Reductive O-formylation of carbon dioxide and alcohols over porous phenanthroline-based polymer supported single iridium atom catalyst
Abstract
The robust O-formylation of alcohols using carbon dioxide to produce valuable alkyl formats is a green method for achieving carbon capture and utilization. However, developing a highly efficient heterogeneous catalyst with outstanding stability remains a significant challenge. Herein, we report a porous phenanthroline-based polymer-supported single-iridium-atom catalyst (Ir/POP-Phen) for the O-formylation of various alcohols using carbon dioxide and molecular hydrogen. This catalyst demonstrates superior catalytic activity and substrate compatibility compared to previous homogeneous and heterogeneous systems. In the synthesis of bulk methyl formate, the turnover number and turnover frequency reach up to 138,216 and 2,880 h-1, respectively. Additionally, other types of alcohols are successfully converted into their corresponding alkyl formates. Notably, the Ir/POP-Phen catalyst exhibits high tolerance to water concentrations of up to 4,000 ppm during the O-formylation process and can be reused for four cycles without a significant decline in catalytic activity. This work offers insights into the rational design of heterogeneous catalysts for the O-formylation of alcohols.
Keywords
INTRODUCTION
Since the industrial revolution, anthropogenic carbon dioxide (CO2) concentrations have risen dramatically from 250 to 415 ppm due to the extensive use of fossil fuels, contributing to global warming, sea-level rise, and extreme weather events[1,2]. Despite its role as a greenhouse gas, CO2 represents a nontoxic, abundant, and renewable carbon feedstock. Carbon capture and utilization (CCU) has thus emerged as a critical strategy for mitigating CO2 emissions while supporting sustainable development in a carbon-constrained economy[3-6]. In particular, the catalytic conversion of CO2 into value-added bulk and fine chemicals has garnered significant scientific and industrial interest[7-11].
Alkyl formates, versatile intermediates in C1 chemistry, are widely used as refrigerants, insecticides, solvents, and platform chemicals for synthesizing formamide, formic acid, and hydrogen energy carriers[12-15]. The green synthesis of alkyl formates via O-formylation of alcohols with CO2 and H2 offers an atom-economical and sustainable pathway, enabling integration with renewable energy systems[16]. Although homogeneous catalysts such as RuCl2(dppe)2 achieve impressive turnover numbers (TONs up to 12,900) and frequencies (TOFs up to 830 h-1) under mild conditions [Supplementary Table 1], their reliance on expensive noble metals, non-recyclable ligands, and poor stability limits practical applications[17-21]. Heterogeneous catalysts, while addressing recyclability, suffer from inferior activity, metal leaching, and stability issues [Supplementary Table 2][22-26]. Additional challenges, including the use of NaBH4 as a reductant[27], supercritical CO2[12,22,26], high pressures, carbonates instead of CO2 and H2 as the substrate[28], and limited alcohol substrate scope[29], further hinder its scalability. Thus, developing efficient, stable, and reusable heterogeneous catalysts for O-formylation under environmentally benign conditions remains imperative.
Porous organic polymers (POPs) have recently gained traction as heterogeneous catalytic platforms due to their high surface areas, tunable pore architectures, thermal stability, and tailored functional groups. These properties facilitate the modest adsorption, diffusion, enrichment of chemical molecules and their induced electronic effects during the reaction process[30,31]. Metal-functionalized POPs have demonstrated exceptional performance in CO2 transformations, including hydroformylation[32], O-/N-formylation[33-36], methanol and formic acid synthesis[37-39], and cyclic carbonate production[40,41]. These materials combine the precise control of active metal centers - akin to homogeneous organometallic complexes - with the benefits of heterogeneous systems, such as facile catalyst recovery and recyclability, thereby achieving enhanced reaction performance. For O-formylation, Sun et al. developed phosphine-based Ru-POP catalysts[34], but susceptibility to phosphine oxidation under operational conditions presents a critical limitation. In contrast, nitrogen-ligand-based POPs offer superior oxygen-resistance stability.
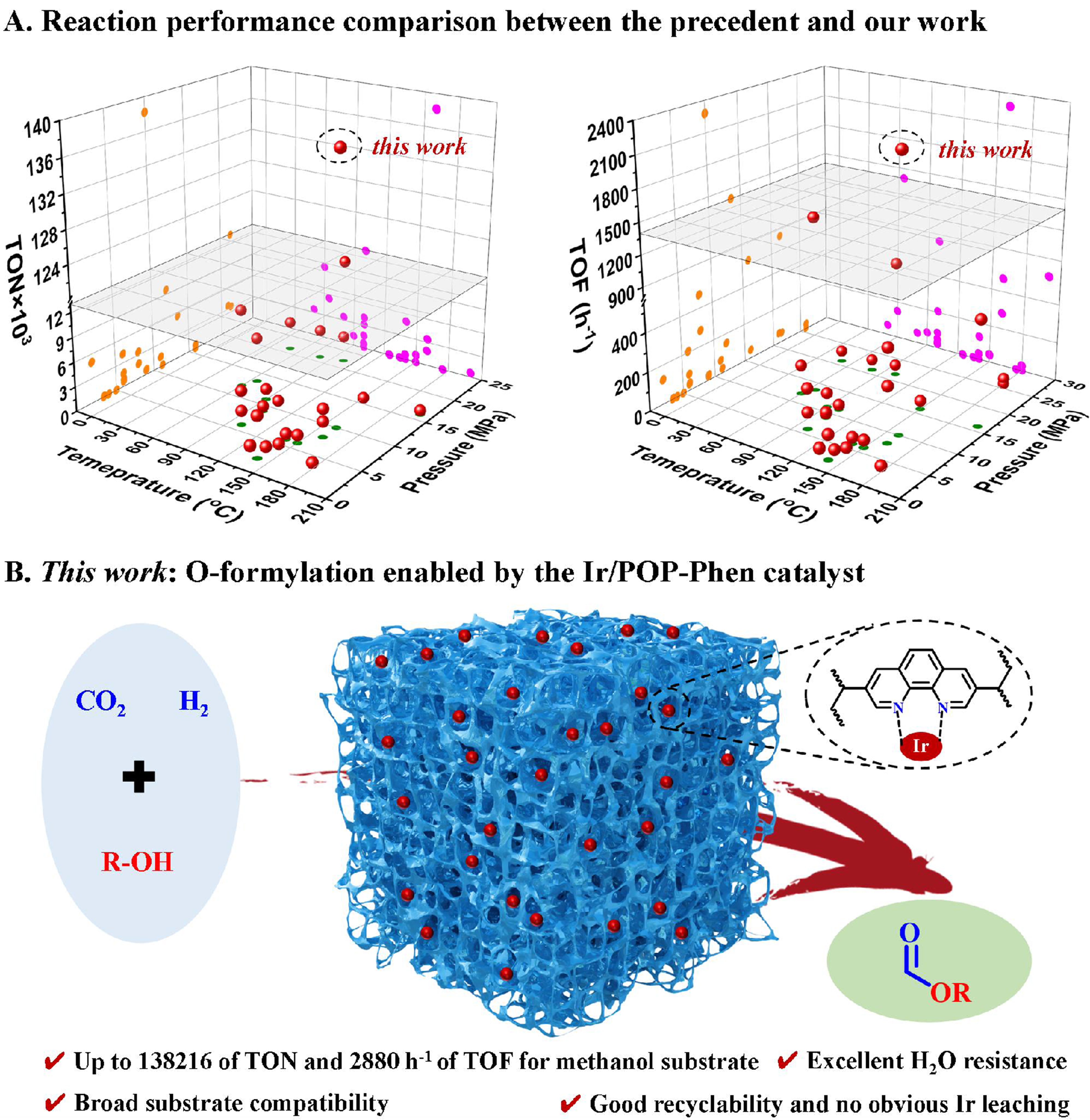
Building on these aforementioned challenges and our long-standing interests in CO2 valorization and POPs design[42,43], we report a nitrogen-rich phenanthroline-based porous polymer-supported single-atom iridium catalyst (Ir/POP-Phen) for the O-formylation of diverse alcohols with CO2 and H2. This system achieves superior catalytic activity and substrate compatibility, surpassing the precedent work in both homogeneous and heterogeneous catalysis [Figure 1A]. For methyl formate synthesis, TONs of 138,216 and TOFs of
Figure 1. (A) 3D plots based on reaction temperature, reaction pressure [P(CO2+H2)], and associated TON or TOF for various reported homo- and heterogeneous catalysts applied for O-formylation of methanol (see Supplementary Tables 1 and 2 for details); (B) O-formylation of alcohols catalyzed by the Ir/POP-Phen catalyst. TON: Turnover number; TOF: turnover frequency; Ir/POP-Phen: porous phenanthroline-based polymer-supported single-iridium-atom catalyst.
