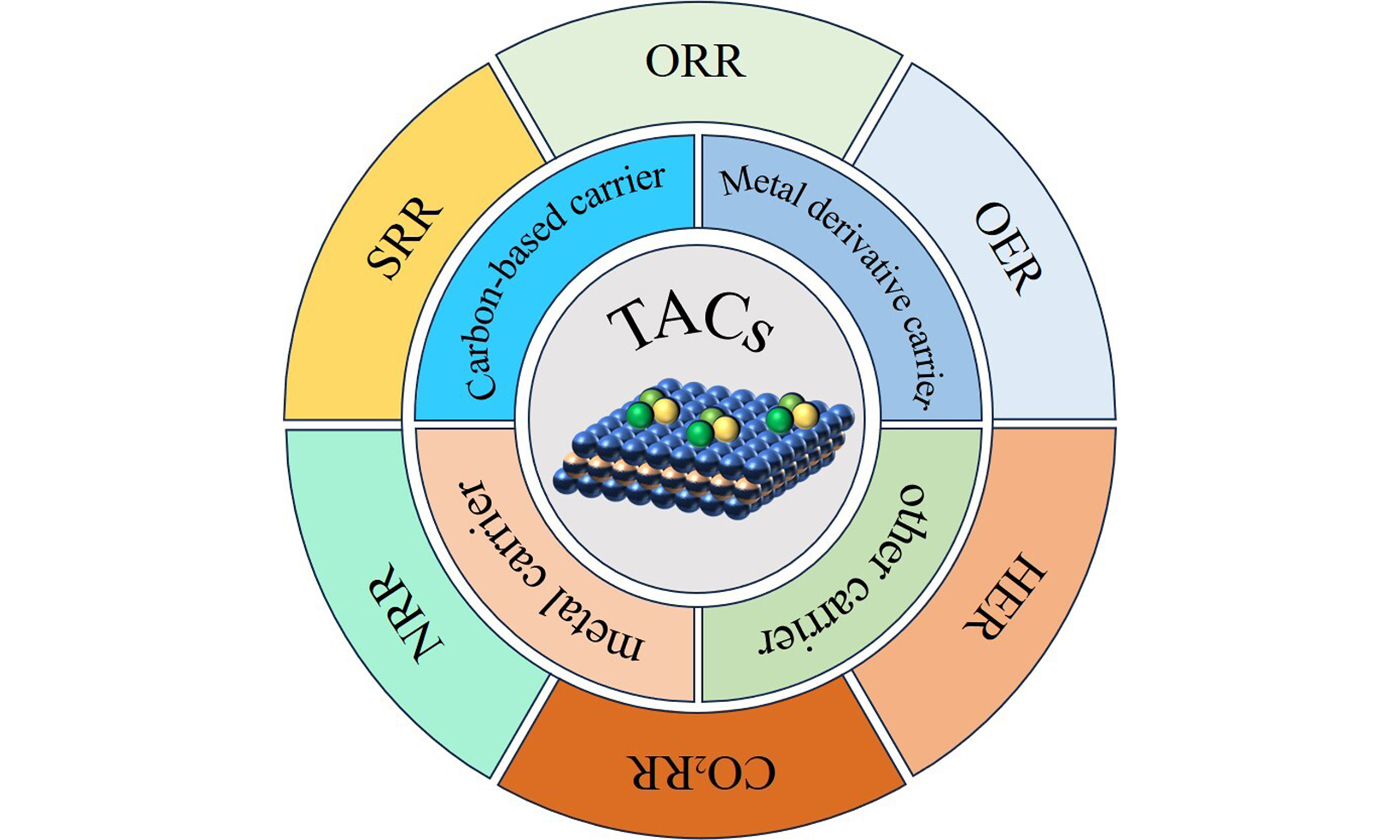
Triatomic catalysts: from design strategies to energy applications
Abstract
Materials science has now progressed to the fine atomic scale, demonstrating a broad spectrum of applications in photocatalysis, electrocatalysis, and thermal catalysis. Triatomic catalysts (TACs) have attracted considerable attention, primarily because of their ultra-high atomic utilization efficiency, as well as their remarkable catalytic activity and selectivity. TACs currently face multiple challenges that affect their durability, efficiency, and stability. The high surface energy of atoms and limited loading capacity further restrict their practical applications. To address these issues and meet the strict requirements for catalyst applications, researchers have devoted themselves to developing novel metal TACs with features of cost-effectiveness, high stability, excellent activity, high loading capacity, and maintained selectivity. This review systematically summarizes the recent progress in TACs, covering preparation methods, support material selection, and application fields, with an emphasis on the role of carriers in influencing TAC performance. Furthermore, the potential of TACs in energy conversion reactions, such as oxygen reduction reaction, CO2 reduction reaction, and N2 reduction reaction, is discussed. The existing challenges of TACs and key directions for future research in this field are also elaborated in a systematic manner.
Keywords
INTRODUCTION
Catalysts play a pivotal role in material synthesis, environmental remediation, and various other applications by enabling the regulation of reaction rates without being consumed in the process. With recent breakthroughs in nanoscience and materials science, atomic-scale catalysts have garnered significant research attention. Since the formal introduction of the concept of atomic catalysts in 2011, these catalysts have rapidly advanced and garnered vital global attention[1-13], especially single-atom catalysts (SACs), diatomic catalysts (DACs) and triatomic catalysts (TACs). The essential differences among TACs, DACs and SACs lie in the number of atoms at the active sites and the cooperative mechanisms. SACs achieve high atomic utilization with isolated single atoms, but a single active site is difficult to regulate multi-step reactions[14]. DACs enhance the reaction process through the electronic coupling of dual atoms; however, the configuration and electronic regulation are still limited. TACs form multi-active-site cooperativity such as linear and triangular configurations, which have great electronic delocalization (metal bonds and carrier coordination). The triatomic sites have the dynamic stability of anti-aggregation, breaking the limitations of single and dual-atom systems[15-17]. In particular, demonstrating high activity and multifunctionality in multi-electron complex reactions such as the CO2 reduction reaction (CO2RR) and the N2 reduction reaction (NRR). Through multi-atom cooperativity and heteronuclear design, TACs can simultaneously regulate the adsorption energies of multiple intermediates, such as optimizing the adsorption strengths of
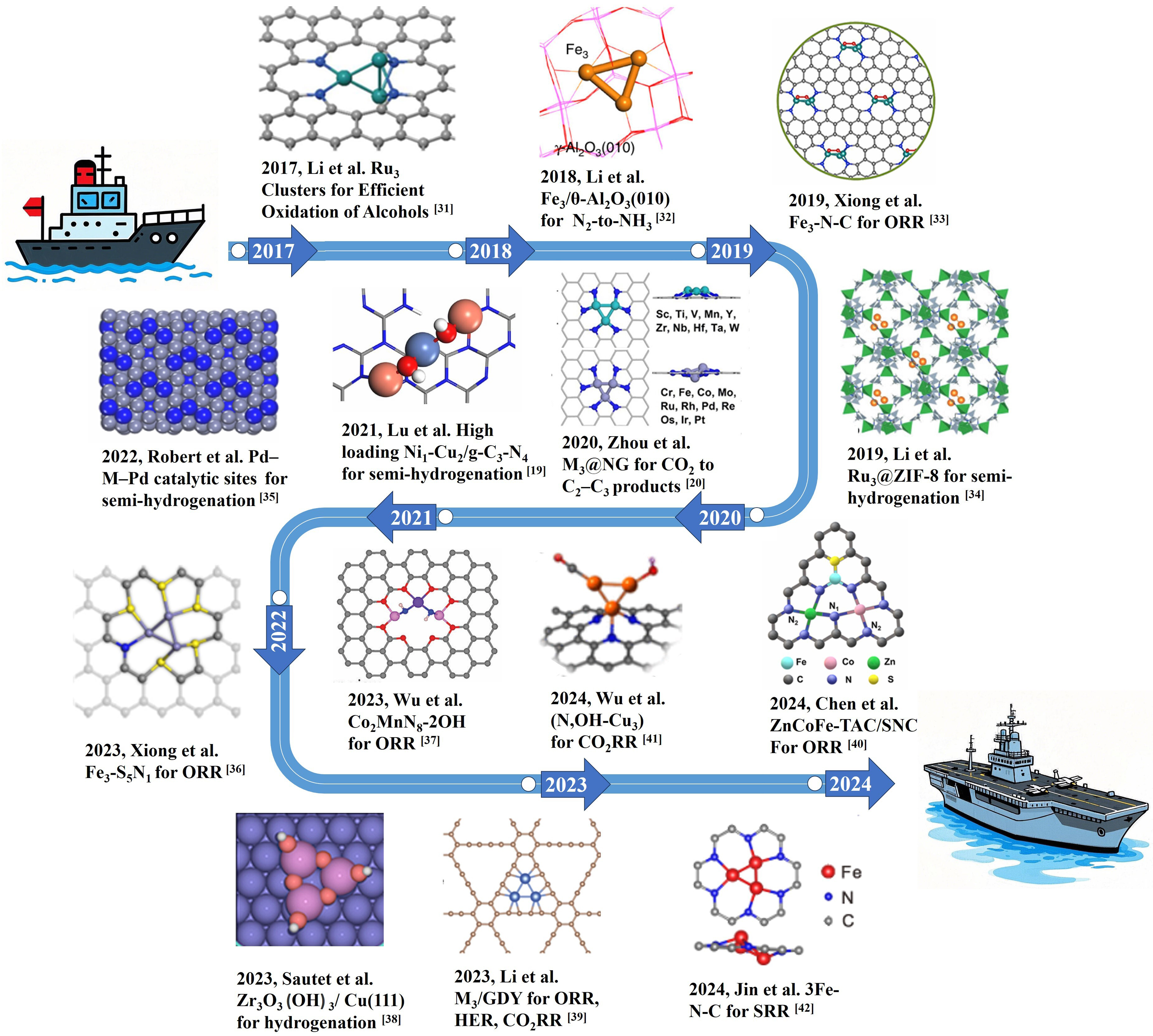
Nevertheless, notable progress has been made in the synthesis of TACs. Figure 1 illustrates key advancements in TAC preparation from 2017 to 2024. Researchers have achieved significant breakthroughs in preparation techniques, structural design, theoretical innovation, and applications including CO2RR, semi - hydrogenation reactions, and beyond[22,23,34-45]. This review mainly introduces some important achievements in the development of TAC so far in terms of carrier, preparation and application, such as the TACs with different functions prepared by Gu et al. on different carriers and all possess great performance[22,23,34-45]. Metal TACs primarily consist of three metal atoms, along with their coordination environments and carriers. Several factors influence the performance of these catalysts, including metal loading, doping with various metals, the presence of polymetallic active centers, synergistic interactions between adjacent active sites, local coordination environments featuring unsaturated metal atoms, and the desorption of intermediates[46]. Carriers can be broadly classified into carbon-based materials such as graphene[22,37,47,48], carbon nanotubes (CNTs)[49,50], metal oxides such as metal-organic frameworks (MOFs), metallic carriers, case in point Pt[25,47], and others such as g-C3N4[51]. The synergistic effects and interactions of these carriers significantly impact catalytic efficiency. Therefore, selecting appropriate carriers is a pivotal step in the preparation process[22,52]. Moreover, ensuring the stability of highly loaded TACs is challenging due to the complex preparation process and the difficulty in precisely adjusting the number of atoms during synthesis[47]. Research on TACs remains in its early stages, and achieving precise synthesis continues to pose vital challenges. In this review, we systematically summarize the key factors affecting the catalytic performance of TACs, the rational selection of support materials, and their practical applications in reactions such as CO2RR and NRR, and offer perspectives on the future development prospects of TACs.
PREPARATION OF TACS
The selection of an appropriate carrier is crucial for the preparation and performance of catalysts, as it significantly influences key factors such as electronic structure modulation, interface effects, and the regulation of catalytic active sites. Metal atoms dispersed on a suitable carrier to form an appropriate coordination environment can exhibit optimal catalytic performance[21]. The catalytic efficiency of TACs is influenced by parameters such as surface area, dispersion, pore size, morphology, and the interaction between the carrier and metal atoms[50,53]. To date, the main types of carriers can be categorized into carbon-based materials, metal derivatives and metallic supports. Carbon-based supports possess a high specific surface area, superior electronic conductivity, and remarkable mechanical stability, all of which significantly enhance the intrinsic catalytic activity[54,55]. Nevertheless, they may have inadequate thermal stability and potential interactions with metal atoms, which could unfavorably modify the catalytic properties[56]. In contrast, metal supports, while offering robust structural integrity and improved metal dispersion, may exhibit limited effectiveness in electronic tuning, thereby potentially restricting their activity in certain reactions[57]. Conversely, metal oxide supports can establish strong metal-support interactions that stabilize the metal catalysts, thereby augmenting selectivity[58-61]. The rational design of the carrier is the core strategy to solve the contradiction between high loading of metal atoms and anti-aggregation. Its core lies in achieving compatibility between atomic-level dispersion and high density through multi-scale coordinated regulation of electronic, geometric and dynamic aspects[54]. By doping with heteroatoms such as N, S, P and through defect engineering such as vacancies and edge sites can construct strong metal-carrier electronic interactions[39,62,63]. The surface energy can be vitally reduced and the migration barrier enhanced by regulating the position of the d-band center of metal sites, thus inhibiting the tendency of atomic diffusion and agglomeration. These contribute to the construction of high-density anchored active sites, thereby increasing the loading amount of metal atoms[19,64,65]. For instance, when using smooth CNTs as a carrier, the oxygen extraction reaction (OER) and oxygen reduction reaction (ORR) performance of Ni-Ru@Fe/C@CNT are crucially superior to those of Pt/C and RuO2[66]. This improvement can be attributed to the distinctive architecture of CNTs when combined with Fe. The carbon-encapsulated Fe/Fe3C nanoparticles resemble tentacle-like structures on the surface of Fe/C@CNT. These structures not only enhance the exposed surface area, thereby facilitating the adhesion of active materials, but also improve the dispersion and stability of Ni- and Ru-based compounds. Additionally, others, such as the surface-modified Ti3C2Tx carriers[67] and nitrogen-doped high-loading catalyst carriers polymeric carbon nitride (PCN)[68], have also provided different insights for the preparation of TACs and the design of carriers. In recent years, momentous advancements have been made in the development of TACs and their corresponding carriers.
