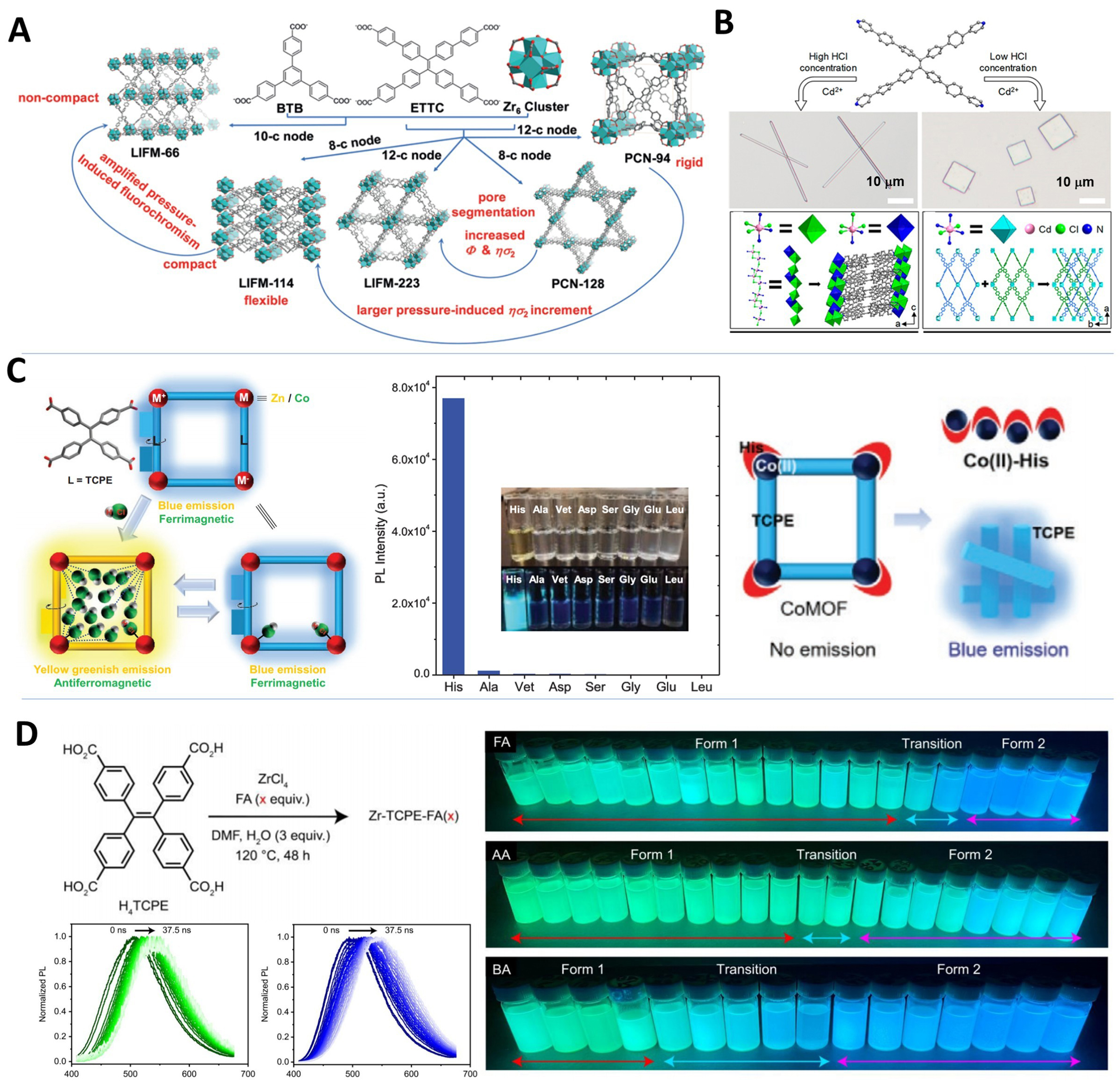
fig12
Figure 12. (A) Structural characteristics of the five Zr-MOFs (PCN-94, PCN-128, LIFM-66, LIFM-114, and LIFM-223)[85], Copyright 2019, Wiley-VCH; (B) Synthesis routes, microscopy images, and the structures of the FJU-96 microwires and FJU-97 microplates[151], Copyright 2020, American Chemical Society; (C) Schematic illustration of [M+–L–M-–L–M]∞ configured AIE MOFs for HCl vapor induced reversible luminescence and magnetic switch, and the fluorescence responsiveness of CoMOF towards different amino acids[50], Copyright 2021, Wiley-VCH; (D) Synthesis of Zr-TCPE-FA(x), monitoring spectral diffusion in Zr-TCPE-FA(50) and Zr-TCPE-FA(200), and images of Zr-TCPE samples with UV light on[56], Copyright 2023, American Chemical Society. MOFs: Metal–organic frameworks; PCN: porous coordination network; LIFM: Lehn Institute of Functional Materials; FJU: Fujian Normal University; AIE: aggregation-induced emission; HCl: hydrogen chloride; CoMOF: cobalt-based metal–organic framework; TCPE: tetrakis(4-carboxyphenyl)ethylene; FA: formic acid; UV: ultraviolet; BTB: benzene tribenzoate; ETTC: 4’,4’’’,4’’’’’,4’’’’’’’-(ethene-1,1,2,2-tetrayl)tetrakis(([1,1’-biphenyl]-4-carboxylic acid)); DMF: N,N-dimethylformamide; AA: acetic acid; BA: benzoic acid.