EXPERIMENTAL
Preparation of the POP-Phen
First, 0.80 g of 3,8-divinyl-1,10-phenanthroline (2v-Phen) was dissolved in 8 mL of dimethylformamide (DMF), followed by the addition of 20 mg of 2,2′-azobis(2-methylpropionitrile) (AIBN). The mixture was transferred into an autoclave, stirred at room temperature for 0.5 h, and then heated to 100 oC for 24 h without stirring. After the polymerization was finished, the resulting solid was filtered, washed with tetrahydrofuran (THF, 20 mL × 3) and Et2O (20 mL × 3), and dried under vacuum at room temperature. Finally, the POP-Phen was obtained as a yellow solid.
Preparation of the M/POP-Phen
As a typical run, 0.16 g of POP-Phen was swelled in 3 mL of DMF for 1 h, followed by dropwise adding the Ir solution (32 mg of IrCl3·3H2O dissolved in 3 mL of DMF) under vigorous stirring. The mixture continued to stir at room temperature for 24 h. After the immobilization of Ir was finished, the resulting solid was filtered, washed with THF (20 mL × 3) and Et2O (20 mL × 3), and dried under vacuum at room temperature. Finally, the Ir/POP-Phen was obtained as a brown solid. Other kinds of POP-Phen supported metal catalysts were obtained by replacing 32 mg of IrCl3·3H2O with 26 mg of Pd(CH3CN)Cl2, 26 mg of
Gram-scale preparation of the Ir/POP-Phen
First, 1.6 g of POP-Phen was swelled in 30 mL of DMF for 1 h, followed by dropwise adding the Ir solution (320 mg of IrCl3·3H2O dissolved in 30 mL of DMF) under vigorous stirring. The mixture continued to stir at room temperature for 24 h. After the immobilization of Ir was finished, the resulting solid was filtered, washed with THF (200 mL × 3) and Et2O (200 mL × 3), and dried under vacuum at room temperature. Finally, the Ir/POP-Phen was obtained as a brown solid.
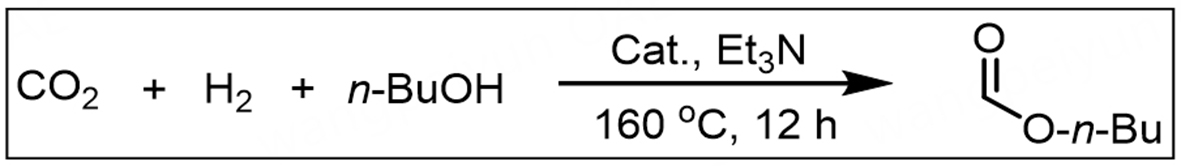
O-formylation of n-BuOH
The as-prepared Ir/POP-Phen catalyst (1.9 mg containing 0.83 µmol of Ir), n-BuOH (48 mL), and Et3N
Recycling tests of the Ir/POP-Phen
The as-prepared Ir/POP-Phen catalyst (38 mg containing 16.6 µmol of Ir), n-BuOH (48 mL), and Et3N
The spent Ir/POP-Phen catalyst was washed with MeOH (8.0 mL × 3) and THF (8.0 mL × 3), and dried at 60 oC under vacuum. Then the spent Ir/POP-Phen catalyst was used for the next run. The Ir contents of the reused catalyst and filtrate after each run were determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES).
More detailed information, including synthesis methods and materials, is presented in the Supplementary Materials.
RESULTS AND DISCUSSION
Catalyst characterization
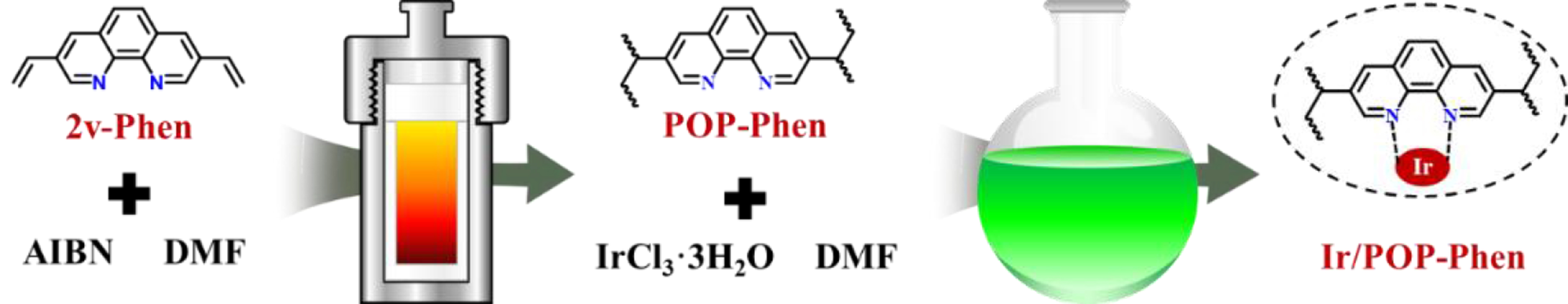
The Ir/POP-Phen catalyst was synthesized as follows [Figure 2]: 2v-Phen underwent hydrothermal polymerization at 100 oC in DMF using AIBN as a radical initiator, yielding the POP-Phen. Subsequently, Ir/POP-Phen was prepared by immobilizing an IrCl3·H2O precursor onto the POP-Phen support via wet impregnation at room temperature. Additional phenanthroline-based polymer-supported metal catalysts were synthesized using analogous protocols [Supplementary Materials].
Figure 2. Schematic diagram for the synthesis of the Ir/POP-Phen catalyst. Ir/POP-Phen: Porous phenanthroline-based polymer-supported single-iridium-atom catalyst.