Carbon-based carriers
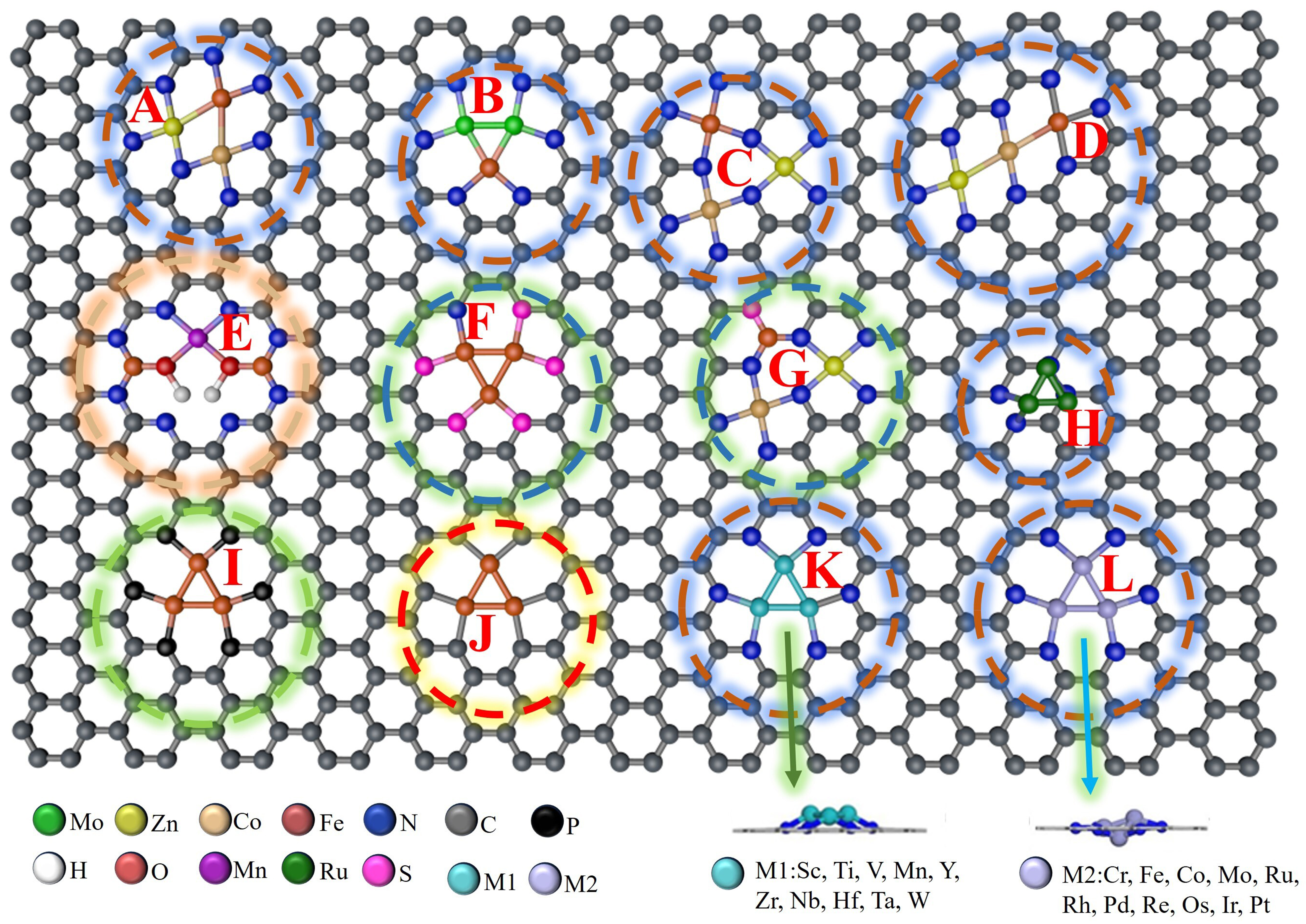
Carbon-based materials, such as graphene, exhibit distinctive advantages including high electrical conductivity, low manufacturing costs, and facile structural modulation. These properties facilitate efficient electron transfer and enhance catalytic performance. Furthermore, graphene is recognized as an environmentally friendly carrier and is extensively utilized in the preparation of TACs[46]. Graphyne, another environmentally friendly carrier, is also widely used in TACs synthesis. Due to its sp2 orbital hybridization, graphyne enhances the interaction between trimetallic atoms and its structure, thereby improving stability. Additionally, CNTs, porous activated carbon, and nano-carbon spheres are excellent carbon-based materials for TACs. Moreover, different coordination environments significantly impact the actual performance of these catalysts. Here, we summarize some general constructions of carbon-based carriers in Figure 2[23,34,40,43,46,69-73], representing various coordination environments and metal atoms.
Figure 2. Schematic illustration of TAC interface structures in carbon-based carriers: (A-D, H, K, L) Carbon and Nitrogen-coordinated atomic interface structures; (E) Carbon, Nitrogen, and hydroxyl (OH)-coordinated atomic interface structures; (F and G) Carbon, Nitrogen, and Sulfur-modified atomic interface structures; (I) Carbon and Phosphorus-doped atomic interface structures; (J) Carbon-coordinated atomic interface structures[23,34,40,43,46,69-73]. TAC: Triatomic catalyst.
The stability of carbon-based carriers is enhanced by the incorporation of metal atoms, which effectively prevents the aggregation of active sites induced by surface energy and facilitates the expression of their synergistic effects[51-53]. Furthermore, introducing dopant atoms into these carriers can modify their electronic structure, potentially improving the catalytic performance of TACs[22,46,47,54,55]. Density functional theory (DFT) calculations have been employed to investigate the effects of boron (B) and nitrogen (N) doping on graphene[56,57], revealing that lithium metal TACs loaded on B- and N-doped graphene significantly optimize enzymatic reactions. Additionally, a novel method has been reported for modifying the oxy tropism of catalysts by varying the loading of transition metals (TMs), leading to the synthesis of mesoporous N-doped carbon nanosheets riddled with ultrafine molybdenum carbide nanoparticles
The advantages of defects are that they can significantly increase the density of the active site, regulate the adsorption free energy, optimize the adsorption and activation process of the reactants, optimize the electronic structure, and enhance the electron transfer efficiency between the metal active center and the reactants, thus significantly improving the overall performance of the catalysts[74,75]. Carbon-based carriers possess a high density of defects that can be artificially introduced through methods such as in situ doping and post-modification[59]. In this context, researchers have successfully immobilized free Pt atoms on CN defects, transforming them into highly reactive Pt species. This modification resulted in significantly enhanced activity in the semi-hydrogenation reaction compared to the original state[60]. Additionally, studies have shown that graphene with inherent or artificially induced defects can improve the catalytic activity of TACs via synergistic effects[25]. These investigations have elucidated the mechanism by which defects enhance catalytic performance and proposed strategies for modulating defect sites to alter the coordination environment, thereby increasing the number of active sites in TACs[19,46,61].
The preparation of carbon-based carriers is both straightforward and safe. For example, Tsai et al. synthesized nitrogen-doped carbon (NC) through the pyrolysis of zeolitic imidazolate frameworks (ZIFs) and SiO2[76]. Meanwhile, DFT calculations were also utilized to predict the conformational relationship between metal triatoms and nitrogen-doped graphene and to examine the coordination mechanism, providing valuable insights for the precise fabrication of efficient carriers and TACs[77]. Furthermore, porous carbon is commonly utilized as a carrier owing to its extensive pore structure and abundant active sites for attachment[67,68]. The predominant preparation method currently employed is activator-induced pyrolysis[69,70].
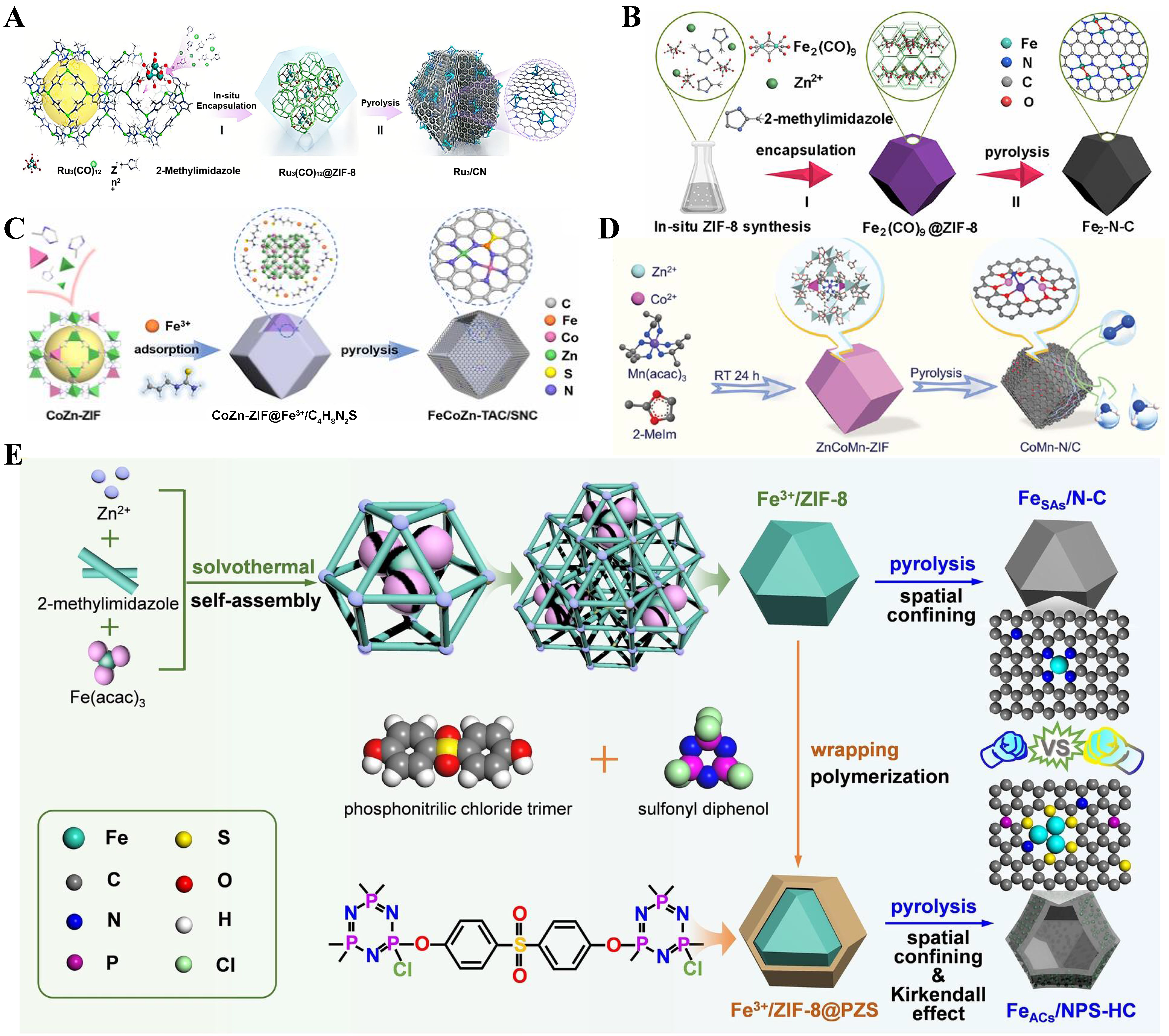
To date, significant progress has been made in the preparation of TACs supported on carbon-based materials. Notably, Gu et al. successfully synthesized Ru3/CN[22]. This was achieved by using Ru3(CO)12 as the precursor, which was stabilized through a nitriding process involving precursor separation via cages and a pyrolysis method. Specifically, molecular sieve ZIFs were employed for the cage separation of the precursor, followed by heat treatment. Additionally, a combination of first-principles computational analysis showed that the well-distributed Ru3 clusters contributed to the highly efficient catalytic activity of the effective sites [Figure 3A][78]. In addition, controlling the number of metal atoms can also be regulated to prepare three-atom catalysts, by encapsulating a specific number of Fe compounds such as Fe2(CO)9 in ZIF-8 and performing pyrolysis can synthesis Fe2/N-C, which was can also be used to synthesized Fe3/N-C by
Figure 3. (A) The preparation of Ru3/CN[34], Copyright 2017, American Chemical Society; (B) The preparation of Fe2-N-C[36], Copyright 2019 Elsevier Inc; (C) The preparation of FeCoZn-TAC/SNC[49], Copyright 2022, American Chemical Society; (D) The preparation of CoMn-N/C[40], Copyright 2023 Wiley-VCH GmbH; (E) The preparation of FeSAs/N-C and FeACs/NPS-HC[39], Copyright 2023 Wile-VCH GmbH. TAC: Triatomic catalyst.
