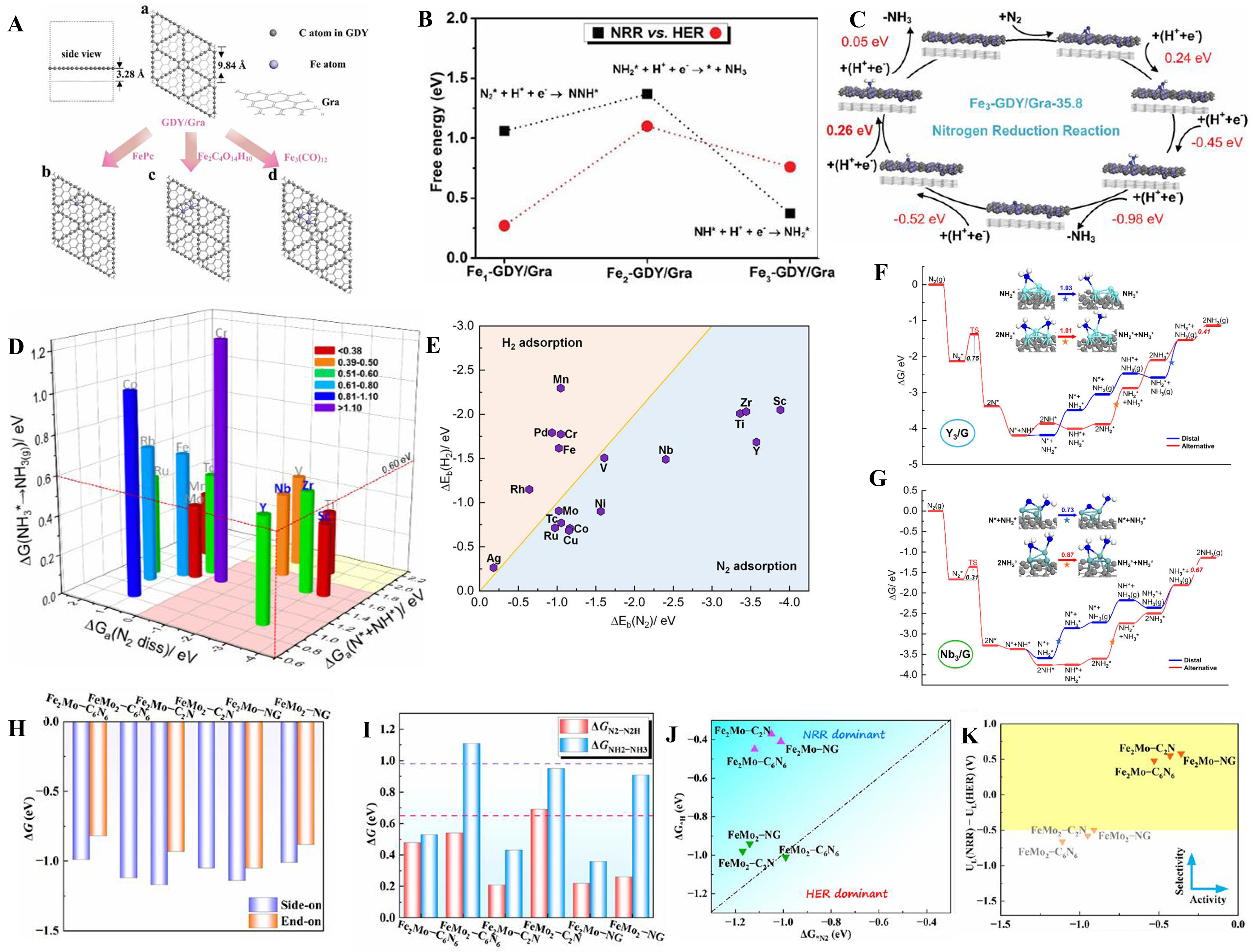
fig7
Figure 7. (A) The synthetic strategy of Fex-GDY/Gra; (B) Comparisons of free energies of PLS for the NRR and HER on Fex-GDY/Gra; (C) Reaction path and free energy of Fe3-GDY/Gra with respect to NRR[140], Copyright 2020 The Royal Society of Chemistry; (D) The free energy of transition state for N2 dissociation, hydrogenation barrier of *N + *NH formation, and desorption energy of NH3 on14 three-atom metal clusters; (E) Binding energy of M3 metal clusters on N2 and H2; (F) Free energy diagram of NRR reaction on Y3/G; (G) Free energy diagram of NRR reaction on Nb3/G[26], Copyright 2022, American Chemical Society; (H) Two modes of N2 adsorption free energy on FexMoy-CNs; (I) Preliminary and final reaction free energies on different FexMoy-CNs; (J) Reaction free energy tendency model on different FexMoy-CNs; (K) The diagram of UL(NRR)−UL(HER) vs. UL(NRR) relationship and the colored area shows better performance[70]. PLS: Potential-limiting step; NRR: N2 reduction reaction; HER: hydrogen evolution reaction.