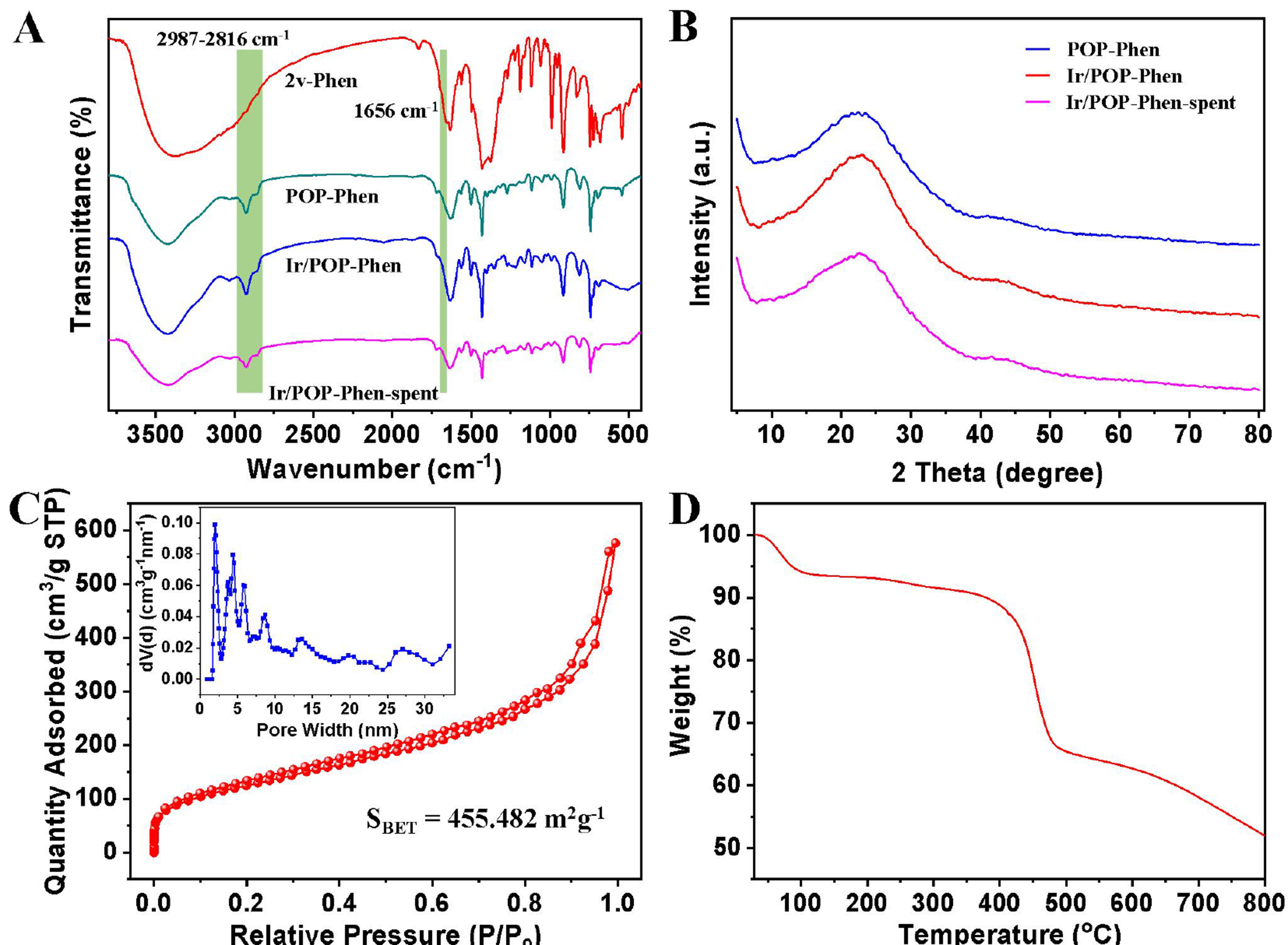
The catalyst’s structure was characterized using multiple techniques. Fourier-transform infrared (FT-IR) spectroscopy [Figure 3A] revealed the disappearance of the vinyl C=C stretching vibration at 1,656 cm-1 in POP-Phen compared to the 2v-Phen monomer, accompanied by new alkyl C–H stretching vibrations at 2,987-2,816 cm-1, confirming successful polymerization. No structural changes were observed in Ir/POP-Phen compared to POP-Phen, indicating that Ir immobilization does not alter the polymer framework. Remarkably, the FT-IR spectrum of the recycled catalyst (Ir/POP-Phen-spent) remained consistent with the fresh catalyst, demonstrating robust chemical stability. Powder X-ray diffraction (PXRD) analysis [Figure 3B] showed no distinct crystalline peaks for POP-Phen or Ir/POP-Phen, confirming the amorphous nature of the polymer and high dispersion of Ir species on the support. The absence of Ir-derived peaks in Ir/POP-Phen-spent further confirmed no metal aggregation during recycling, underscoring the catalyst’s structural integrity.
Figure 3. (A) FT-IR spectra of the 2v-Phen, POP-Phen, Ir/POP-Phen, and Ir/POP-Phen-spent; (B) PXRD patterns of the POP-Phen, Ir/POP-Phen, and Ir/POP-Phen-spent; (C) N2 sorption isotherm, and pore size distribution of the Ir/POP-Phen calculated by NLDFT; (D) TG curve of the Ir/POP-Phen. FT-IR: Fourier-transform infrared; POP-Phen: Ir/POP-Phen: porous phenanthroline-based polymer-supported single-iridium-atom catalyst; NLDFT: nonlocal density functional theory; TG: thermogravimetric.
Nitrogen sorption isotherms were conducted to characterize the structural properties of the POP catalysts
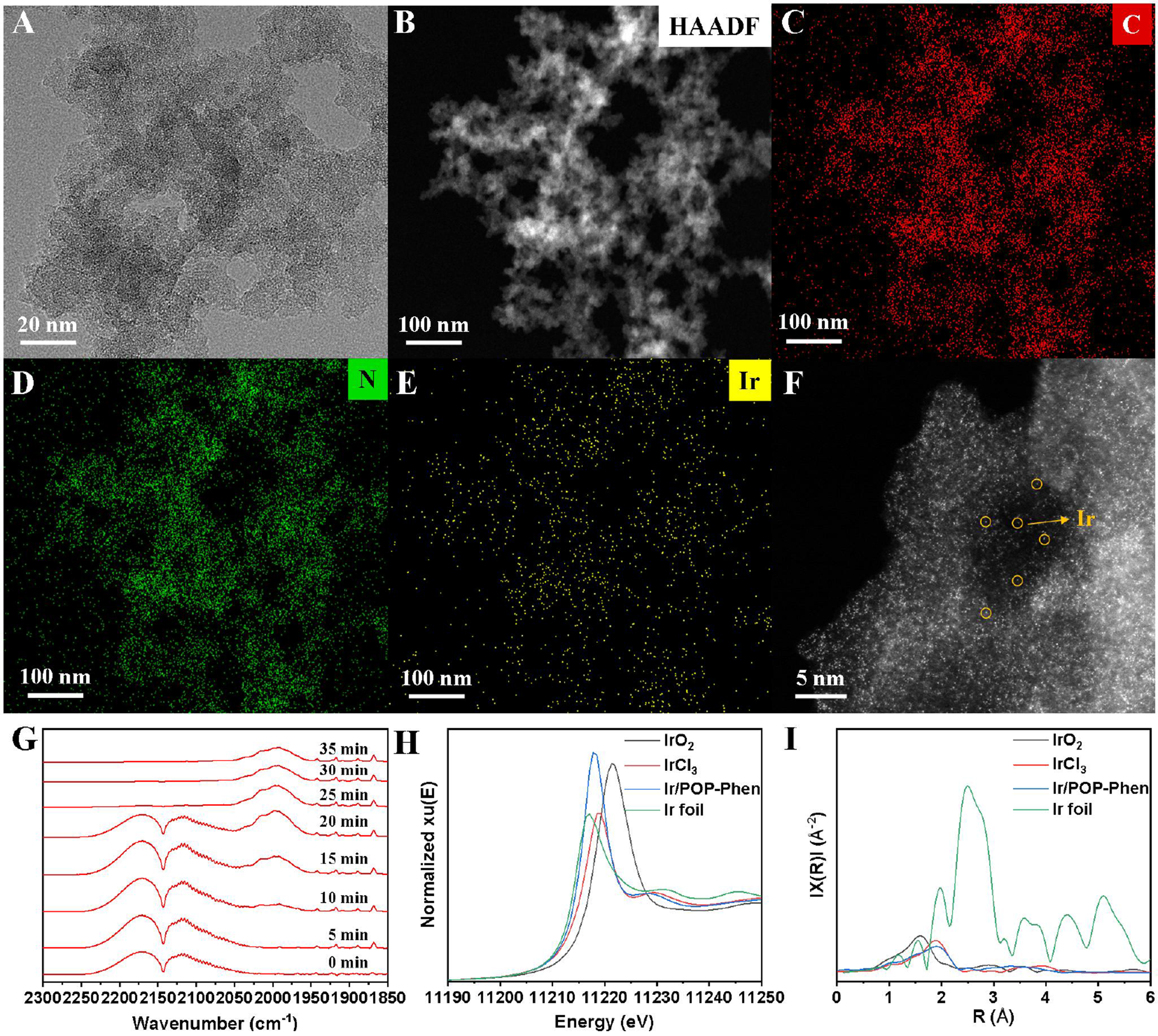
For Ir/POP-Phen, layered structure and spherical morphologies were observed by SEM and TEM images
Figure 4. Structural characterization of the Ir/POP-Phen. (A) HR-TEM images of the Ir/POP-Phen; (B-E) EDX elemental mapping of the Ir/POP-Phen; (F) Abreaction-corrected HAADF-STEM image of the Ir/POP-Phen; (G) In-situ CO absorbed FT-IR spectra of the Ir/POP-Phen; (H) XANES spectra of Ir K-edge; (I) EXAFS Fourier transformed spectra. Ir/POP-Phen: Porous phenanthroline-based polymer-supported single-iridium-atom catalyst; HR-TEM: high-resolution-transmission electron microscopy; EDX: energy-dispersive X-ray; HAADF-STEM: high-angle-annular-dark-field scanning transmission electron microscopy; FT-IR: Fourier-transform infrared; XANES: X-ray absorption near-edge structure; EXAFS: extended X-ray absorption fine structure.