Metal and metal derivative carriers
In recent years, substantial research efforts have focused on metal and metal-derived carriers. The coordination environment of metals and their derivatives can accurately modulate the electronic structure of metal centers. This modulation alters the activity and selectivity of catalysts via strong electronic interactions and electron transfer processes[79]. Through metal bond connection, not only is there a stronger adsorption stability effect, but also a better synergistic catalytic effect can be achieved[80]. For derivative carriers, by loading different groups, the coordination structure can be precisely regulated and the active sites can be optimized, thereby improving the selectivity and activity of the catalyst[81], such as TiO2[82],
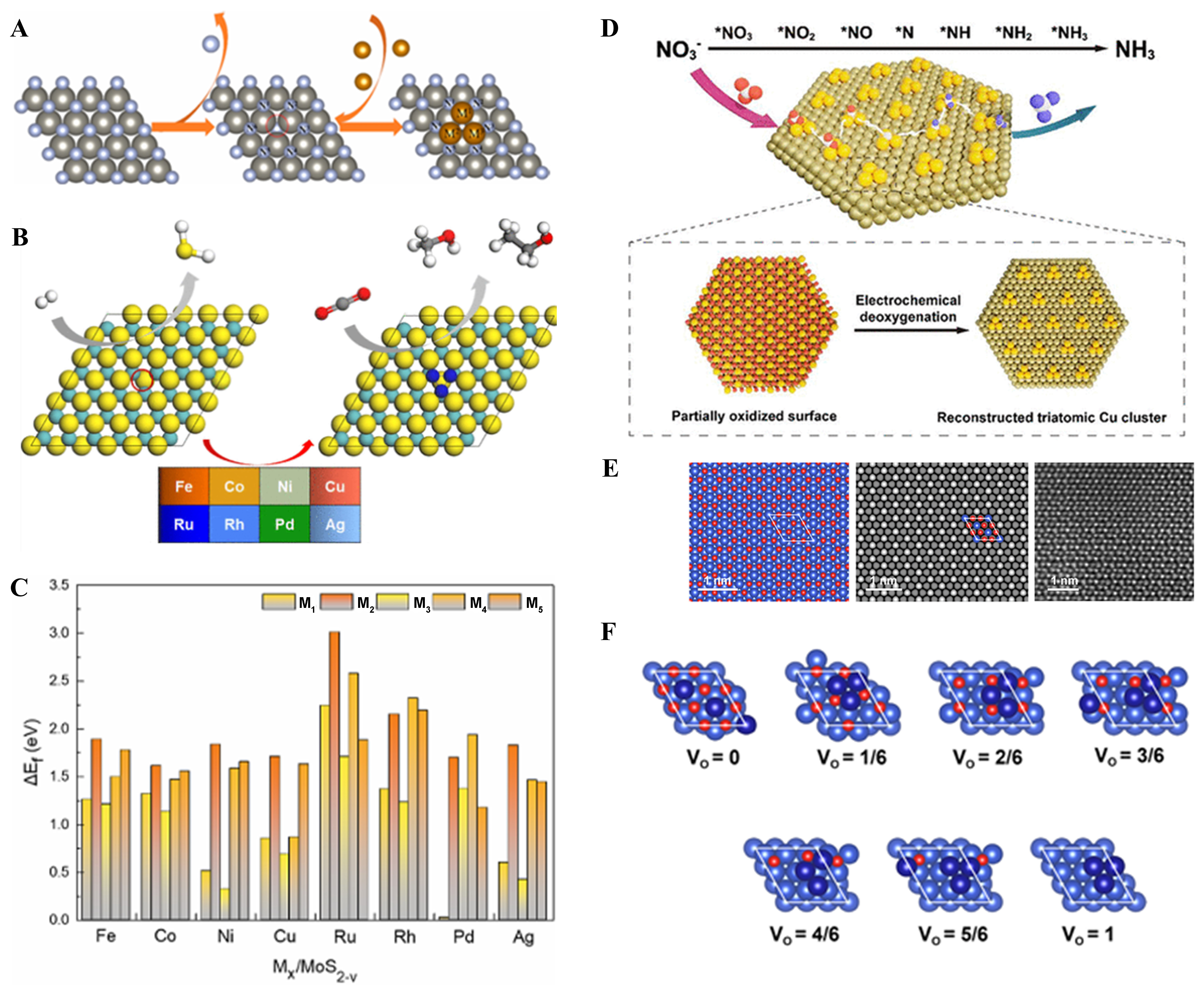
Figure 4. (A) Schematic diagram of partially oxidized Cu surface reconfiguration to form triatomic clusters; (B) Cu(111) surface, from left to right are model, simulated STM and experimental TEM images respectively; (C) Diagram of a stepwise deoxidation process with oxygen atom removal rate from 0 to 1[98], Copyright 2022, American Chemical Society; (D) Structural construction of
The metal carriers utilized in TACs predominantly include copper (Cu), platinum (Pt), and silver (Ag). Although metal carriers are less commonly employed compared to carbon-based carriers, they offer substantial catalytic efficiency. For instance, Cu-based carriers are used in CO2 reduction processes; however, their selectivity is often limited. Consequently, researchers are actively seeking ways to modify the structures of metal-based carriers and explore the intricacies of catalytic reaction steps to enhance selectivity[92-95]. Researchers conducted both experimental and computational studies to investigate the doping of copper-based materials with various metals[96]. Their approach aimed to modify the potential structure, introduce defects, and refine the surface microstructure, ultimately enhancing the selectivity of copper-based catalysts. Researchers also investigated the kinetics of the catalytic process by examining modifications to the Cu-based carrier and varying the reaction conditions using computational and alternative methods. They found that the designed triatomic Zr clusters formed a sub-stable conformation with the Cu carrier, which significantly accelerated the CO2 hydrogenation reaction[41]. It was reported that by doping other elements onto a copper base, the formation of Cu alloys could be stimulated. The developed Cu-NSA alloys outperformed pure copper carrier catalysts in the CO2RR, offering valuable insights into the advancement of metal-carrier TACs[97]. For example, Wu et al. prepared Cu3/Cu(111) metal TAC by electrochemical deoxidation and surface reconstruction on partially oxidized Cu nanosheets with uniform surface [Figure 4D], which was a simple and convenient method[98]. And they constructed the Cu(111) structure model in which the bright one is Cu atom and the shade is O atom in Figure 4E. In Figure 4F, the researchers simulated the oxygen atom removal process that the V0 from 0-1 which suggested that when the oxygen atoms were completely eaten, a triatomic cluster was formed, showing a convenient way to form TACs.
The development of metal and metal derivative carriers for TACs has witnessed substantial progress. Recent research has emphasized both theoretical and experimental innovations, such as heteroatom doping and surface modification. The interaction between metal atoms and the metal components on the carrier has also become one of the main breakthrough points for in-depth research and innovation of TACs. These approaches aim to enhance the applicability of TACs by improving their structural properties, thereby making them significant in various applications within the field.
Other types of carriers
Among the types of carriers used for the preparation of TACs, in addition to the carbon-based, metal-derivative, and metal-based carriers described above, this section will also discuss other types of carriers. Common supports such as SiO2 provide a stable, low-coordination environment that facilitates the dispersion of active sites and minimizes aggregation[99]. Other supports such as graphitic carbon nitride offer unique advantages due to their porous structure, tunable electronic properties, and ability to provide nitrogen-rich coordination sites that enhance metal-support interactions. Others, such as covalent organic frameworks (COFs)[100], introduce additional complexity through their well-defined pore structures and customizable active sites, allowing for precise control over the local coordination environment[101]. These carriers can influence the electronic structure of the catalyst through ligand effects or charge transfer, thereby modulating catalytic performance. However, the precise coordination environment and its impact on catalytic mechanisms remain areas of active research, particularly for novel supports where the interplay between geometry, electronic structure, and reactivity is not yet fully understood[102]. Recently, researchers have found that the layered double hydroxide (LDH) acts as a carrier that can provide a stable coordination environment for the catalyst[103]. Its layered structure and cation-exchange capacity enable the uniform dispersion of metal atoms, forming active sites while preventing metal agglomeration. The LDH framework promotes electron transfer, enhancing the catalyst’s activity and selectivity. Additionally, the hydroxide groups and electronic properties of LDH stabilize the catalyst and improve its efficiency in polyolefin hydrogenolysis. Also, researchers have synthesized atomically dispersed triatomic alloys using a microwave-assisted alcohol reduction technique. This method employed three distinct metal salts along with ethylene glycol and the secondary reducing agent NaBH4. The resulting triatomic alloys were subsequently immobilized on SiO2. The final TACs exhibited valence activity ratios of up to 3.353 min-1, significantly surpassing those of monometallic catalysts[89].
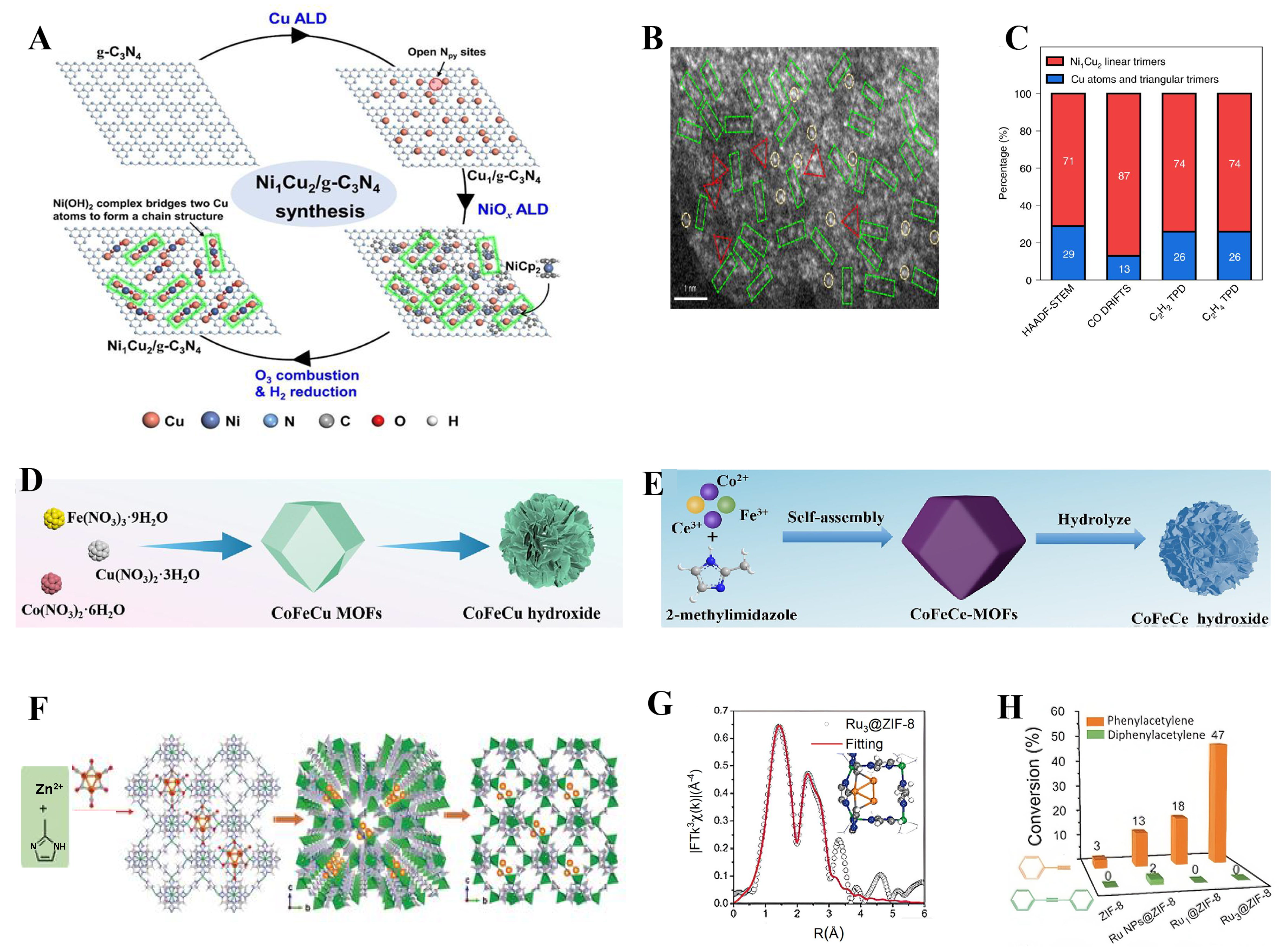
Carriers such as g-C3N4 possess a unique layered structure and a large specific surface area, providing abundant catalytic active sites that help improve the activity and selectivity of the catalyst. And g-C3N4 has good chemical and thermal stability, which can maintain catalytic performance under harsh reaction conditions. Its good electrical conductivity and photothermal properties make it suitable for photocatalytic and electrocatalytic reactions, and improve the efficiency of the reaction. For example, Gu et al. prepared Ni1Cu2/g-C3N4 by burning ozone and hydrogen reduction after loading Cu and Ni onto g-C3N4 by atomic layer deposition (ALD) method [Figure 5A and B][22]. The linear trimer increases sharply to 71% at the expense of single atoms, which decrease to 18%, while the triangular trimer remains at ~11% [Figure 5C]. This indicates that most of the pre-deposited single Cu atoms may be bridged by Ni atoms to form new linear trimers.
Figure 5. (A) Ni1Cu2/g-C3N4 catalyst preparation process; (B) HAADF-STEM image of Ni1Cu2/g-C3N4 in which the yellow dashed circles represent isolated atoms, green rectangular dashed lines represent linear trimers, and red dashed triangles represent triangular trimers. (C) Statistics of single atoms, linear trimers and triangular trimers in Ni1Cu2/g-C3N4 using four different approaches[22], Copyright 2021, Springer Nature Limited; (D) Schematic illustration of the preparation process of the CoFeCu hydroxides[104], Copyright 2024 Elsevier B.V.; (E) The synthesis procedure of CoFeCe three-atom hydroxide[105], Copyright 2023 Elsevier Ltd; (F) Preparation process of
Additionally, a simple method has been applied for synthesizing TACs, where different MOF precursors serve as templates and triatomic hydroxides are obtained via pyrolysis. For example, Chen et al. utilized CoFeCu-MOFs as a template, and obtained CoFeCu hydroxides by ion exchange using a simple wet chemical method [Figure 5D][104]. Shang et al., after obtaining CoFeCe-MOFs by self-assembly, directly prepared CoFeCe three-atom hydroxide by hydrolysis combined with the hydrothermal method
APPLICATION OF METAL TACS
As an innovative and highly efficient catalytic material, TACs have garnered widespread recognition for their ability to be stably anchored on various carriers and exhibit different performances in some areas. Their unique structure and exceptional catalytic performance have facilitated extensive applications in chemical industry and environmental solutions, etc. In this section, we will provide a detailed performance of TACs in CO2RR, NRR, ORR, OER and other applications.
CO2RR
Currently, the development of CO2RR has a vital position in protecting the environment and mitigating the greenhouse effect[94,106-110]. The CO2RR is the reduction of CO2 into single-carbon products, CO[111], CH4[87], CH3OH[41] and multi-carbon products such as C3H6, C2H4[112], C3H6, C2H4[113], CH3CH2OH[114]. The typical process is as follows: (1) CO2 + 8H+ + 8e- → CH4 + 2H2O, (2) CO2 + 2H+ + 2e- → CO + H2O, (3) CO2 + 6H+ + 6e- → CH3OH + H2O, (4) 3CO2 + 12H2O + 18e- → C3H6 + 18OH- and so on. Researchers aim to develop catalysts featuring high selectivity, excellent catalytic activity, and robust stability[115,116].
Based on previous studies[117-122], most researchers favor[107] choosing the cost-effective and uniquely structured Cu as the focus of their research and constructed a stable and efficient Cu3-π structure using DFT calculations, which enhances the understanding of the relationship between aromaticity and catalysis, offering new insights for catalyzing CO2RR. There are also some other insights in CO2RR.