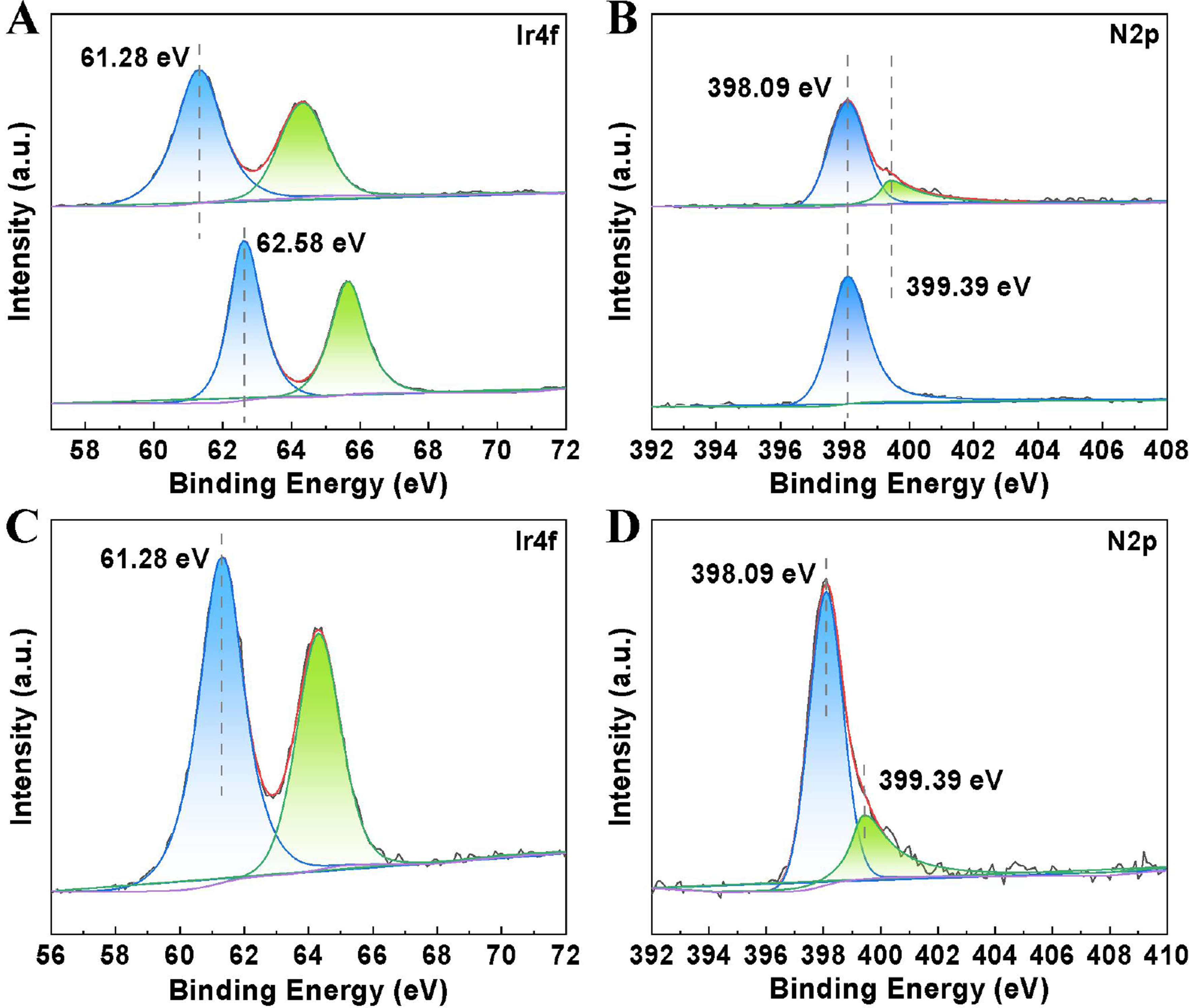
X-ray photoelectron spectroscopy (XPS) was employed to elucidate the chemical state of Ir species and their interaction with the phenanthroline-based polymer [Figure 5]. The Ir 4f binding energy in Ir/POP-Phen appeared at 61.28 eV [Figure 5A], consistent with Ir(III). Notably, this value was 0.3 eV lower than that of the IrCl3·3H2O precursor (62.58 eV), indicating electron donation from phenanthroline nitrogen to Ir and confirming coordination between Ir and the polymer backbone[47]. In addition, compared with the only one peak of N 2p on POP-Phen, the N 2p spectrum of Ir/POP-Phen revealed two distinct peaks at 398.09 and 399.39 eV [Figure 5B], corresponding to free phenanthroline nitrogen and Ir-coordinated phenanthroline nitrogen, respectively. The 1.3 eV upshift in the coordinated nitrogen peak further corroborated electron transfer from nitrogen to Ir, providing direct evidence of metal-ligand interaction. Post-reaction XPS analysis of Ir/POP-Phen-spent showed identical Ir 4f and N 2p profiles to the fresh catalyst [Figure 5C and D], confirming the Ir species retained their +3 oxidation state with no evidence of aggregation during catalytic recycling. This aligns with PXRD and HR-TEM data [Figures 3B and 4A], reinforcing the catalyst’s structural and electronic stability.
Figure 5. Comparison of the XPS spectra. (A) Biding energy comparison of Ir4f between IrCl3·3H2O (bottom) and the Ir/POP-Phen (top); (B) Biding energy comparison of N 2p between POP-Phen (bottom) and the Ir/POP-Phen (top); (C and D) Ir4f and N 2p spectra of the Ir/POP-Phen-spent. XPS: X-ray photoelectron spectroscopy; Ir/POP-Phen: porous phenanthroline-based polymer-supported single-iridium-atom catalyst.
O-formylation application
Optimization of reaction conditions for the O-formylation of n-BuOH with CO2 and H2 was conducted using Ir/POP-Phen as the catalyst. Initial tests with homogeneous IrCl3·3H2O yielded only trace amounts of butyl formate (Table 1, entry 1). Introducing 1,10-phenanthroline (1,10-Phen) as a ligand dramatically enhanced performance, increasing the yield, TON, and TOF to 12.35 mmol, 14,880, and 1,240 h-1, respectively (Table 1, entry 2), attributed to favorable electronic interactions between Ir and 1,10-Phen. To address recyclability of expensive Ir metal and 1,10-Phen ligand, heterogeneous Ir/POP-Phen was synthesized (Table 1, entry 3), achieving superior activity (13.69 mmol yield, 16,494 TON, 1,375 h-1 TOF) due to enhanced mass transfer via hierarchical porosity and CO2 activation by pyridinic nitrogen sites on the polymer catalyst. Comparative studies with other metal-loaded POP-Phen catalysts confirmed Ir’s superiority [Supplementary Table 5]. Reducing Ir/POP-Phen with NaBH4 (Ir/POP-Phen-NaBH4) decreased activity (Table 1, entry 4), likely due to Ir nanoparticle formation and reduced active site accessibility. The in situ encapsulated Ir species within the POP (Ir@POP-Phen) was synthesized via one-pot solvothermal polymerization. When tested in the O-formylation reaction, Ir@POP-Phen achieved a TON of 6,867 and a TOF of 572 h-1 (Table 1, entry 5). Other types of heterogeneous Ir catalysts, prepared by copolymerizing 1,10-Phen with divinylbenzene (DVB) or tris(4-vinylphenyl)amine (p-3vPA), were evaluated (Table 1, entries 6 and 7). However, these systems underperformed compared to the optimized Ir/POP-Phen, attributed to reduced 1,10-Phen ligand density in the polymer matrices. Scaling Ir loading to 11 μmol and extending reaction time to 108 h boosted the yield to 35.5 mmol (Table 1, entry 8). Remarkably, Ir/POP-Phen enabled direct CO2 utilization from industrial flue gas (19.4% CO2), achieving 1.77 mmol yield, 631 TON, and 53 h-1 TOF (Table 1, entry 9).