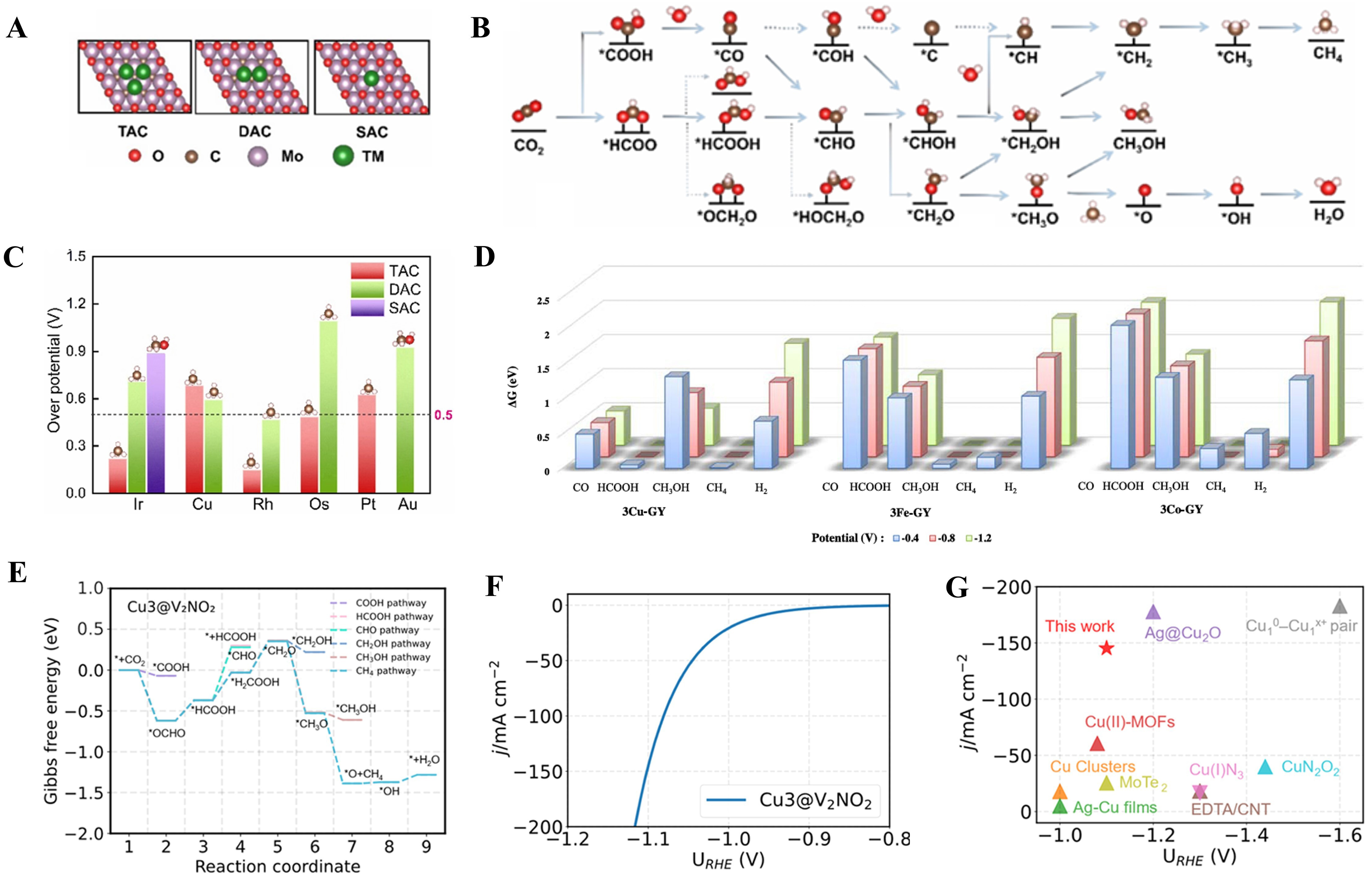
Xiao et al. built a model for embedding homonuclear metal atoms in oxygen vacancies to obtain lower formation energies [Figure 6A][83]. They analyzed the examined pathways for the C1 products shown in Figure 6B. With 0.5 V as the boundary, the higher overvoltage was excluded, leaving Ir TAC, Rh TAC and Os TAC [Figure 6C]. Also, the adsorption energy determines the type and difficulty of their adsorption to a certain extent, so by studying the activation barriers and adsorption energies, it was comprehensively found that Os TAC exhibited the best performance. Zhou et al. investigated the limiting potential at zero point and found that 3Cu-GY, 3Co-GY and 3Fe-GY all had greater adsorption effects on CO2RR[123]. With the increase of potential, C1 product was easily generated in the reaction, and the competitive advantage of
Figure 6. (A) The structure model of TACs, DACs, SACs; (B) Reaction pathways examined for the CO2RR to C1 products; (C) Comparison of overpotential during reaction[83], Copyright 2023 The Royal Society of Chemistry; (D) The ΔGmax in CO2RR to CO, HCOOH, CH3 OH, CH4, and H2 on 3TM-GYs at different V[123], Copyright 2021 Elsevier Ltd; (E) Gibbs free energy for CO2RR to CH4 on Cu3@V2NO2; (F) Simulated polarization curves; (G) Summary of recent advancements for the electrocatalytic conversion of CO2 to CH4 at various applied potential[87], Copyright 2024 Published by Elsevier B.V. TACs: Triatomic catalysts; DACs: diatomic catalysts; SACs: single-atom catalysts; CO2RR: CO2 reduction reaction.
Currently, researchers are working on more ways to design CO2RR catalysts, such as improving the surface structure and catalytic environment[124,125], changing the composition of the electrode-electrolyte interface[126], etc. Not only that, in the design process, considering the development of multi-functional catalysts is also one of the mainstreams; selectively generating different products according to reaction conditions also needs researchers to continue to innovate. Precisely synthesizing highly stable, efficient, and selective CO2RR metal TACs represents the core pursuit of researchers, while also aligning with the critical requirements for industrial-scale production.
NRR
Ammonia (NH3), recognized as the world’s second-largest chemical product by production capacity, serves a crucial function within the domains of chemistry and chemical synthesis. Its role extends beyond mere industrial application, establishing ammonia as a significant renewable energy source[127-130]. Consequently, NH3 is integral not only in chemical manufacturing but also in the development of sustainable fuels and various other innovative applications. The multifaceted utility of ammonia underscores its importance in advancing both chemical science and energy solutions[131,132]. Currently, the development of sustainable and eco-friendly ammonia production methods has become a critical priority. This transition is essential for advancing ecological conservation and mitigating the environmental challenges posed by conventional ammonia synthesis processes[133]. Consequently, advancing green technologies in ammonia synthesis is essential for fostering a more sustainable future within the chemical sector[134]. Currently, the mainstream ammonia production method is the Haber-Bosch (HB) process[135]. However, this process not only consumes high energy but also emits a large amount of carbon dioxide that affects the environment; therefore, exploring green ammonia production methods is increasingly important[30,98,136]. The NRR reaction is the process of reducing nitrogen to ammonia as follows: (1) N2 + 6H+ + 6e- → 2NH3 (acidic environment), (2) 2N2 + 6H2O + 12e- → 4NH3 + 6OH- (alkaline environment). Since N2 is not readily soluble in water and is difficult to dissociate, researchers are exploring methods for efficient nitrogen fixation and nitrogen conversion[135,137-139].
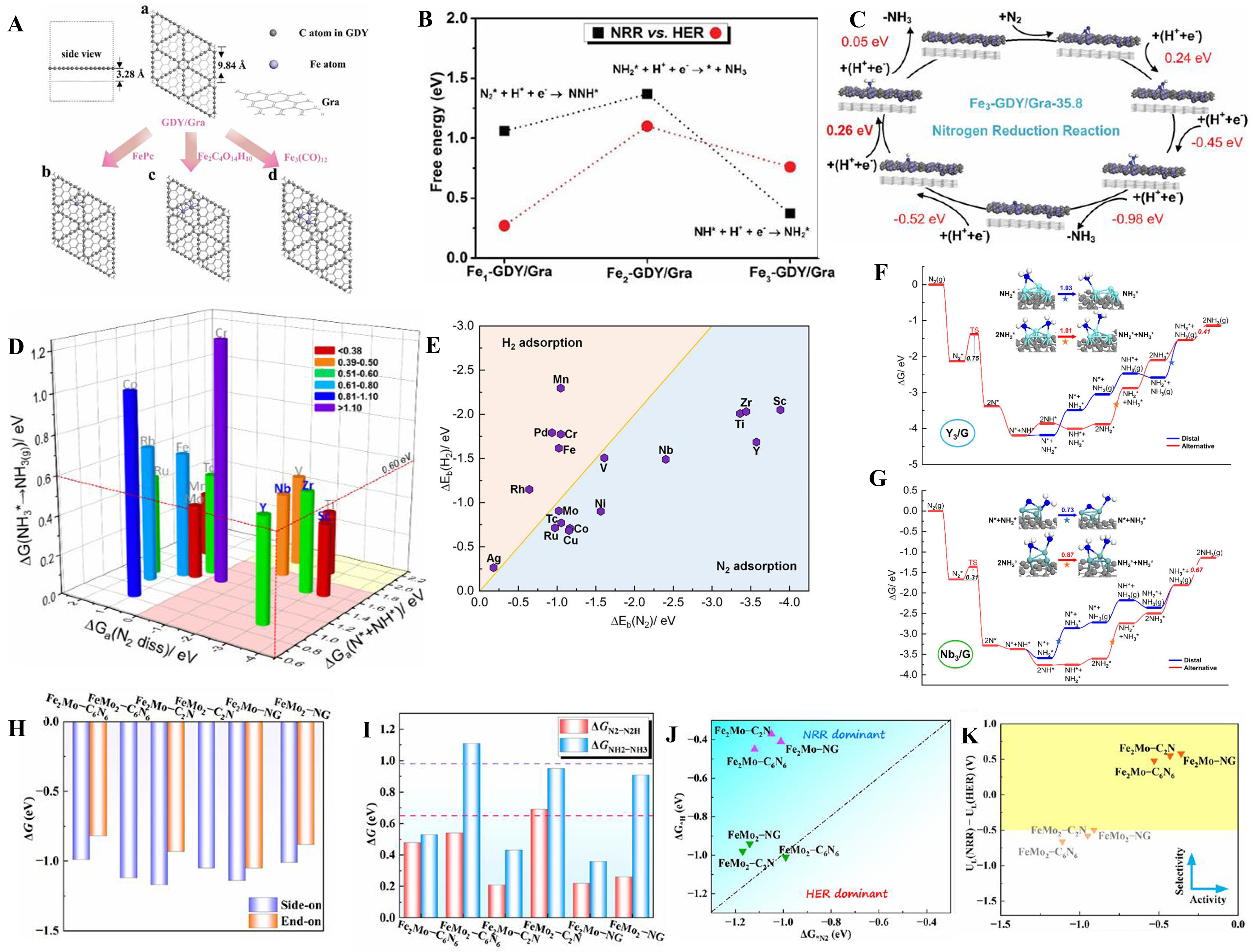
Chen et al. anchored different numbers of metal atoms on the graphdiyne (GDY) to form different atomic catalysts[140] [Figure 7A]. Because hydrogen evolution reactions (HER) often interfere with NRR as complementary reactions[125,126], researchers also analyzed the selectivity for NRR and HER, finding that Fe3-GDY/graphene (Gra) not only had high catalytic activity, but also had high selectivity to NRR [Figure 7B]. It also had low free energy, which further indicated that Fe3-GDY/Gra has high catalytic activity [Figure 7C]. Cui et al. studied the adsorption and desorption energies of 14 trimetallic atomic clusters for the NRR process, and found that Y3, Sc3, Zr3, and Nb3 were ideal NRR catalysts, among which Y3 showed the best catalytic activity [Figure 7D][26]. It could also be confirmed in Figure 7E. Researchers then studied in detail the reaction pathways and activation energies of Y3 and Nb3 catalysts for NRR processes, and concluded that Y3 and Nb3 are ideal NRR catalytic materials, showing high activity and selectivity [Figure 7F and G]. Wang et al. investigated theoretically the N2 adsorption free energy and configuration of FexMoy-CNs, finding that N2 was more easily adsorbed to the side-on configuration [Figure 7H][70]. Figure 7I showed that Fe2Mo-CNs exhibited the best performance among all of catalysts which could also be confirmed in Figure 7J and K that Fe2Mo-CNs had the best activity and selectivity.
Figure 7. (A) The synthetic strategy of Fex-GDY/Gra; (B) Comparisons of free energies of PLS for the NRR and HER on Fex-GDY/Gra; (C) Reaction path and free energy of Fe3-GDY/Gra with respect to NRR[140], Copyright 2020 The Royal Society of Chemistry; (D) The free energy of transition state for N2 dissociation, hydrogenation barrier of *N + *NH formation, and desorption energy of NH3 on14 three-atom metal clusters; (E) Binding energy of M3 metal clusters on N2 and H2; (F) Free energy diagram of NRR reaction on Y3/G; (G) Free energy diagram of NRR reaction on Nb3/G[26], Copyright 2022, American Chemical Society; (H) Two modes of N2 adsorption free energy on FexMoy-CNs; (I) Preliminary and final reaction free energies on different FexMoy-CNs; (J) Reaction free energy tendency model on different FexMoy-CNs; (K) The diagram of UL(NRR)−UL(HER) vs. UL(NRR) relationship and the colored area shows better performance[70]. PLS: Potential-limiting step; NRR: N2 reduction reaction; HER: hydrogen evolution reaction.
There are still many issues to be addressed in NRR. For instance, in the NRR, using 15N2 as the nitrogen source is necessary because 15N is a stable isotope with a relatively low natural abundance (approximately 0.36%), and its unique signal can be detected through nuclear magnetic resonance (NMR) or mass spectrometry (MS)[141,142]. This characteristic enables 15N2 to clearly distinguish the source of reaction products (such as ammonia), thereby avoiding interference from background nitrogen or impurities. In contrast, traditional colorimetric detection methods rely on color changes to quantify ammonia production, but this method is prone to interference from background signals or non-specific reactions, resulting in inaccurate or overestimated results[143]. Therefore, using 15N2 in combination with advanced analytical techniques can provide more reliable experimental data and ensure a deeper understanding of the mechanism of nitrogen reduction reactions[144]. In the same way as in CO2RR and sulfur reduction reaction (SRR), using 13CO2 and 34S will yield better experimental results[145,146].
ORR and OER
With energy and environmental issues gaining global recognition, researchers have shifted their focus to developing high-efficiency eco-friendly devices. Among these, ORR and OER stand out as essential processes in energy conversion and storage systems, such as air batteries, serving as critical core steps in these technologies[76,147-149]. ORR in electrocatalysis refers to the reaction of oxygen at the cathode to gain electrons into a negative valence form, and the reaction is divided into 2-electron and 4-electron reduction modes, which are O2 + 2H+ + 2e- → H2O2 (acidic conditions) and O2 + 2H2O + 4e- → 4OH- (alkaline conditions). OER refers to the process in which water or other oxygen-containing substances undergo an oxidation reaction at the anode, and oxygen precipitates to become oxygen. Usually, the OER is a 4-electron reaction, a reaction such as 2H2O → O2 + 4H+ + 4e- (acidic conditions) and 4OH- → O2 + 2H2O + 4e- (alkaline conditions). In recent years, great insights have been gained into these two reactions[150-153].
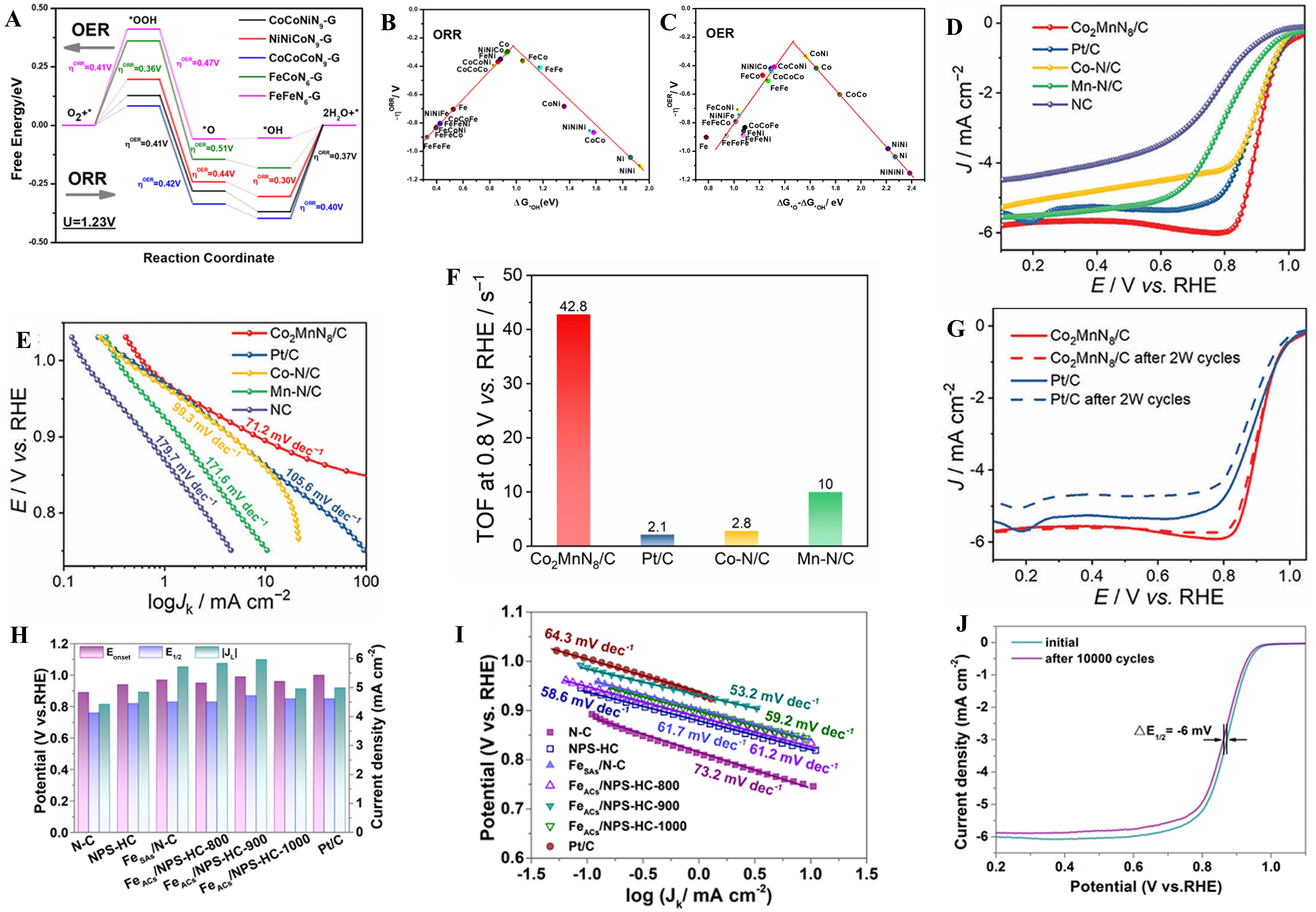
Ma et al. investigated the free energy of a bifunctional TAC under the condition of U = 1.23 V and NiNiCoN9-G showed the best bifunctional ORR and OER activity[46] [Figure 8A]. According to the thermodynamic principle, the lower the overpotential, the better the electrochemical activity of the catalyst, and according to the DFT calculation, it was confirmed that the three-atom catalyst NiNiCoN9-G and CoCoNiN9-G showed excellent bifunctional performance [Figure 8B and C]. Yan et al. tested the linear sweep voltammetry (LSV) of Co2MnN8/C finding that it had onset potential of 1.027 V and half-wave potential of 0.912 V which demonstrated optimal catalytic activity in all reference samples [Figure 8D][40]. And after calculation, Co2MnN8/C exhibited the lowest Tafel slope of 71.2 mV·dec-1 showing the fastest kinetics [Figure 8E]. In atomic catalysts, turnover frequency (TOF) was also an important kinetic index, so by evaluating it, they found that Co2MnN8/C was 42.8 s-1 at 0.8 V, which surpassed other samples in comparison far beyond [Figure 8F]. After 20,000 CV cycles, Co2MnN8/C showed higher stability than Pt/C, which had a wider prospect in application [Figure 8G]. Guo et al. found that after Kirkendall hollowing and triple doping, the TACs showed higher half-wave potential and limiting current density than those directly pyrolyzed from ZIF-8, exhibiting better performance in activity in catalyzing especially FeACs/NPS-HC-900
Figure 8. (A) The ORR and OER free energy diagram of CoCoNiN9-G, NiNiCoN9-G, CoCoCoN9-G, FeCoN6-G and FeFeN6-G under U = 1.23 V; (B and C) The volcano plots of OER and ORR[46], Copyright 2022 Elsevier B.V.; (D) LSV curves for ORR in O2-saturated 0.1 m KOH at 1,600 rpm; (E) Tafel plots; (F) The TOF values Co2MnN8/C, Pt/C, Co-N/C and Mn-N/C at 0.8 V vs. RHE; (G) LSV curves of
Other applications
As for TACs, there are a great bunch of applications that are not described in detail. For example, hydrogenation reaction, nitrogen oxide reduction[91,154] and dehydrogenation reaction, methane oxidation reaction, etc.[31,155,156]. Besides, SRR is also an important application in the field of energy catalysis for TACs. For example, researchers have investigated the prospect of the doped number of Fe ranging from 1 to 3 in SRR; although the final comprehensive performance of 3Fe-N/C is not as good as 2Fe-N/C, it still showed the promising possibility in SRR[45]. Meanwhile, TACs can also be used as nano-enzyme catalysts to build a sensing platform. For example, CoFeCu hydroxides[104], CoFeCe hydroxide[105], FeCoZn-TAC/SNC[157].
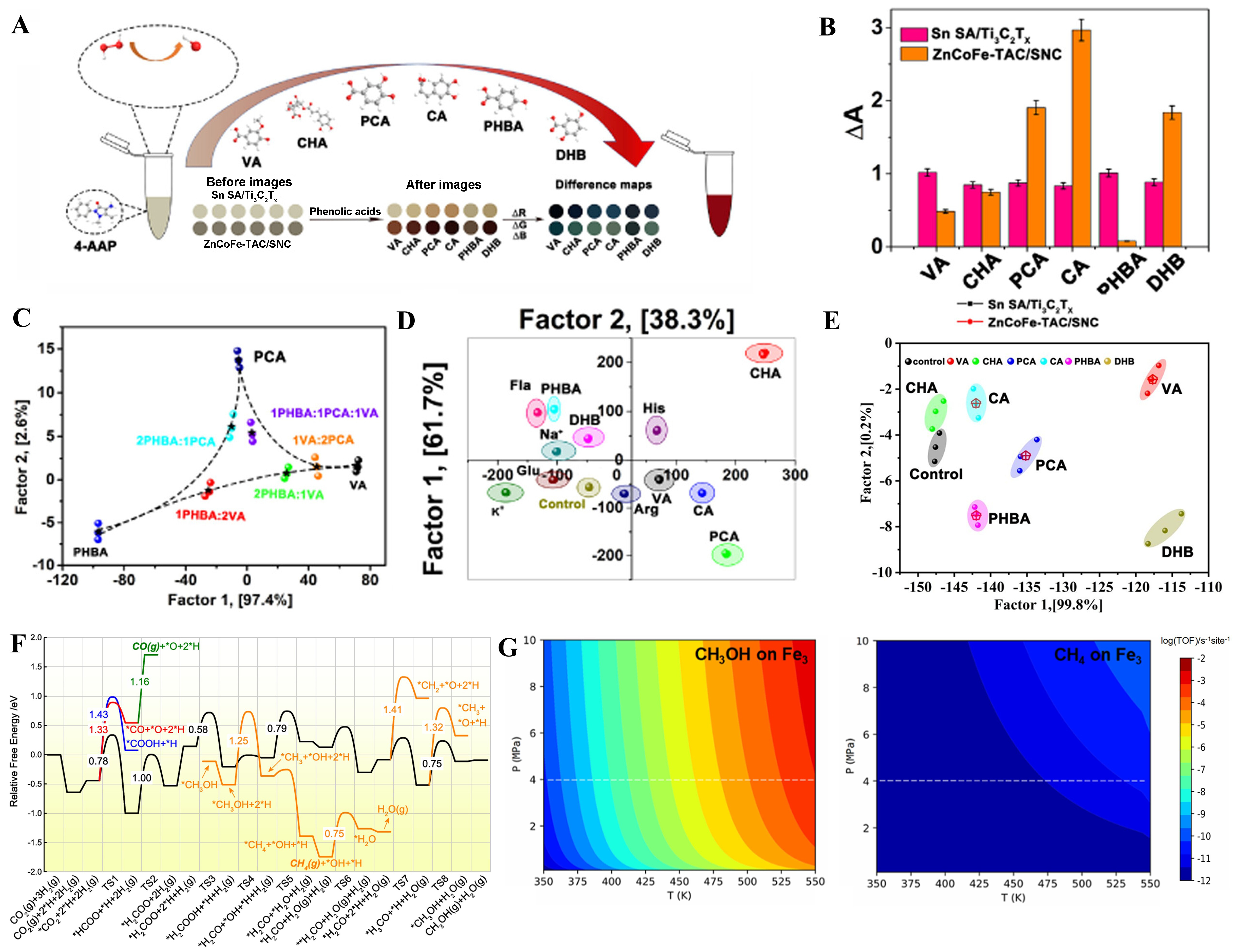
Huang et al. built the mode of the sensor array for discrimination of phenolic acids as we can see in Figure 9A[157]. In Figure 9B, researchers listed the absorbance change of different substances reacting on two catalysts. The FeCoZn/SNC triple-atom catalyst showed a high discrimination at a pure environment [Figure 9C]. And the researchers found that FeCoZn/SNC was able to distinguish correctly even under the influence of higher concentrations of interfering substances and serum environment [Figure 9D and E]. Wang et al. investigated the path and activation energy of CO2 hydrogenation reaction at 523 K on
Figure 9. (A) The mode of the sensor array for discrimination of phenolic acids based on the two nanozymes; (B) Response pattern; (C) LDA scores plot for identifing phenolic acid mixtures; (D) LDA scores plot for iidentifing phenolic acids against interfering substances and the sample without adding any analyte; (E) LDA scores plot for discriminating six phenolic acids at 0.1, 1, and 10 mM in the presence of serum[157], Copyright 2023, American Chemical Society; (F) Reaction mechanism and relative activation energy of CO2 hydrogenation reaction on Fe3/MoS2-v; (G) TOF maps of products at Fe3/MoS2-v[91], Copyright 2023, American Chemical Society. LDA: Linear discriminant analysis; TOF: turnover frequency.
The development of TACs represents another significant advance in the history of catalyst development. TACs play a crucial role in energy and energy storage, among other areas. In the synthesis of TACs, the most common methods include pyrolysis, adsorption, in-situ construction, cage encapsulation and separation-reduction methods, etc.[158,159]. Each of them has its own advantages and disadvantages. For instance, the pyrolysis method decomposes the precursor at high temperature (which generates metal active sites and has the advantages of mature process, controllable structure and high stability), but high temperature is prone to cause aggregation of metal atomic clusters or collapse of the precursor framework, resulting in loss of active sites[160]. The adsorption method anchors metal precursors on the surface of the carrier, which can achieve atomic-level dispersion and is operationally mild[161,162]. However, it is sensitive to the surface properties of the carrier, has limited loading capacity, and its stability is often restricted by subsequent reaction conditions. In contrast, the in-situ construction method dynamically regulates the combination of metal atoms and the carrier through reactions such as electrochemical deposition, which can precisely construct active sites and adapt to complex reaction environments[163]. However, the operation is complex and the cost is relatively high, and it is not suitable for industrial production. For these, we have made a detailed summary in Table 1.