Optimization of the catalysts for O-formylation of n-BuOHa
 | ||||
| Entry | Cat. | Yield (mmol) | TON | TOF (h-1) |
| 1 | IrCl3·3H2O | 0.32 | 386 | 33 |
| 2 | IrCl3·3H2O + 1,10-Phen | 12.35 | 14,880 | 1,240 |
| 3 | Ir/POP-Phen | 13.69 | 16,494 | 1,375 |
| 4 | Ir/POP-Phen-NaBH4 | 2.51 | 3,019 | 252 |
| 5 | Ir@POP-Phen | 5.70 | 6,867 | 572 |
| 6 | Ir/POP-Phen&DVB | 2.66 | 3,205 | 267 |
| 7 | Ir/POP-Phen&p-3vPA | 2.43 | 2,928 | 245 |
| 8 | Ir/POP-Phenb | 35.50 | 3,243 | 30 |
| 9 | Ir/POP-Phenc | 1.77 | 631 | 53 |
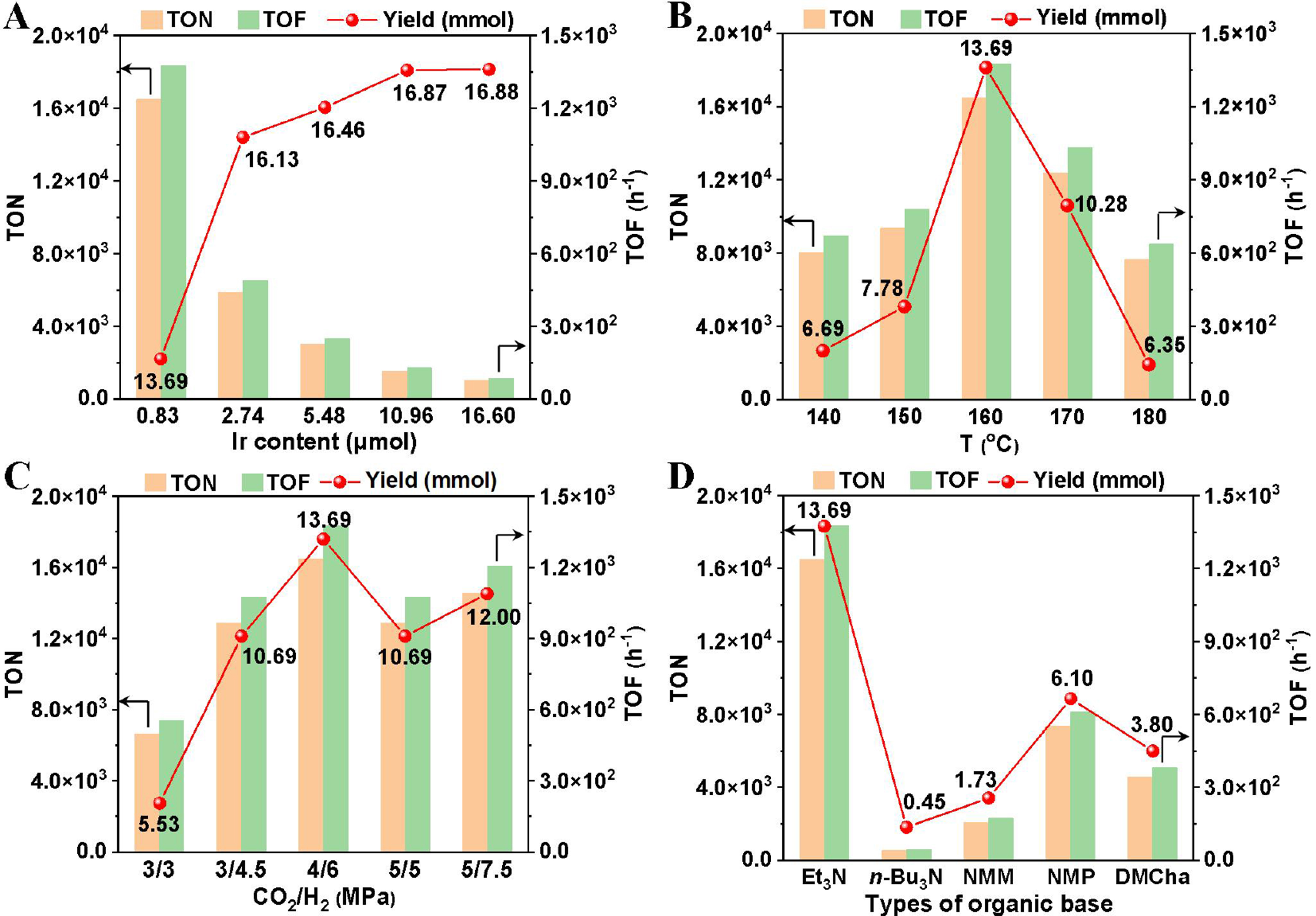
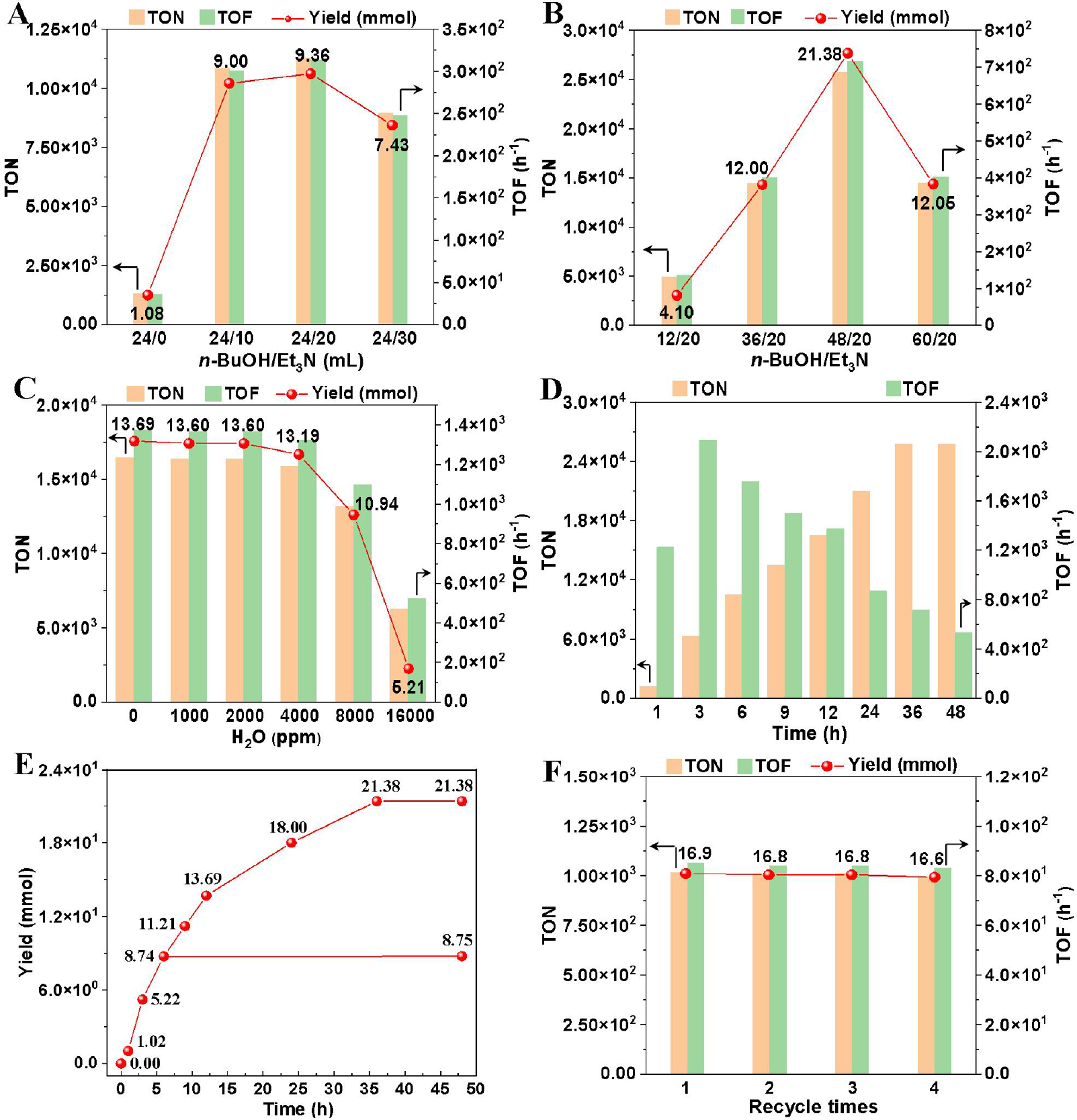
Subsequently, other key reaction parameters for the O-formylation were optimized. Increasing the Ir loading enhanced the butyl formate yield incrementally until it plateaued at 16.60 μmol of Ir [Figure 6A]. However, both TON and TOF declined markedly, indicating a reduced reaction rate at higher Ir loadings. Peak TON and TOF values were achieved at an Ir loading of 0.83 μmol. A volcano-shaped trend emerged with rising reaction temperature (140-180 °C), with 160 °C identified as optimal [Figure 6B]. Systematic evaluation of CO2 and H2 pressures, alongside organic base screening, revealed 4 MPa CO2, 6 MPa H2, and cost-effective Et3N as ideal conditions [Figure 6C and D].