Synthesis and application of TACs
| Applications | Metal | TACs | Ref. | Synthetic method | Performance |
| ORR | Ru | Ru3/CN | [34] | Encapsulation-pyrolysis | Conversion of 2-amino benzyl alcohol up to 100% |
| ORR | Co Mn | Co2MnN8/C | [40] | Adsorption-pyrolysis | Half-wave potential of 0.912 V |
| ORR | Zn Co Fe | ZnCoFe-TAC/SNC | [43] | Wet chemical method | TAC based battery at the max power density of 304 mW·cm-2 |
| ORR | Co Fe | Co2/Fe-N@CHC | [164] | Cage separation-pyrolysis | TAC based battery at max power density of 232.4 mW·cm-2 |
| ORR | Zn Co Fe | ZnCoFe-N-C | [73] | One-step pyrolysis | TAC-based battery capacity at liquid state: 931.8 Wh·kgZn-1, power density (liquid state:137.8 mW·cm-2) |
| ORR | Fe | Fe3-N-C | [36] | Encapsulation-pyrolysis | Half-wave potential of 0.891 V |
| ORR | Fe | FeACs/NPS-HC | [39] | Encapsulation-pyrolysis | A max power density of 172.4 mW·cm-2 |
| CO2RR | Cu | N, OH-Cu3 | [44] | In situ reconstruction | Faradaic efficiency of 74.2% for the CO2 reduction to methane at current density of 300 mA·cm-2 |
| Hydrogenation | Ni Cu | Ni1Cu2/g-C3N4 | [22] | Atom-atomic synthesis | 100% conversion at 170 °C and ethylene selectivity was as high as 90% |
| Hydrogenation | Ru | Ru3@ZIF-8 | [37] | Cage-controlled encap-sulation and reduction | The TOF of Ru3@ZIF-8 achieves 360 h-1 with the styrene selectivity being 97% |
| Degradation | Fe | Fe3-N-C | [165] | Package pyrolysis | Pseudo-first-order kinetics with reaction rate constant of 0.034 s-1 for the activation of PMS |
| NRR | Cu | Cu(111) | [98,166] | Surface reconstruction | Ammonia yield of 2.16 mg·mgcat-1·h-1, max efficiency of 81.1% at -0.5 V |
| NRR | Pd Au Cu | PdAuCuNPs | [167] | One-pot method | The turnover rates of NPs are 3.353 min-1·$-1 |
| Enzymatic reaction | Fe Co Zn | FeCoZn-TAC/SNC | [157] | Adsorption-pyrolysis | Clustering separation of six different phenolic acids at concentrations of 0.1 μM to 1 mM |
| Enzymatic reaction | Co Fe Cu | CoFeCu hydroxides | [104] | Wet chemical method | 3.69 × 10-8 M·s-1 toward TMB with 2.22 mM and limits of 0.9, and 2.96 μM for nitrite |
| Enzymatic reaction | Co Fe Ce | CoFeCe hydroxide | [105] | Wet chemical method | Limit of detection of 0.092 μM for GSH detection |
CONCLUSION AND OUTLOOK
The growing urgency of environmental and energy challenges demands significant progress in green renewable energy technologies. Among emerging solutions, electrocatalytic conversion of emissions from human activities has attracted global attention. A key factor in optimizing these conversions is catalyst selection, which significantly affects reaction efficiency and kinetics. Catalysts play an integral role in accelerating reaction rates and promoting efficient substrate transformation. TACs, characterized by their abundant active sites, hold great promise for energy conversion and material synthesis applications. Their exceptional catalytic performance stems from several key attributes: (1) ultra-high atomic utilization; (2) unique atomic configurations; (3) synergistic effects arising from the interaction between the carrier and metal atoms; and (4) interatomic interactions within the metals.
This review provides a detailed review of the carriers and applications of TACs, and conducts an analysis. Although significant advancements have been made in TACs development, challenges persist in areas such as increasing the atomic load rate and promoting industrial production. One primary challenge is product selectivity. Though TACs often exhibit remarkable selectivity for specific products, they may also produce a diverse array of by-products. Addressing this issue requires meticulous adjustments to reaction conditions, a deeper understanding of reaction pathways, and the implementation of more effective separation techniques. Moreover, the preparation of TACs poses significant challenges due to the high surface energy of metal atoms, which leads to aggregation and hinders achieving high metal loading rates. Ongoing research must focus on developing innovative preparation methods to mitigate surface energy issues and enhance loading efficiency.
In terms of support selection, current supports mainly consist of carbon-based materials, metals, metal derivatives, MOFs and other similar compounds. With the increasing focus on green chemistry and environmental sustainability, it is necessary to give priority to the use of safe, non-toxic and cost-effective supports. In addition, factors such as the number of anchoring sites, preparation methods, support structures and their synergistic effects with metal atoms should also be taken into account. Enhancing the interaction between metal sites and carriers can stabilize the catalysts and prevent aggregation. Selecting supports with high functional anchoring sites and optimizing synthesis methods can effectively increase the loading rate while maintaining catalyst performance.
Characterization techniques for atomic catalysts differ significantly from those employed for conventional materials. Predominantly, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are utilized to investigate surface morphology and microstructure at the atomic and nanoscale levels. Techniques such as X-ray absorption spectroscopy (XAS), X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD) provide insights into the elemental composition, distribution, and valence states of the materials. X-ray absorption fine structure (XAFS) is a powerful tool based on synchrotron radiation for studying the local atomic or electronic structure of materials. It is most widely applied in the structural analysis of atomic catalysts. Furthermore, through the use of aberration-corrected electron microscopy, there are also some in-situ characterization techniques. These techniques are mutually supportive with theoretical calculations and experimental verifications. Despite the contributions of existing characterization methods to the advancement of TACs, challenges related to accuracy, mechanistic understanding, and the high costs of current technologies pose significant barriers. Therefore, the development of new, cost-effective, and precise characterization tools remains crucial.
In conclusion, the progression of TACs presents a formidable challenge that necessitates a systematic and rational strategy. Researchers must prioritize the development of catalysts that are not only economically viable but also demonstrate long-term stability. Integrating simulations with advanced material characterization techniques allows for a comprehensive exploration of the complex reaction mechanisms. As for the synthesis process of TACs, more meticulous research is required to seek for substrates and precursors with better performance, more suitable carriers, and to establish a clear structure-activity relationship. These are all very worthy research topics at present.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study and performed the whole work writing: Chen, W.; Shang, H.; Gao, Y.; Jiang, S.
Revised and directed parts of the writing manuscript: Chen, W.; Shang, H.; Liu, K.; Gao, Y.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Grant Nos. 22201262 to Shang, H. and 22375019 to Chen, W.), Natural Science Foundation of Henan (252300421175) and University Natural Science Research Project of Anhui Province (Grant No. 2024AH040186 to Gao, Y.).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Liang, X.; Fu, N.; Yao, S.; Li, Z.; Li, Y. The progress and outlook of metal single-atom-site catalysis. J. Am. Chem. Soc. 2022, 144, 18155-74.
2. Liu, Y.; Guo, C.; Wu, G.; et al. Uniformly dispersed bismuth metal nano catalyst modified carbon cloth electrode for iron-chromium flow battery. Nano. Res. Energy. 2025, 4, e9120135.
3. Talib, S. H.; Jiang, X.; Feng, S.; Zhao, M.; Yu, Q. Theoretical catalytic performance of single-atom catalysts M1/PW12O40 for alkyne hydrogenation materials. Nano. Res. Energy. 2024, 3, e9120128.
4. Li, J.; Zhang, L.; Doyle-Davis, K.; Li, R.; Sun, X. Recent advances and strategies in the stabilization of single-atom catalysts for electrochemical applications. Carbon. Energy. 2020, 2, 488-520.
5. Da, Y.; Jiang, R.; Tian, Z.; Han, X.; Chen, W.; Hu, W. The applications of single-atom alloys in electrocatalysis: progress and challenges. SmartMat 2023, 4, e1136.
6. Wei, Z.; Zhu, Y.; Liu, J.; et al. Recent advance in single-atom catalysis. Rare. Met. 2021, 40, 767-89.
7. Zheng, X.; Li, P.; Dou, S.; et al. Non-carbon-supported single-atom site catalysts for electrocatalysis. Energy. Environ. Sci. 2021, 14, 2809-58.
8. Li, Z.; Chen, Y.; Ji, S.; et al. Iridium single-atom catalyst on nitrogen-doped carbon for formic acid oxidation synthesized using a general host-guest strategy. Nat. Chem. 2020, 12, 764-72.
9. Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical synthesis of single atomic site catalysts. Chem. Rev. 2020, 120, 11900-55.
10. Yu, X.; Wang, D.; Peng, Q.; Li, Y. High performance electrocatalyst: Pt-Cu hollow nanocrystals. Chem. Commun. 2011, 47, 8094-6.
11. Liang, G.; Yang, S.; Wu, C.; et al. Advancing C–C coupling of the electrocatalytic CO2 reduction reaction for C2+ products. J. Mater. Chem. A. 2025, 13, 11210-35.
12. Zhang, X.; Zhang, X.; Wang, X.; Cui, G.; Pan, H.; Sun, W. Engineering spin states of metal sites toward advanced lithium–sulfur batteries. Energy. Environ. Sci. 2025, 18, 3553-67.
13. Guo, Q.; Kong, F.; Yu, X.; et al. An inter-atomic synergistic Co–Zn diatomic catalyst for efficient H2O2 electrosynthesis in neutral and alkaline media. Green. Chem. 2025, 27, 3032-43.
14. Yang, Q.; Liu, H.; Yuan, P.; et al. Single carbon vacancy traps atomic platinum for hydrogen evolution catalysis. J. Am. Chem. Soc. 2022, 144, 2171-8.
15. Tang, C.; Jiao, Y.; Shi, B.; et al. Coordination tunes selectivity: two-electron oxygen reduction on high-loading molybdenum single-atom catalysts. Angew. Chem. Int. Ed. Engl. 2020, 59, 9171-6.
16. Qu, Z.; He, G.; Zhang, T.; et al. Tricoordinated single-atom cobalt in zeolite boosting propane dehydrogenation. J. Am. Chem. Soc. 2024, 146, 8939-48.
17. Pan, F.; Zhang, H.; Liu, K.; et al. Unveiling active sites of CO2 reduction on nitrogen-coordinated and atomically dispersed iron and cobalt catalysts. ACS. Catal. 2018, 8, 3116-22.
18. Zhou, X.; Tamtaji, M.; Zhou, W.; Goddard, W. A. 3rd.; Chen, G. Nonprecious triple-atom catalysts with ultrahigh activity for electrochemical reduction of nitrate to ammonia: a DFT screening. ACS. Appl. Mater. Interfaces. 2025, 17, 4854-64.
19. Zhang, J.; Song, Z.; Yao, X.; et al. Precisely constructing asymmetric triple atoms for highly efficient electrocatalysis. Chem. 2025, In Press.
20. Li, Y.; Liu, Y.; Guo, M.; et al. Advances in atomically dispersed catalysts for water splitting. Adv. Funct. Mater. 2025, 35, 2425056.
21. Chen, Z.; Chen, L. X.; Yang, C. C.; Jiang, Q. Atomic (single, double, and triple atoms) catalysis: frontiers, opportunities, and challenges. J. Mater. Chem. A. 2019, 7, 3492-515.
22. Gu, J.; Jian, M.; Huang, L.; et al. Synergizing metal-support interactions and spatial confinement boosts dynamics of atomic nickel for hydrogenations. Nat. Nanotechnol. 2021, 16, 1141-9.
23. Pei, W.; Zhou, S.; Zhao, J.; Xu, X.; Du, Y.; Dou, S. X. Immobilized trimeric metal clusters: a family of the smallest catalysts for selective CO2 reduction toward multi-carbon products. Nano. Energy. 2020, 76, 105049.
24. Gao, Q.; Pillai, H. S.; Huang, Y.; et al. Breaking adsorption-energy scaling limitations of electrocatalytic nitrate reduction on intermetallic CuPd nanocubes by machine-learned insights. Nat. Commun. 2022, 13, 2338.
25. Liu, J.; Xu, H.; Zhu, J.; Cheng, D. Understanding the pathway switch of the oxygen reduction reaction from single- to double-/triple-atom catalysts: a dual channel for electron acceptance-backdonation. JACS. Au. 2023, 3, 3031-44.
26. Cui, C.; Zhang, H.; Cheng, R.; Huang, B.; Luo, Z. On the nature of three-atom metal cluster catalysis for N2 reduction to ammonia. ACS. Catal. 2022, 12, 14964-75.
27. Zhang, D.; Gong, L.; Ma, J.; Wang, X.; Zhang, L.; Xia, Z. Disperse multimetal atom-doped carbon as efficient bifunctional electrocatalysts for oxygen reduction and evolution reactions: design strategies. J. Phys. Chem. C. 2020, 124, 27387-95.
28. Chen, S.; Zhang, T.; Han, J.; et al. Interface engineering of Fe-Sn-Co sulfide/oxyhydroxide heterostructural electrocatalyst for synergistic water splitting. Nano. Res. Energy. 2024, 3, e9120106.
29. Zhang, F.; Gong, L.; Liu, M.; et al. Nature of C-C coupling and strategy of tuning the catalytic activity of Cu-N-C catalysts for electro-reduction of CO2 to ethanol. Nano. Energy. 2024, 127, 109699.
30. Yu, Z.; Gu, M.; Wang, Y.; Li, H.; Chen, Y.; Wei, L. Recent progress of electrochemical nitrate reduction to ammonia on copper-based catalysts: from nanoparticles to single atoms. Adv. Energy. Sustain. Res. 2024, 5, 2300284.
31. Liu, C.; Liu, J. Computational study on graphdiyne supported PdxCuy clusters as potential catalysts for formic acid dehydrogenation. Int. J. Hydrogen. Energy. 2024, 79, 248-57.
32. Guo, Q.; Yuan, R.; Zhao, Y.; Yu, Y.; Fu, J.; Cao, L. Performance of nitrogen-doped carbon nanoparticles carrying FeNiCu as bifunctional electrocatalyst for rechargeable zinc-air battery. Small 2024, 20, e2400830.
33. Li, H.; Wang, P.; Zhu, C.; et al. Triple-helical self-assembly of atomically precise nanoclusters. J. Am. Chem. Soc. 2022, 144, 23205-13.
34. Ji, S.; Chen, Y.; Fu, Q.; et al. Confined pyrolysis within metal-organic frameworks to form uniform Ru3 clusters for efficient oxidation of alcohols. J. Am. Chem. Soc. 2017, 139, 9795-8.
35. Liu, J. C.; Ma, X. L.; Li, Y.; Wang, Y. G.; Xiao, H.; Li, J. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 2018, 9, 1610.
36. Ye, W.; Chen, S.; Lin, Y.; et al. Precisely tuning the number of Fe atoms in clusters on N-doped carbon toward acidic oxygen reduction reaction. Chem 2019, 5, 2865-78.
37. Ji, S.; Chen, Y.; Zhao, S.; et al. Atomically dispersed ruthenium species inside metal-organic frameworks: combining the high activity of atomic sites and the molecular sieving effect of MOFs. Angew. Chem. Int. Ed. Engl. 2019, 58, 4271-5.
38. Dasgupta, A.; He, H.; Gong, R.; et al. Atomic control of active-site ensembles in ordered alloys to enhance hydrogenation selectivity. Nat. Chem. 2022, 14, 523-9.
39. Guo, X.; Shi, J.; Li, M.; et al. Modulating coordination of iron atom clusters on N,P,S triply-doped hollow carbon support towards enhanced electrocatalytic oxygen reduction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202314124.
40. Yan, X.; Liu, D.; Guo, P.; et al. Atomically dispersed Co2MnN8 triatomic sites anchored in N-doped carbon enabling efficient oxygen reduction reaction. Adv. Mater. 2023, 35, e2210975.
41. Kumari, S.; Alexandrova, A. N.; Sautet, P. Nature of zirconia on a copper inverse catalyst under CO2 hydrogenation conditions. J. Am. Chem. Soc. 2023, 145, 26350-62.
42. Liu, J.; Xiao, H.; Zhao, X.; et al. Computational prediction of graphdiyne-supported three-atom single-cluster catalysts. CCS. Chem. 2023, 5, 152-63.
43. Chen, C.; Chai, J.; Sun, M.; et al. An asymmetrically coordinated ZnCoFe hetero-trimetallic atom catalyst enhances the electrocatalytic oxygen reaction. Energy. Environ. Sci. 2024, 17, 2298-308.
44. Pan, F.; Fang, L.; Li, B.; et al. N and OH-immobilized Cu3 clusters in situ reconstructed from single-metal sites for efficient CO2 electromethanation in bicontinuous mesochannels. J. Am. Chem. Soc. 2024, 146, 1423-34.
45. Cai, G.; Lv, H.; Zhang, G.; et al. A volcano correlation between catalytic activity for sulfur reduction reaction and Fe atom count in metal center. J. Am. Chem. Soc. 2024, 146, 13055-65.
46. Ma, C.; Feng, J.; Xia, C.; et al. Theoretical insights into multi-metal atoms embedded nitrogen-doped graphene as efficient bifunctional catalysts for oxygen reduction and evolution reactions. Appl. Surf. Sci. 2022, 605, 154714.
47. Wang, H.; Li, J.; Zhu, H. Ultimate structures in catalysis: single atoms, subnano-clusters, and electrons. Sci. China. Mater. 2023, 66, 4521-41.
48. Jiao, L.; Jiang, H. Metal-organic frameworks for catalysis: fundamentals and future prospects. Chin. J. Catal. 2023, 45, 1-5.
49. Wu, R.; Sun, M.; Liu, X.; et al. Oxidase-like ZnCoFe three-atom nanozyme as a colorimetric platform for ascorbic acid sensing. Anal. Chem. 2022, 94, 14308-16.
50. Xing, D.; Xu, C.; Wang, Y.; Li, J. Heterogeneous single-cluster catalysts for selective semihydrogenation of acetylene with graphdiyne-supported triatomic clusters. J. Phys. Chem. C. 2019, 123, 10494-500.
51. Pei, W.; Hou, L.; Yu, X.; et al. Graphitic carbon nitride supported trimeric metal clusters as electrocatalysts for N2 reduction reaction. J. Catal. 2024, 429, 115232.
52. Qiao, F. Photoelectrocatalytic hydrogen production: hydrogen production principle, performance optimization strategy, application and prospect. Nano. Res. Energy. 2025, 4, e9120132.
53. Li, J.; Chen, C.; Xu, L.; et al. Challenges and perspectives of single-atom-based catalysts for electrochemical reactions. JACS. Au. 2023, 3, 736-55.
54. Li, Z.; Wang, H.; Gao, Y. Computational study of tri-atomic catalyst-loaded two-dimensional graphenylene for overall water splitting. Catalysts 2025, 15, 296.
55. Su, J.; Musgrave, C. B.; Song, Y.; et al. Strain enhances the activity of molecular electrocatalysts via carbon nanotube supports. Nat. Catal. 2023, 6, 818-28.
56. Gao, Z.; Li, A.; Liu, X.; et al. Shielding Pt/γ-Mo2N by inert nano-overlays enables stable H2 production. Nature 2025, 638, 690-6.
57. Gates, B. C.; Katz, A.; Liu, J. Nested metal catalysts: metal atoms and clusters stabilized by confinement with accessibility on supports. Precis. Chem. 2023, 1, 3-13.
58. Deng, Z.; Liu, Y.; Lin, J.; Chen, W. Rational design and energy catalytic application of high-loading single-atom catalysts. Rare. Met. 2024, 43, 4844-66.
59. Deng, Z.; Guo, Y.; Sun, Z.; Lin, J.; Zhai, H.; Chen, W. Electrocatalytic organic transformation reactions in green chemistry: exploring nanocrystals and single atom catalysts. Nano. Res. 2024, 17, 9326-44.
60. Chen, C.; Sun, M.; Wang, K.; Li, Y. Dual-metal single-atomic catalyst: the challenge in synthesis, characterization, and mechanistic investigation for electrocatalysis. SmartMat 2022, 3, 533-64.
61. Wei, J.; Tang, H.; Sheng, L.; et al. Site-specific metal-support interaction to switch the activity of Ir single atoms for oxygen evolution reaction. Nat. Commun. 2024, 15, 559.
62. Ma, Y.; Liu, X.; Tang, M.; et al. Waste eggshell-derived N, P, S tri-doped core-shell catalysts for efficient Fenton-like catalysis. Chem. Eng. J. 2022, 440, 135879.
63. Yang, N.; Li, L.; Li, J.; Ding, W.; Wei, Z. Modulating the oxygen reduction activity of heteroatom-doped carbon catalysts via the triple effect: charge, spin density and ligand effect. Chem. Sci. 2018, 9, 5795-804.
64. Li, S.; E, J.; Zhao, X.; et al. Hetero-trimetallic atom catalysts enable targeted ROS generation and redox signaling for intensive apoptosis and ferroptosis. Adv. Mater. 2025, 37, e2417198.
65. Yuan, L.; Li, X.; Li, G.; et al. Gram-scale preparation of tri-coordinated single-atom catalysts for CO2 electrolysis in large-scale membrane electrode assembly. Adv. Sci. 2025, 12, e2500368.
66. Gao, T.; Li, X.; Chen, X.; et al. Ultra-fast preparing carbon nanotube-supported trimetallic Ni, Ru, Fe heterostructures as robust bifunctional electrocatalysts for overall water splitting. Chem. Eng. J. 2021, 424, 130416.
67. Zhao, X.; Li, W. P.; Cao, Y.; et al. Dual-atom Co/Ni electrocatalyst anchored at the surface-modified Ti3C2Tx MXene enables efficient hydrogen and oxygen evolution reactions. ACS. Nano. 2024, 18, 4256-68.
68. Wang, Y.; Li, C.; Han, X.; et al. General negative pressure annealing approach for creating ultra-high-loading single atom catalyst libraries. Nat. Commun. 2024, 15, 5675.
69. Huang, J.; Hu, S.; Liu, M.; et al. Single-, double-, and triple-atom catalysts on PC6 for nitrate reduction to ammonia: a computational screening. Electrochim. Acta. 2024, 504, 144915.
70. Wang, S.; Zhao, T.; Yan, L. Tailoring of three-atom metal cluster catalysts for ammonia synthesis. Catalysts 2023, 13, 869.
71. Liu, Z.; Ma, A.; Wang, Z.; et al. Single-cluster anchored on PC6 monolayer as high-performance electrocatalyst for carbon dioxide reduction reaction: first principles study. J. Colloid. Interface. Sci. 2024, 669, 600-11.
72. Shi, X.; Li, Y.; Zhang, S.; et al. Precious trimetallic single-cluster catalysts for oxygen and hydrogen electrocatalytic reactions: theoretical considerations. Nano. Res. 2023, 16, 8042-50.
73. Lin, X.; Li, Q.; Hu, Y.; et al. Revealing atomic configuration and synergistic interaction of single-atom-based Zn-Co-Fe trimetallic sites for enhancing oxygen reduction and evolution reactions. Small 2023, 19, e2300612.
74. Wang, W.; Zheng, Y.; Hu, Y.; Liu, Y.; Chen, S. Intrinsic carbon defects for the electrosynthesis of H2O2. J. Phys. Chem. Lett. 2022, 13, 8914-20.
75. Zhu, J.; Mu, S. Defect engineering in carbon-based electrocatalysts: insight into intrinsic carbon defects. Adv. Funct. Mater. 2020, 30, 2001097.
76. Tsai, J.; Hong, W.; Pourzolfaghar, H.; Wang, W.; Li, Y. A Fe-Ni-Zn triple single-atom catalyst for efficient oxygen reduction and oxygen evolution reaction in rechargeable Zn-air batteries. Chem. Eng. J. 2023, 460, 141868.
77. Jin, H.; Ha, M.; Kim, M. G.; Lee, J. H.; Kim, K. S. Engineering Pt coordination environment with atomically dispersed transition metal sites toward superior hydrogen evolution. Adv. Energy. Mater. 2023, 13, 2204213.
78. Zhang, J. P.; Zhang, Y. B.; Lin, J. B.; Chen, X. M. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001-33.
79. Jia, J.; Li, Z.; Sang, Z.; et al. High-throughput design of single-atom catalysts with nonplanar and triple pyrrole-N coordination for highly efficient H2O2 electrosynthesis. Angew. Chem. Int. Ed. Engl. 2025, 64, e202421864.
80. Liu, L.; Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981-5079.
81. Holby, E. F.; Taylor, C. D. Activity of N-coordinated multi-metal-atom active site structures for Pt-free oxygen reduction reaction catalysis: role of *OH ligands. Sci. Rep. 2015, 5, 9286.
82. Cui, D.; Li, Y.; Pan, K.; et al. NO hydrogenation to NH3 over FeCu/TiO2 catalyst with improved activity. Front. Chem. Sci. Eng. 2023, 17, 1973-85.
83. Xiao, J.; Liu, Z.; Wang, X.; Li, F.; Zhao, Z. Homonuclear multi-atom catalysts for CO2 electroreduction: a comparison density functional theory study with their single-atom counterparts. J. Mater. Chem. A. 2023, 11, 25662-70.
84. Li, F.; Tian, Y.; Su, S.; et al. Theoretical and experimental exploration of tri-metallic organic frameworks (t-MOFs) for efficient electrocatalytic oxygen evolution reaction. Appl. Catal. B. Environ. 2021, 299, 120665.
85. Li, Y.; Zuo, S.; Wu, X.; et al. Design of hybrid zeolitic imidazolate framework-derived material with C-Mo-S triatomic coordination for electrochemical oxygen reduction. Small 2021, 17, e2003256.
86. Sementa, L.; Barcaro, G.; Fortunelli, A. Analogy between homogeneous and heterogeneous catalysis by subnanometer metal clusters: ethylene oxidation on Ag trimers supported on MgO(1 0 0). Inorg. Chim. Acta. 2015, 431, 150-5.
87. Wang, Y.; Zhang, Y.; Ma, N.; et al. High-selectivity CO2-to-CH4 electrochemical reduction on copper trimer: a theoretical insight. Surf. Interfaces. 2024, 50, 104498.
88. Zhang, S.; Chen, M.; Zhao, X.; et al. Advanced noncarbon materials as catalyst supports and non-noble electrocatalysts for fuel cells and metal–air batteries. Electrochem. Energ. Rev. 2021, 4, 336-81.
89. Yang, M.; Cui, Z.; DiSalvo, F. J. Mesoporous chromium nitride as a high performance non-carbon support for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2013, 15, 7041-4.
90. Chen, S.; Gao, Y.; Wang, W.; Prezhdo, O. V.; Xu, L. Prediction of three-metal cluster catalysts on two-dimensional W2N3 support with integrated descriptors for electrocatalytic nitrogen reduction. ACS. Nano. 2023, 2, 1522-32.
91. Wang, G.; Jiang, X.; Jiang, Y.; Wang, Y.; Li, J. Screened Fe3 and Ru3 single-cluster catalysts anchored on MoS2 supports for selective hydrogenation of CO2. ACS. Catal. 2023, 13, 8413-22.
92. Kim, D.; Kley, C. S.; Li, Y.; Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2-C3 products. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 10560-5.
93. Kuhl, K. P.; Cave, E. R.; Abram, D. N.; Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy. Environ. Sci. 2012, 5, 7050.
94. Li, L.; Hasan, I. M. U.; Farwa,
95. Chu, S.; Kang, C.; Park, W.; et al. Single atom and defect engineering of CuO for efficient electrochemical reduction of CO2 to C2H4. SmartMat 2022, 3, 194-205.
96. Xiao, C.; Zhang, J. Architectural design for enhanced C2 product selectivity in electrochemical CO2 reduction using Cu-based catalysts: a review. ACS. Nano. 2021, 15, 7975-8000.
97. Liu, S.; Wang, Y.; Norwood, S. Discovering effective descriptors for CO2 electroreduction to predict the catalysts with different selectivity. J. Phys. Chem. C. 2021, 125, 4550-8.
98. Wu, K.; Sun, C.; Wang, Z.; et al. Surface reconstruction on uniform Cu nanodisks boosted electrochemical nitrate reduction to ammonia. ACS. Mater. Lett. 2022, 4, 650-6.
99. Chen, J.; Shakouri, M.; Alizadeh, M.; et al. A catalytic sites contiguity study on atomically-dispersed ZnO-Cu/SiO2 catalysts to improve methanol formation from CO2 hydrogenation. Can. J. Chem. Eng. 2025.
100. Fu, P.; Chen, C.; Wu, C.; et al. Covalent organic framework stabilized single CoN4Cl2 site boosts photocatalytic CO2 reduction into tunable syngas. Angew. Chem. Int. Ed. Engl. 2025, 64, e202415202.