Figure 6. Optimization of the reaction conditions. General reaction conditions: n-BuOH (48 mL, 524 mmol), total pressure (10 MPa) of CO2 and H2 (CO2:H2 = 4:6), Ir dosage (0.83 μmol), Et3N (20 mL), reacted at 160 °C for 12 h. Yield, TON, and TOF were determined by GC analysis using m-xylene as the internal standard. (A) Screening of the Ir dosage of the Ir/POP-Phen; (B) Screening of the reaction temperature; (C) Screening of the pressure of CO2 and H2; (D) Screening of the organic bases. TON: Turnover number; TOF: turnover frequency; GC: gas chromatography; Ir/POP-Phen: porous phenanthroline-based polymer-supported single-iridium-atom catalyst; NMM: 4-methylmorpholine, NMP: 1-methylpyrrolidine, DMCha: N, N-dimethylcyclohexylamine.
Furthermore, the amounts of n-BuOH and Et3N were optimized, identifying 48 mL of n-BuOH and 20 mL of Et3N as the optimal conditions [Figure 7A and B]. The O-formylation reaction produces H2O as a by-product, whose accumulation can perturb the reaction equilibrium and diminish catalytic efficiency[29]. Consequently, evaluating the impact of H2O concentration on performance was critical. Remarkably, the Ir/POP-Phen catalyst exhibited robust activity even at H2O concentrations up to 4,000 ppm, underscoring its exceptional water tolerance [Figure 7C].
Figure 7. Optimization of other reaction parameters and recycle tests of the Ir/POP-Phen. General reaction conditions: n-BuOH (48 mL, 524 mmol), total pressure (10 MPa) of CO2 and H2 (CO2:H2 = 4:6), Ir dosage (0.83 μmol), Et3N (20 mL), reacted at 160 °C for 12 h. Yield, TON, and TOF were determined by GC analysis using m-xylene as the internal standard. (A and B) Screening of the amount of n-BuOH and Et3N; (C) Screening of the amount of H2O added; (D and E) Kinetic study and hot-filtration experiments; (F) Recyclability of the Ir/POP-Phen. Ir/POP-Phen: Porous phenanthroline-based polymer-supported single-iridium-atom catalyst; TON: turnover number; TOF: turnover frequency; GC: gas chromatography.
A kinetic study was conducted to elucidate the relationship between reaction performance and time
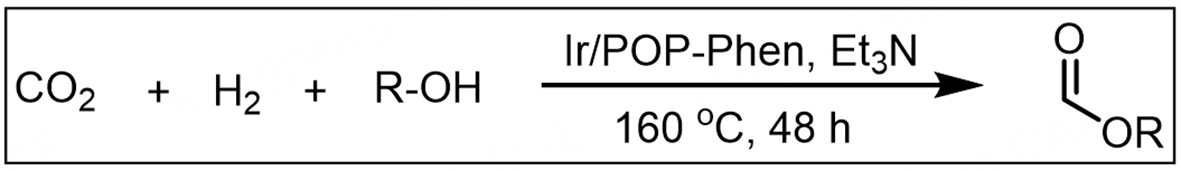
Substrate compatibility was systematically investigated to evaluate the universality of Ir/POP-Phen, addressing a notable gap in prior studies of alcohol scope for O-formylation[14,48]. The catalyst demonstrated exceptional performance in synthesizing bulk industrial chemicals: methyl formate (114.72 mmol yield, TON = 138,216, TOF = 2,880 h-1) and ethyl formate (33.28 mmol yield, TON = 40,096, TOF = 835 h-1) were produced efficiently from O-formylation of methanol and ethanol, respectively (Table 2, entries 1 and 2). Long-chain aliphatic alcohols (Table 2, entries 3-5) and sterically hindered alcohols (Table 2, entries 6 and 7) exhibited reduced activity, likely due to pore size limitations restricting diffusion of bulky molecules. Notably, benzyl formate - a key flavoring agent and solvent in the fragrance industry[49] - was synthesized from O-formylation of benzyl alcohol with high efficiency (Table 2, entry 8), highlighting the catalyst’s versatility.
Substrate scope for O-formylationa
 | ||||
| Entry | Substrate | Yield (mmol) | TON | TOF (h-1) |
| 1 | 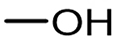 | 114.72 | 138,216 | 2,880 |
| 2 | 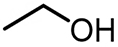 | 33.28 | 40,096 | 835 |
| 3 | 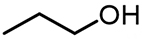 | 17.31 | 20,855 | 434 |
| 4 | 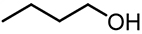 | 21.38 | 25,759 | 536 |
| 5 |  | 6.33 | 7,627 | 159 |
| 6 | 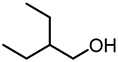 | 7.11 | 8,567 | 179 |
| 7 | 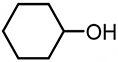 | 5.35 | 6,446 | 135 |
| 8 | 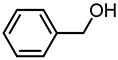 | 5.35 | 6,446 | 135 |
Mechanism discussion
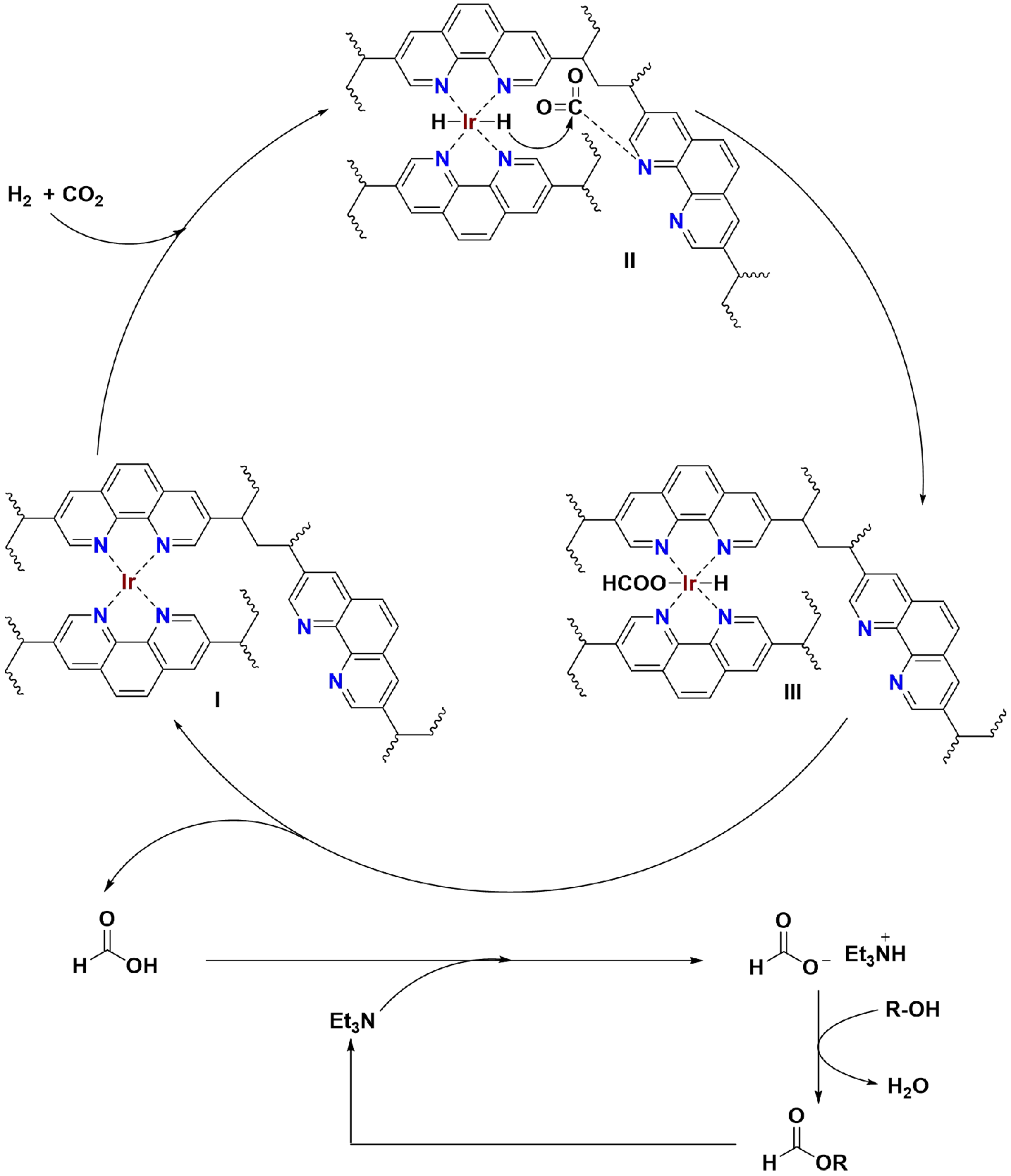
Building on experimental results and prior mechanistic studies[33,34,50,51], a plausible reaction mechanism for alkyl formate synthesis over Ir/POP-Phen is proposed [Figure 8]. The catalytic cycle initiates with H2 dissociation at the phenanthroline-coordinated Ir complex (I), while free phenanthroline sites adsorb and activate CO2. This generates an Ir-hydride intermediate (II), a recognized prerequisite for CO2 hydrogenation. Subsequent CO2 insertion into the Ir–H bond forms a metal-bound formate species (III). Reductive elimination between the Ir-hydride and Ir-formate of III releases formic acid, regenerating the active Ir complex (I). The liberated formic acid is trapped by Et3N, forming a HCOO-·Et3NH+ ion pair, which undergoes esterification with alcohols to yield alkyl formates, releasing Et3N for subsequent cycles.