101. Chen, Y.; Zhuo, H.; Pan, Y.; Liang, J.; Liu, C.; Li, J. Triazine COF-supported single-atom catalyst (Pd1/trzn-COF) for CO oxidation. Sci. China. Mater. 2021, 64, 1939-51.
102. Dong, C.; Li, Y.; Cheng, D.; et al. Supported metal clusters: fabrication and application in heterogeneous catalysis. ACS. Catal. 2020, 10, 11011-45.
103. Chu, M.; Tu, W.; Zhuang, Z.; et al. Efficient polyolefin upcycling over single-atom alloy catalyst. CCS. Chem. 2025, 7, 2451-64.
104. Chen, Q.; Yang, X.; Mao, T.; Xiao, J. Spatially confining copper atom in flowerlike-CoFe hydroxides for smartphone-based ratiometric colorimetric dual-mode detection of nitrite. Microchem. J. 2024, 201, 110578.
105. Shang, H.; Zhang, X.; Ding, M.; Zhang, A.; Wang, C. A smartphone-assisted colorimetric and photothermal probe for glutathione detection based on enhanced oxidase-mimic CoFeCe three-atom nanozyme in food. Food. Chem. 2023, 423, 136296.
106. Tang, T.; Bai, X.; Wang, Z.; Guan, J. Structural engineering of atomic catalysts for electrocatalysis. Chem. Sci. 2024, 15, 5082-112.
107. Yan, Y.; Chen, J.; Wang, Z.; et al. Novel flexible aromatic Cu3 metal-organic π-cluster for electrocatalytic CO2 reduction reaction. Surf. Interfaces. 2024, 48, 104349.
108. Ouyang, Y.; Shi, L.; Bai, X.; Li, Q.; Wang, J. Breaking scaling relations for efficient CO2 electrochemical reduction through dual-atom catalysts. Chem. Sci. 2020, 11, 1807-13.
109. Gandionco, K. A.; Kim, J.; Bekaert, L.; Hubin, A.; Lim, J. Single-atom catalysts for the electrochemical reduction of carbon dioxide into hydrocarbons and oxygenates. Carbon. Energy. 2024, 6, e410.
110. Xie, Z.; Hwang, S.; Chen, J. G. Reduction-induced metal/oxide interfacial sites for selective CO2 hydrogenation. SmartMat 2023, 4, e1201.
111. Wang, J.; Zheng, X.; Wang, G.; et al. Defective bimetallic selenides for selective CO2 electroreduction to CO. Adv. Mater. 2022, 34, e2106354.
112. Tamtaji, M.; Kwon, S.; Musgrave, C. B. 3rd.; Goddard, W. A. 3rd.; Chen, G. Reaction Mechanism of rapid CO electroreduction to propylene and cyclopropane (C3+) over triple atom catalysts. ACS. Appl. Mater. Interfaces. 2024, 16, 50567-75.
113. Zhao, Z. H.; Zheng, K.; Huang, N. Y.; et al. A Cu(111)@metal-organic framework as a tandem catalyst for highly selective CO2 electroreduction to C2H4. Chem. Commun. 2021, 57, 12764-7.
114. Chen, J.; Wang, T.; Li, Z.; et al. Recent progress and perspective of electrochemical CO2 reduction towards C2-C5 products over non-precious metal heterogeneous electrocatalysts. Nano. Res. 2021, 14, 3188-207.
115. Liu, S.; Yin, Y.; Yang, J.; et al. Temperature-dependent pathways in carbon dioxide electroreduction. Sci. Bull. 2025, 70, 889-96.
116. Liu, J.; Li, P.; Jia, S.; et al. Electrocatalytic CO2 hydrogenation to C2+ alcohols catalysed by Pr–Cu oxide heterointerfaces. Nat. Synth. 2025, 4, 730-43.
117. Gawande, M. B.; Goswami, A.; Felpin, F. X.; et al. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722-811.
118. Jiang, K.; Cai, W. Disentangling distinct Cu surface sites for electrocatalytic CO2-to-CO and CO-to-C2+ conversion. Sci. China. Chem. 2024, 67, 1047-8.
119. Bagger, A.; Ju, W.; Varela, A. S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 reduction: a classification problem. Chemphyschem 2017, 18, 3266-73.
120. Liu, M.; Liu, S.; Xu, Q.; et al. Dual atomic catalysts from COF-derived carbon for CO2 RR by suppressing HER through synergistic effects. Carbon. Energy. 2023, 5, e300.
121. Wang, D.; Chen, Z.; Wu, Y.; et al. Structurally ordered high-entropy intermetallic nanoparticles with enhanced C–C bond cleavage for ethanol oxidation. SmartMat 2023, 4, e1117.
122. Yang, X.; Li, Q.; Chi, S.; Li, H.; Huang, Y.; Cao, R. Hydrophobic perfluoroalkane modified metal-organic frameworks for the enhanced electrocatalytic reduction of CO2. SmartMat 2022, 3, 163-72.
123. Zhou, S.; Cao, S.; Wei, S.; et al. Triple-atom catalysts 3TM-GYs (TM = Cu, Fe, and Co; GY = graphyne) for high-performance CO2 reduction reaction to C1 products. Appl. Mater. Today. 2021, 25, 101245.
124. Todorova, T. K.; Schreiber, M. W.; Fontecave, M. Mechanistic understanding of CO2 reduction reaction (CO2RR) toward multicarbon products by heterogeneous copper-based catalysts. ACS. Catal. 2020, 10, 1754-68.
125. Zhan, C.; Dattila, F.; Rettenmaier, C.; et al. Key intermediates and Cu active sites for CO2 electroreduction to ethylene and ethanol. Nat. Energy. 2024, 9, 1485-96.
126. Banerjee, S.; Gerke, C. S.; Thoi, V. S. Guiding CO2RR Selectivity by compositional tuning in the electrochemical double layer. Acc. Chem. Res. 2022, 55, 504-15.
127. Iqbal, M. S.; Yao, Z.; Ruan, Y.; et al. Single-atom catalysts for electrochemical N2 reduction to NH3. Rare. Met. 2023, 42, 1075-97.
128. He, C.; Zhang, Y.; Wang, J.; Fu, L. Anchor single atom in h-BN assist NO synthesis NH3: a computational view. Rare. Met. 2022, 41, 3456-65.
129. Zhang, Y.; Li, S.; Zheng, W.; Wang, X. Computational design of catalysts for ammonia synthesis. Nano. Res. Energy. 2023, 2, e9120068.
130. Yang, X.; Nash, J.; Anibal, J.; et al. Mechanistic insights into electrochemical nitrogen reduction reaction on vanadium nitride nanoparticles. J. Am. Chem. Soc. 2018, 140, 13387-91.
131. Macfarlane, D. R.; Cherepanov, P. V.; Choi, J.; et al. A roadmap to the ammonia economy. Joule 2020, 4, 1186-205.
132. Lin, L.; Wei, F.; Jiang, R.; Huang, Y.; Lin, S. The role of central heteroatom in electrochemical nitrogen reduction catalyzed by polyoxometalate-supported single-atom catalyst. Nano. Res. 2023, 16, 309-17.
133. Guo, H.; Zhang, P.; Huang, S.; et al. Achilles’ heel of single atom catalysts towards practical PEMFC application: degradation mechanisms and regulatory strategies. Nano. Res. Energy. 2025, 4, e9120144.
134. Wang, L.; Xia, M.; Wang, H.; et al. Greening ammonia toward the solar ammonia refinery. Joule 2018, 2, 1055-74.
135. Cheng, R.; Cui, C. N.; Luo, Z. X. Reduction of dinitrogen to ammonia on doped three-atom clusters Nb2M (M = Sc to Cu & Y to Ag). Rare. Met. 2024, 43, 3810-8.
136. Smith, C.; Hill, A. K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy. Environ. Sci. 2020, 13, 331-44.
137. Li, M.; Xie, Y.; Song, J.; Yang, J.; Dong, J.; Li, J. Ammonia electrosynthesis on carbon-supported metal single-atom catalysts. Chin. J. Catal. 2024, 60, 42-67.
138. Kerpal, C.; Harding, D. J.; Lyon, J. T.; Meijer, G.; Fielicke, A. N2 activation by neutral ruthenium clusters. J. Phys. Chem. C. 2013, 117, 12153-8.
139. Ghoshal, S.; Pramanik, A.; Sarkar, P. Towards H2O catalyzed N2-fixation over TiO2 doped Run clusters (n = 5, 6): a mechanistic and kinetic approach. Phys. Chem. Chem. Phys. 2021, 23, 1527-38.
140. Chen, Z. W.; Chen, L. X.; Jiang, M.; et al. A triple atom catalyst with ultrahigh loading potential for nitrogen electrochemical reduction. J. Mater. Chem. A. 2020, 8, 15086-93.
141. Yang, L.; Feng, S.; Zhu, W. Achieving reaction pathway separation for electrochemical nitrate fixation on triatomic catalysts: a new mechanism. J. Hazard. Mater. 2023, 441, 129972.
142. Fu, X.; Zhang, J.; Kang, Y. Recent advances and challenges of electrochemical ammonia synthesis. Chem. Catal. 2022, 2, 2590-613.
143. Liu, Y.; Zhang, H.; Jiang, Y.; et al. Sulfonic acid-functionalized spiropyran colorimetric gas-sensitive aerogel for real-time visual ammonia sensing. Chem. Eng. J. 2025, 511, 162160.
144. Andersen, S. Z.; Čolić, V.; Yang, S.; et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 2019, 570, 504-8.
145. Kong, D.; Moon, P. J.; Lui, E. K. J.; Bsharat, O.; Lundgren, R. J. Direct reversible decarboxylation from stable organic acids in dimethylformamide solution. Science 2020, 369, 557-61.
146. Liu, X.; Wang, P.; Zhang, Q.; et al. Synthesis of MoS2/Ni3S2 heterostructure for efficient electrocatalytic hydrogen evolution reaction through optimizing the sulfur sources selection. Appl. Surf. Sci. 2018, 459, 422-9.
147. An, Q.; Bo, S.; Jiang, J.; et al. Atomic-level interface engineering for boosting oxygen electrocatalysis performance of single-atom catalysts: from metal active center to the first coordination sphere. Adv. Sci. 2023, 10, e2205031.
148. Lu, Z.; Wang, B.; Hu, Y.; et al. An isolated zinc-cobalt atomic pair for highly active and durable oxygen reduction. Angew. Chem. Int. Ed. Engl. 2019, 58, 2622-6.
149. Pang, M.; Yang, M.; Zhang, H.; et al. Synthesis techniques, mechanism, and prospects of high-loading single-atom catalysts for oxygen reduction reactions. Nano. Res. 2024, 17, 9371-96.
150. Gong, H.; Liang, X.; Sun, G.; et al. Insight into role of Ni/Fe existing forms in reversible oxygen catalysis based on Ni-Fe single-atom/nanoparticles and N-doped carbon. Rare. Met. 2022, 41, 4034-40.
151. Xu, H.; Huang, C.; Shuai, T.; et al. Noble metal-free N-doped carbon-based electrocatalysts for air electrode of rechargeable zinc-air battery. Sci. China. Mater. 2023, 66, 2953-3003.
152. Qin, S.; Li, K.; Cao, M.; et al. Fe-Co-Ni ternary single-atom electrocatalyst and stable quasi-solid-electrolyte enabling high-efficiency zinc-air batteries. Nano. Res. Energy. 2024, 3, e9120122.
153. Yin, Z.; Li, Y.; Ye, Y.; et al. Sp/sp2 carbon ratio-driven high-throughput screening of electrocatalytic nitrogen reduction performance on transition metal single-atom catalysts. Rare. Met. 2024, 43, 5781-91.
154. Xie, K.; Xu, K.; Liu, M.; Song, X.; Xu, S.; Si, H. Catalysts for selective hydrogenation of acetylene: a review. Mater. Today. Catal. 2023, 3, 100029.
155. Han, B.; Zhang, R. Unraveling the role of active site Rh aggregation form in tuning the catalytic performance of ethane direct dehydrogenation. Mol. Catal. 2023, 547, 113397.
156. Cai, S.; Zhang, W.; Yang, R. Emerging single-atom nanozymes for catalytic biomedical uses. Nano. Res. 2023, 16, 13056-76.
157. Huang, J.; Gu, H.; Wang, G.; Wu, R.; Sun, M.; Chen, Z. Visual sensor arrays for distinction of phenolic acids based on two single-atom nanozymes. Anal. Chem. 2023, 95, 9107-15.
158. Li, M.; Wu, T.; Wang, H.; et al. Atomic insights into dynamic integration of morphology and surface chemistry on the growth mechanism of ternary Pt-based nanocatalysts. Cell. Rep. Phys. Sci. 2025, 6, 102396.
159. Pu, T.; Ding, J.; Zhang, F.; et al. Dual atom catalysts for energy and environmental applications. Angew. Chem. Int. Ed. Engl. 2023, 62, e202305964.
160. Wang, G.; Zhang, M.; Zhang, G.; et al. Novel approach of diffusion-controlled sequential reduction to synthesize dual-atomic-site alloy for enhanced bifunctional electrocatalysis in acidic and alkaline media. Adv. Funct. Mater. 2024, 34, 2308876.
161. Zhao, L.; Zhang, Y.; Huang, L. B.; et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10, 1278.
162. Babucci, M.; Sarac Oztuna, F. E.; Debefve, L. M.; et al. Atomically dispersed reduced graphene aerogel-supported iridium catalyst with an iridium loading of 14.8 wt %. ACS. Catal. 2019, 9, 9905-13.
163. Yin, S.; Yi, H.; Liu, M.; et al. An in situ exploration of how Fe/N/C oxygen reduction catalysts evolve during synthesis under pyrolytic conditions. Nat. Commun. 2024, 15, 6229.
164. Wang, Z.; Jin, X.; Zhu, C.; et al. Atomically dispersed Co2-N6 and Fe-N4 costructures boost oxygen reduction reaction in both alkaline and acidic media. Adv. Mater. 2021, 33, e2104718.
165. Li, M.; Chen, J.; Wu, W.; Wu, S.; Xu, L.; Dong, S. Diatomic Fe-Fe catalyst enhances the ability to degrade organic contaminants by nonradical peroxymonosulfate activation system. Nano. Res. 2023, 16, 4678-84.
166. Xu, Y.; Xie, M.; Zhong, H.; Cao, Y.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].