CONCLUSIONS
In summary, a single-atom iridium catalyst supported on a phenanthroline-based POP (Ir/POP-Phen) was successfully developed for the synthesis of value-added alkyl formates via O-formylation of diverse alcohols using CO2 and H2 as feedstocks. The catalyst demonstrated excellent activity and broad substrate scope, achieving unprecedented TONs (TON = 138,216) and frequencies (TOF = 2,880 h-1) for methyl formate synthesis - surpassing those of previously reported homogeneous and heterogeneous catalytic systems. Notably, Ir/POP-Phen exhibited robust performance even at H2O concentrations up to 4,000 ppm, highlighting its practical potential under industrially relevant conditions. Recycling tests confirmed outstanding stability, with no significant activity loss or Ir leaching (< 0.1 ppm) observed over multiple cycles. Comprehensive characterization (FT-IR, PXRD, N2 sorption, TGA, SEM, TEM, XPS) validated the catalyst’s structural integrity and single-atom Ir coordination. This work advances the rational design of POP-based catalysts for CO2 valorization and provides a viable strategy for enhancing carbon CCU efficiency in chemical manufacturing.
DECLARATIONS
Authors’ contributions
Developed the project: Shi, F.; Cui, X.
Designed and performed most of the experiments and data analysis: Zhao, K.
Participated in part of the experiments: He, D.; Ni, H.; Wang, H.; Liu, C.; Yang, D.; Gao, X.; Zhang, J.; Liu, H.
Supervised the project: Cui, X.; Shi, F.
Prepared a manuscript draft: Zhao, K.
Contributed to the final manuscript: Cui, X.; Shi, F.
Availability of data and materials
The detailed materials and methods in the experiment were listed in the Supplementary Materials. Other raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This study was supported by the National Natural Science Foundation of China (U22A20393, 22372180, 22402212 and 22202216), the Natural Science Foundation of Gansu Province (25JRRA473), the PetroChina Lanzhou Petrochemical Company (LZSH-2025-JS-14), CAS Project for Young Scientists in Basic Research (Grant No. YSBR-050), the Major Project of Gansu Province (21ZD4WA021, 22ZD6GA003 and 23ZDFA016), the Science and Technology Planning Project of Lanzhou City (2023-1-15), and International Partnership Program of Chinese Academy of Sciences (037GJHZ2023010FN).
Conflicts of interest
Gao, X. and Zhang, J. are affiliated with PetroChina Lanzhou Petrochemical Company. Liu, H. is affiliated with PetroChina Lanzhou Chemical Research Center. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Álvarez, A.; Bansode, A.; Urakawa, A.; et al. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 2017, 117, 9804-38.
2. Sun, R.; Liao, Y.; Bai, S.; et al. Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: from nanoscale to single atom. Energy. Environ. Sci. 2021, 14, 1247-85.
3. Mac Dowell, N.; Fennell, P. S.; Shah, N.; Maitland, G. C. The role of CO2 capture and utilization in mitigating climate change. Nature. Clim. Change. 2017, 7, 243-9.
4. Ramirez, A.; Gong, X.; Caglayan, M.; et al. Selectivity descriptors for the direct hydrogenation of CO2 to hydrocarbons during zeolite-mediated bifunctional catalysis. Nat. Commun. 2021, 12, 5914.
5. Li, N.; Zhang, X.; Shi, M.; Zhou, S. The prospects of China’s long-term economic development and CO2 emissions under fossil fuel supply constraints. Resour. Conserv. Recycl. 2017, 121, 11-22.
6. Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: a systematic review of two decades of research from 1995 to 2017. Sci. Total. Environ. 2019, 649, 31-49.
7. Guan, C.; Pan, Y.; Ang, E. P. L.; et al. Conversion of CO2 from air into formate using amines and phosphorus-nitrogen PN3P-Ru(II) pincer complexes. Green. Chem. 2018, 20, 4201-5.
8. Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J. G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem. Rev. 2020, 120, 7984-8034.
9. Liang, H. Q.; Beweries, T.; Francke, R.; Beller, M. Molecular catalysts for the reductive homocoupling of CO2 towards C2+ compounds. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200723.
10. Zhao, S.; Liang, H. Q.; Hu, X. M.; Li, S.; Daasbjerg, K. Challenges and prospects in the catalytic conversion of carbon dioxide to formaldehyde. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204008.
11. Wang, D.; Sun, X.; Yin, H.; Dong, H.; Liu, H.; Zhang, Y. Tuning selectivity of CO2 hydrogenation via support composition modification adjusted the activity reduction of H species over Ce1-xPrxO2-δ-supported metal (Ru, Rh) nanoclusters. ACS. Catal. 2024, 14, 10060-76.
12. Jessop, P. G.; Hsiao, Y.; Ikariya, T.; Noyori, R. Homogeneous catalysis in supercritical fluids: hydrogenation of supercritical carbon dioxide to formic acid, alkyl formates, and formamides. J. Am. Chem. Soc. 1996, 118, 344-55.
13. Federsel, C.; Boddien, A.; Jackstell, R.; et al. A well-defined iron catalyst for the reduction of bicarbonates and carbon dioxide to formates, alkyl formates, and formamides. Angew. Chem. Int. Ed. Engl. 2010, 49, 9777-80.
14. Lemmens, V.; Vanbergen, T.; O’Rourke, G.; Marquez, C.; De Vos, D. E. Cascade catalysis for the hydrogenation of carbon dioxide to methyl formate using a molecular Ru–phosphine complex and the metal–organic framework UiO-66 as heterogeneous acid. ACS. Appl. Energy. Mater. 2023, 6, 9153-8.
15. Sang, R.; Wei, Z.; Hu, Y.; et al. Methyl formate as a hydrogen energy carrier. Nat. Catal. 2023, 6, 543-50.
16. Scott, M.; Westhues, C. G.; Kaiser, T.; et al. Methylformate from CO2 : an integrated process combining catalytic hydrogenation and reactive distillation. Green. Chem. 2019, 21, 6307-17.
17. Kröcher, O.; Köppel, R. A.; Baiker, A. Highly active ruthenium complexes with bidentate phosphine ligands for the solvent-free catalytic synthesis of N,N-dimethylformamide and methyl formate. Chem. Commun. 1997, 453-4.
18. Darensbourg, D. J.; Ovalles, C.; Pala, M. Homogeneous catalysts for carbon dioxide/hydrogen activation. Alkyl formate production using anionic ruthenium carbonyl clusters as catalysts. J. Am. Chem. Soc. 1983, 105, 5937-9.
19. Ziebart, C.; Federsel, C.; Anbarasan, P.; et al. Well-defined iron catalyst for improved hydrogenation of carbon dioxide and bicarbonate. J. Am. Chem. Soc. 2012, 134, 20701-4.
20. Gowrisankar, S.; Federsel, C.; Neumann, H.; et al. Synthesis of stable phosphomide ligands and their use in Ru-catalyzed hydrogenations of bicarbonate and related substrates. ChemSusChem 2013, 6, 85-91.
21. Westhues, N.; Belleflamme, M.; Klankermayer, J. Base‐free hydrogenation of carbon dioxide to methyl formate with a molecular ruthenium‐phosphine catalyst. ChemCatChem 2019, 11, 5269-74.
22. Yu, K. M.; Yeung, C. M.; Tsang, S. C. Carbon dioxide fixation into chemicals (methyl formate) at high yields by surface coupling over a Pd/Cu/ZnO nanocatalyst. J. Am. Chem. Soc. 2007, 129, 6360-1.
23. Wu, C.; Zhang, Z.; Zhu, Q.; Han, H.; Yang, Y.; Han, B. Highly efficient hydrogenation of carbon dioxide to methyl formate over supported gold catalysts. Green. Chem. 2015, 17, 1467-72.
24. Ayodele, O. B.; Tasfy, S. F. H.; Zabidi, N. A. M.; Uemura, Y. Co-synthesis of methanol and methyl formate from CO2 hydrogenation over oxalate ligand functionalized ZSM-5 supported Cu/ZnO catalyst. J. CO2. Util. 2017, 17, 273-83.
25. Corral-Pérez, J. J.; Bansode, A.; Praveen, C. S.; et al. Decisive role of perimeter sites in silica-supported Ag nanoparticles in selective hydrogenation of CO2 to methyl formate in the presence of methanol. J. Am. Chem. Soc. 2018, 140, 13884-91.
26. Corral-Pérez, J. J.; Copéret, C.; Urakawa, A. Lewis acidic supports promote the selective hydrogenation of carbon dioxide to methyl formate in the presence of methanol over Ag catalysts. J. Catal. 2019, 380, 153-60.
27. Gastelu, G.; Savary, D.; Hulla, M.; Ortiz, D.; Uranga, J. G.; Dyson, P. J. Autocatalytic O-formylation of alcohols using CO2. ACS. Catal. 2023, 13, 2403-9.
28. Federsel, C.; Ziebart, C.; Jackstell, R.; Baumann, W.; Beller, M. Catalytic hydrogenation of carbon dioxide and bicarbonates with a well-defined cobalt dihydrogen complex. Chemistry 2012, 18, 72-5.
29. Kerry Yu, K. M.; Tsang, S. C. A study of methyl formate production from carbon dioxide hydrogenation in methanol over a copper zinc oxide catalyst. Catal. Lett. 2011, 141, 259-65.
30. Kramer, S.; Bennedsen, N. R.; Kegnæs, S. Porous organic polymers containing active metal centers as catalysts for synthetic organic chemistry. ACS. Catal. 2018, 8, 6961-82.
31. Song, K. S.; Fritz, P. W.; Coskun, A. Porous organic polymers for CO2 capture, separation and conversion. Chem. Soc. Rev. 2022, 51, 9831-52.
32. Wang, G.; Jiang, M.; Sun, Z.; et al. Synergistic effect between monophosphine species for regioselective hydroformylation of olefin with CO2. Chem. Eng. J. 2023, 476, 146332.
33. Corral‐Pérez, J. J.; Billings, A.; Stoian, D.; Urakawa, A. Continuous hydrogenation of carbon dioxide to formic acid and methyl formate by a molecular iridium complex stably heterogenized on a covalent triazine framework. ChemCatChem 2019, 11, 4725-30.
34. Sun, R.; Kann, A.; Hartmann, H.; Besmehn, A.; Hausoul, P. J. C.; Palkovits, R. Direct synthesis of methyl formate from CO2 with phosphine-based polymer-bound Ru catalysts. ChemSusChem 2019, 12, 3278-85.
35. Wang, G.; Jiang, M.; Ji, G.; et al. Bifunctional heterogeneous Ru/POP catalyst embedded with alkali for the N-formylation of amine and CO2. ACS. Sustainable. Chem. Eng. 2020, 8, 5576-83.
36. Shen, Y.; Zheng, Q.; Chen, Z. N.; et al. Highly efficient and selective N-formylation of amines with CO2 and H2 catalyzed by porous organometallic polymers. Angew. Chem. Int. Ed. Engl. 2021, 60, 4125-32.
37. Hlatshwayo, Z. T.; Doremus, J. G.; Mcgrier, P. L. Hydrosilylative reduction of CO2 to formate and methanol using a cobalt porphyrin‐based porous organic polymer. ChemCatChem 2022, 14, e202200783.
38. Zhang, Z.; Zhang, L.; Yao, S.; et al. Support-dependent rate-determining step of CO2 hydrogenation to formic acid on metal oxide supported Pd catalysts. J. Catal. 2019, 376, 57-67.
39. Ma, W.; Xiong, W.; Hu, J.; Geng, J.; Hu, X. Highly efficient catalysts for CO2 hydrogenation to formic acid in water catalyzed by hydrophobic porous polymers containing stable metal–hydride. Green. Chem. 2024, 26, 4192-8.
40. Wang, W.; Li, C.; Yan, L.; Wang, Y.; Jiang, M.; Ding, Y. Ionic liquid/Zn-PPh3 integrated porous organic polymers featuring multifunctional sites: highly active heterogeneous catalyst for cooperative conversion of CO2 to cyclic carbonates. ACS. Catal. 2016, 6, 6091-100.
41. Wang, W.; Wang, Y.; Li, C.; Yan, L.; Jiang, M.; Ding, Y. State-of-the-art multifunctional heterogeneous POP catalyst for cooperative transformation of CO2 to cyclic carbonates. ACS. Sustainable. Chem. Eng. 2017, 5, 4523-8.
42. Wu, Y.; Wang, T.; Wang, H.; Wang, X.; Dai, X.; Shi, F. Active catalyst construction for CO2 recycling via catalytic synthesis of N-doped carbon on supported Cu. Nat. Commun. 2019, 10, 2599.
43. Zhao, K.; Wang, H.; Wang, X.; et al. Confinement of atomically dispersed Rh catalysts within porous monophosphine polymers for regioselective hydroformylation of alkenes. J. Catal. 2021, 401, 321-30.
44. Lu, Y.; Wang, J.; Yu, L.; et al. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts. Nat. Catal. 2019, 2, 149-56.
45. Cheng, D.; Wang, M.; Tang, L.; et al. Catalytic synthesis of formamides by integrating CO2 capture and morpholine formylation on supported iridium catalyst. Angew. Chem. Int. Ed. Engl. 2022, 61, e202202654.
46. Wei, X.; Zhang, X.; Jiang, Y.; et al. Ionic liquid boosting N-formylation of amines on polyoxometalate-stabilizing iridium single-atom catalysts. J. Catal. 2024, 433, 115493.
47. Gunasekar, G. H.; Yoon, S. A phenanthroline-based porous organic polymer for the iridium-catalyzed hydrogenation of carbon dioxide to formate. J. Mater. Chem. A. 2019, 7, 14019-26.
48. Yadav, M.; Linehan, J. C.; Karkamkar, A. J.; van der Eide, E.; Heldebrant, D. J. Homogeneous hydrogenation of CO2 to methyl formate utilizing switchable ionic liquids. Inorg. Chem. 2014, 53, 9849-54.
49. Sá, A. G. A.; Meneses, A. C. D.; Araújo, P. H. H. D.; Oliveira, D. D. A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends. Food. Sci. Technol. 2017, 69, 95-105.
50. Lee, B.; Gong, E.; Kim, M.; et al. Electronic interaction between transition metal single-atoms and anatase TiO2 boosts CO2 photoreduction with H2O. Energy. Environ. Sci. 2022, 15, 601-9.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].